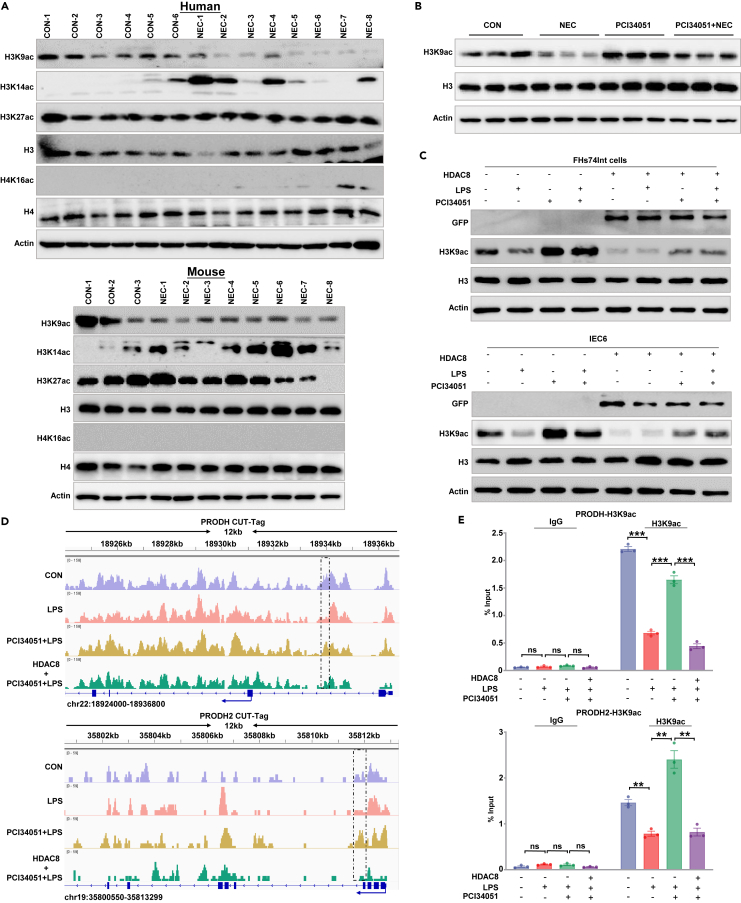
Figure 6.
HDAC8 maintains arginine metabolism enzymes by regulating H3K9ac activity
(A) Western blotting analysis was performed to assess the relative expression levels of genes known to be substrates of HDAC8 in healthy controls and NEC samples obtained from humans (upper panel) and mice (lower panel).
(B) Western blotting analysis was performed to assess the relative expression levels of genes known to be substrates of HDAC8 in IECs isolated from mice. Three independent experiments were performed.
(C) The protein levels of HDAC8, H3K9ac, H3K14ac, H3K27ac, and H4K16ac were analyzed by Western blotting in FHs74Int and IEC6 cells transfected with control lentivirus and HDAC8 lentivirus with 10 μmol/L PCI34051 incubation for 24 h and 10 μg/mL LPS for 48 h.
(D) H3K9ac-binding profiles on the targeted gene promoter detected by CUT-Tag in FHs74Int cells.
(E) ChIP analysis of H3K9ac in the PRODH and PRODH2 promoters under the conditions of PCI34051 (10 μmol/L for 24 h) or LPS (10 μg/mL for 48 h) treatment in control or HDAC8 stably overexpressing FHs74Int cells. The results are expressed as the means ± SEMs. Three independent experiments were performed. ∗∗p < 0.01, ∗∗∗p < 0.001. ns means no significance. Statistical significance was tested by two-tailed one-way analysis of variance test (E).