Abstract
Background
Lenvatinib was approved for use in unresectable hepatocellular carcinoma (uHCC) in Japan in 2018. Patients with diverse clinical characteristics receive lenvatinib treatment in clinical practice. Thus, it is crucial to evaluate the safety and effectiveness of lenvatinib in real-world clinical settings.
Objective
This study aimed to evaluate the real-world safety and effectiveness of lenvatinib for uHCC in clinical practice in Japan.
Patients and Methods
Between July 2018 and January 2019, patients with uHCC who were administered lenvatinib for the first time were enrolled in this prospective, multicenter, observational post-marketing study (NCT03663114). Patients were orally administered lenvatinib and followed up for 12 months. For safety, adverse drug reactions (ADRs) were evaluated. For effectiveness, the objective response rate (ORR) was calculated to evaluate tumor response. Overall survival (OS) was estimated using the Kaplan–Meier method.
Results
Data of 703 patients (median age, 73 years; 80.2% males) were analyzed. The median (range) treatment duration was 25.3 (0.3–68.9) weeks. The mean ± standard deviation initial dose was 7.37 ± 1.65 mg in patients with body weight < 60 kg and 10.43 ± 2.49 mg in those with body weight ≥ 60 kg. ADRs (any grade) were reported in 84.9% of the patients, with Grade ≥ 3 ADRs reported in 42.5% of the patients. The most common ADRs (> 10%) were decreased appetite, fatigue, hypertension, proteinuria, palmar-plantar erythrodysesthesia, hypothyroidism, and diarrhea. The median OS of the 703 patients was 498.0 days. In 494 patients assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST), the ORR was 39.5% (95% confidence interval: 35.1–43.9%). Patients with better liver or renal function at baseline achieved significantly higher ORR than those with worse liver or renal function.
Conclusions
In patients with uHCC in real-world clinical practice in Japan, treatment with lenvatinib was generally well tolerated, and no new safety concerns were identified. The ORR and median OS were similar to or better than the results of the Japanese subset of the global Phase III REFLECT trial. Our results demonstrated that clinically meaningful treatment responses were achieved with lenvatinib in real-world clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-022-00348-w.
Key Points
| Real-world safety and effectiveness of lenvatinib were evaluated in patients with uHCC in routine clinical practice in Japan. |
| No new safety concerns were observed. The risk of hepatic encephalopathy did not increase in clinical settings compared to clinical trials. |
| The median OS was 498.0 days, and clinically meaningful treatment responses were observed, with an objective response rate of 39.5% and disease control rate of 78.9% using mRECIST. |
Introduction
Liver cancer is the sixth most common cancer and the third leading cause of cancer deaths worldwide [1]. In Japan, it is estimated that about 40,050 people will develop liver cancer annually from 2020 to 2024, with an average of 23,390 deaths each year [2]. Locoregional curative therapy is available for early-stage hepatocellular carcinoma (HCC), but HCC frequently recurs and progresses to unresectable advanced tumors. For such unresectable HCC (uHCC), molecular-targeted therapy is an important treatment option [3]. Sorafenib, an oral multi-kinase inhibitor, had long been the only molecular-targeted drug for almost 10 years, since 2007. However, other kinase inhibitors such as lenvatinib and regorafenib have been developed in the last few years, and immune checkpoint inhibitors have also come into use [4]. As such, systemic therapy for uHCC has advanced in recent years.
Lenvatinib is an oral multi-kinase inhibitor that targets vascular endothelial growth factor (VEGF) receptors 1–3, fibroblast growth factor (FGF) receptors 1–4, platelet-derived growth factor receptor α, RET, and KIT [5–7]. A preclinical study demonstrated its antitumor activity against HCC tumor cells by inhibiting FGF signaling pathways and tumor angiogenesis [8]. Furthermore, a global Phase III REFLECT trial in patients with uHCC demonstrated the non-inferiority of lenvatinib to sorafenib in terms of overall survival (OS) (median, 13.6 vs. 12.3 months), with secondary endpoints (e.g., progression-free survival and objective response) favoring lenvatinib over sorafenib [9]. Based on this result, lenvatinib was approved for uHCC treatment in Japan in March 2018 and recommended as first-line systemic therapy in addition to sorafenib until recently, when atezolizumab plus bevacizumab became the first-line therapy if indicated, in the updated guidelines in 2021 [10].
Although clinical trials demonstrated the efficacy and safety of lenvatinib in a Japanese population [9, 11, 12], their strict inclusion and exclusion criteria limited the generalizability of these trials’ results. Since patients with diverse clinical characteristics might be treated with lenvatinib in clinical practice, it is crucial to assess the safety and effectiveness of lenvatinib under routine clinical conditions. It is also important to accumulate data on some safety concerns observed in clinical trials, such as hepatic encephalopathy [11], to better understand the safety profiles of this treatment.
Therefore, to evaluate the real-world safety and effectiveness of lenvatinib using data from a sufficiently large sample size, we conducted a prospective post-marketing study of lenvatinib in patients with uHCC in clinical practice in Japan. We evaluated treatment effectiveness using the best overall response, i.e., objective response rate (ORR) and disease control rate (DCR). This study was conducted as part of pharmacovigilance activities, as required by the Japanese Pharmaceutical and Medical Devices Agency.
Methods
Study Design
This was a prospective, multicenter, observational post-marketing study of lenvatinib (Lenvima®; Eisai Co., Ltd., Tokyo, Japan) in patients with uHCC in Japan (ClinicalTrials.gov Trial Registration ID: NCT03663114). This study was planned as a “Drug Use Investigation” in compliance with the Good Post-Marketing Study Practice and related guidelines in Japan and employed a prospective design, which helped reduce information and reporting bias. The study protocol was submitted to the Japanese Pharmaceutical and Medical Devices Agency before conducting the study. Among institutions adopting the study drug, those who agreed to participate entered into a contract with the sponsor and participated in the study. Patients were enrolled at these institutions between July 2018 and January 2019. This study conformed to the provisions of the Declaration of Helsinki and the Pharmaceutical Affairs Law and ministerial ordinance on Good Post-Marketing Study Practice in Japan.
