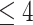Abstract
Background
Malnutrition in childhood has lasting consequences; its effects not only last a lifetime but are also passed down from generation to generation such as short stature, school-aged children are the most vulnerable section of the population and require special attention, including nutrition.
Method
We searched Medline through PubMed, Scopus, and Web of Science to identify all observational studies published before Jun 2022. Observational studies with a pediatric population aged 5–18 years that evaluated risk estimate with 95% confidence intervals the relationship between dietary diversity and undernutrition (wasting, stunting, and thinness) were included. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) were followed.
Results
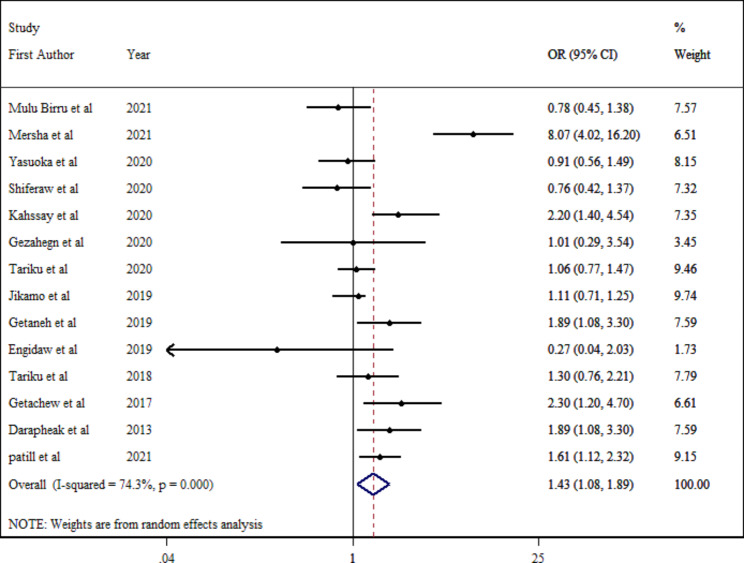
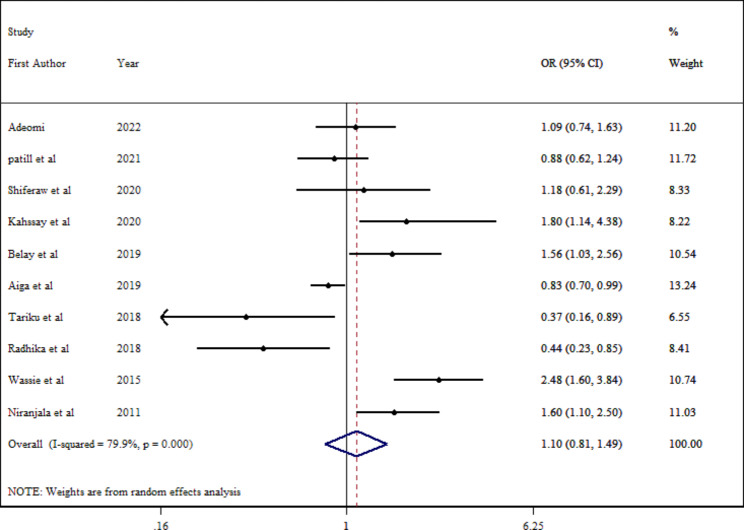
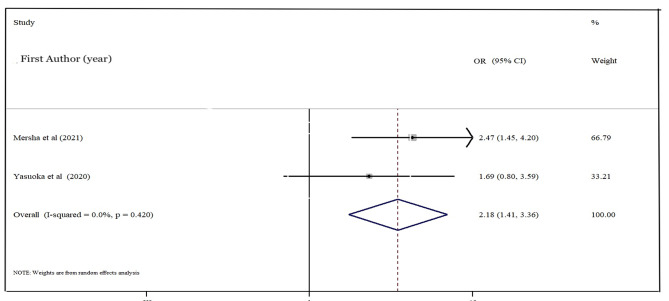
This is a first systematic review and meta-analysis with a total of 20 studies were eligible (n = 18 388). Fourteen data evaluated stunting resulting in a pooled effect size estimated odds ratio of 1.43 (95% CI: 1.08–1.89; p = 0.013). Ten data evaluated Thinness resulting in a pooled effect size estimated odds ratio of 1.10 (95% CI: 0.81–1.49; P = 0.542). Two studies were revealed wasting with a odds ratio of 2.18 (95% CI: 1.41–3.36; p-value < 0.001).
Conclusion
According to the conclusions of this meta-analysis of cross-sectional studies, inadequate dietary diversity increases the risk of undernutrition in growth linear but not in thinness in school-aged children. The findings of this analysis suggest that initiatives that support improvements to the diversity of children’s diets to reduce the risk of undernutrition may be warranted in LMICs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-023-04032-y.
Keywords: Dietary diversity score (DDS), Undernutrition, School-aged children, Stunting, Thinness
Introduction
Malnutrition is a term that refers to energy or nutritional deficiency, as well as excess or imbalanced nutrition intake. There are three distinct forms of malnutrition: (1) malnutrition due to micronutrient deficiency, (2) malnutrition due to micronutrient deficiency and obesity, (3) undernutrition classified as wasting (low weight-to-height ratio), stunting (low height-to-age ratio), and underweight (low weight-for-age) [1].
