Abstract
Sleep-disordered breathing (SDB), characterized by specific underlying physiological mechanisms, comprises obstructive and central pathophysiology, affects nearly 1 billion individuals worldwide, and is associated with excessive cardiopulmonary morbidity. Strong evidence implicates SDB in cardiac arrhythmogenesis. Immediate consequences of SDB include autonomic nervous system fluctuations, recurrent hypoxia, alterations in carbon dioxide/acid-base status, disrupted sleep architecture, and accompanying increases in negative intrathoracic pressures directly affecting cardiac function. Day-night patterning and circadian biology of SDB-induced pathophysiological sequelae collectively influence the structural and electrophysiological cardiac substrate, thereby creating an ideal milieu for arrhythmogenic propensity. Cohort studies support strong associations of SDB and cardiac arrhythmia, with evidence that discrete respiratory events trigger atrial and ventricular arrhythmic events. Observational studies suggest that SDB treatment reduces atrial fibrillation recurrence after rhythm control interventions. However, high-level evidence from clinical trials that supports a role for SDB intervention on rhythm control is not available. The goals of this scientific statement are to increase knowledge and awareness of the existing science relating SDB to cardiac arrhythmias (atrial fibrillation, ventricular tachyarrhythmias, sudden cardiac death, and bradyarrhythmias), synthesizing data relevant for clinical practice and identifying current knowledge gaps, presenting best practice consensus statements, and prioritizing future scientific directions. Key opportunities identified that are specific to cardiac arrhythmia include optimizing SDB screening, characterizing SDB predictive metrics and underlying pathophysiology, elucidating sex-specific and background-related influences in SDB, assessing the role of mobile health innovations, and prioritizing the conduct of rigorous and adequately powered clinical trials.
Keywords: AHA Scientific Statements, arrhythmia, atrial fibrillation, autonomic, hypoxia, sleep apnea
Sleep-disordered breathing (SDB) is characterized by alterations in breathing during sleep. SDB subtypes relevant to this scientific statement include obstructive sleep apnea (OSA), central sleep apnea (CSA), and CSA–Cheyne-Stokes breathing (CSB). SDB adversely affects cardiovascular and neuroendocrine physiology, quality of life, and mood and thus is an important clinical and public health problem. OSA, in particular, is highly prevalent, affecting an estimated 1 billion adults worldwide1; it continues to be largely undiagnosed,2 especially among racial and ethnic groups that have faced historic and systemic marginalization.3 Immediate sequelae of SDB include repetitive episodes of hypoxia, intrathoracic pressure alterations (in OSA), autonomic fluctuations, hypercapnia, and disturbed sleep architecture. Over time, SDB physiological stresses result in sustained biological effects, culminating in cardiovascular substrate alterations, increasing risk for cardiac arrhythmogenesis, and thus drives the focus of this scientific statement (Figure 1).
Figure 1. Multilayered pathophysiology and temporality of sleep-disordered breathing and cardiac arrhythmia.
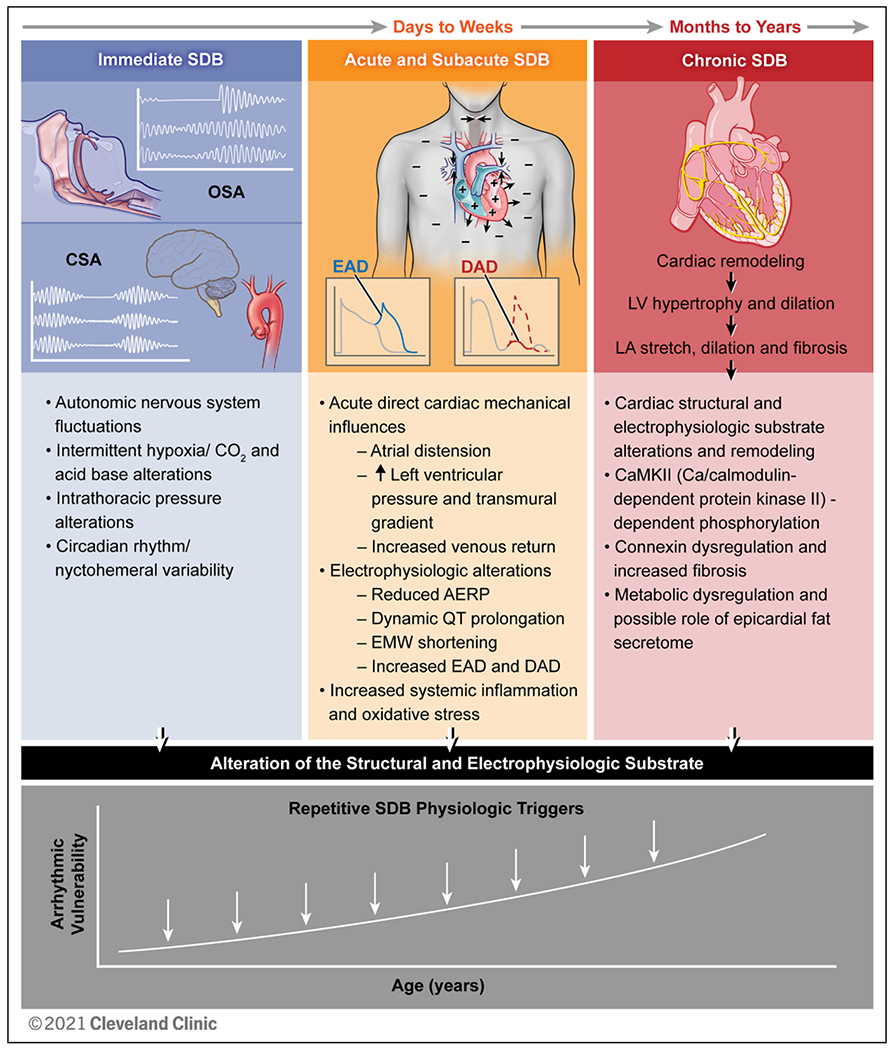
Immediate, subacute, and chronic sleep-disordered breathing (SDB) pathophysiology contributing to cardiac arrhythmogenesis. SDB includes obstructive sleep apnea (OSA) characterized by upper airway collapse and central sleep apnea (CSA) with abnormalities in hypoxic ventilatory mechanisms (carotid chemoreceptors) and carbon dioxide chemosensitivity (medullary chemoreception). Immediate SDB effects include autonomic nervous system fluctuations, repetitive intermittent hypoxia and carbon dioxide alterations, intrathoracic pressure alterations, and circadian variability. Acute and subacute SDB influences over days to weeks lead to repetitive direct cardiac mechanical influences, resulting in atrial distention, increased left ventricular (LV) pressure and transmural gradient, and increased venous return, as well as electrophysiological alterations, including reduced atrial effective refractory period (AERP), dynamic QT prolongation, electromechanical window (EMW) shortening, increased delayed afterdepolarizations (DADs), and early afterdepolarizations (EADs), as well as increased systemic inflammation and oxidative stress. Chronic SDB influences include cardiac structural and electrophysiological remodeling, with data supporting Ca/calmodulin–dependent protein kinase II (CaMKII)–dependent phosphorylation, connexin dysregulation, increased fibrosis, and a potential role of metabolic dysregulation and epicardial fat secretome. Over time, with increasing age, these SDB-induced pathophysiological effects on the cardiac substrate enhance arrhythmia vulnerability. LA indicates left atrial. Reprinted with permission from the Cleveland Clinic Center for Medical Art & Photography. Copyright © 2022. All rights reserved.
In epidemiological studies, some of the strongest associations of SDB and cardiac outcomes are with cardiac arrhythmias, which are associated with both OSA and CSA. The demonstration that discrete respiratory events can trigger arrhythmia, combined with longitudinal associations between SDB and incident arrhythmias, provides temporally consistent support for causal associations. Clinical and experimental animal studies have identified discrete SDB-related mechanistic arrhythmogenic triggers, the attenuation of which appears to ameliorate these effects of SDB. Uncontrolled studies suggest that SDB treatment reduces arrhythmia recurrence after rhythm control interventions such as catheter ablation and cardioversion in atrial fibrillation (AF). These findings support the need to consider SDB in cardiac arrhythmia risk factor modification. However, there are also clear bidirectional associations (cardiac disease can exacerbate SDB), and most existing data are observational, underscoring the need for rigorous, adequately powered, randomized interventional trials.
The goals of this scientific statement are to increase knowledge and awareness of the existing science relating SDB to cardiac arrhythmias (AF, ventricular tachyarrhythmias [VTAs], sudden cardiac death [SCD], and bradyarrhythmias) specific to epidemiology, risk factors, health inequities, mechanistic underpinnings, and integration of SDB as a risk factor in lifestyle modification strategies in clinical care models for cardiac arrhythmia. Given that the majority of the existing literature is focused on SDB and AF, this document concentrates on AF, with dedicated sections for VTAs and bradyarrhythmias. The content reflects data synthesis relevant for clinical practice and identifies current knowledge gaps and priorities for future scientific directions for the research community.
This scientific statement provides considerations and suggestions for best clinical practice but not formal clinical recommendations. The summary statements represent expert consensus requiring at least 80% agreement among the writing group members. Detailed literature searches were conducted by the writing group using PubMed, Web of Science, and Scopus restricted to the English language to identify relevant original articles, guidelines, statements, and review articles to inform the content presented. The document was peer reviewed by official external reviewers representing experts in epidemiology and clinical, translational, and experimental research focused on SDB or cardiac arrhythmia.
SDB DEFINITIONS
OSA is characterized by repetitive occlusion or narrowing of the upper airway, resulting in apneas and hypopneas, that is, the absence or reduction, respectively, of inspiratory airflow for ≥10 seconds.4 With the use of in-laboratory polysomnography or home sleep apnea testing (HSAT), OSA is defined as an apnea-hypopnea index (AHI; or respiratory event index when HSAT is used) of ≥5 events/h with typical symptoms including daytime sleepiness or an AHI ≥15 events/h regardless of symptoms.4 Hypopneas, reflecting partial upper airway collapse, are identified when associated with oxygen desaturations (typically ≥3% or 4%) or microarousals from sleep. Notably, daytime sleepiness, a symptom often used to screen for OSA, poorly correlates with the presence and severity of SDB in cardiac disease, including arrhythmia.5
CSA is characterized by a transient cessation of or decrease in ventilatory effort generated by the pontomedullary respiratory pacemaker during sleep. This results from a reduction in the partial pressure of CO2 below the apneic threshold, resulting in breathing cessation. CSA is defined by >50% of the apneas/hypopneas classified as central.4 CSB is a form of CSA often occurring in heart failure and characterized by heightened ventilatory chemosensitivity resulting in alternating crescendo-decrescendo apneic and hyperpneic ventilatory periods, which can lead to decreased intrathoracic pressure during periods of hyperventilation. There are likely bidirectional associations between cardiac dysfunction and CSA and CSB. Cardiac influences include delayed circulation times and ventilatory instability attributable to poor left ventricular function contributing to CSA and CSB; conversely, SDB via its attendant hypoxia and sympathetic nervous system activation may exacerbate cardiac dysfunction.
