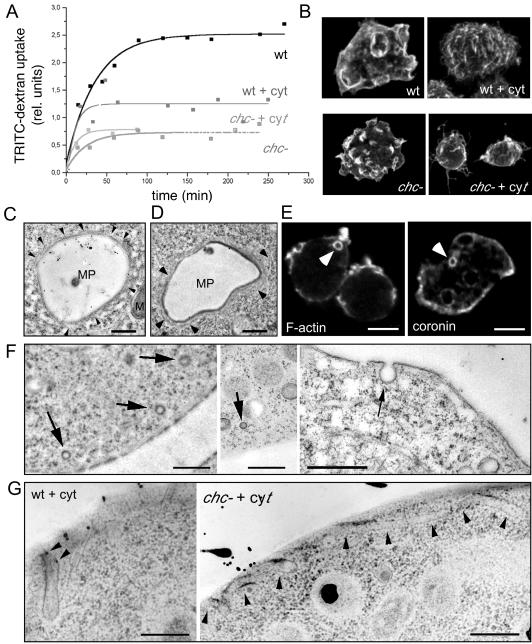
Figure 3.
Uptake mechanisms. (A) Uptake of fluorescently labeled dextran as fluid phase marker in wild-type (wt) and chc− cells; chc− knockout cells showed severe defects in the uptake of fluid phase compared with wild-type cells, as described previously (O'Halloran and Anderson, 1992). Fluid phase endocytosis in wild-type cells was ∼50% reduced after disruption of the cytoskeleton with 10 μM cytochalasin A (wt + cyt), whereas fluid phase endocytosis in chc− cells (chc− + cyt) was not impaired by the same drug treatment. (B) Immunofluorescence staining of the surface of cytochalasin-treated and nontreated wild-type and chc− cells. (C and D) Ultrastructure of big endocytic vacuoles (∼4 μm in diameter) in wild-type D. discoideum cell fed with HRP. These newly formed macropinosomes (MPs) still have an actin cytoskeletal coat, as evidenced by the presence of thin filamentous structures (arrowheads) M, mitochondrium. Bar, 1 μm. (E) Phalloidin staining visualized the actin cortex and intracellular macropinocytic vacuoles (arrowhead) with their F-actin coat (F-actin). Immunofluorescence localization of coronin, an actin-binding protein enriched in the actin cortex, and on macropinocytic vacuoles (arrowhead). Bar, 10 μm. (F) Thin sections of rapidly frozen wild-type cells present small, presumably coated vesicles near the plasma membrane (left) and only noncoated vesicles directly budding from the plasma membrane (right). Bars, 1 μm. (G) EM of cytochalasin A-treated wild-type and chc− cells showed extended tubular structures invaginating from the plasma membrane (arrowheads). Bars, 0.5 μm.