Malnutrition in childhood has lasting consequences; its effects not only last a lifetime but are also passed down from generation to generation, such as; high absenteeism, early dropout, unsatisfactory classroom performance, delayed cognitive development, short stature, reduced work capacity, and poor reproductive performance and health problem that are among the most common causes of low school enrolment [2–4]. School age is a time of significant physical, mental, and emotional development for children in this age group [5]. Their social engagement also expands beyond their immediate family, posing an additional risk due to less nutritional care and support. As with children under five, school-aged children are the most vulnerable section of the population and require special attention, including nutrition [1, 6]. Adolescent females are more susceptible than boys due to their specific physiological traits, such as menarche, and deep-rooted gender norms leading to intra-household disparities in food consumption [7].
Thinness has been embraced as a more accurate predictor of recent nutritional deprivation in older children, such as insufficient energy, protein, or micronutrients, than underweight [8]. According to the date data revealed in 2022, 21% of school-aged children were stunted, 12.5% were thin, and 24% were wasted [9]. Despite economic growth in emerging countries, malnutrition is widespread [10]. Children in low- and middle-income nations have a higher risk of malnutrition due to poverty and a lack of food [11]. Children’s physiology and the family and community’s effect on their behavior may all play a role in the child’s optimal growth and nutritional status [12].
A dietary variety score, a simple count of food types consumed by an individual over the preceding 24 h, is regarded as a proxy for an individual’s nutrient-adequacy diet [13]. Insufficient nutritional intake among school-aged children may have short-term and long-term detrimental effects on health, including postponed physical development and impaired cognitive development in adolescence [14], and an elevated risk of cardiometabolic disorders in adulthood [15].
Dietary variety is a crucial sign of a high-quality diet and is especially important for school-aged children whose bodies have high nutrient needs [16]. Numerous studies conducted in African countries have demonstrated that a lack of dietary diversity relates to malnutrition, whereas a diverse diet can enhance overall nutritional status [17–19]. However, some studies have not observed any association between dietary diversity and undernutrition [20–22].
Therefore, this systematic review and meta-analysis of published cross-sectional studies synthesized data about the connection between diet diversity and the risk of wasting, stunting, and thinness in school-aged children because to the best of our knowledge no study has explained this debate.
Method
This study complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [23]. Data were double-screened for full-text publications, and two reviewers worked separately from selection through data extraction (MZ and BZ). A third reviewer resolved any issues that arose (LA).
Search strategy
We carefully searched MEDLINE (through PubMed), Scopus, and Web of Science without regard for language restrictions until Jun 8th, 2022. Dietary diversity, DDS, Dietary diversity score, wasting, underweight, and stunting (Table S1) were used in the search approach. All relevant papers were identified by searching published reference lists, and the corresponding author was contacted via email to request access to the full study text. All publications were kept in an EndNote library (version 20 for Windows) to facilitate the referral process.
Inclusion and exclusion criteria
To set inclusion criteria, two independent reviewers examined all fields of research discovered by the search strategy, which were as follows: (1) children over the age of five, (2) observational design studies (cross-sectional, cohort, or case-control studies), and (3) reported hazard ratios (HR), relative risks (RR), or odds ratios (OR) with 95% confidence intervals (CI) to determine the relationship between dietary diversity and the risk of wasting, stunting, and thinness. These were the exclusion criteria: (1) children under the age of 5, (2) maternal or paternal dietary variety, (3) animal studies, (4) not in English, (5) review articles, opinions, editorials, or letters, as well as unpublished studies or abstracts, (6) Intervention studies, (7) populations with acute diseases such as cancer, cystic fibrosis, and others, and (8) statistical analyses revealed correlation coefficients rather than estimated risk.
Study selection
The screening process for studies was conducted independently by two reviewers, as previously reported. Two reviewers first did a title-abs screening, and then the remaining titles likely to be included were reviewed in full-text so that no relevant work was overlooked. Two reviewers independently did full text screening on every potentially relevant paper discovered.
Data extraction
In addition to the impact size estimations, the following factors were gathered: (1) Child age range (years), (2) child gender, (3) dietary measurement, (4) the number of participants, (5) research publication year, (6) design of the study (cross-sectional or longitudinal), (7) quality of studies, (8) country of origin, and (9) any modifications to their study.
If two reports utilized the same data set, we chose the one with the greatest sample size and the statistical analysis. We utilized the adjusted measure if both unadjusted and adjusted effect sizes were available.
Quality assessment
Two reviewers assessed the relevant cross-sectional studies using the updated Newcastle–Ottawa quality assessment scale (Table S2).
Quality of studies was assessed as follows: poor (3), reasonable (4–6), and strong (7). The revised Newcastle–Ottawa quality rating scale for cross-sectional research awarded a maximum score of 10 to cross-sectional studies. This is a representative tool used for observational studies to evaluate studies in three sections: study selection, comparability, and outcome. The overall score was measured by summarizing each score.
Grading the evidence
We evaluated the overall degree of confidence in the evidence for each relationship using the revised Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) method. GRADE evaluations were carried out separately by the authors (MZ and BZ).
Data analysis
Risk estimates for undernutrition were calculated in two ways: using the dietary diversity score (DDS; greatest score vs. lowest score) or using the minimal diet diversity (MDD). The relevant SEs of ORs 24 were then determined using the method SE log (RR) = [SElog (OR) log (RR)]/log (OR). The natural logarithms of the ORs, as well as their SEs and 95% CIs, were calculated.