The distributions of central and obstructive respiratory events may vary not only macro-longitudinally, but also during a single night due to differences in cardiac function and fluid balance. In particular, with recumbency, there is rostral fluid redistribution, resulting in upper airway edema, which contributes to a predisposition to obstructive events. However, pulmonary congestion also can provoke hyperventilation, CO2 reduction below the apneic threshold, resulting in central respiratory events.
It is increasingly recognized that the pathophysiological bases for SDB are heterogeneous, with individuals differing in the extent to which underlying risk is mediated by anatomic risk factors and by several physiological risk factors, including low cortical arousal threshold, poor upper airway muscle responsiveness, increased collapsibility, and elevated loop gain (biomarker of ventilatory instability). There is active interest in deriving these novel metrics of SDB physiology, referred to as endotypes, from routine polysomnography and using them to direct personalized therapies.6 For example, individuals with high loop gain (common in heart failure) appear to respond well to supplemental oxygen. Additional metrics also have recently been developed to better characterize SDB subtypes associated with cardiac disease. For instance, higher heart rate response to discrete respiratory events predicts cardiovascular disease and incident heart failure7 and is being investigated as a predictor of AF.
SDB, SHARED CARDIAC ARRHYTHMIA RISK FACTORS, AND HEALTH INEQUITIES
SDB and AF share many risk factors, including increasing age, male sex, and obesity. Although not yet well studied, some of these factors also influence SDB subtypes that may result in differences in propensity to adverse outcomes and predict responsiveness to alternative treatments. An improved understanding of how phenotypic and genotypic differences influence SDB-related AF holds promise for informing personalized risk factor identification and treatment approaches.
Although the emerging aging and obesity epidemics contribute to the increasing prevalence of OSA and AF, observed associations remain even after these factors are accounted for.8 Obesity and SDB commonly coexist, often interact, and may have similar consequences; however, delineation of shared and unique pathways in AF remains unclear. The magnitude of association of SDB with AF (hazard ratio, 2.18 [95% CI, 1.34–3.54]) is greater than for obesity and AF (hazard ratio, 1.49 [95% CI, 1.67–1.87]), suggesting that SDB may be a stronger AF driver than obesity.9 OSA contributes to hypertension (an AF risk) through sympathetic nervous system excitation and vascular remodeling. Heart failure, often associated with CSA, shares overlapping, multidirectional relationships with SDB and cardiovascular risk and disease. Under these circumstances, cardiac arrhythmias can arise from comorbid risk attributable to SDB as a result of neurohumoral and hemodynamic alterations, as well as changes in sympathetic drive and cardiac structure that can affect underlying electrophysiology.
Recognition of health inequities and intersection with race and ethnicity in sleep disorder risk, screening, and diagnostic and therapeutic approaches such as anticoagulation use in cardiac arrhythmia10 carries high public health relevance and implications. Despite an elevated cardiovascular risk burden, racial and ethnic groups that have faced historic and systemic marginalization have a lower incidence of AF compared with White individuals.11 However, people in those racial and ethnic groups with AF frequently experience long-lasting and more frequent symptomatic AF episodes, less aggressive care, and higher stroke risk and mortality.11
Anatomic OSA risk factors, including craniofacial morphology and patterns of adiposity, tend to vary by background ancestry,12 which may influence endotypic features of SDB and the efficacy of specific OSA treatments. For example, Black women participants of the MESA (Multi-Ethnic Study of Atherosclerosis) have been shown to have a shorter duration of apneas and hypopneas, a marker of a low arousal threshold, and thus may tolerate continuous positive airway pressure (CPAP) less well but benefit from medications that increase arousal threshold.13 Whether shorter event duration modulates AF risk is not yet known. In addition, Black, Hispanic, and Chinese adults have a higher prevalence of short sleep duration, also an AF risk factor, compared with their White counterparts.14 Some data suggest that Black individuals with SDB are at greater risk for AF than White adults,15 potentially reflecting a longer duration of untreated SDB. These studies demonstrate the importance of considering potential racial and ethnic differences in SDB and its association with AF, including potentially modifiable environmental and social factors15a,15b that influence differences in SDB physiology and responsiveness to treatment and other sleep (eg, insufficient sleep duration) and general health (eg, diabetes, obesity) comorbidities, as well as marked differences in socioeconomic factors that influence access to and quality of care and overall health. Together, these considerations highlight the importance of addressing populations who have faced such marginalization and are at greatest need for prioritization of SDB prevention, identification, and management to target reduction of AF and associated morbidity.
AF: EPIDEMIOLOGY OF SDB AND SLEEP DISRUPTION
Community-based and clinical cohort studies of SDB and cardiac arrhythmia subtypes provide valuable insights into their interrelationships and the factors that influence their associations.
SDB, Sleep Disruption, and AF in Community-Based Studies
The estimated prevalence of OSA in patients with AF is higher relative to controls: 21% to 74% compared with 3% to 49%.16 Large-scale community-based cohorts indicate a strong and consistent association of nocturnal cardiac arrhythmias with SDB, that is, a nearly 5-fold higher odds of AF in moderate to severe SDB versus without after both matching and adjusting for confounding of obesity and underlying cardiovascular risk.17
CSA is strongly associated with 5.3- to 6.5-year excess risk of consequential AF even after accounting for self-report of heart failure.18,19 The lack of objective cardiac function measures in this study, however, limits the ability to assess causality. On the other hand, OSA (more than CSA) modifies sympathovagal balance, as demonstrated by ECG-based heart rate variability measures from polysomnography that are associated with an increased incidence of AF over 8.0±2.6 years.20 Findings support direct and indirect roles of central and obstructive SDB subtypes, respectively, and differential autonomic response profiles on long-term AF development. An overview of key observational studies characterizing SDB indices in relation to atrial arrhythmia is provided in Table 1 (Section 1).
Table 1.
Select Observational Studies of SDB and Cardiac Arrhythmia
| Reference | Type of study | Sample size/duration of study follow-up if applicable | Purpose | Results |
|---|---|---|---|---|
| AF (Section 1) | ||||
| May et al,18 2016 | Prospective | n=843 Follow-up, 6.5±0.7 y | Investigating SDB indicators to be a predictor of incident AF in the Outcomes of Sleep Disorders in Older Men multicenter population-based cohort | CSB (OR, 2.27 [95% CI, 1.13–4.56]) and central apnea (OR, 2.58 [95% CI, 1.18–5.66]) were found to predict an increased risk of AF in older men. Strongest findings were in older patients, in whom overall SDB also increased AF risk. |
| Tung et al,19 2017 | Prospective | n=2912 Follow-up, 5.3 y | Providing data from a prospective cohort, the Sleep Heart Health Study, that distinguishes the associations of OSA from CSA with AF | CSA (OR, 3.00 [95% CI, 1.40–6.44]) and CSB (OR, 1.83 [95% CI, 0.95–3.54]) were associated with AF; OSA was not associated with AF. |
| Cadby et al,21 2015 | Retrospective | n=6841 Follow-up, 11 y | Establishing whether OSA is a risk factor for AF independent of obesity and other established risk factors from a large, longitudinal clinical cohort | Independent association of incident AF with OSA severity and diagnosis: AHI >5/h: HR, 1.55 (95% CI, 1.21–2.00); log(AHI+1): HR, 1.15 (95% CI, 1.06–1.26); and log(time with Sao2 <90%)+1: HR, 1.12 (95% CI, 1.06–1.19). |
| Gami et al,22 2007 | Retrospective | n=3542 Follow-up, 4.7 y | Identifying OSA and obesity as independent predictors of incidence AF | Independent risk factors for incident AF in individuals <65 y of age include magnitude of nocturnal oxygen desaturation and obesity: decrease in nocturnal oxygen saturation (per 0.5-U log change): HR, 3.29 (95% CI, 1.35–8.04). |
| Holmqvist et al,23 2015 | Retrospective | n=10 132 Follow-up, up to 2 y | Comparing patients with and without OSA and the likelihood of progressing to more persistent forms of AF, requiring more hospitalizations, or having worse outcomes | Patients with OSA had a higher rate of hospitalization and rates of mortality, AF progression, and major adverse cardiovascular events similar to those of patients without OSA; patients with OSA had a higher risk of hospitalization (HR, 1.12 [95% CI, 1.03–1.22]; P=0.0078). |
| Ventricular tachyarrhythmia/SCD (Section 2) | ||||
| Gami et al,24 2013 | Prospective | n=10 701 Follow-up, 5.3 y | Determining whether there is an association between OSA and SCD in a clinical cohort | OSA is a risk factor for SCD: lowest nocturnal Sao2 (per 10% decrease: HR, 1.14; P=0.029). SCD was predicted by AHI >20 (HR, 1.60), mean nocturnal O2 saturation <93% (HR, 2.93), and lowest nocturnal O2 saturation <78% (HR, 2.60; all P<0.0001). |
| Mehra et al,17 2009 | Cross-sectional (nested exposed-un-exposed study) | n=566 | Determining the prevalence of nocturnal cardiac arrhythmias in patients with SDB in the Sleep Heart Health Study | Those with SDB have 2- to 4-fold higher odds of complex arrhythmias; more common in subjects with SDB compared with those without SDB: 5.3% vs 1.2% (P=0.004) for NSVT; 25.0% vs 14.5% (P=0.002) for complex ventricular ectopy. |
| Reshetnik et al,25 2019 | Prospective | n=202 Follow-up, 11.3 mo | Observing the prevalence of CSA and SDB in the subacute phase of AMI and assessing the impact of the severity of SDB on the prevalence of NSVT | There is an independent association of severe SDB with a higher risk of NSVT. AHI >23/h was independently associated with higher risk of NSVT in subacute AMI. |
| Gami et al,26 2005 | Retrospective | n=112 | Determining the rate of SCD in individuals with OSA | People with SCD between midnight and 6 AM had higher AHI than those who experienced it at different times of day. Relative risk of SCD in OSA from midnight to 6 AM was 2.57 (95% CI, 1.87–3.52). |
| Bradyarrhythmia (Section 3) | ||||
| Selim et al,27 2016 | Cross-sectional | n=697 | Using a clinic-based population with multiple cardiovascular comorbidities and severe SDB to determine whether SDB is associated with cardiac arrhythmia | There is an independent association between SDB and nocturnal cardiac arrhythmias; an increase in SDB severity is associated with an increased risk of any cardiac arrhythmia, including bradyarrhythmias: 2-fold odds of intraventricular conduction delay (2.50 [95% CI, 1.58–3.95]; P=0.001). |
| Becker et al,28 1998 | Cross-sectional | n=239 | Determining the prevalence of heart block in those with SA | There was a strong correlation between the prevalence of bradyarrhythmias and OSA severity and the degree of nocturnal desaturation. Occurrence of bradycardic arrhythmias was clearly linked to apnea severity. None of the patients with an AHI <60/h developed heart block, whereas 17 of the 97 with an AHI of at least 60/h did. |
| Garrigue et al,29 2007 | Cross-sectional | n=98 | Determining the prevalence of SAS in patients with pacemakers | High prevalence was seen between patients with pacemakers and undiagnosed SAS. |
AF indicates atrial fibrillation; AHI, apnea-hypopnea index; AMI, acute myocardial infarction; CSA, central sleep apnea; CSB, Cheyne-Stokes breathing; HR, hazard ratio; NSVT, nonsustained ventricular tachycardia; OR, odds ratio; OSA, obstructive sleep apnea; SA, sleep apnea; Sao2, oxygen saturation <90%; SAS, sleep apnea syndrome; SCD, sudden cardiac death; and SDB, sleep-disordered breathing.
