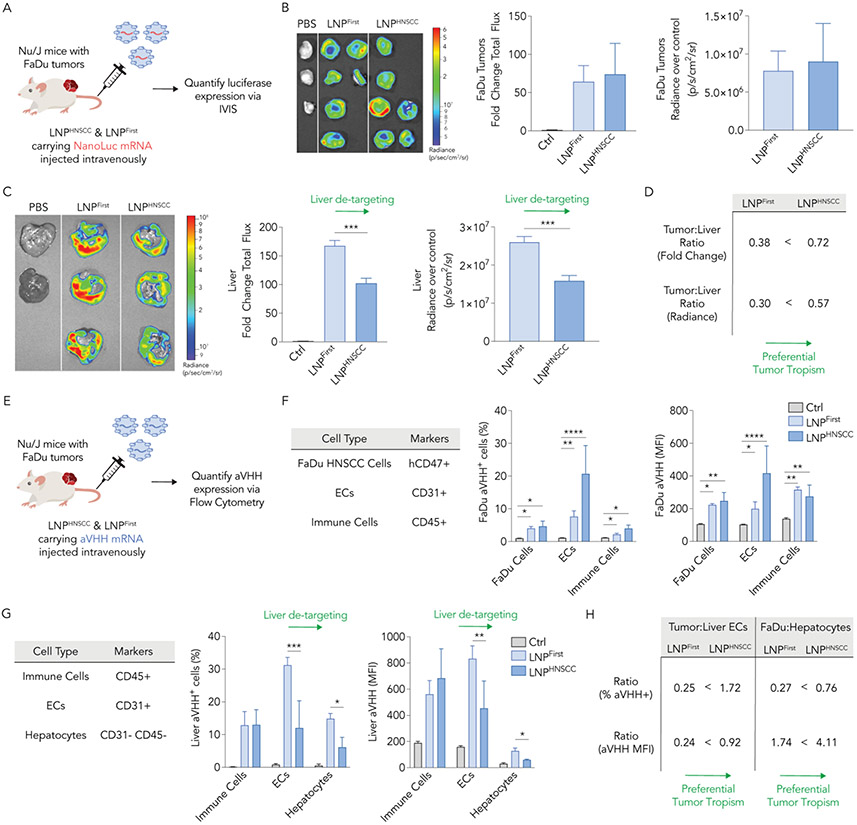
Fig. 4. LNPHNSCC shows preferential systemic delivery of mRNA to human solid tumors while de-targeting the liver in vivo.
(A) LNPHNSCC and LNPFirst, the winner LNP of the first screen, were formulated with an anchored NanoLuc mRNA and administered intravenously to Nu/J mice carrying FaDu xenograft tumors at 2 mg of RNA/kg. Thirty-six hours later, luciferase expression was quantified via whole-organ imaging in (B) FaDu tumors and (C) livers. Fold change total flux and radiance over control organs’ background (p/s/cm2/sr) in tumors were comparable while LNPHNSCC had lower NanoLuc expression in the livers, demonstrating de-targeted delivery to the liver. (D) Tumor tropism was quantified by computing the tumor-to-liver ratio using fold change and radiance over control for both LNPs, demonstrating LNPHNSCC had superior tumor preferential delivery. (E) LNPHNSCC and LNPFirst were formulated with an aVHH mRNA and administered intravenously to Nu/J mice carrying FaDu xenograft tumors at 2 mg of RNA/kg. Sixteen hours later, aVHH protein expression (% transfected and MFI) was quantified via flow cytometry in (F) FaDu tumors (human FaDu cells, ECs, and immune cells) and (G) livers (immune cells, ECs, and hepatocytes). LNPHNSCC and LNPLlver both functionally delivered aVHH mRNA to tumor cell types comparably while LNPHNSCC had lower aVHH expression in liver ECs and hepatocytes, demonstrating de-targeted delivery to the liver. (H) Tumor tropism was quantified by computing the tumor-to-liver ECs and FaDu-to-hepatocyte ratios using % transfected and MFI for both LNPs, demonstrating LNPHNSCC had superior tumor preferential delivery ECs: Endothelial cells, MFI: Mean fluorescence intensity. Two-way ANOVA, Tukey’s multiple comparisons test, *P < 0,05, **P < 0.01, ***P < 0.001, ****P < 0.001, average ± S.D.