Abstract
Background:
The association between dyslipidaemia and breast cancer remains controversial, especially regarding the dynamic changes in lipid levels.
Objectives:
This study aimed to elucidate the role of serum lipid levels and the changes in disease outcomes in patients with breast cancer.
Methods:
The lipid profiles of patients with breast cancer who underwent surgery between 2013 and 2017 were retrospectively reviewed. The lipid profiles comprised triglyceride (TG), total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein. Serum lipid levels were categorized into three groups based on the tertiles. The Wilcoxon test was used to compare changes in serum lipid levels during follow-up. Hazard ratios (HRs) for survival outcomes were estimated using a multivariate Cox regression analysis.
Results:
A total of 3499 women diagnosed with nonmetastatic invasive breast cancer were included in this study, with a median follow-up of 60.4 months. We confirmed that each 1-tertile increased TG at baseline [HR = 1.19, 95% confidence interval (CI) 1.02–1.39] and 1-year follow-up (HR = 1.46, 95% CI 1.07–1.98) led to worse relapse-free survival (RFS). A lower risk of disease relapse was observed with each 1-tertile upregulation in HDL at 1-year follow-up (HR = 0.72, 95% CI 0.56–0.92). Receiving systemic therapies tends to induce an elevation in plasma lipid levels 1 year after surgery, especially in terms of TG. Regarding the prognostic value of dynamic changes in lipid levels, patients with sustained high levels of TG had poorer RFS (HR = 1.90, 95% CI 1.16–3.11), whereas maintaining high levels of HDL led to better survival (HR = 0.60, 95% CI 0.37–0.97).
Conclusion:
High TG at baseline and during follow-up was associated with worse disease outcome in early breast cancer patients. Systemic treatment would lead to an elevation of serum lipid levels. Patients with sustained high HDL level at 1-year follow-up after surgery had a superior prognosis, warranting further clinical evaluation.
Keywords: breast cancer, high-density lipoprotein, prognosis, serum lipid, triglycerides
Introduction
According to GLOBOCAN 2020, breast cancer in women has surpassed lung cancer and ranked first in cancer incidence worldwide, with an estimated 2.3 million new cases per year. 1 Breast cancer is the leading cause of cancer-related mortality among women. Thus, efforts are constantly being made to explore convenient and effective predictors of disease outcomes that can guide timely interventions to improve patient survival.
Lipids are the main structural and functional components of cell membranes and play vital roles in cell signal transduction, inflammation, and energy storage. Reprograming of lipid metabolism has been identified as a new hallmark of cancer. It enhances the propagation of oncogenic signaling via growth factor pathways and contributes to tumor proliferation and invasion.2–4 Epidemiological studies have shown that high-fat diet is associated with an increased risk of many malignancies. 5 Lipids also serve as prognostic biomarkers for several types of cancer, including breast cancer. 6
Dyslipidaemia refers to increased levels of triglycerides (TG), total cholesterol (TC), and low-density lipoprotein (LDL) and decreased levels of high-density lipoprotein (HDL). 7 It is a component of metabolic syndrome, a type of multifactorial metabolic disease taking into account the presence of at least three factors, including abdominal obesity/high body mass index (BMI), insulin resistance, hypertension, hypertriglyceridemia, and low HDL. 8 And dyslipidaemia is related to the occurrence and development of breast tumors. 9 Generally, high levels of “bad” serum lipid (TG, TC, and LDL) and low levels of “good” serum lipid (HDL) are associated with poor prognoses of breast cancer.10–14 However, conflicting results indicate that patients with hyperlipidemia can achieve better clinical outcomes.15,16 Further studies are needed to elucidate the prognostic potential of serum lipid levels.
Currently, serum lipid levels are not routinely monitored in patients with breast cancer. In addition, data on changes in lipid levels during the follow-up period and their association with disease outcomes are still lacking. Based on our previous study, some patients with different metabolic conditions showed different response to treatments and outcomes, Therefore, we conducted this retrospective analysis to explore the potential prognostic value of metabolic syndrome, especially the circulating lipid levels and their changes, on breast cancer patients’ survival, which may prompt us the importance and feasibility to monitor patients’ basal metabolism to make intervene in time.
Materials and methods
Study population
Data of patients diagnosed with invasive breast cancer who underwent surgical treatment between 2013 and 2017 at the Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, were retrospectively reviewed. Patients who met the following criteria were included: (1) female sex; (2) absence of distant metastasis; (3) complete clinicopathological and follow-up information; and (4) medical records of serum lipid profiles within 1 week before surgery. Patients who received neoadjuvant therapy or were diagnosed with de novo stage IV breast cancer were excluded. Cohort y0 comprised all the eligible patients. Among cohort y0, those with available records of serum lipid at 1 year after surgery and no disease relapse within the first year were further classified as cohort y1. This study was approved by the independent Ethical Committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments.
Laboratory examinations
Serum lipid levels, including TG, TC, HDL, and LDL, of the enrolled patients were measured using fasting blood samples collected before surgery and during follow-up. Laboratory tests were conducted on fresh blood samples obtained from the Department of Clinical Laboratory at Ruijin Hospital. Lipid levels were then categorized into three groups by tertile values in the cohort y0 as cutoffs: TG (<0.95, ⩾0.95 and <1.44, ⩾1.44), TC (<4.43, ⩾4.43 and <5.27, ⩾5.27), HDL (<1.21, ⩾1.21 and <1.49, ⩾1.49), and LDL (<2.61, ⩾2.61 and <3.29, ⩾3.29). Histopathological information, including tumor size, lymph node status, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, and Ki-67 index, was determined by the Department of Pathology. The molecular classification was divided into five categories. Luminal A subtype: ER+, PR ⩾ 20%, HER2–, and Ki67 < 14%; Luminal B (HER2−) subtype: ER+, HER2−, and PR < 20% or Ki67 ⩾ 14%; Luminal B (HER2+) subtype: ER+ and HER2+; HER2 + subtype: ER−, PR−, and HER2+; and (triple-negative breast cancer) TNBC subtype: ER−, PR−, and HER2–. 17
Outcomes
Follow-up information for all patients was collected during outpatient reviews and telephone interviews. The primary end point was relapse-free survival (RFS), defined as the period from the date of surgery to local recurrence, distant metastasis, or death as a result of any cause, or time at censor. The secondary endpoint overall survival (OS) was calculated from the date of surgery to death from any cause or the last follow-up. 18
Statistical analysis
The Wilcoxon test was used to compares changes in serum lipid levels during follow-up. A Cox proportional hazards regression model with a 95% confidence interval (CI) was used to calculate the association between serum lipid levels and prognosis in patients with breast cancer. Age, BMI, menopausal status, ER status, PR status, HER2 status, Ki67, tumor size, lymph node status, and systemic therapy (chemotherapy, endocrine therapy, and targeted therapy) were included in the multivariable analyses. Interactive analyses were performed to evaluate whether these associations varied across subgroups.