Patients and Treatment
All patients with uHCC who were lenvatinib-naïve and who agreed to participate were centrally registered using an electronic data capture (EDC) system within 14 days of the initial administration of lenvatinib.
Lenvatinib was orally administered once daily according to the package insert, and patients were followed up for 12 months (the observation period). The standard dose was 12 mg/day for patients with body weight ≥ 60 kg and 8 mg/day for those with body weight < 60 kg, but the dose could be reduced at physicians’ discretion. For patients who completed or discontinued treatment within 12 months of treatment initiation, the survival status was followed up until the end of the 12-month observation period. In case of loss of follow-up due to death or transfer to another hospital, the observation period ended at the time when the patient was lost to follow-up.
Data Collection
Data of all registered patients during the 12-month observation period were collected using case report forms (CRFs) via the EDC system. Data collected included the following: baseline demographic and clinical characteristics (including laboratory test results), treatment history for HCC, treatment status with lenvatinib (e.g., dosage, treatment duration, dose modifications), co-medications (e.g., preventive medications for hepatic encephalopathy), tumor assessments by imaging findings, survival outcomes (alive or dead), and adverse events (AEs). The assessment schedule is summarized in Supplementary Table S1.
Using serum albumin and total bilirubin values, albumin-bilirubin (ALBI) score, a measure for assessing liver function, was calculated using the following formula: ALBI score = (log10 bilirubin [μmol/L] × 0.66) + (albumin [g/L] × − 0.085) [13]. Then, modified ALBI (mALBI) grade was determined as follows: an ALBI score of ≤ − 2.60 = Grade 1; > − 2.60 to < − 2.27 = Grade 2a; ≥ − 2.27 to ≤ − 1.39 = Grade 2b; and > − 1.39 = Grade 3 [14]. For renal function, the estimated glomerular filtration rate (eGFR) was calculated using the following formula: eGFR (mL/min/1.73 m2) = 194 × serum creatinine (mg/dL)−1.094 × age−0.287 (× 0.739 if female). The relative dose intensity (RDI) was calculated as the ratio of the total dosage delivered to the standard dosage (i.e., the treatment duration [day] multiplied by 12 mg for patients weighing ≥ 60 kg or by 8 mg for those weighing < 60 kg).
Assessments
Safety
Adverse events were assessed for a period from initial administration to 14 days after the last administration of lenvatinib. Adverse events, for which a causal relationship with lenvatinib could not be denied, were defined as adverse drug reactions (ADRs). Hepatic encephalopathy was an ADR of our particular interest; therefore, the time to its onset and recovery/remission and its outcome were also evaluated. Adverse drug reactions were classified according to the Japanese version of the Medical Dictionary for Regulatory Activities (MedDRA/J) version 23.1, and severity was graded according to the Japanese version of the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Effectiveness
We evaluated the OS and tumor response rates. Overall survival was defined as the time from lenvatinib treatment initiation to death. Tumor response was evaluated using imaging data and classified into the following five categories by attending physicians, according to response criteria, including but not limited to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [15]: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and not evaluable (NE). The best treatment response observed among the three periods (i.e., from treatment initiation to 3 months, from 3 to 6 months, and from 6 to 12 months) was defined as the “best overall response.” Based on the patients’ best overall response, the ORR was defined as the proportion of patients with CR and PR, and the DCR as the proportion of patients with CR, PR, and SD.
Statistical Analyses
The patients’ baseline characteristics, treatment status, ADRs, and tumor responses were descriptively summarized. Overall survival was estimated using the Kaplan–Meier method. To further analyze the safety and effectiveness outcomes in terms of the three patient characteristics of interest, the incidence proportions of ADRs of Grade ≥ 3 and ORRs were analyzed by age, liver function (according to Child-Pugh class and mALBI grade), and renal function (according to eGFR) at baseline. The cut-off age was set at 65 years; anyone at this age or above is defined as an older person in Japan. The cut-off eGFR was set at 45 mL/min/1.73 m2, which is the level of moderate impairment of renal function. Comparisons between categories were made using the chi-square test or Fisher’s exact test.
Furthermore, multivariate logistic regression analyses were performed to explore factors associated with (1) treatment duration, (2) ADRs that led to treatment discontinuation, and (3) ORR, using the following baseline factors as explanatory variables (using stepwise selection with selection criteria of p < 0.05): age, sex, body mass index, Eastern Cooperative Oncology Group performance status (ECOG PS), bile duct invasion, portal vein invasion, maximum tumor size, intrahepatic lesions, extrahepatic lesions, previous tyrosine kinase inhibitor (TKI) therapy, number of previous transcatheter arterial chemoembolization (TACE) procedures, previous hepatic arterial infusion chemotherapy (HAIC), mALBI grade, eGFR, alpha-fetoprotein (AFP) level, and concomitant therapy/medications for HCC (hepatectomy, ablation, radiation therapy, chemotherapy, embolization, and HAIC).
Additionally, factors associated with the onset of hepatic encephalopathy were explored using a multivariate logistic regression model, considering the following factors in addition to those above (excluding body mass index and eGFR) as explanatory variables: etiology of HCC; concomitant diseases considered as a risk for hepatic encephalopathy (cirrhosis, portosystemic shunt, hepatic encephalopathy, constipation, dehydration, infection, gastrointestinal bleeding, and esophageal varices); history of these diseases (those considered to be a risk for hepatic encephalopathy); use of medications for prevention of hepatic encephalopathy; use of medications considered as a risk for hepatic encephalopathy-related AEs (diuretics and analgesics); and use of medications requiring precautions for co-administration with lenvatinib (P-gp inhibitors and CYP3A/P-gp inducers).
