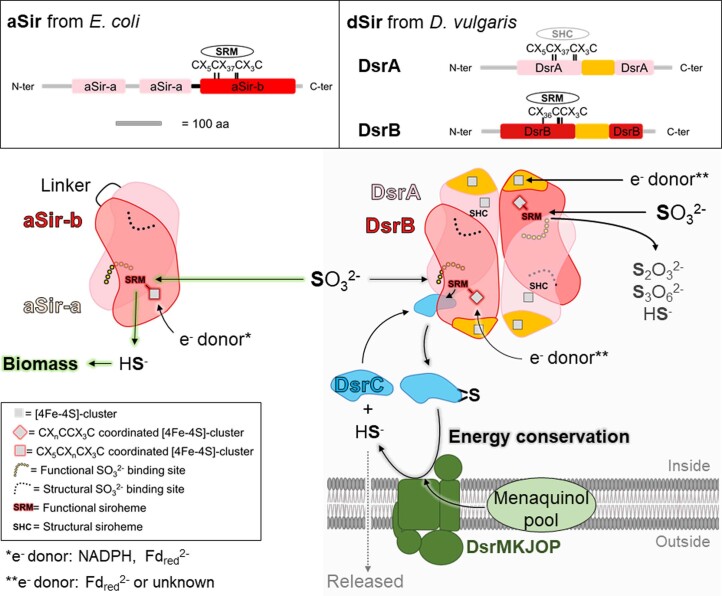
Extended Data Fig. 1. Structural and functional organization of assimilatory (aSir) and dissimilatory (dSir refer here as DsrAB) sulfite reductases.
Distinct and conserved domains in aSir as well as dSir are shown in the top panel. The [4Fe-4S]-cluster binding motifs in the proximity of the siroheme or sirohydrochlorin are highlighted. Bottom panel: aSirs (left) are functional monomers that probably evolved through a gene duplication event, where one gene lost its cluster binding motif. The N-terminal half abbreviated as aSir-a (light pink) has a structural function and the C-terminal half abbreviated as aSir-b (red) harbours the active [4Fe-4S]-siroheme. aSirs indirectly use electrons from NADPH (bacteria) or directly via a [2Fe-2S]-cluster containing ferredoxin (plants) to reduce SO32− to HS− in a six-electron reduction reaction11,35. The produced sulfide will be used for sulfur assimilation. dSirs (right) are composed of two DsrA (light pink) and two DsrB (red) subunits and receive electrons from reduced ferredoxins (Fdred2−) or so far unknown donors15. In absence of DsrC (cyan), DsrAB turns SO32− to thionates (that is S2O32−, S3O62−) and HS−. In presence of DsrC, the intermediate sulfur species bound on the siroheme is transferred to DsrC. In the case of Desulfovibrio species, the membrane DsrMKJOP complex (green) fully reduces the DsrC-trisulfide (4 electrons transfer) probably by using the menaquinol pool and generates DsrC and HS− via the trisulfide pathway, a key process for energy conservation15.