All statistical analyses were performed using IBM SPSS Statistics software (version 26.0; SPSS Inc., Chicago, IL, USA). A two-sided p value <0.05 was considered statistically significant.
Results
Characteristics of study populations in cohort y0 and y1
A total of 3757 patients were reviewed, and 3499 patients were included in cohort y0. Among the general population, 1616 patients with available lipid information at 1-year follow-up were enrolled in cohort y1 (Supplemental Figure S1). The clinical characteristics and metabolic features are shown in Table 1.
Table 1.
Clinicopathological and metabolic characteristics of patients in cohort y0 and cohort y1.
| Characteristic | Cohort y0 | Cohort y1 |
|---|---|---|
| (N = 3499) | (N = 1616) | |
| Age, years | ||
| ⩽55 | 1760 (50.3) | 716 (44.3) |
| >55 | 1739 (49.7) | 900 (55.7) |
| BMI, kg/m2 | ||
| <24 | 2168 (62.0) | 991 (61.3) |
| ⩾24 | 1331 (38.0) | 625 (38.7) |
| Menopausal status | ||
| Premenopausal | 1346 (38.5) | 552 (34.2) |
| Postmenopausal | 2153 (61.5) | 1064 (65.8) |
| Histological types | ||
| IDC | 3127 (89.4) | 1436 (88.9) |
| ILC | 99 (2.8) | 48 (3.0) |
| Others | 273 (7.8) | 132 (8.1) |
| Histological grade | ||
| I | 253 (7.2) | 130 (8.0) |
| II | 1575 (45.0) | 763 (47.2) |
| III | 1185 (33.9) | 483 (29.9) |
| Unknown | 486 (13.9) | 240 (14.9) |
| Tumor size (cm) | ||
| ⩽2 | 2079 (59.4) | 1015 (62.8) |
| >2 | 1420 (40.6) | 601 (37.2) |
| Lymph node status | ||
| N− | 2347 (67.1) | 1107 (68.5) |
| N+ | 1152 (32.9) | 509 (31.5) |
| ER status | ||
| Positive | 2545 (72.7) | 1317 (81.5) |
| Negative | 954 (27.3) | 299 (18.5) |
| PR status | ||
| Positive | 2101 (60.0) | 1100 (68.1) |
| Negative | 1398 (40.0) | 516 (31.9) |
| HER2 status | ||
| Positive | 840 (24.0) | 324 (20.0) |
| Negative | 2659 (76.0) | 1292 (80.0) |
| Ki67 | ||
| <14% | 1212 (34.6) | 616 (38.1) |
| ⩾14% | 2287 (65.4) | 1000 (61.9) |
| Subtypes | ||
| Luminal A | 714 (20.4) | 378 (23.4) |
| Luminal B (HER2−) | 1454 (41.6) | 756 (46.8) |
| Luminal B (HER2+) | 388 (11.1) | 187 (11.6) |
| HER2+ | 452 (12.9) | 137 (8.5) |
| TNBC | 491 (14.0) | 158 (9.7) |
| Hypertensiona | ||
| Yes | 909 (26.0) | 448 (27.9) |
| No | 2590 (74.0) | 1168 (72.1) |
| Diabetes mellitusa | ||
| Yes | 256 (7.3) | 122 (7.6) |
| No | 3243 (92.7) | 1494 (92.4) |
| Metabolic syndromea | ||
| Yes | 426 (12.2) | 242 (15.0) |
| No | 3073 (87.8) | 1374 (85.0) |
| Metabolic abnormalitiesa | ||
| 0 | 1393 (39.8) | 506 (31.3) |
| 1 | 1037 (29.6) | 499 (30.9) |
| 2 | 639 (18.3) | 490 (30.3) |
| 3 | 306 (8.7) | 189 (11.7) |
| 4 | 102 (2.9) | 47 (2.9) |
| 5 | 18 (0.5) | 6 (0.4) |
BMI, body mass index; HER 2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Metabolic characteristics.
In cohort y0, 2153 (61.5%) patients were postmenopausal. A total of 1331 (38.0%) patients had BMI ⩾ 24 kg/m2. Regarding the pathological stage, pT1 tumor accounted for 59.4% (2079 patients) and lymph node-positive disease accounted for 32.9% (1152 patients). ER-positive and HER2-positive tumors were found in 2545 (72.7%) and 840 (24.0%) patients, respectively. Lipid levels were classified into three categories based on the tertile values. A history of hypertension or diabetes mellitus was found in 909 (26.0%) and 256 (7.3%) patients, respectively. A total of 426 patients (12.2%) had metabolic syndrome. In cohort y1, 625 (38.7%) patients had BMI ⩾ 24 kg/m2. Postmenopausal patients accounted for 65.8% (1064 patients). A total of 1015 (62.8%) patients had tumors ⩽2 cm, and 509 (31.5%) patients had lymph node metastasis. ER-positive and HER2-positive tumors accounted for 81.5% and 20.0%, respectively. A total of 242 (15.0%) patients had metabolic syndrome.