To examine homogeneity, the I2 Index, Cochran’s Q statistic, and the associated P-value for heterogeneity were utilized. To detect publication bias, visual inspection of funnel plots and Egger’s tests for funnel plot asymmetry were performed. We were able to investigate consistency in the pooled data by removing one trial at a time and re-estimating the pooled OR. All statistical analyses were performed using STATA 11.0 software (STATA Corp.). Statistical significance was defined as P values less than 0.05 [24].
Results
The flow diagram depicts the search strategy and results (Fig. 1). During the first search, 5478 studies were discovered. A full-text review of 143 articles was conducted. The final analysis comprised 20 studies ranging in age from 5 to 18 years.
Fig. 1.
PRISMA diagram of selection process
Systematic review
The review examined the following data released between 2011 and 2022: 2011 (n = 1), 2013 (n = 1), 2015 (n = 1), 2017 (n = 1), 2018 (n = 2), 2019 (n = 4), 2020 (n = 5), 2021 (n = 3), 2022 (n = 1). The features of the included studies are shown in Table 1. Among the studies included, six were done on girls, while the remaining were conducted on both sexes. Except for seven studies performed in other countries (Madagascar (n = 1) [25], India (n = 2) [26, 27], Sri Lanka (n = 2) [28, 29], Cambodia (n = 1), and Nigeria (n = 1)), the rest were conducted in Ethiopia. Except for two studies that used 7-day recall [20] and 30 days-FFQ [30], and one study did not reveal the dietary assessment index [31], the reminder utilized 24-hour recall for dietary assessment. With only three studies with HIV-positive children [21, 31, 32], all the studies investigated healthy children’s populations. Stunting, wasting, and thinness was identified using a height-for-age z-score (HAZ) of -2 SD, a weight-for-length z-score (WLZ) of -2 SD, and a weight-for-age z-score (WAZ) of -2 SD SDs below the WHO population median, respectively.
Table 1.
Summary Characteristics of Included Studies
| Author, Year | Country | Age | Sex | Health status | Sample size |
Diet index assessment | Comparison | No. of variables in adjustments | Outcome | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
|
Adeomi et al. 2022 [30] |
Nigeria | 6–19 years old | G/B | Healthy | 1200 | 30 days-FFQ |
DDS < 4 food groups VS. DDS ≥ 4 food groups |
5 | Thinness | 10 |
|
Mulu Birru et al. 2021 [41] |
Ethiopia | 14–15 years old | G/B | Healthy | 364 | 24-h recall |
DDS ≥ 5 food groups vs. DDS < 5 food groups |
26 | Stunting | 10 |
|
Mersha et al. 2021 [17] |
Ethiopia | 10–19 years old | G | Healthy | 706 | 24-h recall |
DDS ≥ 5 food groups vs. DDS < 5 food groups |
12 | Stunting/ Wasting | 10 |
|
Patil et al. 2021 [27] |
India | 16–18 years old | G | Healthy | 586 | 24-h recall |
DDS < 3 food groups vs. DDS ≥ 3 food groups |
NOT Adjusted | Stunting/ Thinness | 7 |
|
Yasuoka et al. 2020 [21] |
Ethiopia | 6–15 years old | G/B | HIV | 298 | 24-h recall |
DDS 5–7 groups vs. DDS 0–4 groups |
10 | Stunting/ Wasting | 8 |
|
Shiferaw et al. 2020 [32] |
Ethiopia | 10–19 years old | G/B | HIV | 260 | 24-h recall |
DDS < 6 food groups vs. DDS ≥ 6 food groups |
NOT Adjusted | Stunting/ Thinness | 7 |
|
Kahssay et al. 2020 [18] |
Ethiopia | 10–19 years old | G | Healthy | 340 | 24-h recall |
DDS < 4 food groups vs. DDS ≥ 4 food groups |
10 | Stunting/ Thinness | 10 |
|
Gezahegn et al. 2020 [31] |
Ethiopia | 5–15 years old | G/B | HIV | 405 | Not reported |
DDS < 4 food groups vs. DDS ≥ 4 food groups |
5 | Stunting | 10 |
|
Tariku et al. 2019 [55] |
Ethiopia | 10–19 years old | G | Healthy | 1550 | 24-h recall |
DDS < 4 food groups vs. DDS ≥ 4 food groups |
NOT Reported | Stunting | 7 |
|
Jikamo et al. 2019 [20] |
Ethiopia | 13–17 years old | G/B | Healthy | 2084 | 7days-recall |
DDS DDS > 9–12 food groups |
S: 6 T:7 |
Stunting | 10 |
|
Getaneh et al. [19] 2019 |
Ethiopia | 6–14 years old | G/B | Healthy | 523 | 24-h recall |
DDS ≥ 7 food groups vs. DDS < 4 food groups |
4 | Stunting | 10 |
| Engidaw et al. 2019 [56] | Ethiopia | 10–19 | G | Healthy | 423 | 24-h recall |
DDS DDS > 6food groups |
4 | Stunting | 8 |
|
Belay et al. 2019 [48] |
Ethiopia | 5–14 years old | G/B | Healthy | 848 | 24-h recall |
DDS < 4 food groups vs. DDS ≥ 4 food groups |
4 | Thinness | 8 |
|
Aiga et al. 2019 [25] |
Madagascar | 5–14 years old | G/B | Healthy | 205 | 24-h recall |
DDS DDS > 9–12 food groups |
16 | Thinness | 10 |
|
Tariku et al. 2018 [57] |
Ethiopia | 6–14 years old | G/B | Healthy | 389 | 24-h recall |
DDS < 4 food groups vs. DDS ≥ 4 food groups |
4 | Stunting/ Thinness | 10 |
|
Radhika et al. 2018 [26] |
India | 10–19 years old | G | Healthy | 3930 | 24-h recall |
DDS > 5 food groups vs. DDS ≤ 5 food groups |
