Abstract
Aims
In bradycardia patients treated with dual-chamber pacing, we aimed to evaluate whether pacing with atrioventricular (AV) delay management [AV hysteresis (AVH)], compared with standard pacing with fixed AV delays, reduces unnecessary ventricular pacing percentage (VPP) and is associated with better clinical outcomes. Main study endpoints were the incidence of heart failure hospitalizations (HFH), persistent atrial fibrillation (AF), and cardiac death.
Methods and results
Data from two identical prospective observational studies, BRADYCARE I in the USA and BRADYCARE II in Europe, Africa, and Asia, were pooled. Overall, 2592 patients (75 ± 10 years, 45.1% female, 50% with AVH) had complete clinical and device data at 1-year follow-up and were analysed. Primary pacing indication was sinus node disease (SND) in 1177 (45.4%), AV block (AVB) in 974 (37.6%), and other indications in 441 (17.0%) patients. Pacing with AVH, compared with standard pacing, was associated with a lower 1-year incidence of HFH [1.3% vs. 3.1%, relative risk reduction (RRR) 57.5%, P = 0.002] and of persistent AF (5.3% vs. 7.7%, RRR = 31.1%, P = 0.028). Cardiac mortality was not different between groups (1.0% vs. 1.4%, RRR = 27.8%, P = 0.366). Pacing with AVH, compared with standard pacing, was associated with a lower (P < 0.001) median VPP in all patients (7% vs. 75%), in SND (3% vs. 44%), in AVB (25% vs. 98%), and in patients with other pacing indications (3% vs. 47%).
Conclusion
Cardiac pacing with AV delay management via AVH is associated with reduced 1-year incidence of HFH and persistent AF, most likely due to a reduction in VPP compared to standard pacing.
Keywords: AV hysteresis, Pacemaker, Ventricular pacing, Heart failure, AV block, Sinus node dysfunction
Graphical Abstract
Graphical abstract.
What’s new?
In patients with indications for persistent dual-chamber cardiac pacing, incidence of heart failure hospitalizations (HFH) and persistent atrial fibrillation were significantly lower in patients programmed with atrioventricular (AV) delay management via AV hysteresis (AVH) compared with patients with standard pacing with fixed AV delay.
A previous study showed reduction of persistent atrial fibrillation, comparing pacing with AVH and pacing with fixed AV delay, in patients with sinus node disease. Our results expand those findings to the general population of bradycardia patients and show that AVH is also associated with reduced incidence of HFH.
While it was expected that the use of AVH would lower ventricular pacing in patients with sinus node disease, our analyses also show ventricular pacing reduction in patients with high-degree AV block; this suggests that AV conduction disease may often be intermittent and paroxysmal in these patients.
Introduction
High rates of right ventricular pacing (RVP) are increasingly recognized as harmful to pacemaker patients, being associated with increased rates of atrial fibrillation (AF), ischaemic stroke, heart failure (HF), and death.1,2 Consistent with these observations, international guidelines3,4 suggest the use of algorithms to promote intrinsic conduction, at least in patients without atrioventricular (AV) conduction block, but also caution that stretching AV pacing intervals in patients with prolonged AV conduction can cause AV dyssynchrony, diastolic mitral regurgitation, AF, and HF. To avoid the potentially deleterious effects of long-term RVP, alternative approaches have been promoted, such as a wider use of cardiac resynchronization therapy (CRT) or conduction system pacing; however, the supportive evidence is still limited.4
Several algorithms have been proposed to reduce unnecessary ventricular pacing, and their operations are based on two different mechanisms: (i) progressive prolongation of the AV pacing delay [AV hysteresis (AVH)] and (ii) AAI-DDD pacing mode. Both mechanisms have DDD back-up when needed. Both AVH and AAI-DDD modes have been shown to reduce ventricular pacing in clinical trials.5 While the use of these algorithms was initially proposed in sinus node disease (SND) patients, recent data from two controlled studies6,7 indicated a benefit even in patients with AV block (AVB) as their pacemaker indication.5 While the use of AVH has been shown to significantly reduce persistent AF,8 a recent meta-analysis questions the clinical impact of AV management through the AAI-DDD mode in pacemaker patients.9
To shed more light on this important controversy, we pooled the data of two prospective observational studies, BRADYCARE I in the USA and BRADYCARE II in Europe and Asia, which evaluated the clinical benefit of AVH algorithms to reduce incidence of persistent AF, HF hospitalizations (HFH), and cardiac death.
Methods
Study design
Individual patient data from two multicentre, observational studies with 1-year follow-up post-implant, BRADYCARE I (Clinicaltrial.gov NCT01062126) and BRADYCARE II (Clinicaltrial.gov NCT02577887), were pooled to perform this analysis. The two studies were identical in design and mainly differed because BRADYCARE I included a population from the USA and BRADYCARE II included a population from Europe, Africa, and Asia. The studies were conducted in compliance with Good Clinical Practice and the Declaration of Helsinki. Investigational review board or ethics committee approval was obtained before study initiation. All patients signed an informed consent prior to participating in any study-related activities.
Eligibility criteria
Patients were eligible to participate in the studies if they had a standard indication for a pacemaker, were implanted with a dual-chamber pacemaker capable of AV sequential pacing, were within 30 days of implant, ≥18 years of age, and were able to provide written informed consent. Exclusion criteria included life expectancy of less than 1 year and current or planned pregnancy.
