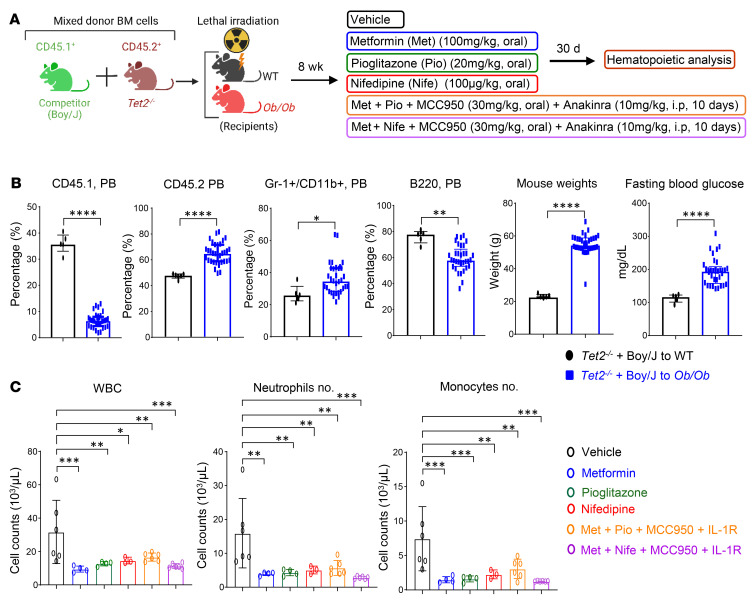
Figure 8. Combination of metformin, nifedipine, MCC950, and anakinra treatment reduces Tet2–/– myeloid cells in Ob/Ob mice.
(A) Schematic of the competitive BMT assay and drug treatment. (B) Percentages of CD45.1, CD45.2, and myeloid Gr-1+/CD11b+ cells and B220+ B cells in the PB of the indicated recipients over 8 weeks of BMT, and body weights and fasting blood glucose levels in the indicated recipients over 8 weeks of BMT. (C) PB WBC, neutrophil, and monocyte counts in the indicated recipients after 30 days of the indicated drug treatments (n = 3–6 mice per group). Data are shown as the mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001, by 2-tailed Student’s t test (B) or 1-way ANOVA (C).