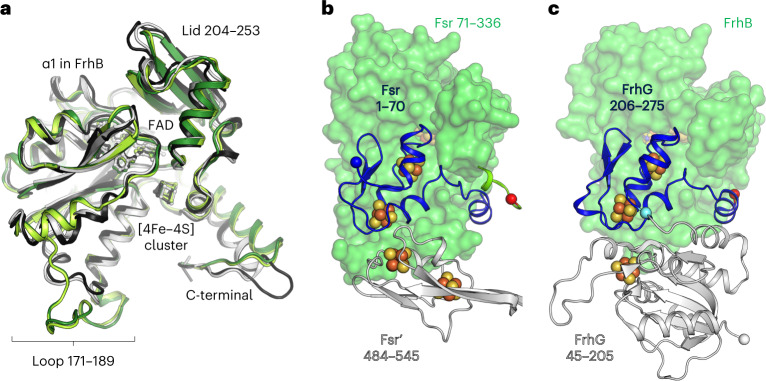
Fig. 2. Comparison of the F420H2-oxidase domain between Fsr and Frh.
a, Superposition of the F420H2-oxidase domain in Fsr (MjFsr in dark green, MtFsr in light green) with FrhB from M. barkeri (black, PDB 6QGR) and FrhB from M. marburgensis (white, PDB 4OMF). The extended loops 171–189 in MjFsr and MtFsr are highlighted, as well as the lid, which is static in the Frh structures, but more flexible in Fsr (Extended Data Fig. 4b,c). b, Representation of MtFsr F420H2-oxidase domain (green surface) and its N-terminal ferredoxin domain (blue cartoon residues 1–70). The N terminus of Fsr and C terminus from the F420H2-oxidase domain are highlighted by blue and red spheres, respectively. The inserted ferredoxin domain, provided by the opposing monomer (Fsr′), is shown in white cartoon representation. c, Arrangement of FrhB (green surface) with FrhG (cartoon) from M. marburgensis (PDB 4OMF). The N-terminal part (45–205) of FrhG is colored in white and its C-terminal part (206–275), structurally equivalent to the N-terminal ferredoxin domain of Fsr, is colored in blue. The cyan ball highlights the connection between both FrhG parts.