Fig. 2.
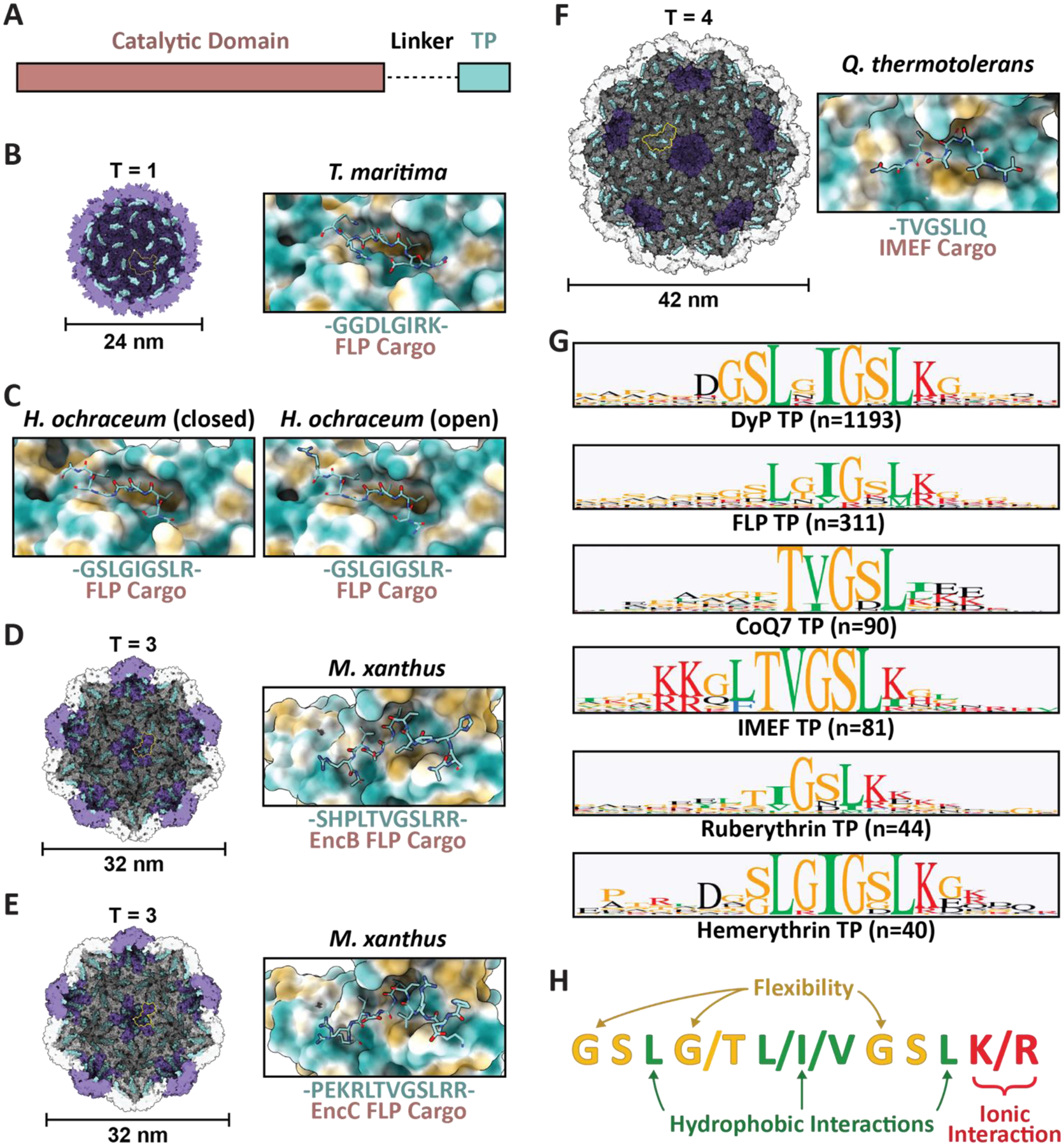
Family 1 cargo loading is mediated by specific TP-shell interactions. (A) Schematic representation of Family 1 cargo components, including the catalytic domain (pink), proline- and glycine-rich flexible linker (dash), and targeting peptide (turquoise). (B) Cutaway view of the T. maritima T1 encapsulin shell (PDB: 3DKT) with one shell protein subunit highlighted (yellow) and the encapsulin (purple) and GGDLGIRK TP of the FLP cargo (turquoise) shown in surface representation (left) and zoomed-in view of the conserved binding pocket (hydrophobic representation) with the resolved residues of the bound TP shown in stick representation (turquoise; right). (C) Zoomed-in view of the H. ochraceum T1 encapsulin TP-shell interaction (PDB: 7OE2) highlighting the binding pocket and the GSLGIGSLR TP sequence of the FLP cargo as found in the closed (left) and open (right) pentamer conformations of the shell. (D) Cutaway view of the M. xanthus T3 encapsulin shell (PDB: 7S2T) with one shell protein subunit highlighted (yellow) and the SHPLTVGSLRR TP (turquoise) of the EncB FLP cargo shown in surface representation (left). A zoomed-in view of the TP-shell interaction is shown on the right. (E) Similar overview of the M. xanthus T3 shell interaction with the PEKRLTVGSLRR TP of the EncC FLP cargo (PDB: 7S4Q). (F) Analogous overview of the Q. thermotolerans T4 shell interaction with the TVGSLIQ TP of the IMEF cargo (PDB: 6NJ8). (G) Consensus sequences for TPs from each of the major Family 1 cargo classes after alignment via Clustal Omega 1.2.3 with 20 residues centred on the consensus peak or, when limited by sequence length, using the last 20 C-terminal residues; visualized using GraphPad Prism v9.0.2; n, number of cargo sequences used. (H) Schematic of general binding mode for Family 1 TPs. Figures created using ChimeraX (Goddard et al., 2018). TP, targeting peptide; PDB, protein data bank; FLP, ferritin-like protein; IMEF, iron-mineralizing encapsulin-associated firmicute.