Baseline serum lipids and prognosis
During a median follow-up time of 60.4 months, a total of 281 endpoint events were involved, including 178 relapses and 103 deaths. Among them, 12 deaths were caused by non-breast cancer. Therefore, 95.73% of RFS events and 88.35% of OS events were directly driven by breast cancer. In general, increasing tertiles of baseline TG were associated with poor prognosis of breast cancer [hazard ratio (HR) = 1.22, 95% CI 1.06–1.41, p = 0.01] (data not shown). However, no significant association was observed between baseline TC, HDL, or LDL levels and prognosis.
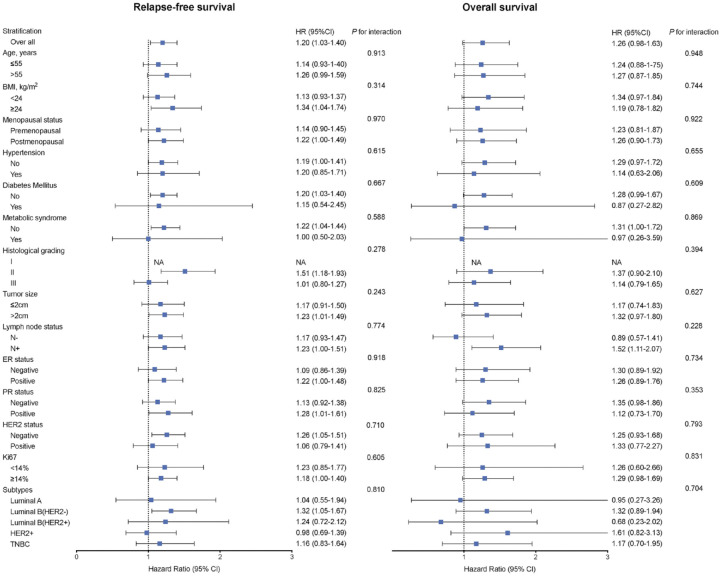
Multivariate analysis demonstrated that patients with the highest tertile of baseline TG had the worst RFS (HR = 1.44, 95% CI 1.05–1.96, p = 0.02) and OS (HR = 1.64, 95% CI 0.96–2.78, p = 0.07) compared to those with the lowest levels of TG. The corresponding HR of each 1-tertile increase in baseline TG was 1.19 (95% CI 1.02–1.39, p = 0.02) for RFS and 1.26 (95% CI 0.98–1.63, p = 0.07) for OS (Table 2). The estimated 5-year RFS rates were 93.1%, 91.7%, and 90.1% for patients in the low, intermediate, and high tertiles of baseline TG, and the estimated 5-year OS rates were 97.7%, 96.4%, and 96.3%, respectively (Table 2). The impact of other possible factors in RFS and OS was shown in Supplemental Table S1. Furthermore, subgroup analysis demonstrated that the relationship was consistent across different stratifications according to age, BMI, menopausal status, history of hypertension, diabetes mellitus, metabolic syndrome, and histopathological features (p for interaction >0.1; Figure 1).
Table 2.
Multivariable Cox regression analysis with RFS and OS according to tertiles of serum lipids at baseline.
| Parameter | Relapse-free survival | 5-Year RFS (%) | Overall survival | 5-Year OS (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Cases | HR (95% CI) | p | Cases | HR (95% CI) | p | |||
| TG, mmol/L | |||||||||
| <0.95 | 1145 | 73 | 1.00 (Ref.) | 93.1 | 24 | 1.00 (Ref.) | 97.7 | ||
| ⩾0.95, <1.44 | 1176 | 95 | 1.28 (0.94–1.75) | 0.12 | 91.7 | 36 | 1.43 (0.84–2.43) | 0.19 | 96.4 |
| ⩾1.44 | 1178 | 113 | 1.44 (1.05–1.96) | 0.02 | 90.1 | 43 | 1.64 (0.96–2.78) | 0.07 | 96.3 |
| Each 1-tertile increase | 1.19 (1.02–1.39) | 0.02 | 1.26 (0.98–1.63) | 0.07 | |||||
| TC, mmol/L | |||||||||
| <4.43 | 1157 | 92 | 11.00 (Ref.) | 92.2 | 39 | 11.00 (Ref.) | 96.5 | ||
| ⩾4.43, <5.27 | 1173 | 92 | 1.04 (0.77–1.39) | 0.81 | 91.5 | 33 | 0.86 (0.53–1.38) | 0.52 | 96.7 |
| ⩾5.27 | 1169 | 97 | 1.12 (0.83–1.52) | 0.45 | 91.2 | 31 | 0.77 (0.47–1.27) | 0.30 | 97.2 |
| Each 1-tertile increase | 1.06 (0.91–1.23) | 0.45 | 0.88 (0.68–1.13) | 0.30 | |||||
| HDL, mmol/L | |||||||||
| <1.21 | 1163 | 98 | 1.00 (Ref.) | 91.6 | 38 | 1.00 (Ref.) | 96.5 | ||
| ⩾1.21, <1.49 | 1150 | 101 | 11.08 (0.82–1.43) | 0.57 | 90.5 | 39 | 1.04 (0.67–1.64) | 0.85 | 96.2 |
| ⩾1.49 | 1186 | 82 | 0.92 (0.69–1.25) | 0.60 | 92.7 | 26 | 0.76 (0.46–1.26) | 0.29 | 97.6 |
| Each 1-tertile increase | 0.97 (0.83–1.12) | 0.63 | 0.88 (0.69–1.13) | 0.32 | |||||
| LDL, mmol/L | |||||||||
| <2.61 | 1165 | 94 | 1.00 (Ref.) | 91.9 | 38 | 1.00 (Ref.) | 96.5 | ||
| ⩾2.61, <3.29 | 1163 | 86 | 0.91 (0.67–1.22) | 0.53 | 92.2 | 31 | 0.79 (0.49–1.29) | 0.35 | 96.9 |
| ⩾3.29 | 1171 | 101 | 11.10 (0.82–1.48) | 0.54 | 90.8 | 34 | 0.82 (0.50–0.34) | 0.43 | 96.9 |
| Each 1-tertile increase | 1.05 (0.90–1.22) | 0.53 | 0.90 (0.70–1.16) | 0.42 | |||||
HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; OS, overall survival; RFS, relapse-free survival; TC, total cholesterol; TG, triglyceride.
