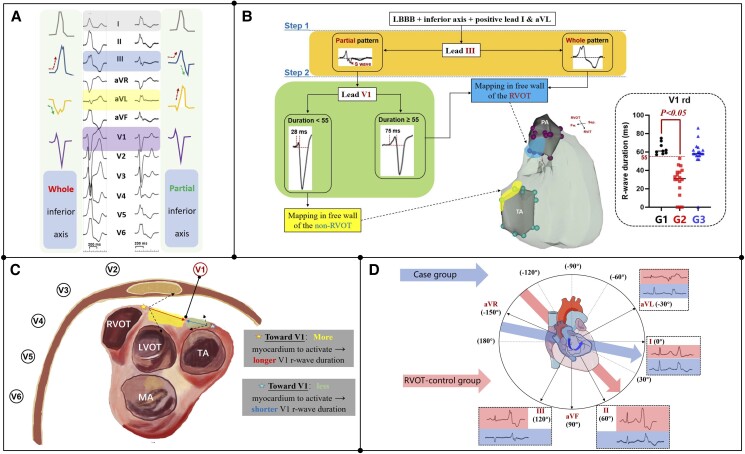
Graphical Abstract
Graphical Abstract.
(A) Ventricular arrhythmias (VAs) originating around the right pulmonary sinus (PRS) in different types of inferior axis ECG. (B) Electrocardiographic localization of VAs with inferior axis and LBBB pattern; G1, case group; G2, non-RVOT control group; G3, RVOT control group. (C) Hypothesis of the longer R-wave duration during PRS-VAs in lead-V1. (D) The electric axis of the control and case group during sinus rhythm. Fw., free wall; LBBB, left bundle branch block; PA, pulmonary artery; RVOT/LVOT, right/left ventricular outflow tract; Sep., septum; TA/MA, tricuspid/mitral annulus.
Keywords: Ventricular arrhythmias, Right ventricular outflow tract, Inferior axis, ECG diagnosis, Mapping
A ‘whole inferior axis’ with a unidirectional positive R-wave in all three inferior leads and a negative QS-wave in lead-aVR/aVL indicates that the ventricular arrhythmia (VA) originates from the right ventricular outflow tract (RVOT).1–3 Additionally, a ‘partial inferior axis (PIA)’ with a negative S-wave in lead-III and a positive R-wave in lead-aVL indicates that the VA originates from the right ventricular non-outflow tract region.4 However, some atypical cases may show discordance between electrocardiographic classification and three-dimensional mapping. The aim of this study is to explore the target distribution and electrophysiological characteristics of RVOT-VAs with the PIA.
The study was approved (No. 2021-1523) by the ethics committee of Fuwai Hospital. We performed a case-control study in which 9 consecutive RVOT-VA patients (among 1247 RVOT-VAs) with PIA deviation and monomorphism were enrolled as the case group from January 2014 to July 2021. Two control groups (selected during the same period as the case group) were matched at a ratio of 1:2:2. Eighteen cases were enrolled in the RVOT control group, which were matched according to the site of VA origin in the RVOT, and 18 cases were enrolled in the non-RVOT control group, which were matched according to the characteristics of limb lead electrocardiogram (ECG) for right-sided non-RVOT origin VAs with a PIA.
A total of 45 patients were enrolled in the present study. Although, female patients accounted for more than 50% in both the case group and the RVOT control group, they comprised a smaller proportion in the non-RVOT control group (88.9% vs. 66.7 vs. 27.8%, P < 0.05). In the case group, all the ablation targets were located around the pulmonary right sinus.
The ECG comparisons between the case group and RVOT control group are mainly reflected in the limb leads: lead-III of the case group had an ‘RS’ pattern, and the RVOT control group had an ‘R’ pattern with a higher R-wave amplitude (0.48 ± 0.21 vs. 0.92 ± 0.35 mV, P = 0.002). In addition, the ratio of R-wave amplitudes (lead-III/lead-II) in the case group was lower than that in the RVOT control group (0.60 ± 0.11 vs. 0.79 ± 0.11, P < 0.001). A positive R-wave in lead-aVL was more common in the case group (100% vs. 5.6%, P < 0.001).