SDB and AF in Clinical Cohort Studies
Nocturnal hypoxia is a particularly important risk factor for incident AF development in clinical SDB cohorts made up of patients with sleep-related symptoms. This may be explained by a higher prevalence of overnight hypoxia in clinic-based versus community-based samples.8 In a report of 8256 subjects, after controlling for a range of confounders, including underlying pulmonary disease, nocturnal hypoxia (but not AHI) predicted incident AF (hazard ratio, 2.47 [95% CI, 1.64–3.71]).30
Temporality of SDB Influences and AF
In patients with paroxysmal AF, SDB is more severe in those with a higher AF burden than in those with a lower AF burden (75% versus 43%).31 Data from technologically advanced pacemakers capable of detecting both SDB and AF onset and burden provide support for a causal association between SDB and AF.32 In the VARIOSA-AF study (Variability of Sleep Apnea Severity and Risk of Atrial Fibrillation), nights with the highest SDB severity had twice the likelihood of ≥1 hour of AF the following day compared with nights with the lowest SDB severity.32 Alternatively, AF episodes did not predict respiratory events. These studies characterize directionality (ie, SDB burden predicts AF occurrence) and strongly suggest a cause-effect relationship between SDB and AF.
Other Sleep Disturbances and AF
Beyond SDB, and often attributable to SDB, sleep curtailment and sleep architectural alterations may modulate arrhythmogenic risk. In a multicohort report, frequent nocturnal awakenings were associated with a 33% increased risk of AF over an 11.6-year median follow-up.33 Insomnia was associated with a 36% increased risk of incident AF in 14 million California residents over a median 3.9-year follow-up.33 In the MESA (Multi-Ethnic Study of Atherosclerosis) cohort, shorter duration of slow wave sleep (N3) and lower sleep efficiency and arousal index were associated with AF.34 A clinic-based study further identified short sleep duration as an important factor for incident AF.35 Moreover, healthy sleep (chronotype, sleep duration, insomnia, snoring, and daytime sleepiness composite) confers a lower risk of AF (comparing extreme categories: hazard ratio, 0.71 [95% CI, 0.64–0.80]) over an 11-year median follow-up.36
AF: SDB PATHOPHYSIOLOGY
Day-night patterning of SDB-related pathophysiological sequelae involves interplay of autonomic fluctuations, intermittent hypoxia and hypercapnia, and alterations in intrathoracic and thus intracardiac pressures collectively operating to alter the structural and electrophysiological substrate, thus creating an ideal milieu for arrhythmogenic propensity.37
Autonomic Nervous System Mechanisms
Evidence implicates autonomic effects as a key facilitator of cardiac arrhythmogenesis in SDB. Obstructive respiratory events lead to parasympathetic activation through the diving reflex immediately followed by a sympathetic surge arising from hypoxia, arousal, and inhibition of pulmonary stretch.38 Increasing inspiratory effort against the collapsed pharynx during obstructive apneas further contributes to increased sympathetic nerve activity.38 Apnea-induced vagal influences followed by sympathetic activation, the latter most marked at apneic termination, may trigger and, if repetitive, maintain AF. Inducibility of AF by OSA is attenuated by ablation of right pulmonary vein ganglionated plexi, combined pharmacological-neurohumoral blockade, autonomic modulation with renal sympathetic denervation, or low-level vagosympathetic trunk stimulation, thus providing consistent evidence of the role of the autonomic nervous system in SDB-induced AF.38 Autonomic nervous system mechanisms may operate through Ca/calmodulin–dependent protein kinase II–dependent phosphorylation of sodium channels to induce atrial arrhythmogenesis in SDB.16 Ca/calmodulin–dependent protein kinase II represents a potential mediator of cellular clock function and of coupling between morning and evening behavioral rhythms.16
Intermittent Hypoxia and Carbon Dioxide Fluctuations
Chronic intermittent hypoxia enhances AF vulnerability through atrial effective refractory period shortening (increases AF inducibility) with sensitivity to parasympathetic activation and sympathetic potentiation.38 Intermittent deoxygenation-reoxygenation results in increases in reactive oxygen species, vascular inflammation, and rises in blood pressure, all of which contribute to myocardial damage and cardiac remodeling.38 Limited data suggest the role of CO2 fluctuations in AF generation. Transition from hypercapnia to eucapnia around apneic events increases AF vulnerability through return of the atrial effective refractory period to baseline and persistence of atrial conduction time prolongation.39 Hypocapnia specific to CSA-CSB may increase arrhythmogenic propensity through increased electrical instability.40
Intrathoracic Pressure Alterations
Obstructive apnea, caused by upper airway collapse, results in intrathoracic pressure swings, causing myocardial stretch and changes in intracardiac transmural pressure gradients.39 In healthy humans, intrathoracic pressure changes induced by a Mueller maneuver increase postganglionic sympathetic nerve activity by >200%, along with a 14% increase in mean blood pressure after apnea episodes.8 Intrathoracic pressure swings reproducibly induce transient shortening in the atrial effective refractory period and enhance AF inducibility by sympathovagal coactivation.39
Circadian Rhythm Influences and Nyctohemeral (Day-Night) Patterning
The central circadian clock directly affects the electrophysiology of the heart and arrhythmogenesis through the autonomic nervous system, and the local cardiac clock may exert influence through ion channel expression, thus influencing the arrhythmic substrate.41 Although little is known about circadian contributions to the observed nocturnal predominance of arrhythmia in SDB, it is likely that factors driving arrhythmic vulnerability to hypoxia and sympathetic-vagal activation are modulated by central circadian mechanisms. These include regulating the timing and duration of rapid eye movement sleep, the sleep stage during which autonomic instability is most evident and when obstructive apneas are most likely to occur. There is strong evidence of clock gene regulation of atrial function, for example, the cardiac K+ channels Kv1.5 and 4.2, and of key intermediary mechanisms linking SDB to arrhythmogenic mechanisms such as inflammation, ion channel expression, and cardiac autonomic activity.42
Alteration of the Cardiac Substrate
Long-standing, untreated SDB may lead to progressive structural and electrical atrial remodeling, creating a dynamic substrate for progression and perpetuation of cardiac arrhythmias16 (Figure 1). Nocturnal AF paroxysms are temporally related to individual respiratory obstructive events in close proximity, suggesting that acute transient arrhythmogenic changes during apneas may contribute to alteration of the cardiac substrate.43 High-frequency intermittent deoxygenation-reoxygenation and hypercapnia result in atrial conduction abnormalities arising from connexin dysregulation and increased fibrosis in the atria.44 Electrophysiological atrial mapping during AF ablation in OSA identifies a higher likelihood of low-voltage areas and abnormal electrograms in both atria and increased atrial fibrosis with reduced atrial conduction velocities, all indicative of OSA-induced structural and electrical remodeling.45 Overall, common mechanistic pathways of systemic inflammation and oxidative stress arising from SDB physiological effects, and implicated in AF progression, may play a role in electrophysiological and structural cardiac remodeling.8 The epicardial fat secretome facilitates atrial substrate progression and myocardial fibrosis.46 Because OSA is associated with increased epicardial adipose tissue, likely through hypoxia-induced inflammatory remodeling of adipose depots, this represents a biologically plausible mechanism of atrial arrhythmogenesis.47
APPROACH TO SCREENING AND EVALUATION OF SDB IN AF
It is critical that health care professionals and the public be aware of the role of SDB as a triggering event for cardiac arrhythmias and contributor to a substrate for AF maintenance and progression.45,46 The likelihood of detecting SDB is high in patients with relevant symptoms (eg, snoring, witnessed apneas), in those with difficult-to-treat or nondipping hypertension, and in people with AF, particularly persistent AF.48 Identifying SDB is important to improve sleep satisfaction, sleep difficulties, and quality of life.
SDB occurs in 21% to 74% of people with AF, underscoring the importance of identifying this highly prevalent physiological risk stressor in AF.49 Symptom-based OSA screening questionnaires have limited predictive value in patients with AF, given the paucity of daytime sleepiness among people with AF.5 A validated OSA screening instrument for patients with paroxysmal AF holds promise. Specifically, incorporating NABS (Neck Circumference, Age, Body Mass Index, and Snoring) had improved discriminative ability (area under the receiver-operating curve, 0.82 [95% CI, 0.68–0.92]) compared with commonly used instruments such as STOP-BANG (Snoring, Tiredness, Observed Apnea, Pressure [High Blood Pressure], Body Mass Index, Age, Neck Circumference, and Gender, area under the receiver-operating curve, 0.73 [95% CI, 0.55–0.87).50 A recent study validated the oxygen desaturation index (frequency of oxygen desaturations) derived from simple overnight oximetry against the polysomnography-derived AHI as an accessible and reliable screening tool to rule out moderate to severe OSA (AHI ≥15; area under the receiver-operating curve, 0.95 [95% CI, 0.93–0.97]).51 No reliable CSA screening tools are available.