NOT Reported | Thinness | 8 |
|
Getachew et al. 2017 [58] |
Ethiopia | 7–14 years old | G/B | Healthy | 511 | 24-h recall |
DDS < 4 food groups vs. DDS ≥ 4 food groups |
4 | Stunting | 10 |
|
Wassie et al. 2015 [28] |
Sri Lanka | 10–19 years old | G | Healthy | 1281 | 24-h recall |
DDS < 3 food groups vs. DDS > 6 food groups |
4 | Thinness | 10 |
|
Darapheak et al. 2013 [59] |
Cambodia | 6–14 years old | G/B | Healthy | 523 | 24-h recall |
DDS ≥ 7 food groups vs. DDS < 4 food groups |
8 | Stunting | 9 |
|
Niranjala et al. 2011 [29] |
Sri Lanka | 13–16 years old | G/B | Healthy | 205 | 24-h recall |
DDS > 5 food groups vs. DDS ≤ 5 food groups |
10 | Thinness | 9 |
MDD: Minimum Dietary Diversity, DDS: Dietary Diversity Score, S: Stunting, W: Wasting, B: boy, G: girl
Fourteen effect sizes reported stunting, ten effect sizes reported thinness, and only two studies revealed wasting.
Quality assessment
According to our findings, all the included studies received a high-quality score (Table S2). As previously described, we used the NOS scale to assess the quality of studies and sum up the total score above 7.
Meta-analyses
Dietary diversity and stunting
Fourteen effect sizes were included in the final analysis with 8539 individuals. Participants with inadequate dietary diversity had a 43% higher (pooled OR: 1.43; 95% CI: 1.08–1.89; p = 0.013) estimated odds of stunting compared to those with adequate diet diversity (Fig. 2). Due to considerable between-study heterogeneity (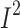 = 74.3%; PQ−test <0.001), subgroup analysis was conducted based on different parameters (Table 2). According to the stratified analysis by location, the pooled estimates for the association between dietary diversity and stunting remained significant in studies conducted in the Asian continent (OR:1.69; 95% CI: 1.24–2.29; p-value < 0.001), studies with a more than 500 population (OR:1.86; 95% CI: 1.25–2.78; p-value = 0.002), and studies were conducted on healthy individuals (OR:1.61; 95% CI: 1.17–2.21; p-value = 0.004). In addition, subgroup analysis indicated that heterogeneity was removed in studies with less equal than 500 individuals (
= 74.3%; PQ−test <0.001), subgroup analysis was conducted based on different parameters (Table 2). According to the stratified analysis by location, the pooled estimates for the association between dietary diversity and stunting remained significant in studies conducted in the Asian continent (OR:1.69; 95% CI: 1.24–2.29; p-value < 0.001), studies with a more than 500 population (OR:1.86; 95% CI: 1.25–2.78; p-value = 0.002), and studies were conducted on healthy individuals (OR:1.61; 95% CI: 1.17–2.21; p-value = 0.004). In addition, subgroup analysis indicated that heterogeneity was removed in studies with less equal than 500 individuals (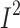 = 45.9%; PQ−test =0.086), and studies were conducted in both groups of sex (
= 45.9%; PQ−test =0.086), and studies were conducted in both groups of sex (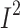 = 46.0%; PQ−test =0.063).
= 46.0%; PQ−test =0.063).
Fig. 2.
Estimated odds of stunting among school-aged children
Table 2.
Stratified analysis of the association between dietary diversity and undernutrition
| Number of studies | Odds Ratio (95% CI) | P value | P-heterogeneity | I2 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stunting | Thinness | Stunting | Thinness | Stunting | Thinness | Stunting | Thinness | Stunting | Thinness | |
| Overall | 14 | 10 | 1.43 (1.08–1.89) | 1.10 (0.81–1.49) | < 0.001 | 0.542 | < 0.001 | < 0.001 | 74.3% | 79.9% |
| Sample size | ||||||||||
| ≤ 500 | 7 | 5 | 1.04 (0.73–1.47) | 1.05 (0.68–1.64 | 0.834 | 0.821 | 0.086 | 0.002 | 45.9% | 76.5% |
| > 500 | 7 | 5 | 1.86 (1.25–2.78) | 1.13 (0.70–1.84) | 0.002 | 0.611 | < 0.001 | 0.001 | 82.4% | 83.4% |
| Continent | ||||||||||
| Africa | 12 | 7 | 1.37 (0.98–1.92) | 1.20 (0.81–1.77) | 0.061 | 0.365 | < 0.001 | < 0.001 | 76.8% | 81.8% |
| Asia | 2 | 3 | 1.69 (1.24–2.29) | 0.89 (0.81–1.49) | < 0.001 | 0.718 | 0.637 | 0.003 | 0.0% | 82.8% |
| Sex | ||||||||||
| Girl | 9 | 4 | 1.87 (0.94–3.71) | 1.16 (0.57–2.36) | 0.117 | 0.684 | < 0.001 | < 0.001 | 87.2% | 87.5% |
| Both | 5 | 6 | 1.22 (0.95–1.56) | 1.06 (0.76–1.48) | 0.075 | 0.714 | 0.063 | 0.002 | 46.0% | 73.0% |
| Health status | ||||||||||
| Healthy | 11 | 9 | 1.61 (1.17–2.21) | 1.09 (0.79–1.51) | 0.004 | 0.604 | < 0.001 | < 0.001 | 77.1% | 82.0% |
| HIV | 3 | 1 | 0.86 (0.60–1.23) | 1.18 (0.61–2.29) | 0.407 | 0.624 | 0.868 | - | 0.0% | - |
We visually inspected the funnel plot for asymmetry for stunting and dietary diversity. The funnel plot (Figure S1) appeared significant for publication bias (Egger test intercept; p = 0.001).
