Figure 3: Nucleation of the AIM2, pyrin, and non-canonical inflammasomes.
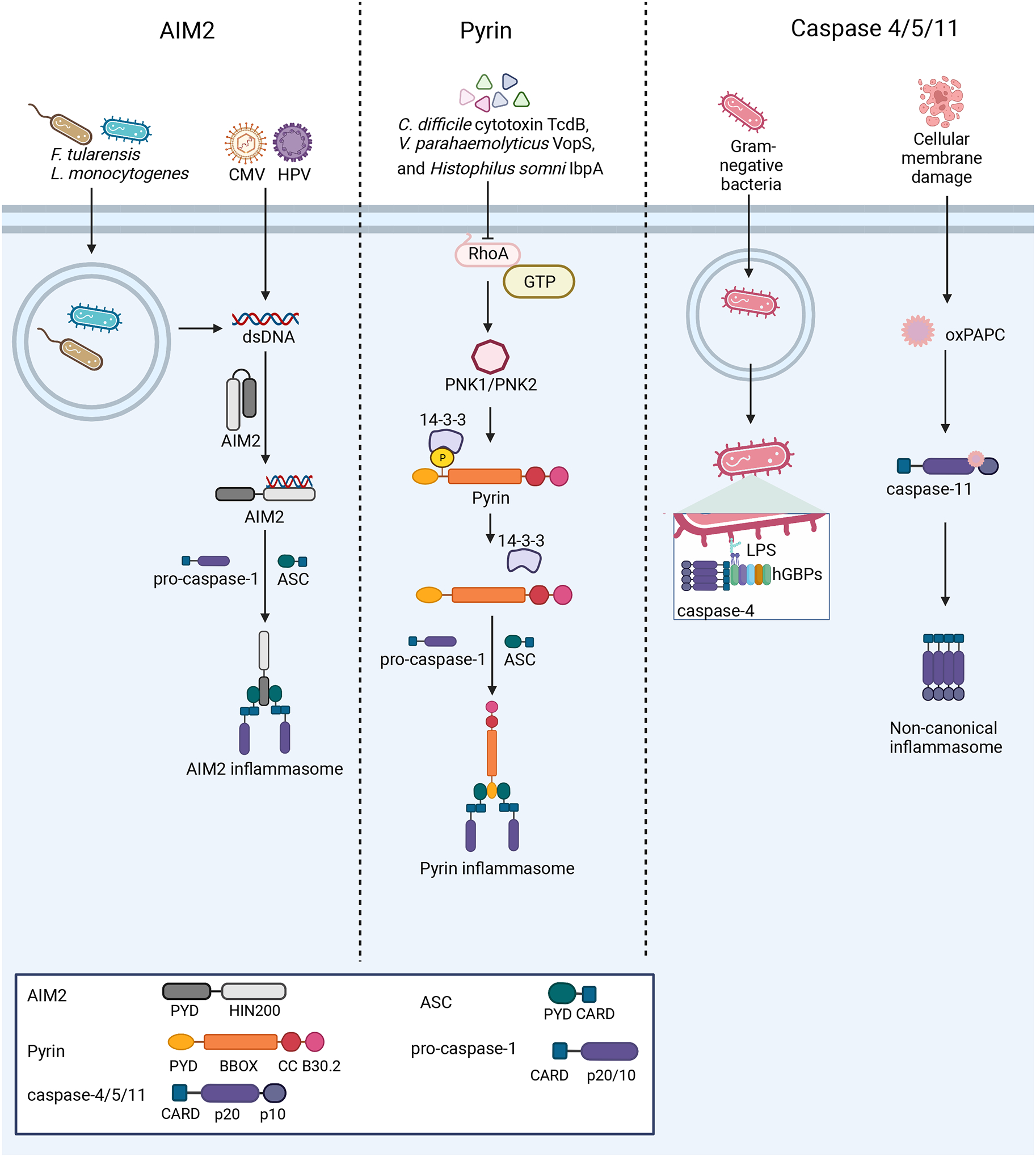
Left: AIM2 detects dsDNA from a variety of sources, including bacterial and viral infections. Upon dsDNA binding, AIM2 oligomerizes along the dsDNA and interacts with ASC to form the inflammasome. Center: At steady-state, pyrin is kept in an inactive conformation through phosphorylation by PKN1/2 and interaction of 14-3-3 proteins with this PTM. Several bacterial toxins activate the pyrin inflammasome through inactivation of the RhoA GTPase, which in turn causes pyrin to be dephosphorylated and nucleate inflammasome formation. Right: Murine caspase-11 and human caspases-4/5 act as PRRs. Guanylate-binding proteins (GBPs) assemble on the surface of Gram-negative bacteria after escape from the vacuole, exposing Lipid A moieties from LPS. This GBP coat then serves as a platform for caspase-4 recruitment and activation186–188. The binding of LPS or oxPAPC by these caspases promotes their activation and downstream inflammasome formation.