Figure 1.
Multivariable adjusted hazard ratio (95% CI) of RFS and OS according to each 1-tertile increase in TG across stratifications of clinical characteristics.
OS, overall survival; RFS, relapse-free survival; TG, triglyceride.
Dynamic changes of serum lipids and the association with prognosis
Systemic therapies can alter lipid profiles. Notably, the concentration of TG increased significantly in all therapy modalities (p < 0.001). Meanwhile, endocrine therapy and endo-chemotherapy led to an increase in plasma HDL concentration. Among patients without adjuvant treatment, no significant change in serum lipid levels was observed (Supplemental Figure S2). When using tertile lipid levels to categorize patients into three groups, 736 patients (45.5%) in the TG group underwent a change. A total of 106 patients (6.6%) changed from the lowest to the highest tertile, and four patients (0.2%) changed in reverse. Regarding HDL levels, 642 (39.7%) patients showed a shift in the lipid-level category. There were 56 patients (3.5%) with elevated HDL levels from the lowest to the highest tertiles, and HDL levels were downregulated in 26 patients (1.6%; Supplemental Figure S3). Univariable and multivariable analysis showed that BMI ⩾ 24 kg/m2 (ORfor high TG = 1.97, 95% CI 1.58–2.44, p < 0.01; ORfor low HDL = 1.778 95% CI 1.41–2.25, p < 0.01) and history of hypertension (OR for high TG = 1.53, 95% CI 1.18–1.97, p < 0.01; ORfor low HDL = 1.56, 95% CI 1.20–2.03, p < 0.01) were significant independent predictors of both high TG (in tertile 3) and low HDL (in tertile 1; Table 3).
Table 3.
Univariable and multivariable analysis on odds ratio (95% CI) of high TG and low HDL according to major characteristics at 1-year follow-up.
| Characteristic | High TG (in tertile 3) | Low HDL (in tertile 1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| OR (95% CI) | p | OR (95% CI) | P | OR (95% CI) | p | OR (95% CI) | p | |
| Age, years | ||||||||
| ⩽55 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | ||||
| >55 | 1.60 (1.31–1.95) | <0.01 | 0.77 (0.55–1.09) | 0.14 | 1.41 (1.13–1.77) | <0.01 | 0.99 (0.68–1.44) | 0.96 |
| BMI, kg/m2 | ||||||||
| <24 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | ||||
| ⩾24 | 2.29 (1.86–2.82) | <0.01 | 1.97 (1.58–2.44) | <0.01 | 2.03 (1.62–2.54) | <0.01 | 1.78 (1.41–2.25) | <0.01 |
| Menopausal status | ||||||||
| Premenopausal | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | ||||
| Postmenopausal | 1.98 (1.61–2.44) | <0.01 | 2.00 (1.40–2.85) | <0.01 | 1.52 (1.19–1.93) | <0.01 | 1.17 (0.79–1.74) | 0.43 |
| Hypertension | ||||||||
| No | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | ||||
| Yes | 5.00 (3.91–6.40) | <0.01 | 1.53 (1.18–1.97) | <0.01 | 1.90 (1.50–2.40) | <0.01 | 1.56 (1.20–2.03) | <0.01 |
| Diabetes mellitus | ||||||||
| No | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | ||||
| Yes | 1.79 (1.21–2.64) | <0.01 | 1.24 (0.82–1.87) | 0.31 | 2.14 (1.47–3.11) | <0.01 | 1.64 (1.11–2.43) | 0.01 |
| Chemotherapy | ||||||||
| No | 1.00 (Ref.) | 1.00 (Ref.) | ||||||
| Yes | 1.02 (0.83–1.26) | 0.86 | 1.27 (1.00–1.60) | 0.05 | 1.11 (0.88–1.41) | 0.39 | 1.26 (0.97–1.65) | 0.08 |
| Endocrine therapy | ||||||||
| No | 1.00 (Ref.) | 1.00 (Ref.) | ||||||
| Yes | 1.17 (0.91–1.51) | 0.22 | 1.24 (0.94–1.63) | 0.13 | 0.90 (0.68–1.19) | 0.45 | 0.93 (0.69–1.27) | 0.66 |
| Targeted therapy | ||||||||
| No | 1.00 (Ref.) | 1.00 (Ref.) | ||||||
| Yes | 0.86 (0.67–1.11) | 0.26 | 0.89 (0.67–1.19) | 0.43 | 1.07 (0.80–1.43) | 0.65 | 1.05 (0.77–1.45) | 0.75 |
HDL, high-density lipoprotein; HR, hazard ration; TG, triglyceride.
Regarding the prognostic value of changes in lipid levels, patients with TG maintained at high levels had the worst RFS (HR = 1.90, 95% CI 1.16–3.11, p = 0.01). The estimated 5-year RFS rates were 94.5, 92.9, 95.1, and 91.4% for patients with changes in TG from tertile 1/2 to tertile 1/2, tertile 1/2 to tertile 3, tertile 3 to tertile 1/2, and tertile 3 to tertile 3, respectively. In addition, sustained high levels of HDL were associated with the lowest risk of disease relapse (HR = 0.60, 95% CI 0.37–0.97, p = 0.04; Table 4). And the estimated 5-year RFS were 90.9, 94.9, 88.2, and 94.2% for patients with changes in HDL from tertile 1 to tertile 1, tertile 1 to tertile 2/3, tertile 2/3 to tertile 1, and tertile 2/3 to tertile 2/3. No correlations were observed between OS and changes in TG or HDL levels.
