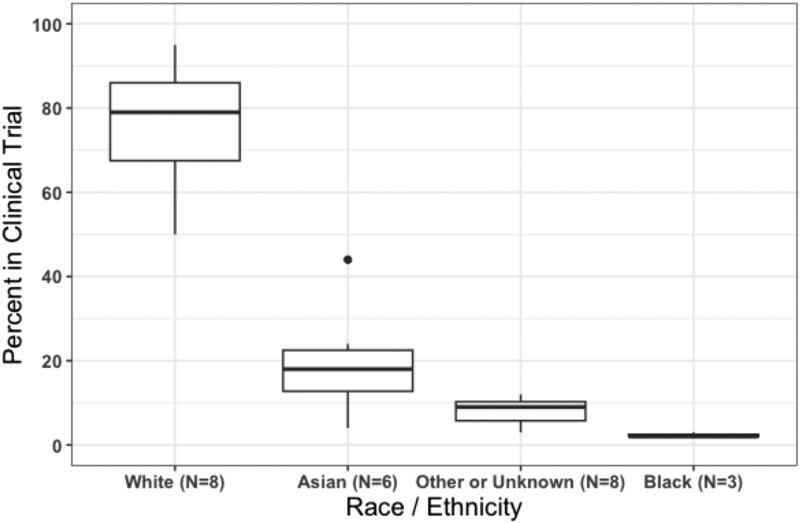
Figure 1.
Race/ethnicity specific percentage reported in clinical trials that led to the US Food and Drug Administration (FDA)-approval of 10 systemic therapies for the treatment of advanced thyroid cancers. Only 8 of the clinical trials included any data on race/ethnicity: the phase 3 DECISION trial (sorafenib), phase 3 SELECT trial (lenvatinib), phase 3 ZETA trial (vandetanib), phase 3 COSMIC-311 trial (cabozantinib), phase 3 EXAM trial (cabozantinib), phase 1/2 LIBRETTO-001 study (selpercatinib), phase 1/2 ARROW study (pralsetinib), and the phase 2 ROAR basket study (dabrafenib/trametinib) [20-27]. The clinical trials that led to FDA approval of Entrectinib and Larotrectinib did not include race/ethnicity data on the patients with thyroid cancer who enrolled in the studies [28-31]. The EXAM trial and ZETA trial reported race as a dichotomous variable (White vs non-White, and White vs other race, respectively) [20, 26]—we included the latter categories in “other or unknown” in this figure.