Fig. 4.
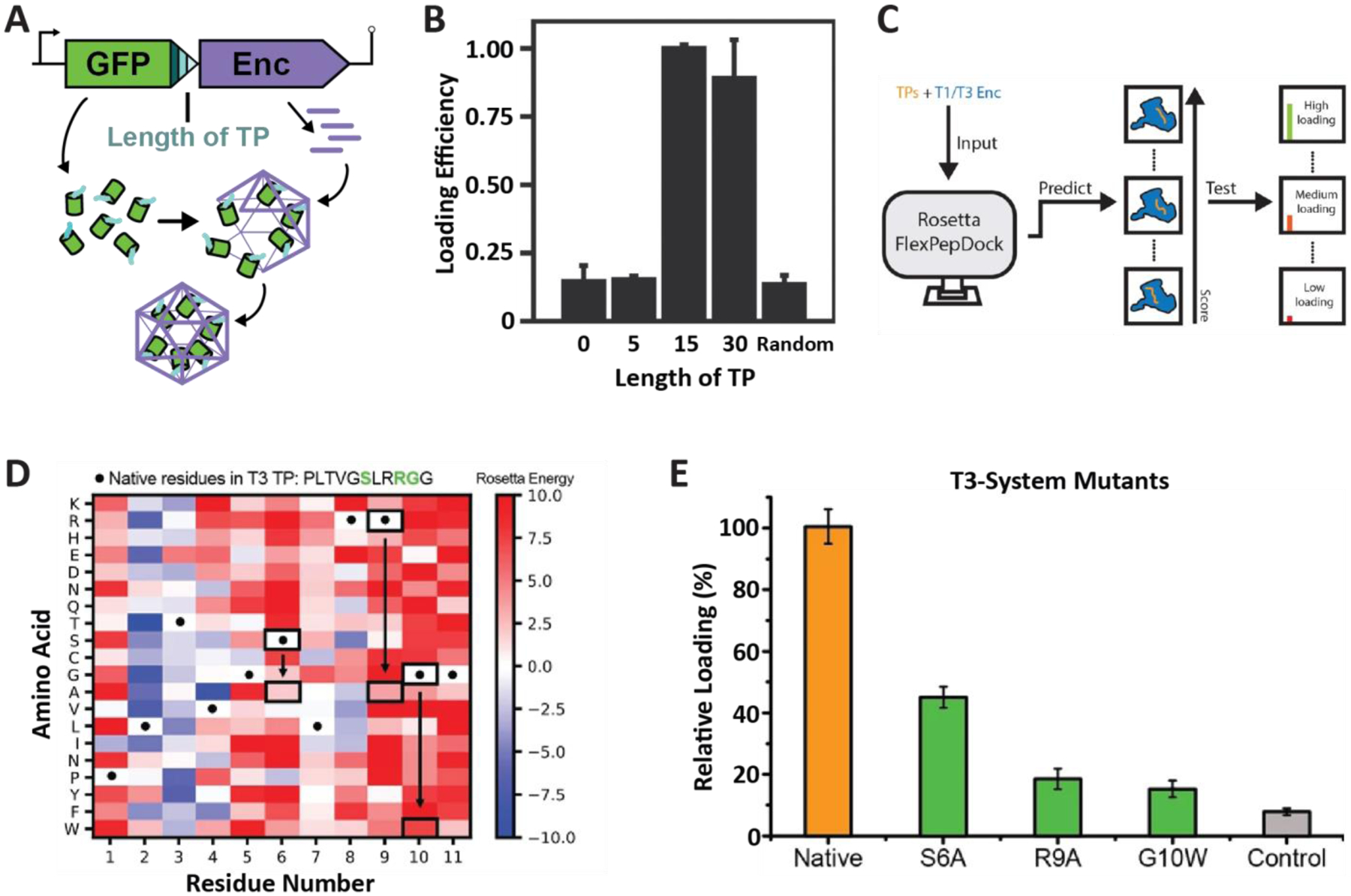
Characterization of T1 and T3 encapsulin targeting peptides. (A) Operon design of TP-fused sfGFP and the corresponding T. maritima encapsulin used for heterologous co-expression and downstream cargo loading analysis. Different TP truncations are highlighted. (B) Comparison of normalized sfGFP fluorescence in purified encapsulins to investigate the influence of TP truncation on cargo loading highlighting that the 15 C-terminal residues are sufficient for maximal cargo encapsulation. (A) and (B) adapted with permission from reference35. Copyright 2016 American Chemical Society (ACS). (C) Schematic of computational flexible docking and experimental workflow used to predict and analyze the relative strength of TP-shell binding in single residue TP mutants. (D) Heat map of computational point mutations with the color gradient representing the Rosetta Energy score (blue, improved binding; red, worse binding). (E) Experimental analysis of cargo loading for the three TP mutants highlighted in green in panel (D) highlighting that most single residue substitutions lead to decreased cargo encapsulation. (C)-(E) adapted with changes with open access permission from reference54 via a creative common license (https://creativecommons.org/licenses/by/4.0/). sfGFP, super folder green fluorescent protein.