Although the gold standard for OSA diagnosis is in-laboratory polysomnography (particularly in those with comorbidity), it is resource intensive with limited accessibility compared with HSAT. Because OSA is the most common form of SDB in people with AF, HSAT may be sufficient to diagnose OSA in a large proportion of patients with AF. A prospective study comparing the diagnostic accuracy of a level 3 HSAT (recording of oximetry, heart rate, airflow, and respiratory effort) against polysomnography in an AF population validated HSAT for all levels of OSA severity with excellent diagnostic accuracy, thereby demonstrating its utility as a diagnostic tool in AF.52 In-laboratory polysomnography is generally recommended in those with cardiopulmonary and neurological comorbidities, particularly in people with AF with concomitant heart failure who are predisposed to mixed or predominant CSA or CSB or in patients who develop CSA during CPAP treatment (referred to as treatment-emergent CSA). Wearable mobile health devices measuring concomitant sleep and cardiac physiological parameters, some using artificial intelligence approaches for novel predictive analytics, are emerging and likely to have an increased role in the diagnostics and management of sleep disorders in AF.53
Because intraindividual night-to-night variability of respiratory events and AHI is high,54 repeat sleep testing should be performed if suspicion for SDB remains high or SDB cannot be fully excluded despite initial negative testing. Because synchronous heart rhythm monitoring is standard during polysomnography and sometimes used with HSATs, examining these data can help identify and validate sleep apnea events as a major triggering event of repetitive AF episodes. Alternatively, frequent nocturnal paroxysmal AF episodes should foster targeted investigation of SDB.32
AF SECONDARY PREVENTION: TREATMENT OF SDB
Existing SDB studies have focused on secondary AF prevention with little to no data on primary AF prevention. In a historical study of Holter monitoring in patients with severe SDB, those with nocturnal arrhythmias, including AF, showed resolution of arrhythmias after treatment with tracheostomy.31
SDB is reported in 24% to >50% of those with AF undergoing catheter ablation and is associated with increased AF recurrence.55–57 Treatment of OSA with CPAP may mitigate the recurrence of AF. In a registry study of 10 132 patients with AF, CPAP treatment was associated with a reduced likelihood of progression to permanent AF in the sample with AF and OSA.58 Those with severe SDB were also less likely to respond to anti-arrhythmic drug therapy compared to those with milder degrees of SDB.58 Accruing data support a reduction of AF recurrence after direct current cardioversion or pulmonary vein isolation in SDB with CPAP treatment.16 Those with untreated OSA have a higher likelihood of manifesting residual AF triggers after pulmonary vein antral isolation, with these triggers likely to be responsive to OSA-induced autonomic perturbations in promoting postablation development of AF.59
Three meta-analyses have been conducted (sample size range, n=1087–3743), showing (1) consistent findings of OSA and greater risk of AF recurrence after catheter ablation, (2) use of CPAP resulting in a 42% reduction in AF recurrence, and (3) a 57% increased risk of AF recurrence in untreated OSA.55–57 Certain subgroups may realize more favorable outcomes; that is, meta-regression analysis supports the benefits of CPAP, which were more pronounced in younger, obese, and male patients.56 Although findings are consistent, studies are limited by observational and retrospective design. A challenge with the interpretation is the healthy user effect, that is, those who are adherent to the use of CPAP may be adherent to other lifestyle behaviors. Furthermore, most studies suffer from a lack of clarity of the specific pathophysiological subtype of SDB represented in these cohorts, that is, OSA versus CSA, and a lack of detailed sleep endophenotyping to identify mechanistic risk factors.
Randomized controlled clinical trial data examining the effects of SDB treatment on AF are limited. The SAVE study (Sleep Apnea Cardiovascular Endpoints) with a primary composite cardiovascular end point (including AF) did not show a reduction in incident AF, although the study was underpowered and not designed to examine AF as a primary outcome.60 In the ARREST-AF study (Aggressive Risk Factor Reduction Study for Atrial Fibrillation) and LEGACY study (Long-Term Effect of Goal Directed Weight Management on Atrial Fibrillation Cohort: A 5 Year Follow-Up Study), an aggressive risk factor reduction intervention targeting OSA in addition to weight management and lifestyle modification of cardiovascular risk factors significantly reduced AF burden after catheter ablation.61,62
A small randomized controlled clinical trial (n=25 of 1757 screened) enrolling individuals with a range of OSA (AHI >5) without excessive sleepiness and AF after cardioversion showed no benefit of CPAP in preventing AF recurrence.63 In addition to the small sample size, AF recurrence was captured by 12-lead ECG versus continuous monitoring, which may have led to underappreciation of self-terminating asymptomatic AF episodes.63 Another randomized controlled clinical trial (n=108) enrolled those with moderate to severe SDB (AHI ≥15, mainly obstructive events) without high levels of daytime sleepiness (Epworth Sleepiness Scale score <15) with a left ventricular ejection fraction ≥45% and body mass index ≤40 kg/m2. A CPAP run-in period and an implantable loop recorder were used. No difference in 3-month AF burden in those randomized to CPAP versus those randomized to supportive care was observed.64 The AF burden in this study was lower than anticipated. Relative to the mean time of 34% projected in the power calculation, the mean time in AF at baseline versus the past 3 months was 5.6% and 4.1% in the CPAP group and 5.0% and 4.3% in the control group, respectively. Therefore, the ability to detect a 25% difference between the groups was limited.64 The follow-up period also may have been too short to observe a significant CPAP treatment benefit, and the study design perhaps excluded patients more likely to benefit from CPAP.64
Although compelling experimental and observational data support a strong magnitude of association of OSA and AF and improvement in AF outcomes with OSA treatment, clinical trials, albeit with limitations as outlined, have not borne out these results. Therefore, definitive conclusions as to whether OSA treatment improves AF outcomes remain unclear. Table 2 (Section 1) provides an overview of studies examining the impact of SDB treatment on AF.
Table 2.
Select Interventional Studies of SDB and Cardiac Arrhythmia
| Reference | Type of study | Sample size, n | Duration of follow-up | Outcome | Additional results |
|---|---|---|---|---|---|
| AF (Section 1) | |||||
| McEvoy et al,65 2016 | Randomized controlled | 2717 | 3.7 y | Although not powered to examine AF as a primary outcome, there was no reduction in AF as a component of the composite outcome in response to CPAP therapy. | CPAP was shown to significantly reduce only daytime sleepiness and snoring. It also improved mood and health-related quality of life. |
| Caples et al,63 2016 | Randomized controlled | 25 of 1757 screened | ≤1 y | No difference was detected between those treated with usual care vs PAP in AF recurrence: AF recurred in 25% of patients in the PAP and control groups, at 129.0±166.5 d vs 109.3±73.2 d, respectively (P=0.98). | Of the 34 patients who underwent polysomnography for this study, 25 had an AHI >5/h; 13 were randomized to usual care and 12 to PAP therapy. There were no differences in ESS score at follow-up. |
| Traaen et al,64 2021 | Randomized controlled | 579 | 5 mo | CPAP treatment in patients with SA and paroxysmal AF did not result in a statistically significant reduction in the burden of AF. | A decrease in mean time in AF from 5.6% (baseline) to 4.1% during the past 3 mo of CPAP was shown. In the control group, mean time in AF decreased from 5.0% to 4.3%. |
| Holmqvist et al,23 2015 | Retrospective | 10 132 | Up to 2 y | AF was less common in patients who were treated with CPAP. Patients with CPAP treatment were less likely to progress to more permanent forms of AF compared with patients without CPAP (HR, 0.66 [95% CI, 0.46–0.94]; P=0.021). | No significant difference was shown in cardiovascular or hospitalization outcomes between patients with OSA who were being treated with or without CPAP. |
| Qureshi et al,56 2015 | Meta-analysis | 4516 | None (did not restrict their outcome to any specific follow-up period) | CPAP decreases the risk of recurrence of AF in patients with OSA. | Study showed a 44% decreased risk of recurrence of AF attributable to CPAP use. |
| 8 studies | |||||
| Li et al,55 2014 | Meta-analysis | 2851 | None | Catheter ablation was improved in patients with OSA who underwent CPAP therapy, similar to those without OSA. Higher rates of AF were associated with the presence of OSA after catheter ablation. | A 31% greater risk of AF recurrence was seen after successful catheter ablation in patients with OSA vs those without OSA. A 57% increased risk of AF was found. Recurrence was seen in nonusers of CPAP. CPAP users had a relative risk of 1.25, similar to that of patients without OSA. |
| 5 studies | |||||
| Shukla et al,57 2015 | Meta-analysis | 1087 | None | CPAP users had a significant reduction in AF recurrence vs nonusers. Of those who underwent PVI, lower risk of AF recurrence was associated with CPAP use. | CPAP use was statistically significant for those who underwent catheter ablation with PVI and those managed solely medically. |
| 7 studies | |||||
| Ventricular tachyarrhythmia (Section 2) | |||||
| Seyis et al,66 2018 | Observational perspective | 80 | 2 wk, 1 mo, 2 mo, 3 mo, 4.5 mo, 6 mo | CPAP treatment in patients with HF and OSAS reduced levels of PVC and NT-proBNP levels. | At 6 mo, CPAP treatment significantly decreased frequency of PVC, QTc, Tp-e, QTc dispersion, and Tp-e/QTc ratio. In the control group, no significant change was seen. |
| Javaheri67 2000 | Observational prospective | 29 | 1 night | First-night nCPAP reduced ventricular irritability and eliminated disordered breathing in 55% of patients with SA and HF. | A decrease was seen in couplets and nocturnal PVCs in patients whose SA responded to CPAP. VTAs did not significantly change in patients whose SA did not respond to CPAP. |
| Ryan et al,68 2005 | Randomized controlled | 18 | 1 mo | Patients with HF and OSA who are treated by CPAP have a reduction in VPBs and other VTAs. | CPAP treatment group experienced a reduction in AHI and urinary norepinephrine concentrations and 58% reduction in the frequency of VPBs during their sleep, as well as an increase in minimum O2 saturation. Control group did not experience any significant changes. |
| Bradyarrhythmia (Section 3) | |||||
| Abe et al,69 2010 | Observational prospective | 316 | 3.9 wk (average) | A significant relationship was demonstrated between several cardiac disorders and OSA. Trial showed the large-scale efficacy of CPAP by preventing OSA-associated arrhythmias in a large population of Japanese patients. | CPAP treatment reduced sinus bradycardia and sinus pauses. |
| Becker et al,70 1995 | Observational | 239 | 17 mo | CPAP eliminated atrioventricular block and sinus arrest in 12 of the 17 patients with OSA. Three patients experienced a reduction in episodes. One patient had a continued increase in frequency. | A significant decrease was seen in the number of episodes of heart block, from 1575 before therapy to 165 during nCPAP. Twelve patients experienced complete prevention of heart block by nCPAP. In 3 patients, there was a 71%–97% reduction in the second night of treatment in the number of heart blocks. An increase in block frequency was seen in 2 patients, 1 of which was reversed after 4 wk on nCPAP, but the other had persistence. |
AF indicates atrial fibrillation; AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; HF, heart failure; nCPAP, nasal continuous positive airway pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OSA, obstructive sleep apnea; OSAS, obstructive sleep apnea syndrome; PAP, positive airway pressure; PVC, premature ventricular contraction; PVI, pulmonary vein isolation; SA, sleep apnea; SDB, sleep-disordered breathing; VPBs, ventricular premature beats; and VTA, ventricular tachyarrhythmia.
AF and SDB Guideline Recommendations
European Society of Cardiology guideline recommendations71 suggest prudent treatment of comorbidities, including SDB, to improve outcomes in patients with AF and that it may be reasonable to implement CPAP treatment to reduce recurrent AF and to optimize AF treatment results72 (Table 3, Section 2).
Table 3.
