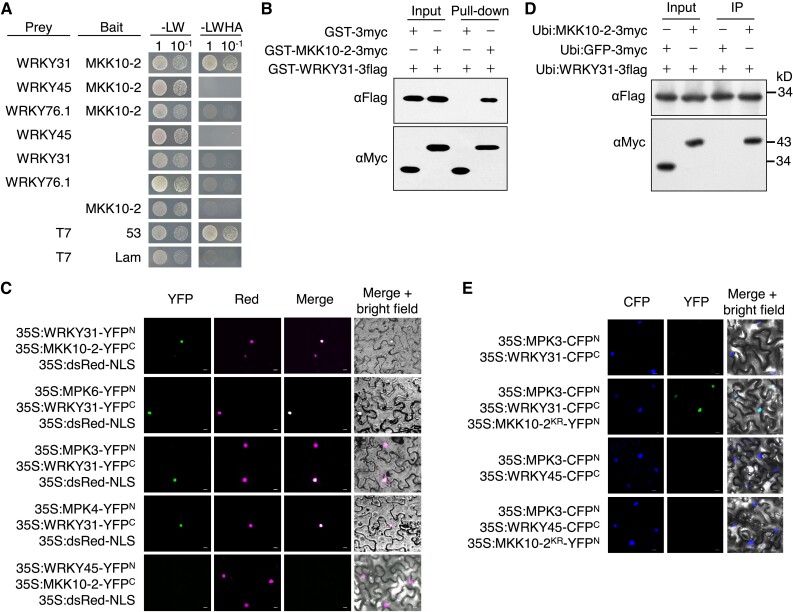
Figure 1.
OsMKK10-2 and OsMPKs interact with OsWRKY31. A) OsWRKY31, OsWRKY45, OsWRKY76.1, and OsMKK10-2 were fused to the Gal4 activation domain (prey) or DNA-binding domain (bait). The resulted plasmids in appropriate combinations were introduced into yeast cells and then incubated in synthetic dropout medium without Leu and Trp (-LW) or lacking Leu, Trp, His, and adenine (-LWHA) and photographed 3 d after plating. Yeast cells containing AD-T7 plus BD-53, and AD-T7 plus BD-Lam plasmids were used as the positive and negative controls, respectively. B) OsMKK10-2 and OsWRKY31 were sandwiched with GST and 3×Myc (GST-MKK10-2-3myc) or GST and 3×Flag (GST-WRKY31-3flag) tags. Purified protein (each about 1 μg) was combined accordingly and incubated at 4 °C for 2 h in immunoprecipitation buffer. The protein mixture was precipitated with anti-c-Myc agarose affinity gels, separated on 10% SDS-PAGE gels, and detected by immunoblots with αMyc and αFlag antibodies. C) OsWRKY31, OsWRKY45, OsMKK10-2, OsMPK3, OsMPK4, and OsMPK6 were fused in frame with the YFP N-terminal region (YFPN) or the YFP C-terminal region (YFPC). The Agrobacteria with combined plasmids were infiltrated into the leaves of N. benthamiana. Confocal images were taken at 2 d after the infiltrations. Red fluorescence signals indicative of the nuclei were from co-infiltrated 35S:dsRed-NLS (NLS: nuclear localization signal). D) Plasmids of Ubi:MKK10-2-3myc, Ubi:WRKY31-3flag, and Ubi:GFP-3flag control were introduced separately in A. tumefaciens and co-expressed in appropriate combinations in leaves of N. benthamiana. Total proteins were extracted and immunoprecipitated with anti-c-Flag affinity gels. The precipitates were subjected for immunoblots with αMyc and αFlag antibodies. E) OsWRKY31 or OsWRKY45 and OsMPK3 were fused with CFPC and CFPN, respectively. OsMKK10-2KR, a kinase-dead mutant with Lys81 to Arg81 substitution, was fused with YFPN. Images photographed at 2 d after the infiltrations are CFP (left), YFP (middle), and merged with bright field (right). Bar = 10 μm.