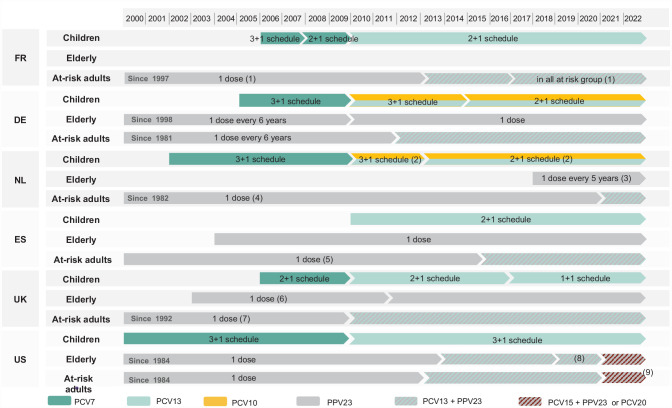
Figure 2.
Overview of pneumococcal vaccine recommendations in children, elderly, and adult risk groups.
PCV, Pneumococcal conjugate vaccine; PPV, Pneumococcal polysaccharide vaccine.
(1) Additional dose of PPV23 could be administered after 5 years. (2) PCV10 won national tender and is the only vaccine used in the national childhood immunization program. (3) Revaccination recommended every 5 years in adults from 60 to 75 years of age. (4) Revaccination after 5 years is desirable in people with asplenia, sickle cell disease, liquor leakage; should be consider for Hodgkin disease; may be considered for non-Hodgkin lymphoma. (5) Additional dose of PPV23 could be administered after 5 years for some immunocompromised persons. (6) In March 2011, the JCVI issued a statement for the discontinuation of the PPV23 elderly vaccination program. In July 2011, the JCVI finally recommended to maintain this vaccination strategy. (7) Additional dose of PPV23 should be administered after 5 years in people with long-term immunosuppression, chronical kidney disease and asplenia. (8) In 2019, the elderly recommendation was modified and PCV13 administration was recommended based on shared clinical decision-making. (9) Repeat PPV23 after 5 years for those with immunocompromising conditions.