Intervention
Since the studies were observational in nature, clinicians programmed the pacemakers per their standard practice. Patients were categorized into two groups, named the AVH group or standard pacing group, based on the status of the AVH feature, ON or OFF, respectively. Subjects with a change in the status of this feature during the study period were excluded from the analysis.
Atrioventricular hysteresis algorithm
The AVH algorithm used in the BRADYCARE I and II studies was the Ventricular Intrinsic Preference (VIP™) algorithm (Abbott, Sylmar, CA, USA). This is an AV search hysteresis algorithm which searches for and promotes intrinsic AV conduction. When the VIP™ algorithm is activated, the device periodically (every 30 s) extends the sensed AV (SAV) and paced AV (PAV) delays by a programmable value (e.g. by 150 ms) for a number of programmed search cycles (e.g. three cycles) to search for intrinsic conduction.10 Additionally, when three consecutive R-waves occur at the programmed SAV or PAV delays, VIP™ will extend the SAV/PAV delays by the programmed value. If an R-wave is sensed during the extended AV delay, the ventricular pulse is inhibited, and the SAV/PAV delays will remain extended until VP occurs. If intrinsic conduction does not occur during this extended AV delay, the device reverts to the programmed static AV delay. With the VIP™ algorithm, SAV/PAV delays are limited to programmable maximum value (in any case lower than 350 ms). The long AV delay is maintained until a programmable number of cycles with absent ventricular-sensed events (i.e. continuous need for ventricular pacing). The VIP™ feature is disabled during atrial high-rate events and mode switches.
Study objectives and endpoints
The primary objective of this analysis was to evaluate whether dual-chamber pacing with AV delay management (AVH algorithm), compared with standard pacing with fixed AV delays, reduces unnecessary RVP percentage and improves clinical outcomes. Main study endpoints were the incidences of cardiac death, HFH, and persistent AF. Incidences of cardiac death and HFH were evaluated in the whole cohort of 2592 patients on the basis of clinical outcomes data collected during follow-up visits. Data about AF incidence and burden, as percentage of time spent in AF by the patient, were extracted from device diagnostics. Incidence of persistent AF was evaluated in the subgroup of 2039 patients who had no previous history of persistent/permanent AF at implant. This choice was derived from the objective of evaluating the deleterious effect of unnecessary RVP on the progression of atrial disease. Patients were diagnosed with persistent AF if they spent a given percentage of the 12-month observation period in AF; this per cent threshold was set according to the results of the MINERVA trial11 that showed that in patients who developed persistent AF, the median AF burden was ≥50% in SND patients and ≥30% in patients with other pacing indications. Appropriateness of persistent AF diagnosis, which was based on device data, was confirmed by verifying that when the device detected AF occurrence, the investigators reported AF events in the follow-up case report forms.
The secondary endpoint of our study was ventricular pacing percentage (VPP) which was evaluated in the two study groups (pacing with AVH vs. pacing with fixed AV delays) and according to pacing indication.
Data collection
Demographics, primary indication for pacemaker implant, medical history, and medication information were recorded at the time of enrolment. Patients who had both SND and AVB were classified as AVB patients. Clinical outcomes and device data, such as AF burden and VPP, were collected at the scheduled and unscheduled follow-up visits throughout the 1-year observation period. Reported clinical outcomes were verified through monitoring of data source documentation. Device data included information about all variables relevant to the pacemaker function: advanced feature usage (e.g. AVH programming), stored electrocardiograms, device-detected cardiac events (e.g. AF), and device-related measures (e.g. VPP).
Statistical analysis
Individual participant data from both studies, BRADYCARE I and BRADYCARE II, were pooled and analysed. Following best practices to perform pooled analysis,12 we verified that the two studies were identical in terms of inclusion/exclusion criteria, follow-up schedules, and the data recorded at each visit. We compared the patient’s baseline characteristics of the two pooled studies to verify that the two studies included similar patient populations, and we compared the incidences of the studied endpoints in the two pooled studies. Sensitivity analyses did not show significant statistical differences between the two studies; therefore, no further adjustments were made to account for combining the study data.
Patient characteristics were summarized via descriptive statistics, including mean and standard deviation, or median with the interquartile range, for continuous variables, and counts and percentages for categorical/nominal variables, as appropriate. The 1-year incidence of clinical endpoints was expressed in percentage with 95% confidence intervals. The difference of clinical endpoint incidences when comparing patients with AVH and patients with standard pacing was expressed as relative risk reduction (RRR). Since the BRADYCARE studies did not randomize the use of AVH, when comparing clinical endpoint incidences between patients with AVH and patients with standard pacing, we verified that baseline characteristics were similar in the compared patient groups.
Statistical comparisons were performed through Student’s t-test for normally distributed variables and by nonparametric tests for variables with skewed distributions. A chi-square proportion test was performed to compare incidences of the clinical endpoints. All tests were two-sided, and a P-value < 0.05 was considered statistically significant.
Analyses were performed using SAS or the statistics toolbox in MATLAB R2018a.
Results
Subject disposition
A total of 5490 patients were enrolled in the two BRADYCARE studies with a standard indication for pacemaker implant, respectively, 3389 (61.7%) in BRADYCARE I and 2101 (38.3%) in BRADYCARE II. In the BRADYCARE I study, consecutive patient recruitment started in February 2010 and lasted till September 2011 and the completion date was in September 2012. In the BRADYCARE II study, consecutive patient recruitment started in July 2015 and lasted till November 2016, and the completion date was in November 2017.
The main characteristics of patients included in the two studies have been already described in the clinicaltrial.gov repository and are reported in table A of the Supplementary material online, Materials.