The ECG comparisons between the case group and non-RVOT control group are mainly reflected in the precordial leads: the R-wave duration of lead-V1 was significantly longer in the case group (63.4 ± 6.4 vs. 28.4 ± 15.9 ms, P < 0.001). Detailed comparisons of the ECG are shown in Table 1. A cut-off value of 55 ms (area under curve: 1; P < 0.001) for R-wave duration in lead-V1 could entirely predict the RVOT origin with an accuracy of 100%.
Table 1.
Comparison of ECG characteristics among different groups
| ECG characteristics during Vas | Case-RVOT (n = 9) | Comparison 1 | Comparison 2 | ||
|---|---|---|---|---|---|
| Control-non-RVOT (n = 18) | P value | Control-RVOT (n = 18) | P value | ||
| Lead-I | |||||
| ‘R’ pattern, n (%) | 9 (100) | 18 (100) | >0.999 | 17 (94.4) | >0.999 |
| *R-wave amplitude, mV | 0.64 ± 0.14 | 0.56 ± 0.26 | 0.418 | 0.33 ± 0.15 | <0.001 |
| Lead-II | |||||
| ‘R’ pattern, n (%) | 9 (100) | 13 (72.2) | 0.136 | 18 (100) | >0.999 |
| R-wave amplitude, mV | 0.80 ± 0.33 | 0.78 ± 0.34 | 0.920 | 1.15 ± 0.35 | 0.020 |
| Lead-III | |||||
| ‘RS’ pattern, n (%) | 9 (100) | 15 (83.3) | 0.529 | 0 | <0.001 |
| R-wave amplitude, mV | 0.48 ± 0.21 | 0.37 ± 0.22 | 0.222 | 0.92 ± 0.35 | 0.002 |
| Lead-III/II | 0.60 ± 0.11 | 0.45 ± 0.23 | 0.032 | 0.79 ± 0.11 | <0.001 |
| Lead-aVR: Q-wave amplitude, mV | 0.62 ± 0.21 | 0.64 ± 0.22 | 0.859 | 0.70 ± 0.21 | 0.374 |
| Lead-aVL | |||||
| ‘QR/R’ pattern in aVL, n (%) | 9 (100) | 17 (94.4) | >0.999 | 1 (5.6) | <0.001 |
| R-wave amplitude in aVL, mV | 0.41 ± 0.11 | 0.40 ± 0.24 | 0.899 | — | — |
| Lead-aVF: R-wave amplitude, mV | 0.61 ± 0.28 | 0.58 ± 0.29 | 0.739 | 1.0 ± 0.34 | 0.004 |
| Lead-V1 | |||||
| ‘RS’ pattern | 9 (100) | 15 (83.3) | 0.529 | 17 (94.4) | >0.999 |
| R-wave amplitude, mV | 0.28 ± 0.10 | 0.18 ± 0.14 | 0.062 | 0.24 ± 0.13 | 0.378 |
| R-wave duration, ms | 63.4 ± 6.4 | 28.4 ± 15.9 | <0.001 | 57.9 ± 16.7 | 0.349 |
| Lead-V2 | |||||
| R-wave amplitude, mV | 0.60 ± 0.35 | 0.31 ± 0.19 | 0.008 | 0.48 ± 0.18 | 0.315 |
| R-wave duration, ms | 67.2 ± 8.6 | 39.9 ± 16.7 | <0.001 | 65.1 ± 11.1 | 0.612 |
| QRS duration, ms | 182.6 ± 15.6 | 170.4 ± 20.3 | 0.130 | 183.7 ± 16.9 | 0.864 |
RVOT, right ventricular outflow tract; Vas, ventricular arrhythmias. Bold P values represent less than 0.05.
