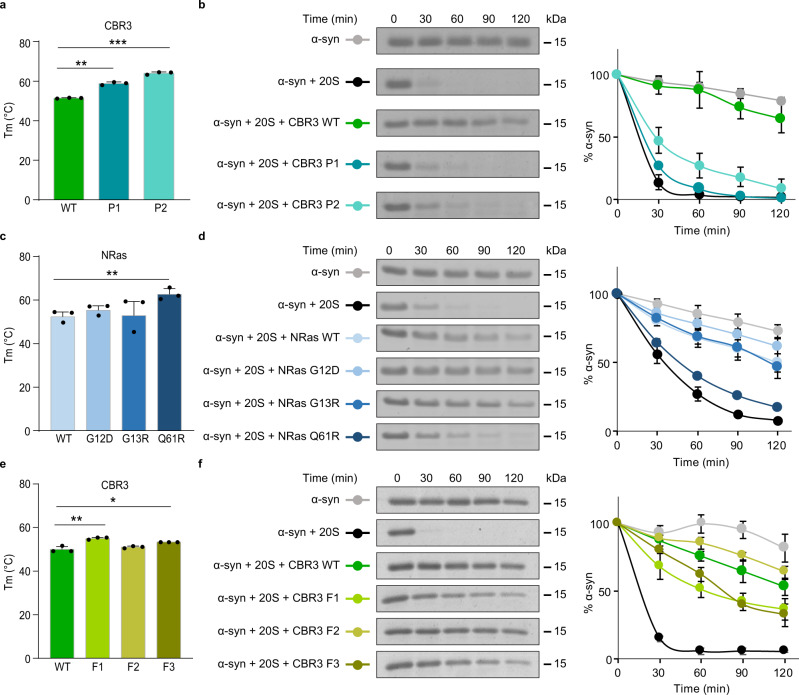
Fig. 4. CCRs function requires a balance between structural rigidity and flexibility.
a, c, e Melting temperatures (Tm) measured for CBR3 PROSS (P) and FuncLib (F) designs and NRas cancer-associated mutants. Each bar represents the mean values of three independent experiments. Measurements were subjected to one-tailed Student’s t-test analysis, *p < 0.05, **p < 0.01, ***p < 0.001. (a ** represents p-value = 0.0017, *** represents p-value = 0.0004). (c ** represents p-value = 0.0043). (e ** represents p-value = 0.0078, * represents p-value = 0.0229). Error bars represent SD. b, d, f Time-dependent degradation assays using α-synuclein (α-syn) as the 20S proteasome substrate in the presence of CBR3 b PROSS and f FuncLib designs, and d cancer-associated NRas mutants. Averaged quantification of three independent experiments is displayed on the right; error bars represent SD. 20S proteasomes were purified from rat livers. Given the variability that occurs due to age, sex, genetics, and health conditions of the animals, batch effects are observed. Therefore, the same batch of purified 20S proteasomes was used for all repeats of a particular experiment (for each panel). In spite of the batch effect that influences degradation kinetics, the difference in the CCR activity of the WT proteins and mutational variants is clearly detected. Source data are provided with this paper.