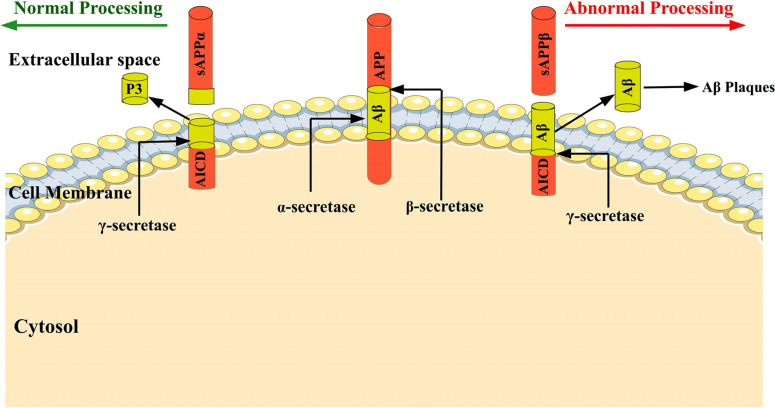
Fig. (1).
The amyloid precursor protein (APP) is a transmembrane protein, and during normal physiological condition, 90% of the APP is cleaved through α and γ-secretase and forms soluble p3 and APP intracellular domain (AICD) fragments. When a small percentage of APP molecules enter the β-secretase route, APP is cleaved by β-secretase. It results in the formation of β-APPs and membrane-bound C99 peptides. The γ-secretase cleaves the C-terminal membrane-bound C99 peptide within the transmembrane domain to produce two primary isoforms of Αβ with 40 and 42 amino acid lengths which are responsible for Αβ plaques formation.