For the purpose of our analyses, from this patient cohort, we included 2592 patients who had complete clinical outcomes data and complete device data regarding VPP and AF burden throughout the 1-year observation period. This patient subgroup was composed by 1544 (60%) BRADYCARE I patients and 1048 (40%) BRADYCARE II patients. Baseline characteristics of these 2592 patients are shown in Table 1. In particular, the primary indication for pacing was SND in 1177 (45.4%), AVB in 974 (37.6%), and other indications in 441 (17.0%), where other indications comprised syncope (defined as recurrent syncope caused by carotid sinus stimulation or symptomatic recurrent syncope associated with documented bradycardia or syncope that is not determined to be due to AVB when other likely causes were excluded), familial condition (conditions with a high risk for bradycardia such as long QT syndrome), prevention and termination of tachyarrhythmias by pacing (symptomatic recurrent SVT or sustained pause-dependent VT where the efficacy of pacing was thoroughly documented), and pacemaker replacement.
Table 1.
Baseline characteristics in the analysed patient cohort
| Patient characteristics | Whole cohort 2592 patients |
Standard pacing 1296 patients |
AV hysteresis 1296 patients |
P-value standard vs. AV hysteresis |
|---|---|---|---|---|
| Age | 75 ± 10 | 76 ± 12 | 74 ± 12 | ns |
| Gender (female) | 1168/2592 (45.1%) | 555/1296 (42.8%) | 613/1296 (47.3%) | 0.022 |
| Primary indication | ns | |||
| SND | 1177/2592 (45.4%) | 629 (48.5%) | 548 (42.3%) | |
| AV block | 974/2592 (37.6%) | 444 (34.2%) | 530 (40.9%) | |
| Other | 441/2592 (17.0%) | 223 (17.2%) | 218 (16.8) | |
| Atrial fibrillation | 1352/2592 (52.2%) | 741/1296 (57.2%) | 705/1296 (59.4%) | ns |
| Hypertension | 1917/2592 (74.0%) | 946/1296 (73.0%) | 971/1296 (74.9%) | ns |
| Diabetes | 644/2592 (24.8%) | 328/1296 (25.3%) | 316/1296 (24.4%) | ns |
| Cardiomyopathy | 469/2592 (18.1%) | 241/1296 (18.6%) | 228/1296 (17.6%) | ns |
| Myocardial infarction | 255/2590 (9.8%) | 125/1294 (9.6%) | 130/1296 (10.0%) | ns |
| NYHA class III or IV | 724/2289 (31.6%) | 371/1144 (32.4%) | 353/1145 (30.8%) | ns |
| LVEF (mean ± st dev) | 58 ± 10 | 57 ± 10 | 58 ± 10 | ns |
| LVEF < 50% | 250/1856 (13.5%) | 133/938 (14.2%) | 117/918 (12.7%) | ns |
AV, atrioventricular; LVEF, left ventricle ejection fraction; ns, not significantly different (in particular P-value was >0.15 for all comparisons when P = ns); NYHA, New York Heart Association; SND, sinus node disease.
Atrioventricular block was present in 974/2592 (37.6%) patients, 72/2592 (2.8%) had first-degree AVB, 120/2592 (4.6%) had second-degree type 1 AVB, 252/2592 (9.7%) had second-degree type 2 AVB, and 530/2592 (20.4%) had third-degree AVB.
Paroxysmal AF was present in 799/2592 (30.8%), persistent AF in 223/2592 (8.6%), and permanent AF in 330/2592 (12.7%).
The AVH feature was enabled and maintained in 1296 (50%) of patients, while the other 1296 (50%) patients were continuously programmed with standard DDD pacing with fixed AV delays. Comparison of patients’ baseline characteristics in the two study groups (standard DDD pacing vs. AVH pacing) is shown in Table 1.
Ventricular pacing lead was positioned in the right ventricular apex in 1735 (66.9%) of patients, in the right ventricular septum in 670 (25.8%) of patients, and in other right ventricular sites in 187 (7.2%) of patients.
The AF Suppression™ algorithm, designed to pace the atrium at rates faster than the intrinsic atrial rate to overdrive and suppress paroxysmal or persistent AF, was enabled in a minority (15) (0.6%) of patients, eight in the standard DDD pacing group and seven in the AVH pacing group.
Ventricular pacing percentage
Patients with the AVH algorithm programmed ON were associated with significantly lower VPP, compared with patients with standard pacing. This was observed in both the total population and in all the patient subgroups, as shown in Table 2. In particular, the median VPP with AVH enabled was 3% in patients with a SND pacing indication, and it was 25% in patients with AVB.
Table 2.
Percentage of ventricular pacing according to pacing indication and AVH programming
| Patient cohort | Median ventricular pacing percentage (interquartile range) | ||
|---|---|---|---|
| Standard pacing | AV hysteresis | P-value | |
| All pacing indications | 75% (20–99) | 7% (1–48) | P < 0.0001 |
| Sinus node disease | 44% (6–76) | 3% (1–21) | P < 0.0001 |
| AV block | 98% (56–99) | 25% (2–74) | P < 0.0001 |
| Other | 47% (8–92) | 3% (1–19) | P < 0.0001 |
AV, atrioventricular.
Importantly, the median VPP in first-degree AVB was 75% with standard pacing and 33% with AVH; in the second-degree AVB type, it was 98% with standard pacing and 20% with AVH; in second-degree AVB type 2, it was 97% with standard pacing and 26% with AVH; and in third-degree AVB, it was 98% with standard pacing and 22% with AVH.
