Table 3.
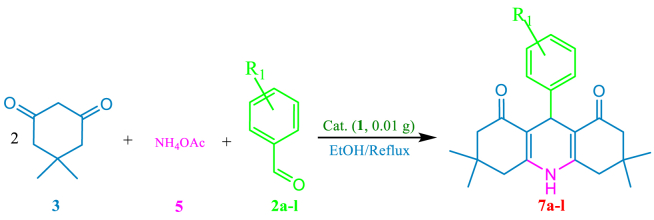
Synthesis of PHA derivatives 5a-l from different aldehydes 2a-l catalyzed by the Cs/ECH-TMA organocatalyst (1) under optimized conditions.a
| Entry | Aldehyde 2 | Product 7 | Time (min) | Yield (%) | M.P/Obs. (oC) | M.P/Rep. (oC) |
|---|---|---|---|---|---|---|
| 1 | 4-Chlorobenzaldehyde (2a) | 5a | 25 | 95 | 306–308 | 303 ‒ 305 [98] |
| 2 | 2,4-Dichlorobenzaldehyde (2b) | 5b | 25 | 94 | 319–322 | 321 [99] |
| 3 | 4-Nitrobenzaldehyde (2c) | 5c | 40 | 84 | 301–303 | 302 ‒ 305 [100] |
| 4 | 4-Hydroxy-3-methoxybenzaldehyde (2d) | 5d | 35 | 88 | 293–296 | 295 ‒ 298 [101] |
| 5 | 3-Nitrobenzaldehyde (2e) | 5e | 40 | 80 | 292–294 | 294 ‒ 296 [102] |
| 6 | 4-Florobenzaldehyde (2f) | 5f | 25 | 96 | 246–248 | 246 ‒ 248 [103] |
| 7 | Benzaldehyde (2g) | 5g | 25 | 92 | 190–191 | 189 ‒ 191 [103] |
| 8 | 2-Chlorobenzaldehyde (2h) | 5h | 25 | 92 | 222–224 | 221 ‒ 222 [104] |
| 9 | 4-Methoxybenzaldehyde (2i) | 5i | 25 | 86 | 295–297 | 296 ‒ 298 [105] |
| 10 | 4-Hydorxybenzaldehyde (2j) | 5j | 25 | 87 | 303–305 | >300 [106] |
| 11 | 4-Methylbenzaldehyde (2k) | 5k | 25 | 89 | 300–302 | >300 [106] |
| 12 | 2-Bromobenzaldehyde (2l) | 5l | 25 | 90 | 253–255 | 252 ‒ 254 [107] |
bAll products are known and their structures were established from their spectral data and melting points compared to authentic samples or literature values.
cIsolated yields.
Reaction conditions: Dimedone (3, 2.0 mmol), aryl aldehydes (2, 1.0 mmol), NH4OAc (5, 1.2 mmol) and the Cs/ECH-TMA (1, 10 mg) in EtOH under reflux conditions.