We supposed that the negative S-wave in lead-III may have been related to the preferential conduction that was activated counterclockwise from the free wall to the septum of the right ventricle, which was generated a left-sided inferior–superior vector (negative S-wave in the terminal QRS of lead-III). Additionally, the RVOT with a lower position tends left axis deviation may be the cause of the PIA. This eccentric ECG pattern may challenge the current ECG classification for VAs from the right ventricle.5 Ventricular arrhythmias with a right PIA were mostly described as non-outflow tract origins such as the superior-lateral tricuspid annulus, the parietal band, and the right anterior papillary muscle, which is located far away from the RVOT. Based on the analysis of the case group and the control groups, the RVOT-VAs with a PIA have similar ECG characteristics of precordial leads to RVOT-VAs with a whole inferior axis and similar ECG characteristics of limb leads to non-RVOT-VAs with the PIA. The present study summarized an ECG algorithm for VAs with an inferior axis, left bundle branch abnormality (LBBB)-type (‘RS’ pattern in lead-V1), and R-wave in lead-aVL/I (Graphical Abstract).
In conclusion, VAs with the PIA and LBBB-type pattern may originate from the RVOT. Among those VAs, longer R-wave duration in lead-V1 could be used for the differential diagnosis.
Acknowledgements
None.
Contributor Information
Sixian Weng, Department of Cardiology, The Key Laboratory of Geriatrics, Beijing Institute of Geriatrics, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Hospital, National Center of Gerontology of National Health Commission, No.1 Da Hua Road, Dongcheng District, Beijing 100730, China; Department of Cardiology, State Key Laboratory of Cardiovascular Disease, Cardiovascular Institute, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, and Peking Union Medical College, No.167 Beilishi Road, Xicheng District, Beijing 100037, China.
Haiyang Xie, Department of Cardiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510000, China; Department of Cardiology, State Key Laboratory of Cardiovascular Disease, Cardiovascular Institute, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, and Peking Union Medical College, No.167 Beilishi Road, Xicheng District, Beijing 100037, China.
Xiaogang Guo, Department of Cardiology, State Key Laboratory of Cardiovascular Disease, Cardiovascular Institute, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, and Peking Union Medical College, No.167 Beilishi Road, Xicheng District, Beijing 100037, China.
Qi Sun, Department of Cardiology, State Key Laboratory of Cardiovascular Disease, Cardiovascular Institute, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, and Peking Union Medical College, No.167 Beilishi Road, Xicheng District, Beijing 100037, China.
Fang Wang, Department of Cardiology, The Key Laboratory of Geriatrics, Beijing Institute of Geriatrics, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Hospital, National Center of Gerontology of National Health Commission, No.1 Da Hua Road, Dongcheng District, Beijing 100730, China.
Min Tang, Department of Cardiology, State Key Laboratory of Cardiovascular Disease, Cardiovascular Institute, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, and Peking Union Medical College, No.167 Beilishi Road, Xicheng District, Beijing 100037, China.
Funding
The study was supported by the National High Level Hospital Clinical Research Funding (BJ-2022-117) and National Natural Science Foundation of China (U1913210).
Data Availability
The summary data are available from the corresponding author on reasonable request.
References
- 1. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri Net al. . 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: executive summary. Europace 2020;22:450–95. [DOI] [PubMed] [Google Scholar]
- 2. Weng S, Tang M, Zhou B, Ding L, Yu F, Qi Yet al. . Spatial distribution of idiopathic ventricular arrhythmias originating around the pulmonary root: lessons from intracardiac echocardiography. JACC Clin Electrophysiol 2022;8:665–76. [DOI] [PubMed] [Google Scholar]
- 3. Sorgente A, Farkowski MM, Iliodromitis K, Guerra JM, Jubele K, Chun JKRet al. . Contemporary clinical management of monomorphic idiopathic premature ventricular contractions: results of the European Heart Rhythm Association survey. Europace 2022;24:1006–14. [DOI] [PubMed] [Google Scholar]
- 4. Van Herendael H, Garcia F, Lin D, Riley M, Bala R, Cooper Jet al. . Idiopathic right ventricular arrhythmias not arising from the outflow tract: prevalence, electrocardiographic characteristics, and outcome of catheter ablation. Heart Rhythm 2011;8:511–8. [DOI] [PubMed] [Google Scholar]
- 5. Enriquez A, Baranchuk A, Briceno D, Saenz L, Garcia F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm 2019;16:1538–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The summary data are available from the corresponding author on reasonable request.