Overall Knowledge Gaps, Existing Guideline Recommendations, and Current Document Consensus Statements of SDB by Cardiac Arrhythmia Subtype
| Overarching research priorities to address knowledge gaps (Section 1) | |
| Mechanisms Investigate molecular, genetic, and multiomic determinants of SDB-related pathways in cardiac arrhythmia risk Discern the independent and interdependent mechanistic pathways by which SDB, nocturnal hypoxia, and sleep disorders contribute to onset of and worsening of cardiac bradyarrhythmias and tachyarrhythmias relative to obesity, metabolic factors, and the epicardial fat secretome Clarify the role of intermittent hypoxia, hypercapnia, and hypocapnia, acid-base alterations, cardiopulmonary hemodynamics, and autonomic fluctuations occurring with SDB with regard to precipitation and triggering of arrhythmias Understand the contribution of SDB endophenotypes, that is, low cortical arousal threshold, poor muscle responsiveness, high loop gain, and upper airway critical closing pressure, on cardiac arrhythmogenicity Study the SDB contribution to chronic remodeling of structural and electrophysiologic substrate as it relates to worsening of cardiac arrhythmias Enhance understanding of the role of non-SDB sleep disorders such as insomnia, periodic limb movements during sleep, and circadian disruption in cardiac arrhythmogenesis Clarify the effects of acute changes in the vagus and sympathetics that are affected by SDB and cause arrhythmias Screening Identify the most effective and cost-effective tools and strategies to screen for OSA and CSA in patients with cardiac arrhythmias, with a focus on understanding the role of symptom subtypes and the role of physiological biomarkers derived from polysomnography, for example, delta heart rate change with respiratory events Conduct clinical trials to compare effectiveness of various SDB screening strategies, that is, questionnaire-based approaches, oximetry, and wearable devices, and examine effectiveness of detection of clinically significant levels of SDB burden responsiveness to SDB interventions to reduce cardiac arrhythmia Diagnostics Identify optimal and most efficient SDB diagnostic approaches, leveraging remote continuous and simultaneous monitoring, including mobile technologies, allowing collection of longitudinal data, temporal patterns, and nyctohemeral patterning of sleep and cardiac rhythm, which may improve the understanding of the relationship of SDB to arrhythmias Prediction Leverage and apply signal processing analysis and machine learning algorithms to multiparameter physiological signals collected during polysomnography (including ECG) to identify high-risk biomarker clusters and to investigate the role of these clusters to predict cardiac arrhythmia outcomes Therapeutic interventions Clarify utility of sleep disorder interventions for primary and secondary prevention for cardiac arrhythmias Design adequately powered, rigorously conducted randomized clinical trials involving CPAP and novel interventions (eg, neurostimulation therapies [upper airway neurostimulation for OSA and phrenic nerve stimulation for CSA], oral appliance, supplemental oxygen, pharmacologics) to treat SDB to determine whether these interventions reduce arrhythmia, improve quality of life, and improve arrhythmia-related morbidity such as stroke in AF Identify optimal timing of SDB treatment across the evolution of cardiac arrhythmogenesis and remodeling for reduction of cardiac arrhythmia, for example, across the spectrum of paroxysmal, persistent, and long-standing persistent AF Develop tools to create innovative study designs, leveraging existing data for propensity-matched analyses and clinical trials using crossover or adaptive designs Evaluate the effectiveness of integrated multidisciplinary care models in clinical trials on cardiac arrhythmia clinical outcomes Subgroup social vulnerabilities and biological susceptibilities Identify, understand, and address racial and ethnic inequities in the epidemiology of AF, associations with various sleep dimensions, and differential exposure to social and environmental determinants, as well as receipt of optimal health care treatment (eg, anticoagulant therapy, treatments for SDB)10 Investigate sex-specific differences in relation to SDB endophenotype (eg, women have lower arousal threshold) and how this influences cardiac arrhythmogenesis and SDB treatment responsiveness to reduce cardiac arrhythmia burden Understand sleepy and nonsleepy phenotypes of SDB in relationship to cardiac arrhythmias and risk of morbidity and mortality, as well as treatment responsiveness Elucidate the role of SDB subtypes, that is, obstructive vs central apnea phenotypes, in relation to cardiac arrhythmia outcomes Clarify and identify novel endophenotypes (eg, postapneic heart rate response, SA-specific hypoxic burden) in cardiovascular disease to grasp the role of biomarkers specific to cardiac arrhythmias and treatment responsiveness to inform design of clinical trials and personalized treatment approaches |
|
| AF (Section 2) | |
| Existing guideline recommendations | Opportunistic screening for AF should be considered (ESC, ESC guidelines, Class IIac).73 Optimal management of OSA may be considered to reduce AF incidence, AF progression, AF recurrences, and symptoms (Class IIbc).73 OSA treatment should be optimized to reduce AF recurrences and to improve AF treatment results (Class IIac).73 SDB management should be part of a combined risk factor management approach in patients with AF (high evidence, ESC AF guidelines, Class IB).73 For patients with overweight or obesity and AF, weight loss combined with risk factor modification is recommended (ACC/AHA/HRS, COR I, LOE B-R).74 Screening (by polysomnography) and management of SA are recommended in individuals with recurrent symptomatic AF (GRADE quality of evidence: moderate; GRADE strength of recommendation: strong; CSANZ).75 Inform patients with OSA that there is a greater risk of developing AF and subsequent risk of stroke and death. Assess by anamnesis (snoring, daytime fatigue) the possibility of OSA. Refer to specialized clinic as needed (consensus, EHRA).76 Sleep study or overnight oximetry should be performed in most patients because typical symptoms are less prevalent and screening questionnaires are less accurate in the AF population.77 In patients with established AF or at high risk of developing AF, we recommend a systematic approach to the identification of traditional modifiable cardiovascular risk factors and conditions associated with AF, with strict guideline-adherent management to reduce major cardiovascular events (strong recommendation; high-quality evidence) and to prevent recurrence of the arrhythmia or to decrease its symptom burden (CCS/CHRS, strong recommendation; low-quality evidence).77 |
| Current report Best practices consensus statements |
Given the high prevalence of SDB in AF, screening for OSA in AF should be considered particularly in those at increased risk for OSA, that is, those with obesity, hypertension, and predominance of nocturnal AF episodes. We suggest that there is limited clinical utility of sleepiness in AF screening, given the variable predictive ability of self-reported sleepiness for SDB and that its role specifically in SDB with AF has not been established. Other symptom-based questionnaires used alone (without objective assessments) also have not shown strong predictive ability in this patient population. In-laboratory polysomnography should be considered in patients with substantive comorbidities, that is, particularly in people with AF and heart failure with mixed or predominant CSA, as well as pulmonary (eg, pulmonary hypertension) and neurological disease (eg, stroke). SDB management may be considered as part of a multidisciplinary team, with an integrated lifestyle modification, risk factor management, and patient-centric approach to reduce the risk of recurrent AF. Active engagement of the patient with shared medical decision making should be encouraged with technology to support comprehensive care for lifestyle modification, anticoagulation, and rate/rhythm control. For those with SDB, treatment of SDB should be considered part of lifestyle modification strategies to reduce the risk of AF, to prevent its progression, and to improve patient-reported outcomes. |
| Ventricular tachyarrhythmias/SCD (Section 3) | |
| Existing guideline recommendations | The presence of SDB and reduced oxygen saturation may be considered a risk factor for SCD in subjects with SDB (ESC guidelines, Class IIb).78 |
| Current report Best practices consensus statements |
Screening and diagnostics for SDB and nocturnal hypoxia should be considered in those with VTA and as a risk factor for SCD. In those with SDB, treatment of SDB and nocturnal hypoxia (eg, CPAP therapies, supplemental oxygen) should be considered in those with VTA and those at risk for SCD. |
| Bradyarrhythmias (Section 4) | |
| Existing guideline recommendations | In patients with documented or suspected bradycardia or conduction disorder during sleep, screening for symptoms of SA syndrome is recommended with subsequent confirmatory testing directed by clinical suspicion (ACC/AHA/HRS guideline Level I COR, LOE B-NR).79 In patients with sleep-related bradycardia or conduction disorder and documented OSA, treatment directed specifically at the SA (eg, CPAP and weight loss) is recommended (ACC/AHA/HRS guideline Level I COR, LOE B-NR).79 In patients who have previously received or are being considered for a permanent pacemaker for bradycardia or conduction disorder, screening for SA syndrome is reasonable (ACC/AHA/HRS guideline Level IIa COR, LOE B-NR).79 In patients with sleep-related sinus bradycardia or transient sinus pauses occurring during sleep, permanent pacing should not be performed unless other indications for pacing are present (ACC/AHA/HRS guideline Level III COR, LOE C-LD).79 |
| Current report Best practices consensus statements |
In those with profound or severe nocturnal bradyarrhythmia, that is, bradycardia, atrioventricular conduction delay, or prolonged sinus pauses, SDB screening with follow-up diagnostic testing as indicated should be considered to assess for improvement in bradyarrhythmia. As sleep medicine resources and patient medical stability allow, this could be considered before pacemaker placement. In those being considered for permanent pacemaker placement for bradyarrhythmia, assessment for SDB is reasonable as a potential targetable risk factor, particularly in those with predominant nocturnal (or sleep-time) bradyarrhythmia episodes. In those with profound or severe nocturnal bradyarrhythmia and SDB, treatment of SDB can be considered. |
ACC/AHA/HRS indicates American College of Cardiology/American Heart Association/Heart Rhythm Society; AF, atrial fibrillation; CCS/CHRS, Canadian Cardiovascular Society/Canadian Heart Rhythm Society; COR, Class of Recommendation; CPAP, continuous positive airway pressure; CSA, central sleep apnea; CSANZ, Cardiac Society of Australia and New Zealand; EHRA, European Heart Rhythm Association; ESC, European Society of Cardiology; GRADE, Grading of Recommendations Assessment, Development and Evaluation; LOE, Level of Evidence; OSA, obstructive sleep apnea; SA, sleep apnea; SCD, sudden cardiac death; SDB, sleep-disordered breathing; and VTA, ventricular tachyarrhythmia.
VTAs AND SDB
VTAs and SDB Epidemiology
A 2-fold higher odds of nonsustained ventricular tachycardia and 50% increased odds of complex ventricular ectopy in SDB are observed in epidemiological studies, along with graded, monotonic increases in the prevalence of complex ventricular ectopy.8 The association of nocturnal hypoxia and increased SDB has been specifically identified in older men.8 The immediate influences of apneic and hypopneic events as triggers of nonsustained ventricular tachycardia episodes indicate acute on chronic effects.80
A longitudinal study (n=10 701) with an average 5.3-year follow-up identified nocturnal hypoxia (mean nocturnal oxygen saturation and nadir nocturnal oxygen saturation) as an independent risk factor for SCD.81 In CSA, including CSB, increases in ventricular ectopy occur during the hyperpneic phase, when chemostimulation, blood pressure, and heart rate reach their peak, rather than the apneic phase.31 In heart failure, both OSA and CSA are predictors of sleep-specific lethal VTA.31
Increased degree of CSB (>20% of the recording time) was associated with higher frequency of VTA (>30 premature ventricular complexes per hour), particularly during sleep,82 in patients with heart failure with reduced ejection fraction (left ventricular ejection fraction ≤45%) in the SERVE-HF trial (Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure).60 However, the role of VTA on the adaptive servoventilation–related increased risk of cardiovascular death treated for predominant CSA is unclear.82
Case-crossover studies implicate respiratory events and periodic limb movements associated with electroencephalographic microarousals as immediate temporal triggers for VTA.43,83 Moreover, a nocturnal predilection of SCD is noted with OSA.80 Aligned with these observational studies, small clinical trials support a reduction in VTA burden with OSA treatment, particularly in heart failure.31 However, larger trials are needed to justify benefit of OSA treatment. An overview of key observational studies characterizing SDB indices in relation to VTA/SCD is provided in Table 1 (Section 2), and Table 2 (Section 2) provides an overview of studies examining the impact of SDB treatment on VTA.