Clinical outcomes as a function of atrioventricular delay management programming
The 1-year incidences of cardiac death, HFH, and persistent AF, as estimated in the pooled analyses, are shown in Table 3. These incidences were also separately estimated in the two BRADYCARE studies and are reported in table B of the Supplementary material online, Materials.
Table 3.
Incidence rate [95% confidence interval (CI)] of study endpoints according to pacing indication and AVH programming
| Patients | Endpoint | Standard pacing | AV hysteresis | RRR | P-value |
|---|---|---|---|---|---|
| Whole cohort | Cardiac death | 18/1296 1.4% (0.8–2.2) % |
13/1296 1.0% (0.5–1.7) % |
27.8% | 0.366 |
| Whole cohort | HF hospitalizations |
40/1296
3.1% (2.2–4.2) % |
17/1296
1.3% (0.8–2.1) % |
57.5% | 0.002 |
| Whole cohort a | Persistent AF |
67/870
7.7% (6.0–9.7) % |
62/1169
5.3% (4.1–6.7) % |
31.1% | 0.028 |
| SND | Cardiac death | 8/548 1.5% (0.6–2.9) % |
5/629 0.8% (0.3–1.8) % |
45.5% | 0.276 |
| SND | HF hospitalizations |
21/548
3.8% (2.4–5.8) % |
11/629
1.7% (0.9–3.1) % |
54.4% | 0.028 |
| SND a | Persistent AF |
32/251
12.7% (8.9–17.5) % |
41/522
7.9% (5.7–10.5) % |
38.4% | 0.029 |
| AV block | Cardiac death | 8/530 1.5% (0.7–3.0) % |
6/444 1.4% (0.5–2.9) % |
10.5% | 0.836 |
| AV block | HF hospitalizations | 10/530 1.9% (0.9–3.4) % |
5/444 1.1% (0.4–2.6) % |
40.3% | 0.337 |
| AV block a | Persistent AF |
22/488
4.5% (2.8–6.7) % |
8/434
1.8% (0.8–3.6) % |
60.0% | 0.023 |
| Other | Cardiac death | 2/218 0.9% (0.1–3.3) % |
2/223 0.9% (0.1–3.2) % |
2.2% | 0.982 |
| Other | HF hospitalizations |
9/218
4.1% (1.9–7.7) % |
1/223
0.4% (0.0–2.5) % |
89.1% | 0.009 |
| Othera | Persistent AF | 13/131 9.9% (5.4–16.4) % |
13/213 6.1% (3.3–10.2) % |
32.3% | 0.193 |
AF, atrial fibrillation; AV, atrioventricular; HF, heart failure; RRR, risk rate ratio; SND, sinus node disease.
Patients without history of persistent/permanent AF at inclusion. Bold values indicate all the endpoints with P < 0.05.
The incidence of cardiac death was not different between groups (1.0% in the AVH group vs. 1.4% in the standard pacing group, RRR = 27.8%, P = 0.366).
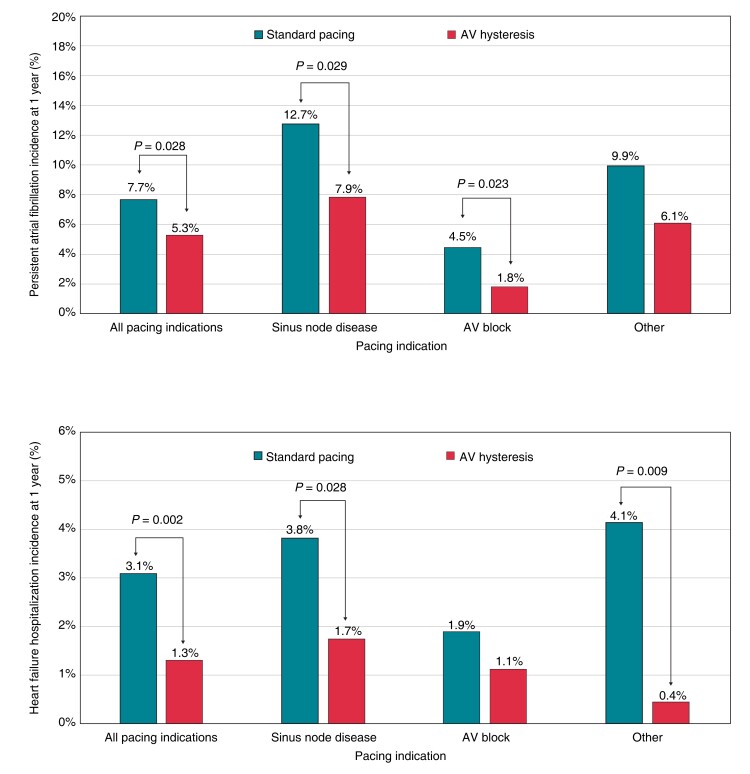
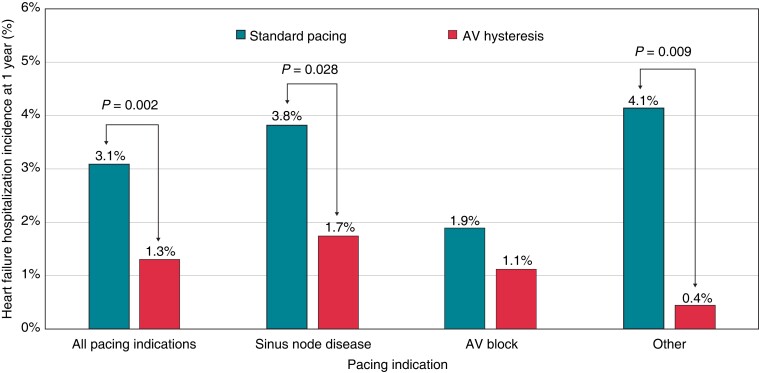
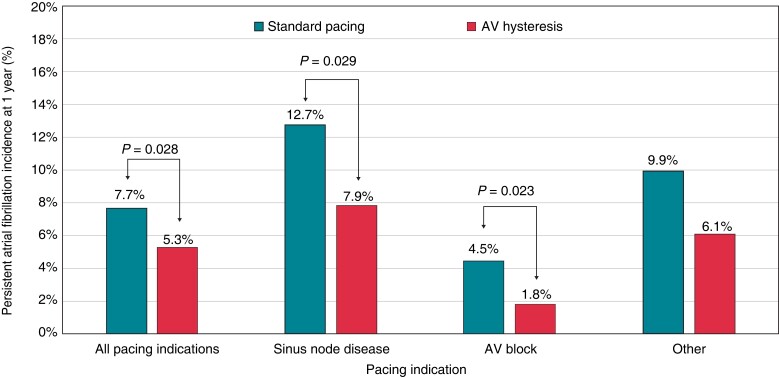
The incidence of HFH was significantly lower in the AVH group compared with the standard pacing group (1.3% vs. 3.1%, RRR = 57.5%, P = 0.002). This reduction, observed in the whole population, was also confirmed in SND patients and in patients with other pacing indications; however, only a trend towards reduction was observed in AVB patients (Figure 1). The history of persistent or permanent AF in the whole population of 2492 patients was associated with a higher risk of HFH, which occurred in 3.8% patients with AF and in 1.8% patients without AF (chi-square proportion test, P = 0.0032). Heart failure hospitalizations among patients with standard DDD pacing occurred in 4.7% patients with persistent or permanent AF and in 2.3% patients without AF (chi-square proportion test, P = 0.019) while in patients with AVH, pacing occurred in 0.8% patients with persistent or permanent AF and in 1.4% patients without AF (P = ns). Incidence