Table 4.
Multivariable Cox regression analysis with RFS according to the changes of tertiles of lipids.
| Parameter | Patients | Cases | HR (95% CI) | p | 5-Year RFS (%) |
|---|---|---|---|---|---|
| TG, mmol/La | |||||
| Tertile 1/2–tertile 1/2 | 648 | 31 | 1.00 (Ref.) | 94.5 | |
| Tertile 1/2–tertile 3 | 412 | 29 | 1.50 (0.89–2.51) | 0.13 | 92.9 |
| Tertile 3–tertile ½ | 74 | 3 | 0.74 (0.23–2.45) | 0.63 | 95.1 |
| Tertile 3–tertile 3 | 482 | 38 | 1.90 (1.16–3.11) | 0.01 | 91.4 |
| TC, mmol/L b | |||||
| Tertile 1/2–tertile 1/2 | 746 | 48 | 1.00 (Ref.) | 93.2 | |
| Tertile 1/2–tertile 3 | 271 | 23 | 1.46 (0.88–2.42) | 0.15 | 90.3 |
| Tertile 3–tertile 1/2 | 158 | 10 | 1.28 (0.64–2.57) | 0.49 | 90.6 |
| Tertile 3–tertile 3 | 441 | 21 | 0.79 (0.46–1.35) | 0.38 | 94.7 |
| HDL, mmol/L c | |||||
| Tertile 1–tertile 1 | 296 | 27 | 1.00 (Ref.) | 90.9 | |
| Tertile 1–tertile 2/3 | 216 | 10 | 0.60 (0.29–1.25) | 0.17 | 94.9 |
| Tertile 2/3–tertile 1 | 129 | 17 | 1.40 (0.76–2.60) | 0.28 | 88.2 |
| Tertile 2/3-–tertile 2/3 | 975 | 48 | 0.60 (0.37–0.97) | 0.04 | 94.2 |
| LDL, mmol/L d | |||||
| Tertile 1/2–tertile 1/2 | 726 | 48 | 1.00 (Ref.) | 92.7 | |
| Tertile 1/2–tertile 3 | 286 | 19 | 0.98 (0.57–1.70) | 0.95 | 92.9 |
| Tertile 3–tertile 1/2 | 154 | 11 | 1.24 (0.63–2.43) | 0.53 | 92.4 |
| Tertile 3–tertile 3 | 450 | 24 | 0.85 (0.51–1.43) | 0.55 | 94.2 |
Hyphen indicated changes in lipid tertiles.
TG tertiles: 1: <0.95 mmol/L, 2: ⩾0.95, and <1.44 mmol/L, 3: ⩾1.44 mmol/L.
TC tertiles: 1: <4.43 mmol/L, 2: ⩾4.43, and <5.27 mmol/L, 3: ⩾5.27 mmol/L.
HDL tertiles: 1: <1.21 mmol/L, 2: ⩾1.21, and <1.49 mmol/L, 3: ⩾1.49 mmol/L.
LDL tertiles: 1: <2.61 mmol/L, 2: ⩾2.61, and <3.29 mmol/L, 3: ⩾3.29 mmol/L.
HR, hazard ratio; HDL, high-density lipoprotein; HR, hazard ration; LDL, low-density lipoprotein; OS, overall survival; RFS, relapse-free survival; TC, total cholesterol; TG, triglyceride.
Serum lipids at 1-year follow-up and prognosis
A total of 102 RFS events were observed in cohort y1, with a median follow-up time of 51.9 months. In general, increasing tertiles of TG at 1-year follow-up was associated with elevated risk of disease relapse (HR = 1.42, 95% CI 1.06–1.91, p = 0.02), and HDL was related to more favorable clinical outcomes (HR = 0.70, 95% CI 0.56–0.89, p < 0.01; data not shown). No significant relationship with OS was observed.
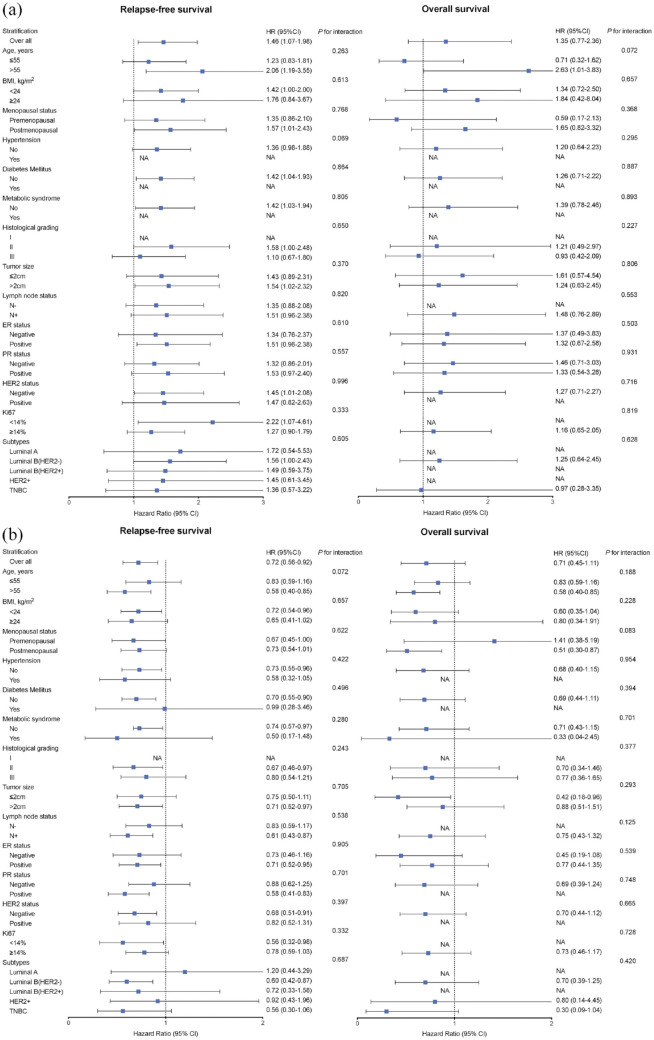
In multivariable analysis, high TG levels were associated with poor RFS with an HR of 1.46 (95% CI 1.07–1.98, p = 0.02) for each 1-tertile increase at 1-year follow-up. Moreover, the risk of disease relapse decreased (HR = 0.72, 95% CI 0.56–0.92, p = 0.01) with each 1-tertile increase in HDL (Table 5). The estimated 5-year RFS rates were 94.0%, 93.5%, and 90.6% for patients in the low, intermediate, and high tertiles of TG at 1-year follow-up and 88.9%, 93.1%, and 93.0% in patients in the three tertiles of HDL, respectively (Table 5). The correlation between TG or HDL levels and clinical outcomes was consistent across all subgroups (p for interaction >0.1; Figure 2). However, no significant relationship was observed between follow-up serum lipid levels and OS.
