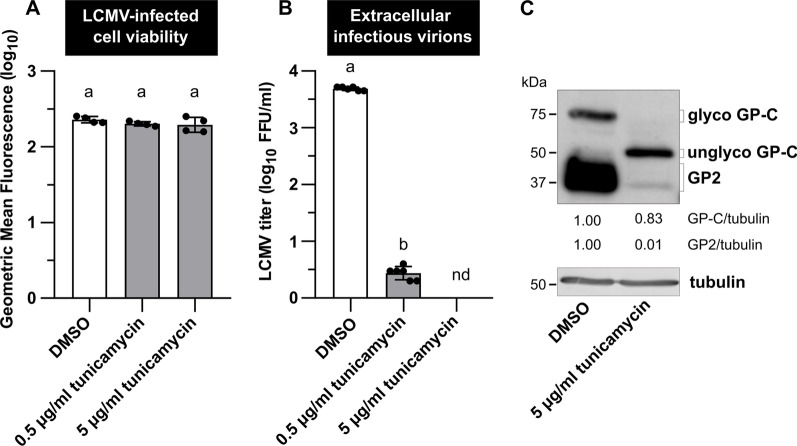
Fig. 9.
N-glycosylation of the LCMV GP-C is impaired in the presence of tunicamycin. MRC-5 cells were infected with LCMV at an MOI of 3. After adsorption, the inoculum was removed and replaced with complete medium supplemented with 0.5% DMSO or 0.5–5 µg/ml tunicamycin. A Twenty-four hours after medium exchange, cell viability was measured by LIVE/DEAD™ Fixable Dead Cell Stain Kit. Data from four independent experiments are shown as a scatter of individual values of geometric mean fluorescence intensity. Mean and SD are shown as column and error bars, respectively. B Supernatants were collected 24 h after medium exchange and LCMV titers were determined by a focus-forming assay. Data from six independent experiments are shown as a scatter of individual values. Mean and SD are shown as column and error bars, respectively. Statistical differences between groups were analyzed using one-way ANOVA and Tukey’s post hoc test (A) or unpaired t-test with Welch’s correction (B). Different letters above the columns indicate significant differences (P ≤ 0.05), while identical letters indicate no significant difference. C Whole-cell extracts were harvested 24 h after media exchange and used for the immunoblot analysis of viral glycoprotein expression levels using anti-GP2 antibody. The signals obtained with anti-α-tubulin antibody were used as loading controls. The ratios of GP-C/tubulin and GP2/tubulin were determined based on the densitometric analysis of protein abundance. Final values are expressed relative to control (DMSO), which was set to 1. FFU/ml, focus-forming units per milliliter; nd, not detected; GP2, glycoprotein 2; glyco/unglyco GP-C, glycosylated/unglycosylated glycoprotein precursor complex