of persistent AF was significantly lower in the AVH group compared with the standard pacing group (5.3% vs. 7.7%, RRR = 31.1%, P = 0.028). Specifically, persistent AF occurred in 7.9% of SND patients with AVH and in 12.7% of SND patients with standard pacing (RRR = 38.4%, P = 0.029). In AVB patients, persistent AF occurred in 1.8% of those with AVH and in 4.5% of patients with standard pacing (RRR = 60.0, P = 0.023), as shown in Figure 2. Of the whole study population, 2039 patients without a history of persistent/permanent AF were evaluated to compare new persistent AF between the AVH and standard pacing groups. The baseline characteristics were homogeneous between the two study groups (Tables C, D, and E of the Supplementary material online, Materials).
Figure 1.
Incidence of the heart failure hospitalizations at 1 year according to pacing indication and AV hysteresis programming. AV, atrioventricular.
Figure 2.
Incidence of persistent atrial fibrillation at 1 year according to pacing indication and AV hysteresis programming. AV, atrioventricular.
Safety endpoints
Incidence of adverse events in the BRADYCARE I and BRADYCARE II studies has been already described in the respective clinicaltrial.gov repositories. In our patient population, safety endpoints and adverse events were not different between patients with AVH or standard pacing; in particular, syncopal events were very rare and not different in the two groups [2/1296 (0.15%), standard vs. 1/1296 (0.08%) AVH].
Discussion
BRADYCARE studies represent two of the largest international studies on pacemaker patients performed in the last decade and provide information about a contemporary population managed in real-world practice. This analysis provides strong evidence about the clinical value of the automatic AV management algorithm (AVH) in pacemaker patients. The mechanism behind this observed reduction in persistent AF and HFH is not entirely clear but may be due to improved AV synchrony when reduction in RVP is successfully achieved.
Heart failure hospitalizations and atrioventricular hysteresis
Several studies1,2,13 have associated HFH with high and unnecessary RVP, suggesting that non-physiological RVP pacing may induce inter- and intra-ventricular dyssynchrony and AV dyssynchrony and may cause pacing-induced cardiomyopathy.
Our results clearly show that the minimization of RVP is strongly associated with a reduction in HFH at 1 year [1.3% (AVH) vs. 3.1% (standard pacing); RRR = 57.5%, P = 0.002] in the overall population. Furthermore, this clinical benefit was observed in SND patients (1.7% vs. 3.8%, RRR = 54.4%, P = 0.028) and in patients with ‘other’ as their pacing indication (0.4% vs. 4.1%, RRR = 89.1%, P = 0.009). Interestingly, HFH incidence in AVB patients was low [1.1% (AVH) vs. 1.9% (standard pacing), RRR = 40.3%, P = 0.337]. This result may be evaluated in the context of the short observation period of our study. The observed low incidences of HFH in AVB patients are similar to those observed in large pacemaker trials1,9 and may be explained by the fact that many patients enrolled in our study had normal left ventricular function and may tolerate ventricular pacing in a 1-year horizon.