VTAs and SDB Pathophysiology
Impaired baroreflex mechanisms in SDB appear to increase VTA and SCD through increased sympathetic activation and parasympathetic withdrawal.84 Hypoxia and acidosis result in early afterdepolarizations and triggered activity, which can lead to increased VTA.8 Acute upper airway obstruction results in transient dynamic QT interval prolongation, contributing to electromechanical window shortening and increased ventricular ectopy likely through alterations in intrathoracic pressures.85 Because most SDB occurs during the night, factors that influence circadian rhythms may modify risk for VTA. Molecular pathways governing the endogenous circadian rhythm and enhancing vulnerability to VTA have been described; that is, deficiency or gain of function of Krüppel-like factor 15 (Klf15) results in loss of rhythmic QT variation and abnormal repolarization and thereby increases risk for VTA and SCD.86
VTAs and SDB Guideline Recommendations
A Class IIb recommendation from the European Society of Cardiology guidelines indicates that the presence of sleep apnea and hypoxia may be considered a risk factor for SCD in those with SDB.78 It is recommended that the presence of OSA should be included in risk stratification for SCD.78 Moreover, it is stated that there is no evidence suggesting a deviation from the standard management of VTA in patients with SDB and that the value of CPAP for VTA and SCD prevention is still undefined78 (Table 3, Section 3).
BRADYARRHYTHMIAS AND SDB
Bradyarrhythmia and SDB Epidemiology
The most common cardiac arrhythmias during sleep and in SDB are bradyarrhythmias, including sinus bradycardia, sinus pauses, and first-degree and Mobitz I second-degree atrioventricular block. The prevalence of profound nocturnal sinus bradycardia in SDB is 7.2% to 40%, of second-or third-degree atrioventricular block is 1.3% to 13.3%, and of sinus pauses is 3.3% to 33%.79 Bradyarrhythmias have long been observed in OSA with resolution after tracheostomy.79 Epidemiological data indicate a higher percentage of atrioventricular block (first and second degree: 1.8% versus 0.3% and 2.2% versus 0.9%, respectively) in severe OSA, but without statistically significant differences, perhaps attributable to competing risk factors or mitigated autonomic response in this older cohort.81
Limited data from a small cohort with severe OSA and 50% prevalence of severe nocturnal bradycardic episodes support long-term benefit of OSA treatment with CPAP on the reduction of median number of bradycardic events based on insertable loop recorder monitoring over a 16-month follow-up period.81
An overview of key observational studies characterizing SDB indices in relation to bradyarrhythmia is provided in Table 1 (Section 3), and Table 2 (Section 3) provides an overview of studies examining the impact of SDB treatment on bradyarrhythmia.
Bradyarrhythmia and SDB Pathophysiology
Prolonged apneas and accompanying hypoxia result in enhanced parasympathetic tone that becomes more pronounced during rapid eye movement sleep, a sleep state associated with further enhancement of parasympathetic influences.84 Absence of ventilation during apneic events results in vagotonic hypoxic stimulation of the carotid body, resulting in bradycardia.87 Interindividual variability of susceptibility to bradyarrhythmia likely depends on the severity of hypoxia, inherent hypoxic chemosensitivity, and hypoxic influences on the sinoatrial node.87
Bradyarrhythmia and SDB Guideline Recommendations
The European Society of Cardiology guidelines recommend that SDB should be considered in the differential diagnosis of bradyarrhythmias.78 American Heart Association Class I recommendations include the following: (1) Patients with documented or suspected bradycardia or conduction disorder during sleep should be screened for SDB; and (2) patients with sleep-related bradycardia or conduction disorder and documented SDB should undergo treatment of SDB.79 In addition, in patients who have previously received or are being considered for a permanent pacemaker for bradycardia or conduction disorder, SDB screening is reasonable79 (Table 3, Section 4).
STEPWISE CLINICAL CARE MODELS OF INTEGRATION OF SDB RISK IN CARDIAC ARRHYTHMIA MANAGEMENT
Integrated health care delivery models are needed to provide SDB screening, diagnostics, and management in cardiac arrhythmias. This requires close interdisciplinary collaborations between electrophysiologists and sleep medicine specialists using patient-centric, team-based approaches inclusive of clinicians and sleep technologists. Effective care approaches require active engagement of the patient with shared medical decision-making, a multidisciplinary team approach, technology to support integrated care, a comprehensive approach to lifestyle modification, and anticoagulation and rate and rhythm control as indicated88 (Figure 2).
Figure 2. Patient-centric, integrated stepped care model of SDB and cardiac arrhythmia.

A multidisciplinary team–based care approach in a patient-centered model leveraging technology to support sleep-disordered breathing (SDB) diagnostics and management in people with cardiac arrhythmia incorporating the following steps can be considered: initiation of (1) guideline-directed therapy, (2) SDB screening, (3) SDB diagnostics as indicated, (4) SDB treatment as indicated, and (5) cardiac arrhythmia risk factor and SDB management with the goal of follow-up to reduced cardiac arrhythmia–related morbidity and maintain sinus rhythm in atrial fibrillation (AF), as well as (6) teaching and self-care support. App indicates application; CSA, central sleep apnea; EMR, electronic medical record; HSAT, home sleep apnea testing; NABS, Neck Circumference/Age/Body Mass Index/Snoring; NC, neck circumference; OSA, obstructive sleep apnea; PAP, positive airway pressure; PSG, polysomnogram; STOP-BANG, Snoring/Tiredness/Observed Apnea/Pressure (High Blood Pressure)/Body Mass Index/Age/Neck Circumference/Gender; and VTA, ventricular tachyarrhythmia. *Please refer to Table 3 for reference to sources for guideline recommendations for cardiac arrhythmia management. Reprinted with permission from the Cleveland Clinic Center for Medical Art & Photography. Copyright © 2022. All rights reserved.
SUMMARY, GAPS, AND FUTURE DIRECTIONS
The interplay of SDB, sleep disorders, and cardiac arrhythmia is complex and linked through multifactorial mechanisms. Strong preclinical data implicate SDB-induced autonomic responses; however, repetitive episodes of hypoxia, intrathoracic pressure alterations, and increased systemic inflammation and free radicals also play a role in adverse cardiac remodeling over time, thereby enhancing arrhythmogenic risk. Observational data support strong associations of SDB and arrhythmia, as well as immediate and long-term temporal, graded monotonic relationships.
Treatment of SDB in many observational studies is associated with improved AF outcomes. However, these findings have not been confirmed in randomized controlled clinical trials, although trials to date have been limited by modest sample size, inadequate power, short duration of follow-up, or patient selection. Sleep quality, sleep duration, sleep disruptions, and SDB each may be important in the pathogenesis of AF, potentially representing a novel target for preventing occurrence, recurrence, and progression of AF. International societies suggest that it is reasonable to conduct OSA screening in patients with AF in those with risk factors to ensure optimization of AF treatment strategies. Effective approaches to screen for SDB in AF are recommended; however, ideal strategies remain unclear. Society recommendations also support consideration of SDB as a risk for VTA and bradyarrhythmias (Table 3).
Epidemiological studies integrating detailed cardiac function and measures of visceral adiposity are needed to better discern the interrelationships of SDB, other sleep disorders, and metabolic mechanisms that contribute to cardiac arrhythmia. More effective screening strategies and diagnostic paradigms that enable patients with cardiac arrhythmias to be evaluated for underlying mechanistic features that influence responsiveness to treatment and risk for arrhythmias are needed to better direct the right treatment to the right patient. Understanding the heterogeneity of SDB pathophysiological contributions (eg, extent of hypoxia burden, autonomic responses) and the trajectory of arrhythmia development over time is essential to inform risk stratification and clinical trials. Better diagnostic approaches for distinguishing obstructive from central apneas, including methods that can be used over time to understand longitudinal trends, are needed to inform our understanding of which phenotypes are the most appropriate targets for improving cardiac arrhythmia outcomes.
With little innate biological or genetic basis, race and ethnicity are social constructs shaped by the sociopolitical and economic societal forces that can manifest into biological consequences. Racial and ethnic inequities in the epidemiology of AF and its association with various sleep dimensions warrant additional research, for example, facilitating earlier recognition/diagnosis and management of SDB in disadvantaged populations. Sex-specific differences in clinical presentation of SDB and tailoring of diagnostic strategies given recognized sex-specific differences in respiratory event arousal thresholds, for example, also deserve further study. Leverage of mobile health innovations and integrated models of care to facilitate SDB diagnosis in relation to arrhythmia burden is a priority area for investigation.
Last, an important gap that is of high priority is the need for adequately powered, rigorously conducted clinical trials involving CPAP and newer interventions (eg, neurostimulation therapies, supplemental oxygen, drugs) to treat SDB in patients with cardiac arrhythmias. The use of methods for optimizing treatment adherence, as well as adaptive and enrichment designs, may be particularly useful given the likelihood that treatment responses differ across people. Because large multicenter clinical trials to effectively understand SDB interventions are not imminent, large administrative databases and claims data may offer interim useful insights. Critical to the success of these clinical trials is enhanced phenotyping to facilitate the inclusion of individuals most likely to benefit and respond to therapy to improve cardiac arrhythmia outcomes. Innovative study designs leveraging existing data for propensity-matched analyses and clinical trials using crossover or adaptive designs should be carefully considered.
Acknowledgments
The authors sincerely thank Anne Leonard, MPH, RN, CCRC, FAHA, senior science and medicine advisor, Office of Science Operations, American Heart Association National Center, and Connie Land, assistant managing editor, Scientific Publishing, Office of Science Operations, American Heart Association National Center, for their support and guidance during the preparation of this statement. They thank Jessica Love, research observer, for her assistance with assembly of the tables.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on March 21, 2022, and the American Heart Association Executive Committee on April 27, 2022. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com.