Dietary diversity and thinness
Ten effect sizes were included in the final analysis with 9434 individuals that failed to establish a statistically significant association (pooled OR = 1.10; 95% CI: 0.81–1.49; P = 0.542) (Fig. 3). There was considerable heterogeneity among studies (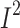 = 79.9%; PQ−test <0.001). Due to considerable between-study heterogeneity, Subgroup analysis was conducted based on different parameters (Table 2). According to the stratified analysis, the pooled estimates for the association between dietary diversity and stunting thinness remained insignificant in all groups. Moreover, sample size, continent, health status, and sex subgroups were identified as a source of heterogeneity. We were able to estimate the funnel plot’s asymmetry based on a visual examination of stunting thinness and dietary diversity. The funnel plot (Figure S2) was not significant for publication bias (Egger test intercept; p = 0.121).
= 79.9%; PQ−test <0.001). Due to considerable between-study heterogeneity, Subgroup analysis was conducted based on different parameters (Table 2). According to the stratified analysis, the pooled estimates for the association between dietary diversity and stunting thinness remained insignificant in all groups. Moreover, sample size, continent, health status, and sex subgroups were identified as a source of heterogeneity. We were able to estimate the funnel plot’s asymmetry based on a visual examination of stunting thinness and dietary diversity. The funnel plot (Figure S2) was not significant for publication bias (Egger test intercept; p = 0.121).
Fig. 3.
Estimated odds of thinness among school-aged children
Dietary diversity and wasting
Two studies were included in the final analysis with 1004 individuals. According to the result, the participant with the inadequate dietary diversity had two times higher (pooled OR: 2.18; 95% CI: 1.41–3.36; p-value < 0.001) (Fig. 4).
Fig. 4.
Estimated odds of wasting among school-aged children
Sensitivity analysis
Sensitivity analyses were performed for evaluated effect sizes (i.e., ORs for stunting and thinness) by removing data from the meta-analytic model. There was no sensitivity among all studies (Figure S3, S4).
Certainty of evidence
GRADE was performed for evaluated the certainty of studies. The result of GRADE showed that the certainty of both outcome stunting and thinness is very low while wasting is moderate (Table S3).
Discussion
Concerning survey data, our meta-analysis and systematic evaluation of cross-sectional research comprised 18,388 participants from 20 studies. According to the findings of this study, school-aged children with a limited diet are more likely to have a higher estimated pooled risk of stunted growth and wasting. However, there is no association between a limited diet and thinness.
Dietary Diversity (DD) is the variety of foods or food categories ingested throughout a certain time period [33]. Diverse foods are the greatest way to guarantee dietary sufficiency and are an excellent source of macro and micronutrients [33]. Dietary variables are linked to an increased risk of chronic illnesses and malnutrition, and both international and national recommendations call for increasing dietary variety [33]. Dietary diversity is vital for satisfying energy and other basic nutritional needs, especially for individuals at risk of nutrient deficiencies, such as children from low-income families. Inadequate dietary variety and higher nutritional needs have been linked to an increased risk of poor development trajectory in children, which becomes more obvious as they grow [34–36].
School-aged is a period of great change [37]. Depending on socioeconomic factors, the transformation could take several years [37–39]. Even within a particular culture, adolescents are not a uniform population; their growth, maturity, and lifestyles vary widely [37–39].
A lack of variety in one’s diet has been linked to an increased risk of short height in adolescents. This might be due to a cereal-based monotonous diet with inadequate quality, amount, and frequency of feeding that does not meet micronutrient needs for child development, such as iron, vitamin B12, folate, and other necessities [40]. In addition, it may be due to low nutrient intake during infancy and childhood that substantially influences the linear growth of the prescribed height as the relevant age [22]. Chronic and cumulative food shortages, lack of essential medication and fuel, infrastructure damage from conflicts, and poor economic conditions may pose significant problems to the nutritional health of teenage in later life [22].
Due to our analysis, dietary diversity has not been linked to thinness which is different from another study that discovered low dietary diversity, three times more likely to become thin [41]. This might be because of insufficient data on the thinness we have had. Studies have shown that nutritional deficiencies due to loss of menstruation, irregular eating habits, and food preferences are common causes of underweight girls in transition [42, 43]. This might be due to changes in eating habits due to menstrual signs and symptoms, peer influence on food preferences, and the presence of monotonous family nutrition due to common rations may contribute to reduced weight gain, and directly affect BMI in adolescent girls [22].
