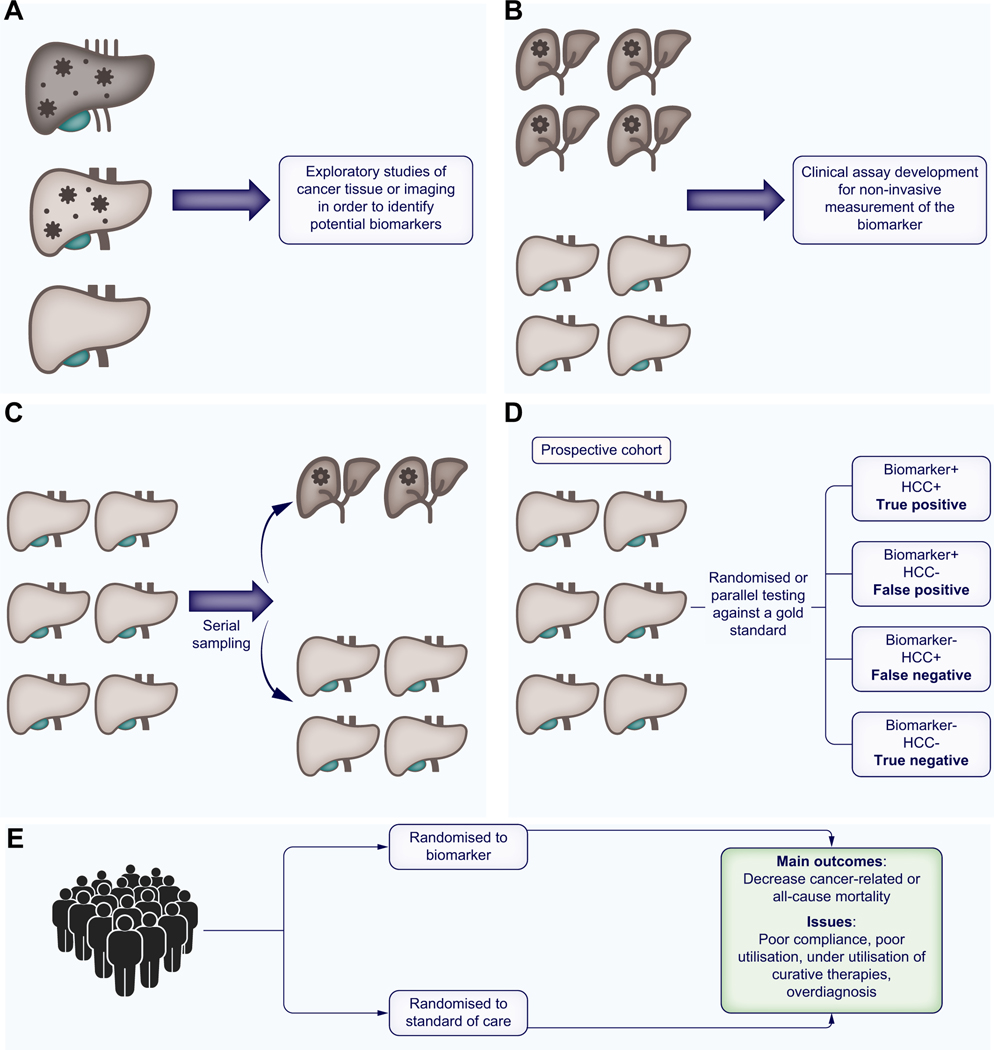
Fig. 1. Phases of biomarker validation.
(A) Phase I - preclinical exploratory to identify candidate biomarkers; (B) Phase II – clinical assay validation using a case-control design; (C) Phase III – longitudinal prospective-specimen collection in at-risk patients, with retrospective blinded-evaluation of biomarker performance; (D) Phase IV - prospective cohort studies or clinical utility trial where the biomarker is tested against a gold standard; (E) Phase V – cancer control studies to determine the impact of biomarker screening on cancer mortality. HCC, hepatocellular carcinoma.