Figure 2.
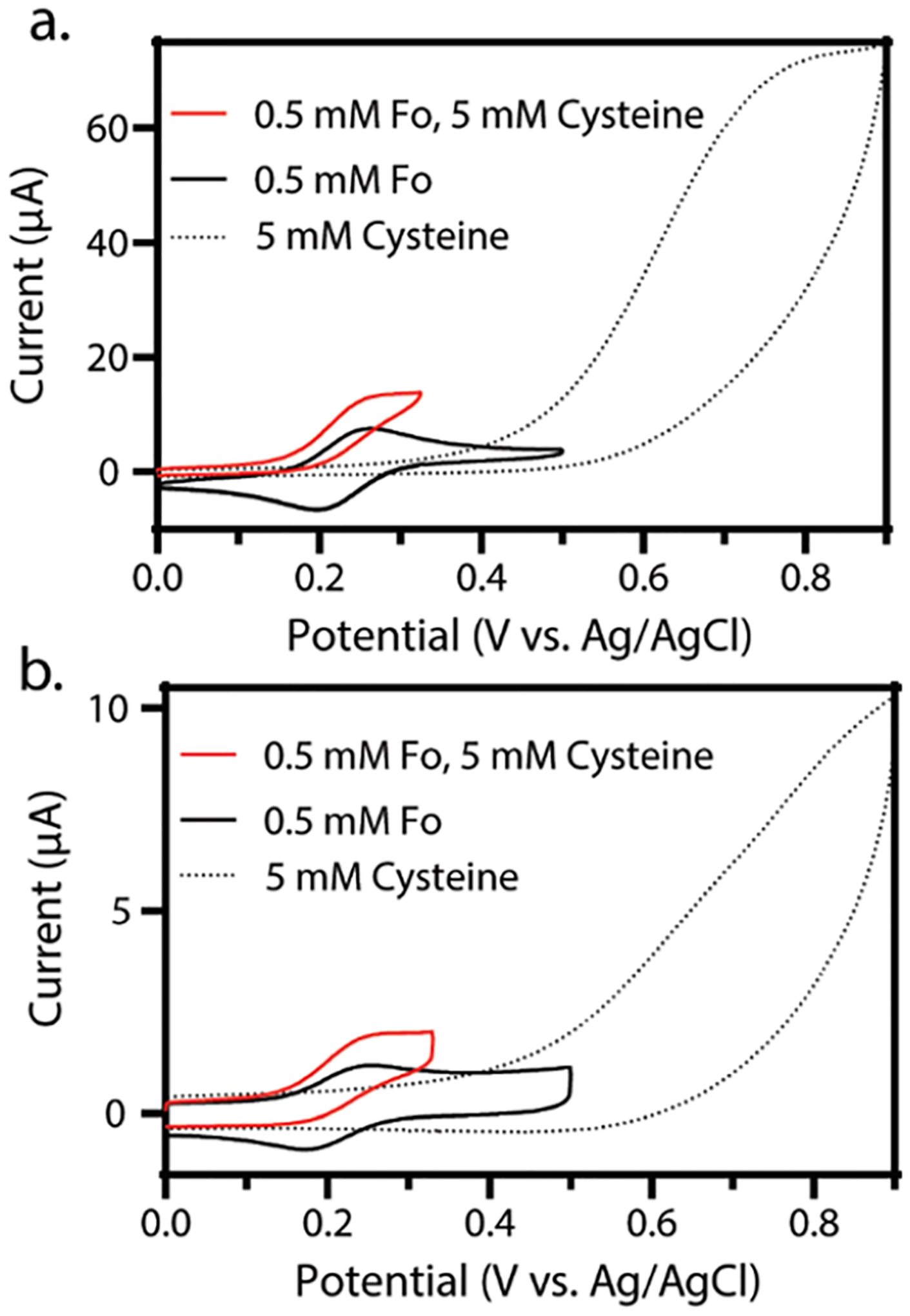
Overlaid cyclic voltammograms showing the voltammetry of hexacyanoferrate(II) (black), cysteine (black dotted), and hexacyanoferrate(II) in the presence of cysteine (red) in 50 mM PB, 0.5 M KCl (pH = 6.4). (a) Bulk, continuous water—cyclic voltammetry of 0.5 mM hexacyanoferrate(II) (black), 5 mM cysteine (black dots), and 0.5 mM hexacyanoferrate(II) and 5 mM cysteine (red). (b) Microliter water droplet—cyclic voltammetry of 0.5 mM hexacyanoferrate(II) (black), 5 mM cysteine (black dots), and 0.5 mM hexacyanoferrate(II) and 5 mM cysteine (red). For all voltammograms in this figure, three electrodes were used: working, reference, and counter electrodes of glassy carbon, Ag/AgCl, and platinum coil, respectively. The scan rate for all experiments is 0.1 V/s, and in line with IUPAC convention, the anodic current is plotted as positive.