Due to rapid development and increasing nutritional needs throughout adolescence, a low frequency of meals will cause them to become underweight or thin [41]. School-aged children’s thinness is strongly linked to food insecurity at home [44, 45]. Household food insecurity may be linked to a lack of food variety, translating to an insufficient supply of all necessary nutrients [46]. This is consistent with cross-sectional research on 2016 children in Bangladesh [41] and 656 homes with young women in Kenya [42]. Dietary diversity can be used to diagnose food insecurity, according to the FANTA experiment [47]. However, our study confirmed the link between food variety and stunting; a survey in Jimma is in a different line from our research [20].
However, our stratified analysis failed to show a significant association between sex and stunting and thinness, other studies indicated that stunting is significantly higher in female adolescents [20, 48, 49]. This might be due to variation in maturation time in boys and girls, for which girls reached maturation earlier than boys [49]. Adolescence is a period of significant physiological, sexual, neurological, and behavioral changes, and it provides the groundwork for assuming adult tasks and responsibilities, including the move to employment and financial independence, as well as the establishment of lifelong relationships [50].
As this duration is a phase of fast growth, proper nutrition is essential to reach full growth potential, and failure to acquire appropriate food may result in delayed and stunted linear growth and poor organ remodeling [51]. The duration and length of body composition changes are intimately related to sexual development; hence, dietary requirements rely more on sexual maturity than on chronological age [52].
Our stratified analysis showed a significant relationship between stunting and studied conducted in Asia. This might be because more low- and middle-income countries (LMICs) are from the Asian continent, and various research from LMICs have shown a lack of nutritional variety and inadequacy [53, 54]. Since that short stature and HAZ are indicators of a child’s long-term nutritional status, children living in these countries with persistent micronutrient deficiencies were more likely to be stunted.
The current studies have many strengths. To the best of our knowledge, this is the first systematic review and meta-analysis to evaluate the risk estimations for the relationship between dietary variety and undernutrition in school-aged children, and both groups of sex were included. The meta-analysis had a reasonable sample size, indicating that it improved the power of the meta-analysis to detect meaningful conclusions and precise estimates. Finally, all studies included in this review were deemed high-quality based on the assessment tool.
However, limitations should be considered when evaluating the findings of this meta-analysis. We used dietary diversity as a proxy for the overall nutritional quality of the child’s diet. Since this study did not account for the number of foods consumed, it is impossible to determine with certainty if the foods consumed corresponded to the recommended dietary intake. Considerable heterogeneity was found in the primary analysis. However, a subgroup-stratified analysis was performed to evaluate potential causes for heterogeneity. Most studies used a 24-hour dietary recall assessment, which is subject to erroneous categorization and reporting bias and may not capture all relevant dietary data. Finally, all studies were cross-sectional, meaning causality cannot be established, and there remains a limited understanding of the longitudinal relationship between dietary diversity and undernutrition.
Conclusion
According to the conclusions of this meta-analysis of cross-sectional studies, inadequate dietary diversity increases the risk of undernutrition in growth linear and wasting but not in thinness in school-aged children. The findings of this analysis suggest that initiatives that support improvements to the diversity of children’s diets to reduce the risk of undernutrition may be warranted in LMICs. However, future research, specifically longitudinal cohorts, and clinical trials, with age-classification are needed to confirm these findings and determine the mechanisms responsible for the association between dietary diversity and undernutrition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Leila Azadbakht, she is the recipient of a grant from Tehran University of Medical Sciences for this study.
Author contributions
Mobina Zeinalabedin is the first author who designed the study and independently did the literature search, data screening, data extraction, and quality evaluation. Additionally, she wrote the manuscript. Behzad Zamani designed the study and independently did the literature search, data screening, data extraction, and quality evaluation and performed the statistical analysis. Additionally, he wrote the manuscript. Dr. Ensieh Nasli Esfahani provided feedback on the manuscript and revising it critically for important intellectual content. Dr. Leila Azadbakht supervised the study and designed the study and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This study is supported by Tehran University of Medical Sciences (grant number: 1400-2-212-54588).
Data Availability
The [supplementary Material] data used to support the findings of this study are included within the article.
Declarations
Ethics approval and consent to participate
Given that this was a meta-analysis, no ethical approval was necessary.
Consent for publication
Not applicable.
Competing interests
The authors declared no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Available from: https://www.who.int/news-room/fact-sheets/detail/malnutrition.
- 2.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7(Suppl 3):5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 Lancet, 2016. 388(10053): p. 1659–1724. [DOI] [PMC free article] [PubMed]
- 4.Black RE, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava A, et al. Nutritional status of school-age children - a scenario of urban slums in India. Arch Public Health. 2012;70(1):8. doi: 10.1186/0778-7367-70-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Federal Democratic Republic of Ethiopia, National School Health and Nutrition Strategy. Ministry of Education. 2012; Available from: https://planipolis.iiep.unesco.org/sites/default/files/ressources/ethiopia_national_school_health_nutrition_strategy.pdf.
- 7.Chaparro, C., Oot, L. and Sethuraman, K. Nutrition Profile Nutrition Profile. 2014; Available from: https://www.fantaproject.org/sites/default/files/download/Laos-Nutrition-Profile-Apr2014.pdf.
