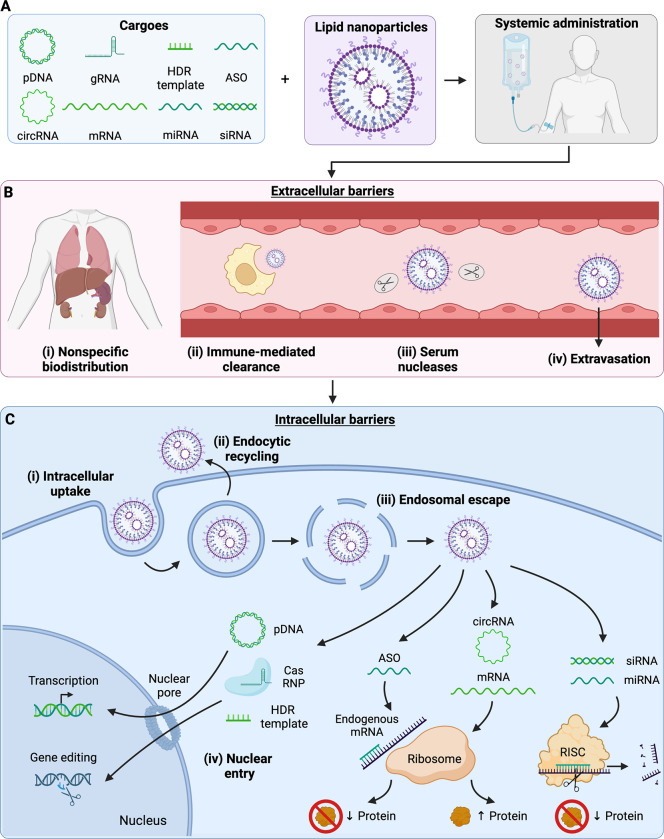
Fig 1. Extracellular and intracellular barriers to nucleic acid delivery.
A. Lipid nanoparticles are versatile drug carriers and can be employed to encapsulate diverse nucleic acid cargoes, including plasmid DNA (pDNA), messenger RNA (mRNA), and circular RNA (circRNA) for gene delivery; antisense oligomers (ASOs), microRNA (miRNA), and small interfering RNA (siRNA) for gene silencing; and guide RNA (gRNA), mRNA, and homology-directed repair (HDR) templates for gene editing applications. B. Following systemic administration, nucleic acid therapeutics face extracellular barriers including nonspecific biodistribution, clearance by phagocytic immune cells, cargo degradation by serum nucleases, and extravasation across endothelial barriers. C. Upon reaching the cell, nucleic acid therapeutics face intracellular barriers, the first of these being intracellular uptake. Following successful uptake into endosomes, nucleic acid therapeutics must avoid endocytic recycling to stay within the cell and escape the endolysosomal pathway to reach the cytoplasm. Once inside the cytoplasm, siRNA and miRNA cargoes can associate with the RNA-induced silencing complex (RISC) to recognize and cleave endogenous mRNA, reducing target protein production. Similarly, ASOs in the cytoplasm can bind to complementary sequences within endogenous mRNA, resulting in reduced protein expression through mechanisms including steric hindrance at the ribosome. Assuming proper structure and chemical composition, exogenous mRNA and circRNA can be translated at the ribosome to produce the encoded protein of interest. Other cargoes require nuclear translocation to accomplish their function. pDNA requires nuclear entry to enable its transcription to mRNA and subsequent translation. CRISPR-associated (Cas) ribonucleoplexes (RNPs) consisting of gRNA and translated Cas protein must enter the nucleus to access genomic DNA and perform their gene editing function. In the case of gene knock-in using HDR, DNA templates must also make their way into the nucleus. Created with BioRender.com.