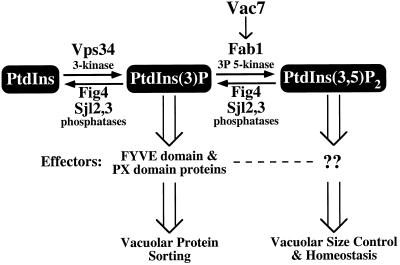
Figure 8.
Model for regulation of PtdIns(3,5)P2 synthesis and degradation. PtdIns is phosphorylated by the PtdIns 3-kinase Vps34 to generate PtdIns(3)P, which is essential for vacuolar protein sorting through interactions with FYVE and PX domain-containing proteins. PtdIns(3)P can be further phosphorylated by the PtdIns(3)P 5-kinase Fab1 to produce PtdIns(3,5)P2. Fab1 kinase activity is regulated by the upstream activator Vac7. The generation of PtdIns(3,5)P2 is essential for the maintenance of vacuolar size control and homeostasis through interactions with unknown effectors. Fig4, through its Sac1 polyphosphoinositide phosphatase domain, functions with Sj12 and Sjl3, in regulating PtdIns(3,5)P2 levels by mediating the turnover of this lipid.