- 8.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser, 1995. 854: p. 1–452. [PubMed]
- 9.Khan, D.S.A., et al., Nutritional Status and Dietary Intake of School-Age Children and early adolescents: systematic review in a developing Country and Lessons for the global perspective. Frontiers in Nutrition, 2022. 8. [DOI] [PMC free article] [PubMed]
- 10.Müller O, Krawinkel M. Malnutrition and health in developing countries. Cmaj. 2005;173(3):279–86. doi: 10.1503/cmaj.050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) ‘Obesity and overweight. 2011; Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 12.Liu, J. and A. Raine, Nutritional status and social behavior in preschool children: the mediating effects of neurocognitive functioning. Matern Child Nutr, 2017. 13(2). [DOI] [PMC free article] [PubMed]
- 13.Yvette Fautsch Macías, P.G., Guidelines for assessing nutrition-related knowledge, attitudes and practices. 2014.
- 14.Patton GC, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet. 2016;387(10036):2423–78. doi: 10.1016/S0140-6736(16)00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Victora CG, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verger EO, et al. Dietary diversity indicators and their Associations with Dietary Adequacy and Health Outcomes: a systematic scoping review. Adv Nutr. 2021;12(5):1659–1672. doi: 10.1093/advances/nmab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mersha, J., A. Tariku, and K.A. Gonete, Undernutrition and Associated factors among School adolescent girls attending schools in Mirab-Armachiho District, Northwest Ethiopia. Ecology of Food and Nutrition, 2021. [DOI] [PubMed]
- 18.Kahssay, M., L. Mohamed, and A. Gebre, Nutritional Status of School Going Adolescent Girls in Awash Town, Afar Region, Ethiopia Journal of Environmental and Public Health, 2020. 2020. [DOI] [PMC free article] [PubMed]
- 19.Getaneh, Z., et al., Prevalence and determinants of stunting and wasting among public primary school children in Gondar town, northwest, Ethiopia. BMC Pediatrics, 2019. 19(1). [DOI] [PMC free article] [PubMed]
- 20.Jikamo, B. and M. Samuel, Does dietary diversity predict the nutritional status of adolescents in Jimma Zone, Southwest Ethiopia? BMC Research Notes, 2019. 12(1). [DOI] [PMC free article] [PubMed]
- 21.Yasuoka, J., et al., Nutritional status and dietary diversity of school-age children living with HIV: a cross-sectional study in Phnom Penh, Cambodia. BMC Public Health, 2020. 20(1). [DOI] [PMC free article] [PubMed]
- 22.Engidaw, M.T. and A.D. Gebremariam, Prevalence and associated factors of stunting and thinness among adolescent somalian refugee girls living in eastern somali refugee camps, somali regional state, Southeast Ethiopia. Conflict and Health, 2019. 13(1). [DOI] [PMC free article] [PubMed]
- 23.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boston R, Sumner A. STATA: a statistical analysis system for examining Biomedical Data. Advances in experimental medicine and biology. 2003;537:353–69. doi: 10.1007/978-1-4419-9019-8_23. [DOI] [PubMed] [Google Scholar]
- 25.Aiga, H., et al., Risk factors for malnutrition among school-aged children: a cross-sectional study in rural Madagascar. BMC Public Health, 2019. 19(1). [DOI] [PMC free article] [PubMed]
- 26.Radhika, M.S., et al., Dietary and nondietary determinants of nutritional status among adolescent girls and adult women in India, in Annals of the New York Academy of Sciences. 2018. p. 5–17.
- 27.Patil, S., et al., Stunting is A Reflection of Poor Dietary Diversity Among Adolescent Girls in Rural KONKAN Region (DERVAN-6) 2021.
- 28.Wassie, M.M., et al., Predictors of nutritional status of Ethiopian adolescent girls: a community based cross sectional study. BMC Nutrition, 2015. 1(1).
- 29.Niranjala AMS, Gunawardena NS, Infant Nutritional status of adolescent females in estates in Haliela, Sri Lanka. Child, & Adolescent Nutrition. 2011;ICAN(5):260–267. doi: 10.1177/1941406411420368. [DOI] [Google Scholar]
- 30.Adeomi, A.A., A. Fatusi, and K. Klipstein-Grobusch, Food Security, Dietary Diversity, dietary patterns and the double burden of malnutrition among school-aged children and adolescents in two nigerian States. Nutrients, 2022. 14(4). [DOI] [PMC free article] [PubMed]
- 31.Gezahegn D, et al. Predictors of stunting among pediatric children living with HIV/AIDS, Eastern Ethiopia. International Journal of Public Health Science. 2020;9(2):82–89. [Google Scholar]
- 32.Shiferaw H, Gebremedhin S. Undernutrition among HIV-Positive adolescents on antiretroviral therapy in Southern Ethiopia. Adolesc Health Med Ther. 2020;11:101–111. doi: 10.2147/AHMT.S264311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy, G., T. Ballard, and M.C. Dop, Guidelines for measuring household and individual dietary diversity. 2011: Food and Agriculture Organization of the United Nations.