Persistent atrial fibrillation and atrioventricular hysteresis
The incidence of persistent AF was significantly lower in the AVH group compared with the standard pacing group in the overall population (5.3% vs. 7.7%, RRR = 31.1%, P = 0.028). In particular, persistent AF occurred in 1.8% of AVB patients with AVH and in 4.5% of AVB patients with standard pacing (RRR = 60.0, P = 0.023). This finding has high importance because specific data on the clinical benefit of reducing ventricular pacing in AVB patients are scarce and because it also reinforces the proposal to shift to a pacing paradigm implementing AV delay management in patients with intermittent and paroxysmal AVB.5
As for SND patients in our study, persistent AF occurred in 12.7% patients with standard pacing and in 7.9% patients with AVH (RRR = 38.4%, P = 0.029). In the SavePACE trial,8 which followed SND patients for a mean follow-up period of 1.7 years, the incidence of persistent AF was equal to that observed in our studies [12.7% in the dual-chamber rate-modulated (DDDR) arm and 7.9% in the arm treated with algorithms to reduce RVP]. Interestingly, the algorithm used for reducing unnecessary ventricular pacing in 90% of patients in the SavePACE trial was an AVH algorithm (Medtronic Search AV), an algorithm similar to the one used here.
Several algorithms have been proposed to reduce unnecessary ventricular pacing, and their operation is based on two different mechanisms: (i) progressive prolongation of the AV pacing delay (AVH) and (ii) AAI-DDD pacing mode, with both mechanisms having DDD back-up when needed. Both AVH and AAI-DDD modes have been proven in terms of technical efficacy to reduce RVP in several studies.5
Several randomized studies have tested the benefit of AAI-DDD switch algorithms in pacemaker patients.6,7,11,14,15 Even if none of these studies found a significant reduction of the study primary endpoints when comparing AAI-DDD switch algorithms with standard DDD pacing, nevertheless, the ANSWER trial6 showed a 51% risk reduction in experiencing cardiac death or HFH and a 30% risk reduction in experiencing cardiovascular hospitalizations when using the SafeR algorithm instead of standard DDD pacing.
Furthermore in the SavePACE trial,8 which showed persistent AF reduction using AVH (Medtronic Search AV), the AAI-DDD switch algorithm (Medtronic MVP) used in 10% of study patients was not associated with persistent AF reduction when compared with standard DDDR pacing. A recent meta-analysis of these studies confirmed that despite a reduction in ventricular pacing, AAI-DDD algorithms failed to affect all-cause death, all-cause hospitalizations, and persistent AF in patients with preserved left ventricular function9, which constitutes an unexpected result after the clear evidence of deleterious effect of long-term ventricular pacing.
The results here should be interpreted in the context of several similar recent clinical trials.11,16,17 In the MINERVA trial, the MVP algorithm failed to show a benefit compared with DDDR pacing,11 but secondary analyses of that trial16 showed that MVP was associated with a lower risk of persistent AF compared with the DDDR mode in patients with PR < 180 ms and that MVP was associated with a higher risk of persistent AF compared with the DDDR mode in patients with PR ≥ 180 ms. In the DANPACE trial,17 a higher occurrence of AF was observed in patients with a PR interval longer than 180 ms when treated with AAI pacing compared with standard DDDR. The MVP trial18 also observed worse clinical outcomes (HFH and death) in patients with long PR interval treated with MVP compared with DDDR pacing. All these trials strongly suggest that AAI pacing or AAI-DDD switch algorithms, without limitations in the longest allowed AV delays, are less effective in patients with prolonged PR intervals and AV conduction. Programming that allows for markedly prolonged AV delays and does not correct first-degree AVB can worsen mitral regurgitation, shorten diastolic filling, and cause pacemaker syndrome.19 According to these findings, in patients with prolonged AV conduction, AVH algorithms or AAI-DDD algorithms limited to AV delays shorter than 250 ms may be more physiological and improve clinical outcomes.
Unnecessary ventricular pacing and atrioventricular hysteresis
The AVH algorithm tested in our analyses was associated with a significantly lower VPP in all pacing indications (Table 2). The significant reduction in median VPP also observed in patients with AVBs—from 98% [interquartile range (IQR) = 56–99%] with standard pacing to 25% (IQR = 2–74%) with AVH—suggests that in a relevant proportion of patients with AV conduction disturbance, the AV conduction may be more dynamic than previously thought, possibly with significant circadian and/or monthly variations. Interestingly, VPP was reduced with AVH pacing, compared with standard DDD pacing, for all sub-types of AVB. Recently Auricchio and Ellenbogen5 have suggested that implementation of an algorithm enabling RVP reduction is warranted not only in SND patients but also in patients with intermittent and paroxysmal AVB instead of programming fixed AV pacing intervals.
The AVH algorithm used in our studies was tested in two previous randomized studies10,20 that showed a significant reduction of unnecessary ventricular pacing compared with standard DDDR pacing. Pakarinen and Toivonen10 showed that VIP™ reduces VPP in SND patients with both intact and compromised AV conduction. Among 389 SND patients, 30.1% had intact AV conduction (PR interval was ≤210 ms on ECG and 1 : 1 AV conduction during atrial pacing up to 120 bpm with PR interval ≤ 350 ms), and 69.9% had intermittent AVB. The mean VPP at 12 months was 9.6% by VIP™ compared to 51.8% with standard AV settings in patients with intact AV conduction (P < 0.0001) and 28.0% vs. 78.9% (P < 0.0001) in patients with compromised AV conduction. In a smaller study, Yadav et al.20 showed similar results; 80 patients were classified to either an intact AV conduction or compromised AV conduction and were then randomized (1 : 1) to the VIP™ algorithm or to standard DDDR with SAV and PAV delays programmed at 150 and 180 ms, respectively. The mean VPP evaluated at 12-month post-implantation follow-ups in the VIP™ ON vs. DDDR groups were 15% vs. 68% (P < 0.01) in the intact AV conduction groups and 39% vs. 97% (P < 0.001) in the compromised AV conduction groups.