Table 5.
Multivariable Cox regression analysis with RFS and OS according to tertiles of serum lipids at 1-year follow-up.
| Parameter | RFS | 5-Year RFS (%) | OS | 5-Year OS (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Cases | HR (95% CI) | p | Cases | HR (95% CI) | p | |||
| TG, mmol/L | |||||||||
| <0.95 | 235 | 11 | 1.00 (Ref.) | 94.0 | 2 | 1.00 (Ref.) | 98.5 | ||
| ⩾0.95, < 1.44 | 487 | 23 | 1.16 (0.57–2.38) | 0.85 | 93.5 | 9 | 2.37 (0.50–11.27) | 0.28 | 98.2 |
| ⩾1.44 | 894 | 68 | 1.78 (0.92–3.45) | 0.07 | 90.6 | 20 | 2.48 (0.56–11.06) | 0.23 | 97.9 |
| Each 1-tertile increase | 1.46 (1.07–1.98) | 0.02 | 1.35 (0.77–2.36) | 0.30 | |||||
| TC, mmol/L | |||||||||
| < 4.43 | 422 | 30 | 1.00 (Ref.) | 92.4 | 8 | 1.00 (Ref.) | 97.5 | ||
| ⩾4.43, < 5.27 | 482 | 28 | 0.70 (0.41–1.18) | 0.18 | 94.1 | 9 | 0.71 (0.27–1.88) | 0.49 | 97.9 |
| ⩾5.27 | 712 | 44 | 0.82 (0.51–1.34) | 0.44 | 93.0 | 14 | 0.76 (0.30–1.90) | 0.55 | 97.7 |
| Each 1-tertile increase | 0.92 (0.72–1.19) | 0.52 | 0.89 (0.46–1.42) | 0.61 | |||||
| HDL, mmol/L | |||||||||
| <1.21 | 425 | 44 | 1.00 (Ref.) | 88.9 | 13 | 1.00 (Ref.) | 96.8 | ||
| ⩾1.21, <1.49 | 511 | 26 | 0.53 (0.33–0.86) | 0.01 | 93.1 | 10 | 0.71 (0.31–1.63) | 0.41 | 97.4 |
| ⩾1.49 | 680 | 32 | 0.53 (0.33–0.85) | 0.01 | 93.0 | 8 | 0.50 (0.20–1.24) | 0.13 | 98.5 |
| Each 1-tertile increase | 0.72 (0.56–0.92) | 0.01 | 0.71 (0.45–1.11) | 0.13 | |||||
| LDL, mmol/L | |||||||||
| <2.61 | 432 | 33 | 1.00 (Ref.) | 92.1 | 10 | 1.00 (Ref.) | 97.2 | ||
| ⩾2.61, <3.29 | 448 | 26 | 0.67 (0.39–1.13) | 0.13 | 93.5 | 6 | 0.39 (0.14–1.11) | 0.08 | 98.6 |
| ⩾3.29 | 736 | 43 | 0.70 (0.43–1.15) | 0.16 | 93.7 | 15 | 0.57 (0.24–1.35) | 0.20 | 97.5 |
| Each 1-tertile increase | 0.85 (0.66–1.09) | 0.19 | 0.79 (0.50–1.25) | 0.31 | |||||
HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; OS, overall survival; RFS, relapse-free survival; TC, total cholesterol; TG, triglyceride.
Figure 2.
Multivariable adjusted hazard ratio (95% CI) of RFS since 1-year follow-up according to each 1-tertile increase in TG (a) and HDL (b) across stratifications of clinical characteristics.
HDL, high-density lipoprotein; RFS, relapse-free survival; TG, triglyceride.
Discussion
In the current study, we comprehensively analyzed the role of serum lipids at two time points, as well as changes in the risk of disease relapse in 3499 patients with invasive breast cancer. Our study demonstrated that high TG levels at both baseline and 1 year after surgery increased the risk of recurrence, whereas high HDL levels served as a protective metabolic marker and were associated with longer disease outcomes. Systemic treatment, especially endocrine therapy, causes an increase in circulating serum lipid levels, providing additional prognostic value. Sustained high TG or low HDL levels during the disease course are associated with unfavorable outcomes.
Considering the influence of systemic treatment on lipid metabolism, we found that patients who received systemic therapies tended to have increased serum lipid levels. Changes in lipid profiles are primarily caused by endocrine therapy. Wasan et al. suggested that patients treated with tamoxifen had significantly decreased TC and LDL and increased TG and HDL due to its estrogen-like properties. 19 Consequently, these would lead to venous thrombosis, fatty liver, and lipid dysfunction as common adverse effects. 20 Regarding aromatase inhibitors (AI), Elisaf et al. reported that 8–16 weeks of AI in postmenopausal women increased TC and LDL levels. 21 Indeed, the circulating estrogen level is closely related to lipid metabolism, including hepatic lipid accumulation, lipogenesis as well as the inflammation of adipose tissue.22,23 Several previous studies have shown that patients receiving chemotherapy have worse lipid metabolism, manifested as increased TG, TC, and LDL concentrations and decreased HDL levels; these changes are greater in premenopausal patients than in postmenopausal populations. 24 The use of glucocorticoids in combination with chemotherapy may explain this finding. It also enhances systemic oxidative stress, resulting in lipid peroxidation in the liver. In addition, chemotherapy-induced suppression of ovarian function and chemotherapy-related metabolic disorders may contribute to changes in serum lipid levels.25,26 In general, our results suggested that the detection of plasma lipid is reasonable in patients with breast cancer, particularly those receiving endocrine therapy or chemotherapy.