- 34.Wondimagegne Z, et al. Child feeding practice and primary Health Care as Major Correlates of Stunting and Underweight among 6- to 23-Month-Old Infants and Young Children in Food-Insecure Households in Ethiopia. Current developments in nutrition. 2020;4:nzaa137. doi: 10.1093/cdn/nzaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam AHMS, et al. Farm diversification and food and nutrition security in Bangladesh: empirical evidence from nationally representative household panel data. Food Security. 2018;10(3):701–720. doi: 10.1007/s12571-018-0806-3. [DOI] [Google Scholar]
- 36.Msc B, et al. Significance of wild vegetables in micronutrient intakes of women in Vietnam: an analysis of food variety. Asia Pacific Journal of Clinical Nutrition. 2001;10:21–30. doi: 10.1046/j.1440-6047.2001.00206.x. [DOI] [PubMed] [Google Scholar]
- 37.Jaworska N, MacQueen G. Adolescence as a unique developmental period. J Psychiatry Neurosci. 2015;40(5):291–3. doi: 10.1503/jpn.150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentine G. Boundary crossings: transitions from Childhood to Adulthood. Children’s Geographies. 2003;1:37–52. doi: 10.1080/14733280302186. [DOI] [Google Scholar]
- 39.Akseer, N., et al., Global and regional trends in the nutritional status of young people: a critical and neglected age group, in Annals of the New York Academy of Sciences. 2017. p. 3–20. [DOI] [PubMed]
- 40.Eicher-Miller HA, et al. Food insecurity is associated with iron deficiency anemia in US adolescents. Am J Clin Nutr. 2009;90(5):1358–71. doi: 10.3945/ajcn.2009.27886. [DOI] [PubMed] [Google Scholar]
- 41.Mulu Birru, G., et al., Malnutrition in School-Going Adolescents in Dessie Town, South Wollo, Ethiopia Journal of Nutrition and Metabolism, 2021. 2021. [DOI] [PMC free article] [PubMed]
- 42.Anwar, S., P. Deshmukh, and B. Garg, Epidemiological Correlates of Nutritional Anemia in adolescent girls of rural Wardha. Indian Journal of Community Medicine, 2006. 31.
- 43.Kishore, J., National Health Programs of India. In: Century publications: New Delhi. 2006. p. p. 82–4.
- 44.Motbainor, A., A. Worku, and A. Kumie, Stunting is associated with food diversity while wasting with food insecurity among underfive children in East and West Gojjam Zones of Amhara Region, Ethiopia. PLoS ONE, 2015. 10(8). [DOI] [PMC free article] [PubMed]
- 45.Rose, E.S., et al., Determinants of undernutrition among children aged 6 to 59 months in rural Zambézia Province, Mozambique: results of two population-based serial cross-sectional surveys. BMC Nutrition, 2015. 1(1). [DOI] [PMC free article] [PubMed]
- 46.Ali D, et al. Household food insecurity is associated with higher child undernutrition in Bangladesh, Ethiopia, and Vietnam, but the effect is not mediated by child dietary diversity. Journal of Nutrition. 2013;143(12):2015–2021. doi: 10.3945/jn.113.175182. [DOI] [PubMed] [Google Scholar]
- 47.Swindale, A., and Paula Bilinsky, Household Dietary Diversity Score (HDDS) for Measurement of Household Food Access: Indicator Guide (v.2). Washington, D.C.: FHI 360/FANTA 2006.
- 48.Belay E, et al. Prevalence and determinants of pre-adolescent (5–14 years) acute and chronic undernutrition in Lay Armachiho District, Ethiopia. Int J Equity Health. 2019;18(1):137. doi: 10.1186/s12939-019-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damie, T., M. kbebew, and A. Teklehaymanot, Nutritional status and associated factors among school adolescent in Chiro Town, West Hararge, Ethiopia. Gaziantep Medical Journal, 2015. 21.
- 50.Farella Guzzo, M. and G. Gobbi, Parental Death During Adolescence: A Review of the Literature Omega (Westport), 2021: p. 302228211033661. [DOI] [PubMed]
- 51.Das JK, et al. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Ann N Y Acad Sci. 2017;1393(1):21–33. doi: 10.1111/nyas.13330. [DOI] [PubMed] [Google Scholar]
- 52.Manna I. Growth Development and Maturity in Children and Adolescent: relation to Sports and physical activity. American Journal of Sports Science and Medicine. 2022;2(5A):48–50. doi: 10.12691/ajssm-2-5A-11. [DOI] [Google Scholar]
- 53.Nichols MS, et al. Decreasing trends in overweight and obesity among an australian population of preschool children. Int J Obes (Lond) 2011;35(7):916–24. doi: 10.1038/ijo.2011.64. [DOI] [PubMed] [Google Scholar]
- 54.Rokholm B, Baker JL, Sørensen TI. The levelling off of the obesity epidemic since the year 1999–a review of evidence and perspectives. Obes Rev. 2010;11(12):835–46. doi: 10.1111/j.1467-789X.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 55.Tariku A, et al. Stunting and its determinants among adolescent girls: findings from the Nutrition Surveillance Project, Northwest Ethiopia. Ecology of Food and Nutrition. 2019;58(5):481–494. doi: 10.1080/03670244.2019.1636793. [DOI] [PubMed] [Google Scholar]
- 56.Engidaw MT, Gebremariam AD. Prevalence and associated factors of stunting and thinness among adolescent somalian refugee girls living in eastern somali refugee camps, somali regional state, Southeast Ethiopia. Conflict and Health. 2019;13(1):17. doi: 10.1186/s13031-019-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tariku, E.Z., et al., Prevalence and factors associated with stunting and thinness among school-age children in Arba Minch Health and demographic surveillance site, Southern Ethiopia. PLoS ONE, 2018. 13(11). [DOI] [PMC free article] [PubMed]
- 58.Getachew, T. and A. Argaw, Intestinal helminth infections and dietary diversity score predict nutritional status of urban schoolchildren from southern Ethiopia. BMC Nutrition, 2017. 3(1).
- 59.Darapheak, C., et al., Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. International Archives of Medicine, 2013. 6(1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The [supplementary Material] data used to support the findings of this study are included within the article.