A p-value of < 0.05 was considered as the level of statistical significance. All statistical analyses were performed using the Statistical Analysis System (SAS) Release 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics
In total, 713 patients were registered at 137 institutions, of whom CRFs were obtained from 708 patients. Case report forms were not collected from five patients because there was either no lenvatinib administration after registration (n = 2) or refusal of data input (n = 3). Of the remaining 708 patients, another five patients were excluded for the following reasons: registered after the registration period (protocol violation) (n = 2), administration of lenvatinib for non-target disease (n = 1), no administration of lenvatinib (n = 1), and uncertain AE status (i.e., physicians’ confirmation could not be obtained for the safety information provided by spontaneous reports, and there were no other AEs reported; n = 1). Data from the remaining 703 patients were analyzed in this study.
The patients’ baseline characteristics are summarized in Table 1. The median (range) age was 73 (25–94) years, and 80.2% were male. The most common etiology was hepatitis C virus infection (40.8%). Patients were mainly classified as Barcelona Clinic Liver Cancer (BCLC) stage B (41.4%) or C (47.2%). Portal vein invasion was present in 23.5% of all patients: by the degree of invasion, 4.4% had Vp1 (invasion at the 3rd or more peripheral portal branch), 7.4% had Vp2 (at the 2nd portal branch), 8.5% had Vp3 (at the 1st portal branch), and 3.1% had Vp4 (at the main portal branch). Most patients were classified as Child-Pugh class A (88.8%), but 10.4% were classified as Child-Pugh class B and 0.3% as Child-Pugh class C. Overall, 513 (73.0%) patients previously received TACE, and 44.6% of these patients had previous TACE procedures one or two times, 31.2% had three to five times, 15.0% had six to nine times, and 6.8% had ≥10 times. Of all patients, 18.6% had a history of TKI therapy.
Table 1.
Baseline demographic and clinical characteristics of patients
| Characteristics | Total (n = 703) | |
|---|---|---|
| Age, years | 73 | [25‒94] |
| Male, n (%) | 564 | (80.2) |
| Body weight, kg, n (%) | ||
| < 60 | 323 | (45.9) |
| ≥ 60 | 380 | (54.1) |
| Time from the onset of primary HCC, years | 2.5 | [0.0‒20.6] |
| < 0.5 | 135 | (19.2) |
| ≥ 0.5, < 1 | 65 | (9.2) |
| ≥ 1, < 2 | 87 | (12.4) |
| ≥ 2, < 3 | 69 | (9.8) |
| ≥ 3, < 5 | 97 | (13.8) |
| ≥ 5, < 10 | 121 | (17.2) |
| ≥ 10 | 65 | (9.2) |
| Unknown | 64 | (9.1) |
| ECOG PS, n (%) | ||
| 0 | 533 | (75.8) |
| 1 | 148 | (21.1) |
| 2 | 11 | (1.6) |
| ≥ 3 | 5 | (0.7) |
| Unknown | 6 | (0.9) |
| BCLC stage, n (%) | ||
| 0/A | 59 | (8.4) |
| B | 291 | (41.4) |
| C | 332 | (47.2) |
| D | 11 | (1.6) |
| Unknown | 10 | (1.4) |
| Portal vein invasion, n (%) | ||
| No | 520 | (74.0) |
| Yes | 165 | (23.5) |
| Unknown | 18 | (2.6) |
| Extrahepatic lesions, n (%) | ||
| No | 450 | (64.0) |
| Yes | 229 | (32.6) |
| Unknown | 24 | (3.4) |
| Child-Pugh class, n (%) | ||
| A | 624 | (88.8) |
| B | 73 | (10.4) |
| C | 2 | (0.3) |
| Unknown | 4 | (0.6) |
| Etiology, n (%) | ||
| Hepatitis B virus | 137 | (19.5) |
| Hepatitis C virus | 287 | (40.8) |
| Alcohol | 167 | (23.8) |
| NAFLD/NASH | 88 | (12.5) |
| Others | 12 | (1.7) |
| Unknown | 59 | (8.4) |
| Treatment history, n (%) | ||
| Surgerya | 227 | (32.3) |
| Radiofrequency ablationb | 270 | (38.4) |
| Radiation therapyc | 93 | (13.2) |
| TKI therapy | 131 | (18.6) |
| TACE | 513 | (73.0) |
| HAIC | 74 | (10.5) |
| AFP level, ng/mL, n (%) | ||
| < 200 | 432 | (61.5) |
| ≥ 200 | 240 | (34.1) |
| Unknown | 31 | (4.4) |
Data are median [min‒max] or n (%). Percentages may not add up to 100.0 because of rounding
AFP alpha-fetoprotein, BCLC stage Barcelona Clinic Liver Cancer stage, ECOG PS Eastern Cooperative Oncology Group performance status, HAIC hepatic arterial infusion chemotherapy, HCC hepatocellular carcinoma, NAFLD/NASH non-alcoholic fatty liver disease/non-alcoholic steatohepatitis, TACE transcatheter arterial chemoembolization, TKI tyrosine kinase inhibitor
aStatus was unknown for one patient
bStatus was unknown for one patient
cStatus were unknown for two patients
Treatment Status
A majority of patients started lenvatinib at a body weight-based standard dose of 8 mg in 80.5% of patients with body weight < 60 kg, and 12 mg in 68.2% of patients with body weight ≥ 60 kg (Table 2). The median (range) treatment duration of all patients was 25.3 (0.3–68.9) weeks, with 50.8% of patients having a treatment period of >24 weeks. Overall, 528 (75.1%) patients discontinued treatment within 12 months, with similar discontinuation rates regardless of body weight category. The proportion of patients with an RDI ≥ 80% was 42.1% in patients weighing < 60 kg, in contrast to 24.2% in those weighing ≥ 60 kg.
Table 2.
