Abstract
Purpose
To evaluate disease progression and associated vision changes in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD) in 1 eye and GA or neovascular AMD (nAMD) in the fellow eye using a large dataset from routine clinical practice.
Design
Retrospective analysis of clinical data over 24 months.
Subjects
A total of 256 635 patients with GA from the American Academy of Ophthalmology (Academy) IRIS® Registry (Intelligent Research in Sight) Registry (January 2016 to December 2017).
Methods
Patients with ≥ 24 months of follow-up were grouped by fellow-eye status: Cohort 1, GA:GA; Cohort 2, GA:nAMD, each with (subfoveal) and without subfoveal (nonsubfoveal) involvement. Eyes with history of retinal disease other than AMD were excluded. Sensitivity analysis included patients who were managed by retina specialists and had a record of imaging within 30 days of diagnosis.
Main Outcome Measures
Change in visual acuity (VA), occurrence of new-onset nAMD, and GA progression from nonsubfoveal to subfoveal.
Results
In total, 69 441 patients were included: 44 120 (64%) GA:GA and 25 321 (36%) GA:nAMD. Otherwise eligible patients (57 788) were excluded due to follow-up < 24 months. In both GA:GA and GA:nAMD cohorts, nonsubfoveal study eyes had better mean (standard deviation) VA at index (67 [19.3] and 66 [20.3] letters) than subfoveal eyes (59 [23.9] and 47 [26.9] letters), and 24-month mean VA changes were similar for nonsubfoveal (−7.6 and −6.2) and subfoveal (−7.9 and −6.5) subgroups. Progression to subfoveal GA occurred in 16.7% of nonsubfoveal study eyes in the GA:GA cohort and 12.5% in the GA:nAMD cohort. More new-onset study-eye nAMD was observed in the GA:nAMD (21.6%) versus GA:GA (8.2%) cohorts. Sensitivity analysis supported the robustness of the observations in the study.
Conclusions
This retrospective analysis describes the natural progression of GA lesions and the decline in VA associated with the disease.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Keywords: Geographic atrophy, Macular degeneration, Retrospective analysis
Geographic atrophy (GA) is the advanced form of age-related macular degeneration (AMD).1, 2, 3 Over 5 million patients worldwide are estimated to have GA, with 1 million in the United States alone.4,5 Although neovascular AMD (nAMD) can be successfully treated with VEGF inhibitors, the treatment of GA remains a substantially unmet need. Geographic atrophy is defined by the irreversible loss of retinal structures, including photoreceptor cells, retinal pigment epithelium, and choriocapillaris.6 Pathophysiological studies show that GA often begins in the regions away from the fovea and, over time, spreads to cover the center of the fovea; however, the rates and dynamics of disease progression vary considerably from person to person.6 It is important to note that GA and nAMD can coexist in the same eye.
Loss of visual acuity (VA) in GA has been reported in numerous prospective and retrospective natural history studies.6, 7, 8, 9 Irreversible decline in VA has been shown to accompany GA lesion growth, although the relationship between lesion growth and VA decline is complex, owing to GA lesion characteristics such as location, focality, and degree of foveal involvement as well as limitations in instruments used to measure visual function.8,10,11 Data from the prospective Proxima A and B studies showed rapid GA lesion expansion and an associated decline in vision.12 A retrospective study of 40 543 patients from the United Kingdom showed that the type and stage of AMD in the fellow eye affected rate of disease progression in the study eye. Additionally, factors including age, female sex, and presence of cardiovascular conditions tended to predispose patients to higher risk of disease progression to advanced AMD, according to a multivariate analysis.13
With the wide-ranging functional impact of GA, there is a need for additional data from a large patient population that represent the natural course of GA outside the context of a controlled clinical study. Understanding the natural history and patterns of GA progression over time from a large-scale, representative registry may help the timely diagnosis and management of patients with GA.
To characterize the GA population and disease progression in everyday clinical practice, we conducted a retrospective analysis of the Academy’s IRIS registry.
Methods
Study Design
This was a retrospective analysis of clinical data recorded in the IRIS Registry. The IRIS registry houses information from 60 electronic health record (EHR) data systems from > 15 000 clinicians in ophthalmic practices and > 70 million unique patients, including those without insurance, with adequate security per Health Insurance Portability and Accountability Act laws. According to the guidance regarding methods for deidentification of Protected Health Information in accordance with the Health Insurance Portability and Accountability Act Privacy Rule, neither Institutional Review Board approval nor exemption is required because (1) the research and analysis was conducted on anonymized data in accordance with the deidentification standard promulgated under 45 CFR § 164.514; and (2) no research was conducted on human subjects. No patient authorization is required because this study was certified as conducted on anonymized, deidentified data used for research purposes by Verana Health.
