Figure 2.
Potential for the field to build off of INTEGRATE-Pain accomplishments.
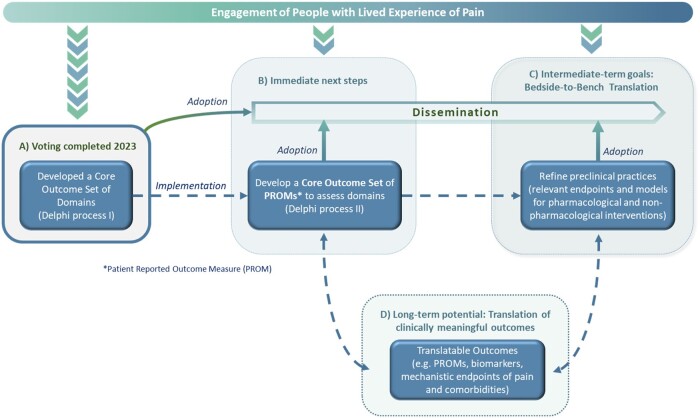
INTEGRATE-Pain has completed one major effort and has additional short- and long-term goals. Because partnering with people who have lived experience of pain improves the relevance and impact of initiatives related to pain research and care, these stakeholders have been included early on and throughout the entire process of INTEGRATE-Pain s initiatives, deliverables, and events. A: In early 2023, INTEGRATE-Pain completed the final round of a Delphi process to achieve consensus on Core Outcome Sets (COS) of domains for pain research. B: The immediate next steps for consortium members will be to disseminate the results of the Delphi process to interested stakeholders. This includes two journal articles in preparation, presentations at scientific meetings, webinars for research, clinical, and advocacy organizations, and educational materials for people with lived experience of pain that will be co-produced with lived experience expert members of our Advisory Committee. To ultimately implement the COS, consortium members or other stakeholders, will determine an appropriate set of patient-reported outcomes (PROM). C: These COS can inform ongoing efforts for bedside-to-bench back-translation to refine condition-specific preclinical models and validate clinically relevant endpoints to better model the experience of pain in humans. D: To advance the field in the long-term, collaborative pre-clinical and clinical approaches of INTEGRATE-Pain and the international pain community might further improve clinically meaningful outcomes for pain conditions and comorbidities. The model undertaken by the INTEGRATE-Pain Consortium integrates deliverables from all levels of pain research and pain care to ultimately produce and deliver clinically meaningful outcomes for people living with pain.