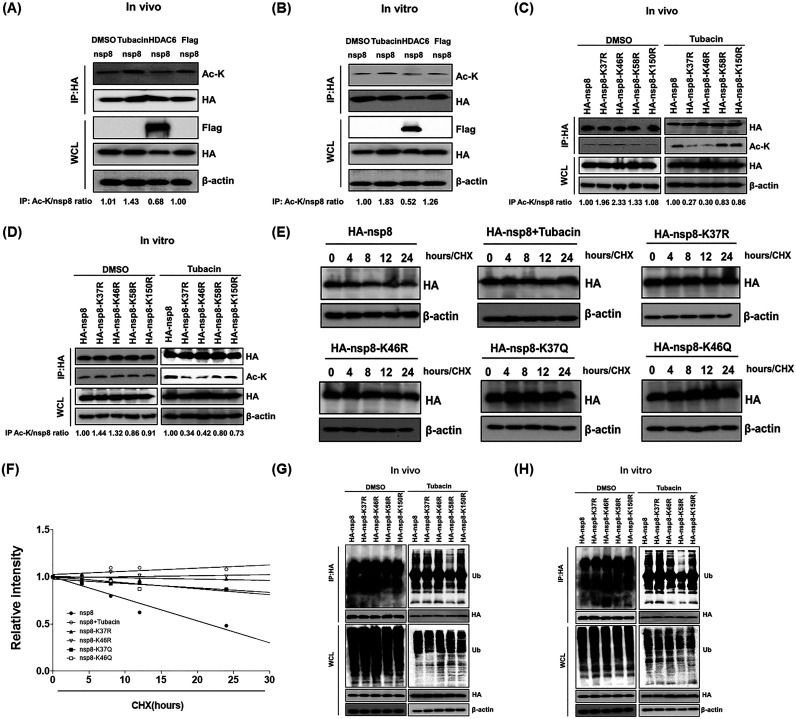
FIG 6.
Determination of nsp8 acetylation sites at K37 and K46, and its ubiquitination at K58. (A) HEK-293T cells were transfected with pCAGGS-HA-nsp8 and pCAGGS-Flag-HDAC6 or empty vector, and then treated with DMSO or tubacin. The cell samples were collected and subjected to a Co-IP assay with anti-HA antibodies and Western blotting for detecting the acetylation level of nsp8 in vivo. (B) The cell lysates with nsp8 expression were immunoprecipitated with anti-HA antibodies and then the immunoprecipitation complexes were incubated with DMSO, tubacin, or the lysates of cell with HDAC6 expression or empty vector transfection for 8 h in vitro, followed by treatment as described in panel A and Western blotting assay. (C) HEK-293T cells were transfected with pCAGGS-HA-nsp8 or expression plasmids encoding nsp8 mutants and then treated with DMSO or tubacin for 8 h in vivo, followed by a Co-IP assay with anti-HA antibodies and Western blotting as described in panel A. (D) The cell lysates with the expression of HA-nsp8 or HA-nsp8 mutants were immunoprecipitated with anti-HA antibodies, and then the immunoprecipitation complexes were incubated with DMSO or tubacin for 8 h in vitro, followed by treatment as described in panel A and Western blotting assay. (E) HEK-293T cells were transfected with pCAGGS-HA-nsp8 or expression plasmids encoding each of nsp8 mutants and then left untreated or treated with tubacin, followed by treatment with CHX (10 μg/mL). The cells were collected at different time points (0, 4, 8, 12, and 24 h) and subjected to a Western blotting assay. (F) Calculated relative half-lives of HA-nsp8 or HA-nsp8 mutants from panel E. The relative intensity was plotted versus time. (G) HEK-293T cells were transfected with expression plasmids encoding HA-nsp8 or HA-nsp8 mutants and then treated with DMSO or tubacin for 8 h in vivo as described in panel A. The cell lysates were subjected to a Western blotting assay for detecting the ubiquitination level of HA-nsp8 and its mutants. (H) The cell lysates with the expression of HA-nsp8 or HA-nsp8 mutants were immunoprecipitated with anti-HA antibodies and then the immunoprecipitation complexes were incubated with DMSO or tubacin for 8 h in vitro, followed by detection of the ubiquitination level of HA-nsp8 and its mutants via Western blotting as described in panel G.