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Reena Mehra | Cleveland Clinic Foundation | NIH*; American Academy of Sleep Medicine* | None | None | None | None | None | None |
| Mina K. Chung | Cleveland Clinic | AHA†; NIH† | None | None | None | None | None | None |
| Dobromir Dobrev | University Duisburg–Essen Institute of Pharmacology (Germany) | None | None | None | None | None | None | None |
| Chandra L. Jackson | National Institutes of Health | None | None | None | None | None | None | None |
| Vaishnavi Kundel | Icahn School of Medicine at Mount Sinai | None | None | None | None | None | None | None |
| Dominik Linz | Maastricht University Medical Centre (Netherlands) | None | None | None | None | None | None | None |
| Brian Olshansky | University of Iowa Hospitals and Clinics | None | None | None | None | None | AstraZeneca DSMB* | None |
| Nancy S. Redeker | Yale School of Nursing | None | None | None | None | None | None | None |
| Susan Redline | Brigham and Women’s Hospital | NIH (multiple NIH grants on sleep)† | None | None | None | None | Apnimed, Inc†; Eli Lilly*; Jazz Pharma* | None |
| Prashanthan Sanders | University of Adelaide and Royal Adelaide Hospital Centre for Heart Rhythm Disorders (Australia) | None | None | None | None | None | None | None |
| Virend K. Somers | Mayo Clinic | NIH (funds to institution)†; Sleep Number (funds to institution)†; Medtronic (equipment to institution)†; Itamar (equipment to institution)† | None | None | None | None | Bayer*; Jazz Pharmaceuticals†; Respicardia*; Huxley Medical*; ResMed* | None |
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Martin R. Cowie | Royal Brompton Hospital (United Kingdom) | Abbott (Research Fellowship grant to my department)† | None | Abbott*; Medtronic* | None | None | AstraZeneca†; Abbott*; Medtronic* | None |
| Jose A. Joglar | UT Southwestern Medical Center | None | None | None | None | None | None | None |
| Chiadi Ndumele | Johns Hopkins University | None | None | None | None | None | None | None |
| Jean-Louis Pépin | Centre Hospitalier Universitaire Sleep Laboratory and EFCR Grenoble University Hospital (France) | None | None | None | None | None | None | None |
| Craig L. Phillips | University of Sydney (Australia) | None | None | None | None | None | None | None |
REFERENCES
- 1.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DA, Guo N, Rueschman M, Wang R, Wilson JG, Redline S. Prevalence and correlates of obstructive sleep apnea among African Americans: the Jackson Heart Sleep Study. Sleep. 2018;41:zsy154. doi: 10.1093/sleep/zsy154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadhim K, Middeldorp ME, Elliott AD, Jones D, Hendriks JML, Gallagher C, Arzt M, McEvoy RD, Antic NA, Mahajan R, et al. Self-Reported Day-time Sleepiness and Sleep-Disordered Breathing in Patients With Atrial Fibrillation: SNOozE-AF. Can J Cardiol. 2019;35:1457–1464. doi: 10.1016/j.cjca.2019.07.627 [DOI] [PubMed] [Google Scholar]
- 6.Edwards BA, Redline S, Sands SA, Owens RL. More than the sum of the respiratory events: personalized medicine approaches for obstructive sleep apnea. Am J Respir Crit Care Med. 2019;200:691–703. doi: 10.1164/rccm.201901-0014TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra A, Ayappa I, Ayas N, Collop N, Kirsch D, McArdle N, Mehra R, Pack AI, Punjabi N, White DP, et al. Metrics of sleep apnea severity: beyond the AHI. Sleep. 2021;44:zsab030. doi: 10.1093/sleep/zsab030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May AM, Van Wagoner DR, Mehra R. Obstructive sleep apnea and cardiac arrhythmogenesis: mechanistic insights. Chest. 2017;151:225–241. doi: 10.1016/j.chest.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol. 2014;64:710–721. doi: 10.1016/j.jacc.2014.06.1169 [DOI] [PubMed] [Google Scholar]
- 10.Essien UR, Kim N, Hausmann LRM, Mor MK, Good CB, Magnani JW, Litam TMA, Gellad WF, Fine MJ. Disparities in anticoagulant therapy initiation for incident atrial fibrillation by race/ethnicity among patients in the Veterans Health Administration system. JAMA Netw Open. 2021;4:e2114234. doi: 10.1001/jamanetworkopen.2021.14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volgman AS, Bairey Merz CN, Benjamin EJ, Curtis AB, Fang MC, Lindley KJ, Pepine CJ, Vaseghi M, Waldo AL, Wenger NK, et al. Sex and race/ethnicity differences in atrial fibrillation. J Am Coll Cardiol. 2019;74:2812–2815. doi: 10.1016/j.jacc.2019.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17:213–222. doi: 10.1111/j.1440-1843.2011.02082.x [DOI] [PubMed] [Google Scholar]
- 13.Borker PV, Reid M, Sofer T, Butler MP, Azarbarzin A, Wang H, Wellman A, Sands SA, Redline S. Non-REM apnea and hypopnea duration varies across population groups and physiologic traits. Am J Respir Crit Care Med. 2021;203:1173–1182. doi: 10.1164/rccm.202005-1808OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghazi L, Bennett A, Petrov ME, Howard VJ, Safford MM, Soliman EZ, Glasser SP. Race, sex, age, and regional differences in the association of obstructive sleep apnea with atrial fibrillation: Reasons for Geographic and Racial Differences in Stroke study. J Clin Sleep Med. 2018;14:1485–1493. doi: 10.5664/jcsm.7320 [DOI] [PMC free article] [PubMed] [Google Scholar]; 15a. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, et al. ; on behalf of the American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]; 15b. Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, Pita MA, Potharaju KA, Tamura K, Wallen GR. Social determinants of cardiovascular disease. Circ Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linz D, Nattel S, Kalman JM, Sanders P. Sleep apnea and atrial fibrillation. Card Electrophysiol Clin. 2021;13:87–94. doi: 10.1016/j.ccep.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May AM, Blackwell T, Stone PH, Stone KL, Cawthon PM, Sauer WH, Varosy PD, Redline S, Mehra R; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med. 2016;193:783–791. doi: 10.1164/rccm.201508-1523OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung P, Levitzky YS, Wang R, Weng J, Quan SF, Gottlieb DJ, Rueschman M, Punjabi NM, Mehra R, Bertisch S, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc. 2017;6:e004500. doi: 10.1161/JAHA.116.004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raman D, Kaffashi F, Lui LY, Sauer WH, Redline S, Stone P, Cawthon PM, Stone KL, Ensrud KE, Ancoli-Israel S, et al. Polysomnographic heart rate variability indices and atrial ectopy associated with incident atrial fibrillation risk in older community-dwelling men. JACC Clin Electrophysiol. 2017;3:451–460. doi: 10.1016/j.jacep.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadby G, McArdle N, Briffa T, Hillman DR, Simpson L, Knuiman M, Hung J. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest. 2015;148:945–952. doi: 10.1378/chest.15-0229 [DOI] [PubMed] [Google Scholar]
- 22.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060 [DOI] [PubMed] [Google Scholar]
- 23.Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, et al. ; ORBIT-AF Investigators. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation: results from the Outcomes Registry for Bette1r Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647–654.e2. doi: 10.1016/j.ahj.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 24.Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, Herges RM, Howard DE, Somers VK. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reshetnik A, Puppe S, Bonnemeier H. Central sleep apnoea and arrhythmogenesis after myocardial infarction: the CESAAR study. Front Cardiovasc Med. 2019;6:108. doi: 10.3389/fcvm.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832 [DOI] [PubMed] [Google Scholar]
- 27.Selim BJ, Koo BB, Qin L, Jeon S, Won C, Redeker NS, Lampert RJ, Concato JP, Bravata DM, Ferguson J, et al. The association between nocturnal cardiac arrhythmias and sleep-disordered breathing: the DREAM study. J Clin Sleep Med. 2016;12:829–837. doi: 10.5664/jcsm.5880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker HF, Koehler U, Stammnitz A, Peter JH. Heart block in patients with sleep apnoea. Thorax. 1998;53(suppl 3):S29–S32. doi: 10.1136/thx.53.2008.s29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrigue S, Pépin JL, Defaye P, Murgatroyd F, Poezevara Y, Clémenty J, Lévy P. High prevalence of sleep apnea syndrome in patients with long-term pacing: the European Multicenter Polysomnographic Study. Circulation. 2007;115:1703–1709. doi: 10.1161/CIRCULATIONAHA.106.659706 [DOI] [PubMed] [Google Scholar]
- 30.Kendzerska T, Gershon AS, Atzema C, Dorian P, Mangat I, Hawker G, Leung RS. Sleep apnea increases the risk of new hospitalized atrial fibrillation: a historical cohort study. Chest. 2018;154:1330–1339. doi: 10.1016/j.chest.2018.08.1075 [DOI] [PubMed] [Google Scholar]
- 31.Di Fusco SA, Pignalberi C, Santini L, Colivicchi F, Santini M. Arrhythmias and sleep apnea: physiopathologic link and clinical implications. J Interv Card Electrophysiol. 2020;57:387–397. doi: 10.1007/s10840-020-00707-z [DOI] [PubMed] [Google Scholar]
- 32.Linz D, Brooks AG, Elliott AD, Nalliah CJ, Hendriks JML, Middeldorp ME, Gallagher C, Mahajan R, Kalman JM, McEvoy RD, et al. Variability of sleep apnea severity and risk of atrial fibrillation: the VARIOSA-AF study. JACC Clin Electrophysiol. 2019;5:692–701. doi: 10.1016/j.jacep.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 33.Christensen MA, Dixit S, Dewland TA, Whitman IR, Nah G, Vittinghoff E, Mukamal KJ, Redline S, Robbins JA, Newman AB, et al. Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15:1289–1295. doi: 10.1016/j.hrthm.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon Y, Gharib SA, Biggs ML, Jacobs DR Jr, Alonso A, Duprez D, Lima J, Lin GM, Soliman EZ, Mehra R, et al. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2015;70:873–879. doi: 10.1136/thoraxjnl-2014-206655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genuardi MV, Ogilvie RP, Saand AR, DeSensi RS, Saul MI, Magnani JW, Patel SR. Association of short sleep duration and atrial fibrillation. Chest. 2019;156:544–552. doi: 10.1016/j.chest.2019.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Zhou T, Ma H, Huang T, Gao X, Manson JE, Qi L. Healthy sleep patterns and risk of incident arrhythmias. J Am Coll Cardiol. 2021;78:1197–1207. doi: 10.1016/j.jacc.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowie MR, Linz D, Redline S, Somers VK, Simonds AK. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:608–624. doi: 10.1016/j.jacc.2021.05.048 [DOI] [PubMed] [Google Scholar]
- 38.Tavares L, Lador A, Valderrábano M. Sleep apnea and atrial fibrillation: role of the cardiac autonomic nervous system. Methodist Debakey Cardiovasc J. 2021;17:49–52. doi: 10.14797/ZYUT2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Lévy P, Kalman JM, Sanders P. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3:532–540. doi: 10.1001/jamacardio.2018.0095 [DOI] [PubMed] [Google Scholar]
- 40.Javaheri S, Corbett WS. Association of low PaCO2 with central sleep apnea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann Intern Med. 1998;128:204–207. doi: 10.7326/0003-4819-128-3-199802010-00006 [DOI] [PubMed] [Google Scholar]
