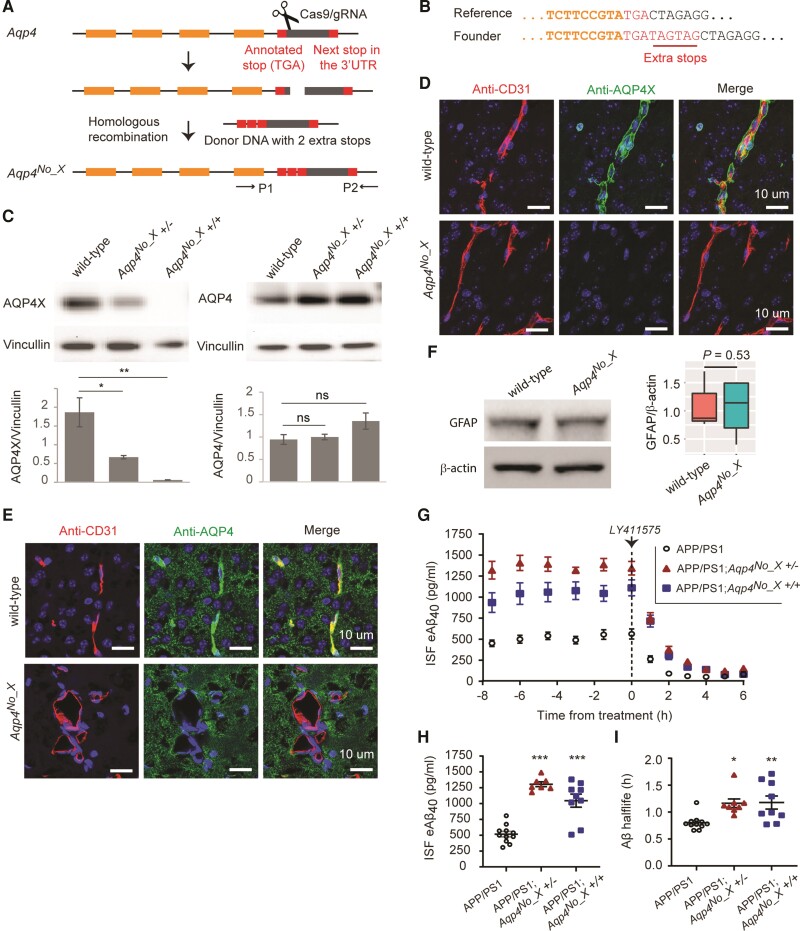
Figure 1.
AQPX regulates clearance of Aβ. (A) CRISPR–Cas9 for generating the Aqp4No_X mouse. A double-strand break is made using a gRNA and two extra stop codons are introduced using a donor oligo for homologous recombination. P1 and P2 indicate primers designed to screen the founders. (B) Genotyping of founder mice. Illumina sequencing read from a founder is shown. (C) Western blot to validate mice. Note that anti-AQP4 recognizes both normal and readthrough AQP4s and hence continues to generate signal in the Aqp4No_X mouse. n = 3, t-test. (D and E) Characterization of Aqp4No_X mice. Perivascular AQP4, i.e. AQP4X, is lost while parenchymal AQP4 is intact in mutant mice. CD31 marks endothelial cells. Blue = nuclear staining with DAPI. (F) No gliosis is observed in mutant mice, as apparent from GFAP western blot. n = 3, t-test. (G) Steady-state exchangeable hippocampal ISF Aβ (eAβ) was measured longitudinally using in vivo microdialysis for 7.5 h followed by administration of a γ-secretase inhibitor (LY411575, 3 mg/kg intraperitoneally) then Aβ measured hourly for an additional 6 h. (H) Steady-state ISF eAβ concentration in APP/PS1 Aqp4 wild-type mice is 516.0 ± 42.8 pg/ml and significantly elevated to 1305 ± 38.82 pg/ml and 1045 ± 102.9 in Aqp4No_X +/− and +/+, respectively. Concentrations are not significantly different between +/− and +/+. (I) ISF eAβ elimination half-life in APP/PS1 Aqp4 wild-type mice is 0.80 + 0.04 h and significantly prolonged to 1.16 ± 0.08 and 1.18 ± 0.12 h in Aqp4No_X +/− and +/+ mice, respectively. Data presented as mean ± SEM. See Supplementary Table 1 for n and statistical tests. See Supplementary Fig. 4 for full images of blots used to prepare C and F. ***P ≤ 0.0001; **P ≤ 0.01; *P ≤ 0.05.