Clinical implications
The observation that the AVH algorithm significantly reduced the median VPP in all the evaluated pacemaker cohorts, regardless of pacing indication (Table 2), without safety issues, forms the theoretical basis for proposing AVH not only to SND patients but possibly to all pacemaker patients. With this perspective, it is time to change the current pacing paradigm and attempt to also reduce ventricular pacing frequency in patients with paroxysmal AVBs.5
Despite the fact that at pacemaker implant, AV conduction disturbance type—persistent or intermittent—may not be fully known, AVH algorithms seem to be a safe choice because they allow both to reduce the risk of pacing-induced cardiac dyssynchrony and to address the bradycardia indication by providing AV synchrony with good trans-mitral left ventricle (LV) filling when needed.
The use of algorithms to reduce unnecessary ventricular pacing is indicated by AHA/ACC/HRS3 and ESC4 Guidelines in SND patients and suggested for use in patients with intermittent AVB (while taking into account the possibility that prolonged AV conduction in severe first-degree AVB could be disadvantageous from a haemodynamic point of view, possibly being associated with inappropriate timing of atrial and ventricular contraction and/or causing diastolic mitral regurgitation, symptoms, and AF).
Limitations
The BRADYCARE studies were not randomized trials, and therefore, they are subject to all of the limitations of observational studies; selection biases or confounding factors cannot be excluded. Importantly, the baseline characteristics of the two study cohorts—AVH pacing and standard DDD pacing—were very similar (Table 1 and Supplementary material online, Table C, D, E of Materials). This suggests that the choice of the pacing mode was likely not driven by different patient characteristics and supports the possibility that the results of our analyses were also not biased by major differences in the patients’ characteristics.
Our data did not allow us to compare the pharmacological therapy between the two study groups.
We recognize the fact that we analysed a subgroup (2592) of the whole cohort (5490) of patients included in the BRADYCARE I and BRADYCARE II studies. The selection of the 2592 patients was based on the need to have complete clinical outcomes data and complete device data throughout the 1-year observation period. The comparison of the baseline characteristics of the 2592 analysed patients and the 5490 whole cohort patients (Supplementary material online, Table F of Materials) shows that the two populations were quite homogeneous.
While the concept of reduction of unnecessary ventricular pacing may be applied also to patients wearing implantable cardioverter defibrillators, the results of our analyses apply to patients with indications for dual-chamber cardiac pacing.
The evaluated cohort of BRADYCARE I and II studies was not dimensioned to evaluate the impact of AVH in all the subgroups of patients identified according to all baseline characteristics.
While higher incidences of HFH and AF are usually associated with higher mortality risk, our data did not show higher incidence of death in the standard pacing group. The 1-year observation period of our study possibly limited the possibility to observe long-term deleterious effects of unnecessary ventricular pacing on the risk of death.
BRADYCARE studies were observational registries with source data verification monitoring activities which warranted coherent reporting from the Hospital Medical Records to the study data collection system. We cannot exclude under-reporting of clinical outcomes data, but we hypothesize that, if that occurred, that would have impacted both patients with and without AVH and therefore should not have biased study results.
Conclusions
In patients indicated to permanent dual-chamber cardiac pacing, incidence of HFH and incidence of persistent AF were significantly lower in patients programmed with an AV delay management via AVH compared with patients with standard pacing.
While it is expected that the use of AV delay managed by AVH is associated with lower VPP in patients with SND, our analyses also showed a reduction of ventricular pacing in a relevant proportion of patients with high-degree AVBs, suggesting that in these patients conduction disease may be intermittent and paroxysmal during follow-up.
Supplementary Material
Acknowledgements
We would like to thank the investigators involved in both studies for their diligent efforts in gathering the study data and the internal and external reviewers for their meaningful contributions to the development of this manuscript. We acknowledge the work and contribution to our research of Craig Barnett, investigator of BRADYCARE I study, who passed away in 2012.
Contributor Information
Martin Arnold, Cardiology Department, Friedrich-Alexander-Universität, Erlangen, Germany.
Mark Richards, Cardiology Department, Yakima Heart and Vascular, Yakima, WA, USA.
Antonio D’Onofrio, Cardiology Department, UOSD di Elettrofisiologia, Studio e Terapia delle Aritmie A.O.R.N. ‘Ospedali dei Colli’ Osp Monaldi, Napoli, Italy.
Brett Faulknier, Cardiology, West Virginia University, Vero Beach, FL, USA.
Michele Gulizia, Cardiology Department, Azienda Ospedaliera Garibaldi Nesima, Catania, Italy.
Ranjan Thakur, Cardiology Department, Thoracic Cardio Healthcare Foundation, Lansing, MI, USA.
Yasushi Sakata, Cardiology Department, Osaka University Hospital, Osaka, Japan.
Wenjiao Lin, Biostatistics, Abbott, Sylmar, CA, USA.
Annalisa Pollastrelli, Medical Affairs, Abbott, Rome, Italy.
Andrea Grammatico, Medical Affairs, Abbott, Rome, Italy.
Angelo Auricchio, Clinical Electrophysiology Unit, Cardiocentro Ticino Institute, Lugano, Switzerland.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena University Hospital, Via del Pozzo, 71, Modena 41124, Italy.