- 41.Black N, D’Souza A, Wang Y, Piggins H, Dobrzynski H, Morris G, Boyett MR. Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm. 2019;16:298–307. doi: 10.1016/j.hrthm.2018.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernardi J, Aromolaran KA, Zhu H, Aromolaran AS. Circadian mechanisms: cardiac ion channel remodeling and arrhythmias. Front Physiol. 2020;11:611860. doi: 10.3389/fphys.2020.611860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monahan K, Storfer-Isser A, Mehra R, Shahar E, Mittleman M, Rottman J, Punjabi N, Sanders M, Quan SF, Resnick H, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54:1797–1804. doi: 10.1016/j.jacc.2009.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwasaki YK, Kato T, Xiong F, Shi YF, Naud P, Maguy A, Mizuno K, Tardif JC, Comtois P, Nattel S. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol. 2014;64:2013–2023. doi: 10.1016/j.jacc.2014.05.077 [DOI] [PubMed] [Google Scholar]
- 45.Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, Antic N, Thornton A, Saint DA, McEvoy D, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–327. doi: 10.1016/j.hrthm.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 46.Benjamin EJ, Al-Khatib SM, Desvigne-Nickens P, Alonso A, Djoussé L, Forman DE, Gillis AM, Hendriks JML, Hills MT, Kirchhof P, et al. Research priorities in the secondary prevention of atrial fibrillation: a National Heart, Lung, and Blood Institute virtual workshop report. J Am Heart Assoc. 2021;10:e021566. doi: 10.1161/JAHA.121.021566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mariani S, Fiore D, Barbaro G, Basciani S, Saponara M, D’Arcangelo E, Ulisse S, Moretti C, Fabbri A, Gnessi L. Association of epicardial fat thickness with the severity of obstructive sleep apnea in obese patients. Int J Cardiol. 2013;167:2244–2249. doi: 10.1016/j.ijcard.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 48.Desteghe L, Hendriks JML, Heidbuchel H, Potpara TS, Lee GA, Linz D. Obstructive sleep apnoea testing and management in atrial fibrillation patients: a joint survey by the European Heart Rhythm Association (EHRA) and the Association of Cardiovascular Nurses and Allied Professions (ACNAP). Europace. 2021;23:1677–1684. doi: 10.1093/europace/euab109 [DOI] [PubMed] [Google Scholar]
- 49.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E [DOI] [PubMed] [Google Scholar]
- 50.May AM, Wang L, Kwon DH, Van Wagoner DR, Chung MK, Dalton JE, Mehra R. Sleep apnea screening instrument evaluation and novel model development and validation in the paroxysmal atrial fibrillation population. Int J Cardiol Heart Vasc. 2020;31:100624. doi: 10.1016/j.ijcha.2020.100624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linz D, Kadhim K, Brooks AG, Elliott AD, Hendriks JML, Lau DH, Mahajan R, Gupta AK, Middeldorp ME, Hohl M, et al. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int J Cardiol. 2018;272:155–161. doi: 10.1016/j.ijcard.2018.07.124 [DOI] [PubMed] [Google Scholar]
- 52.Mohammadieh AM, Sutherland K, Kanagaratnam LB, Whalley DW, Gillett MJ, Cistulli PA. Clinical screening tools for obstructive sleep apnea in a population with atrial fibrillation: a diagnostic accuracy trial. J Clin Sleep Med. 2021;17:1015–1024. doi: 10.5664/jcsm.9098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varma N, Cygankiewicz I, Turakhia MP, Heidbuchel H, Hu YF, Chen LY, Couderc JP, Cronin EM, Estep JD, Grieten L, et al. 2021 ISHNE/HRS/ EHRA/APHRS expert collaborative statement on mHealth in arrhythmia management: digital medical tools for heart rhythm professionals: from the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia-Pacific Heart Rhythm Society. Circ Arrhythm Electrophysiol. 2021;14:e009204. doi: 10.1161/CIRCEP.120.009204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roeder M, Bradicich M, Schwarz EI, Thiel S, Gaisl T, Held U, Kohler M. Night-to-night variability of respiratory events in obstructive sleep apnoea: a systematic review and meta-analysis. Thorax. 2020;75:1095–1102. doi: 10.1136/thoraxjnl-2020-214544 [DOI] [PubMed] [Google Scholar]
- 55.Li L, Wang ZW, Li J, Ge X, Guo LZ, Wang Y, Guo WH, Jiang CX, Ma CS. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace. 2014;16:1309–1314. doi: 10.1093/europace/euu066 [DOI] [PubMed] [Google Scholar]
- 56.Qureshi WT, Nasir UB, Alqalyoobi S, O’Neal WT, Mawri S, Sabbagh S, Soliman EZ, Al-Mallah MH. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol. 2015;116:1767–1773. doi: 10.1016/j.amjcard.2015.08.046 [DOI] [PubMed] [Google Scholar]
- 57.Shukla A, Aizer A, Holmes D, Fowler S, Park DS, Bernstein S, Bernstein N, Chinitz L. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol. 2015;1:41–51. doi: 10.1016/j.jacep.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 58.Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers VK, on behalf of the American Heart Association Council on Clinical Cardiology; Council on Peripheral Vascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Stroke Council; and Council on Cardiovascular Surgery and Anesthesia. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association [published correction appears in Circulation. 2021;145:e775]. Circulation. 2021;144:e56–e67. doi: 10.1161/CIR.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 59.Siontis KC, Oral H. Atrial fibrillation and obstructive sleep apnea: beyond the pulmonary veins. Circ Arrhythm Electrophysiol. 2017;10:e005890. doi: 10.1161/CIRCEP.117.005890 [DOI] [PubMed] [Google Scholar]
- 60.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, et al. Adaptive servoventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 62.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 63.Caples SM, Mansukhani MP, Friedman PA, Somers VK. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol. 2019;278:133–136. doi: 10.1016/j.ijcard.2018.11.100 [DOI] [PubMed] [Google Scholar]
- 64.Traaen GM, Aakerøy L, Hunt TE, Øverland B, Bendz C, Sande LØ, Aakhus S, Fagerland MW, Steinshamn S, Anfinsen OG, et al. Effect of continuous positive airway pressure on arrhythmia in atrial fibrillation and sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2021;204:573–582. doi: 10.1164/rccm.202011-4133OC [DOI] [PubMed] [Google Scholar]
- 65.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, et al. ; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 66.Seyis S, Usalan AK, Rencuzogullari I, Kurmuş Ö, Gungen AC. The effects of continuous positive airway pressure on premature ventricular contractions and ventricular wall stress in patients with heart failure and sleep apnea. Can Respir J. 2018;2018:2027061. doi: 10.1155/2018/2027061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–397. doi: 10.1161/01.cir.101.4.392 [DOI] [PubMed] [Google Scholar]
- 68.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60:781–785. doi: 10.1136/thx.2005.040972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abe H, Takahashi M, Yaegashi H, Eda S, Tsunemoto H, Kamikozawa M, Koyama J, Yamazaki K, Ikeda U. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart Vessels. 2010;25:63–69. doi: 10.1007/s00380-009-1164-z [DOI] [PubMed] [Google Scholar]
- 70.Becker H, Brandenburg U, Peter JH, Von Wichert P. Reversal of sinus arrest and atrioventricular conduction block in patients with sleep apnea during nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:215–218. doi: 10.1164/ajrccm.151.1.7812557 [DOI] [PubMed] [Google Scholar]
- 71.Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, Noseworthy PA, Pack QR, Sanders P, Trulock KM; on behalf of the American Heart Association Electrocardiography and Arrhythmias Committee and Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Lifestyle and Cardiometabolic Health. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. doi: 10.1161/CIR.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 72.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm. 2017;14:e445–e494. doi: 10.1016/j.hrthm.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 73.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 74.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2019;140:e285]. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 75.Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani H, et al. ; NHFA CSANZ Atrial Fibrillation Guideline Working Group. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. 2018;27:1209–1266. doi: 10.1016/j.hlc.2018.06.1043 [DOI] [PubMed] [Google Scholar]
- 76.Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, Van Gelder IC, Halle M, Kudaiberdieva G, Lane DA, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation. Eur J Prev Cardiol. 2017;24:4–40. doi: 10.1177/2047487316676037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, et al. ; Members of the Secondary Panel. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. doi: 10.1016/j.cjca.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 78.Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. ; ESC Scientific Document Group. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- 79.Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382–e482. doi: 10.1161/CIR.0000000000000628 [DOI] [PubMed] [Google Scholar]
- 80.Riaz S, Bhatti H, Sampat PJ, Dhamoon A. The converging pathologies of obstructive sleep apnea and atrial arrhythmias. Cureus. 2020;12:e9388. doi: 10.7759/cureus.9388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geovanini GR, Lorenzi-Filho G. Cardiac rhythm disorders in obstructive sleep apnea. J Thorac Dis. 2018;10(suppl 34):S4221–S4230. doi: 10.21037/jtd.2018.12.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fisser C, Bureck J, Gall L, Vaas V, Priefert J, Fredersdorf S, Zeman F, Linz D, Wöhrle H, Tamisier R, et al. Ventricular arrhythmia in heart failure patients with reduced ejection fraction and central sleep apnoea. ERJ Open Res. 2021;7:00147–02021. doi: 10.1183/23120541.00147-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.May AM, May RD, Bena J, Wang L, Monahan K, Stone KL, Barrett-Connor E, Koo BB, Winkelman JW, Redline S, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Individual periodic limb movements with arousal are temporally associated with nonsustained ventricular tachycardia: a casecrossover analysis. Sleep. 2019;42:zsz165. doi: 10.1093/sleep/zsz165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Padeletti M, Zacà V, Mondillo S, Jelic S. Sleep-disordered breathing increases the risk of arrhythmias. J Cardiovasc Med (Hagerstown). 2014;15:411–416. doi: 10.2459/JCM.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linz B, Sattler SM, Flethoj M, Hotbjerg Hansen ME, Hesselkilde EM, Saljic A, Wirth K, Linz D, Tfelt-Hansen J, Jespersen T. Arrhythmogenic mechanisms of acute obstructive respiratory events in a porcine model of drug-induced long QT. Heart Rhythm. 2021;18:1384–1391. doi: 10.1016/j.hrthm.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 86.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leung RS. Sleep-disordered breathing: autonomic mechanisms and arrhythmias. Prog Cardiovasc Dis. 2009;51:324–338. doi: 10.1016/j.pcad.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 88.Hendriks JM, Heidbüchel H. The management of atrial fibrillation: an integrated team approach: insights of the 2016 European Society of Cardiology guidelines for the management of atrial fibrillation for nurses and allied health professionals. Eur J Cardiovasc Nurs. 2019;18:88–95. doi: 10.1177/1474515118804480 [DOI] [PubMed] [Google Scholar]