Study Participants
The study included patients who were ≥ 50 years of age at the index date with an International Classification of Diseases (ICD)-10 diagnosis code for GA in ≥ 1 eye between January 1, 2016 and December 31, 2017. The start date was selected based on the availability of the ICD-10 code for GA, confirming disease severity and eye laterality. As the ICD-10 codes use the phrases “with subfoveal involvement” and “without subfoveal involvement,” the terms “subfoveal” and “nonsubfoveal” have been used to describe lesion location, respectively. Included patients had advanced AMD in both eyes (GA in 1 eye and either GA or nAMD in the other). The eye with GA was selected as the study eye in patients with unilateral GA; in patients with bilateral GA, the eye without subfoveal involvement was selected as the study eye; and in cases where neither or both had subfoveal GA, the better-seeing eye was selected. Patients were excluded if they had nAMD in the study eye before GA diagnosis (< 3 years before the study index date), history of any other retinal condition, missing demographic information, or < 24 months of follow-up. To examine how disease in the fellow eye affected outcome measures in the study eye, patients were categorized according to the presence of bilateral GA (GA:GA, Cohort 1), or fellow-eye nAMD (GA:nAMD, Cohort 2).
For the sensitivity analysis, patients who were managed by retina specialists from the index diagnosis of GA and had a record of imaging within 30 days of diagnosis were included. For inclusion in this analysis, patients were also required to have a record of imaging that was a Current Procedural Terminology code for either OCT or fundus autofluorescence within 30 days of a progression event.
Study End Points
End points included change in VA over time, categorical VA decline at 12 and 24 months, and mean time to severe visual impairment in all the cohorts. All Snellen scores used to measure VA were converted to logarithm of the minimum angle of resolution first for analysis and then to ETDRS letters. Change in VA over time was analyzed by VA values observed at baseline and at 12 (± 45 days) and 24 (± 45 days) months. Visual acuity decline at 12 and 24 months was also measured in terms of clinically relevant vision end points, including loss of driving ability (worse than 20/40 in the study eye) and legal blindness (20/200 or worse in the study eye).
In study and fellow eyes, disease progression was defined as either a change from nonsubfoveal to subfoveal GA or development of new-onset nAMD, based on ICD-10 codes. In patients who developed nAMD, those receiving anti-VEGF treatment and the intervals of treatment were recorded.
Statistical Analysis
Statistical comparisons for VA values were not performed because the VA assessments were a function of data availability in this dataset. Comparisons between groups based on atrophy location and fellow-eye involvement were performed using 2-sample t tests (normal distribution) or Wilcoxon rank sum tests (nonnormal distribution) for 2-group continuous variable comparisons. For more than 2-group comparisons, analysis of variance was used. To find heterogeneity between each cohort for comparisons of categorical variables, chi-square test was used. Worsening of VA was calculated by stratifying patients according to baseline VA. A logistic multivariable analysis was performed to analyze factors associated with follow-up < 24 months.
Results
Patient Characteristics
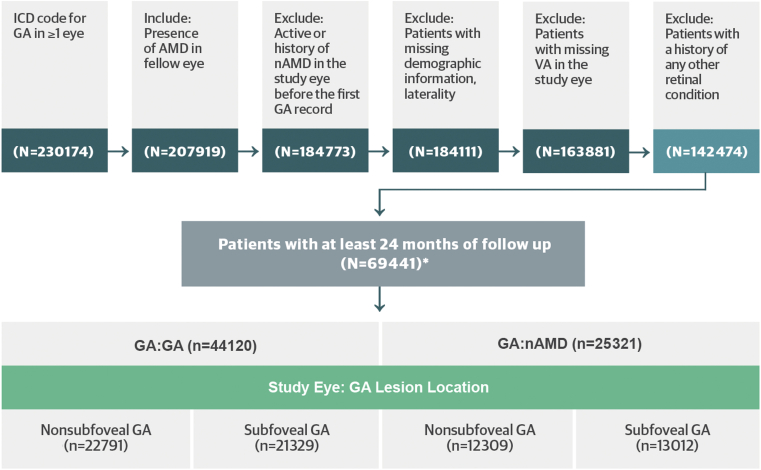
A total of 256 635 patients with GA were identified from the IRIS Registry during the index period, and 69 441 patients met inclusion criteria. Among the included population, 44 120 patients (63.5%) had bilateral GA (GA:GA; Cohort 1) and 25 321 patients (36.5%) had nAMD in the fellow eye (GA:nAMD; Cohort 2) (see Fig 1 and Table 1 for further details).
Figure 1.
Patient disposition.
∗Includes only patients with geographic atrophy (GA) or neovascular age-related macular degeneration (nAMD) in the fellow eye
AMD = age-related macular degeneration; CNV = choroidal neovascularization; EMR = electronic medical records; ICD = International Classification of Diseases; VA = visual acuity.
Table 1.
Patient Demographics and Treating Provider of Patients with ≥ 24 Months of Follow-up
| GA:GA (n = 44 120) | GA:nAMD (n = 25 321) | |||
|---|---|---|---|---|
| Age, Mean (SD) | 81.38 (8.68) | 82.58 (7.90) | ||
| Sex, n (%) | ||||
| Female | 29 685 | 67 | 16 916 | 67 |
| Male | 14 435 | 33 | 8405 | 33 |
| Race, n (%) | ||||
| White | 37594 | 85 | 22 368 | 88 |
| Black | 506 | 1 | 133 | 0.5 |
| Asian | 571 | 1 | 195 | 0.8 |
| Other | 163 | 0.4 | 64 | 0.3 |
| Unknown | 5286 | 12 | 2561 | 10 |
| Treating provider, n (%) | ||||
| Retina specialist | 24 297 | 55 | 21 871 | 86 |
| General ophthalmologist | 8770 | 20 | 1898 | 7 |
| Other specialist (e.g., glaucoma) | 7953 | 18.0 | 1113 | 4 |
| Optometrist | 2789 | 6 | 323 | 1 |
| Unknown | 311 | 0.7 | 116 | 0.5 |
GA = geographic atrophy; nAMD = neovascular age-related macular degeneration; SD = standard deviation.