Supplementary material
Supplementary material is available at Europace online.
Funding
The BRADYCARE I and II were sponsored by St. Jude Medical, now part of Abbott, that provided technical support for study management and data management. As for the analyses of the BRADYCARE I and II pooled data, presented in this manuscript, Abbott has provided technical support for data management and statistical analysis. The sponsor had no role in the collection of clinical data, in the interpretation of data, and in the decision to submit the article for publication. All authors have read and agree to the manuscript as written.
Data availability
Some of the data described in this manuscript are available in Clinicaltrial.gov and can be accessed with the following trials’ identifiers NCT0106212 and NCT02577887. Other data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KLet al. . Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. [DOI] [PubMed] [Google Scholar]
- 2. Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation 2006;113:2082–8. [DOI] [PubMed] [Google Scholar]
- 3. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MRet al. . 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019;140:e382–482. [DOI] [PubMed] [Google Scholar]
- 4. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. . 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. [DOI] [PubMed] [Google Scholar]
- 5. Auricchio A, Ellenbogen KA. Reducing ventricular pacing frequency in patients with atrioventricular block: is it time to change the current pacing paradigm? Circ Arrhythm Electrophysiol 2016;9:e004404. [DOI] [PubMed] [Google Scholar]
- 6. Stockburger M, Boveda S, Moreno J, Da Costa A, Hatala R, Brachmann Jet al. . Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J 2015;36:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thibault B, Ducharme A, Baranchuk A, Dubuc M, Dyrda K, Guerra PGet al. . Very low ventricular pacing rates can be achieved safely in a heterogeneous pacemaker population and provide clinical benefits: the CANadian Multi-Centre Randomised Study-Spontaneous AtrioVEntricular Conduction pReservation (CAN-SAVE R) Trial. J Am Heart Assoc 2015;4:e001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick Det al. . Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med 2007;357:1000–8. [DOI] [PubMed] [Google Scholar]
- 9. Shurrab M, Healey JS, Haj-Yahia S, Kaoutskaia A, Boriani G, Carrizo Aet al. . Reduction in unnecessary ventricular pacing fails to affect hard clinical outcomes in patients with preserved left ventricular function: a meta-analysis. Europace 2017;19:282–8. [DOI] [PubMed] [Google Scholar]
- 10. Pakarinen S, Toivonen L. Minimizing ventricular pacing by a novel atrioventricular (AV) delay hysteresis algorithm in patients with intact or compromised intrinsic AV conduction and different atrial and ventricular lead locations. Ann Med 2013;45:438–45. [DOI] [PubMed] [Google Scholar]
- 11. Boriani G, Tukkie R, Manolis AS, Mont L, Pürerfellner H, Santini Met al. . Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J 2014;35:2352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol 1999;28:1–9. [DOI] [PubMed] [Google Scholar]
- 13. Sharma AD, Rizo-Patron C, Hallstrom AP, O’Neill GP, Rothbart S, Martins JBet al. . Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005;2:830–4. [DOI] [PubMed] [Google Scholar]
- 14. Davy JM, Hoffmann E, Frey A, Jocham K, Rossi S, Dupuis JMet al. . Near elimination of ventricular pacing in SafeR mode compared to DDD modes: a randomized study of 422 patients. Pacing Clin Electrophysiol 2012;35:392–402. [DOI] [PubMed] [Google Scholar]
- 15. Chen S, Chen K, Tao Q, Zheng L, Shen F, Wu Set al. . Reduction of unnecessary right ventricular pacing by managed ventricular pacing and search AV+ algorithms in pacemaker patients: 12 month follow-up results of a randomized study. Europace 2014;16:1595–602. [DOI] [PubMed] [Google Scholar]
- 16. Boriani G, Pieragnoli P, Botto GL, Puererfellner H, Mont L, Ziacchi Met al. . Effect of PR interval and pacing mode on persistent atrial fibrillation incidence in dual chamber pacemaker patients: a sub-study of the international randomized MINERVA trial. Europace 2019;21:636–44. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen JC, Thomsen PE, Højberg S, Møller M, Riahi S, Dalsgaard Det al. . Atrial fibrillation in patients with sick sinus syndrome: the association with PQ-interval and percentage of ventricular pacing. Europace 2012;14:682–9. [DOI] [PubMed] [Google Scholar]
- 18. Sweeney MO, Ellenbogen KA, Tang AS, Whellan D, Mortensen PT, Giraldi Fet al. . Managed Ventricular Pacing Versus VVI 40 Pacing Trial Investigators. Atrial pacing or ventricular backup-only pacing in implantable cardioverter-defibrillator patients. Heart Rhythm 2010;7:1552–60. [DOI] [PubMed] [Google Scholar]
- 19. Barold SS, Herweg B. Conventional and biventricular pacing in patients with first-degree atrioventricular block. Europace 2012;14:1414–9. [DOI] [PubMed] [Google Scholar]
- 20. Yadav R, Jaswal A, Chennapragada S, Kamath P, Hiremath SMS, Kahali Det al. . Effectiveness of Ventricular Intrinsic Preference (VIP™) and ventricular AutoCapture (VAC) algorithms in pacemaker patients: results of the validate study. J Arrhythm 2016;32:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some of the data described in this manuscript are available in Clinicaltrial.gov and can be accessed with the following trials’ identifiers NCT0106212 and NCT02577887. Other data underlying this article will be shared on reasonable request to the corresponding author.