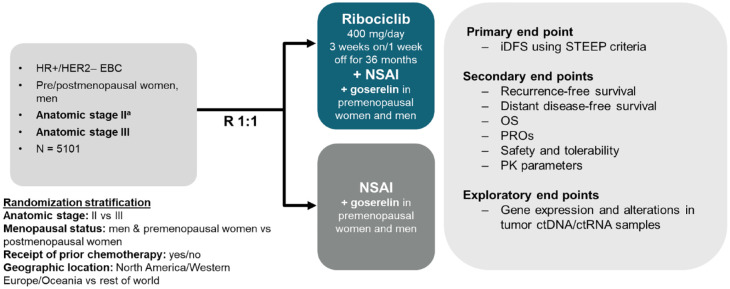
Figure 1.
Trial design.
aStage IIB or IIA that is either: N1 or N0 with: Grade 3 or Grade 2 with any of the following criteria: Ki67 ⩾ 20%, or Oncotype DX Breast Recurrence Score ⩾26, or categorized as high risk via Prosigna/PAM50, MammaPrint or EndoPredict EPclin Risk Score.
ctDNA, circulating tumor DNA; ctRNA, circulating tumor RNA; EBC, early nonmetastatic breast cancer; HER2−, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive; iDFS, invasive disease–free survival; NSAI, nonsteroidal aromatase inhibitor; OS, overall survival; PK, pharmacokinetic; PRO, patient-reported outcome; R, randomization; STEEP, Standardized Definitions for Efficacy End Points.