The relationship between lipid profile and breast cancer has been extensively studied. Theoretically, tumor cells require more structural lipids for membrane synthesis, lipid signaling, and inflammation activation. In preclinical studies, breast cancer cell lines showed altered lipid metabolism compared to normal mammary epithelial cells. 27 Our results indicated that high TG levels were related to poor outcomes in patients with early-stage breast cancer. Similarly, Harborg et al. demonstrated that dyslipidaemia is associated with an increased risk of breast cancer recurrence and metastasis. 27 In addition, the results of a competitive risk analysis showed that patients with higher TG levels had an increased risk (HR = 1.87) of breast cancer-specific mortality. 28 However, studies by Li et al. as well as Jung et al. have indicated that high TG levels could be a favorable prognostic factor for breast cancer.12,15 A plausible reason for the difference lies in that cardiovascular-associated deaths due to elevated TG levels would reduce the occurrence of breast cancer-related adverse events. Another possible mechanism is the use of lipid-lowering agents such as statins.9,29
From another aspect, HDL, conventionally considered a “good lipid,” plays a protective role in patients with breast cancer in this study. Li 12 and Lofterød et al. 13 observed consistent results, especially among patients with TNBC. Gutpa et al. also found that low HDL levels were associated with an increased risk of TNBC (OR = 2.67). 30 In addition, literature also reported that women with type 2 diabetes mellitus had an increased risk of breast cancer, partly due to the formation of functionally defective HDL particles. The underlying mechanism mainly involves the prevention of lipid peroxidation.31–33 These results further support the idea that blood lipid-level testing, as a convenient and efficient tool, might be included in the routine strategy for patients with breast cancer, while its accuracy and definite value for prediction should be validated prospectively. Moreover, the role of lipid-lowering agents could be studied in well-designed randomized trials before their use become novel standard strategies.
Furthermore, the effects of changes in plasma lipid levels on breast cancer prognosis have rarely been described. We analyzed the relationship between the dynamic changes in serum lipid levels during follow-up and long-term patient outcomes. In terms of TG, the risk of disease relapse increased when TG remained at a high level in the patients’ circulation. HDL had the opposite effect, and more favorable survival was observed in patients with constantly high levels of HDL. This further strengthened our views on the potential role of serum lipids in the clinical outcomes of patients with breast cancer and emphasized the value of detecting serum lipids during follow-up. Actually, circulating tumor DNA is a promising liquid biopsy to monitor recurrence.34,35 While the definite prediction value remains to be validated by more clinical trials before it become routine practice. And its cost is still high to afford so far.
The strengths of the present study include the relatively large sample size and abundant follow-up information. We demonstrated, for the first time, the relationship between changes in lipid levels and breast cancer prognosis. However, this study had several limitations. First, selection biases and confounding effects may have been inevitable owing to the retrospective design of our study, although we used multivariate analysis to narrow the influence. Second, due to incomprehensive information collection and loss of data, the information on the use of lipid-lowering agents like statins and blood lipid levels at multiple time points during follow-up was insufficient. Therefore, the accuracy and definite predictive value of these findings were limited. Finally, the relatively few events number may limit the power of detecting statistically significant associations between serum lipid levels and clinical outcomes.
In conclusion, our study indicated that lipid abnormality and its change during disease course in early breast cancer patients was associated with disease outcomes. Particularly, high TG levels at baseline and after 1-year follow-up was associated with worse outcomes. Systemic treatment for breast cancer could cause an elevation of serum lipid levels. Early breast cancer patients with sustained high HDL level at 1-year follow-up after surgery may have a superior prognosis, which warrants further clinical evaluation.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231177004 for Association of serum lipid levels and clinical outcomes in early breast cancer patients by Shuwen Dong, Jing Yu, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors appreciate Ms. Yidong Du for maintaining the SJTU-BCDB.
Footnotes
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shuwen Dong, Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Jing Yu, Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Xiaosong Chen, Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, 197 Ruijin Er Road, Shanghai 200025, China.
Kunwei Shen, Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, 197 Ruijin Er Road, Shanghai 200025, China.
Declarations
Ethics approval and consent to participate: This study was approved by the independent Ethical Committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (Ethics Acceptance No. 20191101). All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. Given the anonymized nature of the data and the retrospective nature of this study, the requirement for formal consent was waived by the IEC of Ruijin Hospital.
Consent for publication: Not applicable.
Author contribution(s): Shuwen Dong: Conceptualization; Data curation; Formal analysis; Investigation; Software; Visualization; Writing – original draft; Writing – review & editing.
Jing Yu: Methodology; Project administration; Validation; Writing – review & editing.
Xiaosong Chen: Conceptualization; Funding acquisition; Project administration; Resources; Validation; Writing – review & editing.
Kunwei Shen: Conceptualization; Funding acquisition; Project administration; Resources; Supervision; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by funds from the National Natural Science Foundation of China (grant no. 82072937, 82072897, 82002773), the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (grant no. 20172007), and the Beijing Breast Disease Society. The funding agencies had no role in the study design, data collection, data analysis, interpretation, and writing of the report.
The authors declare that there is no conflict of interest.
Availability of data and material: The anonymized datasets used in this study can be available upon reasonable request to the corresponding author. Supplemental material for this article is available online.