Dosage and treatment status with lenvatinib
| Total (n = 703) | Body weight | |||||
|---|---|---|---|---|---|---|
| < 60 kg (n = 323) | ≥ 60 kg (n = 380) | |||||
| Initial dose of lenvatinib, mg | ||||||
| Mean ± SD | 9.02 ± 2.63 | 7.37 ± 1.65 | 10.43 ± 2.49 | |||
| 12 | 265 | (37.7) | 6 | (1.9) | 259 | (68.2) |
| 8 | 353 | (50.2) | 260 | (80.5) | 93 | (24.5) |
| 4 | 85 | (12.1) | 57 | (17.6) | 28 | (7.4) |
| Treatment duration, weeks | ||||||
| Median [min‒max] | 25.3 | [0.3‒68.9] | 21.9 | [0.3‒68.9] | 28.2 | [0.3‒65.4] |
| ≤ 4 | 85 | (12.1) | 40 | (12.4) | 45 | (11.8) |
| > 4 to 8 | 71 | (10.1) | 34 | (10.5) | 37 | (9.7) |
| > 8 to 16 | 112 | (15.9) | 55 | (17.0) | 57 | (15.0) |
| > 16 to 24 | 78 | (11.1) | 44 | (13.6) | 34 | (8.9) |
| > 24 to 36 | 89 | (12.7) | 38 | (11.8) | 51 | (13.4) |
| > 36 | 268 | (38.1) | 112 | (34.7) | 156 | (41.1) |
| Dose modifications at 12 months | ||||||
| Continuing with no dose interruption or reduction | 32 | (4.6) | 17 | (5.3) | 15 | (3.9) |
| Continuing with dose reduction | 46 | (6.5) | 22 | (6.8) | 24 | (6.3) |
| Continuing with dose interruption | 13 | (1.8) | 6 | (1.9) | 7 | (1.8) |
| Continuing with dose reduction and interruption | 84 | (11.9) | 29 | (9.0) | 55 | (14.5) |
| Discontinued | 528 | (75.1) | 249 | (77.1) | 279 | (73.4) |
| RDI during treatment, % | ||||||
| ≥ 80 | 228 | (32.4) | 136 | (42.1) | 92 | (24.2) |
| 60 to < 80 | 155 | (22.0) | 50 | (15.5) | 105 | (27.6) |
| 40 to < 60 | 173 | (24.6) | 88 | (27.2) | 85 | (22.4) |
| < 40 | 147 | (20.9) | 49 | (15.2) | 98 | (25.8) |
Data are presented as n (%) unless otherwise stated. Percentages may not add up to 100.0 because of rounding
RDI relative dose intensity, SD standard deviation
Multivariate logistic regression analysis revealed that ECOG PS score, portal vein invasion, previous HAIC, mALBI grade, eGFR, AFP level, and concomitant therapy/medications for HCC were significantly associated with treatment duration (Supplementary Table S2).
Safety
ADRs
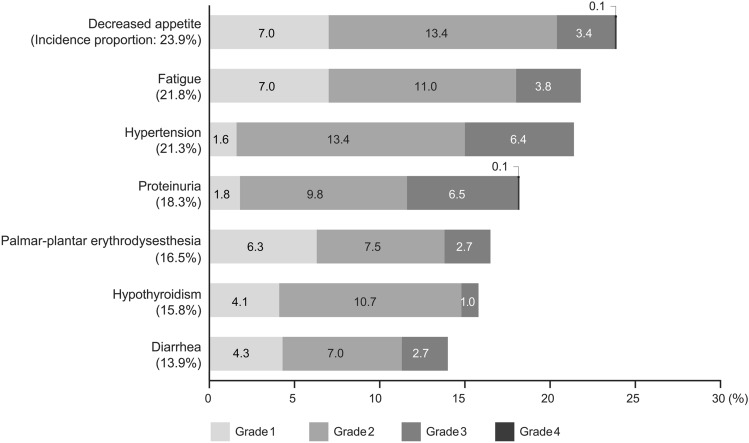
Adverse drug reactions were reported in 84.9% (n = 597) of all patients. The five most common ADRs (any grade) were decreased appetite (23.9%), fatigue (21.8%), hypertension (21.3%), proteinuria (18.3%), and palmar-plantar erythrodysesthesia (16.5%) (Fig. 1). Adverse drug reactions of Grade ≥ 3 were reported in 42.5% (n = 299) of all patients. In terms of liver function, statistically significant differences were observed between mALBI grades, with higher ADRs of Grade ≥ 3 in patients with higher mALBI grades at baseline (mALBI Grade ≥ 2a vs 1, 46.1% vs 35.6%, p = 0.010; ≥ 2b vs ≤ 2a, 49.6% vs 38.3%, p = 0.003; Table 3), whereas no significant differences were observed between Child-Pugh classes. There were no significant differences between the age groups. In our additional analysis, the incidence proportion did not increase even among patients aged ≥ 75 years (44.5% in those aged 65–75 years and 42.9% in those aged ≥ 75 years; Chi-square test, p = 0.354). The occurrence of Grade ≥ 3 ADRs also did not differ significantly depending on renal function according to eGFR (Table 3).
Fig. 1.
Incidence proportions of common ADRs by grade. Common ADRs with incidence proportions of 10 % or higher were displayed. ADRs adverse drug reactions
Table 3.
Incidence proportions of ADRs of Grade ≥ 3 by age, liver function, and renal function
| Variable | Category | Total | Patients with ADRs of Grade ≥ 3 | p-valuea | |
|---|---|---|---|---|---|
| n | (%) | ||||
| All | 703 | 299 | (42.5) | ||
| Age (years) | < 65 | 117 | 43 | (36.8) | 0.183 |
| ≥ 65 | 586 | 256 | (43.7) | ||
| Child-Pugh class | A | 624 | 260 | (41.7) | 0.140 |
| B or C | 75 | 38 | (50.7) | ||
| mALBI grade | 1 or 2a | 415 | 159 | (38.3) | 0.003* |
| 2b or 3 | 276 | 137 | (49.6) | ||
| 1 | 216 | 77 | (35.6) | 0.010* | |
| 2a, 2b, or 3 | 475 | 219 | (46.1) | ||
| eGFR (mL/min/1.73 m2) | ≥ 45 | 615 | 263 | (42.8) | 1.000 |
| < 45 | 72 | 31 | (43.1) | ||
ADR adverse drug reaction, eGFR estimated glomerular filtration rate,
mALBI grade, modified albumin-bilirubin grade
aFisher’s exact test
*p < 0.05
Of 703 patients, treatment discontinuation due to ADRs occurred in 231 patients (298 events), dose reduction due to ADRs in 220 patients (329 events), and dose interruption due to ADRs in 208 patients (336 events). Despite ADRs, treatment was continued without dose modification in 288 patients (593 events). The common ADRs that led to treatment discontinuation (incidence proportions ≥ 1.5%) were decreased appetite (6.3%), fatigue (4.7%), proteinuria (3.3%), hepatic encephalopathy (2.4%), and diarrhea (1.8%).
Multivariate logistic regression analysis revealed that patients with the following factors were significantly more likely to have ADRs that led to treatment discontinuation: female sex, ECOG PS score ≥ 1, no history of previous TKI therapy, previous TACE procedures ≥ 3 times, mALBI Grade ≥ 2b, and no concomitant therapy/medications for HCC (Supplementary Table S3).
Hepatic Encephalopathy
During the study, hepatic encephalopathy was reported in 5.4% (n = 38) of all patients. At baseline, nine patients were comorbid with hepatic encephalopathy. Of the 38 patients who developed this ADR during the study, two patients developed Grade 1, 18 developed Grade 2, 15 developed Grade 3, and 3 developed Grade 4 hepatic encephalopathy. The median (range) time to onset was 10.0 (3‒281) days, with over 60% of the initial events occurring within two weeks after the initiation of treatment (Fig. 2). While lenvatinib was discontinued due to this ADR in 17 patients, 8 patients continued treatment after dose interruption, 3 continued with dose reduction, and 9 continued without dose modification (in case of patients with multiple events, one treatment status was adopted according to the following order of priority: discontinuation > dose interruption > dose reduction > no dose modification). The remaining 1 patient was not categorized in any status because treatment had been discontinued before the event occurred. Regarding the outcome, 12 patients recovered, 21 were remitted, 4 were unrecovered during the observation period, and 1 was unknown; the median (range) time to recovery/remission was 8.0 (2‒127) days.
Fig. 2.
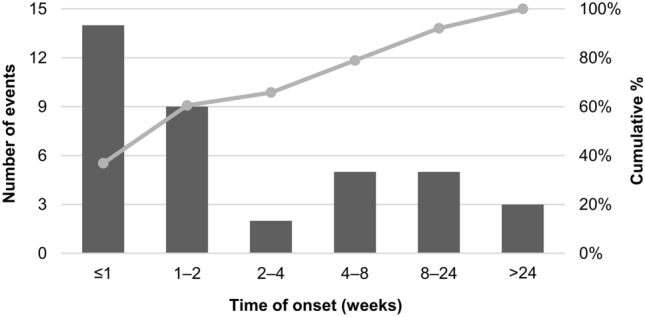
Time of onset of hepatic encephalopathya after the treatment initiation (n = 38). aIn the case of patients with multiple events of hepatic encephalopathy, the time of onset of the first event was counted
In multivariate logistic regression analysis, the presence of extrahepatic lesions was significantly negatively associated with hepatic encephalopathy. In contrast, patients with baseline mALBI Grade ≥ 2b and those who used preventive medications for hepatic encephalopathy had significantly higher odds ratios for hepatic encephalopathy than those with mALBI Grade 1 and those who did not use these medications, respectively (Supplementary Table S4). During this study, 14.4% (n = 101) of the study population were prescribed medications for preventing hepatic encephalopathy: the most commonly used medications were lactulose (7.1%, n = 50), followed by rifaximin (5.0%, n = 35).
Effectiveness
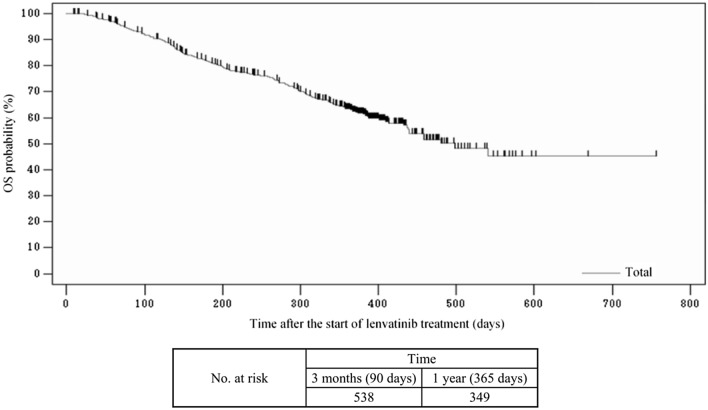
The median OS of the 703 patients was 498.0 days (Fig. 3). Of the overall analysis population, 622 patients underwent tumor assessments based on imaging findings. Of these, 494 patients were assessed according to the mRECIST criteria, and their best overall responses are summarized in Table 4. For these 494 patients, ORR was 39.5% (95% confidence interval [CI]: 35.1–43.9) and DCR was 78.9% (95% CI: 75.1–82.5).
Fig. 3.
Kaplan–Meier estimates of overall survival (OS) in all patients
Table 4.
Best overall responses in patients with assessments using with mRECIST criteria
| Assessment using mRECIST | ||
|---|---|---|
| (n = 494) | ||
| Best overall response, n (%) | ||
| Complete response | 36 | (7.3) |
| Partial response | 159 | (32.2) |
| Stable disease | 195 | (39.5) |
| Progressive disease | 104 | (21.1) |
| Not evaluable | 0 | (0.0) |
| ORRa, % (95 % CI) | 39.5 | (35.1–43.9) |
| DCRb, % (95 % CI) | 78.9 | (75.1–82.5) |
CI confidence interval, DCR disease control rate, mRECIST modified Response Evaluation Criteria in Solid Tumors, ORR objective response rate
aORR = proportion of patients with complete response and partial response
bDCR = proportion of patients with complete response, partial response, and stable disease
In subgroup analysis, significantly higher ORRs were achieved in patients with baseline mALBI Grade 1 than in those with mALBI Grade ≥ 2a (49.4% vs 34.6%; p = 0.002), and patients with baseline eGFR ≥ 45 mL/min/1.73 m2 achieved higher ORR than in those with eGFR < 45 mL/min/1.73 m2 (40.5% vs 21.4%; p = 0.019), while no statistically significant differences were observed between age groups and Child-Pugh classes (Table 5).
Table 5.
Objective response rates by age, liver function, and renal function
| Variable | Category | Total | Patients with objective responsea | p-valueb | |
|---|---|---|---|---|---|
| n | (%) | ||||
| All | 494 | 195 | (39.5) | ||
| Age (years) | < 65 | 91 | 32 | (35.2) | 0.406 |
| ≥ 65 | 403 | 163 | (40.4) | ||
| Child-Pugh class | A | 438 | 178 | (40.6) | 0.101 |
| B or C | 53 | 15 | (28.3) | ||
| mALBI grade | 1 or 2a | 301 | 128 | (42.5) | 0.070 |
| 2b or 3 | 187 | 64 | (34.2) | ||
| 1 | 156 | 77 | (49.4) | 0.002* | |
| 2a, 2b, or 3 | 332 | 115 | (34.6) | ||
| eGFR (mL/min/1.73 m2) | ≥ 45 | 442 | 179 | (40.5) | 0.019* |
| < 45 | 42 | 9 | (21.4) | ||
eGFR estimated glomerular filtration rate, mALBI grade modified albumin-bilirubin grade
*p < 0.05
aObjective response was defined as complete response and partial response
bFisher’s exact test
In the 494 patients assessed according to the mRECIST criteria, multivariate logistic regression analysis revealed that patients without extrahepatic lesions and those with higher mALBI grade at baseline were significantly less likely to achieve objective response than those without these factors (Supplementary Table S5).
Discussion
In clinical practice, patients with diverse clinical characteristics beyond the patient criteria for clinical trials receive treatment; thus, it is crucial to evaluate the safety and effectiveness of treatment in patients in real-world clinical settings. In this prospective, observational post-marketing study, we analyzed the data of 703 patients who were treated with lenvatinib in Japan. Their demographic characteristics, including male predominancy, were similar to those of previous Japanese studies [16–18] and the present study included patients with more advanced tumors or worse liver function, such as patients with invasion to the bile duct or main portal vein and those with Child-Pugh class ≥B, in addition to patients with previous systemic therapy. Given that these patients were not included in the REFLECT trial [9], this study will provide important data to physicians in this field. Overall, this study demonstrated the clinically meaningful antitumor activity of lenvatinib with acceptable tolerability, corroborating the results of recent, relatively small observational studies in Japan [16, 17, 19].
In this study, most patients (84.9%) experienced ADRs, and 42.5% reported ADRs of Grade ≥ 3, which is consistent with the results of a previous observational study with a median observation period of 12.2 months (any grade, 83.0%; Grade ≥ 3, 48.9%) [20]. The common ADRs observed in the present study were decreased appetite, fatigue, hypertension, proteinuria, palmar-plantar erythrodysesthesia, hypothyroidism, and diarrhea, which are known toxicities of VEGF receptor inhibitors [21, 22] and have been commonly reported in previous studies of lenvatinib [12, 16, 17, 20, 23]. No new safety concerns were identified. Interestingly, we found that women had a higher chance of ADRs that led to treatment discontinuation than men. The primary ADRs that led to discontinuation were loss of appetite and fatigue, in line with previous findings [20], and these symptoms probably involved less muscle mass, that is, sarcopenia. In general, women have lower muscle mass and a smaller dietary intake than men. This may predispose women to a more severe appetite loss or fatigue compared with men, which can lead to treatment discontinuation.
In Phase II and III trials, hepatic encephalopathy was the most common serious AE reported in lenvatinib-treated patients [11, 24]. Therefore, we paid particular attention to this ADR. The occurrence of hepatic encephalopathy in the present study (5.4%) was comparable to the global data of the REFLECT trial (5%) [24] and less frequent compared to previous reports in Japan (10.1–13.2%) [11, 19, 20]. Most patients (33 of 38) recovered or were remitted during the follow-up period, with adequate management. Our results provided important data indicating that the overall risk of hepatic encephalopathy was within the range of existing knowledge and did not increase in clinical settings. However, as suggested in our exploratory analysis, patients at higher risk may still have chances to develop it, even if they receive preventive medications; thus, it would still be important for physicians to be cautious of this ADR, especially for high-risk patients. We also observed that 3 of 9 patients with comorbid hepatic encephalopathy at baseline developed or exacerbated this condition during the study. Given the much higher risk of this ADR in these patients relative to that in the overall study population (33.3% vs 5.4%), lenvatinib should be administered with extra caution to those who already have this comorbid condition.
As shown in Table 3, our subgroup analyses demonstrated that the occurrence of ADRs of Grade ≥ 3 was significantly higher in patients with worse liver function, as assessed by the mALBI grade. A similar trend was observed in patients with worse liver function according to the Child-Pugh class (B/C vs A), although the differences were not statistically significant (50.7% vs 41.7%; p = 0.140). Patients with impaired liver function are more likely to be subject to increased drug exposure [25], which may have been responsible for the greater ADR risk in these patients. We also found that mALBI grade was a significant factor for ADRs that led to treatment discontinuation, as reported previously [20], and more previous TACE procedures, which suggests worsened liver functional reserve [26], was another significant factor (Supplementary Table S3). Given these findings, administration of lenvatinib, especially in patients with worse liver function according to the mALBI grade, should be performed with extra caution for ADR risks, mindful of proper dose modifications, and management of ADRs.
As for the effectiveness of lenvatinib, meaningful improvements were observed, with an ORR of 39.5% and a DCR of 78.9%. Although direct comparison may not be appropriate because of different study methods, the ORR in this study was better than that in the Japanese subset of the REFLECT trial (29.6%) [12] and within the range of previous real-world data (30.4–42.1%) [16–19, 26]. The median OS was similar to the Japanese subset data of the REFLECT trial (16.6 vs 17.6 months [12]), which was longer than the trial’s global data (13.6 months) [9]. Furthermore, subgroup analyses showed that patients with worse liver function, as assessed by the mALBI grade, achieved a significantly lower ORR than those with better liver function, and the same was true for renal function (Table 5). Patients with more impaired liver or renal function at baseline probably received lower dose intensity, which may have contributed to the low treatment efficacy in these patients [27]. Moreover, given that the high mALBI grade and low eGFR were both significant factors for a short treatment period (Supplementary Table S2), the earlier treatment discontinuation may also have influenced the lower achievement of objective response in these patients. However, possible confounding factors should also be considered for the eGFR result as it was not a significant factor for objective response in multivariate analysis.
Our multivariate analysis revealed that the absence of extrahepatic lesions and higher mALBI grade at baseline (i.e., greater impairment of liver function) were significantly associated with low achievement of objective response. Unfortunately, the exact reason is unknown for the higher ORR in patients without extrahepatic lesions than in those with such lesions. One possibility may be that, as suggested previously, some differences in enhancement patterns of intrahepatic nodules, rather than the overall staging, might be more responsible for the differences in their treatment responses [28], although this hypothesis requires further investigation. As for the relationship between mALBI and ORR, similar results were reported in previous lenvatinib studies suggesting that mALBI grade, or an ALBI score, may be a good predictor of ORR [29, 30]. It was also reported that baseline mALBI grade was significantly associated with OS and progression-free survival [31–33]. Therefore, it may be beneficial for physicians to assess mALBI grade to identify patients with better liver functional reserve, who would be more likely to benefit from lenvatinib treatment. As mALBI grade is a simple index based only on two serological parameters, it can easily be used in clinical practice. Moreover, our results also suggested that treatment with lenvatinib may better be initiated early before patients’ liver function deteriorates due to, for instance, repeated TACE.
This study provides a real-world picture of lenvatinib treatment in clinical practice. We found that 25.3% of patients started lenvatinib at a lower dose than the body weight-based standard dose. Although the proportion of patients starting with the standard dose was higher in this study than previously reported (73.8% vs 48.9% [34]), our results revealed that some patients do not start with a full dose in Japan. This trend probably reflects the careful attitudes of physicians about the use of this new agent, considering that the study was conducted in the early days of its approval.
This study has several limitations. First, tumor responses could not be evaluated in all patients because of the absence of assessment and the different criteria used in routine practice, despite the mRECIST criteria being the principal criteria used in this study. Tumor response evaluated using different criteria cannot be combined; thus, the effectiveness assessment was restricted to 494 patients (70.3% of the study population) using the mRECIST criteria, which conformed to the criteria used in clinical trials. Second, as this was an observational study conducted in clinical practice, tumor response was not evaluated according to the uniform criteria. Furthermore, no result confirmation or independent imaging review was performed, which limits the reliability or accuracy of the obtained tumor evaluation results. Such a potential bias in the accuracy of assessments may have influenced the ORR. Third, this was a single-arm study with no control group. Finally, data regarding safety were collected for only 12 months; long-term safety should be further investigated in the future. Nevertheless, this study provided important data on the safety profiles and treatment outcomes of lenvatinib based on a large sample size, which will be valuable to physicians treating this patient population.
Conclusions
In conclusion, this study demonstrated that treatment with lenvatinib was generally well tolerated with no new safety concerns, and that clinically meaningful treatment responses were achieved in patients with uHCC in real-world clinical practice in Japan. Given that patients with good liver function may be more likely to respond to lenvatinib and continue treatment, it may be beneficial for physicians to assess mALBI grade at baseline to identify these potential candidates.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical writing support was provided by Clinical Study Support, Inc. (Nagoya, Japan), under contract with Eisai Co., Ltd.
Declarations
Funding
This work was supported by Eisai Co., Ltd. (Tokyo, Japan).
Conflict of Interest
J. Furuse received honoraria from Ono Pharmaceutical, Bayer, Eisai, Eli Lilly Japan, MSD, Yakult Honsha, Chugai Pharma, Novartis Pharma, AstraZeneca, Pfizer, Takeda, Taiho Pharmaceutical, Sanofi, Mylan EPD, EA Pharma, Kyowa Hakko Kirin, Daiichi Sankyo, Teijin Pharma, Servier Japan, and Incyte Biosciences Japan; and research grants from Ono Pharmaceutical, MSD, Merck Bio, J-Pharma, Taiho Pharmaceutical, Takeda, Chugai Pharma, AstraZeneca, Yakult Honsha, Eisai, Daiichi Sankyo, Mochida, Sanofi, Sumitomo Dainippon, Bayer, Astellas, and Incyte Biosciences Japan. N. Izumi received honoraria from Eisai, Chugai Pharmaceutical, Takeda Pharmaceutical, Eli Lilly, and Bayer. K. Motoyoshi is an employee of Eisai Co., Ltd. M. Kudo received honoraria from Eisai, Bayer, MSD, EA Pharma, Eli Lilly, Chugai Pharmaceutical, and Ono Pharmaceutical; and research grants from Eisai, Takeda Pharmaceutical, Otsuka Pharmaceutical, Taiho Pharmaceutical, EA Pharma, Gilead Sciences, AbbVie, Sumitomo Dainippon Pharma, Chugai Pharmaceutical, and Ono Pharmaceutical. K. Motomura, Y. Inaba, Y. Katamura, Y. Kondo, and K. Yabushita declare that they have no conflict of interest.
Author Contributions
J. Furuse and K. Motoyoshi contributed to the conception and design of this study. All authors contributed to data interpretation. K. Motoyoshi contributed to the manuscript drafting. All the authors critically reviewed the manuscript for important intellectual content and approved the final version for publication.
Ethics Approval
This study was conducted as part of pharmacovigilance activities as required by the Japanese Pharmaceutical and Medical Devices Agency. Therefore, no ethical approval was required. The study was performed in accordance with the provisions of the Declaration of Helsinki, Pharmaceutical Affairs Law, and Ministerial Ordinance on Good Post-Marketing Study Practice in Japan.
Informed Consent
Informed consent was obtained from all participants included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.World Health Organization, International Agency for Research on Cancer. Liver cancer fact sheet. 2022. https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. Accessed 24 May 2022.
- 2.Cancer Information Service, National Cancer Center Japan. Causes and contributions of cancer in Japanese people: latest estimates and future forecasts. 2022. https://ganjoho.jp/reg_stat/statistics/data/dl/excel/cancer_prediction(2015-2039).xlsx. Accessed 24 May 2022.
- 3.Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M. Recent advances in systemic therapy for hepatocellular carcinoma in an aging society: 2020 update. Liver Cancer. 2020;9:640–662. doi: 10.1159/000511001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014 doi: 10.1155/2014/638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Matsuki M, Hoshi T, Yamamoto Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641–2653. doi: 10.1002/cam4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 10.The Japan Society of Hepatology. In: Clinical Practice Guidelines for Hepatocellular Carcinoma 2021. 5th ed. Tokyo: Kanehara; 2021. (Japanese).
- 11.Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512–519. doi: 10.1007/s00535-016-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita T, Kudo M, Ikeda K, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55:113–122. doi: 10.1007/s00535-019-01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiraoka A, Kumada T, Tsuji K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer. 2019;8:121–129. doi: 10.1159/000488778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 16.Ogushi K, Chuma M, Uojima H, et al. Safety and efficacy of lenvatinib treatment in Child-Pugh A and B patients with unresectable hepatocellular carcinoma in clinical practice: a multicenter analysis. Clin Exp Gastroenterol. 2020;13:385–396. doi: 10.2147/CEG.S256691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya K, Kurosaki M, Sakamoto A, et al. The real-world data in Japanese patients with unresectable hepatocellular carcinoma treated with lenvatinib from a nationwide multicenter study. Cancers (Basel). 2021 doi: 10.3390/cancers13112608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatanaka T, Kakizaki S, Nagashima T, et al. Lenvatinib for hepatocellular carcinoma patients with nonviral infection who were unlikely to respond to immunotherapy: a retrospective, comparative study. Oncology. 2021;99:641–651. doi: 10.1159/000517494. [DOI] [PubMed] [Google Scholar]
- 19.Maruta S, Ogasawara S, Ooka Y, et al. Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer. 2020;9:382–396. doi: 10.1159/000507022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimose S, Iwamoto H, Niizeki T, et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers (Basel). 2020 doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42:3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Schmidinger M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl. 2013;11:172–191. doi: 10.1016/j.ejcsup.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada T, Kumada T, Hiraoka A, et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: a multicenter analysis with propensity score matching. Hepatol Res. 2020;50:75–83. doi: 10.1111/hepr.13427. [DOI] [PubMed] [Google Scholar]
- 24.Nair A, Reece K, Donoghue MB, et al. FDA supplemental approval summary: lenvatinib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2021;26:e484–e491. doi: 10.1002/onco.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shumaker R, Aluri J, Fan J, et al. Influence of hepatic impairment on lenvatinib pharmacokinetics following single-dose oral administration. J Clin Pharmacol. 2015;55:317–327. doi: 10.1002/jcph.398. [DOI] [PubMed] [Google Scholar]
- 26.Piscaglia F, Ogasawara S. Patient selection for transarterial chemoembolization in hepatocellular carcinoma: importance of benefit/risk assessment. Liver Cancer. 2018;7:104–119. doi: 10.1159/000485471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirino S, Tsuchiya K, Kurosaki M, et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS ONE. 2020 doi: 10.1371/journal.pone.0231828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura Y, Kobayashi M, Shindoh J, et al. Pretreatment heterogeneous enhancement pattern of hepatocellular carcinoma may be a useful new predictor of early response to lenvatinib and overall prognosis. Liver Cancer. 2020;9:275–292. doi: 10.1159/000505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueshima K, Nishida N, Hagiwara S, et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: a multicenter study. Cancers (Basel). 2019 doi: 10.3390/cancers11070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeki I, Yamasaki T, Yamashita S, et al. Early predictors of objective response in patients with hepatocellular carcinoma undergoing lenvatinib treatment. Cancers (Basel). 2020 doi: 10.3390/cancers12040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraoka A, Kumada T, Atsukawa M, et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med. 2019;8:3719–3728. doi: 10.1002/cam4.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchigami A, Imai Y, Uchida Y, et al. Therapeutic efficacy of lenvatinib for patients with unresectable hepatocellular carcinoma based on the middle-term outcome. PLoS ONE. 2020 doi: 10.1371/journal.pone.0231427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuma M, Uojima H, Hiraoka A, et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: a multicenter analysis. Hepatol Res. 2021;51:201–215. doi: 10.1111/hepr.13592. [DOI] [PubMed] [Google Scholar]
- 34.Hatanaka T, Kakizaki S, Nagashima T, et al. Analyses of objective response rate, progression-free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: A multicenter retrospective study. Hepatol Res. 2020;50:382–395. doi: 10.1111/hepr.13460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.