Both cohorts were balanced with respect to race (as classified in the IRIS Registry database), age, and sex. Notably, 57 788 otherwise eligible patients were excluded due to follow-up of < 24 months (Table 2).
Table 2.
Characteristics of Patients with < 24 Months of Follow-up
| < 24 mos Follow-up (n = 57 788) | ≥ 24 mos Follow-up (n = 84 686) | P | |
|---|---|---|---|
| Age, Mean (SD) | 85.03 (8.66) | 81.37 (8.48) | < 0.0001 |
| VA at index, study eye; mean ETDRS letters, (SD) | 48.97 (26.73) | 56.87 (25.37) | < 0.0001 |
| Visits to retina specialist, mean, SD | |||
| first year post-index | 2.91 (2.59) | 4.09 (3.26) | |
| second year post-index | 2.16 (1.83) | 4.08 (3.18) | |
| Disease classification, n (%) | < 0.0001 | ||
| GA:GA, nonsubfoveal | 18 288 (31.6) | 24 476 (28.9) | |
| GA:GA, subfoveal | 19 375 (33.5) | 22 631 (26.7) | |
| GA:nAMD, nonsubfoveal | 4921 (8.5) | 11 798 (13.9) | |
| GA:nAMD, subfoveal | 6548 (11.3) | 12320 (14.5) |
GA = geographic atrophy; nAMD = neovascular age-related macular degeneration; SD = standard deviation; VA = visual acuity.
In an analysis of treatment provider type, 55.1% (24 297) of the patients in the GA:GA group (Cohort 1) were seen by a retina specialist, 19.9% (8770) by a general ophthalmologist, 18% (7953) by other subspecialists, and 6.3% (2789) by an optometrist. In comparison, 86.4% (21 871) of the patients in the GA:nAMD group (Cohort 2) were seen by a retina specialist, 7.5% (1898) were seen by a general ophthalmologist, 4.4% (1113) were seen by other subspecialists, and 1.3% (323) were seen by an optometrist.
Changes in VA
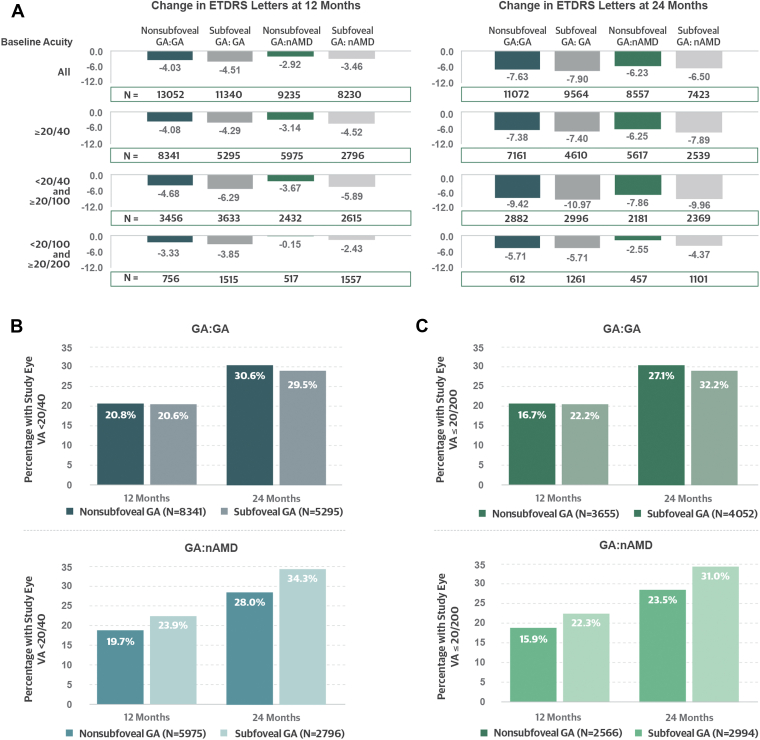
In Cohort 1 (GA:GA), mean (standard deviation) VA at baseline was 63 (22.0) letters. Study eyes with nonsubfoveal GA had better mean VA at index (67 [19.3] letters) than those with subfoveal GA (59 [23.9] letters). However, over 24 months, mean changes in VA were similar for both the nonsubfoveal and subfoveal lesion subgroups, with a loss of 7.6 and 7.9 letters, respectively. In Cohort 2 (GA:nAMD), mean (standard deviation) VA at baseline was 56 (25.7) letters. Visual acuity at index was likewise better for the nonsubfoveal than subfoveal GA subgroups (mean 66 [20.3] vs. 47 [26.9] letters, respectively), and similar changes were seen at 24 months, with a mean loss of 6.2 and 6.5 letters (Table 3, Fig 2A).
Table 3.
Visual Acuity in the Study Eye at Index and by Cohorts
| Overall GA:GA (n = 44 120) | Nonsubfoveal GA:GA (n = 22 791) | Subfoveal GA:GA (n = 21 329) | Overall GA:nAMD (n = 25 321) | Nonsubfoveal GA:nAMD (n = 12 309) | Subfoveal GA: nAMD (n = 13 012) | |
|---|---|---|---|---|---|---|
| Mean ETDRS letters, (SD)∗ | 63 (22.02) | 67.45 (19.25) | 58.98 (23.87) | 56 (25.72) | 65.69 (20.34) | 46.86 (26.91) |
| Proportion of patients, n (%) | ||||||
| 20/20 or better | 6294 (14) | 3571 (15) | 2723 (12) | 2161 (8) | 1469 (12) | 692 (5) |
| < 20/20 or ≥ 20/40 | 18 628 (42) | 11 354 (49) | 7274 (34) | 9407 (37) | 6295 (51) | 3112 (24) |
| < 20/40 or ≥ 20/100 | 11 144 (25) | 5209 (22) | 5935 (28) | 6354 (25) | 2930 (24) | 3424 (26) |
| < 20/100 or ≥ 20/200 | 3765 (8) | 1179 (5) | 2586 (12) | 2762 (10) | 632 (5) | 2130 (16) |
| < 20/200 | 4289 (9) | 1478 (6) | 2811 (13) | 4637 (18) | 983 (8) | 3564 (28) |
GA = geographic atrophy; nAMD = neovascular age-related macular degeneration; SD = standard deviation.
Snellen was converted to ETDRS letters.
Figure 2.
A, Mean changes in visual acuity (VA) by cohort (including only patients with baseline and either month 12 or 24 VA data available). Cohorts are nonsubfoveal geography atrophy (GA):GA; subfoveal GA:GA; nonsubfoveal GA:neovascular age-related macular degeneration (nAMD); subfoveal GA: nAMD. B, Worsening of VA to < 20/40 among those with VA 20/40 or better at baseline. C, Decline of VA to ≤ 20/200 or worse among those with VA worse than 20/40 but better than 20/200 at baseline.
Categorical Decline in VA
At index in Cohort 1 (GA:GA), 14% of study eyes had VA better than 20/20, and 42% had VA worse than 20/20 but better than 20/40. In Cohort 2 (GA:nAMD), 8% of study eyes had 20/20 vision or better, whereas 37% had vision ranging from 20/20 to 20/40.
Among study eyes with VA of 20/40 or better at baseline, the rates of decline were similar in both cohorts, with 30.2% in the GA:GA cohort and 30.0% in the GA:nAMD cohort progressing to VA worse than 20/40 at 24 months.
Among study eyes with VA worse than 20/40 and better than 20/200 at index, 29.7% of the GA:GA and 27.6% of the GA:nAMD cohorts progressed to VA 20/200 or worse at 24 months. In general, vision loss to VA 20/200 or worse occurred more frequently in eyes with baseline subfoveal GA. Fellow-eye status did not seem to correlate with decline of VA in the study eye (Fig 2B, C).
Progression from Nonsubfoveal to Subfoveal Lesions
In Cohort 1 (GA:GA), 3800 (16.7%) study eyes progressed from nonsubfoveal to subfoveal GA over the follow-up period, with a mean time to progression of 73.6 weeks (16.9 months). In Cohort 2 (GA:nAMD), 1540 (12.5%) study eyes progressed from nonsubfoveal to subfoveal GA during follow-up, with a mean time to progression of 68.2 weeks (15.6 months). At the end of the first 12 months, 7.6% (GA:GA) and 6.0% (GA:nAMD) lesions progressed from nonsubfoveal to subfoveal. At 24 months, these rates were 12.8% and 9.9% for patients in the GA:GA and GA:nAMD groups, respectively.
Development of Neovascular AMD
Overall, occurrence of nAMD was observed in the study eye in 2066 (4.7%) of Cohort 1 (GA:GA) patients and in 3370 (13.3%) of Cohort 2 (GA:nAMD) patients during the first 12 months. The occurrence of study-eye nAMD at 24 months increased to 8.2% (3611) and 21.6% (5476) for patients with GA:GA and GA:nAMD, respectively (Fig 3A).
Figure 3.
A, Occurrence of new-onset neovascular age-related macular degeneration (nAMD) in study eye by cohort. B, Disease progression to subfoveal involvement and occurrence of new-onset nAMD in study eye by cohort. Patients were followed for ≥ 24 months with mean (standard deviation) follow-up of 1001 (164) days and median follow-up of 998 days (interquartile range, 261). Month 12 was measured as (365 + 45-day window). Month 24 was measured as (730 + 45-day window). GA = geographic atrophy.
In patients with bilateral GA, 2608 (11.4%) study eyes with nonsubfoveal and 2176 (10.2%) with subfoveal lesions had new-onset nAMD with a mean time to occurrence of 76.1 weeks (17.5 months) and 72.6 weeks (16.7 months), respectively. In comparison, patients with fellow-eye nAMD developed new-onset nAMD in the study eye more frequently in both the nonsubfoveal (3605 [29.3%]) and subfoveal (3387 [26.0%]) subgroups than in the bilateral GA group, with mean time to occurrence of 70 weeks (16.1 months) and 66.9 weeks (15.3 months), respectively (Fig 3B).
Across cohorts, a majority of study eyes that developed new-onset nAMD had a record of receiving ≥ 1 anti-VEGF injection(s) during the follow-up period. Of study eyes in the GA:GA cohort that developed nAMD, 58% and 57% with baseline nonsubfoveal and subfoveal lesions, respectively, received ≥ 1 anti-VEGF injection. Of study eyes in the GA:nAMD cohort that developed nAMD, 65% and 57% with baseline nonsubfoveal and subfoveal lesions, respectively, received ≥ 1 anti-VEGF injection.
Patients Lost to Follow-up
An incidental but important finding of this analysis was the number of patients who were lost to follow-up. Of the eligible patients, 57 788 (40.5%) were excluded from the primary analysis due to < 24 months of follow-up (Table 2). These excluded patients were, on average, 3.7 years older and had worse VA (49 vs. 57 letters) than those with ≥ 24 months of follow-up (P < 0.0001 for both). A larger proportion of patients in the group with < 24 months of follow-up had vision worse than 20/200 (24.6% vs. 16.8%). This group also had a higher proportion of bilateral GA (65.2% vs. 55.6%), subfoveal involvement in the study eye (33.5% vs. 26.7%), and a lower rate of fellow-eye nAMD (19.8% vs. 28.5%). They were less likely to be managed by a retina specialist (60.7%) than those with ≥ 24 months of follow-up (69.9%). According to a logistic multivariable analysis, patients between 60 and 80 years old, those with a fellow-eye diagnosis of nAMD, those managed by a retina specialist from the start of the follow-up period, and those who had a concomitant glaucoma/cataract diagnosis or procedure were more likely to have ≥ 24 months of follow-up.
Sensitivity Analysis
To confirm these overall observations, we performed a sensitivity analysis among a subgroup of patients who were diagnosed and managed by retina specialists (Table S4, available at www.ophthalmologyscience.org). This subpopulation was relatively balanced between Cohort 1 (GA:GA) and Cohort 2 (GA:nAMD) but had a greater proportion of patients in Cohort 2 (GA:nAMD) than the primary analysis (50% vs. 36%), likely because of retina specialists’ involvement in nAMD management.
In this sensitivity analysis, Cohort 1 (GA:GA) had better mean baseline acuity, and more study eyes in this cohort had nonsubfoveal lesions. At 24 months, Cohort 1 (GA:GA) and Cohort 2 (GA:nAMD) had a mean loss of 7.52 and 6.24 letters, respectively (Fig S4A, available at ophthalmologyscience.org). Eyes with subfoveal involvement also had greater mean and categorical VA decline over both 12 and 24 months than those with nonsubfoveal GA (Fig S4B).
At 24 months, 5891 (25.4%) study eyes in Cohort 1 (GA:GA) and 8423 (39.1%) study eyes in Cohort 2 (GA:nAMD) experienced disease progression to subfoveal involvement or nAMD. Progression from nonsubfoveal to subfoveal GA occurred in 21.8% and 15.0% of eyes in the GA:GA and GA:nAMD groups, respectively. The effect of fellow-eye nAMD on disease progression was also observed in this analysis (Fig S4C).
These findings are consistent with the data from the primary analysis.
Discussion
This analysis of the Academy’s IRIS Registry offers additional insights into the clinical course of GA over a 24-month period. Substantial GA progression, including from nonsubfoveal to subfoveal lesions, associated decline in vision, and development of nAMD were observed in this large dataset.
In the absence of clinical imaging data to confirm GA lesions, a sensitivity analysis using diagnoses from retina specialists was undertaken. Given that retina specialists may have greater access to OCT and other imaging devices used for monitoring GA lesions, this analysis lends credibility to the observations of disease progression. Additionally, presence of an OCT Current Procedural Terminology code was required within 30 days of the GA diagnosis to ensure an imaging procedure was conducted for each GA diagnosis observed. Results from the sensitivity analysis of patients seen by retina specialists were consistent with these overall trends.
The influence of fellow-eye nAMD in this study was notable. The development of nAMD was up to 3 times more frequent in patients with fellow-eye nAMD compared with those with bilateral GA, regardless of GA lesion location. Other research has also shown that the presence of nAMD at baseline in the fellow eye increases the probability of nAMD developing in the study eye.14 The development of neovascular disease in eyes with GA, here and in other studies, suggests that the underlying relationship between these pathological processes requires further elucidation.15 In a prospective cohort study within the Age-Related Eye Disease Study 2, 29% of eyes with incident GA developed nAMD within 4 years.16 A subsequent Age-Related Eye Disease Study 2 report also demonstrated that fellow-eye nAMD increases the chances of developing nAMD in an eye with GA, and in a follow-up, new-onset nAMD occurred within 5 years in 36.4% of eyes with baseline fellow-eye nAMD versus 16% of those without.9,17,18 As observed in this study, patients with fellow-eye nAMD were also more likely to be seen by a retina specialist, probably related to anti-VEGF treatment and associated monitoring, suggesting a gap for patients with GA without nAMD who may benefit from the care of a retina specialist.
In this study, categorical decline to VA worse than 20/40 and 20/200 was measured unilaterally. Because these were patients’ better-seeing eyes, such worsening of vision could potentially render an affected patient ineligible to drive (20/40) or meeting criteria for legal blindness (20/200), substantially impacting their independence and quality of life. It has been well documented that GA affects vision-related quality of life, such as activities of daily living, driving patterns, work schedules, social activities, dependency, and mental health.19, 20, 21, 22, 23 One retrospective analysis showed that 66.7% of patients with bilateral GA became ineligible to drive by a median time of 1.6 years, whereas 16% were legally blind after a median 6.2 years.24
The continued decline of vision, as indicated by the marked deterioration in the first year in our study, highlights the critical need to slow or prevent disease progression in GA. Interestingly, a similar magnitude of vision decline was observed in both nonsubfoveal and subfoveal lesion subgroups, suggesting that visual function may be preserved with future treatments even after lesions enter the fovea. This finding aligns with previous reports suggesting that, although early treatment might be most advantageous, there remains a possibility of vision preservation later in the course of the disease, although the relationship between lesion growth and visual function is not completely understood.8,10,12 At the time of the first clinical encounter in this study, VA was generally not greatly impaired for the patients with GA, particularly for those with nonsubfoveal lesions. This study demonstrates a mean of 15.6 to 16.9 months to foveal encroachment within the 2 cohorts. Considered with previous reports of a median time to foveal encroachment of 2.5 years after GA diagnosis,25 these results suggest an opportunity for early therapeutic intervention to prevent or slow lesion progression into the foveal region.
When considered together with the proportion of patients with < 24 months of follow-up, these data further suggest that, although initial patient assessment may be happening at appropriate times, subsequent care has not been optimal, perhaps due to lack of available therapies and loss to follow-up. Patients with vision loss due to GA may not perceive a benefit to following up with their eyecare provider if they have been informed about the lack of treatment options and the irreversible nature of lesion growth. The results of this study highlight the need for patient education and the importance of follow-up in GA for adequate and ongoing monitoring and vision support and to identify and treat ocular comorbidities.26
This study has several limitations. As a large registry, the IRIS Registry is based on ICD-10 codes and is limited by a lack of clinical images and the likelihood of missing information and documentation errors, all of which are common to data derived from EHRs.27 With large registries such as IRIS, it is impossible to account for miscoding and to verify and validate data quality, which is beyond the scope of this study and could be counted as a limitation.28 In the absence of a direct image analysis, the sensitivity analysis undertaken among patients examined by retina specialists helps address these inherent limitations as care by subspecialists and the presence of imaging data lend credibility to observations of disease progression, and these results support the overall trends observed in the larger population. Additionally, although the IRIS Registry as a whole could be considered representative of the United States population seeking ophthalmic care, this study’s inclusion criteria may be biased toward patients with sufficient access to health care resources to have received 24 months of follow-up. Retrospective database studies necessarily rely mainly on billing codes, which have limited ability to describe wide-ranging medical conditions in detail.29 Because systemic comorbidities and characteristics such as smoking status and family history are not consistently noted in the EHR within an ophthalmology practice setting, these factors could not be analyzed further. In the future, studying such risk factors for progression of GA in a large EHR registry could help identify patients who are most likely to benefit from future therapeutic approaches.
Other substantial limitations include lack of a reading center, leading to diagnostic errors and low-quality evaluations of VA. Also, because the VA data were collected as Snellen scores and converted to ETDRS letters, caution must be exercised in interpreting the precise letter results. Finally, conventional VA testing may not reflect the true extent of visual function loss in patients with foveal-sparing scotomas.30 To further characterize functional progression, patient-reported outcomes, such as reading acuity and speed or use of questionnaires based specifically on low-luminance activities, may be required.31,32
A strength of this study is the inclusion of patients with GA routinely seen by thousands of ophthalmologists in academic and community settings. Unlike other controlled studies with narrowly selected patient populations, our research features a prevalent GA patient population in clinical settings that is diverse across institutions, ages, and pre-existing conditions. Findings from this large study highlight the rapid, irreversible, and clinically meaningful progression of GA lesions and vision decline over a 24-month period.12 Well-designed, prospective studies with longitudinal data are required to study patterns of atrophy development and to further our understanding of disease progression and burden in AMD.33
Acknowledgments
Namrata Saroj, OD, provided critical review of the manuscript. Mark Gallivan, MPH, contributed to study concept and design.
Manuscript no. XOPS-D-22-00234R1.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Presented at the American Academy of Ophthalmology Annual Meeting, November 13-15, 2020 (virtual); the Macula Society Annual Meeting, February 6-7, 2021 (virtual); the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, May 1-7, 2021 (virtual); the 54th Annual Scientific Meeting of the Retina Society, September 29-October 2, 2021, Chicago, Illinois; and the American Society of Retina Specialists (ASRS) Annual Meeting, October 8-12, 2021, San Antonio, Texas.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors have made the following disclosure(s): E.R.: Consultant – Allergan, Apellis, Genentech, Regeneron; Advisory board member – Alimera, Allergan, Genentech, Regeneron.
M.A.K.: Research grants – Regeneron; Consultant – Allergan, Apellis, Genentech, Regeneron; Advisory board member – Alimera, Allergan, Genentech.
A.C.H.: Consultant – Adverum, Aerie, AGTC, Alcon Laboratories Inc, Aldeyra, Allergan, Apellis, Asclepix, Atsena, Beaver-Visitec International Inc, Chengdu Kanghong Biotechnology, Clearside, Genentech, Graybug, Gyroscope, Iveric, Janssen/Johnson & Johnson, Lineage, MeiraGtx, Notal, Ocular Therapeutics, ONL, Regeneron Pharmaceuticals Inc, RegenXBio; Grant support – Aerie, AGTC, Alcon Laboratories Inc, Aldeyra, Allergan, Apellis, Asclepix, Atsena, Chengdu Kanghong Biotechnology, Genentech, Graybug, Gyroscope, Iveric, Janssen/Johnson & Johnson, Lineage, Lumithera, MeiraGtx, Notal, National Eye Institute, Novartis, ProQR Adverum, Regeneron Pharmaceuticals Inc, RegenXBio; Equity – Covalent Medical, LLC, ONL; Patents/royalty – Gyroscope.
M.H.: Employment – Verana Health; Equity interest – Verana Health.
T.H.N.: Employment – Verana Health; Equity interest – Verana Health.
D.J.: Employment – Apellis Pharmaceuticals; Equity interest – Apellis Pharmaceuticals.
A.M.: Employment – Apellis Pharmaceuticals; Equity interest – Apellis Pharmaceuticals.
D.B.: Consultant – Verana Health.
T.L.: Grants – Kodiak, Targeted Therapy Technologies. Consultant – Astellas, Boehringer Ingelheim, Genentech, Kanaph, Nanoscope, Regeneron, Verana Health.
R.R.: Employment – Apellis Pharmaceuticals; Equity interest – Apellis Pharmaceuticals.
N.H.: Research and manuscript preparation support from Apellis Pharmaceuticals; Grants/contracts – Genentech, Gemini, Gyroscope, Notal Vision (payments to Lifelong Vision Foundation); Consultant – 4DMT, AGTC, Apellis, Abbvie/Allergan, Annexon, Bausch and Lomb, Bayer, Biogen, Boehringer, Clearside Biosciences, EyePoint Pharmaceuticals, Genentech, Gemini, Gyroscope, Medpace, Medscape, Nacuity, NGM, Notal Vision, Novartis, Ocuphire, Outlook Therapeutics, Regeneron, Stealth Biosciences, Thea Laboratoires, Vial; Honoria – Abbvie/Allergan, Apellis, Bausch and Lomb, Genentech, Novartis, Regeneron, and Spark; Patent – Katalyst Surgical; Committee member – Editas and Ocuphire; Equity interest – Gemini/Nacuity.
Apellis Pharmaceuticals, Inc, supported the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
HUMAN SUBJECTS: Human subjects were included in this study. According to the guidance regarding methods for de-identification of Protected Health Information in accordance with the Health Insurance Portability and Accountability Act Privacy Rule, neither Institutional Review Board approval nor exemption is required because (1) the research and analysis was conducted on anonymized data in accordance with the de-identification standard promulgated under 45 CFR § 164.514; and (2) no research was conducted on human subjects. No patient authorization is required because this study was certified as conducted on anonymized, deidentified data used for research purposes by Verana Health.
No animals were used in this study.
Author Contributions:
Conception and design: Rahimy, Leng, Ribeiro, Holekamp
Analysis and interpretation: Rahimy, Khan, Ho, Hatfield, Nguyen, Jones, McKeown, Borkar, Leng, Ribeiro, Holekamp
Data collection: Rahimy, Khan, Hatfield, Nguyen, Jones, McKeown, Borkar, Leng, Ribeiro, Holekamp
Obtained funding: N/A
Overall responsibility:
Supplementary Data
References
- 1.Rudnicka A.R., Jarrar Z., Wormald R., et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Rudnicka A.R., Kapetanakis V.V., Jarrar Z., et al. Incidence of late-stage age-related macular degeneration in American Whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85–93.e3. doi: 10.1016/j.ajo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Boyer D.S., Schmidt-Erfurth U., van Lookeren Campagne M., et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37:819–835. doi: 10.1097/IAE.0000000000001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacconi R., Corbelli E., Querques L., et al. A review of current and future management of geographic atrophy. Ophthalmol Ther. 2017;6:69–77. doi: 10.1007/s40123-017-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleckenstein M., Mitchell P., Freund K.B., et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369–390. doi: 10.1016/j.ophtha.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Sunness J.S., Gonzalez-Baron J., Applegate C.A., et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–1779. doi: 10.1016/S0161-6420(99)90340-8. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz-Valckenberg S., Nadal J., Fimmers R., et al. Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica. 2016;235:215–224. doi: 10.1159/000445217. [DOI] [PubMed] [Google Scholar]
- 9.Chew E.Y., Clemons T.E., Agrón E., et al. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. 2014;132:272–277. doi: 10.1001/jamaophthalmol.2013.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heier J.S., Pieramici D., Chakravarthy U., et al. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmol Retina. 2020;4:673–688. doi: 10.1016/j.oret.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Shen L.L., Sun M., Ahluwalia A., et al. Relationship of topographic distribution of geographic atrophy to visual acuity in nonexudative age-related macular degeneration. Ophthalmol Retina. 2021;5:761–774. doi: 10.1016/j.oret.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Holekamp N., Wykoff C.C., Schmitz-Valckenberg S., et al. Natural history of geographic atrophy secondary to age-related macular degeneration: results from the Prospective Proxima A and B clinical trials. Ophthalmology. 2020;127:769–783. doi: 10.1016/j.ophtha.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthy U., Bailey C.C., Scanlon P.H., et al. Progression from early/intermediate to advanced forms of age-related macular degeneration in a large UK cohort: rates and risk factors. Ophthalmol Retina. 2020;4:662–672. doi: 10.1016/j.oret.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Wykoff C.C., Rosenfeld P.J., Waheed N.K., et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology. 2021;128:1325–1336. doi: 10.1016/j.ophtha.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Kaszubski P., Ben Ami T., Saade C., Smith R.T. Geographic atrophy and choroidal neovascularization in the same eye: a review. Ophthalmic Res. 2016;55:185–193. doi: 10.1159/000443209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keenan T.D., Agrón E., Domalpally A., et al. Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology. 2018;125:1913–1928. doi: 10.1016/j.ophtha.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunness J.S., Gonzalez-Baron J., Bressler N.M., et al. The development of choroidal neovascularization in eyes with the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:910–919. doi: 10.1016/S0161-6420(99)00509-6. [DOI] [PubMed] [Google Scholar]
- 18.Klein R., Meuer S.M., Knudtson M.D., Klein B.E. The epidemiology of progression of pure geographic atrophy: the Beaver Dam eye study. Am J Ophthalmol. 2008;146:692–699. doi: 10.1016/j.ajo.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Künzel S.H., Möller P.T., Lindner M., et al. Determinants of quality of life in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61:63. doi: 10.1167/iovs.61.5.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leys A., Zlateva G., Shah S.N., Patel M. Quality of life in patients with age-related macular degeneration: results from the VISION study. Eye (Lond) 2008;22:792–798. doi: 10.1038/sj.eye.6702900. [DOI] [PubMed] [Google Scholar]
- 21.Singh R.P., Patel S.S., Nielsen J.S., et al. Patient-, caregiver-, and eye care professional-reported burden of geographic atrophy secondary to age-related macular degeneration. Am J Ophthal Clin Trials. 2019;2:1–6. [Google Scholar]
- 22.Sarda S.P., Heyes A., Bektas M., et al. Humanistic and economic burden of geographic atrophy: a systematic literature review. Clin Ophthalmol. 2021;15:4629–4644. doi: 10.2147/OPTH.S338253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivaprasad S., Tschosik E.A., Guymer R.H., et al. Living with geographic atrophy: an ethnographic study. Ophthalmol Ther. 2019;8:115–124. doi: 10.1007/s40123-019-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarthy U., Bailey C.C., Johnston R.L., et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:842–849. doi: 10.1016/j.ophtha.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Lindblad A.S., Lloyd P.C., Clemons T.E., et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127:1168–1174. doi: 10.1001/archophthalmol.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J.H., Chang Y.S., Kim J.W. Natural course of patients discontinuing treatment for age-related macular degeneration and factors associated with visual prognosis. Retina. 2017;37:2254–2261. doi: 10.1097/IAE.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 27.Chiang M.F., Sommer A., Rich W.L., et al. The 2016 American Academy of Ophthalmology (Academy) IRIS® Registry (Intelligent Research in Sight) database: characteristics and methods. Ophthalmology. 2018;125:1143–1148. doi: 10.1016/j.ophtha.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Morlet N., Semmens J., et al. Coding accuracy for endophthalmitis diagnosis and cataract procedures in Western Australia. The Endophthalmitis Population Study of Western Australia (EPSWA): second report. Ophthalmic Epidemiol. 2003;10:133–145. doi: 10.1076/opep.10.2.133.13898. [DOI] [PubMed] [Google Scholar]
- 29.Johnson E.K., Nelson C.P. Values and pitfalls of the use of administrative databases for outcomes assessment. J Urol. 2013;190:17–18. doi: 10.1016/j.juro.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunness J.S. Reading newsprint but not headlines: pitfalls in measuring visual acuity and color vision in patients with bullseye maculopathy and other macular scotomas. Retin Cases Brief Rep. 2008;2:83–84. doi: 10.1097/IAE.0b013e31802fa25d. [DOI] [PubMed] [Google Scholar]
- 31.Künzel S.H., Lindner M., Sassen J., et al. Association of reading performance in geographic atrophy secondary to age-related macular degeneration with visual function and structural biomarkers. JAMA Ophthalmol. 2021;139:1191–1199. doi: 10.1001/jamaophthalmol.2021.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owsley C., McGwin G., Jr. Vision-targeted health related quality of life in older adults: patient-reported visibility problems in low luminance activities are more likely to decline than daytime activities. BMC Ophthalmol. 2016;16:92. doi: 10.1186/s12886-016-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz-Valckenberg S. The journey of “geographic atrophy” through past, present, and future. Ophthalmologica. 2017;237:11–20. doi: 10.1159/000455074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.