Reference
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura MT, Yudell BE, Loor JJ.Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 2014; 53: 124–144. [DOI] [PubMed] [Google Scholar]
- 3.Bennett M, Gilroy DW.Lipid mediators in inflammation. Microbiol Spectr 2016; 4: 1–21. [DOI] [PubMed] [Google Scholar]
- 4.Bi J, Ichu TA, Zanca C, et al. Oncogene amplification in growth factor signaling pathways renders cancers dependent on membrane lipid remodeling. Cell Metab 2019; 30: 525–538.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler LM, Perone Y, Dehairs J, et al. Lipids and cancer: emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv Drug Deliv Rev 2020; 159: 245–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larrouy-Maumus G.Lipids as biomarkers of cancer and bacterial infections. Curr Med Chem 2019; 26: 1924–1932. [DOI] [PubMed] [Google Scholar]
- 7.Xing L, Jing L, Tian Y, et al. Epidemiology of dyslipidemia and associated cardiovascular risk factors in northeast China: a cross-sectional study. Nutr Metab Cardiovasc Dis 2020; 30: 2262–2270. [DOI] [PubMed] [Google Scholar]
- 8.Buono G, Crispo A, Giuliano M, et al. Metabolic syndrome and early stage breast cancer outcome: results from a prospective observational study. Breast Cancer Res Treat 2020; 182: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong S, Wang Z, Shen K, et al. Metabolic syndrome and breast cancer: prevalence, treatment response, and prognosis. Front Oncol 2021; 11: 629666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emaus A, Veierød MB, Tretli S, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat 2010; 121: 651–660. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues Dos Santos C, Fonseca I, Dias S, et al. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer 2014; 14: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Tang H, Wang J, et al. The effect of preoperative serum triglycerides and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Breast 2017; 32: 1–6. [DOI] [PubMed] [Google Scholar]
- 13.Lofterød T, Mortensen ES, Nalwoga H, et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Cancer 2018; 18: 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Y, Ding X, Wang J, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers 2015; 30: 200–207. [DOI] [PubMed] [Google Scholar]
- 15.Jung SM, Kang D, Guallar E, et al. Impact of serum lipid on breast cancer recurrence. J Clin Med 2020; 9: 2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozdemir BH, Akcali Z, Haberal M.Hypercholesterolemia impairs angiogenesis in patients with breast carcinoma and, therefore, lowers the risk of metastases. Am J Clin Pathol 2004; 122: 696–703. [DOI] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International expert consensus on the primary therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muss HB, Polley MY, Berry DA, et al. Randomized Trial of standard adjuvant chemotherapy regimens versus capecitabine in older women with early breast cancer: 10-Year update of the CALGB 49907 Trial. J Clin Oncol 2019; 37: 2338–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasan KM, Goss PE, Pritchard PH, et al. The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L). Ann Oncol 2005; 16: 707–715. [DOI] [PubMed] [Google Scholar]
- 20.Song D, Hu Y, Diao B, et al. Effects of tamoxifen vs. Toremifene on fatty liver development and lipid profiles in breast cancer. BMC Cancer 2021; 21: 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elisaf MS, Bairaktari ET, Nicolaides C, et al. Effect of letrozole on the lipid profile in postmenopausal women with breast cancer. Eur J Cancer 2001; 37: 1510–1513. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Yu Q, Shen Q, et al. Black cohosh ameliorates metabolic disorders in female ovariectomized rats. Rejuvenation Res 2016; 19: 204–214. [DOI] [PubMed] [Google Scholar]
- 23.Jelenik T, Roden M.How estrogens prevent from lipid-induced insulin resistance. Endocrinology 2013; 154: 989–992. [DOI] [PubMed] [Google Scholar]
- 24.He T, Wang C, Tan Q, et al. Adjuvant chemotherapy-associated lipid changes in breast cancer patients: A real-word retrospective analysis. Medicine 2020; 99: e21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu J, Wu L, Jiang M, et al. Real-world impact of non-breast cancer-specific death on overall survival in resectable breast cancer. Cancer 2017; 123: 2432–2443. [DOI] [PubMed] [Google Scholar]
- 26.Fredslund SO, Gravholt CH, Laursen BE, et al. Key metabolic parameters change significantly in early breast cancer survivors: an explorative PILOT study. J Transl Med 2019; 17: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harborg S, Ahern TP, Feldt M, et al. Circulating lipids and breast cancer prognosis in the Malmö diet and cancer study. Breast Cancer Res Treat 2022; 191: 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wulaningsih W, Vahdaninia M, Rowley M, et al. Prediagnostic serum glucose and lipids in relation to survival in breast cancer patients: a competing risk analysis. BMC Cancer 2015; 15: 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YR, Ro V, Steel L, et al. Impact of long-term lipid-lowering therapy on clinical outcomes in breast cancer. Breast Cancer Res Treat 2019; 176: 669–677. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A, Saraiya V, Deveaux A, et al. Association of lipid profile biomarkers with breast cancer by molecular subtype: analysis of the MEND study. Sci Rep 2022; 12: 10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray G, Husain SA.Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin Biochem 2001; 34: 71–76. [DOI] [PubMed] [Google Scholar]
- 32.Roberti MP, Arriaga JM, Bianchini M, et al. Protein expression changes during human triple negative breast cancer cell line progression to lymph node metastasis in a xenografted model in nude mice. Cancer Biol Ther 2012; 13: 1123–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holven KB, Retterstøl K, Ueland T, et al. Subjects with low plasma HDL cholesterol levels are characterized by an inflammatory and oxidative phenotype. PLoS One 2013; 8: e78241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015; 7: 302ra133. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Murillas I, Chopra N, Comino-Méndez I, et al. Assessment of molecular relapse detection in early-stage breast cancer. JAMA Oncol 2019; 5: 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231177004 for Association of serum lipid levels and clinical outcomes in early breast cancer patients by Shuwen Dong, Jing Yu, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology