Abstract
Background
There is a large body of evidence evaluating quality improvement (QI) programmes to improve care for adults living with diabetes. These programmes are often comprised of multiple QI strategies, which may be implemented in various combinations. Decision‐makers planning to implement or evaluate a new QI programme, or both, need reliable evidence on the relative effectiveness of different QI strategies (individually and in combination) for different patient populations.
Objectives
To update existing systematic reviews of diabetes QI programmes and apply novel meta‐analytical techniques to estimate the effectiveness of QI strategies (individually and in combination) on diabetes quality of care.
Search methods
We searched databases (CENTRAL, MEDLINE, Embase and CINAHL) and trials registers (ClinicalTrials.gov and WHO ICTRP) to 4 June 2019. We conducted a top‐up search to 23 September 2021; we screened these search results and 42 studies meeting our eligibility criteria are available in the awaiting classification section.
Selection criteria
We included randomised trials that assessed a QI programme to improve care in outpatient settings for people living with diabetes. QI programmes needed to evaluate at least one system‐ or provider‐targeted QI strategy alone or in combination with a patient‐targeted strategy.
‐ System‐targeted: case management (CM); team changes (TC); electronic patient registry (EPR); facilitated relay of clinical information (FR); continuous quality improvement (CQI).
‐ Provider‐targeted: audit and feedback (AF); clinician education (CE); clinician reminders (CR); financial incentives (FI).
‐ Patient‐targeted: patient education (PE); promotion of self‐management (PSM); patient reminders (PR). Patient‐targeted QI strategies needed to occur with a minimum of one provider or system‐targeted strategy.
Data collection and analysis
We dual‐screened search results and abstracted data on study design, study population and QI strategies. We assessed the impact of the programmes on 13 measures of diabetes care, including: glycaemic control (e.g. mean glycated haemoglobin (HbA1c)); cardiovascular risk factor management (e.g. mean systolic blood pressure (SBP), low‐density lipoprotein cholesterol (LDL‐C), proportion of people living with diabetes that quit smoking or receiving cardiovascular medications); and screening/prevention of microvascular complications (e.g. proportion of patients receiving retinopathy or foot screening); and harms (e.g. proportion of patients experiencing adverse hypoglycaemia or hyperglycaemia). We modelled the association of each QI strategy with outcomes using a series of hierarchical multivariable meta‐regression models in a Bayesian framework. The previous version of this review identified that different strategies were more or less effective depending on baseline levels of outcomes. To explore this further, we extended the main additive model for continuous outcomes (HbA1c, SBP and LDL‐C) to include an interaction term between each strategy and average baseline risk for each study (baseline thresholds were based on a data‐driven approach; we used the median of all baseline values reported in the trials). Based on model diagnostics, the baseline interaction models for HbA1c, SBP and LDL‐C performed better than the main model and are therefore presented as the primary analyses for these outcomes. Based on the model results, we qualitatively ordered each QI strategy within three tiers (Top, Middle, Bottom) based on its magnitude of effect relative to the other QI strategies, where 'Top' indicates that the QI strategy was likely one of the most effective strategies for that specific outcome. Secondary analyses explored the sensitivity of results to choices in model specification and priors.
Additional information about the methods and results of the review are available as Appendices in an online repository. This review will be maintained as a living systematic review; we will update our syntheses as more data become available.
Main results
We identified 553 trials (428 patient‐randomised and 125 cluster‐randomised trials), including a total of 412,161 participants. Of the included studies, 66% involved people living with type 2 diabetes only. Participants were 50% female and the median age of participants was 58.4 years. The mean duration of follow‐up was 12.5 months. HbA1c was the commonest reported outcome; screening outcomes and outcomes related to cardiovascular medications, smoking and harms were reported infrequently. The most frequently evaluated QI strategies across all study arms were PE, PSM and CM, while the least frequently evaluated QI strategies included AF, FI and CQI. Our confidence in the evidence is limited due to a lack of information on how studies were conducted.
Four QI strategies (CM, TC, PE, PSM) were consistently identified as 'Top' across the majority of outcomes. All QI strategies were ranked as 'Top' for at least one key outcome. The majority of effects of individual QI strategies were modest, but when used in combination could result in meaningful population‐level improvements across the majority of outcomes. The median number of QI strategies in multicomponent QI programmes was three.
Combinations of the three most effective QI strategies were estimated to lead to the below effects:
‐ PR + PSM + CE: decrease in HbA1c by 0.41% (credibility interval (CrI) ‐0.61 to ‐0.22) when baseline HbA1c < 8.3%;
‐ CM + PE + EPR: decrease in HbA1c by 0.62% (CrI ‐0.84 to ‐0.39) when baseline HbA1c > 8.3%;
‐ PE + TC + PSM: reduction in SBP by 2.14 mmHg (CrI ‐3.80 to ‐0.52) when baseline SBP < 136 mmHg;
‐ CM + TC + PSM: reduction in SBP by 4.39 mmHg (CrI ‐6.20 to ‐2.56) when baseline SBP > 136 mmHg;
‐ TC + PE + CM: LDL‐C lowering of 5.73 mg/dL (CrI ‐7.93 to ‐3.61) when baseline LDL < 107 mg/dL;
‐ TC + CM + CR: LDL‐C lowering by 5.52 mg/dL (CrI ‐9.24 to ‐1.89) when baseline LDL > 107 mg/dL.
Assuming a baseline screening rate of 50%, the three most effective QI strategies were estimated to lead to an absolute improvement of 33% in retinopathy screening (PE + PR + TC) and 38% absolute increase in foot screening (PE + TC + Other).
Authors' conclusions
There is a significant body of evidence about QI programmes to improve the management of diabetes. Multicomponent QI programmes for diabetes care (comprised of effective QI strategies) may achieve meaningful population‐level improvements across the majority of outcomes. For health system decision‐makers, the evidence summarised in this review can be used to identify strategies to include in QI programmes. For researchers, this synthesis identifies higher‐priority QI strategies to examine in further research regarding how to optimise their evaluation and effects. We will maintain this as a living systematic review.
Keywords: Adult; Female; Humans; Male; Middle Aged; Bayes Theorem; Cholesterol, LDL; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Glycated Hemoglobin; Quality Improvement; Retinal Diseases
Plain language summary
Quality improvement strategies for diabetes care: Effects on outcomes for adults living with diabetes
Key messages
‐ Quality improvement programmes can improve diabetes care, especially when multiple strategies are used in combination.
‐ Strategies used in these programmes that lead to the largest improvements in key outcomes in people with diabetes are: case management, team changes, patient education and promotion of self‐management.
Why is improving diabetes care important?
Diabetes, a disorder of how sugar is managed by the body, can lead to complications such as heart disease and blindness. If people with diabetes get the best possible treatment, their risk for these and other diabetes‐related complications will be lowered. Unfortunately, many people with diabetes do not get the best possible treatment.
What are quality improvement strategies?
Quality improvement programmes using different strategies help healthcare professionals improve care. We examined 12 common types of quality improvement strategies.
‐ Four strategies were directed at healthcare professionals: audit and feedback, clinician education, clinician reminders and financial incentives.
‐ Three strategies were directed at people living with diabetes: patient education, patient reminders and promotion of self‐management.
‐ Five strategies involved healthcare organisations: case management, team changes, electronic patient registry, facilitated relay of clinical information and continuous quality improvement.
What did we want to find out?
We wanted to find out which strategies worked best to improve:
‐ blood sugar control (measured using a test called glycated haemoglobin or HbA1c);
‐ blood pressure;
‐ low‐density lipoprotein cholesterol (LDL‐C).
Lower levels on these tests are associated with lower rates of complications such as heart attacks.
We also assessed whether quality improvement strategies improved rates of screening for eye damage (also known as retinopathy) and loss of sensation in the foot (also known as neuropathy). Routine screening for these issues in people living with diabetes is recommended to prevent blindness or amputation, respectively.
What did we do? We searched for randomised trials including adults living with diabetes managed in outpatient settings, which evaluated at least one quality improvement strategy. Although we were interested in strategies directed at people living with diabetes, patient strategies needed to be tested in combination with strategies directed at healthcare organisations or professionals for the study to be included. We summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods, size and other considerations.
What did we find?
We found 553 studies that involved 412,161 people with diabetes up to the year 2019. Studies took place in countries around the world with most being conducted in the USA (231) and in medical settings.
Most studies (367) involved people with type 2 diabetes. Half of the study participants were female. The average age of participants was 57 years. Most studies lasted 12 months.
Studies usually used multiple quality improvement strategies together. Most commonly, studies featured three quality improvement strategies.
Main results
Overall, case management, team changes, patient education and promotion of self‐management appeared to be the most effective quality improvement strategies for diabetes care.
When considering three‐strategy combinations (the median number of quality improvement strategies in multicomponent interventions), the combination of clinician education, promotion of self‐management and patient reminders may lead to the most improvement in blood sugar control in people who begin with lower HbA1c. Whereas the combination of case management, patient education and electronic patient registries may lead to the largest improvement in blood sugar control for people who begin with higher HbA1c.
For blood pressure, people who have lower systolic blood pressure may see the most improvement with the combination of patient education, team changes and promotion of self‐management. People who have higher systolic blood pressure may improve the most with the combination of case management, team changes and promotion of self‐management.
For cholesterol, we found that team changes, patient education and case management may lead to the most improvement in people who already have lower low‐density lipoprotein levels. For those who have higher levels of low‐density lipoprotein, team changes, case management and clinician reminders may lead to the largest improvement.
Patient education, patient reminders and team changes may lead to an increase in retinopathy screening rates. Patient education, team changes and audit and feedback, financial incentives and continuous quality improvement strategies combined may lead to an increase in foot screening rates.
What does this mean?
Clinics can improve their diabetes care by engaging in quality improvement programmes (especially those including case management, team changes, patient education and patient self‐management).
What are the limitations of the evidence?
Many studies did not provide information on everything we were interested in. Most focused on blood sugar control and few studies reported screening rates. We included studies in this review that had important flaws in the way they were conducted, which limits how confident we can be in our findings.
How up‐to‐date is this evidence?
The evidence for this review is up‐to‐date to June 2019, and we have further searched for and screened studies up to September 2021. We are currently working on a living systematic review that will be updated with new evidence at least once a year.
Summary of findings
Summary of findings 1. Case management compared to no case management for diabetes quality improvement.
| Outcome | Anticipated absolute effects (95% CrI) | № of participants (studies) | Certainty | ||||
| Post‐treatment mean with no case management | Difference with case management | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.01% lower (‐0.08 lower to 0.07 higher) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.27% lower (0.39 lower to 0.15 lower) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.35 mmHg lower (1.40 lower to 0.74 higher) | 36,772 (125 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| SBP (> 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 1.89 mmHg lower (3.32 lower to 0.41 lower) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = to 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 1.60 mg/dL lower (3.24 lower to 0.00 higher) | 59,777 (99 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 2.08 mg/dL lower (4.08 lower to 0.09 lower) | 40,766 (87 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| Outcome | N received case management | N did not receive case management | N screened after receiving case management | N screened after not receiving case management | Odds ratio | № of participants (studies) | Certainty |
| Retinopathy screening | 3179 | 35,975 | 2071 | 14,256 | 1.09 (0.66 to 1.78) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 1568 | 27,617 | 1047 | 17,102 | 1.09 (0.59 to 1.83) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+)
Setting: outpatient care
Intervention: case management
Comparison: no case management
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 11.7 (1 to 96)
Baseline > 8.3: 10.6 (1 to 84)
SBP:
Baseline < 136: 11.9 (3 to 96)
Baseline ≥ 136: 13.2 (1 to 60)
LDL:
Baseline < 107: 10.4 (3 to 30)
Baseline ≥ 107: 14.4 (3 to 84)
Retinopathy screening: 12.9 (3 to 24)
Foot screening: 14.1 (12 to 14)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Summary of findings 2. Team changes compared to no team changes for diabetes quality improvement.
| Outcomes | Anticipated absolute effects* (95% CrI) | № of participants (studies) | Certainty of the evidence (GRADE) | ||||
| Post‐treatment mean with no team changes | Difference with team changes | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.11% lower (0.21 lower to 0.02 lower) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.11% lower (0.24 lower to 0.03 higher) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.36 mmHg (130.03 to 131.29) | MD 0.91 mmHg lower (2.10 lower to 0.29 higher) | 36,772 (125 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| SBP > 136 mmHg | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 1.81 mmHg lower (3.30 lower to 0.32 lower) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = to 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 2.24 mg/dL lower (3.97 lower to 0.57 lower) | 59,777 (99 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 3.07 mg/dL lower (5.29 lower to 0.84 lower) | 40,766 (87 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcomes | N received team changes | N did not receive team changes | N screened after receiving team changes | N screened after not receiving team changes | Odds ratio | № of participants (studies) | Certainty of the evidence (GRADE) |
| Retinopathy screening | 2345 | 36,809 | 1641 | 14,686 | 1.60 (0.89 to 2.79) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 1454 | 27,731 | 1011 | 17,138 | 2.01 (0.92 to 4.01) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+)
Setting: outpatient care
Intervention: team changes
Comparison: no team changes
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 14.1 (3 to 96)
Baseline > 8.3: 11.8 (1 to 160)
SBP:
Baseline < 136: 13.8 (3 to 96)
Baseline ≥ 136: 14.9 (1 to 48)
LDL:
Baseline < 107: 11.5 (3 to 30)
Baseline ≥ 107: 16.2 (4 to 160)
Retinopathy screening: 13.8 (6 to 24)
Foot screening: 14.7 (12 to 14)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Summary of findings 3. Electronic patient registries compared to no electronic patient registries for diabetes quality improvement.
| Outcomes | Anticipated absolute effects* (95% CrI) | № of participants (studies) | Certainty of the evidence (GRADE) | ||||
| Post‐treatment mean with no electronic patient registries | Difference with electronic patient registries | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.11% lower (0.20 lower to 0.01 lower) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.17% lower (0.33 lower to 0.02 lower) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.08 mmHg lower (1.47 lower to 1.24 higher) | 36,772 (125 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| SBP (> 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 1.01 mmHg higher (0.96 lower to 2.95 higher) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = to 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 0.20 mg/dL higher (1.69 lower to 2.20 higher) | 59,777 (99 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 2.10 mg/dL higher (0.74 lower to 4.85 higher) | 40,766 (87 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcomes | N received electronic patient registry | N did not receive electronic patient registry | N screened after receiving electronic patient registry | N screened after not receiving electronic patient registry | Odds ratio | № of participants (studies) | Certainty of the evidence (GRADE) |
| Retinopathy screening | 2979 | 36,175 | 1680 | 14,647 | 1.39 (0.68 to 2.43) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 1995 | 27,190 | 673 | 17,476 | 0.95 (0.35 to 2.42) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+)
Setting: outpatient care
Intervention: electronic patient registries
Comparison: no electronic patient registries
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 12.2 (2 to 36)
Baseline > 8.3: 8.2 (3 to 28)
SBP:
Baseline < 136: 13.9 (3 to 160)
Baseline ≥ 136: 16.1 (3 to 60)
LDL:
Baseline < 107: 10.0 (3 to 36)
Baseline ≥ 107: 14.0 (3 to 60)
Retinopathy screening: 15.1 (1 to 30)
Foot screening: 13.4 (1 to 24)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression. Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Summary of findings 4. Clinician education compared to no clinician education for diabetes quality improvement.
| Outcome | Anticipated absolute effects (95% CrI) | № of participants (studies) | Certainty | ||||
| Post‐treatment mean with no clinician education | Difference with clinician education | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.13% lower (0.24 lower to 0.01 lower) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.06% higher (0.15 lower to 0.30 higher) | 51,973 (234 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.26 mmHg higher (1.11 lower to 1.69 higher) | 36,772 (125 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| SBP (> 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 2.05 mmHg higher (0.62 higher to 3.45 higher) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = to 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 1.18 mg/dL higher (0.73 lower to 3.15 higher) | 59,777 (99 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 0.49 mg/dL lower (3.27 lower to 2.39 higher) | 40,766 (87 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcome | N received clinician education | N did not receive clinician education | N screened after receiving clinician education | N screened after not receiving clinician education | Odds ratio | № of participants (studies) | Certainty |
| Retinopathy screening | 23,392 | 15,762 | 8696 | 7631 | 1.16 (0.82 to 1.63) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 10,468 | 18,717 | 7191 | 10,958 | 1.03 (0.75 to 1.47) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population:adults with diabetes (age 18+) Setting: outpatient care Intervention: clinician education Comparison: no clinician education
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 17.9 (1 to 60)
Baseline > 8.3: 12.5 (3 to 26)
SBP:
Baseline < 136: 20.2 (6 to 160)
Baseline ≥ 136: 17.3 (3 to 60)
LDL:
Baseline < 107: 15.3 (6 to 36)
Baseline ≥ 107: 18.6 (10 to 60)
Retinopathy screening: 15.6 (12 to 30)
Foot screening: 14.2 (12 to 21)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI).
CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Groupgrades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations(https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Summary of findings 5. Clinician reminders compared to no clinician reminders for diabetes quality improvement.
| Outcome | Anticipated absolute effects (95% CrI) | № of participants (studies) | Certainty | ||||
| Post‐treatment mean with no clinician reminders | Difference with clinician reminders | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.09% higher (0.02 lower to 0.20 higher) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.09% lower (0.34 lower to 0.15 higher) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.17 mmHg higher (1.82 lower to 1.46 higher) | 36,772 (125 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (> 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 0.20 mmHg lower (2.25 lower to 1.62 higher) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 0.74 mg/dL higher (1.51 lower to 2.98 higher) | 64,072 (109 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 0.38 mg/dL lower (3.05 lower to 2.30 higher) | 41,207 (89 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcome | N received clinician reminders | N did not receive clinician reminders | N screened after receiving clinician reminders | N screened after not receiving clinician reminders | Odds ratio | № of participants (studies) | Certainty |
| Retinopathy screening | 3118 | 36,036 | 1752 | 14,575 | 1.10 (0.70 to 2.09) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 2658 | 26,527 | 903 | 17,246 | 1.30 (0.71 to 2.57) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+) Setting: outpatient care Intervention: clinician reminders Comparison: no clinician reminders
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 14.5 (3 to 36)
Baseline > 8.3: 10.8 (3 to 28)
SBP:
Baseline < 136: 16.5 (3 to 160)
Baseline ≥ 136: 15.4 (6 to 28)
LDL:
Baseline < 107: 15.8 (6 to 36)
Baseline ≥ 107: 14.9 (6 to 36)
Retinopathy screening: 13.9 (1 to 30)
Foot screening: 13.9 (1 to 24)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI).
CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Summary of findings 6. Facilitated relay of information compared to no facilitated relay of information for diabetes quality improvement.
| Outcome | Anticipated absolute effects (95% CrI) | № of participants (studies) | Certainty | ||||
| Post‐treatment mean with no facilitated relay of information | Difference with facilitated relay of information | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.05% lower (0.14 lower to 0.03 higher) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.04% lower (0.18 lower to 0.10 higher) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.32 mmHg lower (1.48 lower to 0.83 higher) | 36,772 (125 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (> 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 0.42 mmHg lower (2.22 lower to 1.41 higher) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 1.20 mg/dL lower (2.91 lower to 0.49 higher) | 59,777 (99 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 0.32 mg/dL higher (2.03 lower to 2.80 higher) | 40,766 (87 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcome | N received facilitated relay | N did not receive facilitated relay | N screened after receiving facilitated relay | N screened after not receiving facilitated relay | Odds ratio | № of participants (studies) | Certainty |
| Retinopathy screening | 1898 | 37,256 | 1058 | 15,269 | 1.51 (0.57 to 3.65) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 638 | 28,547 | 397 | 17,752 | 0.85 (0.35 to 2.16) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+) Setting: outpatient care Intervention: facilitated relay of information Comparison: no facilitated relay of information
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 9.8 (2 to 36)
Baseline > 8.3: 8.8 (1 to 30)
SBP:
Baseline < 136: 9.5 (3 to 36)
Baseline ≥ 136: 11.3 (1 to 30)
LDL:
Baseline < 107: 10.5 (3 to 30)
Baseline ≥ 107: 9.9 (3 to 28)
Retinopathy screening: 11.1 (3 to 18)
Foot screening: 11.5 (6 to 15)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI).
CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Summary of findings 7. Patient education compared to no patient education for diabetes quality improvement.
| Outcome | Anticipated absolute effects (95% CrI) | № of participants (studies) | Certainty | ||||
| Post‐treatment mean with no patient education | Difference with patient education | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.02% higher (0.07 lower to 0.10 higher) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.17% lower (0.30 lower to 0.05 lower) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.71 mmHg lower (1.71 lower to 0.28 higher) | 36,772 (125 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (> to 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 0.12 mmHg lower (1.47 lower to 1.22 higher) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = to 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 1.89 mg/dL lower (3.52 lower to 0.26 lower) | 59,777 (99 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 1.83 mg/dL higher (0.19 lower to 3.86 higher) | 40,766 (87 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcome | N received patient education | N did not receive patient education | N screened after receiving patient education | N screened after not receiving patient education | Odds ratio | № of participants (studies) | Certainty |
| Retinopathy screening | 24,487 | 14,667 | 10,154 | 6173 | 1.76 (1.07 to 2.96) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 2108 | 27,077 | 1540 | 16,609 | 2.32 (1.09 to 5.13) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+) Setting: outpatient care Intervention: patient education Comparison: no patient education
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 12.0 (1 to 96)
Baseline > 8.3: 10.4 (1 to 84)
SBP:
Baseline < 136: 11.3 (3 to 96)
Baseline ≥ 136: 14.1 (1 to 60)
LDL:
Baseline < 107: 9.8 (3 to 30)
Baseline ≥ 107: 12.8 (3 to 84)
Retinopathy screening: 13.3 (6 to 24)
Foot screening: 13.5 (12 to 24)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI).
CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Summary of findings 8. Promotion of self‐management compared to no promotion of self‐management for diabetes quality improvement.
| Outcome | Anticipated absolute effects (95% CrI) | № of participants (studies) | Certainty | ||||
| Post‐treatment mean with no promotion of self‐management | Difference with promotion of self‐management | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.14% lower (0.25 lower to 0.06 lower) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.13% lower (0.24 lower to 0.00 lower) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.53 mmHg lower (1.60 lower to 0.54 higher) | 36,772 (125 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| SBP (> 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 0.69 mmHg lower (2.23 lower to 0.86 higher) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = to 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 0.34 mg/dL lower (2.03 lower to 1.32 higher) | 59,777 (99 RCTs) | ⨁◯◯◯ Very lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 0.23 mg/dL higher (1.94 lower to 2.43 higher) | 40,766 (87 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcome | N received promotion of self‐management | N did not receive promotion of self‐management | N screened after receiving promotion of self‐management | N screened after not receiving promotion of self‐management | Odds ratio | № of participants (studies) | Certainty |
| Retinopathy screening | 2507 | 36,647 | 1858 | 14,469 | 1.29 (0.67 to 2.46) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 1435 | 27,750 | 1133 | 17,016 | 1.28 (0.47 to 3.42) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+) Setting: outpatient care Intervention: promotion of self‐management Comparison: no promotion of self‐management
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 10.6 (1 to 96)
Baseline > 8.3: 9.7 (1 to 60)
SBP:
Baseline < 136: 10.4 (3 to 96)
Baseline ≥ 136: 13.1 (1 to 60)
LDL:
Baseline < 107: 9.6 (3 to 30)
Baseline ≥ 107: 11.9 (3 to 60)
Retinopathy screening: 12.8 (3 to 24)
Foot screening: 13.5 (12 to 24)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI).
CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Summary of findings 9. Patient reminders compared to no patient reminders for diabetes quality improvement.
| Outcome | Anticipated absolute effects (95% CrI) | № of participants (studies) | Certainty | ||||
| Post‐treatment mean with no patient reminders | Difference with patient reminders | ||||||
| HbA1c (< or = to 8.3%) | The mean HbA1c was 7.48% (7.42 to 7.55) | MD 0.14% lower (0.25 lower to 0.03 lower) | 129,327 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| HbA1c (> 8.3%) | The mean HbA1c was 8.70% (8.59 to 8.81) | MD 0.01% lower (0.19 lower to 0.16 higher) | 51,973 (234 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (< or = to 136 mmHg) | The mean SBP was 130.66 mmHg (130.03 to 131.29) | MD 0.45 mmHg higher (1.08 lower to 1.83 higher) | 36,772 (125 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| SBP (> 136 mmHg) | The mean SBP was 138.53 mmHg (137.74 to 139.30) | MD 0.61 mmHg higher (1.28 lower to 2.58 higher) | 59,285 (118 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (< or = to 107 mg/dL) | The mean LDL was 94.46 mg/dL (93.48 to 95.47) | MD 0.70 mg/dL higher (1.15 lower to 2.63 higher) | 59,777 (99 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| LDL (> 107 mg/dL) | The mean LDL was 108.48 mg/dL (107.26 to 109.69) | MD 1.02 mg/dL higher (1.48 lower to 3.53 higher) | 40,766 (87 RCTs) | ⨁⨁◯◯ Lowa,b | |||
| Outcome | N received patient reminders | N did not receive patient reminders | N screened after receiving patient reminders | N screened after not receiving patient reminders | Odds ratio | № of participants (studies) | Certainty |
| Retinopathy screening | 23,703 | 15,451 | 10464 | 5863 | 1.70 (0.79 to 3.57) | 39,154 (58 RCTs) | ⨁◯◯◯ Very lowa,b,c |
| Foot screening | 870 | 28,315 | 442 | 17,707 | 1.39 (0.46 to 3.70) | 29,185 (43 RCTs) | ⨁◯◯◯ Very lowa,b,c |
Patient or population: adults with diabetes (age 18+) Setting: outpatient care Intervention: patient reminders Comparison: no patient reminders
Duration of follow‐up (months) ‐ mean (range):
HbA1c:
Baseline < 8.3: 11.9 (2 to 60)
Baseline > 8.3: 11.1 (3 to 60)
SBP:
Baseline < 136: 9.5 (3 to 13)
Baseline ≥ 136: 14.6 (3 to 60)
LDL:
Baseline < 107: 10.1 (3 to 30)
Baseline ≥ 107: 12.5 (3 to 60)
Retinopathy screening: 13.8 (3 to 30)
Foot screening: 14.0 (6 to 24)
*The risk in the intervention group (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI).
CrI: credible interval; HbA1c: glycated haemoglobin; LDL: low‐density lipoprotein; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; SBP: systolic blood pressure
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations Average baseline risk for each study at baseline was defined as high or low using the median average value for studies as the cutoff.
Reporting of harms was too infrequent and was too variable to properly assess and therefore was not included in the summary of findings tables.
aRefers to the GRADE domain 'inconsistency'. We downgraded all findings for this due to the variation observed in parameter estimates.
bRefers to the GRADE domain 'indirectness'. We downgraded all findings for this due to parameters being estimated predominantly on indirect evidence and due to concerns about the applicability of these findings because of heterogeneity of interventions and populations (https://gdt.gradepro.org/app/handbook/handbook.html).
cRefers to the GRADE domain 'imprecision'. We downgraded only the screening outcome findings due to the small sample sizes for these outcomes, which led to imprecise findings in the meta‐regression.
Background
Description of the condition
Worldwide, diabetes is a leading cause of premature mortality, blindness, renal failure, amputations, and an important contributor to cardiovascular events. Over the past decade, the focus of diabetes care has increasingly shifted from a glucose‐centric approach to one focused on overall risk reduction.
The management of diabetes places burdens on patients, health professionals and health systems: patients are asked to spend time and resources on self‐management; health professionals are asked to titrate treatment and implement best practice guidelines; and health systems are expected to ensure evidence‐based policies are in place and appropriate supports are accessible to patients and their providers to enable the best possible outcomes.
Appropriate treatment (both behavioural and pharmacologic) can reduce the risk of poor outcomes for people living with diabetes (Gæde 2008). However, while guidelines provide evidence‐based recommendations to limit the risk of diabetes complications (e.g. American Diabetes Association 2022; NICE 2022a; NICE 2022b), studies around the world show substantial and persistent gaps in quality of care (Clemens 2021; Fang 2021; Leiter 2019; Mosenzon 2021; Rushforth 2016). This may not be surprising given the complex nature of ideal care for patients living with diabetes. Healthcare systems, health professionals, researchers and patients need to identify quality improvement (QI) programmes to improve the quality of care and reduce the risk of complications.
Description of the intervention
Healthcare systems worldwide are increasingly investing in QI programmes to improve care and outcomes for people living with diabetes. Typically, QI programmes consist of multiple QI strategies that may target or support patients, health professionals and/or system‐level healthcare service changes to promote the implementation of evidence‐based treatments.
For this review, we used an adaptation of the Cochrane Effective Practice and Organisation of Care (EPOC) group taxonomy to characterise the content of the QI programmes that was used in prior versions of this Cochrane Review (see Table 10) (Shojania 2006; Tricco 2012). Specifically, we considered QI programmes that featured one or more of 12 QI strategies targeted at patient, health professional and/or organisational levels to improve diabetes care and outcomes.
1. Taxonomy of quality improvement (QI) strategies.
| Strategy | Definition |
| QI strategies targeting health systems | |
| Case management (CM) |
Any system for co‐ordinating diagnosis, treatment or routine management of patients
(e.g. arrangement for referrals, follow‐up of test results) by a person or multidisciplinary team in collaboration with, or supplementary to, the primary care clinician. For a randomised controlled trial to qualify, the case management has to have happened more than once. If the study calls the intervention ‘case management,’ we classify it as such. Example: Home blood pressure telemonitoring plus frequent telephone‐based nurse case management. The intervention is delivered by HHC nurses who have real‐time access to patients’ EHRs and are in communication with their providers. The nurse case manager had access to the patients’ home BP data via a secure website, where the readings are displayed in easy‐to‐read charts and figures that highlight the control rate for each week. This information was used by the nurse case manager as a basis for counselling sessions with the patient (Grilo 2015). |
| Team changes (TC) |
Changes to the structure or organisation of the primary healthcare team are defined as present if they meet the following criteria:
Example: Professional nurses who successfully completed the educational outreach were authorised by the district manager to prescribe an additional seven medications for NCDs previously restricted to doctors (Fairall 2016). |
| Electronic patient registry (EPR) |
General electronic medical record system or electronic tracking system for patients with diabetes. We do not include websites unless patients were tracked over time. To qualify, the system has to have been part of the clinical trial as an intervention (i.e. not pre‐existing infrastructure unless used more actively). Example: Patients (and their healthcare team) could review laboratory data and recommendations from their physicians and nurses online (Yoon 2008). |
| Facilitated relay of clinical information (FR) |
Clinical information collected from patients and transmitted to clinicians by means other than the existing medical record. We exclude conventional means of correspondence between clinicians. For example, if the results of routine visits with a pharmacist were sent in a letter to the primary care physician, the use of routine visits with a pharmacist counts as a ‘team change,’ but the intervention does not count as ‘facilitated relay.’ However, if the pharmacist issued structured diaries for patients to record self‐monitored glucose values, which were then taken to office visits to review with the primary physician, we count the intervention as facilitated relay. Other examples include electronic or web‐based methods through which patients provide self‐care data and which clinicians reviewed, as well as point‐of‐care testing supplying clinicians with immediate HbA1c values. We include passports, referral systems and dietary information (versus purely clinical information). In general, the patient should be facilitating the relay. To be included, the information must have got to someone with prescribing or ordering ability. For example, if the nurse’s role was expanded to make drug changes, the patient had a portable personal record or ‘diabetes passport,’ and the nurse could directly make a change, we classify the intervention as case management and facilitated relay of clinical information (depending on the study and situation). If the nurse alerted the primary care provider that the patient had run out of drugs, we do not deem this facilitated relay of information because that is a normal part of a nurse’s role. Example: The internet program consisted of a central data repository that the patient or healthcare provider could access via a confidential password. Patients had their own unique profile, where they were able to enter data on BG measurements, diet, exercise, insulin and oral medications (Tjam 2006). |
| Continuous quality improvement (CQI) |
Interventions explicitly identified as involving the techniques of continuous QI, total quality management, or plan‐do‐study‐act, or any iterative process for assessing quality problems, developing solutions to those problems, testing their effects and then reassessing the need for further action. Example: A seven‐step QI process used involved a sequential "tests‐of‐ change" approach (O'Connor 2005). |
| QI strategies targeting health care providers | |
| Audit and feedback (AF) |
Summary of clinical performance of health care delivered by an individual clinician or clinic over a specified period, transmitted back to the clinician (e.g. the percentage of a clinician’s patients who achieved a target HbA1c concentration or who underwent dilated‐eye examinations with a specified frequency). This strategy is strictly based on clinical data and excludes clinical skills. It can include the number of patients with missing tests and dropouts. Example: Physicians received a printed list of all their patients living with diabetes every 4 months, prioritised based on distance from each patient’s A1C or LDL cholesterol goal (O'Connor 2009a). |
| Clinician education (CE) |
Interventions designed to promote increased understanding of principles guiding clinical care or awareness of specific recommendations for a target disorder or population of patients. Subcategories of clinician education include conferences or workshops, distribution of educational materials (e.g. written, video or other), and educational outreach visits (i.e. academic detailing). We exclude teaching how to educate patients, counselling skills, motivational interviewing, self‐directed learning and skills related to the intervention (e.g. teaching how to use the website for the randomised controlled trial). We include all health care providers. If the education was part of the individual’s role (e.g. teaching a case manager about diabetes), we do not categorise it as clinician education. Example: Nurses received half‐day training to review the evidence for patient‐centred consulting and a further full day in which to practice the skills learned. Doctors received only the first half day training (Kinmonth 1998). |
| Clinician reminders (CR) |
Paper‐based or electronic systems intended to prompt a health professional to recall patient‐specific information (e.g. most recent HbA1c value) or to do a specific task (e.g. foot examination). If the strategy was accompanied by a recommendation, we sub‐classify it as decision support (e.g. giving targets to health care providers). An example is a yellow piece of paper clipped to the medical record with the patient’s information on it. This approach has to have been systematic and part of the implementation of the intervention ‐ we exclude ad hoc clinician reminders. Example: A computerised decision support system with diagnostic and treatment algorithms based on the guidelines (Cleveringa 2008). |
| Financial incentives (FI) |
Interventions with positive or negative financial incentives directed at providers (e.g. linked to adherence to some process of care or achievement of some target outcome). This strategy also includes positive or negative financial incentives directed at patients or system‐wide changes in reimbursement (e.g. capitation, prospective payment, or a shift from fee‐for‐service to salary pay structure). Example: pay‐for‐performance programme was designed to create incentives for providers to deliver adequate care, especially regular checkups, for patients with diabetes (Hsu 2014). |
| QI strategies targeting patients | |
| Patient education (PE) |
Interventions designed to promote greater understanding of a target disorder or to teach specific prevention or treatment strategies, or specific in‐person education (e.g. individual or group sessions with diabetes nurse educator, distribution of printed or electronic educational materials). Interventions with education of patients are included only if they also include at least one other strategy related to clinician or organisational change. We do not include occasions of optional education. Example: Patients attended a group educational session (Wagner 2001). |
| Promotion of self‐management (PSM) |
Provision of equipment (e.g. home glucose meters) or access to resources (e.g. system for electronically transmitting home glucose measurements and receiving insulin dose changes based on those data) to promote self‐management. Interventions promoting self‐management are included only if they also include at least one other strategy related to clinician or organisational change. We also include established goals or a print off of a self‐management plan (i.e. did not necessarily require equipment or resources). If the study called the intervention promotion of self‐management, personalised goal‐setting or action‐planning, it is included here. In general, we perceive this as a more active strategy than education of patients.
Example: The intervention group was also given and taught how to use a pill box and a blood glucose meter to conduct self‐monitoring of blood glucose at home and to record their readings (Chung 2014). |
| Patient reminders (PR) |
Any effort (e.g. postcards or telephone calls) to remind patients about upcoming appointments or important aspects of self‐care (e.g. reminders to monitor glucose). Interventions with reminders are included only if they also included at least one other strategy related to clinician or organisational change. If the intervention included case management, patient reminders need to be explicit and to represent an extra task as compared to normal case management. Example: A central database system identified when patients were due for review and generated a letter to the patients (Eccles 2007). |
Pre‐defined QI strategies in previous review versions (Shojania 2006; Tricco 2012).
BG: blood glucose; BP: blood pressure; EHR: electronic health record; HHC: home health care; LDL: low‐density lipoprotein; NCD: non‐communicable disease; QI: quality improvement
In this review, given the focus on health services‐based programmes, and given the existence of numerous other reviews focusing on patient‐oriented strategies to improve diabetes care (Worswick 2013), we included programmes involving patient‐oriented QI strategies (i.e. patient education, promotion of self‐management and patient reminders) only if they also included strategies targeting organisations and/or health professionals.
How the intervention might work
Each QI strategy has a different mechanism of action that may influence the capability, opportunity and/or motivation of patients, healthcare professionals and healthcare systems (Michie 2011) to provide and act upon evidence‐based recommendations. For example, clinician education approaches typically address knowledge gaps of healthcare professionals (motivation). Patient self‐management typically addresses patients' beliefs about capabilities and skills (capability). Case management typically supports patients to access services appropriately (opportunity).
Given that there are often multiple barriers to evidence‐based diabetes care operating at different levels, QI programmes involving different QI strategies have the potential to address multiple barriers through the different mechanisms of action of the included QI strategies. The key challenge is to determine the main barriers in any given context and map these to the QI strategies likely to address them (French 2012).
The minimal clinically important difference for pharmaceutical interventions in diabetes is a reduction in HbA1c of 0.3% to 0.4% (Oh 2021). Given that QI programmes target populations of people living with diabetes, rather than individuals, and given that the decisions to implement these interventions are typically made by health system leaders responsible for large populations of patients rather than individual clinicians within a specific patient encounter, smaller effect sizes than those seen in clinical trials are both expected and can lead to significant population health improvements (Chambers 2013; Rose 1981).
Why it is important to do this review
Those involved in the design and implementation of QI programmes need an up‐to‐date evidence synthesis on the effects of different QI strategies on the quality of diabetes care.
This Cochrane Review updates two previous reviews, one published in 2006 (58 trials) (Shojania 2006) and one published in 2012 (142 trials) (Tricco 2012). These previous reviews suggested that QI programmes could lead to improved patient care and outcomes. They used standard (study‐level) meta‐analytic and meta‐regression approaches that were unable to cleanly unpack the effects of individual QI strategies in multicomponent QI programmes.
This Cochrane Review incorporates trials published since these previous reviews and uses recent advances in arm‐level meta‐regression approaches that allow full use of the data from multi‐arm studies and better reflect the structure of the data (i.e. QI strategies within a multicomponent programme in each arm of each trial) to isolate the contributions of each QI strategy to improvement in diabetes care and outcomes while controlling for the effects of other QI strategies. This allows the review to estimate the isolated effects of QI strategies (for example, what improvement in HbA1c is likely to occur because of the inclusion of a specific QI strategy in a QI programme) and the likely effects of combinations of QI strategies.
The review was registered in PROSPERO (CRD42013005165) and a protocol for this update was published in 2014 (Ivers 2014).
Objectives
To update existing systematic reviews of diabetes QI programmes and apply novel meta‐analytical techniques to estimate the effectiveness of QI strategies (individually and in combination) on diabetes quality of care.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐randomised trials (CRTs) and quasi‐randomised trials. CRTs were only included if they had a minimum of three clusters per arm. For cross‐over trials, we included data from the final time point before cross‐over.
Types of participants
Adults living with type 1 or type 2 diabetes treated in an outpatient setting. We excluded studies involving patients with gestational diabetes. Studies that included mixed populations (e.g. patients with diabetes or hypertension; adolescents and adults with diabetes) were included if the study reported at least one outcome of interest for a subgroup of adults living with diabetes or if adults living with diabetes comprised ≥ 90% of the study sample.
Types of interventions
Any QI programme that included at least one of the below healthcare provider‐ or healthcare system‐targeted QI strategies (see Table 10), as predefined in previous versions of this review (Shojania 2006; Tricco 2012). We included programmes involving patient‐oriented QI strategies (i.e. patient education, promotion of self‐management and patient reminders) only if they also included strategies targeting organisations and/or health professionals. Studies that only included patient‐directed QI strategies were excluded since many other reviews have synthesised evidence specifically for patient‐directed strategies (Captieux 2018; Duke 2009; Liu 2017; Stratton 2000).
Healthcare system‐targeted QI strategies: case management (CM), team changes (TC), electronic patient registry (EPR), facilitated relay of clinical information (FR), continuous quality improvement (CQI).
Healthcare provider‐targeted QI strategies: clinician education (CE), clinician reminders (CR), audit and feedback (AF), financial incentives (FI).
Patient‐targeted QI strategies: patient education (PE), patient reminders (PR), promotion of self‐management (PSM).
Types of outcome measures
We assessed the impact of the QI programmes on 13 outcomes representing four domains including: glycaemic control(mean % glycated haemoglobin (HbA1c));cardiovascular risk factor control (mean systolic blood pressure (SBP), diastolic blood pressure (DBP) or low‐density lipoprotein cholesterol (LDL‐C), proportions of patients on acetylsalicylic acid (ASA), statins or antihypertensives, proportions of patients to have hypertension control or patients who have quit smoking); screening for complications (proportions of patients undergoing retinopathy, foot or renal screening); and harms (proportion of patients experiencing hypoglycaemia or hyperglycaemia) (see Table 11).
2. Outcome definitions.
| Domain | Outcome name | Outcome measure | Data type |
| Glycaemic control | Glycated haemoglobin (HbA1c) | Mean HbA1c | Continuous |
| Vascular risk factor management ‐ outcome | Systolic blood pressure (SBP) | Mean SBP | Continuous |
| Diastolic blood pressure (DBP) | Mean DBP | Continuous | |
| Hypertensive control | Proportion with hypertension control | Dichotomous | |
| Low‐density lipoprotein (LDL) | Mean LDL | Continuous | |
| Smoking cessation | Proportion smoking | Dichotomous | |
| Vascular risk factor management ‐ process | Acetylsalicylic acid (ASA) | Proportion on ASA | Dichotomous |
| Statins | Proportion on statins | Dichotomous | |
| Antihypertensives | Proportion on hypertensives | Dichotomous | |
| Screening for complications | Retinopathy screening | Proportion screened | Dichotomous |
| Foot screening | Proportion screened | Dichotomous | |
| Renal screening | Proportion screened | Dichotomous | |
| Adverse glycaemic events | Hypoglycaemia or hyperglycaemia | Proportion with adverse hypoglycaemic or hyperglycaemic event | Dichotomous |
Primary outcomes
The primary outcomes of interest were:
-
measures related to vascular risk factor management:
glycaemic control, as measured by post‐intervention mean HbA1c (%);
SBP;
LDL‐C;
-
screening for complications;
retinopathy;
foot screening;
renal screening; and
-
harms
hypoglycaemia or hyperglycaemia.
Secondary outcomes
The secondary outcomes of interest were:
-
other measures related to vascular risk factor management:
DBP;
use of aspirin, statins or antihypertensive drugs;
proportions of patients with hypertension control;
proportions of patients after successful smoking cessation.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue 6);
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to 4 June 2019;
Embase (Ovid) 2016 to 4 June 2019;
CINAHL (EBSCOhost) 1981 to 4 June 2019;
Ovid HealthStar 1944 to 2014 (1 July 2010 to 31 December 2014);
EPOC Trials Register (1 July 2010 to 31 December 2014).
The search strategy for the current version of the review covering the period from 2015 to 2019 was developed by the EPOC Information Specialist, Paul Miller. The search strategy contained subject headings and free‐text keyword searches for our key concepts, and methodological filters were applied as appropriate to restrict to RCTs (Lefebvre 2008; Lefebvre 2021). For this update, strategies were applied without date or language restriction on CENTRAL and CINAHL as they had not been searched previously. The Embase search with no language limit was applied, but was restricted by date to 2016 onwards. MEDLINE/PubMed had been searched previously, so for this update the search was restricted by date to 2015 onwards. Previous iterations of the MEDLINE search were limited to English language publications; for this update we ran a MEDLINE search for non‐English language papers without a date restriction. The last update was 4 June 2019.
A top‐up search was conducted on 23 September 2021. The search strategies for the current version of the review are available in Appendix 1 and strategies for previous versions of the review are available in Web Appendix 1.
Searching other resources
We also searched the following trial registers for ongoing studies:
ClinicalTrials.gov (www.clinicaltrials.gov) (4 June 2019);
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch) (4 June 2019).
We also scanned the reference lists of included studies.
Data collection and analysis
Selection of studies
We de‐duplicated the results of the electronic searches and uploaded them into DistillerSR (an online screening and extraction platform) (DistillerSR 2021). Two independent review authors screened the titles and abstracts of records for eligibility using standardised forms; discrepancies were resolved through discussions or, if conflicts remained, with a third senior review author (NI, JMG, KJK, KJS, JL). We pulled and screened the full text of potentially relevant citations through the same process. All new review authors were trained with a pilot round of at least 25 title/abstracts and 10 full texts (chosen randomly) until their screening decisions were considered in good agreement with the independent assessments of senior review members (NI, KJK, KJS, JL). We included and extracted all studies meeting the eligibility criteria.
Data extraction and management
Linking multiple reports
Prior to data extraction, we linked all reports belonging to a single study. We prioritised the extraction of the most recent publication reporting the primary outcome(s) of the study and treated other reports as companion papers. We extracted data from companion papers for relevant secondary outcomes or missing data (for example, additional details on the QI strategies) when available.
Data extraction form
We extracted all data in Excel using detailed extraction sheets for study characteristics (one sheet), coding of QI strategies (one sheet), risk of bias assessment (one sheet) and outcome data (13 sheets; one per outcome). Detailed instructions about extracting all data items were included on the top of each column in Excel. The data extraction form is available online (Web Appendix 2).
Two independent review authors performed data extraction; discrepancies were resolved through discussions or, if conflicts remained, with a third senior review author (KJK, KJS, JL). All data extractors completed a pilot training exercise on a random sample of five articles and these were checked against the extractions of an experienced review member (KJK, KJS, JL). If needed, review authors extracted additional sets of two to three articles until good agreement was obtained with experienced review authors. We extracted study‐level and arm‐level data as described below.
Study level
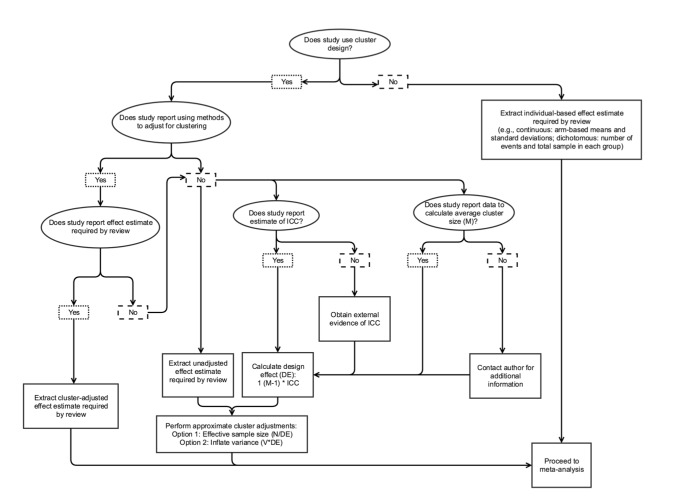
For each study, we extracted information on study name and design, trial registration, country and year of conduct, funding, ethics approval, patient characteristics (age, sex, type of diabetes), study setting and sample size at baseline. For cluster trials, we additionally extracted the number of clusters and providers.
Arm level
QI strategies
See Table 10. For each arm of each study, we extracted data on the presence or absence of each of the 12 QI strategies (and the descriptive text to support the code). We labelled study arms in order of intensity from no QI programme/the least intense QI (Arm 1) to more intensive QI programme (Arm 2 or greater if multi‐arm study).
Outcomes
For continuous outcomes, we extracted the sample size analysed, the group mean and its measure of variance (standard deviation, standard error or other measures) at baseline. If a study reported a median instead of mean, we extracted the median and explored its distribution to see if it could be used in place of the mean (Higgins 2022), and used the median where considered appropriate. If a study reported other measures of variance, we calculated standard deviation from available data using standard methods (Higgins 2022; Hozo 2005). Where we considered values too extreme to be reasonable, we left the value as missing and imputed using the methods described below (see Dealing with missing data).
For dichotomous outcomes, we extracted the sample size analysed and the number of events at baseline. Where only a proportion of events per arm was reported, we calculated the number of events using the sample size analysed.
For all outcomes, we extracted data corresponding to the longest time point of follow‐up (in months).
Additionally, for cluster trials, we extracted details about cluster analysis and the reported intraclass correlation coefficient (ICC) specific to each arm and outcome reported.
Rechecking data
Two review authors (KJK and SN) independently checked the accuracy of the data used in the previous version of the review (up to July 2010) (Tricco 2012). Any imputations of missing variance data were removed in order to be recalculated for the full dataset. The coding of QI strategies was also reassessed for the previous version of the review (up to July 2010) (Tricco 2012) by three review authors (NMI, ACT and KJK) and resulted in coding changes (33 codes removed, 59 codes added, across 142 studies).
Assessment of risk of bias in included studies
We completed risk of bias (RoB) assessment using the Cochrane EPOC RoB tool, which we adapted to assess risk of bias domains for patient and cluster characteristics separately (Web Appendix 3).
Measures of treatment effect
For continuous outcomes, our measure of treatment effect was the mean difference (MD) associated with each QI strategy at follow‐up. For dichotomous outcomes, our measure of treatment effect was the odds ratio (OR) for the desired QI event associated with each QI strategy at follow‐up.
Unit of analysis issues
We used standard methods recommended by Cochrane to identify and correct unit of analysis errors in a systematic review, which we operationalised into the process outlined in Figure 1. To identify and correct unit of analysis errors, we extracted the following study‐ and outcome‐level information for all CRTs:
1.
Process figure for identifying and correcting unit of analysis errors.
Study:
Unit of randomisation
Number of clusters
Whether the study reported using appropriate methods to adjust for clustering (e.g. multilevel models, generalised estimating equations)
Outcome:
Number of patients analysed
Intracluster correlation coefficient (ICC) (per arm if reported; otherwise at the study level)
We modelled studies at the arm level, therefore many studies that may have appropriately adjusted for clustering at the study level (i.e. adjusted for clustering in the estimation of the study‐level mean difference or odds ratio) did not adjust for clustering at the arm level (leading to inflated standard errors of group means for continuous outcomes, effective sample sizes for event and group samples for dichotomous outcomes) and still required adjustment to be included in the arm‐level meta‐regression models.
Where available, we used study‐specific estimates of the ICC to adjust the standard errors of continuous outcomes and sample size of dichotomous outcomes. Where ICCs were unavailable, we imputed ICCs from outcome‐specific posterior distributions of ICCs (Konnyu 2021).
Dealing with missing data
Outcome data
We contacted authors of included studies if the study reported an outcome of interest using a different summary measure than we needed for our extraction (e.g. reported proportion of patients meeting HbA1c targets but not mean HbA1c) or had incomplete data (e.g. reported proportion of patients screened but not analysed sample size). Several authors replied to our requests, allowing for the inclusion of the outcome for the study in analyses. The studies of authors who did not reply within two weeks with additional data were excluded due to 'no outcome data available'.
Variance data (continuous outcomes)
Where an estimate of the standard deviation or standard error could not be extracted (or calculated) as described above, we treated it as missing. We imputed missing data by sampling estimates of the standard error from outcome‐specific uniform distributions with bounds informed by other studies included in the review and content experts. The statistical appendix is available online (Web Appendix 4) and lists selected distributions for modelled outcomes.
Assessment of heterogeneity
We used meta‐regression models (see Data synthesis section) to explore and account for heterogeneity. The base models explore the effect of QI strategies accounting for other QI strategies that may be present in the QI programmes. Where feasible, we extended models to explore the impact of baseline risk. Further iterations of this living review plan to extend meta‐regression models to explore further the impact of additional design, population and setting factors (where data permit).
Assessment of reporting biases
Study level
We assumed different mean effects depending on the presence of different QI strategies delivered in different populations and settings. Given this assumption, a forest plot to detect for publication bias amongst different assumed means would be inappropriate and potentially misleading (Schmid 2020). We therefore did not assess for reporting bias (publication bias) at the study level.
Outcome level
We assessed for presence of outcome reporting bias by comparing the outcomes reported in the Results section of the publication to the outcomes reported in the registered protocol. If no protocol was available, we compared the outcomes reported in the Methods section to those reported in the Results section.
Data synthesis
We fitted a series of hierarchical multivariable random‐effects meta‐regression models (Gelman 2002; Konnyu (in press); Rubin 1992) for three continuous outcomes (HbA1c, SBP, and LDL cholesterol) and three dichotomous outcomes (retinopathy screening, foot screening, smoking cessation), in which we assumed the observed average effect of each included arm to be the additive effect of each QI strategy present. In this way, our models covered three of our four outcome domains (glycaemic control: HbA1c; cardiovascular risk factor control: SBP, LDL cholesterol, smoking cessation; and screening for complications: retinopathy and foot screening). We chose to model SBP rather than DBP, as SBP was reported more commonly and SBP and DBP are highly correlated and relate to the same outcome domain (blood pressure control).
To facilitate stable modelling of strategies and comparison across outcomes with smaller sample sizes (i.e. screening outcomes), we grouped infrequently observed strategies (audit and feedback, continuous quality improvement and financial incentives) in an ‘other’ category across all models. Specified models assumed the post‐intervention mean (or logit proportion) to result from the additive presence of specified QI strategy and the model sought to estimate the additive effect of the presence of each strategy, holding all other strategies constant. We relaxed the strong additive assumption, data permitting, based on hypothesised interactions among strategies and population covariates (described below). We fitted models in a Bayesian framework. Full details of the motivation behind our use of arm‐based multivariable modelling is being published elsewhere (Konnyu (in press)). We cleaned and prepared data for analysis in Stata (StataCorp 2021), and fitted models using Markov chain Monte Carlo (MCMC) methods with the software JAGS (Plummer 2003) called from R. Parameters monitored using the Brooks‐Gelman‐Rubin diagnostic (Brooks 1998; Gelman 1992). All parameters presented had an upper credibility interval Brooks‐Gelman‐Rubin diagnostic of < 1.1. We provide a full statistical appendix that includes model code in Web Appendix 4. To aid in interpretation, we qualitatively ordered QI strategies within three tiers (top, middle, bottom) based on their magnitude of effect relative to the other QI strategies. The number of QI strategies in each tier were not necessarily equal if more/fewer QI strategies had similar clustering of effects.
While the goal of the models is to isolate the estimated independent effect of each QI strategy (holding others constant), QI programmes usually involve combinations of multiple QI strategies. We therefore used the model results to estimate the incremental gain that could potentially be achieved from combining different combinations of QI strategies in hypothetical QI interventions. We constructed these hypothetical future QI interventions based on i) the five most commonly evaluated combinations in the evidence for each outcome (based on empirical studies reviewed) and ii) the five most effective QI strategies (based on model estimates) for each outcome. For dichotomous outcomes, we report illustrative examples of the effect size for these hypothetical combinations assuming different baseline compliance rates with the desired outcome (30%, 50% or 70%).
We did not fit multivariable meta‐regression models for other outcomes that were reported less frequently (DBP, use of ASA, statins or antihypertensives, proportions of patients to have hypertension control, renal screening, harms) due to concerns about model convergence and limitations in interpretation. For these outcomes we initially ran random‐effects meta‐analyses (REMA) in Stata using the meta command with the residual maximum likelihood estimation method (StataCorp 2021), comparing the effect of 'more active QI intervention' versus 'less active/no QI intervention'. As heterogeneity of the REMAs was unsurprisingly quite extreme, we opted to report general trends in the proportion of studies that showed an improvement (comparing most versus least active arms) in study‐specific effect sizes rather than the overall average effect. Full details on REMAs are available in the online statistical appendix (Web Appendix 4).
Subgroup analysis and investigation of heterogeneity
Interactions of QI strategies with effect modifiers
Based on findings from our previous review (Tricco 2012), we hypothesised a differential effect of QI strategies depending on baseline values of the outcome. To explore the impact of baseline values on estimates of the post‐intervention effect associated with each QI strategy, we extended the main additive model for continuous outcomes (HbA1c, SBP and LDL) to include an interaction term between each strategy and average baseline risk for each study. Baseline risk was defined as high or low using the median average value for studies as the cutoff. We used a data‐driven approach for defining thresholds for this review as there is a lack of consensus defining cut‐offs for clinically poor control in the literature. Based on model diagnostics, the baseline interaction models for HbA1c, SBP and LDL performed better than the main model and are therefore presented as the primary analyses for these outcomes. All models, model diagnostics and results for the main model and interaction models for continuous outcomes can be found in the online statistical appendix (Web Appendix 4).
We did not explore effect modification by baseline value for dichotomous outcomes due to data sparsity (i.e. insufficient data points to support the extended model of interactions).
Sensitivity analysis
We performed sensitivity analyses to explore the impact of model assumptions and imputation strategies on synthesised estimates. Specifically, we explored the impact of varying distribution parameters for imputation of missing variance and ICC data and the impact of selecting more or fewer informative priors.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the overall certainty of evidence regarding effectiveness of the QI strategies for the outcomes of interest. We collaboratively rated the certainty of evidence regarding the effects of various QI strategies for that outcome in relation to not receiving that strategy (e.g. receiving case management versus not receiving case management on post‐mean HbA1c). For each outcome, we rated evidence certainty as low, moderate or high based on the GRADE domains as described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2013). As only RCTs were included, the starting point for certainty of evidence was high. We then considered whether downgrading of certainty was needed based on the following:
Risk of bias ‐ based on critical appraisal using the Cochrane risk of bias tool.
Inconsistency ‐ based on model outcomes and model diagnostics.
Indirectness ‐ based on limited direct comparisons of different QI strategies (by the nature of the evidence base and chosen analytic models) and potential limited applicability of evidence due to variation in population and setting (including usual care) across studies.
Imprecision ‐ based on relative sample sizes of outcomes (and number of times strategies were evaluated within outcomes), width of the confidence intervals and whether they included the possibility of a small or null effect.
Other considerations.
We created the summary of findings tables using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2013), along with the Review Manager (RevMan 5.4) table editor (Review Manager 2020). For each QI strategy, we reported the following key outcomes (listed according to priority):
Glycaemic control (HbA1c) (stratified by baseline risk)
SBP (stratified by baseline risk)
LDL‐C (stratified by baseline risk)
Retinopathy screening
Foot screening
The summary of findings tables provide key information about the best estimate of the absolute and relative effects for effective QI strategies for different outcomes, numbers of trials, arms and participants (analysed; accounting for effective sample sizes in CRTs) addressing each outcome, and a rating of overall confidence in the estimates for each outcome (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9). Reporting of harms was too infrequent and too variable and therefore not included in the summary of findings tables.
Results
Description of studies
For a detailed description of trials see the Characteristics of included studies and Characteristics of studies awaiting classification sections. Additional characteristics of included studies are available in Web Appendix 5.
Results of the search
We report a PRISMA diagram for the flow of evidence in the review in Figure 2. The updated search (2010 to 2019) identified an additional 25,626 records of which 8036 were duplicates and removed. During initial screening, we removed an additional 7464 records that failed to meet eligibility requirements. We screened the remaining 10,126 records for eligibility based on titles and abstracts. Of these, we excluded 6819 records. We examined a total of 3307 records at the full‐text level, from which we removed 2882 for not meeting eligibility criteria, leaving 425 records in addition to the 128 records from previously published reviews. The final sample included 553 studies reported in 577 records. For the 'top‐up' search conducted in September 2021, we screened the search results and 42 studies meeting our eligibility criteria are available in Characteristics of studies awaiting classification.
2.
Included studies
We identified a total of 553 studies (428 patient‐RCTs and 125 cluster‐RCTs) involving 1190 study arms, published since 1982. Table 12 lists included studies by type of trial. The 428 patient‐RCTs involved a total of 135,825 patients in 914 study arms. The 125 cluster‐RCTs included 6806 clusters and 276,336 patients in 276 study arms.
3. Included studies by trial design.
Of patients, 50% were female (sex reported in 446 studies); the median age of patients was 58.4 years (reported in 434 studies). Most studies (66%) included patients living with type 2 diabetes only, 15% included patients living with type 2 or type 1 diabetes, and 5% included studies with patients living only with type 1 diabetes. In 14% of studies, the type of diabetes was unclear (see Web Appendix 5).
Of the studies, 47% were conducted in North America (predominantly the US), 25% in Europe and the United Kingdom, 19% in Asia, 5% in Australia and New Zealand, 3% in South America and 1% in Africa. In terms of settings, 54% of studies were undertaken in primary care, 13% in community health clinics, 27% in diabetes‐specific clinics and 4% in non‐medical settings (e.g. community centres, universities) (study setting not reported in 2% of studies).
The frequency of outcomes reported varied. The most commonly reported outcome was mean HbA1c, which was reported in 89% of the trials (492/553), followed by SBP (48%; 265/553), DBP (43%; 236/553) and LDL cholesterol (36%; 199/553). Screening outcomes, and outcomes related to cardiovascular medications, smoking and harms, were reported infrequently. Table 13 lists studies by outcomes reported. The mean duration of follow‐up was 12.5 months (range 1 to 168 months).
4. Included studies by outcomes reported.
The median number of QI strategies amongst all programme arms was three; the median number of QI strategies amongst the most active programme arms was four (range 1 to 9) and the median number of QI strategies amongst the least active programme arms was three. A majority (80%; 721/898) of active programmes had two or more QI strategies (i.e. multicomponent programmes). Of the possible 4095 unique combinations of QI strategies possible (i.e. different programmes comprised of varying combinations of the QI strategies) we observed a total of 220 unique QI programmes. The median frequency that any specific QI combination was evaluated was one (range 1 to 67; interquartile range (IQR) 1 to 3) (Web Appendix 6). The most frequently evaluated QI strategies across all study arms included patient education (PE) (50%; 592/1190), promotion of self‐management (PSM) (45%; 539/1190) and case management (CM) (39%; 461/1190), while the least frequently evaluated QI strategies included audit and feedback (AF) (6%; 67/1190), financial incentives (FI) (3%; 34/1190) and continuous quality improvement (CQI) (2%; 24/1190). Web Appendix 6 summarises the frequency of QI strategies and their combinations across all included studies and within specific outcomes analysed.
Excluded studies
Of the 3307 citations pulled for full‐text review, we excluded 2882 that did not meet eligibility criteria. We listed 27 excluded studies that had been included for data extraction, but were later excluded for not meeting eligibility criteria after closer review in the Characteristics of excluded studies section. Please see Web Appendix 7 for a full list of excluded studies. The most common reasons for exclusion were ineligible design (i.e. not an RCT), evaluation of patient‐level QI strategies only and no reporting of eligible outcomes.
Risk of bias in included studies
Figure 3 and Figure 4 summarise the risk of bias assessment findings for each of the domains. Overall, we judged trials to be at low or unclear risk of bias for most of the domains, except for outcome data reporting, which we judged at higher risk of bias. We provide support for each judgement in the Characteristics of included studies table.
3.

Risk of bias graph
4.

Risk of bias summary
Allocation
We assessed a total of 267/553 studies (48%) to have used appropriate methods for random sequence allocation. Only 17 studies (3%) used non‐random methods and we judged them at high risk of bias for this domain. The rest of the studies provided insufficient information about the sequence generation process to judge the risk of bias (n = 269, 49%). We rated allocation concealment as adequate in 225 studies (41%), either because the unit of allocation was by institution, team or professional and allocation was performed on all units at the start of the study, or a suitable method was used to conceal allocation. We judged 19 studies (3%) at high risk of bias, and more than half of studies (n = 309, 56%) did not provide enough information to assess whether allocation concealment was appropriate.
Blinding
Blinding was not a major issue as most of our outcomes of interest were objective measures. We assessed 76% (419/553) of studies to be low risk of bias; 5% (27/553) to be high risk of bias and 19% (107/553) as unclear risk.
Incomplete outcome data
We assessed 36% of studies (198/553) to be low risk of bias for incomplete outcome data and 13% (72/553) of studies to be unclear. We assessed more than half (51% of studies; 283/553) to be high risk of bias for incomplete outcome data. Cluster trials were at greater risk of patient‐level attrition than patient‐randomised trials.
Selective reporting
We judged 41% of included studies (224/553) as low risk and 35% (195/553) as high risk for selective outcome reporting. The latter had discrepancies between the expected primary outcomes reporting and published data. We judged studies at unclear risk (24%, 132/553) if they lacked a study protocol or trial register entry, or registration was performed retrospectively.
Other potential sources of bias
For the risk of other potential sources of bias, we judged 87% of studies (480/553) to be low risk, 11% (63/553) to be unclear risk and 2% (10/553) to be high risk.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Glycaemic control
Data from 487 randomised controlled trials (RCTs) from 1039 arms contributed to analysis of the HbA1c outcome. The frequency of quality improvement (QI) strategies evaluated across the HbA1c arms was: patient education (PE) 51% (534/1039), promotion of self‐management (PSM) 48% (495/1039), case management (CM) 40% (417/1039), facilitated relay of clinical information (FR) 23% (236/1039), team changes (TC) 21%, (221/1039), electronic patient registry (EPR) 16% (164/1039), clinician education (CE) 13% (137/1039), patient reminders (PR) 12% (125/1039), clinician reminders (CR) 8% (84/1039), audit and feedback (AF) 5% (52/1039), financial incentives (FI) 2% (23/1039) and continuous quality improvement (CQI) 2% (16/1039).
Effects of individual QI strategies
We fitted two models of the HbA1c outcome, one model of the QI strategies alone and one model in which we assessed the interaction of the QI strategies with baseline HbA1c values. Based on our assessments of model diagnostics, the baseline interaction model was the better model and is thus reported here. The main HbA1c model is reported in the online statistical appendix (Web Appendix 4).
There were 501 trial arms with mean baseline HbA1c less than or equal to 8.3% (henceforth labelled as lower baseline HbA1c). In these trial arms, the mean HbA1c was 7.5% (95% credible interval (CrI) 7.4% to 7.6%). There were 500 trial arms with baseline HbA1c > 8.3 (henceforth labelled as higher baseline HbA1c). In these trial arms, the mean HbA1c was 8.7% (95% CrI 8.6% to 8.8%).
As summarised in Table A, for arms with lower study baseline HbA1c, the QI strategies clinician education, promotion of self‐management, team changes, electronic patient registry and patient reminders were associated with the largest reductions in mean HbA1c at follow‐up, while clinician reminders and patient education were not associated with reductions in mean HbA1c at follow‐up.
For arms with higher study baseline HbA1c, the QI strategies case management, patient education, electronic patient registry, team changes, clinician reminders and promotion of self‐management were associated with the largest reductions in mean HbA1c at follow‐up, while clinician education was not associated with reductions in mean HbA1c at follow‐up.
Table A. Effect of QI strategy in arms with lower and higher mean HbA1c at baseline
| Lower baseline HbA1c (≤ 8.3%) | Higher baseline HbA1c (> 8.3%) | |||||
| QI strategy | Post‐intervention meana HbA1c (95% CrI) | Absolute mean difference HbA1c (95% CrI) | Order | Post‐intervention meanb HbA1c (95% CrI) | Absolute mean difference HbA1c (95% CrI) | Order |
| No QI strategy | 7.48 (7.42 to 7.55) | NA | NA | 8.70 (8.59 to 8.81) | NA | NA |
| CM | 7.48 (7.38 to 7.58) | ‐0.01 (‐0.08 to 0.07) | Middle | 8.43 (8.26 to 8.60) | ‐0.27 (‐0.39to‐0.15) | Top |
| TC | 7.37 (7.25 to 7.49) | ‐0.11 (‐0.21to‐0.02) | Top | 8.59 (8.42 to 8.77) | ‐0.11 (‐0.24to0.03) | Top |
| EPR | 7.38 (7.26 to 7.50) | ‐0.11 (‐0.20to‐0.01) | Top | 8.52 (8.33 to 8.71) | ‐0.17 (‐0.33to‐0.02) | Top |
| CE | 7.36 (7.23 to 7.49) | ‐0.13 (‐0.24to‐0.01) | Top | 8.76 (8.52 to 9.02) | 0.06 (‐0.15 to 0.30) | Bottom |
| CR | 7.57 (7.44 to 7.70) | 0.09 (‐0.02 to 0.20) | Bottom | 8.61 (8.33 to 8.87) | ‐0.09 (‐0.34to0.15) | Top |
| FR | 7.43 (7.32 to 7.54) | ‐0.05 (‐0.14 to 0.03) | Middle | 8.66 (8.48 to 8.83) | ‐0.04 (‐0.18 to 0.10) | Middle |
| PE | 7.50 (7.41 to 7.59) | 0.02 (‐0.07 to 0.10) | Bottom | 8.53 (8.39 to 8.67) | ‐0.17 (‐0.30to‐0.05) | Top |
| PSM | 7.34 (7.23 to 7.45) | ‐0.14 (‐0.25to‐0.06) | Top | 8.57 (8.43 to 8.72) | ‐0.13 (‐0.24to0.00) | Top |
| PR | 7.35 (7.21 to 7.48) | ‐0.14 (‐0.25to‐0.03) | Top | 8.69 (8.47 to 8.90) | ‐0.01 (‐0.19 to 0.16) | Middle |
| Otherc | 7.47 (7.36 to 7.58) | ‐0.01 (‐0.11 to 0.08) | Bottom | 8.71 (8.38 to 9.03) | 0.01 (‐0.30 to 0.31) | Middle |
Abbreviations -QI strategies: AF = audit and feedback, CE = clinician education, CM = case management, CQI = continuous quality improvement, CR = clinician reminders, EPR = electronic patient registry, FI = financial interventions, FR = facilitated relay, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes; Other: CrI = credible interval, n = number, QI = quality improvement.
Data included 1001 arms from 468 RCTs. Studies with a study-level mean %HbA1c of 8.3 or less at baseline were defined as lower HbA1c (234 RCTs; 501 arms); all other studies were defined as higher HbA1c (234 RCTs; 500 arms). Frequency of components within the arms of lower and higher studies included: CM (n = 196; n = 213), TC (n = 94; n = 120), EPR (n = 97; n = 62), CE (n = 75; n = 50), CR (n = 49; n = 29), FR (n = 112; n = 120), PE (n = 245; n = 275), PSM (n = 226; n = 262), PR (n = 59; n = 61) and Other (n = 58; n = 22).
The following prior distributions in the Bayesian analyses: post-treatment mean in the absence of intervention (no QI strategy) ~ N(8,100); post-treatment mean when strategy is present ~ N(0,4);main effect of the baseline interaction~N(0,4);statistical interaction coefficient of the modifier with the QI strategy~N(0,4);all standard deviations of the distribution of true effect sizes~U(0,2).
Missing estimates of standard errors were imputed for 315/1001 arms (31%) using a uniform distribution with a minimum of 0 and maximum of 2.
Missing estimates of intracluster correlation coefficients were imputed for 139/159 cluster-RCT arms (87%) using an outcome-specific normal distribution logit_ICC[i,j] ~ dnorm(-2.80043, (1/(0.70553*0.70553))) as described in Konnyu KJ, Taljaard M, Ivers NM, Moher D, Grimshaw JM. Imputing intracluster correlation coefficients from a posterior predictive distribution is a feasible method of dealing with unit of analysis errors in a meta-analysis of cluster RCTs.J Clin Epidemiol. 2021 Nov;139:307-318. doi: 10.1016/j.jclinepi.2021.06.011. Epub 2021 Jun 22. PMID: 34171503.
aPost-intervention mean change in patients with lower baseline HbA1c who did not receive QI strategy: 7.48 (95% CrI 7.42 to 7.55).
bPost-intervention mean change in patients with higher HbA1c who did not receive QI strategy: 8.70 (95% CrI 8.59 to 8.81).
c'Other' is a combined category of infrequently evaluated QI strategy components, AF, CQI and FI.
Effects of combinations of QI strategies
The studies reporting HbA1c evaluated 148 QI programmes involving different combinations of QI strategies. The median frequency of evaluation for each unique QI programme was 2 (range 1 to 63; interquartile range (IQR) 2 to 4) (Web Appendix 6). The median number of QI strategies in the programme arms was 4 (range 1 to 9; IQR 3 to 5). The median number of QI strategies in the control arms was 0 (range 0 to 7; IQR 0 to 1).
Table B provides the estimated effects on HbA1c for: i) the five most commonly evaluated combinations in the evidence and ii) the combined effects of the five most effective QI strategies. We report results separately for populations with lower and higher baseline HbA1c using the estimated most effective QI strategy for these respective populations.
For example, for the first combination, case management + patient education + promotion of self‐management was reported in 31 arms of studies categorised as having lower HbA1c at baseline (< 8.3%) and based on the results produced from the model, the estimated post‐intervention mean associated with this combination would be 7.35 (95% CrI 7.25 to 7.45) representing a reduction of ‐0.13 (95% CrI ‐0.23 to ‐0.04) compared to arms that did not receive a QI strategy. The second group (sequential combinations) represents model‐based estimates of the incremental gain that could be achieved from combining the most effective QI strategies for HbA1c, sequentially (N.B. results for 4th and 5th most effective components for lower HbA1c (PSM and PR) were virtually indistinguishable).
Table B. Model‐based estimated effects of combinations of QI strategies for populations with lower and higher mean HbA1c at baseline
| Lower baseline HbA1c ≤ 8.3% | ||
| QI combination (no. of arms) | Post‐intervention meana HbA1c (95% CrI)b | Absolute mean differencec HbA1c (95% CrI) |
| Five most common combinations of QI strategies observed in included studies reporting HbA1c | ||
| CM + PE + PSM (n = 31) | 7.35 (7.25 to 7.45) | ‐0.13 (‐0.23 to ‐0.04) |
| CM + TC + PE + PSM (n = 12) | 7.24 (7.12 to 7.35) | ‐0.25 (‐0.36 to ‐0.13) |
| CM + FR + PE + PSM (n = 11) | 7.30 (7.18 to 7.41) | ‐0.19 (‐0.30 to ‐0.08) |
| CM + PSM (n = 10) | 7.34 (7.23 to 7.44) | ‐0.15 (‐0.24 to ‐0.06) |
| CM + PE (n = 10) | 7.29 (7.10 to 7.48) | ‐0.19 (‐0.36 to ‐0.03) |
| Sequential combination of the model‐estimated five most effective QI strategies for HbA1c* | ||
| PR + PSM | 7.21 (7.02 to 7.36) | ‐0.28 (‐0.45 to ‐0.13) |
| PR + PSM + CE | 7.08 (6.88 to 7.27) | ‐0.41 (‐0.61 to ‐0.22) |
| PR + PSM + CE + TC | 6.96 (6.75 to 7.17) | ‐0.52 (‐0.73 to ‐0.32) |
| PR + PSM + CE + TC + EPR | 6.86 (6.65 to 7.07) | ‐0.63 (‐0.84 to ‐0.42) |
| Higher baseline HbA1c > 8.3% | ||
| Combinations of QI strategies | Post‐intervention meana HbA1c (95% CrI) d | Absolute mean differencec HbA1c (95% CrI) |
| Five most common combinations of QI strategies observed in included studies reporting HbA1c | ||
| QI combination (no. of arms) | ||
| CM + PE + PSM (n = 31) | 8.13 (7.98 to 8.29) | ‐0.57 (‐0.72 to ‐0.41) |
| CM + TC + PE + PSM (n = 28) | 8.03 (7.87 to 8.18) | ‐0.67 (‐0.82 to ‐0.53) |
| CM + FR + PE + PSM (n = 13) | 8.09 (7.91 to 8.27) | ‐0.61 (‐0.79 to ‐0.43) |
| FR + PSM (n = 10) | 8.53 (8.34 to 8.73) | ‐0.17 (‐0.34 to 0.01) |
| CM + TC + PE (n = 8) | 8.39 (8.20 to 8.59) | ‐0.31 (‐0.49 to ‐0.12) |
| Sequential combination of the model‐estimated five most effective QI strategies for HbA1c | ||
| CM + EPR | 8.25 (8.03 to 8.48) | ‐0.45 (‐0.63 to ‐0.25) |
| CM + EPR + PE | 8.08 (7.85 to 8.32) | ‐0.62 (‐0.84 to ‐0.39) |
| CM + EPR + PE + PSM | 7.96 (7.75 to 8.17) | ‐0.74 (‐0.95 to ‐0.53) |
| CM + EPR + PE + PSM + TC | 7.98 (7.73 to 8.22) | ‐0.72 (‐0.95 to ‐0.50) |
Abbreviations -QI strategies: AF = audit and feedback, CE = clinician education, CM = case management, CQI = continuous quality improvement, CR = clinician reminders, EPR = electronic patient registry, FI = financial interventions, FR = facilitated relay, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes; Other: CrI = credible interval, n = number, QI = quality improvement.
*Model estimates of PSM and PR were virtually identical and thus their order in the sequential combination here is for illustrative purposes.
The following prior distributions were used in the Bayesian analyses: post-treatment mean in the absence of intervention (no QI strategy)~ N(8,100); post-treatment mean when strategy is present ~ N(0,4);main effect of the baseline interaction~N(0,4);statistical interaction coefficient of the modifier with the QI strategy~N(0,4);all standard deviations of the distribution of true effect sizes ~U(0,2).
Missing estimates of standard errors were imputed for 315/1001 arms (31%) using a uniform distribution with a minimum of 0 and maximum of 2.
Missing estimates of intracluster correlation coefficients were imputed for 139/159 cluster-RCT arms (87%) using an outcome-specific normal distribution logit_ICC[i,j] ~ dnorm(-2.80043, (1/(0.70553*0.70553))) as described in Konnyu KJ, Taljaard M, Ivers NM, Moher D, Grimshaw JM. Imputing intracluster correlation coefficients from a posterior predictive distribution is a feasible method of dealing with unit of analysis errors in a meta-analysis of cluster RCTs.J Clin Epidemiol. 2021 Nov;139:307-318. doi: 10.1016/j.jclinepi.2021.06.011. Epub 2021 Jun 22. PMID: 34171503.
aMedian of the posterior distribution of the post-intervention means.
bPost-intervention mean change in patients with lower baseline HbA1c who did not receive QI strategy: 7.48 (95% CrI 7.42 to 7.55).
cMedian of the posterior distribution of the post-intervention absolute mean difference.
dPost-intervention mean change in patients with higher HbA1c who did not receive QI strategy: 8.70 (95% CrI 8.59 to 8.81).
Systolic blood pressure (SBP)
Data from 262 RCTs contributed to 550 arms of data for analysis of the SBP outcome. The frequency of QI strategies evaluated across the SBP arms was: patient education (51%, 281/550), promotion of self‐management (43%, 239/550), case management (41%, 223/550), team changes (23%, 127/550), facilitated relay of clinical information (20%, 108/550), clinician education (18%, 101/550), electronic patient registry (16%, 86/550), patient reminders (13%, 69/550), clinician reminders (10%, 55/550), audit and feedback (7%, 40/550), continuous quality improvement (2%, 13/550) and facilitated relay of information (3%, 15/550).
Effects of individual QI strategies
We fitted two models of the SBP outcome, one model of the QI strategies alone and one model in which we assessed the interaction of the QI strategies with baseline SBP values. Based on our assessments of model diagnostics, the baseline interaction model was the better model and is thus reported here. The main SBP model is reported in the statistical appendix (Web Appendix 4).
There were 263 trial arms with mean baseline SBP less than or equal to 136 mmHg (lower baseline SBP). In these trial arms, the mean SBP was 131 mmHg (95% CrI 130 to 131 mmHg). There were 249 trial arms with mean baseline SBP > 136 mmHg (higher baseline SBP). In these trial arms, the mean SBP was 139 mmHg (95% CrI 138 to 139 mmHg).
As summarised in Table C, for arms with lower study baseline SBP, QI strategies 'Other QI' (audit and feedback, continuous quality improvement, financial incentives combined), case management, patient education, team changes and facilitated relay of clinical information were associated with the largest reductions in mean SBP at follow‐up, while patient reminders and clinician education were not associated with reductions in mean SBP at follow‐up.
For arms with higher study baseline SBP, QI strategies case management, team changes, 'Other QI' and promotion of self‐management were associated with the largest reductions in mean SBP at follow‐up, while clinician education, electronic patient registry and patient reminders were not associated with reductions in mean SBP at follow‐up.
Table C. Effect of QI strategy in arms with lower and higher mean SBP at baseline
| Lower baseline SBP ≤ 136 mmHg | Higher baseline SBP > 136 mmHg | |||||
| QI strategy | Post‐intervention meana SBP mmHg (95% CrI) | Absolute mean difference SBP mmHg (95% CrI) | Order | Post‐intervention meanb SBP mmHg (95% CrI) | Absolute mean difference SBP mmHg (95% CrI) | Order |
| No QI strategy | 130.66 (130.03 to 131.29) | 138.53 (137.74 to 139.30) | ||||
| CM | 130.31 (129.09 to 131.55) | ‐0.35 (‐1.40 to 0.74) | Top | 136.64 (135.00 to 138.30) | ‐1.89 (‐3.32 to ‐0.41) | Top |
| TC | 129.76 (128.38 to 131.11) | ‐0.91 (‐2.10 to 0.29) | Top | 136.72 (135.07 to 138.36) | ‐1.81 (‐3.30 to ‐0.32) | Top |
| EPR | 130.58 (129.07 to 132.04) | ‐0.08 (‐1.47 to 1.24) | Middle | 139.55 (137.46 to 141.59) | 1.01 (‐0.96 to 2.95) | Bottom |
| CE | 130.93 (129.47 to 132.40) | 0.26 (‐1.11 to 1.69) | Bottom | 140.57 (139.06 to 142.08) | 2.05 (0.62 to 3.45) | Bottom |
| CR | 130.49 (128.77 to 132.27) | ‐0.17 (‐1.82 to 1.46) | Middle | 138.32 (136.15 to 140.27) | ‐0.20 (‐2.25 to 1.62) | Middle |
| FR | 130.33 (129.08 to 131.59) | ‐0.32 (‐1.48 to 0.83) | Top | 138.11 (136.16 to 140.06) | ‐0.42 (‐2.22 to 1.41) | Middle |
| PE | 129.95 (128.97 to 130.91) | ‐0.71 (‐1.71 to 0.28) | Top | 138.41 (137.02 to 139.79) | ‐0.12 (‐1.47 to 1.22) | Middle |
| PSM | 130.13 (128.94 to 131.32) | ‐0.53 (‐1.60 to 0.54) | Top | 137.84 (136.16 to 139.52) | ‐0.69 (‐2.23 to 0.86) | Top |
| PR | 131.11 (129.43 to 132.67) | 0.45 (‐1.08 to 1.83) | Bottom | 139.15 (137.05 to 141.26) | 0.61 (‐1.28 to 2.58) | Bottom |
| Otherc | 129.36 (127.80 to 130.89) | ‐1.29 (‐2.79 to 0.08) | Top | 136.92 (135.20 to 138.82) | ‐1.62 (‐3.16 to 0.16) | Top |
Abbreviations -QI strategies: AF = audit and feedback, CE = clinician education, CM = case management, CQI = continuous quality improvement, CR = clinician reminders, EPR = electronic patient registry, FI = financial interventions, FR = facilitated relay, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes; Other: CrI = credible interval, mmHg = millimetres of mercury, QI = quality improvement, SBP = systolic blood pressure
Data included 512 arms from 243 RCTs. Studies with a study-level mean SBP of 136 mmHg or less at baseline were defined as lower baseline SBP (125 RCTs; 263 arms); all other studies were defined as higher baseline SBP (118 RCTs; 249 arms). Frequency of components within the arms of controlled and uncontrolled studies included: CM (n = 116; n = 96), TC (n = 55; n = 66), EPR (n = 46; n = 33), CE (n = 36; n = 58), CR (n = 22; n = 24), FR (n = 59; 41), PE (n = 145; n = 120), PSM (n = 137; n = 90), PR (n = 36; n = 27) and Other (n = 22; n = 30).
The following prior distributions in the Bayesian analyses: post-treatment mean in the absence of intervention (no QI strategy) ~ N(140,100); post-treatment mean when strategy is present ~ N(0,4); main effect of the baseline interaction~N(0,4); statistical interaction coefficient of the modifier with the QI strategy~N(0,4);all standard deviations of the distribution of true effect sizes~U(0,2).
Missing estimates of standard errors were imputed for 183/512 arms (36%) using a uniform distribution with a minimum of 0 and maximum of 7.
Missing estimates of intracluster correlation coefficients were imputed for 82/98 cluster-RCT arms (84%) using an outcome-specific normal distribution logit_ICC[i,j] ~ dnorm(-3.12689, (1/(0.51605 *0.51605))) as described in Konnyu KJ, Taljaard M, Ivers NM, Moher D, Grimshaw JM. Imputing intracluster correlation coefficients from a posterior predictive distribution is a feasible method of dealing with unit of analysis errors in a meta-analysis of cluster RCTs.J Clin Epidemiol. 2021 Nov;139:307-318. doi: 10.1016/j.jclinepi.2021.06.011. Epub 2021 Jun 22. PMID: 34171503.
aPost-intervention mean change in patients with lower baseline SBP who did not receive QI strategy, 130.66 (95% CrI 130.03 to 131.29).
bPost-intervention mean change in patients with higher SBP who did not receive QI strategy, 138.53 (95% CrI 137.74 to 139.30).
c'Other' is a combined category of infrequently evaluated QI strategy components, AF, CQI and FI.
Effects of combinations of QI strategies
The studies reporting SBP evaluated 159 QI programmes involving different combinations of QI strategies. The median frequency of evaluation for each programme was 1 (range 1 to 31; IQR 1 to 2) (Web Appendix 6). The median number of QI strategies in the programme arm was 4 (range 1 to 9; IQR 3 to 5). The median number of QI strategies in the control arms was 0 (range 0 to 5; IQR 0 to 1).
Table D provides the estimated effects on SBP for i) the five most commonly evaluated combinations in the evidence and ii) the combined effect of the five most effective QI strategies. We report results separately for populations with lower and higher baseline SBP using the estimated most effective QI strategies for these respective populations.
For example, for the first combination, case management + patient education + promotion of self‐management was reported in 18 arms of studies categorised as having lower SBP at baseline (≤ 136 mmHg) and based on the results produced from the model, the estimated post‐intervention mean associated with this combination would be 129.07 (95% CrI 127.93 to 130.22) representing a reduction of ‐1.59 (95% ‐2.82 to ‐0.33) compared to arms that did not receive a QI strategy. The second group (sequential combinations) represents model‐based estimates of the incremental gain that could be achieved from combining the most effect QI strategies for SBP, sequentially.
Table D. Model‐based estimated effects of combinations of QI strategies for populations with lower and higher mean SBP at baseline
| Baseline SBP ≤ 136 mmHg | |||
| Combinations of QI strategies (no. of arms) | Post‐intervention meana SBP mmHg (95% CrI)b | Absolute mean differencecSBP mmHg (95% CrI) |
|
| Five most common combinations of QI strategies observed in included studies reporting SBP | |||
| CM + PE + PSM (n = 18) | 129.07 (127.93 to 130.22) | ‐1.59 (‐2.82 to ‐0.33) | |
| CM + TC + PE + PSM (n = 13) | 128.17 (126.96 to 129.40) | ‐2.49 (‐3.77 to ‐1.19) | |
| CM + FR + PE + PSM (n = 87) | 128.75 (127.32 to 130.18) | ‐1.91 (‐3.46 to ‐0.35) | |
| CM + FR + EPR + PE + PSM (n = 5) | 128.67 (126.86 to 130.37) | ‐2.00 (‐3.90 to ‐0.16) | |
| CM + TC + PE + PSM + PR (n = 5) | 128.61 (126.81 to 130.41) | ‐2.06 (‐3.85 to ‐0.23) | |
| Sequential combination of the model‐estimated five most effective QI strategies for SBP | |||
| TC + PE | 129.05 (127.57 to 130.53) | ‐1.62 (‐3.09 to ‐0.13) | |
| TC + PE + PSM | 128.52 (126.89 to 130.09) | ‐2.14 (‐3.80 to ‐0.52) | |
| TC + PE + PSM + CM | 128.17 (126.96 to 129.40) | ‐2.49 (‐3.77 to ‐1.19) | |
| TC + PE + PSM + CM + FR | 127.85 (126.35 to 129.36) | ‐2.81 (‐4.42 to ‐1.20) | |
| Baseline SBP > 136 mmHg | |||
| Combinations of QI strategies (no. of arms) | Post‐intervention meana SBP mmHg (95% CrI)d |
Absolute mean differencec SDP mmHg (95% CrI) |
|
| Five most common combinations of QI strategies observed in included studies reporting SBP | |||
| CM + PE + PSM (n = 12) | 135.84 (134.17 to 137.48) | ‐2.69 (‐4.37 to ‐1.03) | |
| CM + TC + PE + PSM (n = 8) | 134.02 (132.35 to 135.68) | ‐4.51 (‐6.22 to ‐2.80) | |
| CM + TC + PE (n = 5) | 134.71 (132.78 to 136.62) | ‐3.81 (‐5.75 to ‐1.90) | |
| CM + FR + PE + PSM (n = 4) | 135.41 (133.22 to 137.63) | ‐3.12 (‐5.33 to ‐0.86) | |
| CM + TC +FR + PE + PSM (n = 4) | 133.61 (131.43 to 135.75) | ‐4.93 (‐7.15 to ‐2.70) | |
| Sequential combination of the model‐estimated five most effective QI strategies for SBP | |||
| CM + TC | 134.82 (132.99 to 136.66) | ‐3.70 (‐5.41 to ‐2.01) | |
| CM + TC + PSM | 134.14 (132.24 to 136.06) | ‐4.39 (‐6.20 to ‐2.56) | |
| CM + TC + PSM + FR | 133.72 (131.30 to 136.16) | ‐4.81 (‐7.17 to ‐2.40) | |
| CM + TC + PSM + FR + CR | 133.48 (130.48 to 136.53) | ‐5.04 (‐8.02 to ‐1.98) | |
Abbreviations -QI strategies: AF = audit and feedback, CE = clinician education, CM = case management, CQI = continuous quality improvement, CR = clinician reminders, EPR = electronic patient registry, FI = financial interventions, FR = facilitated relay, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes; Other: CrI = credible interval, mmHg = millimetres of mercury, QI = quality improvement, SBP = systolic blood pressure
Missing estimates of standard errors were imputed for 183/512 arms (36%) using a uniform distribution with a minimum of 0 and maximum of 7.
Missing estimates of intracluster correlation coefficients were imputed for 82/98 cluster-RCT arms (84%) using an outcome-specific normal distribution logit_ICC[i,j] ~ dnorm(-3.12689, (1/(0.51605 *0.51605))) as described in Konnyu KJ, Taljaard M, Ivers NM, Moher D, Grimshaw JM. Imputing intracluster correlation coefficients from a posterior predictive distribution is a feasible method of dealing with unit of analysis errors in a meta-analysis of cluster RCTs.J Clin Epidemiol. 2021 Nov;139:307-318. doi: 10.1016/j.jclinepi.2021.06.011. Epub 2021 Jun 22. PMID: 34171503.
The following prior distributions in the Bayesian analyses: post-treatment mean in the absence of intervention (no QI strategy) ~ N(140,100); post-treatment mean when strategy is present ~ N(0,4);main effect of the baseline interaction~N(0,4); statistical interaction coefficient of the modifier with the QI strategy~N(0,4);all standard deviations of the distribution of true effect sizes~U(0,2).
aMedian of the posterior distribution of the post-intervention means.
bPost-intervention mean change in patients with lower baseline SBP who did not receive QI strategy, 130.66 (95% CrI 130.03 to 131.29).
cMedian of the posterior distribution of the post-intervention absolute mean difference.
dPost-intervention mean change in patients with higher baseline SBP who did not receive QI strategy, 138.53 (95% CrI 137.74 to 139.30).
LDL‐C
Data from 198 RCTs included 419 arms of data for analysis of LDL‐C. The frequency of QI strategies evaluated across the LDL‐C arms was: patient education (53%, 222/419), promotion of self‐management (45%, 187/419), case management (41%, 171/419), team changes (24%, 99/419), facilitated relay of clinical information (19%, 81/419), electronic patient registry (17%, 71/419), patient reminders (14%, 57/419), clinician education (13%, 55/419), clinician reminders (10%, 40/419), audit and feedback (8%, 35/419), continuous quality improvement (3%, 11/419) and financial incentives (2%, 9/419).
Effects of individual QI strategies
We fitted two models of the LDL‐C outcome, one model of the QI strategies alone and one model in which we assessed the interaction of the QI strategies with baseline LDL‐C values. Based on our assessments of model diagnostics, the baseline interaction model was the better model and is thus reported here. The main LDL model is reported in the statistical appendix (Web Appendix 4).
There were 211 trial arms with mean baseline LDL‐C ≤ 107 mg/dL (lower baseline LDL‐C). In these trial arms, the mean LDL‐C was 94 mg/dL (95% CrI 93 to 95 mg/dL). There were 183 trial arms with baseline LDL‐C > 107 mg/dL (higher baseline LDL‐C). In these trial arms, the mean LDL‐C was 108 mg/dL (95% CrI 107 to 110 mg/dL).
As summarised in Table E, for arms with lower study baseline LDL‐C, team changes, patient education, case management and facilitated relay of clinical information were associated with the largest reductions in mean LDL‐C at follow‐up, while electronic patient registry, patient reminders, clinician reminders and clinician education were not associated with reductions in mean LDL‐C at follow‐up.
For arms with higher study baseline LDL‐C, team changes, 'Other QI', case management, clinician education and clinician reminders were associated with the largest reductions in mean LDL‐C at follow‐up, while electronic patient registry, facilitated relay of clinical information, promotion of self‐management, patient education and patient reminders were not associated with reductions in mean LDL‐C at follow‐up.
Table E. Effect of QI strategy in arms with lower and higher mean LDL‐C at baseline
| Lower baseline LDL‐C ≤ 107 mg/dL | Higher baseline LDL‐C > 107 mg/dL | |||||
| QI strategy | Post‐intervention meana LDL‐C mg/dL (95% CrI) | Absolute mean differenceb LDL‐C mg/dL (95% CrI) | Order | Post‐intervention meana LDL‐C mg/dL (95% CrI) | Absolute mean differenceb LDL‐C (95% CrI) | Order |
| No QI strategy | 94.46 (93.48 to 95.47) | 108.48 (107.26 to 109.69) | ||||
| CM | 92.86 (91.09 to 94.65) | ‐1.60 (‐3.24to 0.00) | Top | 106.40 (104.07 to 108.72) | ‐2.08 (‐4.08to ‐0.09) | Top |
| TC | 92.22 (90.20 to 94.20) | ‐2.24 (‐3.97to ‐0.57) | Top | 105.41 (102.97 to 107.84) | ‐3.07 (‐5.29to ‐0.84) | Top |
| EPR | 94.66 (92.59 to 96.86) | 0.20 (‐1.69 to 2.20) | Bottom | 110.59 (107.58 to 113.55) | 2.10 (‐0.74 to 4.85) | Bottom |
| CE | 95.64 (93.68 to 97.63) | 1.18 (‐0.73 to 3.15) | Bottom | 107.99 (105.03 to 111.06) | ‐0.49 (‐3.27to 2.39) | Top |
| CR | 95.19 (92.80 to 97.61) | 0.74 (‐1.51 to 2.98) | Middle | 108.11 (105.22 to 110.98) | ‐0.38 (‐3.05 to 2.30) | Top |
| FR | 93.25 (91.38 to 95.14) | ‐1.20 (‐2.91 to 0.49) | Top | 108.82 (106.19 to 111.51) | 0.32 (‐2.03 to 2.80) | Middle |
| PE | 92.57 (90.93 to 94.24) | ‐1.89 (‐3.52 to ‐0.26) | Top | 110.31 (108.27 to 112.35) | 1.83 (‐0.19 to 3.86) | Bottom |
| PSM | 94.12 (92.26 to 96.00) | ‐0.34 (‐2.03 to 1.32) | Middle | 108.71 (106.35 to 111.08) | 0.23 (‐1.94 to 2.43) | Middle |
| PR | 95.18 (93.03 to 97.43) | 0.70 (‐1.15 to 2.63) | Bottom | 109.49 (106.71 to 112.32) | 1.02 (‐1.48 to 3.53) | Bottom |
| Otherc | 95.18 (93.22 to 97.16) | 0.72 (‐1.09 to 2.54) | Bottom | 106.15 (103.21 to 109.04) | ‐2.33 (‐5.07 to 0.35) | Top |
Abbreviations: CM = case management, CE = clinician education, CR = clinician reminders, EPR = electronic patient registry, FR = facilitated relay, , TC = team change, PE = patient education, PR = patient reminders, PSM = promotion of self-management
Abbreviations -QI strategies: AF = audit and feedback, CE = clinician education, CM = case management, CQI = continuous quality improvement, CR = clinician reminders, EPR = electronic patient registry, FI = financial interventions, FR = facilitated relay, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes; Other: CrI = credible interval, LDL-C = low-density lipoprotein cholesterol, mg/dL = milligrams per decilitre, QI = quality improvement
Data included 394 arms from 186 RCTs. Studies with a study-level mean LDL-C of 107 mg/dL or less at baseline were defined as lower baseline LDL-C (99 RCTs; 211 arms); all other studies were defined as higher baseline LDL-C (87 RCTs; 183 arms). Frequency of components within the arms lower baseline LDL-C and higher baseline LDL-C studies included: CM (n = 93; n = 68), TC (n = 50; n = 46), EPR (n = 42; n = 23), CE (n = 27; n = 25), CR (n = 16; n = 22), FR (n = 39; n = 34), PE (n = 110; n = 100), PSM (n = 99; n = 77), PR (n = 28; n = 27) and Other (n = 28; n = 18).
Missing estimates of standard errors were imputed for 137/394 arms (35%) using a uniform distribution with a minimum of 0 and a maximum of 13.
Missing estimates of intracluster correlation coefficients were imputed for 78/90 cluster-RCT arms (87%) using an outcome-specific normal distribution logit_ICC[i,j] ~ dnorm(-3.29464, (1/(0.77787*0.77787))) as described in Konnyu KJ, Taljaard M, Ivers NM, Moher D, Grimshaw JM. Imputing intracluster correlation coefficients from a posterior predictive distribution is a feasible method of dealing with unit of analysis errors in a meta-analysis of cluster RCTs.J Clin Epidemiol. 2021 Nov;139:307-318. doi: 10.1016/j.jclinepi.2021.06.011. Epub 2021 Jun 22. PMID: 34171503.
The following prior distributions in the Bayesian analyses: βο ~ N(100,100); βκ ~ N(0,4); φ~N(0,4); ψκ~N(0,4); all τ~U(0,2).
aPost-intervention mean change in patients with lower baseline LDL-C who did not receive QI strategy, 94.46 (95% CrI 93.48 to 95.47).
bPost-intervention mean change in patients with higher LDL-C who did not receive QI strategy, 108.48 (95% CrI 107.26 to 109.69).
c'Other' is a combined category of infrequently evaluated QI strategy components, AF, CQI and FI.
Effects of combinations of QI strategies
The studies evaluated 125 different unique programmes comprised of the 12 QI strategies. The median frequency of evaluation for each unique programme was 1 (range 1 to 24; IQR 1 to 2) (Web Appendix 6). For studies where LDL‐C was assessed and analysed in the analyses, the median number of QI strategies in the programme arms was 4 (range 1 to 9; IQR 3 to 5). The median number of QI strategies in the control arms was 0 (range 0 to 5; IQR 0 to 1).
Table F provides the estimated effects on LDL‐C for i) the five most commonly evaluated combinations in the evidence and ii) the combined effect of the five most effective QI strategies. We report results separately for populations with lower and higher baseline LDL‐C using the estimated most effective QI strategies for these respective populations.
For example, for the first combination, case management + patient education + promotion of self‐management was reported in 16 arms of studies categorised as having lower LDL‐C at baseline (≤ 107 mg/dL) and based on the results produced from the model, the estimated post‐intervention mean associated with this combination would be 90.63 (95% CrI 88.85 to 92.61) representing a reduction of ‐4.13 (95% CrI ‐6.38 to ‐1.93) compared to arms that did not receive a QI strategy. The second group (sequential combinations) represents model‐based estimates of the incremental gain that could be achieved from combining the most effective QI strategies for LDL‐C, sequentially.
Table F. Model‐based estimated effects of combinations of QI strategies for populations with lower and higher mean LDL‐C at baseline
| Lower baseline LDL‐C ≤107 mg/dL | |||
| Combinations of QI strategies (no. of arms) | Post‐intervention meana LDL‐C mg/dL (95% CrI)b | Absolute mean differencecLDL‐C mg/dL (95% CrI) | |
| Five most common combinations of QI strategies observed in included studies reporting LDL‐C | |||
| CM + PE + PSM (n = 16) | 90.63 (88.85 to 92.41) | ‐3.84 (‐5.77 to ‐1.87) | |
| CM + TC + PE + PSM (n = 7) | 88.38 (86.55 to 90.22) | ‐6.08 (‐8.07 to ‐4.11) | |
| CM + EPR + PE + PSM (n = 5) | 90.85 (88.44 to 93.26) | ‐3.61 (‐6.21 to ‐1.00) | |
| CM + FR + PE + PSM (n = 4) | 89.41 (87.16 to 91.66) | ‐5.05 (‐7.52 to ‐2.60) | |
| CM + TC + PE (n = 4) | 88.73 (86.63 to 90.82) | ‐5.73 (‐7.93 to ‐3.61) | |
| Sequential combination of the model‐estimated five most effective QI strategies for LDL‐C | |||
| TC + PE | 90.33 (88.05 to 92.61) | ‐4.13 (‐6.38 to ‐1.93) | |
| TC + PE + CM | 88.73 (86.63 to 90.82) | ‐5.73 (‐7.93 to ‐3.61) | |
| TC + PE + CM + FR | 87.52 (84.94 to 90.05) | ‐6.94 (‐9.69 to ‐4.33) | |
| TC + PE + CM + FR + PSM | 87.17 (84.90 to 89.39) | ‐7.29 (‐9.76 to ‐4.91) | |
| Higher baseline LDL‐C > 107 mg/dL | |||
| Combinations of QI strategies (no. of arms) | Post‐intervention meana LDL‐C mg/dL (95% CrI)d | Absolute mean differencecLDL‐C mg/dL (95% CrI) | |
| Five most common combinations of QI strategies observed in included studies reporting LDL‐C | |||
| CM + TC + PE + PSM (n = 10) | 105.40 (103.02 to 107.76) | ‐3.07 (‐5.69 to ‐0.51) | |
| CM + PE (n = 8) | 108.23 (105.64 to 110.85) | ‐0.25 (‐2.83 to 2.39) | |
| CM + PE + PSM (n = 7) | 108.47 (105.99 to 110.93) | ‐0.02 (‐2.61 to 2.60) | |
| TC + PE + PSM (n = 4) | 107.47 (104.74 to 110.21) | ‐1.00 (‐3.92 to 1.91) | |
| CM + PE + PSM + PR (n = 4) | 109.49 (106.23 to 112.74) | 1.00 (‐2.31 to 4.33) | |
| Sequential combination of the model‐estimated five most effective QI strategies for LDL‐C | |||
| TC + CM | 103.33 (100.69 to 105.98) | ‐5.15 (‐7.59 to ‐2.70) | |
| TC + CM + CR | 102.96 (99.16 to 106.70) | ‐5.52 (‐9.24 to ‐1.89) | |
| TC + CM + CR + CE | 102.46 (98.09 to 107.01) | ‐6.01 (‐10.38 to ‐1.54) | |
| TC + CM + CR + CE + FR | 102.82 (97.97 to 107.68) | ‐5.65 (‐10.51 to ‐0.81) | |
Abbreviations -QI strategies: AF = audit and feedback, CE = clinician education, CM = case management, CQI = continuous quality improvement, CR = clinician reminders, EPR = electronic patient registry, FI = financial interventions, FR = facilitated relay, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes; Other: CrI = credible interval, LDL-C = low-density lipoprotein cholesterol, mg/dL = milligrams per decilitre, QI = quality improvement
Missing estimates of standard errors were imputed for 137/394 arms (35%) using a uniform distribution with a minimum of 0 and maximum of 13.
Missing estimates of intracluster correlation coefficients were imputed for 78/90 cluster-RCT arms (87%) using an outcome-specific normal distribution logit_ICC[i,j] ~ dnorm(-3.29464, (1/(0.77787*0.77787))) as described in Konnyu KJ, Taljaard M, Ivers NM, Moher D, Grimshaw JM. Imputing intracluster correlation coefficients from a posterior predictive distribution is a feasible method of dealing with unit of analysis errors in a meta-analysis of cluster RCTs.J Clin Epidemiol. 2021 Nov;139:307-318. doi: 10.1016/j.jclinepi.2021.06.011. Epub 2021 Jun 22. PMID: 34171503.
The following prior distributions in the Bayesian analyses: post-treatment mean in the absence of intervention (no QI strategy) ~ N(100,100); post-treatment mean when QI strategy is present ~ N(0,4); main effect of the baseline interaction~N(0,4); ψ~N(0,4); all τ~U(0,2).
aMedian of the posterior distribution of the post-intervention means.
bPost-intervention mean change in patients with lower baseline LDL-C who did not receive QI strategy, 94.46 (95% CrI 93.48 to 95.47).
cMedian of the posterior distribution of the post-intervention absolute mean difference.
dPost-intervention mean change in patients with higher LDL-C who did not receive QI strategy, 108.48 (95% CrI 107.26 to 109.69).
Retinopathy screening
Data were included from 122 arms in 58 RCTs. Frequency of QI strategies included: patient education (32%, 39/122), clinician education (28%, 34/122), case management (23%, 28/122), audit and feedback (20%, 24/122), promotion of self‐management (20%, 24/122), team changes (19%, 23/122), clinician reminders (16%, 20/122), electronic patient registry (12%, 15/122), facilitated relay of clinical information (12%, 15/122), patient reminders (12%, 15/122), continuous quality improvement (6%, 7/122) and financial incentives (2%, 3/122).
Baseline rates of retinopathy screening were generally comparable between arms within studies but varied across studies (0% to 88% screened at baseline (median 42%, IQR 23% to 67%), where reported (n = 81/122 arms)).
Effects of individual QI strategies
Table G provides the odds ratio (OR) and order for the QI strategies. As effects would depend on the baseline rates of screening uptake, we report illustrated examples of the effect size if baseline levels of retinopathy screen were 30%, 50% or 70%. Based on our baseline model, patient education, patient reminders, team changes and facilitated relay of clinical information were associated with the largest improvements in retinopathy screening and case management, clinician education, clinician reminders and 'Other QI' strategies were associated with the lowest improvement in retinopathy screening at follow‐up.
Table G. Effect of QI strategy on proportion of patients who received retinopathy screening
| Predicted mean proportion of patients screened given different baseline control proportions | |||||
| QI strategy | OR (95% CrI)a in patients who received QI strategyb | Order | 30% baseline screening (95% CrI) | 50% baseline screening (95% CrI) | 70% baseline screening (95% CrI) |
| CM | 1.09 (0.66 to 1.78) | Bottom | 0.32 (0.22 to 0.43) | 0.52 (0.40 to 0.64) | 0.72 (0.61 to 0.81) |
| TC | 1.60 (0.89 to 2.79) | Top | 0.41 (0.28 to 0.54) | 0.62 (0.47 to 0.74) | 0.79 (0.67 to 0.87) |
| EPR | 1.39 (0.68 to 2.43) | Middle | 0.37 (0.23 to 0.51) | 0.58 (0.41 to 0.71) | 0.76 (0.61 to 0.85) |
| CE | 1.16 (0.82 to 1.63) | Bottom | 0.33 (0.26 to 0.41) | 0.54 (0.45 to 0.62) | 0.73 (0.66 to 0.79) |
| CR | 1.10 (0.70 to 2.09) | Bottom | 0.32 (0.23 to 0.47) | 0.52 (0.41 to 0.68) | 0.72 (0.62 to 0.83) |
| FR | 1.51 (0.57 to 3.65) | Top | 0.39 (0.20 to 0.61) | 0.60 (0.36 to 0.78) | 0.78 (0.57 to 0.89) |
| PE | 1.76 (1.07 to 2.96) | Top | 0.43 (0.31 to 0.56) | 0.64 (0.52 to 0.75) | 0.80 (0.71 to 0.87) |
| PSM | 1.29 (0.67 to 2.46) | Middle | 0.36 (0.22 to 0.51) | 0.56 (0.40 to 0.71) | 0.75 (0.61 to 0.85) |
| PR | 1.70 (0.79 to 3.57) | Top | 0.42 (0.25 to 0.60) | 0.63 (0.44 to 0.78) | 0.80 (0.65 to 0.89) |
| Other | 1.12 (0.76 to 1.50) | Bottom | 0.32 (0.25 to 0.39) | 0.53 (0.43 to 0.60) | 0.72 (0.64 to 0.78) |
Abbreviations-QI strategies: AF = audit and feedback, CE = clinician education, CM = case management, CQI = continuous quality improvement, CR = clinician reminder, CrI = credible interval, EPR = electronic patient registry, OR = odds ratio, PE = patient education, PR = patient reminder, PSM = promotion of self-management, QI = quality improvement, TC = team change
Data included 122 arms from 58 RCTs. Frequency of QI strategies included: AF (n = 24, 20%), CM (n = 28, 23%), TC (n = 23, 19%), EPR (n = 15, 12 %), CE (n = 34, 28%), CR (n = 20, 16%), FR (n = 15, 12%), PE (n = 39, 32%), PSM (n = 24, 20%), PR (n = 15, 12%), CQI (n = 7, 6%), and FI (n = 3, 2%). For the purpose of analysis, we combined AF, CQI and FI into one 'Other' category (n = 31; 25%).
The following prior distributions in the Bayesian analyses: post-treatment estimate in the absence of intervention ~ N(logit(0.43, 0.95)), post-treatment estimate when QI strategy is present ~ N(0,1.04), all standard deviations of the distribution of true effect sizes~U(0,2).
aMedian of the posterior distribution of the post-intervention OR.
bPost-intervention proportion in patients who did not receive any QI strategy, 0.59 (95% CrI 0.42 to 0.82).
Effects of combinations of QI strategies
Studies that included retinopathy screening as an outcome evaluated 58 unique programmes. The median frequency of evaluation for each unique programme was 1 (range 1 to 6; IQR 1 to 1) (Web Appendix 6). The median number of QI strategies in the programme arms was 3 (range 1 to 7; IQR 2 to 4). The median number of QI strategies in the control arms was 0 (range 0 to 4; IQR 0 to 1).
Table H. Model‐based estimated effects of combinations of QI strategies for retinopathy screening assuming different baseline screening rates
| Predicted mean proportion of patients screened given different baseline control proportions | |||
| Combinations of QI strategies (no. of arms) |
30% baseline screening (95% CrI) |
50% baseline screening (95% CrI) |
70% baseline screening (95% CrI) |
| Most common combinations of QI strategies observed 3 or more times in included studies reporting retinopathy screening | |||
| CM + TC + PE + PSM (n = 6) | 0.63 (0.46 to 0.77) | 0.80 (0.67 to 0.89) | 0.90 (0.82 to 0.95) |
| CM + PE + PSM (n = 4) | 0.52 (0.34 to 0.68) | 0.72 (0.55 to 0.83) | 0.85 (0.74 to 0.92) |
| CE + CR + AF (n = 3)a | 0.37 (0.24 to 0.54) | 0.58 (0.42 to 0.73) | 0.76 (0.63 to 0.87) |
| Sequential combinations of the most effective 5 QI strategies as estimated from the model for retinopathy screening | |||
| PE + PR | 0.57 (0.34 to 0.77) | 0.75 (0.55 to 0.89) | 0.88 (0.74 to 0.95) |
| PE + PR + TC | 0.67 (0.41 to 0.86) | 0.83 (0.62 to 0.94) | 0.92 (0.79 to 0.97) |
| PE + PR + TC + FR | 0.76 (0.44 to 0.93) | 0.88 (0.65 to 0.97) | 0.94 (0.81 to 0.99) |
| PE + PR + TC + FR + EPR | 0.81 (0.49 to 0.95) | 0.91 (0.69 to 0.98) | 0.96 (0.84 to 0.99) |
Abbreviations: CE = clinician education, CM = case management, CR = clinician reminders, CrI = credible interval, EPR = electronic patient registry, FR = facilitated relay, n = number, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes
aEstimated using 'Other' category which included audit and feedback, financial incentives and continuous quality improvement.
Foot screening
Data were included from 89 arms in 43 RCTs. Frequency of QI strategies included: clinician education (29%, 26/89), patient education (28%, 25/89), audit and feedback (22%, 20/89), case management (20%, 18/89), team changes (20%, 18/89), promotion of self‐management (19%, 17/89), clinician reminders (17%, 15/89), electronic patient registry (10%, 9/89), continuous quality improvement (9%, 8/89), facilitated relay of clinical information (7%, 6/89), patient reminders (6%, 5/89) and financial incentives (0%, 0/89). For the purpose of analysis, we combined audit and feedback, continuous quality improvement and financial incentives into one 'Other' category (25%, 23/89).
Baseline rates of foot screening were generally comparable between arms within studies but varied across studies (0% to 90% screened at baseline (median 44% IQR 31% to 60%), where reported (n = 63/89 arms)).
Effects of individual QI strategies
Table I provides the odds ratio and order for the QI strategies. Based on our baseline model, QI strategies patient education, team changes and 'Other QI' were associated with the largest improvements in foot screening and facilitated relay of clinical information, electronic patient registry, clinician education and case management strategies were associated with the lowest improvement in foot screening at follow‐up.
Table I. Effects of QI strategy on proportion of patients who received foot screening
| Predicted proportion of patients screened given different baseline control proportions | |||||
| QI strategy | OR (95% CrI)a in patients who received QI strategyb | Order | 30% baseline screening (95% CrI) | 50% baseline screening (95% CrI) | 70% baseline screening (95% CrI) |
| CM | 1.09 (0.59 to 1.83) | Bottom | 0.32 (0.20 to 0.44) | 0.52 (0.37 to 0.65) | 0.72 (0.58 to 0.81) |
| TC | 2.01 (0.92 to 4.01) | Top | 0.46 (0.28 to 0.63) | 0.67 (0.48 to 0.80) | 0.82 (0.68 to 0.90) |
| EPR | 0.95 (0.35 to 2.42) | Bottom | 0.29 (0.13 to 0.51) | 0.49 (0.26 to 0.71) | 0.69 (0.45 to 0.85) |
| CE | 1.03 (0.75 to 1.47) | Bottom | 0.31 (0.24 to 0.39) | 0.51 (0.43 to 0.59) | 0.71 (0.64 to 0.77) |
| CR | 1.30 (0.71 to 2.57) | Middle | 0.36 (0.23 to 0.52) | 0.56 (0.41 to 0.72) | 0.75 (0.62 to 0.86) |
| FR | 0.85 (0.35 to 2.16) | Bottom | 0.27 (0.13 to 0.48) | 0.46 (0.26 to 0.68) | 0.66 (0.45 to 0.83) |
| PE | 2.32 (1.09 to 5.13) | Top | 0.50 (0.32 to 0.69) | 0.70 (0.52 to 0.84) | 0.84 (0.72 to 0.92) |
| PSM | 1.28 (0.47 to 3.42) | Middle | 0.35 (0.17 to 0.59) | 0.56 (0.32 to 0.77) | 0.75 (0.52 to 0.89) |
| PR | 1.39 (0.46 to 3.70) | Middle | 0.37 (0.16 to 0.61) | 0.58 (0.31 to 0.79) | 0.76 (0.52 to 0.90) |
| Other | 1.55 (1.04 to 2.06) | Top | 0.40 (0.31 to 0.47) | 0.61 (0.51 to 0.67) | 0.78 (0.71 to 0.83) |
Abbreviations: AF = audit and feedback, CE = clinician education, CM = case management, CR = clinician reminder, CrI = credible interval, EPR = electronic patient registry, OR = odds ratio, PE = patient education, PR = patient reminder, PSM = promotion of self-management, QI = quality improvement, TC = team change
Data included 89 arms from 43 RCTs. Frequency of QI strategies included: AF (20, 22%), CM (n = 18, 20%), TC (n = 18, 20%), EPR (n = 9, 10%), CE (n = 26, 29%), CR (n = 15, 17%), FR (n = 6, 7%), PE (n = 25, 28%), PSM (n = 17, 19%), PR (n = 5, 6%), CQI (n = 8, 9%) and FI (n = 0, 0%). For the purpose of analysis, we combined AF, CQI and FI into one 'Other' category (n = 23, 25%).
The following prior distributions in the Bayesian analyses: post-treatment estimate in the absence of intervention ~ N(logit(0.49, 1.01)), post-treatment estimate when QI strategy is present ~ N(0,1.04), all standard deviations of the distribution of true effect sizes~U(0,2).
aMedian of the posterior distribution of the post-intervention OR.
bEstimated post-intervention proportion in patients who did not receive any QI strategy, 0.73 (95% CrI 0.50 to 1.06).
Effects of combinations of QI strategies
Studies that included foot screening as an outcome evaluated 40 different unique programmes. The median frequency of evaluation for each unique programme was 1 (range 1 to 6; IQR 1 to 1 ‐ Web Appendix 6). The median number of QI strategies in the programme arms was 3 (range 1 to 7; IQR 2 to 4). The median number of QI strategies in the control arms was 0 (range 0 to 4; IQR 0 to 1).
Table J. Model‐based estimated effects of combinations of QI strategies for foot screening assuming different baseline screening rates
| Predicted proportion of patients screened given different baseline control proportions | |||
| Combinations of QI strategies (no. of arms) |
30% baseline screening (95% CrI) |
50% baseline screening (95% CrI) |
70% baseline screening (95% CrI) |
| Most common combinations of QI strategies observed 3 or more times in included studies reporting foot screening | |||
| CM + TC + PE + PSM (n = 6) | 0.73 (0.51 to 0.88) | 0.87 (0.70 to 0.95) | 0.94 (0.85 to 0.98) |
| CM + PE + PSM (n = 3) | 0.58 (0.35 to 0.79) | 0.76 (0.56 to 0.90) | 0.88 (0.75 to 0.95) |
| CE + CR + AF (n = 3)a | 0.47 (0.30 to 0.65) | 0.67 (0.50 to 0.81) | 0.83 (0.70 to 0.91) |
| Sequential combinations of the most effective 5 QI strategies as estimated from the model for foot screening | |||
| PE + TC | 0.66 (0.41 to 0.84) | 0.82 (0.61 to 0.93) | 0.91 (0.79 to 0.97) |
| PE + TC + Other | 0.75 (0.50 to 0.90) | 0.88 (0.70 to 0.95) | 0.94 (0.84 to 0.98) |
| PE + TC + Other + PR | 0.81 (0.48 to 0.95) | 0.91 (0.68 to 0.98) | 0.96 (0.83 to 0.99) |
| PE + TC + Other + PR + CR | 0.84 (0.54 to 0.96) | 0.93 (0.73 to 0.98) | 0.97 (0.87 to 0.99) |
Abbreviations: CE = clinician education, CM = case management, CR = clinician reminders, CrI = credible interval, EPR = electronic patient registry, FR = facilitated relay, n = number, PE = patient education, PR = patient reminders, PSM = promotion of self-management, QI = quality improvement, TC = team changes
aEstimated using 'Other' category which included audit and feedback, financial incentives and continuous quality improvement.
Smoking cessation
Data included 93 arms from 46 RCTs. Frequency of strategies included: patient education (48%, 45/89), promotion of self‐management (33%, 31/93), case management (32%, 30/93), clinician education (28%, 26/93), team changes (23%, 21/93), audit and feedback (16%, 15/93), patient reminders (13%, 12/93), clinician reminders (12%, 11/93), electronic patient registry (12%, 11/93), facilitated relay of clinical information (11%, 10/93), continuous quality improvement (2%, 2/93) and financial incentives (2%, 2/93). For the purpose of analysis, we combined audit and feedback, continuous quality improvement and financial incentives into one 'Other' category (19%, 19/93).
Effects of individual QI strategies
Based on our baseline model, we found no association (measured by odds ratios) between any of the QI strategies and post‐intervention rates of smoking cessation. We interpret the lack of an observed effect for this outcome as a negative control, as most studies did not target smoking cessation in their QI intervention.
Effects of combinations of QI strategies
Studies that included smoking cessation as an outcome evaluated 40 different unique programmes. The median frequency of evaluation for each unique programme was 1 (range 1 to 5; IQR 1 to 1). The median number of QI strategies in the programme arms was 4 (range 1 to 8; IQR 3 to 5). The median number of QI strategies in the control arms was 0 (range 0 to 5; IQR 0 to 1).
Other outcomes
Aspirin use
Data included 63 arms from 31 RCTs. Reporting on dosing and delivery of acetylsalicylic acid (ASA) varied widely across studies with some studies not reporting dosing and delivery details at all and others noting specific doses and timing. We observed other variations in definitions of outcome with some studies reporting aspirin and others reporting use of antiplatelet agents, clopidogrel, antithrombotics or various combinations thereof. Baseline rates of patients on ASA were generally comparable between arms within studies but varied across studies (1% to 85% on ASA at baseline (median 47%, IQR 25% to 65%), where reported (n = 44/63 arms)). Frequency of strategies included: audit and feedback (16%, 10/63), case management (29%, 18/63), team changes (29%, 18/63), electronic patient registry (11%, 7/63), clinician education (32%, 20/63), clinician reminders (17%, 11/63), facilitated relay of clinical information (6%, 4/63), patient education (43%, 27/63), promotion of self‐management (24%, 15/63), patient reminders (11%, 7/63), continuous quality improvement (5%, 3/63) and financial incentives (2%, 1/63).
Effects of QI interventions
Amongst trials that reported proportion of patients on ASA, 87% (26/30) reported greater improvement in patients on ASA at follow‐up amongst patients randomised to 'more intensive' QI compared to 'less intensive' QI. Less than half of these effects were statistically significant (43%, 13/30).
Statin use
Data included 107 arms from 52 RCTs. Reporting on type, dosing and delivery of statins varied widely across studies. Baseline rates of patients on statins were generally comparable between arms within studies but varied across studies (0% to 93% on statins at baseline (median 48%, IQR 31% to 67%), where reported (n = 84/107 arms)). Frequency of strategies included: audit and feedback (11%, 12/107), case management (35%, 37/107), team changes (28%, 30/107), electronic patient registry (10%, 11/107), clinician education (29%, 31/107), clinician reminders (15%, 16/107), facilitated relay of clinical information (15%, 16/107), patient education (49%, 53/107), promotion of self‐management (29%, 31/107), patient reminders (10%, 11/107), continuous quality improvement (3%, 3/107) and financial incentives (4%, 4/107).
Effects of QI interventions
Amongst trials that reported proportion of patients on statins, 75% (38/51) reported greater improvement in patients on statins at follow‐up (27% (14/51) statistically significant) amongst patients randomised to 'more intensive' QI compared to 'less intensive' QI.
Antihypertensive drug use
Data included 98 arms from 48 RCTs. Reporting on type, dosing and delivery of antihypertensives varied widely across studies, as did outcome measures used (e.g. multiple studies reported combined outcome measure of diverse antihypertensives). Baseline rates of patients on antihypertensives were generally comparable between arms within studies but varied across studies (0% to 97% on antihypertensives at baseline (median 59% IQR 38% to 77%), where reported (n = 84/98 arms)). Frequency of strategies included: audit and feedback (12%, 12/98), case management (33%, 32/98), team changes (34%, 33/98), electronic patient registry (12%, 12/98), clinician education (23%, 23/98), clinician reminders (12%, 12/98), facilitated relay of clinical information (10%, 10/98), patient education (49%, 48/98), promotion of self‐management (31%, 30/98), patient reminders (9%, 9/98), continuous quality improvement (2%, 2/98) and financial incentives (4%, 4/98).
Effects of QI interventions
Amongst trials that reported proportion of patients on antihypertensives, 69% (33/48) reported greater improvement in patients on antihypertensives at follow‐up (19% (9/48) statistically significant) amongst patients randomised to 'more intensive' QI compared to 'less intensive' QI.
Hypertension control
Data included 158 arms from 76 RCTs. Reporting varied on whether blood pressure control was measured using SBP, DBP, or SBP and DBP combined and what cutoff values were used to define hypertension. Where needed, we flipped the number of patients "without blood pressure control" to ensure all data were formatted to be the number of patients with hypertension control (i.e. the desirable outcome). Baseline rates of patients with hypertension control were generally comparable between arms within studies but varied across studies (0% to 97% (median 33%, IQR 24% to 51%), where reported (n = 119/158 arms)). Frequency of strategies included: audit and feedback (15%, 24/158), case management (36%, 57/158), team changes (30%, 48/158), electronic patient registry (14%, 22/158), clinician education (26%, 41/158), clinician reminders (13%, 20/158), facilitated relay of clinical information (16%, 26/158), patient education (46%, 72/158), promotion of self‐management (34%, 54/158), patient reminders (13%, 21/158), continuous quality improvement (4%, 7/158) and financial incentives (1%, 1/158).
Effects of QI interventions
Amongst trials that reported proportion of patients with blood pressure control, 76% (56/74) reported greater improvement in patients with blood pressure control at follow‐up (24% (18/74) statistically significant) amongst patients randomised to 'more intensive' QI compared to 'less intensive' QI.
Renal screening
Data included 42 arms from 20 RCTs. Studies reported renal screening using various tests and terms (e.g. microalbumin test, serum creatinine, urinanalysis, nephropathy screening). Baseline rates of patients who received screening were generally comparable between arms within studies but varied across studies (0% to 91% screened baseline; (median 39%, IQR 25% to 65%), where reported (n = 30/42 arms)). Frequency of strategies included: audit and feedback (21%, 9/42), case management (19%, 8/42), team changes (19%, 8/42), electronic patient registry (17%, 7/42), clinician education (21%, 9/42), clinician reminders (21%, 9/42), facilitated relay of clinical information (10%, 4/42), patient education (31%, 13/42), promotion of self‐management (19%, 8/42), patient reminders (10%, 4/42), continuous quality improvement (10%, 4/42) and financial incentives (0%, 0/42).
Effects of QI interventions
Amongst trials that reported proportion of patients who received renal screening, 90% (18/20) reported greater improvement in patients screened at follow‐up (75% (15/20) statistically significant) amongst patients randomised to 'more intensive' QI compared to 'less intensive' QI.
Patients who experienced harms
Data included 193 arms from 90 RCTs. Studies reported harms quite variably. Often studies reported the domains of hyper‐ or hypoglycaemia but reported number of events, proportion of patients to experience events, number of symptoms, and various other measures and metrics. Frequency of strategies included: audit and feedback (1%, 2/193), case management (47%, 90/193), team changes (22%, 42/193), electronic patient registry (23%, 43/193), clinician education (15%, 29/193), clinician reminders (8%, 16/193), facilitated relay of clinical information (35%, 68/193), patient education (52%, 10/193), promotion of self‐management (61%, 118/193), patient reminders (15%, 28/193), continuous quality improvement (1%, 1/193) and financial incentives (1%, 1/193).
Effects of QI interventions
Amongst trials that reported proportion of patients experiencing a hypoglycaemic event, 50% (29/58) reported an increase in patients experiencing a hypoglycaemic event at follow‐up (8% (5/58) statistically significant) amongst patients randomised to 'more intensive' QI compared to 'less intensive' QI.
Discussion
Summary of main results
There is a significant body of evidence evaluating quality improvement (QI) strategies for diabetes, which continues to increase year‐on‐year. HbA1c was the most commonly reported outcome; screening outcomes, and outcomes related to cardiovascular medications, smoking and harms, were reported infrequently. The most frequently evaluated QI strategies across all study arms included patient education (50%; 592/1190), promotion of self‐management (45%; 539/1190) and case management (39%; 461/1190), while the least frequently evaluated QI strategies included audit and feedback (6%; 67/1190), financial incentives (3%; 34/1190) and continuous quality improvement (2%; 24/1190). We combined the less frequent strategies audit and feedback, financial incentives and continuous quality improvement into an 'Other' category for all outcomes models to ensure stable results in outcomes with fewer studies and comparability across all models. The majority of studies evaluated combinations of QI strategies; the median number of QI strategies in active programme arms was four and 220 different combinations were evaluated across included studies. Given this, the main focus of this review was to estimate the contributions of individual QI strategies using multivariable meta‐regression that controlled for the presence of co‐occurring QI strategies. The model assumes the effect of QI programmes for each outcome assessed is through the additive effect of each QI strategy present. Assuming the models approximately capture the reality of the studies modelled, the estimates give us a sense of what strategies may be more feasible than others to be implemented in a real‐world setting and what approximate differences in outcomes may be associated with their various combinations (comprised of these estimates) in the future.
Effects of multicomponent QI strategies
Whilst the majority of effects of individual QI strategies were modest, the key finding of this review is that multicomponent QI programmes for diabetes care (comprised of effective QI strategies) may achieve meaningful population‐level improvements across the majority of outcomes. In fact, the effects that may be achieved from multicomponent QI programmes approximate the minimal clinically important difference for pharmaceutical interventions in glycaemic control. While the relative effects appear smaller for lower‐risk populations, and for outcomes such as blood pressure and cholesterol, the potential impact on population health from these interventions may be substantial. As described in Rose's germinal paper describing the Paradox of Prevention, the greatest population gains can be expected from small effects applied across broad populations (Rose 1981). The evidence summarised in this review offers insights into ways to achieve such gains.
Table K highlights the estimated post‐treatment changes associated with the most effective QI strategies for each outcome (split by baseline values for HbA1c, SBP and LDL). Larger effects were estimated when QI strategies are conducted in populations with worse baseline control of glycaemia and cardiovascular risk factors. The estimated effects are likely important at a population level for glycaemic and blood pressure control, retinopathy and foot screening; but not for LDL or smoking.
Table K. Summary of most effective combinations of three QI strategies for each of the key outcomes1
| Outcome | Three most effective QI strategies | Estimated absolute mean difference of three QI strategies combined (natural units) (95% CrI)2 |
| Continuous | ||
|
HbA1c, baseline ≤ 8.3% |
PR + PSM + CE* | ‐0.41 (‐0.61 to ‐0.22) % HbA1c |
|
HbA1c, baseline > 8.3% |
CM + PE + EPR | ‐0.62 (‐0.84 to ‐0.39) % HbA1c |
|
SBP, baseline ≤ 136 mmHg |
TC + PE + PSM | ‐2.14 (‐3.80 to ‐0.52) mmHg |
|
SBP, baseline > 136 mmHg |
CM + TC + PSM | ‐4.39 (‐6.20 to ‐2.56) mmHg |
|
LDL, baseline ≤ 107 mg/dL |
TC + PE + CM | ‐5.73 (‐7.93 to ‐3.61) mg/dL |
|
LDL, baseline > 107 mg/dL |
TC + CM + CR | ‐5.52 (‐9.24 to ‐1.89) mg/dL |
| Dichotomous | Three most effective QI strategies | Estimated proportion screened assuming 50% compliance at baseline |
| Retinopathy screening | PE + PR + TC | 0.83 (0.62 to 0.94) |
| Foot screening | PE + TC + Other | 0.88 (0.70 to 0.95) |
Abbreviations: CE = clinician education, CM = case management, CR = clinician reminder, CrI = credible interval, DBP: diastolic blood pressure, EPR = electronic patient registry, LDL = low-density lipoprotein, PE = patient education, PR = patient reminder, PSM = promotion of self-management, QI = quality improvement, SBP = systolic blood pressure, TC = team change
* Model estimates of PR and PSM were virtually identical and thus their order in the sequential combination here is for illustrative purposes.
1Smoking not included due to the overall lack of benefits from any QI strategy, which we interpret as a negative control for the model used (most studies did not address smoking cessations explicitly in their intervention model compared to other diabetic QI and smoking is a difficult behaviour to change).
2The wide credibility intervals around the point estimates highlight the aim of these analyses to produce a probability distribution rather than ascertain the "correct answer", recognising the limits with causal interpretation with the approach.
Effects for individual QI strategies
Table L summarises the order of QI strategies across different outcomes. All QI strategies were ordered Top for at least one outcome. These results highlight that a wide range of QI strategies can work, indicating the importance of matching programme components to the specific determinants (i.e. barriers and enablers) of the targeted behaviours of the health professionals and patients living with diabetes. However, four QI strategies were consistently ordered as Top across the majority of outcomes:
Team changes (TC) was ordered Top across all outcomes.
Case management (CM) was ordered Top for higher HbA1c, SBP and LDL (but middle for lower HbA1c and bottom for retinopathy and foot screening).
Patient education (PE) was ordered Top for higher HbA1c, lower SBP and LDL, retinopathy and foot screening, Middle for higher SBP and Bottom for lower HbA1c and higher LDL.
Patient self‐management (PSM) was ordered Top for HbA1c and SBP, and Middle for LDL, retinopathy and foot screening.
Case management and team changes both provide more focused attention and/or increased interactions between health professionals and the patient. Case management supports better co‐ordination of diabetes care to support the patient and their primary care team. Team changes involves adding a new team member or changing how teams work together, or revising the roles of team members to address aspects of diabetes care.
Patient education and promotion of self‐management provide education and support for people living with diabetes to understand and manage their condition. Patient education tends to focus on knowledge provision whereas promotion of self‐management focuses on a broader range of behavioural determinants and/or providing further resources for patients to manage their diabetes.
The results suggest that those planning diabetes QI programmes might consider including one of team changes and case management and one of promotion of self‐management and patient education. The specific choice of which strategies to use might depend on the targeted population (e.g. team changes and promotion of self‐management may be the preferred strategies if focusing on HbA1c and SBP, especially in populations with better baseline management of these outcomes) and local resources. As these strategies tend to involve ongoing expenses for human resources, it is important for future studies to consider opportunity costs and economic implications for these approaches.
Of note, the 'Other' category, which combined audit and feedback, financial incentives and continuous quality improvement, was ordered Top for SBP, higher baseline LDL and foot screening, suggesting that further evaluation of the benefits of QI strategies captured within this category may be warranted.
Table L. Order of relative effectiveness of quality improvement strategies, by baseline values for key outcomes1
| Order | ||||||||
| QI strategy | HbA1c ≤ 8.3% | HbA1c > 8.3% | SBP ≤ 136 mmHg | SBP > 136 mmHg | LDL ≤ 107 mg/dL | LDL > 107 mg/dL | Retinopathy screening | Foot screening |
| CM | Middle | Top | Top | Top | Top | Top | Bottom | Bottom |
| TC | Top | Top | Top | Top | Top | Top | Top | Top |
| EPR | Top | Top | Middle | Bottom | Bottom | Bottom | Middle | Bottom |
| CE | Top | Bottom | Bottom | Bottom | Bottom | Top | Bottom | Bottom |
| CR | Bottom | Top | Middle | Middle | Middle | Top | Bottom | Middle |
| FR | Middle | Middle | Top | Middle | Top | Middle | Top | Bottom |
| PE | Bottom | Top | Top | Middle | Top | Bottom | Top | Top |
| PSM | Top | Top | Top | Top | Middle | Middle | Middle | Middle |
| PR | Top | Middle | Bottom | Bottom | Bottom | Bottom | Top | Middle |
| Other | Bottom | Middle | Top | Top | Bottom | Top | Bottom | Top |
Abbreviations: CE = clinician education, CM = case management, CR = clinician reminder, EPR = electronic patient registry, FR = facilitated relay of clinical information,PE = patient education, PR = patient reminder, PSM = promotion of self-management, QI = quality improvement, TC = team change
1Smoking not included due to the overall lack of benefits from any QI strategy.
Overall completeness and applicability of evidence
The massive evidence base summarised in this review contains data from over 500 trials using diverse combinations of QI strategies to improve diabetes care. The prior version of this review, published in 2012, included only 142 trials. Although part of the increase in evidence is due to a more comprehensive search strategy compared to the prior version, we have also observed a rapid increase in trials in the last decade.
Although we report on a range of outcomes relevant to improving the quality of diabetes care and outcomes for people living with diabetes, most of the evidence comes from high‐income settings and the generalisability of these findings to health systems in low‐ and middle‐income settings is uncertain. We did not explore the impact of factors such as gender, race, ethnicity and language in the present review, although we have explored these factors in an earlier version of the review including 278 studies. In that review, we observed that less than a third of included trials (n = 95) had equity‐relevant considerations: 64 trials focused on a disadvantaged population specifically, and 31 trials focused on general diabetes populations but conducted subgroup analyses to assess the extent to which the intervention had a differential effect on disadvantaged subgroups.
Of note, the interventions in the trials disproportionately focused on glycaemic control compared to cardiovascular and screening outcomes. Very few studies reported harms from care. Some important outcomes relevant to diabetes, such as patient experience and mental health, were not captured in this review. Further, our approach does not examine for unintended consequences of the targeted QI programmes, where a focus on specific aspects of care could, theoretically, lead to decline in the delivery or quality for other aspects of care for diabetes or other conditions ‐ especially if resources or capacity for care are limited.
Finally, much of this evidence is from a time with fewer effective drugs and less widespread technology. For example in the last five years, new drugs such as SGLT2 inhibitors and technologies such as continuous glucose monitoring have become more widely available. Current attempts to improve diabetes care would likely focus on the implementation of novel drug classes that reduce cardiovascular risk and/or use of technology that might improve patient safety and quality of life.
Quality of the evidence
The quality of the evidence, as assessed by an adapted version of Cochrane's EPOC risk of bias (RoB) tool, was generally moderate. Most studies were assessed to have unclear or high risk of bias (based on the conservative final study judgement of unclear or high if one or more RoB domains was unclear or high, respectively).
Unfortunately, as many domains were unclear due to the lack of information, it is difficult to distinguish true risk of bias from poor reporting (across RoB domains, the range of studies assessed to be unclear was from 11% to 59% (median 29%)). We assessed many studies to be at low RoB for most domains (median 50%, ranging from 13% to 87%). Conversely, few studies had high RoB for most domains (median 13.5%, ranging from 2% to 51%), except for the domain assessing adequate methods for addressing incomplete outcome data (n = 51% assessed as high RoB).
Potential biases in the review process
We followed best practices for conducting systematic reviews (comprehensive literature searches, dual‐independent screening and extraction, RoB assessment tailored to study design and strength of evidence (SoE) assessment) and applied advanced multivariable meta‐regression models to enhance the utility of the synthesis for stakeholders.
A strength of this update is the expanded search strategy of six databases and two registries with no restrictions on language. However, as our search did not include grey literature, it is possible that some trials were still missed.
We used a standardised taxonomy to code the content of the QI programmes. The taxonomy is an adapted version of the EPOC taxonomy of interventions, first utilised in a version of this review published in 2006 (Shojania 2006), then in 2012 (Tricco 2012), and refined over time. Coding non‐pharmacological treatment content is challenging, and it is possible that our coding resulted in misspecification of the QI content due to poor study reporting, inconsistent terminology or definitions used in the QI field over time, challenges with QI codes (e.g. many instances of case management also qualify as a team changes), or extractor error. We attempted to reduce extractor error by having two review authors code content independently and frequent team discussions to resolve conflicts. We also conducted multiple spot checks of coded QI strategies and re‐coded (via group consensus) as needed to ensure codes for each QI strategy were consistent across the review. We published a protocol describing our approach to operationalising the QI strategies to make our judgements on these codes explicit for others to replicate (Ivers 2014). The fact that the models consistently distinguished between QI strategies like team changes and case management is reassuring, but further work is needed to unpack the effective subcomponents of these complex QI strategies.
An additional aspect of intervention coding relates to our analytic assumptions. Our models assume that intervention content is wholly captured by the QI strategies specifically considered for this review. This may be a strong assumption if interventions included other QI strategies. However, based on feedback with our stakeholder experts and our experience in coding the intervention content, we do not believe we have missed the coding of key intervention content. Indeed, our experience with using one alternative taxonomy to date (the Behaviour Change Taxonomy) is that it allowed us to dig into the sub‐category content in further depth but that this different taxonomy was complementary rather than at odds with the QI taxonomy (Konnyu 2020; Presseau 2015). Further work comparing the coding of complex interventions using diverse taxonomies is needed.
Our analytic models are both a strength and a potential weakness of our review. We used an arm‐based multivariable meta‐regression approach as a means of making the most of the trials to inform future practice and research. While the approach can be criticised for 'breaking randomisation', we are aware of no other method that allows review authors to flexibly account for arm‐ and study‐level factors of interest to stakeholders when the number of components (and, importantly, the number of their possible combinations) is so large, and they are believed to potentially interact with each other, as well as the population and setting in which they are implemented. The aim of our analyses is modest ‐ not to produce casual claims about the 'true' effect of QI components ‐ but to gain a rough estimate of the association of these components with common diabetes outcomes after controlling for the presence of other components and study factors (where feasible). For example, in the continuous outcomes, we found modelling baseline risk greatly improved the specification of the model. However, no model is perfect and a more flexible, fully specified model of interest was not feasible due to limited data (e.g. the number of times a QI strategy was present versus absent for each specific outcome evaluated). Future studies could allow improved model specification and potential shift in findings ‐ particularly for outcomes or QI strategies that were understudied or underreported.
We acknowledge that the thresholds used to distinguish well‐controlled versus less well‐controlled glycaemia, blood pressure and cholesterol do not reflect values commonly used to inform specific clinical decisions. We used a data‐driven approach (i.e. the median of all baseline values observed in the trials) to define these cut‐offs due to a lack of consensus in the literature for defining thresholds for clinically poor control.
We used the Cochrane EPOC RoB tool for this review to maintain continuity with the previous versions of the review. In addition, the Cochrane RoB 2.0 tool only became available in August 2019 (for individual patient randomised trials) and November 2020 for cluster‐randomised trials (which was further revised in March 2021) (i.e. when we had already completed the majority of the work for this version of the review). We would argue that the Cochrane EPOC RoB tool covers similar areas to the revised Cochrane RoB 2.0 tool. Depending on available resources we will consider assessing risk of bias with the Cochrane RoB 2.0 tool for a future update of the living systematic review.
Agreements and disagreements with other studies or reviews
In comparison to the last version of this review (Tricco 2012), advances in synthesis methods and the accumulation of a larger body of evidence have allowed us to better isolate the effects of various QI strategy components. As a result, the isolated effect sizes estimated in this review for individual QI strategies are smaller. We believe this is a more accurate representation of the possible influence of these strategies as their estimates are no longer confounded by the presence of other strategies but rather the models controlled for the presence of co‐occurring components explicitly.
In reality, QI strategies are generally implemented in combinations; the results from our meta‐regressions allow us to borrow information across all studies of different combinations to contribute to the estimate of the individual effects of QI components to provide a more informed estimate of the possible combinations.
Numerous prior reviews have examined the effects of different subsets of programmes seeking to improve care and/or outcomes for people living with diabetes (Worswick 2013). Such reviews cover everything from modalities of care (e.g. telemedicine) to targets of programmes (e.g. patients). In particular, a number of reviews have focused on patient education and support. In general, such programmes (as variously defined) tend to lead to improvements in glycaemic control. The current review is distinguished by its focus on studies with at least one programme component involving health services QI, and featuring one of 12 QI strategies. Of note, other reviews exist that examine the effects of many of these QI strategies over a range of patient presentations and contexts, including audit and feedback (Ivers 2012), clinician education (Forsetlund 2021), clinician reminders or decision support (Arditi 2017; Pantoja 2019; Shojania 2009).
Authors' conclusions
Implications for practice.
This review summarises data from a massive and continually growing body of randomised trials of quality improvement (QI) strategies to support implementation of best practices in diabetes care. The evidence is clear that interventions featuring combinations of QI strategies may improve quality of diabetes care and likely population outcomes. The effects achieved with combinations of top‐three QI strategies are similar to those achieved by adding effective medications ‐ except QI strategies are applied to entire populations rather than to one patient at a time.
The goal of this work was not to provide a single answer about the effects for a given component (or combination of components) in a complex QI intervention, but to provide a rich set of data from which decision‐makers and researchers can come at the literature in different ways. The results suggest that a wide range of QI strategies can work, indicating the importance of matching programme components to the specific determinants (i.e. barriers and enablers) of the targeted behaviour of the health professionals involved in diabetes care and patients living with diabetes, and local context and resources.
For some QI strategies the evidence is strong enough that clinical policymakers might consider ways to implement, depending on the context, targeted outcomes and patient types. Four QI strategies were consistently ordered as Top across the majority of outcomes suggesting that those planning diabetes QI programmes may consider including one of team changes and case management and one of promotion of self‐management and patient education. The specific choice of which strategies to use might depend on the targeted population (e.g. team changes and promotion of self‐management may be the preferred strategies if focusing on mean glycated haemoglobin (HbA1c) and systolic blood pressure (SBP), especially in populations with better baseline management) and local resources. As these strategies tend to involve ongoing expenses for human resources, it is important for future studies to consider opportunity costs and economic implications for these approaches.
Many of the QI strategies considered in this review are now usual care in certain settings, such as electronic patient registry and patient education and promotion of self‐management, but evidence from this review may help optimise how such strategies are operationalised.
These results provide a starting point for selecting combinations of QI strategies, but best practices in evidence‐based intervention design should be followed. Clinical leaders looking to improve quality of diabetes care might consider the results of these analyses alongside careful thought regarding the nature of the problem from a behavioural perspective. Intervention design needs to go beyond considering what is feasible or interesting in a local setting by also considering external evidence (data about what is likely to work for the desired outcomes in that type of setting), behavioural antecedents (ensuring the strategy is tailored for known barriers and enablers) and local requirements (leveraging existing resources to ensure sustainability), amongst other factors.
Implications for research.
It is crucial that the next 500 studies are more strategically designed to avoid research waste; future studies must ask questions for which we do not already have substantial evidence.
Researchers may explicitly seek to build upon the gaps or opportunities identified here:
testing interventions that have to date been evaluated relatively rarely (e.g. audit and feedback, financial incentives, continuous quality improvement);
evaluating previously un‐tested combinations of QI strategies (e.g. evaluating the benefits of adding additional QI strategies to team changes/case management and promotion of self‐management/patient education);
broadening the range of outcomes considered (e.g. more studies exploring the effects of QI strategies on screening and smoking cessation, patient reported outcomes, harms);
including economic evaluation of costs and benefits of combinations of QI strategies;
evaluating QI programmes in specific equity‐seeking populations (e.g. gender, low socioeconomic status) and in novel contexts;
evaluating QI programmes in low‐ and middle‐income settings; and
exploring ways to optimise QI strategies.
To reduce the risk of research waste, future trials to improve diabetes care could be pursued in the context of implementation science laboratories, with a long‐term commitment to sequential trials that inform practice and contribute to a learning health system (Ivers 2016). The design of future studies can be informed by the predictive results from the models in this review that demonstrate the range of plausible effect sizes to plan sample size for such studies.
There are substantial opportunities to learn more from the existing evidence using the dataset collated in this review. There is considerable variation in how QI strategies were operationalised in the included studies. Therefore, a more detailed analysis and assessment of the content (i.e. component behaviour change techniques) (Konnyu 2020; Michie 2013), intensity and delivery mechanisms, especially of complex interventions featuring case management or team changes, might provide both guidance for intervention developers and ideas on how to optimise interventions for further evaluation. Such explorations could help distinguish between required and discretionary aspects of strategies like team changes and shift the literature from black box type descriptions to more granular descriptions of active ingredients that are more readily operationalised. For example, our finding that clinician education is helpful for glycaemic control amongst patients with lower baseline HbA1c but not higher baseline HbA1c suggests a need to better understand the detailed content of such interventions and whether they can be more systematically tailored to the desired behaviour changes. Another example might be taking a theory‐informed lens to the data, as was done in the past for re‐analyses of audit and feedback systematic reviews (Gardner 2010).
Further research is needed to understand the necessary conditions for a given QI strategy to be successful and the economic impacts of various strategies. In addition, further research is needed to understand the effects of QI strategies on the implementation of novel, highly efficacious diabetes medications and technologies.
We plan to make our dataset available in our online repository to facilitate other groups to undertake this research. Please contact us if you have any specific interests.
Finally, given the continued rapid growth of research in this area, we aim to maintain this as a living review (Appendix 2); we are interested in identifying collaborators who may wish to contribute to this effort.
What's new
| Date | Event | Description |
|---|---|---|
| 2 June 2023 | Amended | Author order corrected (sofware error) |
History
Review first published: Issue 5, 2023
Acknowledgements
This review updates two previous prior non‐Cochrane reviews (Shojania 2006; Tricco 2012). We thank all colleagues who contributed to those reviews. We would like to acknowledge Dr. Issa Dahabreh for his contribution to the overall methodological approach and support during the conduct of the review and Pauline Barbeau for her work on the current review. Paul Miller (Cochrane Effective Practice and Organisation of Care (EPOC)) devised and ran the search strategy. In addition, we thank the following people for their help in assessing the non‐English papers: Jaewoo Cha (Korean), Irena Druce (Polish), Adriana Freitas (Portuguese), Metin Gülmezoglu (Turkish), Lorenzo Moja (Italian), Minna Johansson (Swedish), Jordi Pardo Pardo (Spanish), Bernd Richter (German), Hyeong Sik Ahn (Korean), Xiaoqin Wang (Chinese), Norio Watanabe (Japanese), Kazmi S. Zertasha (Korean).
Cochrane EPOC supported the authors in the development of this review. Jeremy Grimshaw is Emeritus Editor of Cochrane EPOC but was not involved in the editorial process or decision‐making for this review update. The following people conducted the editorial process for this update:
Sign‐off Editor (final editorial decision): Lisa Bero, University of Colorado Anschutz Medical Campus
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Jenny Bellorini, c/o Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Alice YY Cheng, Trillium Health Partners and Unity Health Toronto, University of Toronto (clinical/content review); Olga Kozlowska, Oxford Brookes University (clinical/content review); Kavita Singh, Public Health Foundation of India (clinical/content review); Sedra Sheikh Debs and Therese Docherty (consumer reviews), Chang Xu, Anhui Medical University (statistical review); Liz Bickerdike, Cochrane Evidence Production & Methods Directorate (methods review); Robin Featherstone, Cochrane Central Editorial Service and Steve McDonald, Cochrane Australia (search reviews)
Appendices
Appendix 1. Search strategies
The Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library, 2019, Issue 6, Wiley
| No. | Search terms |
| #1 | [mh "patient care management"/OG] |
| #2 | ((compliance or adhere* or training or implement* or recommend* or disseminat* or according) near/3 guideline*):ti,ab |
| #3 | ((financial or economic or physician* or patient*) next incentive*):ti,ab |
| #4 | ("disease management" or "case management"):ti,ab |
| #5 | (care near/1 manage*):ti,ab |
| #6 | ((diabet* or intervention* or program* or detailing or patient or continu* or material or nurs* or physician*) near/6 educat*):ti,ab |
| #7 | reminder*:ti,ab |
| #8 | (quality near/3 improv*):ti |
| #9 | (quality near/3 care):ti |
| #10 | (quality next improv*):ab |
| #11 | total quality:ti,ab |
| #12 | continuous quality:ti,ab |
| #13 | ((disease* or diabet*) next (registry or register)):ti,ab |
| #14 | ((structured or co‐ordinat* or coordinat* or multicomponent or comprehensive or multifaceted or multidisciplinary or multifactorial or "multi‐disciplinary" or "multi‐factorial" or "multi‐faceted" or "multi‐component" or interdisciplinary or "inter‐disciplinary" or integrated or "community‐based" or organi*) near/2 (care or intervention* or approach* or program* or management or healthcare)):ti,ab |
| #15 | (self next (care or manage* or monitor*)):ti,ab |
| #16 | team?:ti,ab |
| #17 | (telecare or telemedic* or telehealth or telemonitor* or telephone* or phone*):ti,ab |
| #18 | ("internet based" or "web based"):ti,ab |
| #19 | (audit or feedback):ti,ab |
| #20 | decision support:ti,ab |
| #21 | ("enhanced care" or "managed care"):ti,ab |
| #22 | ((collaborative or shared or sharing) near/2 care):ti,ab |
| #23 | group next visit?:ti,ab |
| #24 | multitherapy:ti,ab |
| #25 | ((nurs* or pharmac* or specialist?) near/2 program*):ti,ab |
| #26 | ((nurs* or pharmac* or specialist?) next (direct* or manag* or led or intervention?)):ti,ab |
| #27 | [mh "managed care programs"] |
| #28 | [mh "patient‐centered care"] |
| #29 | [mh "reimbursement mechanisms"] |
| #30 | [mh telemedicine] |
| #31 | [mh internet] |
| #32 | [mh telephone] |
| #33 | [mh "education, continuing"] |
| #34 | [mh ^"patient education as topic"] |
| #35 | [mh "self care"] |
| #36 | [mh "decision support systems, clinical"] |
| #37 | [mh "quality improvement"] |
| #38 | [mh "quality assurance, health care"] |
| #39 | [mh ^"quality of health care"] |
| #40 | {or #1‐#39} |
| #41 | [mh "diabetes mellitus"] |
| #42 | diabet*:ti,ab |
| #43 | {or #41‐#42} |
| #44 | #40 and #43 |
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present (2015‐June 4, 2019)
| No. | Search terms |
| 1 | exp patient care management/og |
| 2 | ((compliance or adhere* or training or implement* or recommend* or disseminat* or according) adj3 guideline*).ti,ab. |
| 3 | ((financial or economic or physician* or patient*) adj incentive*).ti,ab. |
| 4 | (disease management or case management).ti,ab. |
| 5 | care manage*.ti,ab. |
| 6 | ((diabet* or intervention* or program* or detailing or patient or continu* or material or nurs* or physician*) adj6 educat*).ti,ab. |
| 7 | reminder*.ti,ab. |
| 8 | (quality adj3 improv*).ti. |
| 9 | (quality adj3 care).ti. |
| 10 | (quality adj improv*).ab. |
| 11 | total quality.ti,ab. |
| 12 | continuous quality.ti,ab. |
| 13 | ((disease* or diabet*) adj (registry or register)).ti,ab. |
| 14 | ((structured or co‐ordinat* or coordinat* or multicomponent or comprehensive or multifaceted or multidisciplinary or multifactorial or multi‐disciplinary or multi‐factorial or multi‐facted or multi‐component or interdisciplinary or inter‐disciplinary or integrated or community‐based or organi*) adj2 (care or intervention or approach or program* or management or healthcare)).ti,ab. |
| 15 | (self care or self manage* or self monitor*).ti,ab. |
| 16 | team?.ti,ab. |
| 17 | (telecare or telemedic* or telehealth or telemonitor* or telephone* or phone*).ti,ab. |
| 18 | (internet based or web based).ti,ab. |
| 19 | (audit or feedback).ti,ab. |
| 20 | decision support.ti,ab. |
| 21 | (enhanced care or managed care).ti,ab. |
| 22 | ((collaborative or shared or sharing) adj2 care).ti,ab. |
| 23 | group visit?.ti,ab. |
| 24 | multitherapy.ti,ab. |
| 25 | ((nurs* or pharmac* or specialist?) adj2 program*).ti,ab. |
| 26 | ((nurs* or pharmac* or specialist?) adj (direct* or manag* or led or intervention?)).ti,ab. |
| 27 | managed care programs/ |
| 28 | patient‐centered care/ |
| 29 | exp reimbursement mechanisms/ |
| 30 | exp telemedicine/ |
| 31 | exp internet/ |
| 32 | exp telephone/ |
| 33 | exp education, continuing/ |
| 34 | patient education as topic/ |
| 35 | exp self care/ |
| 36 | decision support systems, clinical/ |
| 37 | quality improvement/ |
| 38 | quality assurance, health care/ |
| 39 | quality of health care/ |
| 40 | or/1‐39 |
| 41 | exp diabetes mellitus/ |
| 42 | diabet*.ti,ab. |
| 43 | or/41‐42 |
| 44 | exp randomized controlled trial/ |
| 45 | controlled clinical trial.pt. |
| 46 | randomi#ed.ti,ab. |
| 47 | placebo.ab. |
| 48 | randomly.ti,ab. |
| 49 | Clinical Trials as topic.sh. |
| 50 | trial.ti. |
| 51 | or/44‐50 |
| 52 | exp animals/ not humans/ |
| 53 | 51 not 52 |
| 54 | 40 and 43 and 53 |
| 55 | limit 54 to english language |
| 56 | 54 not 55 |
| 57 | (2015* or 2016* or 2017* or 2018* or 2019*).dc,dp,ed,ep,yr. |
| 58 | 54 and 57 |
| 59 | 56 or 58 |
Embase (Ovid) 1974 to present (June 4, 2019)
| No. | Search terms |
| 1 | ((compliance or adhere* or training or implement* or recommend* or disseminat* or according) adj3 guideline*).ti,ab. |
| 2 | ((financial or economic or physician* or patient*) adj incentive*).ti,ab. |
| 3 | (disease management or case management).ti,ab. |
| 4 | care manage*.ti,ab. |
| 5 | ((diabet* or intervention* or program* or detailing or patient or continu* or material or nurs* or physician*) adj6 educat*).ti,ab. |
| 6 | reminder*.ti,ab. |
| 7 | (quality adj3 improv*).ti. |
| 8 | (quality adj3 care).ti. |
| 9 | (quality adj improv*).ab. |
| 10 | total quality.ti,ab. |
| 11 | continuous quality.ti,ab. |
| 12 | ((disease* or diabet*) adj (registry or register)).ti,ab. |
| 13 | ((structured or co‐ordinat* or coordinat* or multicomponent or comprehensive or multifaceted or multidisciplinary or multifactorial or multi‐disciplinary or multi‐factorial or multi‐facted or multi‐component or interdisciplinary or inter‐disciplinary or integrated or community‐based or organi*) adj2 (care or intervention or approach or program* or management or healthcare)).ti,ab. |
| 14 | (self care or self manage* or self monitor*).ti,ab. |
| 15 | team?.ti,ab. |
| 16 | (telecare or telemedic* or telehealth or telemonitor* or telephone* or phone*).ti,ab. |
| 17 | (internet based or web based).ti,ab. |
| 18 | (audit or feedback).ti,ab. |
| 19 | decision support.ti,ab. |
| 20 | (enhanced care or managed care).ti,ab. |
| 21 | ((collaborative or shared or sharing) adj2 care).ti,ab. |
| 22 | group visit?.ti,ab. |
| 23 | multitherapy.ti,ab. |
| 24 | ((nurs* or pharmac* or specialist?) adj2 program*).ti,ab. |
| 25 | ((nurs* or pharmac* or specialist?) adj (direct* or manag* or led or intervention?)).ti,ab. |
| 26 | patient care/ |
| 27 | exp "organization and management"/ |
| 28 | 26 and 27 |
| 29 | reimbursement/ |
| 30 | exp telemedicine/ |
| 31 | internet/ |
| 32 | telephone/ |
| 33 | continuing education/ |
| 34 | patient education/ |
| 35 | exp self care/ |
| 36 | clinical decision support system/ |
| 37 | health care quality/ |
| 38 | or/1‐25,28‐37 |
| 39 | exp *diabetes mellitus/ |
| 40 | diabet*.ti,ab. |
| 41 | 39 or 40 |
| 42 | 38 and 41 |
| 43 | random*.ti,ab. |
| 44 | factorial*.ti,ab. |
| 45 | (crossover* or cross over*).ti,ab. |
| 46 | ((doubl* or singl*) adj blind*).ti,ab. |
| 47 | (assign* or allocat* or volunteer* or placebo*).ti,ab. |
| 48 | crossover procedure/ |
| 49 | single blind procedure/ |
| 50 | randomized controlled trial/ |
| 51 | double blind procedure/ |
| 52 | or/43‐51 |
| 53 | exp animal/ not human/ |
| 54 | 52 not 53 |
| 55 | 42 and 54 |
| 56 | (2016* or 2017* or 2018* or 2019*).dp,dd,dc,yr,em. |
| 57 | 55 and 56 |
| 58 | limit 57 to embase |
CINAHL (EBSCOhost) 1981 to present (June 4, 2019)
| No. | Search terms |
| S1 | (compliance or adhere* or training or implement* or recommend* or disseminat* or according) N3 guideline* |
| S2 | (financial or economic or physician* or patient*) N0 incentive* |
| S3 | disease management or case management or reminder* or self care or self manage* or self monitor* or care manage* or total quality or continuous quality or team? or telecare or telemedic* or telehealth or telemonitor* or telephone* or phone* or internet based or web based or audit or feedback or decision support or multitherapy or group visit? or enhanced care or managed care |
| S4 | (diabet* or intervention* or program* or detailing or patient or continu* or material or nurs* or physician*) N6 educat* |
| S5 | TI (quality N3 improv*) |
| S6 | TI (quality N3 care) |
| S7 | AB (quality N0 improv*) |
| S8 | (disease* or diabet*) N0 (registry or register) |
| S9 | (structured or co‐ordinat* or coordinat* or multicomponent or comprehensive or multifaceted or multidisciplinary or multifactorial or multi‐disciplinary or multi‐factorial or multi‐facted or multi‐component or interdisciplinary or inter‐disciplinary or integrated or community‐based or organi*) N2 (care or intervention or approach or program* or management or healthcare) |
| S10 | (collaborative or shared or sharing) N2 care |
| S11 | (nurs* or pharmac* or specialist?) N2 program* |
| S12 | (nurs* or pharmac* or specialist?) N0 (direct* or manag* or led or intervention?) |
| S13 | MH "Managed Care Programs" |
| S14 | MH "Patient Centered Care" |
| S15 | MH "Reimbursement Mechanisms+" |
| S16 | MH "Telehealth+" |
| S17 | MH "Internet+" |
| S18 | MH "telephone+" |
| S19 | MH "Education, Continuing+" |
| S20 | MH "Patient Education+" |
| S21 | MH "Self Care+" |
| S22 | MH "Decision Support Systems, Clinical" |
| S23 | MH "Quality Improvement" |
| S24 | MH "Quality of Health Care" |
| S25 | MH "Quality Assurance" |
| S26 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 |
| S27 | MH "Diabetes Mellitus+" |
| S28 | TI diabet* or AB diabet* |
| S29 | S27 OR S28 |
| S30 | S26 AND S29 |
| S31 | PT randomized controlled trial |
| S32 | PT clinical trial |
| S33 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) |
| S34 | (MH "Clinical Trials+") |
| S35 | (MH "Random Assignment") |
| S36 | S31 OR S32 OR S33 OR S34 OR S35 |
| S37 | S30 AND S36 |
| S38 | S30 AND S36 Limiters ‐ Exclude MEDLINE records |
ClinicalTrials.gov, (www.clinicaltrials.gov) 2019 (June 4, 2019)
Condition=diabetes AND Other terms = quality improvement
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch), 2019 (June 4, 2019)
Diabet* AND quality
Appendix 2. Planned methods for maintaining this review in the living mode
Justification for the Cochrane living systematic review (LSR) status
Cochrane has identified three criteria that should be met to justify maintaining a LSR. See https://community.cochrane.org/review-production/production-resources/living-systematic-reviews.
Criteria 1. Is the review a priority for decision‐making?
Diabetes is one of the commonest chronic diseases (with increasing incidence globally). People living with diabetes experience significant disruption to their lives and increased morbidity and mortality. Healthcare systems spend significant resources on diabetes care but struggle to ensure that people living with diabetes achieve high‐quality care. Thus, improving the care for people living with diabetes is likely to remain a major health system priority for the foreseeable future. Health system decision‐makers, healthcare professionals and people living with diabetes need robust, up‐to‐date evidence on the effects of different QI approaches.
To ensure the credibility of our review and support evidence informed decision‐making, we believe it is essential that it is regularly updated (at least annually).
Criteria 2. Is there an important level of uncertainty?
Although we have a large number of studies that provide sufficient certainty to report our analyses on selected outcomes, there remains considerable uncertainties about:
Effects of QI components that are relatively infrequent ‐ e.g. we had to collapse audit and feedback, financial interventions and CQI into an 'Other' category).
Effects on some important outcomes ‐ for renal screening the number of studies was limited, and we were unable to use modelling to explore the effects of different QI components.
Effects across different settings ‐ diabetes is a global problem, however the majority of studies conducted to date have been based in North America and may be of limited relevance to non North American settings.
Effects of new diabetes treatment options ‐ there are new treatment approaches that will likely significantly alter the management of diabetes and that may raise new QI challenges (one of our clinician team members thought this was a really important issue). Also, with more data come more opportunities to build more complex models and explore more complex relationships that may better reflect reality.
Hence, we need to maintain the review as a living review to address these remaining uncertainties and advance the nuances of our understanding.
Criteria 3. Will new evidence likely change the conclusions of the review?
Given our response to criteria 2, it seems likely that new evidence may change the conclusions of the review as we gain greater certainty about the effects of QI components for the full range of important outcomes across a more diverse range of healthcare settings and in the face of innovations in diabetes care.
Logistical and practical considerations
We anticipate that there are up between 30 to 40 new studies published each year. Our experience is that performing intermittent updates (e.g. every three years) on large reviews becomes a substantive task often taking many years, and so we get into a vicious cycle of each update taking at least two to three years (by which time the searches are out of date etc). Managing updates as a living review will require us to screen every three months at which time we would anticipate having to organise data extraction of six to eight studies, which is much more manageable. I.e. there is a substantive logistical and practical argument to maintain this as a living review (with analyses likely updated annually).
LSR methodological considerations
Search methods for identification of studies
We will conduct database searches every three months in:
the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library;
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®);
Embase (Ovid);
CINAHL (EBSCOhost).
We will also search the following trial registers for ongoing studies:
ClinicalTrials.gov (www.clinicaltrials.gov);
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch).
We will review our search strategies on an ongoing basis every 12 months, as indexing terms and keywords may change, and new search filters may be published. Such changes will be managed by input from experienced information specialists.
Any new review authors will be trained with a pilot round of at least 25 title/abstracts and 10 full texts (chosen randomly) until their screening decisions are considered in good agreement with independent assessments of senior review team members.
Selection of studies
We will screen any new citations retrieved by the searches using DistillerSR 2021, and independent review authors will undertake dual screening of titles and abstracts, and then full texts.
Prior to data extraction, we will link all reports belonging to a single study. We will prioritise the extraction of the most recent publication reporting the primary outcome(s) of the study and treat other reports as companion papers. We will extract data from companion papers for relevant secondary outcomes or missing data (for example, additional details on the QI strategies) when available.
We will extract all data in Excel using detailed extraction sheets for study characteristics (one sheet), coding of QI strategies (one sheet), risk of bias assessment (one sheet) and outcome data (13 sheets; one per outcome). The data extraction form is available online (Web Appendix 2).
Two independent review authors will perform data extraction; discrepancies will be resolved through discussions or with a third senior review author if conflicts remain. All new data extractors will complete a pilot training exercise on a random sample of five articles, checked against the extractions of an experienced review team member. If needed, new authors will extract an additional set of two to three articles until good agreement with experienced review team members is obtained.
We will group and report abstracted studies in a 'Studies awaiting assessment' table until they are included in the analyses.
Data synthesis
We will follow the same analytical strategy used in the baseline review, updated annually. Briefly we will use a series of hierarchical multivariable random‐effects meta‐regression models (Gelman 2002; Rubin 1992) for three continuous (HbA1c, SBP and LDL cholesterol) and three dichotomous (retinopathy screening, foot screening, smoking cessation) outcomes. To facilitate stable modelling of strategies and comparison across outcomes with smaller sample sizes (i.e. screening outcomes), we will group infrequently observed strategies (audit and feedback, continuous quality improvement and financial incentives) in an ‘other’ category across all models (until sufficient studies have reported these strategies, in which case they will be split out). We will fit models in a Bayesian framework. We will clean and prepare data for analysis in Stata (StataCorp 2021), and fit models using Markov chain Monte Carlo (MCMC) methods with the software JAGS (Plummer 2003) called from R. Parameters, monitored using the Brooks‐Gelman‐Rubin diagnostic (Brooks 1998; Gelman 1992).
Given our findings from the baseline review that the baseline interaction models for HbA1c, SBP and LDL performed better than the main model, we will conduct and present these models as the primary analyses for these outcomes.
To aid in interpretation, we will qualitatively order QI strategies within three tiers (top, middle, bottom) based on their magnitude of effect relative to the other QI strategies.
Future updates of review methods
The LSR approach acknowledges that reviews may cease to need to be ‘living’ over time, as the review findings become stable, or the question is no longer a priority for decision‐makers (Brooker 2019). We will continually monitor the LSR approach, including the likely benefits of and challenges to continuing this methodology for this evidence base, and whether such an approach remains warranted. If the evidence is high‐certainty for all outcomes and all comparisons at that point, meaning further studies are judged very unlikely to impact the effect estimate, we would consider ceasing living mode for this review. If, as is more likely, some or all outcomes for our different QI strategies are not yet certain, we will facilitate discussions within the author team and Cochrane, and we will continue to maintain the review as living.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abuloha 2016.
| Study characteristics | ||
| Methods |
The role of clinical pharmacist in initiation and/or dose adjustment of insulin therapy in diabetic patients in outpatient clinic in Jordan RCT (NA clusters and NA providers), conducted in 1) Study carried out in the endocrine‐outpatient clinic in Jordan University Hospital (JUH), 2) Clinical pharmacists collaborated with physician in the management of insulin therapy in the intervention group 2 arms: 1) Control (usual care and SMBG) (control arm) and 2) Intervention (pharmacist management and SMBG) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 3 Mean age: 55.59 ± 8.02 % Male: 42 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care and SMBG) 1) Promotion of self‐management Intervention arm: (pharmacist management and SMBG) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Harms (hypoglycaemic episodes) |
|
| Funding source | This research was supported by a grant from the Deanship of Academic Research, The University of Jordan, Amman, Jordan | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by asking the patients to draw from a closed envelope of equal even and odd numbers. |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes? |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All P values are above 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 12 patients (out of 100, 12%) were lost from follow‐up (7 from the control group and 5 from the intervention group) as they did not return back to their clinic visits. Numbers and reasons balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective outcome (HbA1c) and subjective outcome (hypoglycaemia). Patients were asked to record hypoglycaemic episodes if any. Patients were aware of their allocated group. Limitation: Some of the outcomes which were measured are based on patients reporting. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Methods match results. |
| Risk of contamination (other bias) | Unclear risk | Both arms received SMBG device. However, only the intervention arm had pharmacist management. It is not excluded that pharmacists' recommendations to physicians changed their approach in managing insulin therapy initiation for the control arm patients as well. |
| Other bias | Low risk | No evidence of other bias |
Adachi 2013.
| Study characteristics | ||
| Methods |
Effects of lifestyle education program for type 2 diabetes patients in clinics: a cluster randomized controlled trial Cluster‐RCT (20 clusters with 20 providers), conducted in primary care clinics in Kanagawa, Japan Two arms: 1) Control group (control arm) and 2) Intervention group (intervention arm) |
|
| Participants | Control arm N: 93 Intervention arm N: 100 Diabetes type: 2 Mean age: 61.3 ± NR % Male: 51.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
|
| Funding source | This study was financially supported by the Ministry of Education, Culture, Sports, Science and Technology in Japan Grant‐in‐Aid for Scientific Research (C) in 2007–2008 (Grant No. 19500693), 2009–2010 (Grant No. 20175128) (Representative: MW), and The Japan Dietetic Association Grant in 2006 (Representative: MA) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used randomisation list, but how they generated this list is not reported. |
| Allocation concealment (selection bias) | High risk | Randomisation list with permutated block size of 2: too predictable of next assignment. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported in text or table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | ~24% lost in control group and ~16% lost in intervention group; also double number of patients could not be contacted in control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | For HbA1c: they said physician collected these data, but did not describe objective laboratory methods. |
| Selective reporting (reporting bias) | Low risk | Outcome matches protocol. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Unclear risk | Potential for selection bias. |
Adair 2013.
| Study characteristics | ||
| Methods |
Improving chronic disease care by adding laypersons to the primary care team. A parallel randomized trial. Patient RCT, conducted in 6 primary care clinics in Minnesota, USA Two arms: 1) Usual care (control arm) and 2) Care guide (intervention arm) |
|
| Participants | Control arm N: 706 Intervention arm N: 1429 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Electronic patient registry 2) Clinician reminders 3) Patient education Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Clinician reminders 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | 1) Anti‐hypertensives (ACE inhibitor or angiotensin II receptor blockers) 2) Retinopathy screening (eye exam) 3) Renal screening (albumin) 4) Glycated haemoglobin 5) Systolic blood pressure 6) Diastolic blood pressure 7) Low‐density lipoprotein 8a) Hypertension control (DBP < 81 mmHg) 8b) Hypertension control (SBP < 141 mmHg) 9) Smoking cessation |
|
| Funding source | Financial support: by the Robina Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…prepared sealed opaque envelopes containing either a purple card (assignment to a care guide) or a gold card (assignment to usual care)… each clinic's envelope were shuffled before delivery and daily thereafter." |
| Allocation concealment (selection bias) | Low risk | Quote: "…prepared sealed opaque envelopes containing either a purple card (assignment to a care guide) or a gold card (assignment to usual care)… each clinic's envelope were shuffled before delivery and daily thereafter." |
| Patient's baseline characteristics (selection bias) | Low risk | Characteristics relatively balanced between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Outcomes relatively balanced between groups. |
| Incomplete outcome data (attrition bias) | Low risk | < 10% losses in each, reasons balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | For blood pressure control, they do not state how this was assessed. In addition, outcome assessors were not blinded. |
| Selective reporting (reporting bias) | Low risk | Outcomes matched protocol. |
| Risk of contamination (other bias) | High risk | Quote: "…the usual care delivered by providers may have been influenced by contact with care guides about other patients." |
| Other bias | High risk | Hawthorne effect. Quote: "…improvement in usual care patients could be related to their knowledge that they were study participants." |
Adams 2015.
| Study characteristics | ||
| Methods |
Supervised pharmacy student‐led medication review in primary care for patients with type 2 diabetes: a randomised controlled pilot study RCT (NA clusters and NA providers), conducted in 1) The consultation took place at the patient’s medical practice (5 Norfolk‐based medical practices). 2) The intervention was given by pairs of pharmacy students supervised by a pharmacist. In United Kingdom. 2 arms: 1) Control (standard care) (control arm) and 2) Intervention (student‐led medication reviews) (intervention arm) |
|
| Participants | Control arm N: 66 Intervention arm N: 67, NA, NA Diabetes type: 2 Mean age: 68.75 ± NR % Male: 63.29 Longest follow‐up: 6.5 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (student‐led medication reviews) 1) Case management 2) Team change 3) Clinician education 4) Facilitated relay of clinical information |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Harms (hypoglycaemia) |
|
| Funding source | This work was supported by the National Institute for Health Research (NIHR) under Research for Patient Benefit grant no. PB‐PG‐0909‐19198 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All recruited patients were randomised to intervention or control (standard care) using an automated randomisation system, developed and controlled by the clinical trials unit, which ensured concealed allocation. Randomisation was undertaken in blocks of 4 to maximise equality of group size. All researchers and clinical staff involved with generating outcome data were blind to participant allocation. |
| Allocation concealment (selection bias) | Low risk | All recruited patients were randomised to intervention or control (standard care) using an automated randomisation system, developed and controlled by the clinical trials unit, which ensured concealed allocation. Randomisation was undertaken in blocks of 4 to maximise equality of group size. All researchers and clinical staff involved with generating outcome data were blind to participant allocation. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1, characteristics balanced between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | See Table 1, outcomes are relatively balanced between groups. |
| Incomplete outcome data (attrition bias) | Low risk | 11 lost to follow‐up out of 133 randomised (8.3%), including 9 lost in intervention arm (reasons not related to intervention). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Low‐risk for these objective outcomes (HbA1c, SBP, DBP). Methods for hyper and hypo‐glycaemia assessment not reported (unclear risk, secondary outcome). The trial was unblinded with the service providers, patients and research all aware of group allocation and intervention content. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol applied on 13 August 2015, study done in 2011 to 2012). Results match methods. |
| Risk of contamination (other bias) | Low risk | Patients do not see each other (individual medication reviews). Pharmacist students and one pharmacist only see patients into intervention arm at the patients' clinic. |
| Other bias | Low risk | No evidence of other bias. |
Adjei 2015.
| Study characteristics | ||
| Methods |
Peer coaches to improve diabetes outcomes in rural Alabama: a cluster randomized trial RCT (NA clusters and NA providers), conducted in 1) The study was carried out at the National Diabetes Management Research Centre (NDMRC) of the University of Ghana Medical School, Korle‐Bu Teaching Hospital, Accra, Ghana. 2) Intervention provided by caregivers/physicians. In Ghana 2 arms: 1) Control (standard paper reminders) (control arm) and 2) Intervention (electronic reminders) (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 100, NA, NA Diabetes type: 4 Mean age: NR ± 11.3 % Male: 35.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard paper reminders) Intervention arm:(electronic reminders) 1) Electronic patient registry 2) Clinician reminder 3) Facilitated relay of clinical information 4) Patient reminders |
|
| Outcomes | 1) Systolic blood pressure 2) Diastolic blood pressure |
|
| Funding source | The study was funded by the staff development fund of the School of Allied Health Sciences, College of Health Sciences, University of Ghana | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not adequately reported. The patients were randomised and matched into intervention and control groups using Microsoft Excel 2007. |
| Allocation concealment (selection bias) | Unclear risk | Not adequately reported ‐ Microsoft Excel 2007. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 shows the baseline characteristics of the study participants. Apart from sex (P = 0.018), baseline demographical characteristics were largely similar for both groups (P = 0.05). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. Outcome data appear very similar between control and intervention at baseline. Text: All the metabolic risk factors (BMI, systolic blood pressure, diastolic blood pressure, pulse rate and fasting plasma glucose) were similar between the intervention group and the control group at baseline (P > 0.05). |
| Incomplete outcome data (attrition bias) | High risk | Table 3. At 6 months, 0 lost to follow‐up in the intervention arm (patients received reminders) but 12 lost to follow‐up out of 100 in control arm (12%). Reasons not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Only patients in the intervention arm received reminders, so contamination bias is not applicable for them. However, it is not excluded that when physicians received prompts for their patients into the intervention group, they also remembered to check for their patients in the control arm. |
| Other bias | Low risk | No evidence of other bias. |
Agarwal 2019.
| Study characteristics | ||
| Methods |
Mobile app for improved self‐management of type 2 diabetes: multicenter pragmatic randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from 3 hospital‐based diabetes education programmes (DEPs) in Ontario, Canada. The 3 recruitment sites included (1) a DEP located in an urban area in a large city centre (> 2 million people), (2) 1 located in a midsize city in a remote area of the province (< 150,000 people), and (3) 1 located in a semi‐urban area surrounding a large city centre (< 600,000 people). These sites serve a diverse range of patients including a large immigrant community, rural patients and a large Aboriginal population. The services of these programmes are complementary to primary care delivered through the patients’ primary care provider (PCP) and usually do not include medication titration. 2) Intervention delivered remotely using a BlueStar mobile app. This app facilitated the transfer of data to the user’s clinician. In Canada 2 arms: 1) Control (WLC: wait‐list control) (control arm) and 2) Intervention (ITG: immediate BlueStar mobile app) (intervention arm) |
|
| Participants | Control arm N: 121 Intervention arm N: 119, NA, NA Diabetes type: 2 Mean age: 51.8 ± NR % Male: 52 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (WLC: wait‐list control) Intervention arm: (ITG: immediate BlueStar mobile app) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Harms (hypoglycaemic episodes) |
|
| Funding source | Sponsor: Women's College Hospital (from protocol) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subject randomisation was computer‐generated and stratified by site, using block sizes of 2 or 4, through REDCap, a Web‐based electronic data entry system at the AHRC. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done in a centralised fashion by the Applied Health Research Centre (AHRC) at the Li Ka Shing Knowledge Institute of St. Michael’s Hospital in Toronto, Canada. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 summarises the demographic characteristics of the study population. There were no significant differences in patient characteristics including age, gender, ethnicity, education and household income. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1) Data reported. No evidence of statistical test done. The average HbA1c level for the study population was 8.96% (SD 1.68) and was similar between the 2 study arms, and the use of insulin was similar between the 2 groups. |
| Incomplete outcome data (attrition bias) | High risk | They have HbA1c data for 172 out of 240 patients randomised at baseline (28% lost) and 146 at 3 months (39% lost). The number of patients lost is 36% in the control group (44/121) and 42% in the intervention group (50/119) at 3 months follow up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome was objectively measured (HbA1c). Hypoglycaemic episodes were patient self‐reported and unlikely that they were blinded (but secondary outcomes). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. Some outcomes are not reported at 6 months follow‐up in the paper (ER visit, hypoglycaemic episodes, hospitalisations, physician visits). |
| Risk of contamination (other bias) | Unclear risk | Patients RCT. Unlikely that control patients had access to the BlueStar mobile app before 3 months. However, since both groups were followed by clinicians from the same DEPs, clinicians could have change their approach with control patients after receiving data from the app of intervention patients. |
| Other bias | Low risk | No evidence of other risk of bias |
Aguiar 2018.
| Study characteristics | ||
| Methods |
Pharmacist‐physician collaborative care model for patients with uncontrolled type 2 diabetes in Brazil: results from a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) This study was conducted at a university hospital‐affiliated secondary care clinic in São Paulo, Brazil. The metabolic disease clinic’s medical staff consists of 3 specialist physicians who follow patients with diabetes, hypertension or dyslipidaemia who have been referred by the primary care physician. Nurses and nutritionists also are part of the outpatient care team. 2) The intervention involved a pharmacist‐physician collaborative care model. In Brazil. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (clinical pharmacist service) (intervention arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 40, NA, NA Diabetes type: 2 Mean age: 61.76 ± 9.2 % Male: 32.88 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (clinical pharmacist service) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein 5a) Hypertension control (SBP < 130 mmHg) 5b) Hypertension control (DBP < 80 mmHg) |
|
| Funding source | This project was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) – Grant Number 2011/11145‐4 and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes). The funders had no role in study design, data collection and analysis, writing of the report or decision to publish. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by pharmacist researcher using a computer‐generated randomised list from the research randomizer program (https://www.randomizer.org/), and followed the allocation sequence according to the referral of the physicians. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by pharmacist researcher using a computer‐generated randomised list from the research randomizer program (https://www.randomizer.org/), and followed the allocation sequence according to the referral of the physicians. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1) All P values above 0.05. Baseline characteristics were similar between the 2 study groups (P > 0.05 for all comparisons) (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1) All outcomes have P values above 0.05. Baseline characteristics were similar between the 2 study groups (P > 0.05 for all comparisons) (Table 1). |
| Incomplete outcome data (attrition bias) | Low risk | 7 lost to follow‐up out of 80 (8.8%). Numbers and reasons reported and balanced. Quote: "Finally, only data of the patients who completed this study were analysed, but the lost to follow‐up was balanced between the groups and no difference was noted for the characteristics of patients and reasons for withdrawal. Some of the patients in this study had no laboratory tests of LDL cholesterol levels (52 available out of 80 = 65%)." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest are objective (HbA1c, SBP, DBP and LDL). The outcome assessors were blinded and unaware to which group the patients had been assigned. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | High risk | Only the patients in the intervention arm received consultations from pharmacists but it is not excluded that physicians changed their care approach with their usual care patients following pharmacists' recommendations about intervention patients. Quote: "In addition, the physicians were not blinded to the clinical pharmacy service, which may have had some effect on the care of patients with diabetes in both study groups." |
| Other bias | Low risk | No evidence of other bias. |
Ahring 1992.
| Study characteristics | ||
| Methods |
Telephone modem access improves diabetes control in those with insulin‐requiring diabetes Patient RCT, conducted in 2 endocrinology clinics, in Canada Two arms: 1) Control (control arm) and 2) Modem (intervention arm) |
|
| Participants | Control arm N: 20 Intervention arm N: 22 Diabetes type: unclear/not reported Mean age: 41.4 ± 15.4 % Male: 48.0 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Aiello 2015 (annual follow‐ups).
| Study characteristics | ||
| Methods |
Assessing the effect of personalized diabetes risk assessments during ophthalmologic visits on glycaemic control: a randomized clinical trial Clustered RCT (25 clusters and 123 providers), conducted in 1) This randomised, multicentre clinical trial was conducted by the Diabetic Retinopathy Clinical Research Network at 42 clinical sites in the United States. 2) Point‐of‐care visits and education by ophthalmologists in the United States 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (visits and education by ophthalmologists) (intervention arm) 2 separate cohorts: participants were included in the cohort with more‐frequent‐than‐annual follow‐ups if at least 1 ophthalmologic visit occurred between baseline and 1 year, otherwise they were included in the cohort withannual follow‐ups |
|
| Participants | Control arm N: 368 Intervention arm N: 388, NA, NA Diabetes type: 3 Mean age: 62.03 ± 14.06 % Male: 42 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (visits and education by ophthalmologists) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | This work was supported by a co‐operative agreement from the National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, and the US Department of Health and Human Services (grants EY14231, EY23207, and EY18817) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in text and in trial protocol (Supplement 1). Randomised in a 1:1 ratio, stratified by site or patient race/ethnicity. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Baseline characteristics by patient were similar between treatment groups and between cohorts (eTable 1 in Supplement 2). |
| Patient's baseline outcomes (selection bias) | Low risk | Means of HbA1c, arterial blood pressure and body mass index look similar at baseline between groups. |
| Incomplete outcome data (attrition bias) | High risk | Total of 264 patients lost out of 1746 at baseline (15%). The 1‐year visit completion rates (excluding deaths) were 88% and 89% in the control and intervention groups, respectively, for the cohort with more‐frequent‐than‐annual follow‐ups and 82% and 85% in the control and intervention groups, respectively, for the cohort with annual follow‐ups. Baseline characteristics were similar when comparing 1‐year completers with non completers (data not shown),with the exception of a higher mean central laboratory HbA1c level in the non‐completers of each cohort/group (8.7% vs 8.4% in non‐completers vs completers, overall). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on March 2011, enrollment was from April 2011 through January 2013, intervention of 12 months). Results match protocol. |
| Risk of contamination (other bias) | Low risk | Clustered‐RCT. However, the lack of an intervention effect in our study could reflect the standard care given by this specialised investigator group, which is highly attuned to evidence‐based retinal care for individuals with diabetes and possibly already providing patient education at a level where the prescribed intervention would not add incremental benefit. |
| Other bias | Low risk | No evidence of other bias |
Aiello 2015 (more‐frequent‐than‐annual follow‐ups).
| Study characteristics | ||
| Methods |
Assessing the effect of personalized diabetes risk assessments during ophthalmologic visits on glycaemic control: a randomized clinical trial Clustered RCT (25 clusters and 123 providers), conducted in 1) This randomised, multicentre clinical trial was conducted by the Diabetic Retinopathy Clinical Research Network at 42 clinical sites in the United States. 2) Point‐of‐care visits and education by ophthalmologists. In United States of America. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (visits and education by ophthalmologists) (intervention arm) 2 separate cohorts: participants were included in the cohort with more‐frequent‐than‐annual follow‐ups if at least 1 ophthalmologic visit occurred between baseline and 1 year, otherwise they were included in the cohort with annual follow‐ups |
|
| Participants | Control arm N: 502 Intervention arm N: 488, NA, NA Diabetes type: 3 Mean age: 64.49 ± NR % Male: 47.54 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (visits and education by ophthalmologists) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | This work was supported by a co‐operative agreement from the National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, and the US Department of Health and Human Services (grants EY14231, EY23207, and EY18817) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in text and in trial protocol (Supplement 1). Randomised in a 1:1 ratio, stratified by site or patient race/ethnicity. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Baseline characteristics by patient were similar between treatment groups and between cohorts (eTable 1 in Supplement 2). |
| Patient's baseline outcomes (selection bias) | Low risk | Means of HbA1c, arterial blood pressure and body mass index look similar at baseline between groups. |
| Incomplete outcome data (attrition bias) | High risk | Total of 264 patients lost out of 1746 at baseline (15%). The 1‐year visit completion rates (excluding deaths) were 88% and 89% in the control and intervention groups, respectively, for the cohort with more‐frequent‐than‐annual follow‐ups and 82% and 85% in the control and intervention groups, respectively, for the cohort with annual follow‐ups. Baseline characteristics were similar when comparing 1‐year completers with non‐completers (data not shown),with the exception of a higher mean central laboratory HbA1c level in the non‐completers of each cohort/group (8.7% vs 8.4% in non completers vs completers, overall). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on March 2011, enrollment was from April 2011 through January 2013, intervention of 12 months). Results match protocol. |
| Risk of contamination (other bias) | Low risk | Clustered‐RCT. However, the lack of an intervention effect in our study could reflect the standard care given by this specialised investigator group, which is highly attuned to evidence‐based retinal care for individuals with diabetes and possibly already providing patient education at a level where the prescribed intervention would not add incremental benefit. |
| Other bias | Low risk | No evidence of other bias |
Al‐Shookri 2012.
| Study characteristics | ||
| Methods |
Effectiveness of medical nutrition treatment delivered by dietitians on glycaemic outcomes and lipid profiles of Arab, Omani patients with type 2 diabetes Patient RCT, conducted in outpatient diabetes clinic in Sultan, Qaboos University in Muscat, Oman Two arms: 1) Usual nutritional care (control arm) and 2) Practice guidelines nutritional care (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 100 Diabetes type: 2 Mean age: 50.7 ± 10.4 % Male: 41.8 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Low‐density lipoprotein |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each participant was given a number, which was put in a box. By random selection, the first 100 numbers were assigned to the group to receive practice guidelines nutritional care and the remaining numbers were assigned to the usual nutritional care group." |
| Allocation concealment (selection bias) | Low risk | Quote: "Each participant was given a number, which was put in a box. By random selection, the first 100 numbers were assigned to the group to receive practice guidelines nutritional care and the remaining numbers were assigned to the usual nutritional care group." |
| Patient's baseline characteristics (selection bias) | Low risk | Table and text. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.632); LDL (P = 0.437). |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol, even though they state that intention‐to‐treat analysis was their primary objective. Baseline based on those analysed. Number and reasons for loss to follow‐up not provided (stated that they did not have the information at follow up to conduct analysis). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c using high performance liquid chromatography, LDL using enzymatic methods on Baxter Paramax. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Alanzi 2018.
| Study characteristics | ||
| Methods |
Evaluation of the effectiveness of mobile diabetes management system with social networking and cognitive behavioural therapy (CBT) for T2D RCT (NA clusters and NA providers), conducted in 1) All participants were from a clinic in Saudi Arabia‐Damman. 2) Mobile diabetes management system operated remotely through the SANAD system. Figure 1 shows that diabetic nurses and CBT therapists were involved in the delivery of the intervention. In Saudi Arabia. 2 arms: 1) Control (conventional diabetes treatment) (control arm) and 2) Intervention (SANAD system) (intervention arm) |
|
| Participants | Control arm N: 10 Intervention arm N: 10, NA, NA Diabetes type: 2 Mean age: NR ± 6.45 % Male: 75 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (conventional diabetes treatment) Intervention arm: (SANAD system) 1) Electronic patient registry 2) Patient education |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | No information of funding source. "Some of the limitations of this study that may affect internal and external validity include the small sample size (n = 20), which was due to limited funding" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1, education lower in control group, but all other characteristics balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1) Baseline HbA1c P value > 0.05 |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient lost in the intervention group (1/10 or 10%). None lost in the control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Patient randomised. Both groups were monitored by the same staff. Unlikely that the control group received the SANAD intervention. |
| Other bias | Low risk | No evidence of other bias. |
Albisser 2007.
| Study characteristics | ||
| Methods |
Averting iatrogenic hypoglycaemia through glucose prediction in clinical practice: progress towards a new procedure in diabetes RCT (NA clusters and NA providers), conducted in 1) Secondary care ‐ the Metabolic Care Center, Greenville, Pennsylvania, United States of America. Telemonitoring via scheduled onscreen reviews. All patients were instructed to self‐measure blood glucose ideally 4 or more times a day and to report all episodes of hypoglycaemia. Clinic visits verified this info. 2) Providers accessed the shared, central database using a custom GUI available from the DDC. According to their workgroup security credentials (username and password), providers had full access to the remote server but were restricted only to the data from their cohort of registered patients. In United States of America 2 arms: 1) Control (absence of predicted glycaemia) (control arm) and 2) Intervention (presence of predicted glycaemia) (intervention arm) |
|
| Participants | Control arm N: 11 Intervention arm N: 11, NA, NA Diabetes type: 1 Mean age: 49.7 ± NR % Male: 63.63 Longest follow‐up: 2 months |
|
| Interventions |
Control arm: (absence of predicted glycaemia) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: (presence of predicted glycaemia) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) Glycated haemoglobin 2) Harms (hypoglycaemia) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to either the prediction group or the control group was then by random number generated at the DDC. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1, characteristics are balanced between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | See Table 1, outcomes are balanced between groups. |
| Incomplete outcome data (attrition bias) | Unclear risk | No report of dropout. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Because accuracy in SMBG testing is paramount to reduce errors in the predicted values, each patient’s methodology was reviewed and their accuracy verified at clinic visits. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Unlikely that control patients were given predictive harms information. |
| Other bias | Low risk | None identified |
Aleo 2015.
| Study characteristics | ||
| Methods |
Improving eye care follow‐up adherence in diabetic patients with ocular abnormalities: the effectiveness of patient contracts in a free, pharmacy‐based eye screening Quasi‐RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from an urban outpatient pharmacy in Philadelphia, PA. Community‐based pharmacy setting to screen for ocular diseases. 2) Eye screening done in community‐based setting. Contract administered by research assistants and the follow‐up was done by an ophthalmologist in adherent patients in United States of America. 2 arms: 1) Control non‐contract group (control arm) and 2) Intervention contract group (intervention arm) |
|
| Participants | Control arm N: NR Intervention arm N: NR, NA, NA Diabetes type: 4 Mean age: 54.7 ± NR % Male: 43.4 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (non‐contract group) 1) Case management 2) Facilitated relay of clinical information 3) Patient reminders Intervention arm: (contract group) 1) Case management 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | 1) Retinopathy screening (fundus exam follow‐up adherence) | |
| Funding source | This study was funded by the Centers for Disease Control and Prevention 5U58DP002655‐02 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The participants were assigned to groups by alternating weeks; the 250 patients who attended screenings during odd weeks were assigned to the contract group and the 250 patients who attended screenings during even weeks were assigned to the non‐contract group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no significant differences in the demographic composition of the contract and non‐contract groups. No P values reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | They initially randomised 500 patients. 113 (22.6%) were found to have DR or another ocular abnormality. Of the 113 participants who had abnormal results, 83 (74.3%) were able to be contacted and complete the 3‐month follow‐up questionnaire regarding their follow‐up eye care utilisation. 30 patients were lost to follow‐up (27%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Outcome self‐reported by patients. Research assistants administered a follow‐up questionnaire to all participants with abnormal findings by telephone 3 months after the screening results were distributed. The questionnaire addressed follow‐up eye care utilisation. Patients who signed a contract agreed to inform research staff if/when they completed an eye care appointment. Nothing about blinding. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. The outcome of interest is not reported at baseline. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. It is unlikely that control patients signed a contract. |
| Other bias | Low risk | None. |
Ali 2012.
| Study characteristics | ||
| Methods |
Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomized controlled study Patient RCT, conducted in 2 community pharmacies in Hertfordshire, United Kingdom Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 23 Intervention arm N: 25 Diabetes type: 2 Mean age: NR ± NR % Male: 50.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein 5a) Harms (hyperglycaemic episodes) 5b) Harms (hypoglycaemic episodes) |
|
| Funding source | This pilot study was funded by grants from the Department of Health, UK and Merck Sharp and Dohme Ltd. Diagnostic equipment kits for measuring HbA1c, blood glucose and lipid profile were provided free of charge by Menarini Diagnostics. No party had involvement in the design, conduct or analysis or preparation of the manuscript. However, Professor Robinson from Merck Sharp and Dohme Ltd helped in the analysis and manuscript preparation but received no consulting fee. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " computer generated randomized list." |
| Allocation concealment (selection bias) | Low risk | Quote: "…list held by the researcher at the School of Pharmacy." |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "There was no significant difference in the demographics between the two groups." Table not provided with P values. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Outcome variables not provided in baseline table. |
| Incomplete outcome data (attrition bias) | Low risk | Only withdrawals were from the intervention group, however they provided reasons and it was related to outcomes. They excluded these 2 from the analysis (per‐protocol) and used imputations for missing variables for those who remained. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described. HbA1c: methods not explicitly described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; outcomes match those in methods. |
| Risk of contamination (other bias) | High risk | Quote: "There was also possibility of contamination of the groups as both were attending the same pharmacies." |
| Other bias | Low risk | Information not available. |
Ali 2016.
| Study characteristics | ||
| Methods |
Effectiveness of a multicomponent quality improvement strategy to improve achievement of diabetes care goals: a randomized, controlled trial RCT (NA clusters and NA providers), conducted in 1) Diabetes clinics in India and Pakistan. CARRS trial sites were selected to include a diverse mix of publicly funded, semiprivate and private outpatient clinics in India and Pakistan. 2) Intervention participants were supported by non‐physician care co‐ordinators (CCs) in addition to their usual physicians. In India and Pakistan. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (multi QIs: non‐physician co‐ordinators and electronic records) (intervention arm) |
|
| Participants | Control arm N: 571 Intervention arm N: 575, NA, NA Diabetes type: 2 Mean age: 54.2 ± 10.9 % Male: 45.9 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (multi QIs: non‐physician co‐ordinators and electronic records) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician reminder 5) Facilitated relay of clinical information 5) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein 5) Hypertension control (< 130/80 mm Hg) 6) Harms (hypoglycaemia) |
|
| Funding source | Financial Support: The CARRS trial was funded in part by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services, under contract HHSN268200900026C, and by UnitedHealth Group, Minneapolis, Minnesota. Several members of the research team at the Public Health Foundation of India and Emory University were supported by the Fogarty International Clinical Research Scholars and Fellows program through grant 5R24TW007988 from the National Institutes of Health, Fogarty International Center through Vanderbilt University, Emory Global Health Institute, and D43 NCDs in India Training Program through award 1D43HD05249 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Fogarty International Center. Ms. Singh is supported by the Fogarty International Center, National Institutes of Health, under award D43TW008332 (ASCEND Research Network). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After baseline assessment, study staff at each clinic accessed each eligible participant's randomisation allocation from a password‐protected, web‐based data management system (Interactive Web Response System). The system randomly assigned participants in blocks of 4, and allocation was stratified by site. |
| Allocation concealment (selection bias) | Low risk | Web‐based data management system (onsite computer system). |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. *All baseline characteristics were similar between treatment groups except those for insulin use. |
| Patient's baseline outcomes (selection bias) | Low risk | Legend Table 1. All baseline characteristics were similar between treatment groups except those for insulin use. |
| Incomplete outcome data (attrition bias) | Unclear risk | Of 1486 participants who were screened, 1146 (575 in the intervention group and 571 in the usual care group) were eligible and were randomly assigned. Median follow‐up was 28 months (range, 22 to 36 months), and 1027 participants (516 in the intervention group and 511 in the usual care group) completed EOS visits (89.6% retention). 189 out of 1146 data are missing for HbA1c (16.5%), 205 for blood pressure (17.9%) and 171 for LDL (14.9%). Numbers and reasons reported and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c, blood pressure, LDL). Self‐reported outcome (hypoglycaemia events, secondary outcome). Participants were asked open‐ended questions about hypoglycaemia. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted in September 2010, participants were enrolled from January 2011 to June 2012, and final follow‐up visits were in July 2014). Data match protocol. Secondary outcomes reported elsewhere. |
| Risk of contamination (other bias) | High risk | Physicians treated patients in both groups. Quote: "Although contamination or spillover may have limited the observed between‐group differences, this pragmatic trial aimed to replicate real‐life settings." |
| Other bias | Low risk | No evidence of other bias. |
Allen 2011.
| Study characteristics | ||
| Methods |
COACH Trial: a randomized controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centres Patient RCT (NA clusters and NA providers), recruited from 2 community health centres (part of Baltimore Medical Systems Incorporated ‐ BMS, a federally qualified community health centre). In USA. 2 arms: (control arm) (intervention arm) |
|
| Participants | Control arm N: 264 Intervention arm N: 261, NA, NA Diabetes type: 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (enhanced usual care (EUC)) 1) Clinician education 2) Clinician reminder 3) Patient education 4) Patient reminder Intervention arm: (Nurse Practitioner/Community Health Worker (NP/CHW)) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
|
| Funding source | This study was supported by the National Heart Lung and Blood Institute, National Institutes of Health grant # R01HL082638 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described: "…participants were randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | Not described: "…participants were randomly assigned." |
| Patient's baseline characteristics (selection bias) | Low risk | In table and text. Quote: "There were no significant differences in sociodemographic and baseline measures between the two groups except for higher total cholesterol and HbA1c levels in the NP/CHW intervention group compared to the EUC." |
| Patient's baseline outcomes (selection bias) | High risk | HbA1c (P = 0.006). |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis with imputations, non‐significant differences in attrition rates, number and reasons for loss to follow‐up provided. Baseline based on those randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: lipids, blood pressure, HbA1c. LDL measured using Friedewald equation. HbA1c measured using liquid chromatography. Blood pressure using Omron digital blood pressure monitor. Blinding not described. |
| Selective reporting (reporting bias) | High risk | Some primary outcomes listed in protocol were not reported in the manuscript: physical activity, smoking cessation, antiplatelet use, beta blockers use, ACE inhibitors use. |
| Risk of contamination (other bias) | High risk | Quote: "…physicians had patients in both the intervention and the EUC groups. This may have resulted in a change in the level of care provided to their patients in the EUC groups…" |
| Other bias | Low risk | Information not available. |
Alotaibi 2016.
| Study characteristics | ||
| Methods |
A mobile diabetes management and educational system for type‐2 diabetics in Saudi Arabia (SAED) RCT (NA clusters and NA providers), conducted in 1) Remote mobile intervention in Tabuk region in The Kingdom of Saudi Arabia, 2) Medical staff, specialist diabetic nurse unit, clinicians. In Saudi Arabia. 2 arms: 1) Control (usual care/traditional monitoring) (control arm) and 2) Intervention (SAED: intelligent diabetes management system) (intervention arm) |
|
| Participants | Control arm N: 10 Intervention arm N: 10, NA, NA Diabetes type: 2 Mean age: 45.15 ± 9.65 % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care/traditional monitoring) Intervention arm: (SAED: intelligent diabetes management system) 1) Case management 2) Electronic patient registry 3) Clinician reminder 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | No report of funding | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1, characteristics balanced between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | See Table 1, LDL slightly higher in control group but not significant. |
| Incomplete outcome data (attrition bias) | Unclear risk | No report of loss or dropout. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c measured objectively. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Mobile intervention ‐ unlikely that the control group received SAED intervention. |
| Other bias | Low risk | None identified. |
Al Mazroui 2009.
| Study characteristics | ||
| Methods |
Influence of pharmaceutical care on health outcomes in patients with Type 2 diabetes mellitus Patient RCT, conducted in an endocrinology and medical outpatient clinic of Zayed Military Hospital, United Arab Emirates Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 120 Intervention arm N: 120 Diabetes type: type 2 Mean age: 49.4 ± 8.3 % Male: 69.7 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "restricted randomization". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | The age, gender, duration of diabetes and family history of diabetes for the 2 groups are presented in Table 1. Statistical analyses indicated that the groups were well matched (P > 0.05 in all cases). No education information. |
| Patient's baseline outcomes (selection bias) | Low risk | Partial text report. Intervention group patients had slightly higher mean fasting blood glucose readings at baseline, but this was not statistically significant (P > 0.05). In the case of HbA1c, the primary outcome measure of the study, mean baseline values in both groups were approximately the same (Table 3). At the baseline assessment, intervention group and control group patients exhibited approximately the same mean systolic and diastolic blood pressure. |
| Incomplete outcome data (attrition bias) | Low risk | 3/120 lost in control, 3/120 lost in intervention. Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of all outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No reported protocol. Methods match outcomes reported. |
| Risk of contamination (other bias) | Low risk | Groups were followed by different personnel, contamination unlikely. |
| Other bias | Low risk | None identified. |
Amendezo 2017.
| Study characteristics | ||
| Methods |
Effects of a lifestyle education program on glycaemic control among patients with diabetes at Kigali University Hospital, Rwanda: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted at the outpatient facility of the largest tertiary centre in Kigali, Rwanda. 2) The study team was composed of 5 physicians, 4 nurses, 3 nutritionists and 2 psychologists in Rwanda 2 arms: 1) Control (standard of care) (control arm) and 2) Intervention (lifestyle modification programme: counselling and education) (intervention arm) |
|
| Participants | Control arm N: 128 Intervention arm N: 123, NA, NA Diabetes type: 3 Mean age: 50.9 ± 12.3 % Male: 30.7 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard of care) Intervention arm: (lifestyle modification program: counselling and education) 1) Team change 2) Patient education |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
|
| Funding source | This study received funding from Sanofi Aventis and Kigali University Teaching Hospital’s Department of Research. Sanofi provided funds for HbA1c and urine albumin/creatinine testing while KUTH paid for development of study materials. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. The recruited participants were randomly assigned to an intervention group or to a control group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. The baseline demographic characteristics were not significantly different between the 2 study groups (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All P values above 0.05. The baseline clinical and laboratory characteristics did not significantly differ between the 2 groups (Table 2). |
| Incomplete outcome data (attrition bias) | High risk | Total of 28 lost to follow‐up out of 251 (11.2%). Reasons and numbers reported, but not balanced (20 lost in control group and 8 in intervention group). Quote: "there was a small differential in the loss to follow‐up between intervention and control arms (8 vs 20 patients respectively). It is however unlikely that this differential loss would have dramatically changed the study conclusions, given the robust and consistent nature of the findings." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c, SBP and DBP). Hospital technicians, who were blinded to the participants’ group assignments, collected these data. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol (protocol first posted on January 2014, the study was completed on November 2013). The protocol does not include blood pressure and weight measures as secondary outcomes. |
| Risk of contamination (other bias) | High risk | The standard of care for diabetes patients in the setting already includes QI intervention (consists of monthly medical follow‐up and individual counselling on dietary habits and lifestyle change, delivered by attending physicians and/or nutritionists as required). The novel training, which diabetic care providers involved in the study received prior to study initiation, likely increased provider knowledge on the role of diet and exercise in diabetic management in both arms of the study and could partly explain the improvements in HbA1c among both participants from interventional and control groups. |
| Other bias | Low risk | No evidence of other bias. |
Amsberg 2009.
| Study characteristics | ||
| Methods |
A cognitive behavior therapy‐based intervention among poorly controlled adult type 1 diabetes patients: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted in 2 diabetes outpatient clinics of 2 university hospitals in Stockholm. 2) The intervention was led by a diabetes specialist nurse (first author) and a psychologist trained in cognitive behavior therapy (CBT, second author). In Sweden. 2 arms: 1) Control (CGMS and routine diabetes care) (control arm) and 2) Intervention (CGMS, cognitive behavior therapy sessions and phone calls) (intervention arm) |
|
| Participants | Control arm N: 48 Intervention arm N: 46, NA, NA Diabetes type: 1 Mean age: 41.2 ± 11.4 % Male: 48.6 Longest follow‐up: 11.1 months |
|
| Interventions |
Control arm: (CGMS and routine diabetes care) 1) Promotion of self‐management Intervention arm: (CGMS, cognitive behaviour therapy sessions and phone calls) 1) Case management 2) Team change 3) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Harms (hypoglycaemia) |
|
| Funding source | This project was supported by grants from: the Health Care Sciences Postgraduate School, Karolinska Institutet; Sophiahemmet University College, the Foundation for Medical Research at Sophiahemmet; the Bert von Kantzow Foundation; and the Swedish Diabetes Federation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. A sex‐stratified randomisation was executed manually by a person not involved in the study. The intention was to get mixed groups of females and males. The randomisation was done in blocks of 16 patients, 8 in each arm. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. All P values above 0.05. Participants’ baseline characteristics are given in Table 2, which demonstrates no significant differences between the 2 groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Total of 25 lost to follow‐up out of 94 (26.6%, 11 dropouts from control group, 14 dropouts from intervention group). Numbers and reasons provided, but reasons not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). Subjective outcome (hypoglycaemia, secondary outcome). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Data match methods. |
| Risk of contamination (other bias) | Low risk | Only the intervention patients received feedback on their CGMS data and had phone calls. Control group unlikely to have received intervention, given usual care and did not come in contact with psychologist. Unsure if diabetes nurse specialists were the same in the intervention group. All patients received basic education and CGMS device. |
| Other bias | Low risk | No evidence of other bias. |
Anderson 2005.
| Study characteristics | ||
| Methods |
Evaluating a problem‐based empowerment program for African Americans with diabetes: results of a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The primary intervention was held in convenient community‐based locations (randomised part). The follow‐up was done at the same place or through phone calls (non‐randomised part). 2) Certified diabetes educators (dietitians and nurses) delivered intervention. In United States of America. 2 arms: 1) Control (wait‐list) (control arm) and 2) Intervention (group education: problem‐based empowerment program) (intervention arm) |
|
| Participants | Control arm N: 114 Intervention arm N: 125, NA, NA Diabetes type: 2 Mean age: 61 ± 11.9 % Male: 18 Longest follow‐up: 1.38 months |
|
| Interventions |
Control arm: (wait‐list) 1) Facilitated relay of clinical information Intervention arm: (group education: problem‐based empowerment programme) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
|
| Funding source | This study was supported by National Institutes of Health Grants R01 DK53994‐01 and the Core(s) of the Michigan Diabetes Research and Training Center (NIH5P60 DK20572) from the National Institute of Diabetes and Digestive and Kidney Diseases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients were randomly assigned to either the intervention group or the wait‐listed control group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 3 shows mean values or percent distributions for demographic variables as well as selected laboratory, psychosocial and health variables at baseline. The intervention and control patients did not differ significantly on any of these measures. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3 shows mean values or percent distributions for demographic variables as well as selected laboratory, psychosocial and health variables at baseline. The intervention and control patients did not differ significantly on any of these measures. |
| Incomplete outcome data (attrition bias) | Low risk | Table 4. They have HbA1c data for 225 patients out of 239 at 6 weeks (5.9% lost), 222 for SBP (7.1% lost) and 220 for DBP (8.0% lost). Reasons and numbers in each arms not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c, SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. They did not report data on HDL, LDL and triglycerides in Table 4 (for each arm) as they did in Table 5 (arms combined). |
| Risk of contamination (other bias) | Unclear risk | The control group improved HbA1c level during the 6 weeks. We believe that both groups changed because of the combination of volunteer bias (patients were better insured and educated and exhibited better self‐management), study effects (increased frequency of providing patients and their physicians data over the one‐year study period), and programme impact (unable to demonstrate a statistically significant impact of the intervention). Although control group did not receive the intervention at the same time as intervention group, "The control group made the decision to improve their diabetes self‐management during the control period." |
| Other bias | Low risk | No evidence of other bias. |
Anderson 2010.
| Study characteristics | ||
| Methods |
Managing the space between visits: a randomized trial of disease management for diabetes in a community health center Patient RCT, conducted in 2 community health centres (largest federally qualified health center) in Connecticut serving largely underserved Hispanic/Latino patients, USA Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 149 Intervention arm N: 146 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
|
| Funding source | Funding for this project was provided by a grant from the Connecticut Health Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomized in groups of 4 by a computerized algorithm…" |
| Allocation concealment (selection bias) | Unclear risk | Not reported (block?). |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "There were no significant differences in the two groups at baseline in regards to sociodemographic variables." Table and text. |
| Patient's baseline outcomes (selection bias) | High risk | HbA1c (P = 0.006). |
| Incomplete outcome data (attrition bias) | High risk | They state this was an intention‐to‐treat analysis, but very confusing since final numbers at month 12 do not match with those in table of outcomes at month 12. Baseline based on those randomised. Number for lost to follow‐up provided, but reasons very vague. Number lost to follow‐up much larger in intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective methods to obtain outcomes not described; blinding not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | High risk | Quote: "..presence of control and intervention patients in the same clinics were additional weaknesses, which may have led to contamination." |
| Other bias | Low risk | Information not available. |
Anderson‐Loftin 2005.
| Study characteristics | ||
| Methods |
Soul food light: culturally competent diabetes education RCT (NA clusters and NA providers), conducted in 1) conducted at a diabetes education centre in a rural SC county 2) Educational classes were taught by a local registered dietician with experience in nutrition therapy for rural black southerners with diabetes. Peer‐professional discussion groups began 1 month after educational classes and were facilitated by a nurse case manager who was certified as a diabetes educator in United States of America 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (dietary self‐management behavioural intervention) (intervention arm) |
|
| Participants | Control arm N: 48 Intervention arm N: 49, NA, NA Diabetes type: 2 Mean age: 57.32 ± 10.62 % Male: 21.65 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (dietary self‐management behavioural intervention) 1) Case management 2) Patient education |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | This study was funded by the National Institute of Nursing Research (1R15NR/DK07651‐01) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to experimental or control groups by the principal investigator based on a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Low risk | The RA who assigned participants identification numbers was blinded to group assignment. |
| Patient's baseline characteristics (selection bias) | High risk | Quote: "Significant differences in duration of diabetes were found between groups at baseline (Table 1). Gender and 3 categories of medication (oral hypoglycaemic agents, insulin, and lipid‐reducing medications) were significant for at least 1 dependent variable. " |
| Patient's baseline outcomes (selection bias) | High risk | See Table 1, differences between groups for HbA1c, LDL, triglycerides. |
| Incomplete outcome data (attrition bias) | High risk | Retention in the experimental group was 78% and 56% in the control group; 32 participants (33%) were lost to attrition. Significant differences (P = 0.03) in attrition between experimental and control groups were observed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Patient randomised. Interaction between groups may have occurred. |
| Other bias | Low risk | None identified. |
Andrews 2011.
| Study characteristics | ||
| Methods |
Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial Patient RCT, conducted in 5 secondary care National Health Service trusts: Taunton and Somerset NHS Foundation trust, University Hospitals Bristol NHS Foundation trust, North Bristol NHS Trust, Gloucestershire Hospitals NHS Trust, and Weston Area Health NHS Trust, United Kingdom. Three arms: 1) Usual care (control arm), 2) Intensive dietary intervention (intervention arm 1) and 3. Intensive dietary intervention and activity (intervention arm 2) |
|
| Participants | Control arm N: 99 Intervention arm 1 N: 248 Intervention arm 2 N: 246 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Team changes 2) Patient education 3) Promotion of self‐management Intervention arm 2: 1) Team changes 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Statins 2) Antihypertensives (any) 3) Glycated haemoglobin 4) Systolic blood pressure 5) Diastolic blood pressure 6) Low‐density lipoprotein |
|
| Funding source | This study was funded by Diabetes UK and the UK Department of Health | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "computer‐generated allocation", but unsure if this included generation of sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "computer‐generated allocation." |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "characteristics of enrolled patients in all groups were similar at baseline", in text but not in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "characteristics of enrolled patients in all groups were similar at baseline." Includes outcomes of interest. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis done, attrition very low, reasons for loss to follow‐up provided and proportions are unlikely to reflect outcomes. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary: HbA1c and blood pressure: measures not described, and blinding of outcome assessor not described. Secondary: outcome assessors not blinded for medication. |
| Selective reporting (reporting bias) | Low risk | Checked protocol, everything proposed was conducted. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Anzaldo‐Campos 2016.
| Study characteristics | ||
| Methods |
Dulce wireless Tijuana: a randomized control trial evaluating the impact of Project Dulce and short‐term mobile technology on glycaemic control in a family medicine clinic in northern Mexico RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from Family Medical Unit #27 of the Instituto Mexicano del Seguro Social (IMSS) in Tijuana, Mexico. 2) Consistent with the Project Dulce (PD) model, PD group included a combination of care management by a multidisciplinary team led by trained clinicians and nurses, as well as a peer‐led group education component. In Mexico. 3 arms: 1) Control (IMSS standard of care) (control arm) and 2) Intervention 1 (Project Dulce only) (intervention arm)3. Intervention 2 (Project Dulce technology‐enhanced) (other arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 99, 102, NA Diabetes type: 2 Mean age: 51.54 ± 13 % Male: 33.2 Longest follow‐up: 10 months |
|
| Interventions |
Control arm: (IMSS standard of care) Intervention arm: (Project Dulce only) 1) Case management 2) Team change 3) Clinician education 4) Patient education 5) Promotion of self‐management Intervention arm: (Project Dulce technology‐enhanced) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician education 5) Clinician reminder 6) Facilitated relay of clinical information 7) Patient education 8) Promotion of self‐management 9) Patient reminders |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
|
| Funding source | Funding for this study was provided by Qualcomm Inc. and Iusacell, with the in‐kind support of all partner organisations: the International Community Foundation, the Universidad Autonoma de Baja California, Fronteras Unidas Pro‐Salud, Entra Health Systems, Scripps Whittier Diabetes Institute, and the Fundacion Internacional de la Comunidad. National Center for Research Resources grant 1UL1 TR001114‐01 supported the researchers at the Scripps Whittier Diabetes Institute. The International Community Foundation, a not‐for‐profit dedicated to expanding philanthropy in Mexico and Latin America, was responsible for managing the funds of this study. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients who agreed to participate were randomly assigned to one of 3 groups: Project Dulce‐only intervention (PD), Project Dulce technology‐enhanced intervention (PD‐TE) or control group (CG). A block randomisation procedure was used to promote homogeneity among groups. Patients entered the study in successive cohorts as they were recruited, every 2 or 3 months. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. The 3 study groups were similar at baseline, except for a larger proportion of women in the PD group (76.8%) compared with the CG (62.0%) and PD‐TE (61.8%) groups (P < 0.05). |
| Patient's baseline outcomes (selection bias) | High risk | LDL, HDL look imbalanced between groups. "There were no statistically significant differences of baseline HbA1c levels among the groups." |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. In total, 37 patients were missing at 6 months (12.3%), 8 in the control group (8.0%), 16 in Dulce‐only group (16.2%) and 13 in the Dulce and technology enhanced group (12.7%). Numbers unbalanced. Reasons partly reported (do not report the number for each reason). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (applied on March 2014, recruitment began in 2011). Results match protocol for our outcomes of interest. |
| Risk of contamination (other bias) | Low risk | The control group had similar interventions compared to the Dulce‐only group. IMSS usually does not provide glucose meters, test strips or cell phones to patients, so the combination of technology tools used in this study for the PD‐TE group can be considered a novel therapeutic approach in this institution. The DiabetIMSS program encouraged patients to participate in monthly visits of approximately 3 hours, where they received educational classes and were evaluated by a nurse and a physician. Thus, patients in the DiabetIMSS group had access to 10 monthly medical group visits during the study. In order to prevent contamination among the 3 groups, different physicians and peer educators provided care for each of the study groups. Also, the classes and visits were offered separately and at different times to prevent contamination across conditions. |
| Other bias | Low risk | No evidence of other bias. |
Aubert 1998.
| Study characteristics | ||
| Methods |
Nurse case management to improve glycaemic control in diabetic patients in a health maintenance organization: a randomized, controlled trial RCT (NA clusters and NA providers), conducted in 1) Jacksonville Health Care Group (JHCG), the largest provider of primary care services for the Prudential HealthCare (PHC) HMO of Jacksonville, Florida 2) The NCM programme was conducted by a registered nurse/certified diabetes educator trained to follow a set of detailed diabetes management algorithms under the direction of a board‐certified family medicine physician and an endocrinologist. In United States of America. 2 arms: 1) Control: usual care (control arm) and 2) Intervention: nurse case management (intervention arm) |
|
| Participants | Control arm N: 67 Intervention arm N: 71, NA, NA Diabetes type: 1 and 2 Mean age: 53.5 ± 10.1 % Male: 40.6 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) 1) Promotion of self‐management Intervention arm: (nurse case management) 1) Case management 2) Team change 3) Clinician reminder 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) Renal screening 2) Glycated haemoglobin 3) Diastolic blood pressure 4) Low‐density lipoprotein |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | The two treatment groups were similar for most characteristics at baseline, but the intervention group had fewer members of ethnic minority groups; 17% of the patients in the intervention group and 8% of those in the usual care group had type 1 diabetes; P values not reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | The 2 treatment groups were similar for most characteristics at baseline, but the intervention group had more smokers and more insulin‐treated patients; P values not reported. |
| Incomplete outcome data (attrition bias) | High risk | Of the 138 members randomised into the study, 100 (72%) provided 12‐month follow‐up data. Reasons for loss not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective renal measures. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol; methods match description |
| Risk of contamination (other bias) | Low risk | Physicians and nurses working with intervention group were not their primary physician but the control group were primarily seeing their primary physicians. |
| Other bias | Low risk | None. |
Augstein 2007.
| Study characteristics | ||
| Methods |
Outpatient assessment of Karlsburg diabetes management system‐based decision support Patient RCT, conducted in Germany. Two arms: 1) CGMS ‐ continuous glucose monitoring system (control arm) and 2) CGMS/KADIS ‐ continuous glucose monitoring system/Karlsburg Diabetes Management System (intervention arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 24 Diabetes type: type 1 and type 2 Mean age: 48.9 ± 13.4 % Male: 55.1 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management Intervention arm: 1) Electronic patient registry 2) Clinician reminders 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | The generous support of this study by Medtronic MiniMed is appreciated. This work was supported by grants from the German Federal Ministry of Education and Research (BMBF; FKZ 03i2711) and from the Ministerium für Bildung, Wissenschaft und Kultur Mecklenburg‐Vorpommern (IDK 97 007 80/SOM and IDK 97 007 80/HSP III). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Avdal 2011.
| Study characteristics | ||
| Methods |
The effects of web‐based diabetes education on diabetes care results Patient RCT, conducted in Dokuz Eylul University in the Endocrine Polyclinic, where diabetes care is provided. In Turkey. Two arms: 1) Control group (control arm) and 2) Experimental group (intervention arm) |
|
| Participants | Control arm N: 61 Intervention arm N: 61 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Electronic patient registry 2) Patient education |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomization was performed using the Minitab 14 package program in the computer environment." |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "The analyses demonstrated that there were no significant differences between two groups in terms of situational factors. ie. age, sex, training, marital status, etc)." |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.456). |
| Incomplete outcome data (attrition bias) | Low risk | No attrition; no outcome data missing. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary: do not describe laboratory methods for HbA1c. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Ayadurai 2018.
| Study characteristics | ||
| Methods |
Structured tool to improve clinical outcomes of type 2 diabetes mellitus patients: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The participating sites were 7 government‐funded primary care clinics in Johor, Malaysia. These practices, known as health clinics (Klinik Kesihatan), provide comprehensive medical care for ambulatory patients. 2) Intervention delivered by 14 pharmacists using the Simpler tool, a structured clinical guidelines tool. In Malaysia. 2 arms: 1) Control (UC: pharmacists providing usual care) (control arm) and 2) Intervention (SC: Simpler care by trained pharmacist) (intervention arm) |
|
| Participants | Control arm N: 77 Intervention arm N: 77, NA, NA Diabetes type: 2 Mean age: 56.67 ± 12.12 % Male: 42.75 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (UC: pharmacists providing usual care) Intervention arm: (SC: Simpler care by trained pharmacist) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Patient reminders |
|
| Outcomes | 1) Lipid lowering drugs 2) Glycated haemoglobin 3) Systolic blood pressure 4) Diastolic blood pressure 5) Low‐density lipoprotein 6a) Hypertension control (SBP ≤ 135 mmHg) 6b) Hypertension control (DBP ≤ 75 mmHg) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients at each of the 7 sites were randomised using overall equal randomisation (1: 1) to either the intervention group, namely to receive Simpler care (SC), or the control group to receive usual care (UC). The randomisation numbers were predetermined using an online random number generator based on a one block randomised block design (http://www.randomization.com/, accessed 10 March 2016). |
| Allocation concealment (selection bias) | Unclear risk | Upon receiving written consent, pharmacists opened the envelopes (opaque?) in ascending order and, depending on the randomisation code, allocated patients to either the intervention or control arm of the study. |
| Patient's baseline characteristics (selection bias) | Unclear risk | There were no significant differences between the SC and UC groups with regard to demographic characteristics, family history, types and number of comorbidities and current employment. There was a significant difference between patients’ overall highest education level between the SC and UC arms; highest education level (P value = 0.028) |
| Patient's baseline outcomes (selection bias) | Low risk | There were no significant differences between the SC and UC groups with regard to clinical parameters. |
| Incomplete outcome data (attrition bias) | High risk | They analysed 55/77 (29% lost) patients randomised in the intervention group and 69/77 (10% lost) in the control group. Unbalanced numbers and reasons. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c (primary outcome), LDL and blood pressure were objectively measured. Method to collect statin data not reported. |
| Selective reporting (reporting bias) | High risk | A registered protocol is available. Protocol outlines primary outcomes as "clinical outcomes and health‐related QOL of patients", which is in line with the manuscript primary outcome of "significant improvement in HbA1c". Protocol secondary outcome was "pharmacists’ compliance", manuscript secondary outcomes were "improved lipid profiles and blood pressure (BP)." There was also an additional analysis in the manuscript, which was not described in the protocol: "Comparison of participants in the Simpler care (SC) and usual care (UC) arms of the study who achieved at least a 1% decrease in HbA1c". They provide guidelines about aspirin therapy, but they do not report it as an outcome. There were no significant changes in the number of antihypertensive medications prescribed at 6 months of the study between the SC and UC arms, but they do not report data about it. Not all HbA1c and lipid results were available for each patient due to laboratory tests not scheduled at the exact time before the trial commenced or at completion. Hence, the values obtained ranged from the previous month to the previous 4 months. |
| Risk of contamination (other bias) | High risk | Patient‐randomised. Quote: "In addition, because patients in both the intervention and control groups were recruited from the same primary health center, “contamination” of the control group could occur. This is because it is possible that the same doctor treating patients in the intervention would be treating the control patients and therefore may use the pharmacist’s recommendations for the intervention group in the control group throughout the trial period. Nevertheless, the effect of any contamination did not mask the improvements seen in the SC group." |
| Other bias | Low risk | No evidence of other risk of bias. |
Ayala 2015.
| Study characteristics | ||
| Methods |
Puentes hacia una mejor vida (Bridges to a Better Life): Outcome of a diabetes control peer support intervention RCT (NA clusters and NA providers), conducted in 1) The Puentes study was conducted in the 3 largest Clinicas de Salud del Pueblo, Inc. (CDSDP) clinics, which are located in Brawley, El Centro, and Calexico, California, USA. And at a federally qualified health centre. 2) Intervention provided by peer leaders. In United States of America. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (peer support/leaders) (intervention arm) |
|
| Participants | Control arm N: 168 Intervention arm N: 168, NA, NA Diabetes type: 2 Mean age: 56.3 ± 11.4 % Male: 37 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (peer support/leaders) 1) Case management |
|
| Outcomes | 1) Retinopathy screening (dilated eye exam) 2) Foot screening 3) Glycated haemoglobin 4) Systolic blood pressure 5) Diastolic blood pressure |
|
| Funding source | Funding for this research was provided by the American Academy of Family Physicians Foundation through the Peers for Progress programme with support from the Eli Lilly and Company Foundation. Puentes hacia una mejor vida (Bridges to a Better Life; “Puentes”) was 1 of 8 international studies funded by Peers for Progress. Puentes was a partnership between a university‐affiliated research institute (Institute for Behavioral and Community Health; IBACH) and Clinicas de Salud del Pueblo, Inc. (CDSDP), an Federally Qualified Health Center (FQHC). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients were then randomised to intervention or usual care. Randomisation was conducted by the study biostatistician and stratified by clinic. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Patients were then randomised to intervention or usual care. Randomisation was conducted by the study biostatistician and stratified by clinic. |
| Patient's baseline characteristics (selection bias) | Low risk | Participant characteristics are shown in Table 1. There was some indication that a greater percentage of intervention participants than usual care participants reported having a personal doctor (P = 0.09), while a greater percentage of usual care participants reported living below poverty thresholds (P = 0.08); no other group differences were observed. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 4, P value above 0.05 for HbA1c, SBP and DBP at baseline. |
| Incomplete outcome data (attrition bias) | High risk | 336 patients randomised, 32 excluded, leading to 304 patients and 60 lost to follow‐up (19.7%). Reasons for loss to follow‐up not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c, SBP, DBP). Health care use including ophthalmology and podiatry visits were abstracted from medical records. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol first posted on July 2014, study start date: February 2009). They do not report the results for changes in cholesterol, HDL, LDL and triglycerides, depression and distress (all listed as secondary outcomes in protocol). |
| Risk of contamination (other bias) | Low risk | The peer leaders only meet with the intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Azizi 2016.
| Study characteristics | ||
| Methods |
Evaluating the effect of web‐based Iranian diabetic personal health record app on self‐care status and clinical indicators: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Patients from one of the endocrinology practice offices in Mashhad city. 2) Patients managed their health information using a web‐based diabetic personal health records (DPHR) app and were able to view their physician’s advices. In Iran. 2 arms: 1) Control (routine care) (control arm) and 2) Intervention (web‐based diabetic personal health records app) (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 36, NA, NA Diabetes type: 2 Mean age: 54.55 ± 7.95 % Male: 49.06 Longest follow‐up: 4 months |
|
| Interventions |
Control arm: (routine care) Intervention arm: (web‐based diabetic personal health records app) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
|
| Funding source | This trial is the first author’s PhD dissertation, which has been supported by a grant (grant # 921835) from Mashhad University of Medical Sciences Research Council | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT protocol for a 2‐arm parallel group with a 1:1 allocation ratio. Patients were randomly allocated into the 2 groups regarding covariate‐adaptive randomisation through SPSS version 21.0 (IBM Corp), by a person with no direct role in the research. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The data look relatively balanced; only difference in employment status (P value = 0.01). The test results demonstrated no significant differences in the distribution of the variables between the intervention and control groups other than the variable of the range of working time with a computer, where participants in the intervention group at the baseline stage had spent more time working with a computer. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 6. P values all greater than 0.05. Quote: "the independent T‐test was utilized to compare the scores of weight, HbA1c, serum creatinine, HDL, LDL, total cholesterol, and triglyceride in control and intervention groups. The test results revealed no significant difference between any of them". Outcome characteristics: family history of diabetes mellitus and type of drug taken are balanced. |
| Incomplete outcome data (attrition bias) | High risk | They excluded 10/36 patients in the intervention group (27.8% lost) and 9/36 in the control group (25.0%). High numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Protocol: The study outcome measures include self‐care status of patients, visit adherence and drug adherence. Nothing about drug adherence in the paper. The protocol does not include clinical indicators reported in the paper (HbA1c, LDL and blood pressure). Publication does not report baseline or intermediate values only average difference. |
| Risk of contamination (other bias) | Unclear risk | The patients were randomly sampled from one private practice unit. Participants and practitioners could not be blinded to DPHR since it was an obvious artefact. Practitioners might have changed their approach with their control patients. In addition, the individuals in both groups were not allowed to exchange DPHR information to avoid contamination of the trial. |
| Other bias | Low risk | No evidence of other bias. |
Babamoto 2009.
| Study characteristics | ||
| Methods |
Improving diabetes care and health measures among Hispanics using community health workers: results from a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited during routine clinic visits at 3 inner‐city family health centres in Los Angeles. For patients in CHW arm, sessions were conducted in accessible community locations, at the clinic, or in the patient’s home. They also received follow‐up calls. Patients in the case management arm were usually seen in the clinic and also had follow‐up calls. 2) Community health workers (CHW arm) and nurses (case management arm) provided the interventions. In United States of America. 3 arms: 1) Control (standard provider care) (control arm), 2) Intervention 1 (community health workers) (intervention arm) and 3) Intervention 2 (case management by nurses) (other arm) |
|
| Participants | Control arm N: 54 Intervention arm N: 75, 60, NA Diabetes type: 2 Mean age: 50 ± 13.66 % Male: 36 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard provider care) 1) Patient education Intervention arm: (community health workers) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (case management by nurses) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | Project funding was provided by the Pfizer Foundation and Pfizer Health Solutions Inc. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The final study sample consisted of 318 (or 189?) patients randomly assigned to the 3 study arms via a random‐number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | There were no significant differences across study groups with respect to age, education and household income; however, when compared with the other groups, the standard provider care group had a greater proportion of females, and the case management group had a greater proportion of patients whose parents had diabetes. |
| Patient's baseline outcomes (selection bias) | Low risk | Baseline clinical indicators and self‐reported health measures indicated a population characterised by poor dietary and exercise habits, as well as poor medication‐taking behaviour, with no significant differences at baseline across study groups (Table 2). |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up was greatest in the standard provider care group. Significantly greater proportions of patients enrolled in the standard provider care group (50%) and the case management group (43%) were lost to follow‐up, as compared with the CHW group (28%, P < 0.05). With respect to age and annual income, no significant differences were observed between programme graduates and those who disenrolled. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Data match methods. |
| Risk of contamination (other bias) | Unclear risk | The standard provider care group, as well as the other randomised groups, may have received higher levels of care during the study period than what they would have normally received in the absence of the intervention. Before study activities began, clinic providers received information about the study objectives... It is conceivable that awareness of the study among clinic providers may have motivated them to be more diligent about the services they offered to study patients. |
| Other bias | Low risk | No evidence of other bias. |
Barcelo 2010.
| Study characteristics | ||
| Methods |
Using collaborative learning to improve diabetes care and outcomes: the VIDA project. Cluster RCT (10 clusters with 43 providers), conducted in 10 public health centres in Xalapa and Veracruz, Mexico. Two arms: 1) Usual care (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 111 Intervention arm N: 196 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 18 months |
|
| Interventions |
Control arm: 1) Electronic patient registry Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Clinician education 5) Patient education 6) Promotion of self‐management 7) Continuous quality improvement |
|
| Outcomes | 1) Retinopathy screening (eye exam) 2) Foot screening 3) Glycated haemoglobin 4) Systolic blood pressure 5) Diastolic blood pressure 6) Hypertension control |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not provided. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Provided in text but not in table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Outcome variables not provided in baseline table. |
| Incomplete outcome data (attrition bias) | Unclear risk | Cannot tell whether an intention‐to‐treat or per‐protocol analysis was conducted. No flow diagram provided with losses to follow‐up; do not know whether losses to follow‐up were similar between both arms. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective laboratory methods not described for all outcomes. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | High risk | Quote: "…avoiding contamination of centers that acted as controls (those centers providing usual diabetes care) was not possible, because of the visability and publicity of the intervention at the local level." |
| Other bias | Low risk | Information not available. |
Baron 2017.
| Study characteristics | ||
| Methods |
A randomised, controlled trial of the effects of a mobile telehealth intervention on clinical and patient‐reported outcomes in people with poorly controlled diabetes RCT (NA clusters and NA providers), conducted in 1) The study took place in a diabetes clinic in East London, United Kingdom (UK). 2) Intervention delivered by a mobile telehealth nurse. In United Kingdom. 2 arms: 1) Control (standard care) (control arm) and 2) Intervention (mobile telehealth nurse) (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 45, NA, NA Diabetes type: 3 Mean age: 57.13 ± NR % Male: 57.24 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (mobile telehealth nurse) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Policy Research Programme of the Department of Health for England | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by a member of the research team upon receipt of the completed baseline questionnaire, and independently of diabetes specialist nurses (DSNs), using an online sequence generator that generated randomised block allocations (blocks of 20). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | With the exception of gender (P = 0.013), there were no statistically significant differences at baseline between groups; number with type 2 diabetes between groups differs slightly (91.1% vs 83.3%). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. No P values under 0.05 for all clinical outcomes. There were no statistically significant differences at baseline between groups. |
| Incomplete outcome data (attrition bias) | Unclear risk | 6 lost in intervention group and 7 lost in control, reasons somewhat balanced (See Figure 1). They have HbA1c and BP data for 40 patients out of 45 at 9 months (11.1% lost) in the intervention group. In the control group, they have HbA1c and BP data for 31 patients out of 36 at 9 months (13.9% lost). Reasons and numbers reported and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c, SBP and DBP). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol (protocol first posted on June 2009, study started on June 2010). The number of hypoglycaemic events are not reported in the paper and other outcomes that are not of interest to us are also not reported (BMI, diabetes self‐care and self‐efficacy, user acceptability, etc.). No data on blood pressure at 3 months. |
| Risk of contamination (other bias) | Unclear risk | Only the patients in the intervention group had mobile telehealth nurse management and education at least every month. However, standard care at the diabetes clinic consisted of follow‐up appointments with a diabetes specialist nurse every 3 to 4 months. |
| Other bias | Low risk | No evidence of other bias. |
Basak 2014.
| Study characteristics | ||
| Methods |
Health promotion for patients with diabetes: health coaching or formal health education? Patient RCT, conducted in outpatient clinics of 2 hospitals in Istanbul, Turkey Two arms: 1) Formal health education (control arm) and 2) Health coaching (intervention arm) |
|
| Participants | Control arm N: 114 Intervention arm N: 83 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 16 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education Intervention arm: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | The research is part of an international project that has two phases. The Turkish phase is presented here and is supported by FDI, and the International Research Fund of University of Copenhagen. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not provided in Table 1; they provide proportions. |
| Incomplete outcome data (attrition bias) | High risk | ~11% lost to follow‐up in control; ~9% lost to follow‐up in intervention; numbers lost and reasons were balanced at first follow‐up, but more losses in control group during second follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: glycaemic control and oral health: how HbA1c was measured was not reported. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | One dentist provided the health education group (control), less likely for contamination. |
| Other bias | Low risk | Information not available. |
Basudev 2016.
| Study characteristics | ||
| Methods |
A prospective randomized controlled study of a virtual clinic integrating primary and specialist care for patients with Type 2 diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) 6 general practices were recruited from 2 London boroughs following a sampling frame based on the following parameters: practice size and type 2 diabetes mellitus population, index of multiple deprivation (IMD) score and the level of exception reporting for both blood pressure and glycated haemoglobin. 2) Clinical review by primary care and specialist diabetes team in United Kingdom 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (virtual clinic) (intervention arm) |
|
| Participants | Control arm N: 115 Intervention arm N: 120, NA, NA Diabetes type: 2 Mean age: 59.84 ± 9.43 % Male: 57.45 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (virtual clinic) 1) Case management 2) Team change 3) Clinician education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
|
| Funding source | The article is funded by Diabetes UK and the Royal College of General Practitioners. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a computerised random number generator (managed by our statistician) following a 1:1 ratio to either usual care or the virtual clinic. |
| Allocation concealment (selection bias) | Low risk | All the primary and secondary care professionals involved in the virtual clinics were blind to the allocation process. |
| Patient's baseline characteristics (selection bias) | Low risk | The only difference observed was in current diabetes care (P = 0.003), with a higher proportion of participants in the virtual clinic group having care from the intermediate or secondary diabetes teams (34% compared with 14% in the control group) (P value not reported for this). |
| Patient's baseline outcomes (selection bias) | Low risk | Clinical characteristics and medication look balanced; no significant differences (P values above 0.05). |
| Incomplete outcome data (attrition bias) | High risk | Figure 2. 88/115 control patients analysed (23.5% dropout rate). 79/93 intervention patients analysed (15% dropout rate). Reasons for dropout: death (5), left practice (7), excluded due to no repeat blood test (29). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | High risk | The study results may have been affected by internal practice contamination; as highlighted, it may be that the overall practice of the health professionals was influenced by the virtual clinic, leading to more general improvements in outcomes. |
| Other bias | Low risk | No evidence of other bias. |
Bebb 2007.
| Study characteristics | ||
| Methods |
A cluster randomised controlled trial of the effect of a treatment algorithm for hypertension in patients with type 2 diabetes Cluster RCT (42 clusters), conducted in practices in Nottingham, United Kingdom Two arms: 1) Control arm (control arm) and 2) Intervention arm (intervention arm) |
|
| Participants | Control arm N: 737 Intervention arm N: 797 Diabetes type: type 2 Mean age: 64.3 ± 9.9 % Male: 59.2 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician education |
|
| Outcomes | 1) Antihypertensives (any) 2) Systolic blood pressure 3) Diastolic blood pressure |
|
| Funding source | Fund source: The study was funded by a grant from the NHS Executive, Trent, UK (RBG00XX7) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Cluster RCT. |
| Provider's baseline characteristics (selection bias) | High risk | Table 2. no P values provided. There were some differences between practices in the 2 arms: practices in the intervention arm were smaller, less likely to have agreed a BP target, and more likely to negotiate BP targets with almost all or many patients. |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. No P values provided. The intervention and control arms were similar for most measures, but there were small differences for sex, ethnic group, years since diagnosis of diabetes, and the proportion with macrovascular complications. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. no P values provided; several rows appear unbalanced |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Bellary 2008.
| Study characteristics | ||
| Methods |
Enhanced diabetes care to patients of South Asian ethnic origin (the United Kingdom Asian Diabetes Study): a cluster randomised controlled trial Cluster RCT (21 clusters), conducted in inner‐city practices in the United Kingdom Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 618 Intervention arm N: 868 Diabetes type: type 2 Mean age: 57.0 ± 11.9 % Male: 52.0 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Team changes 2) Clinician education 3) Patient education |
|
| Outcomes | 1) Statins 2) Antihypertensives (any) 3) Systolic blood pressure 4) Diastolic blood pressure |
|
| Funding source | Pfizer, Sanofi‐Aventis, Servier Laboratories UK, Merck Sharp & Dohme/Schering‐Plough, Takeda UK, Roche, Merck Pharma, Daiichi‐Sankyo UK, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Bristol‐Myers Squibb, Solvay Health Care, and Assurance Medical Society UK | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values provided. Differences observed between groups for sex, age, duration of diabetes and treatment for diabetes were not significant. No education level information. |
| Patient's baseline outcomes (selection bias) | High risk | The proportion of current smokers was much the same in both groups, but more patients in the control group than in the intervention group were ex‐smokers... More intervention than control patients were treated with statins. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Benhamou 2007.
| Study characteristics | ||
| Methods |
One‐year efficacy and safety of web‐based follow‐up using cellular phone in type 1 diabetic patients under insulin pump therapy: the PumpNet study Cross‐over RCT, conducted in diabetology clinic outpatients at a clinic from Grenoble and Toulouse, France Two arms: 1) No‐SMS (control arm) and 2) SMS (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 15 Diabetes type: type I Mean age: 41.3 ± 11.3 % Male: 50.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Harms (severe hypoglycaemia or diabetic ketoacidosis) |
|
| Funding source | This study was supported by research grants from Direction de la Recherche Clinique, CHU Grenoble and from Agir‐aDom. Material was provided by Roche Diagnostics France and by Palm Inc. France | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Do not report numbers of dropouts by group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Benson 2019.
| Study characteristics | ||
| Methods |
Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) telemedicine randomized controlled trial on diabetes optimal care outcomes in patients with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Participants were invited from patient panels in 2 different healthcare systems in rural Minnesota, located 60 to 90 miles from the Minneapolis metropolitan area (New Ulm Medical Center and Hutchinson Health). Both facilities include a hospital and a primary care clinic in a single centre. 2) Phone coaching intervention delivered by registered dietitian nutritionist. In United States of America. 2 arms: 1) Control (usual visits with primary care provider) (control arm) and 2) Intervention (ENHANCED: telephone coaching by dietitians) (intervention arm) |
|
| Participants | Control arm N: 59 Intervention arm N: 61, NA, NA Diabetes type: 2 Mean age: 59.90 ± 10.1 % Male: 55.07 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual visits with primary care provider) 1) Patient education Intervention arm: (ENHANCED: telephone coaching by dietitians) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) Lipid lowering drugs 2) Antihypertensive drug 3) Glycated haemoglobin 4) Low‐density lipoprotein 5) Hypertension control (< 140/90 mmHg) 6) Smoking status |
|
| Funding source | This work was supported by a Diabetes Care and Education Dietetic Practice Group/Academy of Nutrition and Dietetics Foundation Diabetes MNT Outcomes Research award | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to either the intervention or control group using a computer‐generated system that grouped condition assignments into sets of 6 (3 intervention, 3 control). |
| Allocation concealment (selection bias) | Unclear risk | Assignments were into envelopes (opaque?) that were opened at the time of assignment. A study staff member who did not perform the randomisation filled the envelopes. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, top. All P values above 0.05. Quote: "There were no statistically significant differences between the intervention and control groups at baseline with regard to demographics, BMI, comorbidities, HbA1c, or optimal diabetes measures." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1, end. Non‐significant P values for HbA1c, LDL and BMI. Quote: "There were no statistically significant differences between the intervention and control groups at baseline with regard to demographics, BMI, comorbidities, HbA1c, or optimal diabetes measures." |
| Incomplete outcome data (attrition bias) | High risk | Six participants from the intervention group and 8 from the control group were lost to follow‐up. Data from 2 participants were excluded (1 in the intervention group and 1 in the control group) from the results, resulting in a 13% attrition rate (16/120 randomised) by the 12‐month assessment. Number lost balanced, but quite high and the reasons for loss are not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcomes included composite and individual diabetes optimal care goals: haemoglobin A1c (venipuncture, objective), blood pressure (sphygmomanometers, objective), not using tobacco (self‐reported, patients unlikely blinded, subjective), and taking a statin and aspirin (both self‐reported, patients unlikely blinded, both subjective). |
| Selective reporting (reporting bias) | High risk | Registered trial. They only listed HbA1c as primary outcome in the protocol, but they have more primary outcomes in the paper. Quote: "Primary outcomes included composite and individual diabetes optimal care goals: haemoglobin A1c, blood pressure, not using tobacco, and taking a statin and aspirin (as appropriate)". Other outcomes are reported in the paper but are not listed in the protocol. Quote: "Secondary measures included physical activity, breakfast, fruits and vegetables, whole grains, body mass index, low‐density lipoprotein, and medication adherence." |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised trial. All patients were recruited from 2 clinics (inclusion criteria were as follows: designated PCP at the Hutchinson or New Ulm clinic, etc.). Unlikely that control patients were followed by dietitians every month, but they met each other at baseline and at the end of the trial. PCPs were aware of the study goals and may have changed practice as a result. Also, primary care providers might have changed their approach with control patients after receiving communications from the dietitians taking care of intervention patients. |
| Other bias | High risk | Some proactive phone medication titration was conducted by registered nurses independently of this study at one clinic site. |
Bergenstal 2005.
| Study characteristics | ||
| Methods |
Impact of modem‐transferred blood glucose data on clinician work efficiency and patient glycaemic control Patient RCT, self‐management education programme, USA Two arms: 1) Telephone (control arm) and 2) Modem (intervention arm) |
|
| Participants | Control arm N: 23 Intervention arm N: 24 Diabetes type: type 1 and type 2 Mean age: 44.0 ± 15.0 % Male: 38.0 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.2 (1.4), post 7.8 (1.5) Intervention arm: pre 9.2 (1.9), post 8.3 (1.5) |
|
| Funding source | This study was conducted under a grant from Roche Diagnostics Corporation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Time spent by the health care provider receiving and reviewing data. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Both groups received exactly the same intervention. |
| Other bias | Unclear risk | Information not available. |
Bertuzzi 2018.
| Study characteristics | ||
| Methods |
Teleconsultation in type 1 diabetes mellitus (TELEDIABE) RCT (NA clusters and NA providers), conducted in 1) 2 diabetes outpatient centres in Italy, 2) diabetologist, physicians, nutrition and psychological specialists in Italy 2 arms: 1) Control (standard care) (control arm) and 2) Intervention (Teleconsultation ‐ TELEDIABE) (intervention arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 37, NA, NA Diabetes type: 1 Mean age: 34.96 ± 10.07 % Male: 38.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (Teleconsultation ‐ TELEDIABE) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | The Italian Diabetes Foundation ‐ thanks to a financial support received from Intesa Sanpaolo Foundation ‐ covered the overall costs of the project | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with the use of a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | The dropout rate for group A was 1 out of 40 patients (2.5%), and for group B was 2 out of 37 patients (5.4%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure for HbA1c; not sure whether patient‐reported harms. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Paper reported harms, protocol did not list it. Time frame in protocol for change in HbA1c is 3rd to 6th to 9th month, in report time frame is 4, 8 and 12 months. Reduction in acute complications is a secondary outcome in the publication but not in the protocol. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Control patients did not have access to the teleconsultation intervention. |
| Other bias | High risk | The enrollment of patients was stopped at 77 patients: the financial support was stopped due to the prolongation of the activities over the planned timeline. For this reason, a futility analysis was performed. A non‐planned futility analysis was performed when the trial was terminated for lack of funding resources The enrollment of the patients was not concluded due to the economical budget restrictions. Due to some organisational problems, the time for patient enrollment lasted more than expected and they lost the economic support from a private foundation. The study was therefore closed ahead of time. This is the reason why the number of enrolled patients was lower than expected. Secondly, the primary outcome, the superiority of teleconsultation versus standard visit, was not reached. The main outcome was probably a bit ambitious. The possibility that the teleconsultation might be superior to standard visits was probably a difficult challenge: the quality of clinical assistance, the time for the visit and the physicians involved were the same. |
Bian 2012.
| Study characteristics | ||
| Methods |
Influence of multi‐disciplinary team management on psychological health and quality of life of patients with diabetic foot RCT (NA clusters and NA providers), conducted in 1) Endocrine clinic located in a level‐III medical facility in Shandong Province, which complies with World Health Organization Diagnostic Mark. 2) Intervention delivered by a multi‐disciplinary team (diabetes specialists, nurses, podiatrists, nutritionists and doctors in eye, cardiology, vascular surgery, nephrology or neurology departments). In China. 2 arms: 1) Control (conventional foot therapy) (control arm) and 2) Intervention (multidisciplinary team foot management) (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 100, NA, NA Diabetes type: 3 Mean age: 66.87 ± 8.27 % Male: 59 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (conventional foot therapy) Intervention arm: (multidisciplinary team foot management) 1) Case management 2) Team change |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Natural Science Foundation of Shandong Province, grant number: Y2008C128 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. A total of 200 patients with diabetic foot were randomly divided into 2 groups, 100 patients in each group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Methods: No statistical differences regarding age, gender, duration of diabetes, type of DM and levels of diabetic foot. No P values reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. Hba1c level in the intervention group (9.85) appears higher than in the control group (9.22) at baseline. No P values reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No reporting on loss to follow‐up; no mention about ITT or other analysis methods. Seems like all patients were included in analysis according to Table 2. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Patients RCT but unlikely that control patients had multi‐disciplinary management. However, they said that control situation of blood glucose and lipid in 2 groups of patients was improved compared with before. |
| Other bias | Low risk | No evidence of other risk of bias. |
Biermann 2002.
| Study characteristics | ||
| Methods |
Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomised, controlled trial Patient RCT (0 clusters and NA providers), conducted in 1) Intervention delivered at the Institute for Diabetes Research in Munich, Germany. 2) Intervention delivered by physicians specialised in diabetes and experienced in intensified insulin therapy. In Germany. 2 arms: 1) Control (conventional outpatient care) (control arm) and 2) Intervention (telecare management) (intervention arm) |
|
| Participants | Control arm N: 18 Intervention arm N: 30, NA, NA Diabetes type: 1 and 2 Mean age: 30.3 ± 13.5 % Male: NR Longest follow‐up: 8 months |
|
| Interventions |
Control arm: (conventional outpatient care) 1) Patient education 2) Promotion of self‐management Intervention arm: (telecare management) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This work was supported by the MediSense/ Abbott Co., Wiesbaden, Germany | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Similar age, duration of diabetes and distance from diabetes centre. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c of 8.0% for the control group and of 8.3% for the intervention group at baseline. Nothing reported in text about possible difference. |
| Incomplete outcome data (attrition bias) | Unclear risk | Sample size in each arms unbalanced (30 participants in the intervention group and 16 in the control). Both intention‐to‐treat and per‐protocol analysis should be done when evaluating the equivalence of an intervention compared to standard practices. Nothing about the number of patients who completed the study. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. No SD data provided for HbA1c levels. Results match methods. |
| Risk of contamination (other bias) | Low risk | Only the intervention arm used telecare and received call from a specialised physician. Both groups received frequent advice from a health professional about insulin titration either by phone (intervention group) or in‐person (control group). |
| Other bias | Unclear risk | The paper lacks details. |
Bieszk 2016.
| Study characteristics | ||
| Methods |
Act on threes paradigm for treatment intensification of type 2 diabetes in managed care: results of a randomized controlled study with an educational intervention targeting improved glycaemic control RCT (NA clusters and NA providers), conducted in 1) Patients identified through the analysis of administrative claims data (1 May 2011 to 30 April 30) from the Humana database. High‐risk Medicare Advantage with prescription drug coverage (MAPD) members from US (Northeast, Midwest, South and West). Humana is a for‐profit health insurance company with more than 13 million US‐based customers. 2) For the educational intervention, patients and physicians were simultaneously mailed general and targeted information (educational brochures and cover letters). The materials were developed in association with Humana’s in‐house clinical task force and communications team. In United States of America. 2 arms: 1) Control (standard care alone) (control arm) and 2) Intervention (Act on Threes educational intervention and standard care) (intervention arm) |
|
| Participants | Control arm N: 1688 Intervention arm N: 4555, NA, NA Diabetes type: 2 Mean age: 70.41 ± 13.59 % Male: 56.46 Longest follow‐up: 15 months |
|
| Interventions |
Control arm: (standard care alone) Intervention arm: (Act on Threes educational intervention and standard care) 1) Clinician education 2) Facilitated relay of clinical information 3) Patient education |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by Sanofi US | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients were randomized 3:1. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Patient demographics and baseline characteristics were similar for the 2 groups; P values above 0.05; data look balanced. |
| Patient's baseline outcomes (selection bias) | High risk | Patients in the intervention group had a significantly higher mean A1c level at baseline compared with the control group (8.66% vs 8.53%, respectively; P = 0.043). |
| Incomplete outcome data (attrition bias) | High risk | A1c levels were examined for a subgroup of patients. They have post‐intervention data for HbA1c outcome for 539/1688 (68.1% lost) in the control arm and 1503/4555 (67.0%) in the intervention arm. High numbers. They excluded 1977/8220 (24.1%) patients after randomisation. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively measured (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Control patients were only enrolled if the treating physician was not involved in the care of any patients in the intervention group. Communications between control and intervention physicians working in the same clinic might have happened. |
| Other bias | Unclear risk | No external testing or validation of the educational materials was conducted to determine their utility for patients and physicians alike. In this study, the less aggressive treatment goals may have affected patient and physician responses to educational intervention. Both groups improved for proportion of patients with at least 2 A1c tests annually (Figure 2). It is not known what other concomitant interventions the patients received that were not related to this study. |
Bieszk 2017.
| Study characteristics | ||
| Methods |
Personalized care and the role of insulin as a vehicle to optimizing treatments in diabetes care Clustered RCT (NR clusters and 3812 providers), conducted in 1) US‐based HealthCore Integrated Research Database (HIRD). This study was conducted in the United States. Very little information on setting provided likely because this was a mail‐out study and pre‐post information was retrieved via a claims‐based database. 2) Mail‐outs delivered to patients and physicians by study team. In United States of America. 4 arms: 1) Control (no outreach) (control arm) and 2) Intervention 1 (cross‐sectional) (intervention arm). 3) Intervention 2 (longitudinal) (other arm). 4) Intervention 3 (enhanced) (other arm) |
|
| Participants | Control arm N: 658 Intervention arm N: 749, 669, 716 Diabetes type: 2 Mean age: 56.24 ± 12.4 % Male: 63.57 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (no outreach) Intervention arm: (cross‐sectional) 1) Clinician education 2) Patient education Intervention arm: (longitudinal) 1) Clinician education 2) Patient education |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by Sanofi US | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Demographic and clinical baseline characteristics did not differ in a statistically significant way between the 4 cohorts (each of the 3 interventions cohorts compared with the control cohort), whether examined in the sample with the 6‐month follow‐up (data not shown) or with the 12‐month follow‐up. |
| Patient's baseline outcomes (selection bias) | Low risk | Demographic and clinical baseline characteristics did not differ in a statistically significant way between the 4 cohorts (each of the 3 interventions cohorts compared with the control cohort), whether examined in the sample with the 6‐month follow‐up (data not shown) or with the 12‐month follow‐up. |
| Incomplete outcome data (attrition bias) | High risk | A1c results for a pre/post comparison were available for 21% to 26% of patients and may not accurately reflect outcomes for the entire study sample. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | High risk | No protocol, no follow‐up on OAD, only insulin initiation. |
| Risk of contamination (other bias) | Unclear risk | There is a possibility that the study intervention was confounded by other educational materials sent out to physicians around the time of the intervention. |
| Other bias | High risk | Some physicians also provided additional unsolicited and unstructured feedback during the session. This feedback indicated that they believed patients are resistant to starting insulin therapy because of concerns over injections and a feeling that their diabetes must be severe to require insulin therapy. |
Billiard 1991.
| Study characteristics | ||
| Methods |
Telematic transmission of computerized blood glucose profiles for IDDM patients Cross‐over RCT, conducted in France Two arms: 1) Group B ‐ booklet (control arm) and 2) Group A ‐ Telematic (intervention arm) |
|
| Participants | Control arm N: 11 Intervention arm N: 11 Diabetes type: type I Mean age: 32.0 ± 14.0 % Male: 36.4 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 6.8 (1.0), post 6.8 (0.9) Intervention arm: pre 6.7 (1.4), post 6.0 (1.0) |
|
| Funding source | This study was supported by grunts from the Université d'Angers (France) and from Ames‐Bayer‐France | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Both groups bring self‐monitoring of blood glucose values to their physician. |
| Other bias | Unclear risk | Information not available. |
Blackberry 2013.
| Study characteristics | ||
| Methods |
Effectiveness of general practice based, practice nurse led telephone coaching on glycaemic control of type 2 diabetes: the Patient Engagement and Coaching for Health (PEACH) pragmatic cluster randomised controlled trial Cluster RCT (59 clusters), conducted in general practices in Victoria, Australia Two arms: 1) Control group (control arm) and 2) Intervention group (intervention arm) |
|
| Participants | Control arm N: 237 Intervention arm N: 236 Diabetes type: type 2 Mean age: 62.8 ± 10.5 % Male: 57.0 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.1 (1.3), post 7.9 (1.4) Intervention arm: pre 8.0 (1.2), post 7.9 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 138.0 (18.0), post 136.0 (16.0) Intervention arm: pre 139.0 (18.0), post 133.0 (14.0) 3) DBP, mean mmHg (SD) Control arm: pre 79.0 (11.0), post 77.0 (11.0) Intervention arm: pre 79.0 (10.0), post 76.0 (9.0) 4) LDL, mean mg/dL (SD) Control arm: pre 92.8 (32.9), post 87.4 (32.5) Intervention arm: pre 92.8 (34.4), post 85.9 (33.6) 5) Smoking cessation, N smokers (%) Control arm: pre 27 (11), post 23 (12) Intervention arm: pre 30 (13), post 25 (13) |
|
| Funding source | This study was supported by the Australian National Health and Medical Research Council (ID 359374 and 566586). The funder was not involved in the study design, data collection, analysis and interpretation. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Low risk | Not reported, but since cluster then low risk. |
| Provider's baseline characteristics (selection bias) | Low risk | In text but not in tables. |
| Patient's baseline characteristics (selection bias) | Low risk | In text but not in tables. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | ~5% lost to follow‐up in both arms; reasons provided are similar. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: HbA1c; laboratories used HbA1c assay methods aligned with standards. Quality assurance HbA1c assays. Secondary outcomes: objective methods not described, but assessors were blinded. |
| Selective reporting (reporting bias) | Low risk | Outcomes matches protocol. |
| Risk of contamination (other bias) | Low risk | Cluster design minimised risk of contamination in control group. |
| Other bias | Low risk | Information not available. |
Boaz 2009.
| Study characteristics | ||
| Methods |
An automated telemedicine system improves patient‐reported well‐being Patient RCT, conducted in an ambulatory diabetes clinic in Holon, Israel Two arms: 1) Control (control arm) and 2) Telemedicine (intervention arm) |
|
| Participants | Control arm N: 18 Intervention arm N: 17 Diabetes type: type 1 and type 2 Mean age: 63.0 ± 10.0 % Male: 37.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Facilitated relay of clinical information |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.3 (1.6), post 9.6 (1.9) Intervention arm: pre 8.4 (1.4), post 8.5 (1.7) 2) LDL, mean mg/dL (SD) Control arm: pre 100.0 (28.0), post 97.0 (29.0) Intervention arm: pre 121.0 (48.0), post 88.0 (22.0) 3a) Harms (hyperglycaemic events), N (%) Control arm: pre 14 (78), post 15 (83) Intervention arm: pre 14 (82), post 6 (35) 3b) Harms (hypoglycaemic events), N (%) Control arm: pre 7 (39), post 15 (83) Intervention arm: pre 5 (29), post 3 (18) |
|
| Funding source | Equipment was supplied by Medic4all, Israel. However, no financial association exists or was provided by the company for this project or any other project with which the researchers are associated. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Bogner 2010.
| Study characteristics | ||
| Methods |
Integrating type 2 diabetes mellitus and depression treatment among African Americans Patient RCT, conducted in a community‐based primary care practice in West Philadelphia with 12 family physicians, USA Two arms: 1) Usual care (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 29 Intervention arm N: 29 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.3 (2.0), post 7.9 (2.6) Intervention arm: pre 7.3 (2.3), post 6.7 (2.3) |
|
| Funding source | This work was supported by an American Diabetes Association Clinical Research Award and an Institute on Aging, University of Pennsylvania, Pilot Research Grant | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Bogner 2012.
| Study characteristics | ||
| Methods |
Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial Patient RCT, conducted with patients recruited from 3 primary care practices in Philadelphia, Pennsylvania. Study was conducted in a clinical setting. In USA. Two arms: 1) Usual care (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 88 Intervention arm N: 94 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.0 (1.9), post 7.5 (NR) Intervention arm: pre 7.2 (1.8), post 6.5 (NR) |
|
| Funding source | This work was supported by American Diabetes Association Clinical Research Award 1‐09‐CR‐07. Dr Bogner was supported by NIMH grant MH082799 and MH047447. Dr Morales was supported by a NIMH‐mentored Career Development Award (MH073903). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…were randomized within each practice by flip of a coin…" |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "HbA1c (P = 0.51)" |
| Incomplete outcome data (attrition bias) | High risk | Only 2 lost to follow‐up in intervention group ~2% |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c methods described. Assessors were blinded, physicians blinded to those randomised to usual care. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything proposed was completed. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Bohingamu 2019.
| Study characteristics | ||
| Methods |
Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Barwon Health, University Hospital Geelong, Geelong, Australia. 2) The Barwon Health personalised telehealth monitoring programme was staffed during office hours 7 days a week by registered nurses with skills in the management of diabetes, respiratory conditions and community nursing and Lead medical consultants (respiratory and diabetes) for each patient cohort were chosen from senior medical staff of University Hospital Geelong in Australia 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (personalised telehealth monitoring programme) (intervention arm) |
|
| Participants | Control arm N: 69 Intervention arm N: 67, NA, NA Diabetes type: 4 Mean age: 70.42 ± 9.6 % Male: 53.54 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (personalised telehealth monitoring program) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Victorian Government provided funding for the pilot project, with an in‐kind contribution by Barwon Health | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants who met inclusion criteria and agreed to participate were randomised to the intervention or control groups using sequential envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Participants who met inclusion criteria and agreed to participate were randomised to the intervention or control groups using sequential envelopes; no mention of whether envelopes were opaque. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 2. P values > 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. P values provided and below 0.05 for HbA1c. |
| Incomplete outcome data (attrition bias) | Unclear risk | 12/85 (14%) lost in control, 10/86 (12%) lost in intervention group. Balanced and reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that control patients received the remote telehealth intervention. |
| Other bias | High risk | Inadvertently, additional patients were randomised to either intervention or control groups who did not meet the original inclusion criteria (PRaDA score); however, if results were affected, they are likely to be a conservative estimate, as the mean PRaDA score at baseline for the control group was lower than the intervention group, suggesting a lower probability of hospital readmission in the control group. |
Bollyky 2018.
| Study characteristics | ||
| Methods |
Remote lifestyle coaching plus a connected glucose meter with certified diabetes educator support improves glucose and weight loss for people with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Delivered through a 2‐way messaging service (Livongo) by Restore Health. The Livongo program was provided to participants by their employer or health plan at no cost to the participant, California, United States of America; 2) certified diabetes educators. In United States of America. 4 arms: 1) Control (Livongo only) (control arm) and 2) Intervention (Livongo + scale) (intervention arm). 3) Intervention (Livongo + Scale + Light Reform Health Coaching (other arm). 4) Intervention (Livongo + Scale + Intense Reform Health Coaching (other arm) |
|
| Participants | Control arm N: 75 Intervention arm N: 115, 73, 67 Diabetes type: 2 Mean age: 50.3 ± 4.6 % Male: 44.2 Longest follow‐up: 2.76 months |
|
| Interventions |
Control arm: (Livongo only) 1) Case management 2) Promotion of self‐management 3) Patient reminders Intervention arm: (Livongo + scale) 1) Case management 2) Promotion of self‐management 3) Patient reminders Intervention arm: (Livongo + Scale + Light Reform Health Coaching) 1) Case management 2) Promotion of self‐management 3) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | NR ‐ Presumably funded by Livongo Health | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Patient's baseline characteristics (selection bias) | Unclear risk | Baseline characteristics among groups were similar except that the participants randomised to the lightweight coaching intervention were significantly less likely to be on insulin than control participants (P = 0 007). Baseline weight differed as well (P = 0.02). |
| Patient's baseline outcomes (selection bias) | Low risk | Baseline outcomes seem to be balanced. |
| Incomplete outcome data (attrition bias) | High risk | Randomised 454, but only 330 participants reached final analysis. The distribution between the groups of these 124 (37.5%) dropouts is not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | It appears that the Livongo system supports blood glucose reading and is attached to the weight scale. Therefore, the patient was not involved in data input and it was objective. |
| Selective reporting (reporting bias) | Unclear risk | No register protocol; however, outcomes reported in results match those outlined in methods. |
| Risk of contamination (other bias) | Unclear risk | Both lifestyle modification programme groups appeared to be orchestrated by the same Restore Health coaches. The population that had the greatest improvement in glucose control may have received additional outside interventions by their primary care and other health care providers that could have contributed to this finding. |
| Other bias | Low risk | No evidence of other risk of bias. |
Bond 2007.
| Study characteristics | ||
| Methods |
The effects of a web‐based intervention on the physical outcomes associated with diabetes among adults age 60 and older: a randomized trial Patient RCT, conducted in the University of Washington Diabetes Centre and local diabetes fairs in greater Seattle area, USA Two arms: 1) Control (control arm) and 2) Treatment (intervention arm) |
|
| Participants | Control arm N: 31 Intervention arm N: 31 Diabetes type: type 1 and type 2 Mean age: 67.2 ± 6.0 % Male: 55.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.1 (0.9), post 7.1 (1.0) Intervention arm: pre 7.0 (1.1), post 6.4 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 130.0 (13.5), post 131.0 (10.2) Intervention arm: pre 134.0 (15.0), post 128.0 (13.2) 3) DBP, mean mmHg (SD) Control arm: pre 73.0 (7.1), post 73.0 (7.2) Intervention arm: pre 76.0 (7.7), post 70.0 (7.0) |
|
| Funding source | This work for this study was supported by grant K01 NR08506‐03 from the National Institute of Nursing Research | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Bonner 2018.
| Study characteristics | ||
| Methods |
Care management to promote treatment adherence in patients with cognitive impairment and vascular risk factors: a demonstration project RCT (NA clusters and NA providers), conducted in 1) Primary care and Memory Disorders Clinic, Veteran's Affairs, Seattle, Washington, USA. 2) Registered Nurse in United States of America 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (care management) (intervention arm) |
|
| Participants | Control arm N: 17 Intervention arm N: 16, NA, NA Diabetes type: 2 Mean age: 65.2 ± NR % Male: 97 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (care management) 1) Case management 2) Patient education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This work was supported by the U.S. Department of Veterans Affairs, VISN 20, through a Memorandum of Understanding with the VISN 20 Geriatric Research, Education and Clinical Center (GRECC) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to intervention or usual care group using a pseudo‐randomisation procedure (selecting among 3 sealed envelopes, 1 of which contained a usual care assignment and 2 of which contained an intervention assignment). |
| Allocation concealment (selection bias) | Low risk | Participants were assigned to intervention or usual care group using a pseudo‐randomisation procedure (selecting among 3 sealed envelopes, 1 of which contained a usual care assignment and 2 of which contained an intervention assignment). |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values provided and above 0.05 except for 1, which is not applicable to us. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1, P values provided and above 0.05 except for 1, which is not applicable to us. |
| Incomplete outcome data (attrition bias) | Low risk | One participant (1/17 or 6%) in the usual care group was excluded after randomisation due to determination of no cognitive impairment. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Unlikely that control patients received the care management intervention. The intervention primarily used telephone contact in order to minimise travel for participants, although some participants chose to have in‐person visits with the care manager. |
| Other bias | Low risk | No evidence of other risk of bias. |
Bonney 2017.
| Study characteristics | ||
| Methods |
A feasibility study of team‐based primary care for chronic disease management training in rural Australia RCT (clusters and providers), conducted in 1) Rural training practices in Wollongong, Australia. 2) General practitioners, GP registrars and nurses in Australia 2 arms: 1) Control (normal care) (control arm) and 2) Intervention (shared continuity) (intervention arm) |
|
| Participants | Control arm N: 16 Intervention arm N: 14, NA, NA Diabetes type: 2 Mean age: 71.36 ± 12 % Male: 53.33 Longest follow‐up: 8 months |
|
| Interventions |
Control arm: (normal care) Intervention arm: (shared continuity) 1) Case management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | Coast City Country General Practice Training funded the project | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised within each practice to an intervention or control arm of normal care over an 8‐month period. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values provided and above 0.05. There were no significant between‐group differences in adjusted baseline parameters. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1, P values provided and above 0.05. There were no significant between‐group differences in adjusted baseline parameters. |
| Incomplete outcome data (attrition bias) | High risk | 7/30 patients lost to follow‐up; no distinction between groups and reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Likely objective measurements but not explicitly reported. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Outcomes to be measured were not reported in methods, only in results. |
| Risk of contamination (other bias) | Unclear risk | Not reported how patients were followed and if intervention team could have seen control patients. |
| Other bias | Unclear risk | This is a short report, so not much information to go off. Insufficient information to assess if other risks of bias. |
Bosi 2013.
| Study characteristics | ||
| Methods |
Intensive structured self‐monitoring of blood glucose and glycemic control in noninsulin‐treated type 2 diabetes: the PRISMA randomized trial RCT (NA clusters and NA providers), conducted in 1) The trial was conducted at 39 diabetes clinics in Italy. 2) Intervention delivered by the PRISMA Study Group Investigators. In Italy. 2 arms: 1) Control (active control by SMBG) (control arm) and 2) Intervention (SMBG and intensive structured monitoring) (intervention arm) |
|
| Participants | Control arm N: 523 Intervention arm N: 501, NA, NA Diabetes type: 2 Mean age: 60.3 ± 8.3 % Male: 60.24 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (active control by SMBG) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (SMBG and intensive structured monitoring) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | This study was supported by Roche Diagnostics Diabetes Care. The sponsor contributed to the design of the study and provided funding for the conduct of the study, collection, management and analysis of the data. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation ratio was 1:1. A computerised random number generator was used to select random permuted blocks of 4. Details on randomisation restriction and block size were not disclosed to investigators. Randomisation was stratified by the diabetes treatment at enrollment (diet only or diet plus diabetes medications). |
| Allocation concealment (selection bias) | Low risk | Allocation information was sealed in sequentially numbered, opaque envelopes prepared by the clinical research organisation managing the trial. |
| Patient's baseline characteristics (selection bias) | Low risk | Proportions seem similar between controls and interventions. |
| Patient's baseline outcomes (selection bias) | Low risk | Proportions seem similar between controls and interventions. |
| Incomplete outcome data (attrition bias) | High risk | High proportions of excluded patients in both the control (38.6%) and the intervention (53.7%) groups. Numbers not balanced between groups. Reasons reported and the most frequent is related to intervention (non‐compliance with SMBG regimen). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All objective outcomes (HbA1c, SBP, DBP and LDL) except one secondary outcome that is subjective (number of hypoglycaemic events). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on March 2008, study started on March 2008). They did not report some outcomes that are not of interest to us. |
| Risk of contamination (other bias) | High risk | Both groups were followed by their usual providers and also by a PRISMA investigators (increased attention was given to all patients). A further limitation is the use of structured SMBG in both groups. Although SMBG data from AC patients were not made available to clinicians for use in evaluating glycaemic status or in making medication adjustments, the availability of these data to AC patients may have prompted changes in lifestyle behaviours or treatment adherence, potentially leading to improved glycaemic control independent of clinician‐based recommendations, especially in those patients who tested more frequently than the protocol allowed. Also, comprehensive education was provided in both study groups. |
| Other bias | Low risk | None identified. |
Bove 2013.
| Study characteristics | ||
| Methods |
Managing hypertension in urban underserved subjects using telemedicine‐‐a clinical trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from Temple University Medical Center in Philadelphia, Pennsylvania, and Christiana Health Care Center in Wilmington, Delaware, through advertising and communication with churches and community centres. 2) Internet‐ and telephone‐based telemedicine intervention. Physicians received a monthly report via fax on the patient's BP status. A study nurse called patients to remind them to upload their data. In United States of America. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (telemedicine system) (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 35, NA, NA Diabetes type: 4 Mean age: 59.59 ± NR % Male: 34.85 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (telemedicine system) 1) Case management 2) Electronic patient registry 3) Clinician education 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Systolic blood pressure Diastolic blood pressure Hypertension control |
|
| Funding source | The study was funded by the Agency for Healthcare Research and Quality | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive patients were assigned a random number from a random number list. Patients assigned odd numbers were placed in the control group, and patients assigned even numbers were placed in the telemedicine group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table I: no P values. Table II: All P values above 0.05, but only for participants who completed the 6‐month study. No data only for diabetic patients in Tables I and II. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table II. All P values above 0.05, but only for participants who completed the 6‐month study, and no data only for diabetic patients. |
| Incomplete outcome data (attrition bias) | High risk | For diabetic patients only, they have blood pressure data for 34/41 patients in the control group (17.1% lost) and 31/35 for the intervention group (11.4%) at 6 months follow‐up. High and unbalanced numbers. Reasons why some patients did not return not reported (figure). Overall, 206 patients out of the 241 randomised completed the 6‐month study (14.5% lost). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured by patients (SBP, DBP and % at goal BP). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Primary outcomes match between protocol and results. However, many secondary outcomes from the protocol are not reported only for diabetic patients in the paper. Some of the outcomes in the study were not mentioned in the protocol. They had not mentioned in the protocol that they would do a subgroup analysis in diabetic patients. |
| Risk of contamination (other bias) | High risk | Their primary care physicians provided hypertension management for both control and telemedicine participants. Physicians might have changed their approach with their control patients after receiving monthly report via fax for their intervention patients. Control participants were provided with the data from their initial assessment and instructed to contact their primary care provider for further care. The observation that BP was also reduced in the control, usual care participants suggests that any means of engaging individuals in their health care to improve CVD risk will have benefit. |
| Other bias | Low risk | No evidence of other bias. |
Brown 2011.
| Study characteristics | ||
| Methods |
Integrating education, group support, and case management for diabetic Hispanics Patient RCT, conducted with patients from ongoing genetic and epidemiological studies. In USA. Two arms: 1) Control (control arm) and 2) Experimental (intervention arm) |
|
| Participants | Control arm N: 35 Intervention arm N: 48 Diabetes type: type 2 Mean age: 49.3 ± 8.4 % Male: 31.3 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Team changes 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.6 (3.0), post 8.6 (2.0) Intervention arm: pre 9.2 (2.7), post 10.4 (2.8) |
|
| Funding source | The study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, grant no. 5R34DK073286‐01 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | In text but not in table. |
| Patient's baseline outcomes (selection bias) | High risk | HbA1c statistically different. |
| Incomplete outcome data (attrition bias) | Low risk | There do not seem to be any losses. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | No mention of objective laboratory methods; blinding of outcome assessors not described. |
| Selective reporting (reporting bias) | High risk | No protocol. |
| Risk of contamination (other bias) | Low risk | Individuals were grouped according to the area of the county in which they lived to foster neighbourhood support between sessions and reduce the likelihood of contamination. |
| Other bias | High risk | Severe weather may have prohibited true effects of intervention. |
Browne 2016.
| Study characteristics | ||
| Methods |
Building the evidence for integrated care for type 2 diabetes: a pilot study RCT (NA clusters and NA providers), conducted in 1) The study was undertaken at 2 IDEAS sites (Whitehorse Community Health Service (WH) and Knox Community Health Service (KX)) and 2 hospital outpatient clinics in the same geographical region (Box Hill Hospital (BH) and Maroondah Hospital (MA)). 2) Integrated care models. The multidisciplinary team includes an endocrinologist and registrar (from EH or Eastern Health Endocrinology Department) working directly with a diabetes nurse educator, podiatrist and community health nurse (from CH or Carrington Health, previously Whitehorse Community Health Service). In Australia. 2 arms: 1) Control (hospital outpatient clinics) (control arm) and 2) Intervention (IDEAS: Integrated Diabetes Education and Assessment Service) (intervention arm) |
|
| Participants | Control arm N: 29 Intervention arm N: 27, NA, NA Diabetes type: 2 Mean age: 56 ± 13.98 % Male: 68 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (hospital outpatient clinics) Intervention arm: (IDEAS: Integrated Diabetes Education and Assessment Service) 1) Team change 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Funding for this study was provided by the State Department of Health, Victoria, through their Building the Evidence initiative and from the Eastern Health Foundation through a research grant to Professor Gilfillan. The funding model utilises Medicare Benefits Schedule‐funded specialist medical services and core Community Health‐funded allied health professionals, with support for start‐up provided by the state‐funded EH Hospital Admission Risk Program (HARP). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Following verbal consent, a computerised random number generator was used to assign the participant to receive their health care via the intervention condition (IDEAS) or control condition (hospital clinics). After being assigned to a condition, participants were then assigned to the site closest to where they lived. Sites were not randomised and the intervention sites (community health service) and control sites (hospital outpatient clinics) did not have the same setting. Quote: "(The intervention was) undertaken at two IDEAS sites (Whitehorse Community Health Service (WH) and Knox Community Health Service (KX)) and (the control condition was done at) two hospital outpatient clinics in the same geographical region (Box Hill Hospital (BH) and Maroondah Hospital (MA))." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Table 2. Primary treatment has a P value of ≤ 0.01. Quote: "Randomisation was largely successful, with the exception of significant differences on treatment type. It could be argued that participants in the hospital group had more advanced diabetes than those in the IDEAS." |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. HbA1c has a P value of 0.04. Quote: "Randomisation was largely successful, with the exception of significant differences on HbA1c." |
| Incomplete outcome data (attrition bias) | High risk | 11 participants (20%) were lost to follow‐up between Time 1 and Time 2 (IDEAS: n = 5/27 = 19%; hospital: n = 6/29 = 21%). High but balanced numbers. Reasons for loss not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. However, they have pooled data from the RCT and cross‐sectional studies for this outcome: perceived quality of diabetes care (secondary outcome for them and for us), while they did not do this for other outcomes. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. However, after being assigned to a condition, participants were then assigned to the site closest to where they lived. Each site was either a control (hospital outpatient clinics) or intervention site (IDEAS: Integrated Diabetes Education and Assessment Service). Unlikely that intervention was provided at control sites. |
| Other bias | Low risk | No evidence of other bias. |
Browning 2016.
| Study characteristics | ||
| Methods |
Management of type 2 diabetes in China: the Happy Life Club, a pragmatic cluster randomised controlled trial using health coaches Clustered RCT (41 clusters and NR providers), conducted in 1) Study held in a primary healthcare setting in Beijing, China. The context of the intervention site, namely Community Health Stations (CHSs) within a district of Beijing where preventive care, health management, primary medical care, rehabilitation, health education and family planning are offered. 2) Management intervention delivered by health coaches. Health coaching was performed by experienced clinicians (community doctors, nurses and psychologists) from each CHS. In China. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (coach‐led motivational interviewing) (intervention arm) |
|
| Participants | Control arm N: 368 Intervention arm N: 385, NA, NA Diabetes type: 2 Mean age: 63.8 ± 6.24 % Male: 47.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (coach‐led motivational interviewing) 1) Case management 2) Promotion of self‐management 3) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This trial was funded by the Fengtai Health Bureau, Beijing, China, and in‐kind support was provided by Monash University, Australia, and Peking University, China | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | CHSs were randomised into the control or intervention groups, using block randomisation. Participants were sampled by computerised random allocation software that stratified by gender in order to achieve balance. |
| Allocation concealment (selection bias) | Low risk | Cluster‐randomised. In order to minimise selection bias, this process was carried out centrally by an independent person and all CHSs were coded to ensure the randomisation was a blinded process. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Of the 41 randomised Community Health Stations (CHSs, 21 intervention and 20 control), 21 intervention CHSs (372 participants) and 18 control CHSs (296 participants) started participation. No CHSs characteristics are reported at baseline. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 3. Means are similar. The intervention and control groups were similar for all variables at baseline. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Means appear similar between controls and interventions at baseline but no P value are reported and nothing is reported in the text about differences at baseline. "The intervention and control groups were similar for all variables at baseline, and even if statistical differences between groups were observed then the analysis method could have accounted for this by adjusting for baseline scores." |
| Incomplete outcome data (attrition bias) | High risk | Only 295 out of 385 patients were analysed for the primary outcome at 12 months in the intervention group (23.4% lost) and 282 out of 368 in the control group (23.4%). Reasons not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All objective outcomes (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol (protocol first posted in November 2010, study started in June 2011). In the protocol: Outcome measures will be assessed at 6, 12 and 18 months. In the paper: Outcomes were assessed at baseline, 6 and 12 months. They do not report data for homocysteine. |
| Risk of contamination (other bias) | Unclear risk | Cluster‐RCT however, in order to complete the data collection requirements for the study, increased monitoring that deviated from usual care recommendations was necessary. Although glycaemic control did not differentially improve, HbA1c in both groups changed significantly and for the better, as did triglycerides, LDL cholesterol and HDL cholesterol. It can be assumed that this trial served as a catalyst for the revitalisation of primary care delivery to individuals with T2DM. Also, the study received a considerable amount of media attention throughout the intervention phase, which may have resulted in participants and CHS staff altering their usual behaviour. |
| Other bias | Low risk | None. |
Bujnowska‐Fedak 2011.
| Study characteristics | ||
| Methods |
The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland Patient RCT, conducted with patients from general practices in the Lower Silesia region of Poland ‐ home‐based intervention. In Poland. Two arms: 1) Conventional group (control arm) and 2) Telehome group (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician reminders 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.6 (1.7), post 7.4 (1.5) Intervention arm: pre 7.6 (1.5), post 7.4 (1.3) 2) SBP, mean mmHg (SD) Control arm: pre 136.1 (24.0), post 129.1 (24.5) Intervention arm: pre 132.2 (25.3), post 127.2 (23.1) 3) DBP, mean mmHg (SD) Control arm: pre 82.6 (13.1), post 82.1 (11.1) Intervention arm: pre 84.4 (15.7), post 81.4 (12.7) 4a) Harms (hyperglycaemic events), N (%) Control arm: pre NR (NR), post 56 (117) Intervention arm: pre NR (NR), post 38 (81) 4b) Harms (hypoglycaemic events), N (%) Control arm: pre NR (NR), post 19 (40) Intervention arm: pre NR (NR), post 8 (17) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: HbA1c (P = 0.688); SBP/DBP (P = 0.350). |
| Incomplete outcome data (attrition bias) | Low risk | < 10% lost to follow‐up per arm and reasons were similar. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | No blinding described. Method of HbA1c and SBP not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; outcomes match those in methods. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No risk of other bias |
Buysse 2019.
| Study characteristics | ||
| Methods |
Sustainable improvement of HbA(1c) and satisfaction with diabetes care after adding telemedicine in patients on adaptable insulin regimens: Results of the TeleDiabetes randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) recruited during conventional consultation in 2 hospitals (Ghent University Hospital and AZ Nikolaas) in Flanders, Belgium. Intervention delivered via TeleDiabetes software, remotely. 2) Diabetes educator and endocrinologist in Belgium 2 arms: 1) Control (wait‐list) (control arm) and 2) Intervention (TeleDiabetes) (intervention arm) |
|
| Participants | Control arm N: 72 Intervention arm N: 81, NA, NA Diabetes type: 3 Mean age: 37.47 ± 8.44 % Male: 50.18 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (wait‐list) Intervention arm: (TeleDiabetes) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | The study was partially financially supported by Sanofi and Roche Diabetes Care Deutschland GmbH. Patients and participating hospitals could freely use the eConnecta platform. Patients could freely use the blood glucose meters BGstar and iBGstar from Sanofi and the blood glucose meters Accu‐Chek from Roche. Neither staff from Sanofi nor Roche are involved in the study design, data acquisition and analysis, and writing or have any other influence on this final report. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were then ‐ via a computerised randomization table ‐ automatically allocated to the control group (CG) (n = 72) or study group (SG) (n = 81). |
| Allocation concealment (selection bias) | Low risk | Patients were then ‐ via a computerised randomization table ‐ automatically allocated to the control group (CG) (n = 72) or study group (SG) (n = 81). |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values all greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values all greater than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. 12 dropouts in the control group (17%), 7 dropouts in the intervention group (9%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c and harms. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol; no mention of outcome measurements for HbA1c or harms. |
| Risk of contamination (other bias) | High risk | Endocrinologist treated both groups; controls were wait‐listed for 3 months but the trial ran for 2 years so there is a possibility of contamination. |
| Other bias | Low risk | None identified. |
Cagliero 1999.
| Study characteristics | ||
| Methods |
Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin‐treated type 2 diabetic patients. Patient RCT, conducted in diabetes centre at major teaching hospital in Boston, Mass, USA Two arms: 1) Control (control arm) and 2) Immediate assay (intervention arm) |
|
| Participants | Control arm N: 101 Intervention arm N: 100 Diabetes type: type 1 and type 2 Mean age: 49.0 ± 16.0 % Male: 48.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Facilitated relay of clinical information |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (1.6), post 8.3 (NR) Intervention arm: pre 8.7 (1.8), post 8.3 (NR) |
|
| Funding source | This work was supported by a grant from Bayer (Elkhart, Indiana) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Cani 2015.
| Study characteristics | ||
| Methods |
Improvement in medication adherence and self‐management of diabetes with a clinical pharmacy program: a randomized controlled trial in patients with type 2 diabetes undergoing insulin therapy at a teaching hospital RCT (NA clusters and NA providers), conducted in 1) The study was performed in the diabetes outpatient clinic of Hospital das Clinicas da Faculdade de Medicina da Universidade de Sao Paulo, which is a tertiary facility hospital located in the city of Sao Paulo, Brazil. 2) Intervention delivered by pharmacists who gave recommendations to care providers when necessary. In Brazil. 2 arms: 1) Control (standard care) (control arm) and 2) Intervention (individualised pharmacotherapeutic care plan) (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 37, NA, NA Diabetes type: 2 Mean age: 61.74 ± 11.8 % Male: 38.6 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (individualised pharmacotherapeutic care plan) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | We acknowledge Coordenacao de Aperfeicoamento de Pessoal de Nıvel Superior (CAPES) for providing financial support | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information. The group allocations were assigned by simple randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. The group allocations were assigned by simple randomisation. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. The demographic characteristics of the patients are shown in Table 1; the 2 groups were homogeneous with respect to all variables. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. P values above 0.05 for HbA1c. |
| Incomplete outcome data (attrition bias) | Low risk | Figure 1. 8 lost out of 78 (10.3%): 12.2% in the control group (n = 5) and 8.1% in the intervention (n = 3). Reasons reported and balanced (missed appointments). 8 patients were excluded (5 in the control group and 3 in the intervention group) because they missed appointments. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. Our outcome of interest is correctly reported (HbA1c). |
| Risk of contamination (other bias) | Low risk | Clinical pharmacists did not meet with participants in control group. It is not excluded that the providers learned from pharmacists' recommendations about intervention patients and that they changed the usual care for control patients. |
| Other bias | Low risk | No evidence of other bias. |
Carlson 1991.
| Study characteristics | ||
| Methods |
Diabetes care organization, process, and patient outcomes: effects of a diabetes control program Clustered RCT (34 clusters and NR providers), conducted in 1) Diabetes care was evaluated at 34 primary health care centres (PHCC) in Stockholm, Sweden. 2) DETU (Diabetes Education and Training Unit) staff working with the PHCC included a physician (UR), a psychologist (AC), a dietician, a podiatrist and 2 registered nurses. Intervention delivered by DETU staff to the healthcare providers and then by healthcare providers to patients in Sweden 2 arms: 1) Control (nonparticipating centres) (control arm) and 2) Intervention (Diabetes Control Program: CME‐ODE) (intervention arm) |
|
| Participants | Control arm N: 361 Intervention arm N: 434, NA, NA Diabetes type: 4 Mean age: NR ± 8.95 % Male: NR Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (nonparticipating centres) Intervention arm: (Diabetes Control Program: CME‐ODE) 1) Case management 2) Clinician education 3) Patient education |
|
| Outcomes | Retinopathy screening Glycated haemoglobin |
|
| Funding source | This research was supported by grants 7542 and 06615 from the Swedish Medical Research Council, the Karolinska Institute, the Swedish Diabetes Federation, and Stockholm University | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. The centres had been randomly assigned to the 2 groups. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | High risk | No comparison made between control and intervention PHCC. Primary Health Care in Sweden is organised in outpatient clinics. Each primary health care centre (PHCC) is staffed by general practitioners (GP), office nurses (ON), district nurses (DN), nursing assistants, secretaries and, in some centres, laboratory technicians and physiotherapists. While the majority of PHCCs are staffed by 3 to 6 GPs there is considerable variation in size, with some smaller units staffed by 1 GP with 2 to 4 registered nurses (RN, ONs and DNs)/nursing assistants to large centres with 10 or more GPs and 30 or more RNs/nursing assistants. The number of PHCCs in the Stockholm area (1.2 million inhabitants) increased from 46 in 1978 to 116 in 1989. PHCCs serve the people living in a defined geographical area and are generally within easy access of their population. Podiatrists and dieticians are available on a part‐time consultant basis. |
| Patient's baseline characteristics (selection bias) | Low risk | Patients from the 2 groups of health centres (17 intervention and 17 control centres) were similar with regard to treatment: diet only (36%, 39%), oral medication (47%, 45%), insulin treatment (17%, 16%); male/female ratio: (49%/51%, 44%/56%); mean age (M ± SD): (64.3 ± 12.1, 63.9 ± 11.5); and mean duration of disease (M ± SD): (6.4 ± 7.0, 5.5 ± 5.9). Neither were there any differences between the intervention and control groups, respectively, with regard to formal education: high school or above (15%, 12%); or work status: old age pensioner (61%, 58%), working full or part time (26%, 29%) and prematurely retired (14%, 15%). Participating patients in the secondary sample did not differ from the total sample with respect to age, male/female ratio, duration of diabetes and treatment noted in the case records. |
| Patient's baseline outcomes (selection bias) | Low risk | Participating patients in the secondary sample (20% of patients) did not differ from the total sample with respect to latest blood glucose value noted in the case records. |
| Incomplete outcome data (attrition bias) | High risk | The number of lost is 27% in the intervention group and 31% in the control group. Quote: "Seventy‐three percent (n = 317) of subjects selected in the intervention group participated in the study, as did 69% (n = 249) of those from the control group." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c was objectively assessed but eye examination outcome was subjectively assessed (questionnaire filled by patients, secondary outcome). The questionnaire was designed to yield information on the following factors: the date of the most recent eye examination (during last year or earlier). Nothing about patient blinding. |
| Selective reporting (reporting bias) | High risk | No registered or published protocol. Method: Data on height, HbA1c concentration and results of urine testing for proteinuria were obtained from the patients’ case records. Results: They do not report data about height and HbA1c at baseline and urine testing for proteinuria at baseline and post‐intervention. Our conclusions are the result of many correlations, and the problem of mass‐significance should be mentioned. Thus, out of 70 comparisons, 3 to 4 significant findings could have been generated by chance (5% risk level). |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT. |
| Other bias | Low risk | No evidence of other bias. |
Carter 2009.
| Study characteristics | ||
| Methods |
Physician and pharmacist collaboration to improve blood pressure control Clustered RCT (6 clusters and 165 providers), conducted in 1) 6 community‐based family medicine residency programs in Davenport, Des Moines (2 offices), Mason City, Sioux City and Waterloo, Iowa. All study visits with intervention pharmacists occurred in the medical office. 2) Intervention delivered to patients by physician and pharmacist. Clinician education provided by Barry L. Carter. Physicians and pharmacists underwent team‐building exercises conducted by Barry L. Carter and William R. Doucette. In United States of America. 2 arms: 1) Control (usual care: control office) (control arm) and 2) Intervention (physician and pharmacist collaborative model) (intervention arm) |
|
| Participants | Control arm N: 80 Intervention arm N: 38, NA, NA Diabetes type: 4 Mean age: 58.29 ± 8.5 % Male: 41.05 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care: control office) 1) Patient education Intervention arm: (physician and pharmacist collaborative model) 1) Case management 2) Team change 3) Clinician education 4) Patient education 5) Patient reminders 6) Continuous quality improvement |
|
| Outcomes | Hypertension control | |
| Funding source | This study was supported by grant R01 HL070740 from the National Heart, Lung, and Blood Institute. Drs Carter, Doucette and Chrischilles are supported by co‐operative agreement 5U18 HS016094 from the Agency for Healthcare Research and Quality Centers for Education and Research on Therapeutics. Dr Carter and Ms Franciscus are supported by grant HFP 04‐149 from the Center for Research in Implementation in Innovative Strategies in Practice, Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 community‐based family medicine residency programmes in Davenport, Des Moines (2 offices), Mason City, Sioux City and Waterloo, Iowa, randomised to a control group (n = 3) or to an intervention group (n = 3) using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Low risk | Table 1 summarises the demographics and staffing at the participating clinics. The general operations of all 6 sites were similar, and the office served as the model office for a distinct family medicine residency. All 6 programmes met the institutional requirements of the Accreditation Committee for Graduate Medical Education and the programme requirements for family practice set out by the Accreditation Committee for Graduate Medical Education and its Residency Review Committee. All faculty physicians were board certified in family practice. |
| Patient's baseline characteristics (selection bias) | High risk | Table 2. Characteristics not provided for diabetic subsample only. At baseline, patients in the control group were significantly less likely to be married (P = 0.001) and were more likely to have diabetes mellitus (P = 0.001, more than double the number of diabetics in the control group versus the intervention, 80 vs 38 respectively), self‐pay for their care (P = 0.001), have more co‐existing conditions (P = 0.001), have an annual household income below USD $25,000 (P = 0.001), take more antihypertensive medications (P = 0.001) and have a history of myocardial infarction (P = 0.002) or angina (P = 0.003) (Table 2). |
| Patient's baseline outcomes (selection bias) | High risk | Table 3. Baseline outcomes not reported only for diabetic patients. Before the study, there was a wide range of BP control rates (28.6% to 70.0%), higher at the control sites (55.7%) than at the intervention sites (41.9%). |
| Incomplete outcome data (attrition bias) | High risk | They lost 36/210 (17%) patients in the control clinics and 34/192 (18%) patients in the intervention clinics. High but balanced numbers of lost in each group. Quote: "Also, this study had a higher dropout rate than a previous efficacy study". Not specific to diabetic population. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | BP objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. They do not report physician knowledge and physician‐pharmacist relationship in the paper. They do not report data for the passive observation group. |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT. Randomisation at the clinic level. |
| Other bias | Unclear risk | Physicians and pharmacists in the intervention offices decided how to best implement the intervention, and they were not required to perform the suggested intervention visits for this pragmatic trial. Few randomised clinics. |
Carter 2011.
| Study characteristics | ||
| Methods |
A patient‐centric, provider‐assisted diabetes telehealth self‐management intervention for urban minorities Patient RCT, conducted with patients recruited from a primary care practice in Washington, DC, intervention was home‐based, USA Two arms: 1) Control (control arm) and 2) Treatment (intervention arm) |
|
| Participants | Control arm N: 21 Intervention arm N: 26 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.8 (NR), post 7.9 (NR) Intervention arm: pre 9.0 (NR), post 6.8 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 148.0 (NR), post 140.0 (NR) Intervention arm: pre 146.0 (NR), post 139.0 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 86.0 (NR), post 72.0 (NR) Intervention arm: pre 90.0 (NR), post 75.0 (NR) |
|
| Funding source | This research was supported by a National Center on Minority Health and Health Disparities (NCMHD) Research to Reduce Ethnic Disparities in ESRD Export Grant, #5P20MD000512 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using a random numbers table". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Unsure whether 74 recruited were randomised or just considered for eligibility. For the 27 lost to follow‐up reasons not provided. Baseline based on those analysed; ~45% losses. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described. HbA1c and SBP methods not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; outcomes match methods. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias. |
Carter 2018.
| Study characteristics | ||
| Methods |
Cluster‐randomized trial to evaluate a centralized clinical pharmacy service in private family medicine offices Clustered RCT (12 clusters and NR providers), conducted in 1) 12 family medicine offices in Iowa, USA. 2) Clinical pharmacy specialists, providers. In United States of America. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (pharmacist management) (intervention arm) |
|
| Participants | Control arm N: 125 Intervention arm N: 121, NA, NA Diabetes type: 4 Mean age: 63.9 ± NR % Male: 50.36 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (pharmacist management) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician reminder 5) Facilitated relay of clinical information 6) Patient education |
|
| Outcomes | Foot screening | |
| Funding source | Sources of Funding: NHLBI, R01HL116311. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to the pharmacists in the order they were enrolled into the study, independent of medical office. The biostatistician (JDD) randomised offices to avoid contamination that would occur if a physician had participants in both the intervention and control groups. All participants and physicians in a given office received either the intervention or usual care. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT with randomisation occurring at one time. |
| Provider's baseline characteristics (selection bias) | High risk | Table 1. No P values provided. Some characteristics look unbalanced. |
| Patient's baseline characteristics (selection bias) | High risk | Table 2. No P values provided. Some characteristic values look unbalanced. |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. No P values provided. LDL showed a significant difference at baseline between the 2 groups, with a mean of 103.1 mg/dL in the control group and 90.6 mg/dL in the intervention group (P = 0.04). Similarly, baseline hyperlipidaemia was more common in the control group (94.1%) than in the intervention group (86.6%) (P = 0.03). |
| Incomplete outcome data (attrition bias) | Unclear risk | Losses were even between groups and just over 13% in one metric. The greatest attrition for both groups was in Direct Measurement Forms: control (12.4%), intervention (14.1%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Cluster‐randomised. Measurements taken objectively by medical professional. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | The biostatistician (JDD) randomised offices to avoid contamination that would occur if a physician had participants in both the intervention and control groups. All participants and physicians in a given office received either the intervention or usual care. |
| Other bias | Low risk | … there is a possibility that selection bias occurred based on the strategy study co‐ordinators used to identify participants. However, we conducted a comprehensive analysis and found that the probability of selection bias was low. |
Castejon 2013.
| Study characteristics | ||
| Methods |
A community‐based pilot study of a diabetes pharmacist intervention in Latinos: impact on weight and hemoglobin A1c. Patient RCT, conducted in an Hispanic Unity of Florida, a community‐based organization, USA Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: NR Intervention arm N: NR Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 8.2 (0.4), post 8.0 (0.2) Intervention arm: pre 8.3 (0.4), post 7.3 (0.3) 2) SBP, mean mmHg (SE) Control arm: pre 131.0 (4.0), post 126.0 (3.4) Intervention arm: pre 129.0 (3.2), post 126.0 (2.8) 3) DBP, mean mmHg (SE) Control arm: pre 81.8 (2.4), post 80.1 (2.0) Intervention arm: pre 82.2 (2.0), post 79.8 (2.0) 4) LDL, mean mg/dL (SE) Control arm: pre 88.2 (5.5), post 96.8 (5.4) Intervention arm: pre 99.9 (NR), post 91.0 (8.8) |
|
| Funding source | The DLASST research project was funded by the Centers for Medicare & Medicaid Services—CMS 1HOCMS030309‐02 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Differences for: education, income level and length of time living in the USA. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | We do not know how many randomised, but not a lot of people had completed the intervention. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective laboratory methods, HbA1c measured using DCA vantage analyser and lipids. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Chamany 2015.
| Study characteristics | ||
| Methods |
Telephone intervention to improve diabetes control: a randomized trial in the New York City A1c Registry RCT (NA clusters and NA providers), conducted in 1) Intervention done by mailed print education materials and phone calls. 2) Health educators made phone calls. They were supervised by multidisciplinary team (nurse‐certified diabetes educator, internal medicine physician, clinical health psychologist). In United States of America 2 arms: 1) Control (PrO: print materials only) (control arm) and 2) Intervention (Tele/Pr: telephone and print materials) (intervention arm) |
|
| Participants | Control arm N: 498 Intervention arm N: 443, NA, NA Diabetes type: 3 Mean age: 56.3 ± 9.95 % Male: 36.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (PrO: print materials only) 1) Patient education 2) Promotion of self‐management Intervention arm: (Tele/Pr: telephone and print materials) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by R18 DK 078077 and partially by P60 DK 020541. Sponsor: Albert Einstein College of Medicine, Inc. Obtained funding: Drs. Walker, Silver and Chamany. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by opening sealed, opaque envelopes containing a computer‐generated sequence of random assignments. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by opening sealed, opaque envelopes containing a computer‐generated sequence of random assignments. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no substantial differences at baseline in demographic characteristics between the 2 study arms. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c and BMI are balanced between groups. |
| Incomplete outcome data (attrition bias) | High risk | Total of 247 lost to follow‐up (247/941 = 26%). There is more loss in the intervention arm (26 vs 13 in control arm); reasons not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on November 2008 before the analysis of results, patients were recruited between September 2008 and October 2010, 1 year intervention). Results match protocol. |
| Risk of contamination (other bias) | Low risk | Patients received printed materials and phone calls at home. Health educators assigned only to intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Chan 2009.
| Study characteristics | ||
| Methods |
Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study Patient RCT, conducted in a diabetes Centre, China Two arms: 1) Usual care (control arm) and 2) Structured care (intervention arm) |
|
| Participants | Control arm N: 101 Intervention arm N: 104 Diabetes type: type 2 Mean age: 65.0 ± 7.2 % Male: 66.5 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.4 (2.0), post 8.0 (1.6) Intervention arm: pre 8.2 (1.9), post 7.3 (1.3) 2) SBP, mean mmHg (SD) Control arm: pre 144.0 (26.7), post 137.0 (21.0) Intervention arm: pre 145.0 (23.7), post 135.0 (25.0) 3) DBP, mean mmHg (SD) Control arm: pre 74.0 (10.0), post 71.0 (12.0) Intervention arm: pre 74.0 (11.7), post 68.0 (12.0) 4) LDL, mean mg/dL (SD) Control arm: pre 116.0 (38.7), post 109.8 (42.5) Intervention arm: pre 119.9 (42.5), post 96.3 (31.3) 5) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 4 (4), post 22 (27) Intervention arm: pre 4 (4), post 40 (49) |
|
| Funding source | This project was supported by the Hong Kong Government Health Services Research Committee (HSRC/HCPF s121012) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Low risk | Information not available. |
Chan 2012.
| Study characteristics | ||
| Methods |
A pharmacist care program: positive impact on cardiac risk in patients with type 2 diabetes Patient RCT, conducted in a diabetes clinic of Tung Wah Eastern Hospital, Hong Kong (public convalescent hospital), Hong Kong Two arms: 1) Control group (control arm) and 2) Intervention group (intervention arm) |
|
| Participants | Control arm N: 54 Intervention arm N: 51 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Patient reminders |
|
| Outcomes | 1a) Aspirin, N users (%) Control arm: pre 32 (59), post 31 (57) Intervention arm: pre 37 (73), post 38 (75) 1b) Aspirin (clopidogrel), N users (%) Control arm: pre 1 (2), post 3 (6) Intervention arm: pre 1 (2), post 1 (2) 1c) Aspirin (other type of anti‐platelets), N users (%) Control arm: pre 23 (43), post 22 (41) Intervention arm: pre 17 (33), post 17 (33) 2) Statins, N users (%) Control arm: pre 33 (61), post 36 (67) Intervention arm: pre 38 (75), post 39 (76) 3a) Antihypertensives (a‐blocker), N users (%) Control arm: pre 7 (13), post 7 (13) Intervention arm: pre 4 (8), post 5 (10) 3b) Antihypertensives (a2 Agonists), N users (%) Control arm: pre 1 (2), post 1 (2) Intervention arm: pre 2 (4), post 2 (4) 3c) Antihypertensives (ACE inhibitor), N users (%) Control arm: pre 31 (57), post 33 (61) Intervention arm: pre 32 (63), post 29 (57) 3d) Antihypertensives (angiotensin II receptor blockers), N users (%) Control arm: pre 11 (20), post 12 (22) Intervention arm: pre 13 (25), post 15 (29) 3e) Antihypertensives (calcium channel blocker), N users (%) Control arm: pre 25 (46), post 25 (46) Intervention arm: pre 29 (57), post 28 (55) 3f) Antihypertensives (diuretic), N users (%) Control arm: pre 13 (24), post 17 (31) Intervention arm: pre 15 (29), post 13 (25) 3g) Antihypertensives (ß‐blocker), N users (%) Control arm: pre 27 (50), post 30 (56) Intervention arm: pre 27 (53), post 29 (57) 3h) Antihypertensives (vasodilators), N users (%) Control arm: pre 1 (2), post 1 (2) Intervention arm: pre 3 (6), post 3 (6) 4) HbA1c, mean % (SD) Control arm: pre 9.5 (1.8), post 9.1 (NR) Intervention arm: pre 9.7 (1.4), post 8.1 (NR) 5) SBP, mean mmHg (SD) Control arm: pre 138.0 (19.0), post 134.8 (NR) Intervention arm: pre 141.0 (24.0), post 134.5 (NR) 6) DBP, mean mmHg (SD) Control arm: pre 74.0 (11.0), post 73.3 (NR) Intervention arm: pre 75.0 (11.0), post 72.2 (NR) 7) LDL, mean mg/dL (SD) Control arm: pre 107.1 (28.6), post 106.0 (NR) Intervention arm: pre 101.3 (32.9), post 87.4 (NR) 8) Smoking cessation, N smokers (%) Control arm: pre 4 (7), post 4 (7) Intervention arm: pre 4 (8), post 3 (6) |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: the School of Pharmacy, The Chinese University of Hong Kong and the Diabetes Research Fund, Diabetes Hong Kong | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…and sealed in envelopes labeled with consecutive numbers. The envelopes were opened in the clinic in ascending manner…" Opaque envelopes? |
| Patient's baseline characteristics (selection bias) | Low risk | Characteristics balanced between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up; intention‐to‐treat analysis. Baseline based on those randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Quote: "Blinding of participants and investigators was not possible in our study". Unsure if pharmacist was outcome collector. Objective methods not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias |
Chan 2014.
| Study characteristics | ||
| Methods |
Effects of telephone‐based peer support in patients with type 2 diabetes mellitus receiving integrated care. A randomized clinical trial Patient RCT, conducted in 3 publicly funded hospital‐based diabetes centres, China Two arms: 1) JADE (control arm) and 2) JADE + PEARL (intervention arm) |
|
| Participants | Control arm N: 316 Intervention arm N: 312 Diabetes type: type 2 Mean age: 54.7 ± 9.3 % Male: 56.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Electronic patient registry 2) Clinician reminders 3) Facilitated relay of clinical information 4) Promotion of self‐management 5) Patient reminders Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Clinician reminders 5) Facilitated relay of clinical information 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.2 (1.6), post 7.9 (NR) Intervention arm: pre 8.2 (1.7), post 7.9 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 135.0 (19.0), post 132.3 (NR) Intervention arm: pre 136.0 (19.0), post 132.8 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 80.0 (11.0), post 76.2 (NR) Intervention arm: pre 80.0 (10.0), post 76.4 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 111.0 (31.7), post 121.0 (NR) Intervention arm: pre 112.1 (31.3), post 123.4 (NR) |
|
| Funding source | This study was supported by the Asia Diabetes Foundation, partially funded by an educational grant by Merck, and the American Academy of Family Physicians Foundation Peers for Progress Program, funded by the Eli Lilly and Company Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, opaque and sealed envelopes. |
| Patient's baseline characteristics (selection bias) | Low risk | General obesity P = 0.047; all other characteristics balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | ~8.2% lost to follow‐up in control; ~5.1% lost to follow‐up in intervention; reasons seem balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: HbA1c, no mention of how it was measured. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Low risk | Information not available. |
Chao 2015.
| Study characteristics | ||
| Methods |
The effect of integrated health management model on the health of older adults with diabetes in a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Outpatients who received clinic services from the Nanjing district hospital endocrinology department. 2) Intervention implemented by specifically trained community health service centre staff, managers and related researchers in China 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (integrated health management model) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 69.6 ± 10.2 % Male: 49 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (integrated health management model) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This study was funded by the National Natural Science Foundation of China (Grant Number 81273189, 30771837) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 100 older adults with type 2 diabetes were randomly allocated to either the management or the control group in a 1:1 ratio using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. The differences of general conditions and health indices between the management and the control groups were not statistically significant. This suggested that the 2 groups were balanced and comparable at the baseline level. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All P values are above 0.05 for all objective measurement health indices. The differences of general conditions and health indices between the management and the control groups were not statistically significant. This suggested that the 2 groups were balanced and comparable at the baseline level. |
| Incomplete outcome data (attrition bias) | Low risk | After 18 months, there were no dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest are objective (SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Fasting blood sugar and blood triglyceride were measured by the clinical test centre of the hospital. The authors do not report any data about triglyceride. |
| Risk of contamination (other bias) | Low risk | The community health service centre staff, managers and related researchers who delivered the intervention only saw patients in the intervention arms and it looks like no communication was made with patients' physicians. |
| Other bias | Low risk | No evidence of other bias. |
Chao 2019.
| Study characteristics | ||
| Methods |
Enhanced self‐efficacy and behavioral changes among patients with diabetes: cloud‐based mobile health platform and mobile app service RCT (NA clusters and NA providers), conducted in 1) Case‐group patients participated (n = 62) in a 1‐hour training and employed the IPMF system when they visited outpatient departments, Taipei City, Taiwan. Remotely delivered mobile intervention 2) Physician, health educator, nutritionist, care manager and service consultant in Taiwan 2 arms: 1) Control (traditional care) (control arm) and 2) Intervention (interactive personalised management framework) (intervention arm) |
|
| Participants | Control arm N: 59 Intervention arm N: 62, NA, NA Diabetes type: 2 Mean age: 63.71 ± 11.61 % Male: 61 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (traditional care) Intervention arm: (interactive personalised management framework) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This study was funded by the National Natural Science Foundation of China (Grant Number 81273189, 30771837). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. No P values provided. No comparison between groups available. |
| Patient's baseline outcomes (selection bias) | Low risk | Baseline outcomes listed in Table 1. |
| Incomplete outcome data (attrition bias) | High risk | A total of 97 patients completed both the pre‐assessment and post‐assessment (case group, n = 49, retention rate = 82%; control group, n = 48, retention rate = 81%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c, BP. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that the control participants received access to the mobile intervention. |
| Other bias | Low risk | None identified. |
Charpentier 2011.
| Study characteristics | ||
| Methods |
The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients Patient RCT, conducted in 17 hospital sites in France Three arms: 1) Control group (control arm), 2) Electronic logbook alone (intervention arm 1) and 3. Electronic logbook + teleconsultation (intervention arm 2) |
|
| Participants | Control arm N: 61 Intervention arm 1 N: 60 Intervention arm 2 N: 59 Diabetes type: type I Mean age: 33.8 ± 12.9 % Male: 36.7 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information Intervention arm 1: 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm 2: 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.9 (0.9), post 9.1 (1.2) Intervention arm 1: pre 9.2 (1.1), post 8.6 (1.1) Intervention arm 2: pre 9.1 (1.1), post 8.4 (1.0) 2) Harms (severe hypoglycaemia events), N (%) Control arm: pre NR (NR), post 3 (5) Intervention arm 1: pre NR (NR), post 3 (5) Intervention arm 2: pre NR (NR), post 1 (2) |
|
| Funding source | This study was sponsored by Sanofi‐Aventis, Orange and CERITD. Financial disclosures are listed in the Supplementary Data. Voluntis provided the Diabeo software, and Orange (Paris, France) provided the smartphone and telephone lines; Sanofi‐Aventis (Bridgewater, NJ) and CERITD funded the study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Chen 2016.
| Study characteristics | ||
| Methods |
Pharmaceutical care of elderly patients with poorly controlled type 2 diabetes mellitus: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted at the Nantou Hospital (a 421‐bed district hospital), Ministry of Health and Welfare, Taiwan, ROC, which participates in the Pay‐for‐Performance (P4P) Program for DM care in Taiwan. 2) Pharmaceutical care was provided by a certified diabetes‐educator pharmacist. In Taiwan. 2 arms: 1) Control (standard care) (control arm) and 2) Intervention (pharmaceutical care) (intervention arm) |
|
| Participants | Control arm N: 61 Intervention arm N: 58, NA, NA Diabetes type: 2 Mean age: 72.46 ± 9.1 % Male: 50 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (pharmaceutical care) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by a Grant from the Department of Health, Executive Yuan, Taiwan (ROC) (100‐MID‐08) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random numbers generated by SAS 9.2 (for Windows), the case manager assigned patients to the intervention or control group before the nurse referred patients to the pharmacist. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values about patient characteristics are above 0.05. The demographic characteristics of the groups were very similar (Table 1). |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. All our outcomes of interest have P values above 0.05. However, weight has a P value of 0.02 and BMI has a P value of 0.059. |
| Incomplete outcome data (attrition bias) | High risk | Information shown in Figure 1 does not match with text. Quote text: "One hundred patients completed the study (control group, 50/61; intervention group, 50/58) (Fig. 1)". It looks like 119 patients (61+58) were randomised (and not 100 as shown in figure 1) and 19 were lost to follow‐up (total of 16.0%, 18.0% in control and 13.8% in intervention groups). Reasons provided but not by arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registered protocol (first posted on Oct. 2011, recruitment started in August 2011 (results), but they also state, in the methods, that 71.9 % of patients in the Nantou Hospital were enrolled in 2010). In the protocol, they intended to look at Mini‐Mental State Examination (MMSE) score change and Taiwan Geriatric Depression Scale (GDS) score change but no data are reported 3 months after baseline in the paper. Also, they added cost analysis in the paper (Table 3). Also, they measured blood pressure at baseline but not after the intervention. |
| Risk of contamination (other bias) | High risk | In the P4P programme, (all) patients’ HbA1c levels were measured every 3 or 6 months during follow‐up. Pharmacists interacted with physicians, so it is not excluded that physicians may have changed their care to the patients in the control arm. All patients were recruited from one hospital. Numerous pharmacists are qualified DM educators and are involved in the P4P program (standard care). |
| Other bias | Low risk | No evidence of other bias. |
Cho 2006.
| Study characteristics | ||
| Methods |
Long‐term effect of the Internet‐based glucose monitoring system on HbA1c reduction and glucose stability: a 30‐month follow‐up study for diabetes management with a ubiquitous medical care system Patient RCT, conducted in Kangnam St Mary's Hospital Diabetes Centre, South Korea Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 40 Diabetes type: type 2 Mean age: 53.0 ± 8.9 % Male: 61.3 Longest follow‐up: 30 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.5 (1.3), post 7.4 (1.3) Intervention arm: pre 7.7 (1.5), post 6.7 (0.9) |
|
| Funding source | This work was supported by Korea Research Foundation Grant KRF‐2004‐041 ‐EOO 148 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Cho 2009.
| Study characteristics | ||
| Methods |
Mobile communication using a mobile phone with a glucometer for glucose control in Type 2 patients with diabetes: as effective as an Internet‐based glucose monitoring system Patient RCT, conducted in outpatient clinic of the Diabetes Centre in Kangnam St Mary's Hospital, South Korea Two arms: 1) Internet group (control arm) and 2) Phone group (intervention arm) |
|
| Participants | Control arm N: 37 Intervention arm N: 38 Diabetes type: type 2 Mean age: 48.2 ± 12.3 % Male: 78.3 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.6 (1.9), post 6.9 (1.1) Intervention arm: pre 8.3 (2.3), post 7.1 (1.1) |
|
| Funding source | This work was supported by the Ministry of Information and Communication (noninvasive glucose‐monitoring project), the Seoul R&D programme and the Ministry of Commerce, Industry and Energy (Energy‐IT project). We also thank the Healthpia Company for their donation of diabetes phones and test strips. Jae‐Hyoung Cho and Hye‐Chung Lee contributed equally to this work. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Cho 2011a.
| Study characteristics | ||
| Methods |
Effectiveness and safety of a glucose data‐filtering system with automatic response software to reduce the physician workload in managing type 2 diabetes Patient RCT, conducted with patients registered with Seoul St. Mary's Hospital, Korea Two arms: 1) Control group (control arm) and 2) SAVE group (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 38 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Electronic patient registry 2) Clinician reminders 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.4 (0.7), post 7.7 (1.3) Intervention arm: pre 7.3 (0.7), post 7.7 (0.9) 2) LDL, mean mg/dL (SD) Control arm: pre 104.4 (34.8), post 92.8 (19.3) Intervention arm: pre 96.7 (23.2), post 92.8 (19.3) |
|
| Funding source | The work was supported by a Seoul R&D project grant and the Ministry for Health, Welfare and Family Affairs (A080872) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…by adaptive randomization." Unclear since they could have used minimisation. |
| Allocation concealment (selection bias) | High risk | You can predict the next assignment when you use adaptive randomisation. |
| Patient's baseline characteristics (selection bias) | High risk | Quote: "BMI (P = 0.012); duration of diabetes in years (P = 0.031); triglycerides (P = 0.028)." |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "HbA1c (P = 0.1989); LDL (P = 0.789)." |
| Incomplete outcome data (attrition bias) | High risk | They state intention‐to‐treat analysis, but they excluded n = 4 who dropped out of the analysis. They provide numbers for dropout and the numbers are equal across arms, but reasons are not provided. Baseline based on randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Secondary outcome: HbA1c, objective laboratory methods not described, outcome assessor not described. |
| Selective reporting (reporting bias) | High risk | < 2005 approach used since no protocol; they listed HbA1c as secondary outcome in methods, but they also provide other items such as LDL, HDL, cholesterol, etc. in the results. |
| Risk of contamination (other bias) | High risk | Although physician was seeing both types of patients, he only provided feedback to those who used the SAVE programme, but how is this guaranteed? |
| Other bias | Low risk | Information not available. |
Cho 2011b.
| Study characteristics | ||
| Methods |
Effects on diabetes management of a health‐care provider mediated, remote coaching system via a PDA‐type glucometer and the internet Patient RCT, conducted in 6 healthcare posts associated with Chung‐Ju City (about 150 km from Seoul), South Korea Two arms: 1) Control group (control arm) and 2) Intervention group (intervention arm) |
|
| Participants | Control arm N: 35 Intervention arm N: 36 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Team changes 2) Facilitated relay of clinical information 3) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.0 (1.0), post 7.8 (1.1) Intervention arm: pre 8.0 (0.8), post 7.5 (0.9) |
|
| Funding source | The study was funded by the Seoul R&D Project | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "The baseline characteristics of the two groups were similar except for blood pressure…" |
| Patient's baseline outcomes (selection bias) | High risk | SBP (P < 0.001); DBP (P = 0.002). |
| Incomplete outcome data (attrition bias) | High risk | Approximately 11% dropout rate in intervention group, and reasons not provided for losses to follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described, HbA1c objective laboratory methods not described. |
| Selective reporting (reporting bias) | High risk | Secondary outcome listed in protocol, not listed in paper. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Cho 2017.
| Study characteristics | ||
| Methods |
An Internet‐based health gateway device for interactive communication and automatic data uploading: clinical efficacy for type 2 diabetes in a multi‐centre trial RCT (NA clusters and NA providers), conducted in 1) Internet‐based communication and data management device. outpatient clinics of the Diabetes Center of Seoul St. Mary’s Hospital, the Seoul Asan Hospital’s Diabetes Center, and Kangbook Samsung Hospital’s Diabetes Center. 2) For the programme, the staff consisted of 2 nurses and 2 diabetologists per institute. There was a separate centre for nutrition and exercise counselling, which included 3 dietitians and 3 exercise experts. The medical team provided consultation to patients who decided to receive additional individualised education for lifestyle management in South Korea. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (Internet‐based communication) (intervention arm) |
|
| Participants | Control arm N: 240 Intervention arm N: 244, NA, NA Diabetes type: 2 Mean age: 53.15 ± 8.92 % Male: 63.43 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education 2) Promotion of self‐management Intervention arm: (Internet‐based communication) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: this work was supported by the Korean Ministries of Health and Welfare and of Trade, Industry and Energy. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to the intervention or control group using adaptive randomisation based on the stratified block randomisation design |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values > 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values > 0.05. |
| Incomplete outcome data (attrition bias) | Unclear risk | 444/484 (91.7%) completed the study. Unclear as to how many were lost in each arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Some of the outcomes (BUN, Cr) are not reported in the results. |
| Risk of contamination (other bias) | Unclear risk | The patients were followed with the same physicians in both arms. As conventional management in the outpatient clinic, patients in the control group visited the diabetes centre and were provided with recommendations about medication, medication dosage and lifestyle modification from the diabetologist. |
| Other bias | Low risk | None. |
Choe 2005.
| Study characteristics | ||
| Methods |
Proactive case management of high‐risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial Patient RCT, conducted in an university affiliated primary care internal medicine clinic, USA Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 39 Intervention arm N: 41 Diabetes type: type 2 Mean age: 51.6 ± 10.1 % Male: 47.5 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 26 (74) Intervention arm: pre NR (NR), post 38 (97) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 22 (63) Intervention arm: pre NR (NR), post 36 (92) 3) HbA1c, mean % (SD) Control arm: pre 10.2 (1.7), post 9.3 (2.1) Intervention arm: pre 10.1 (1.8), post 8.0 (1.4) |
|
| Funding source | Funding for the clinical pharmacist was provided by the University of Michigan College of Pharmacy | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | High risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Choudhry 2018.
| Study characteristics | ||
| Methods |
Effect of a remotely delivered tailored multicomponent approach to enhance medication taking for patients with hyperlipidemia, hypertension, and diabetes: the STIC2IT cluster randomized clinical trial Clustered RCT (14 clusters and 250 providers), conducted in 1) This trial was conducted at Atrius Health, a large multi specialty medical group, Newton, Massachusetts. Remotely delivered tailored multicomponent intervention. 2) Intervention conducted by a staff clinical pharmacist. In United States of America. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (telephone consultation by pharmacist) (intervention arm) |
|
| Participants | Control arm N: 242 Intervention arm N: 246, NA, NA Diabetes type: 4 Mean age: 59.80 ± 9.60 % Male: 54.85 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (telephone consultation by pharmacist) 1) Case management 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This research was supported by a grant from the National Heart, Lung, and Blood Institute to Brigham and Women’s Hospital (R01 HL 117918) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From supplemental file: within the resultant 4 blocks, practices were then randomised in a 1:1 ratio to intervention or control using a random number generator. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. Randomisation occurred at the level of the primary care practice sites. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No comparison done between groups, but they did block randomisation (unlikely that an imbalance happen). Quote: "Because the practice sites differ from each other, simple cluster randomization may have resulted in imbalances in patient or provider factors that could potentially bias outcome assessment. Therefore, we categorized the practice sites based on their size (i.e., small or large, based on the number of patients receiving care at each site) and whether clinical pharmacists at the sites offered disease management counseling directly to patients (i.e., yes or no). Within the resultant 4 blocks, practices were then randomized in a 1:1 ratio to intervention or control using a random number generator." |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 note: Standardised mean differences between intervention and control for age and race/ethnicity were greater than 0.1. Text: Intervention patients were slightly older and less likely to be of white race/ethnicity. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. HbA1c data reported at baseline. Table note: Standardised mean differences between intervention and control for age and race/ethnicity were greater than 0.1; otherwise, there were no significant differences between treatment arms. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of lost not reported. Outcomes were evaluated using intention‐to‐treat principles and multiple imputation for missing values. We used electronic health record data to evaluate clinical outcomes, and any missing or inaccurate data, even if non differential, would have biased treatment effects to the null. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively assessed. |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol. Do not report diastolic blood pressure for patients with hypertension in the paper. All other outcomes match. |
| Risk of contamination (other bias) | Low risk | Clustered‐RCT. We chose to use cluster‐randomisation at the practice level to minimise contamination by clinical pharmacist and primary care clinician. |
| Other bias | High risk | From the supplemental file, some major changes to the protocol were done: 1) Changing randomisation process from the level of the physician to the level of the practice site. 2) Increasing the number of patients included in the study has increased from 2000 to 3000, as this is roughly the number of eligible patients at our practice sites. 3) Changing the enrollment number target from 3000 to 4,080 patients to reflect findings from the first few months of enrollment. |
Christian 2008.
| Study characteristics | ||
| Methods |
Clinic‐based support to help overweight patients with type 2 diabetes increase physical activity and lose weight Patient RCT, conducted in large urban‐based community health centres, USA Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 155 Intervention arm N: 155 Diabetes type: type 2 Mean age: 53.2 ± 11.0 % Male: 33.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Clinician reminders 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.3 (1.9), post 7.8 (NR) Intervention arm: pre 8.1 (2.0), post 7.9 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 132.3 (17.4), post 127.6 (NR) Intervention arm: pre 131.8 (17.0), post 129.3 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 77.8 (9.6), post 75.3 (NR) Intervention arm: pre 76.6 (10.5), post 74.0 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 105.8 (38.8), post 102.1 (NR) Intervention arm: pre 100.2 (32.1), post 85.6 (NR) |
|
| Funding source | This research was supported by grant 5R44DK060272‐3 from the US National Institute of Diabetes and Digestive and Kidney Diseases to PHCC LP, Pueblo, Colorado | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Chung 2014.
| Study characteristics | ||
| Methods |
Effects of a pharmaceutical care model on medication adherence and glycemic control of people with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Any person with T2DM who visited the diabetes clinic of a major teaching hospital during the recruitment period was requested to participate in this study. Participants met with the pharmacist at the hospital's pharmacy and they also received phone call from the pharmacist. 2) Participants in the intervention group received PC from an experienced pharmacist. In Malaysia. 2 arms: 1) Control (standard pharmacy service) (control arm) and 2) Intervention (pharmaceutical care (PC) model) (intervention arm) |
|
| Participants | Control arm N: 121 Intervention arm N: 120, NA, NA Diabetes type: 2 Mean age: 59.10 ± 8.72 % Male: 43.98 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard pharmacy service) Intervention arm: (pharmaceutical care (PC) model) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | We acknowledge the University of Malaya for funding this project under grant PG 138‐2012B. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. People allocated at random to the control (n = 121) or intervention (n = 120) groups. Participants were allocated at random to the control or intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values higher than 0.05. At baseline, there were no significant differences in demographic data and other characteristics, medication adherence, or glycaemic levels between participants in the control and intervention groups (Tables 1 and 2; Figures 1 and 2). |
| Patient's baseline outcomes (selection bias) | Low risk | Tables 1, 2 and 3. Figures 1 and 2. All P values greater than 0.05. At baseline, there were no significant differences in demographic data and other characteristics, medication adherence, or glycaemic levels between participants in the control and intervention groups (Tables 1 and 2; Figures 1 and 2). |
| Incomplete outcome data (attrition bias) | Low risk | Looks like they lost no patients at 12 months. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Participants in the intervention group received pharmaceutical care (PC) from a pharmacist, whereas those in the control group were provided standard pharmacy services, which consisted of dispensing the medications and providing brief instructions on how to take them. Not clear if the same pharmacist met with patients in both groups. There may also be cross‐contamination between participants in the control and intervention groups that could not be avoided, as they were attending the same clinic. Control participants may have discussed the study with the intervention participants and obtained some information regarding their disease conditions and medications. Therefore, the effects of the PC intervention may have been diluted. |
| Other bias | Low risk | No evidence of other risk of bias. |
Chwastiak 2018.
| Study characteristics | ||
| Methods |
Use of a mental health center collaborative care team to improve diabetes care and outcomes for patients with psychosis RCT (NA clusters and NA providers), conducted in 1) The study was conducted in two Community Mental Health Center (CMHCs) in King County, Washington, which together provide comprehensive behavioral health services to over 2000 low‐income residents of Seattle. 2) Care was provided by a CMHC‐based team that included a CMHC nurse care manager, a CMHC psychiatrist, the advanced practice registered nurse who provided primary care onsite at the CMHC and an endocrinologist consultant. In United States of America. 2 arms: 1) Control (usual care) (control arm) and 2) Intervention (Collaborative Care Team) (intervention arm) |
|
| Participants | Control arm N: 17 Intervention arm N: 18, NA, NA Diabetes type: 2 Mean age: 51.26 ± 10.78 % Male: 65.7142857142857 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Collaborative Care Team) 1) Case management 2) Team change 3) Electronic patient registry 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein Smoking status |
|
| Funding source | The authors gratefully acknowledge support from grant 5R21DK096286‐02 (U.S. NIH Grant/Contract) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Participants were randomised at a rate of 1:1, and randomisation was stratified based on baseline treatment with insulin or with clozapine or olanzapine. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Supplemental Table 2. All P values above 0.05. Quote: "There were no statistically significant baseline demographic or clinical differences between the groups [see online supplement]." |
| Patient's baseline outcomes (selection bias) | Low risk | Supplemental Table 2. All P values above 0.05. Quote: "There were no statistically significant baseline demographic or clinical differences between the groups [see online supplement]." |
| Incomplete outcome data (attrition bias) | High risk | Primary outcome data were available for 29 participants (out of 35 randomised, 17% lost). 4/18 patients lost in the intervention group (22%) and 2/17 in the control group (12% lost). High and unbalanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective primary outcomes (HbA1c, SBP and LDL). Smoking likely patient self‐reported but secondary outcome. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Protocol: Both process and outcome measures will be evaluated at baseline, and at 3‐ and 6‐month follow‐up visits for the 40 participants enrolled in this feasibility trial. They only report 3 months follow‐up in the paper. In addition, they added many outcomes in the paper (triglycerides, BMI, smoking, PHQ‐9, etc.) |
| Risk of contamination (other bias) | Unclear risk | All patients were followed at two CMHC where all clinical visits and team meetings for the intervention group were conducted. However, it is unlikely that patients in the control group had a nurse care manager co‐ordinating team care. |
| Other bias | Low risk | No evidence of other risk of bias. |
Ciria de Pablo 2008.
| Study characteristics | ||
| Methods |
Control of risk factor in diabetic patients in secondary prevention. MIRVAS Study RCT (NA clusters and NA providers), conducted in 1) Multifactorial and intensive intervention done in a specific consultation clinic at La Princesa University Hospital, Madrid, Spain. 2) Intervention delivered by specialist/trained nurses and usual physicians. In Spain. 2 arms: 1) Control (conventional healthcare) (control arm) and 2) Intervention (multifactorial and intensive intervention) (intervention arm) |
|
| Participants | Control arm N: 33 Intervention arm N: 38, NA, NA Diabetes type: 2 Mean age: 65.91 ± NR % Male: 74.6 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (conventional healthcare) Intervention arm: (multifactorial and intensive intervention) 1) Case management 2) Clinician education 3) Patient education |
|
| Outcomes | Anti‐platelet drugs Lipid lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Smoking status |
|
| Funding source | Only found information in the protocol. Sponsor type: Hospital/treatment centre. Funder name: La Princesa University Hospital (Hospital Universitario de La Princesa), Biomedical Research Foundation (Fundación de Investigación Biomédioca) (Spain). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method to generate the sequence not reported. Quote: "Patients are randomly assigned to each group and by blocks (type of pathology: AMI or stroke)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. For the distribution of baseline cardiovascular episodes, CVRF, dyslipidaemia, vascular history and hypertension, groups are similar (P values = NS). For age and gender, they only compare diabetics with non‐diabetics, and not control vs intervention. Quote: "The mean age of both groups showed no significant differences (65.91 years in diabetics compared to 64.99 years in non‐diabetics). The gender distribution also does not differ significantly: 74.6% of diabetic males compared to 74.4% of non‐diabetic males." |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. They only report data for tobacco use at baseline, P value above 0.05. Quote: "The mean basal lipid values (determined in the first 24 hours after admission) of the different subgroups (diabetics vs. non‐diabetics and diabetics assigned to intervention vs. diabetics assigned to control) showed no significant differences". Quote: "Baseline BP, Hba1c, weight and abdominal perimeter were not available." |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on the number of patients lost in each group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Method to collect drug prescribed is not reported. Tobacco use is likely self‐reported, so subjective outcome (Quote: "You are considered a smoker if you have used any type of tobacco in the previous six months"). HbA1c, LDL and blood pressure were objectively collected. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol not referred to in the paper. From the protocol, MIRVAS study was designed to last 3 years, and here they report the results after 1 year. Data reported in Table 4 (HbA1c, BP, LDL, etc.) were not planned to be reported at 1 year. |
| Risk of contamination (other bias) | Unclear risk | Patients randomised from a single clinic at La Princesa University Hospital. It is unclear if the same physicians were following patients in both groups. If so, the physicians might have applied the intensive treatment to control patients. Unlikely that control patients met with the nurses. |
| Other bias | High risk | Diabetic subgroup analysis was not the main objective of the study. |
Clancy 2003.
| Study characteristics | ||
| Methods |
Group visits in medically and economically disadvantaged patients with type 2 diabetes and their relationships to clinical outcomes Patient RCT, conducted in an adult medical care centre at the University of South Carolina, USA Two arms: 1) Control (control arm) and 2) Group visit (intervention arm) |
|
| Participants | Control arm N: 61 Intervention arm N: 59 Diabetes type: type 2 Mean age: 54.0 ± 10.4 % Male: 21.7 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.6 (NR), post 9.7 (NR) Intervention arm: pre 10.3 (NR), post 9.5 (NR) 2) LDL, mean mg/dL (SD) Control arm: pre 122.0 (NR), post 116.0 (NR) Intervention arm: pre 123.5 (NR), post 107.5 (NR) |
|
| Funding source | Ibis work was supported by the Improving Chronic Illness Care program (funded by The Robert Wood Johnson Foundation) and South Carolina Excellence Initiative for Eliminating Disparities in Healthcare program (funded by the Agency for Healthcare Research and Quality) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer program for randomisation. |
| Allocation concealment (selection bias) | High risk | Patient told the doctor which intervention randomised to. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Do not report baseline characteristics by group, only overall in Table 1. |
| Patient's baseline outcomes (selection bias) | Low risk | Report in the text that HbA1c, etc. was similar for 2 groups. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 study participant dropped out. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Unclear if the same physician was seeing participants in both groups; may have changed treatment behaviour |
| Other bias | Low risk | Information not available. |
Clancy 2007.
| Study characteristics | ||
| Methods |
Group visits: promoting adherence to diabetes guidelines Patient RCT, conducted in an Adult Primary Care Centre, Medical University of South Carolina, USA Two arms: 1) Control patients (control arm) and 2) Group patients (intervention arm) |
|
| Participants | Control arm N: 90 Intervention arm N: 96 Diabetes type: type 2 Mean age: 56.0 ± NR % Male: 28.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre NR (NR), post 43 (48) Intervention arm: pre NR (NR), post 78 (81) 2) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 48 (53) Intervention arm: pre NR (NR), post 72 (75) 3) Foot screening, N screened (%) Control arm: pre NR (NR), post 25 (28) Intervention arm: pre NR (NR), post 62 (65) 4) Renal screening (microalbumin), N screened (%) Control arm: pre NR (NR), post 30 (33) Intervention arm: pre NR (NR), post 40 (42) 5) HbA1c, mean % (SE) Control arm: pre 8.9 (0.2), post 9.0 (0.3) Intervention arm: pre 9.3 (0.2), post 9.1 (0.2) |
|
| Funding source | This project was supported by grant number 5 P01 HS10871 from the Agency for Healthcare Research and Quality, a grant from The Robert Wood Johnson Foundation, Princeton, New Jersey, and 1R21NS043569 from National Institutes of Health/NINDS | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Cleveringa 2008.
| Study characteristics | ||
| Methods |
Combined task delegation, computerized decision support, and feedback improve cardiovascular risk for type 2 diabetic patients: a cluster randomized trial in primary care Cluster‐RCT (55 clusters), conducted in primary care practices throughout The Netherlands Two arms: 1) Control (control arm) and 2) Intervention (intervention arm) |
|
| Participants | Control arm N: 1692 Intervention arm N: 1699 Diabetes type: type 2 Mean age: 65.1 ± 11.2 % Male: 49.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Case management 3) Clinician reminders 4) Patient reminders |
|
| Outcomes | 1) HbA1c 2) SBP 3) DBP 4) LDL 5) Smoking cessation |
|
| Funding source | For this study we received an unrestricted grant from Pfizer B.V. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported |
| Patient's baseline characteristics (selection bias) | High risk | Data is provided for age and gender in the Table 1 and the authors mention no differences between the groups, but no data on education. Significant difference in smoking and history of cardiac diseases. |
| Patient's baseline outcomes (selection bias) | Low risk | HDL cholesterol levels in Table 1 seem fine (1.36 vs 1.32). |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Clifford 2002.
| Study characteristics | ||
| Methods |
A randomised controlled trial of a pharmaceutical care programme in high‐risk diabetic patients in an outpatient clinic Patient RCT, conducted in a diabetes clinic in Australia Two arms: 1) Control (control arm) and 2) PCP ‐ pharmaceutical care programme (intervention arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 48 Diabetes type: type 1 and Type 2 Mean age: 60.5 ± 12.0 % Male: 55.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (1.6), post 8.1 (1.6) Intervention arm: pre 8.4 (1.4), post 8.2 (1.5) |
|
| Funding source | This study was funded by The Society of Hospital Pharmacists of Australia, Pharmacia and Upjohn Contemporary Therapeutics Research Grant | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | They report that "hypertension, dyslipidaemia and polypharmacy were inclusion criteria that did not apply to all patients, these data were not analysed further". |
| Risk of contamination (other bias) | Unclear risk | The doctors were aware of the allocation and could have treated the pharmaceutical care programme (PCP) group differently. Also, not sure if the pharmacist had exposure to the control group (it is not reported). |
| Other bias | Unclear risk | Information not available. |
Clifford 2005.
| Study characteristics | ||
| Methods |
Effect of a pharmaceutical care program on vascular risk factors in type 2 diabetes: the Fremantle Diabetes Study Patient RCT, conducted with adults from the Fremantle Diabetes Study, Australia Two arms: 1) Usual care (control arm) and 2) PC ‐ Pharmaceutical Care (intervention arm) |
|
| Participants | Control arm N: 99 Intervention arm N: 99 Diabetes type: type 2 Mean age: 70.4 ± 7.7 % Male: 52.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 50 (57), post 52 (59) Intervention arm: pre 51 (55), post 54 (59) 2) Antihypertensives (any), N users (%) Control arm: pre 66 (75), post 74 (84) Intervention arm: pre 69 (75), post 81 (88) 3) HbA1c, median % (SD) Control arm: pre 7.1 (1.1), post 7.1 (NR) Intervention arm: pre 7.5 (0.9), post 7.0 (NR) 4) SBP, mean mmHg (SD) Control arm: pre 156.0 (25.0), post 149.0 (NR) Intervention arm: pre 157.0 (22.0), post 143.0 (NR) 5) DBP, mean mmHg (SD) Control arm: pre 77.0 (11.0), post 75.0 (NR) Intervention arm: pre 77.0 (10.0), post 72.0 (NR) |
|
| Funding source | The Raine Foundation, University of Western Australia, funded the FDS. R.M.C. was the recipient of a National Health and Medical Research Council of Australia PhD scholarship | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Consecutive allocation. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Diabetes duration, etc. |
| Patient's baseline outcomes (selection bias) | High risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Cohen 2011.
| Study characteristics | ||
| Methods |
Pharmacist‐led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes Patient RCT, conducted with patients selected from VA Medical Center's electronic medical record system, USA Two arms: 1) Controls (control arm) and 2) Cases (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 53 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 36 (73), post 40 (82) Intervention arm: pre 37 (74), post 43 (86) 2a) Antihypertensives (ACE inhibitor or angiotensin II receptor blockers), N users (%) Control arm: pre 38 (78), post 37 (76) Intervention arm: pre 40 (80), post 45 (90) 2b) Antihypertensives (calcium channel blocker), N users (%) Control arm: pre 12 (24), post 12 (24) Intervention arm: pre 12 (24), post 17 (34) 2c) Antihypertensives (Diuretic), N users (%) Control arm: pre 14 (29), post 16 (33) Intervention arm: pre 24 (48), post 27 (54) 2d) Antihypertensives (ß‐blocker), N users (%) Control arm: pre 21 (43), post 23 (47) Intervention arm: pre 22 (44), post 25 (50) 3) HbA1c, mean % (SD) Control arm: pre 8.1 (1.4), post 7.9 (NR) Intervention arm: pre 7.8 (1.0), post 7.4 (NR) 4) SBP, mean mmHg (SD) Control arm: pre 136.1 (16.5), post 135.3 (NR) Intervention arm: pre 136.1 (16.8), post 126.9 (NR) 5) LDL, mean mg/dL (SD) Control arm: pre 110.7 (37.2), post 99.2 (NR) Intervention arm: pre 96.1 (25.4), post 86.7 (NR) 6) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 16 (33), post 16 (33) Intervention arm: pre 12 (24), post 29 (58) |
|
| Funding source | This study was funded by the Sandra A. Daugherty Foundation (principal investigator, Dr Wu) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | LDL (P = 0.024). |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those analysed, numbers and reasons for loss to follow‐up provided, but numbers excluded due to revoked consent disproportionate, after that numbers lost to follow‐up are the same. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: controlled hypertension. Secondary outcome: SBP, HbA1c, LDL. Objective methods not described. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias. |
Cohen 2019.
| Study characteristics | ||
| Methods |
Pharmacist‐led telehealth disease management for patients with diabetes and depression RCT (NA clusters and NA providers), conducted in 1) Providence VA Medical Center, Providence RI. Remote Teleheath study. 2) Pharmacists in United States of America 2 arms: 1) Control (nurse‐led telehealth/usual care) (control arm) and 2) Intervention (pharmacist‐led telehealth) (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 15, NA, NA Diabetes type: 3 Mean age: 61.8 ± 9.6 % Male: 93.35 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (nurse‐led telehealth/usual care) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician reminder 5) Facilitated relay of clinical information 6) Promotion of self‐management 7) Patient reminders Intervention arm: (pharmacist‐led telehealth) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician reminder 5) Facilitated relay of clinical information 6) Patient education Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: this work was supported by the Federal Services Junior Investigator Research Grant Program – American Health‐System Pharmacy | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to the pharmacist‐led telehealth arm or the usual care with nurse‐led telehealth arm via a coin toss. |
| Allocation concealment (selection bias) | Low risk | Coin toss. No way to manipulate. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values greater than 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. BP measurements significantly different; HbA1c was P = 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 1 lost in control, 2 lost in intervention. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol; methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Nurses and pharmacists worked in the same clinic together. |
| Other bias | Unclear risk | The depression scoring system used was changed midway through the study. The study initially used PHQ‐9, but was modified and changed to the CES‐D scale to capture depression changes more easily. This resulted in less comparable depression scores in each group. |
Crasto 2011.
| Study characteristics | ||
| Methods |
Multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: The Microalbuminuria Education and Medication Optimisation (MEMO) Study Patient RCT, conducted in primary care practices and specialist diabetes clinics in Leicestershire, United Kingdom Two arms: 1) Control group (control arm) and 2) Education Medication Optimisation ‐ EMO (intervention arm) |
|
| Participants | Control arm N: 95 Intervention arm N: 94 Diabetes type: type 2 Mean age: 61.5 ± 10.5 % Male: NR Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 65 (68), post 58 (69) Intervention arm: pre 80 (85), post 82 (95) 2) Statins, N users (%) Control arm: pre 74 (78), post 74 (88) Intervention arm: pre 77 (82), post 80 (93) 3) Antihypertensives (ACE inhibitor or angiotensin II receptor blockers), N users (%) Control arm: pre 84 (88), post 77 (92) Intervention arm: pre 89 (95), post 86 (100) 4) HbA1c, mean % (SD) Control arm: pre 8.0 (1.6), post 7.9 (NR) Intervention arm: pre 7.9 (1.4), post 7.2 (NR) 5) SBP, mean mmHg (SD) Control arm: pre 136.0 (16.0), post 138.1 (NR) Intervention arm: pre 139.0 (16.0), post 130.0 (NR) 6) DBP, mean mmHg (SD) Control arm: pre 77.0 (12.0), post 76.4 (NR) Intervention arm: pre 76.0 (12.0), post 69.9 (NR) 7) LDL, mean mg/dL (SD) Control arm: pre 85.1 (30.9), post 83.9 (NR) Intervention arm: pre 81.2 (23.2), post 64.6 (NR) 8a) Harms (hypoglycaemic events, grade 1: mild), N (%) Control arm: pre NR (NR), post 31 (35) Intervention arm: pre NR (NR), post 39 (44) 8b) Harms (hypoglycaemic events, grade 2: moderate), N (%) Control arm: pre NR (NR), post 27 (30) Intervention arm: pre NR (NR), post 11 (12) 8c) Harms (hypoglycaemic events, grade 3: severe), N (%) Control arm: pre NR (NR), post 6 (7) Intervention arm: pre NR (NR), post 0 (0) |
|
| Funding source | The MEMO study was funded by a fellowship grant provided by Kidney Research, UK. The study is supported by the NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRC) for Leicestershire, Northamptonshire & Rutland, University Hospitals of Leicester. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Unclear risk | No P values provided. |
| Patient's baseline outcomes (selection bias) | Unclear risk | P values not provided and no in‐text description. |
| Incomplete outcome data (attrition bias) | Low risk | Per‐protocol analysis, baseline based on those randomised. Numbers and reasons for loss to follow‐up provided and seem balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Outcome assessors were blinded. HbA1c measured using liquid chromatography, LDL by Friedwald formula. |
| Selective reporting (reporting bias) | Unclear risk | No data on outcomes. |
| Risk of contamination (other bias) | High risk | Quote: "Since some participants were also recruited from specialist clinic settings, it is conceivable that some participants in the control group would have received aggressive treatment." |
| Other bias | Low risk | Information not available. |
Crowley 2013.
| Study characteristics | ||
| Methods |
The cholesterol, hypertension, and glucose education (CHANGE) study: results from a randomized controlled trial in African Americans with diabetes Patient RCT, conducted in 2 clinics in Durham, NC, USA Two arms: 1. Usual care (control arm) and 2. Nurse intervention (intervention arm) |
|
| Participants | Control arm N: 177 Intervention arm N: 182 Diabetes type: type 2 Mean age: NR ± NR % Male: 28.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 8.0 (0.1), post 7.9 (0.1) Intervention arm: pre 8.0 (0.1), post 7.8 (0.1) 2) SBP, mean mmHg (SE) Control arm: pre 138.8 (0.9), post 134.7 (1.4) Intervention arm: pre 136.8 (0.9), post 137.6 (1.3) 3) LDL, mean mg/dL (SE) Control arm: pre 99.1 (2.2), post 95.5 (2.8) Intervention arm: pre 99.1 (2.2), post 96.5 (2.8) |
|
| Funding source | This research was supported by grants from the Robert Wood Johnson Foundation Disparities Research for Change program and the Kate B. Reynolds Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block‐randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed randomisation assignments in sequentially numbered, opaque, identical envelopes. |
| Patient's baseline characteristics (selection bias) | Unclear risk | They state notable for inadequate literacy and annual income, but do not provide data in table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | ~3% lost to follow‐up in control group; ~6% lost to follow‐up in intervention group, but reasons for losses to follow‐up not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcomes: HbA1c, SBP, LDL, objective laboratory methods not described. |
| Selective reporting (reporting bias) | Low risk | Outcome matches protocol. |
| Risk of contamination (other bias) | High risk | n = 3 in control group mistakenly received the intervention, but analysed in control arm. |
| Other bias | Low risk | Information not available. |
Crowley 2016.
| Study characteristics | ||
| Methods |
Practical telemedicine for veterans with persistently poor diabetes control: a randomized pilot trial RCT (NA clusters and NA providers), conducted in 1) Partnered with Durham Veterans Health Administration (VHA) Home Telehealth (HT) programme nurses to create Advanced Comprehensive Diabetes Care (ACDC). VHA has implemented HT programmes nationwide, for which all veterans with poor diabetes control qualify. 2) Existing VHA clinical staff delivered the intervention. Used 2 HT nurses (A.T.M. or S.K.) and a single physician (M.J.C.) for intervention implementation. If veterans endorsed depressive symptoms, 2 study psychiatrists (J.A.W. or J.Z.) were involved. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (ACDC: Advanced Comprehensive Diabetes Care) (intervention arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 25, NA, NA Diabetes type: 2 Mean age: 60 ± 5.63 % Male: 96 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (ACDC: Advanced Comprehensive Diabetes Care) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Harms |
|
| Funding source | ACDC was supported by grant RRP 12‐458 from the Veterans Affairs Diabetes QUERI. M.J.C. is supported by Career Development Award 13‐261 from the Veterans Affairs Health Services Research and Development. H.B.B. is supported by Research Career Scientist award RCS 08‐027 from the Veterans Affairs Health Service Research and Development. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated, blocked sequence, a research assistant randomised consenting veterans in an unblinded fashion. |
| Allocation concealment (selection bias) | Low risk | Using a computer‐generated, blocked sequence, a research assistant randomised consenting veterans in an unblinded fashion. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Randomisation groups were generally well balanced. More White in ACDC group (52% vs 32% in control group). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Randomisation groups were generally well balanced. |
| Incomplete outcome data (attrition bias) | Low risk | 46 of 50 veterans (n = 23 in each group) completed their 6‐month assessment (Figure 1). Loss of 8% in each group. Low and balanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP. Harms self‐report and objective EHR review. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Addition of BP outcome in paper when comparing to protocol. In addition, authors performed a subgroup analysis of particularly "engaged" vs "non‐engaged" participants to display efficacy of intervention under optimal compliance. |
| Risk of contamination (other bias) | Low risk | Patient RCT but unlikely that control patients used telemedicine system or received calls from Home Telehealth nurses. |
| Other bias | Low risk | None. |
Cummings 2019.
| Study characteristics | ||
| Methods |
Randomized trial of a tailored cognitive behavioral intervention in type 2 diabetes with comorbid depressive and/or regimen‐related distress symptoms: 12‐month outcomes from COMRADE RCT (NA clusters and NA providers), conducted in 1) The study took place in a large academic family medicine practice in the southeastern US that provides primary care to a large rural population. The practice is a training site for residents, medical students and other learners and delivers care to a diverse population of African American and Caucasian patients. 2) The behavioural intervention was delivered by a team of trained behavioural providers working together, including a nurse care manager who provided small changes lifestyle coaching, a psychologist and clinical health psychology doctoral student who provided CBT sessions including elements of problem‐solving therapy (PST) where indicated, and a community health worker (CHW) who provided navigation and social support. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (CBT: cognitive behavioural therapy, plus lifestyle counselling) (intervention arm) |
|
| Participants | Control arm N: 72 Intervention arm N: 67, NA, NA Diabetes type: 2 Mean age: 52.6 ± 6.58 % Male: 22.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (CBT: cognitive behavioural therapy, plus lifestyle counselling ) 1) Case management 2) Team change 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure |
|
| Funding source | The authors acknowledge funding support from East Carolina University | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation used a blocked randomisation process, involving a computer‐generated allocation sequence with allocation concealment from treating providers, with eligible patients randomised in blocks of 4 to the intervention or control group. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. Randomisation used a blocked randomisation process, involving a computer‐generated allocation sequence with allocation concealment from treating providers. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All patient characteristics have P values higher than 0.05. Quote: "Mean age, percent female and percent African American were not significantly different at baseline." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All outcomes (including HbA1c and SPB) have P values higher than 0.05, except for the medication adherence score (P = 0.036). Quote: "There was a difference in self‐reported medication adherence scores at baseline, with the intervention group reporting modestly lower scores (poorer adherence)." |
| Incomplete outcome data (attrition bias) | High risk | 10/72 lost in the control group (14%) and 9/67 in the intervention group (13%). Quite high numbers and the reasons for loss are not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c and SBP were objectively assessed. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. They do not report DBP. Quote: "The principal outcome measure, change in HbA1c, and secondary continuous outcome measures (e.g., changes in RRD score, PHQ‐9 score, weight, and blood pressure) from baseline to 12 months of follow‐up were compared." They do not report hypoglycaemia events. Quote: "Potential for side effects were monitored approximately quarterly by the nurse care manager during face‐to‐face and telephone follow‐up, with particular attention to the potential for hypoglycaemia associated with insulin and sulfonylurea drugs." In the protocol, they wanted to look at 6 and 12 months follow‐up, but they only report 12 months follow‐up in the paper (Table 2). They added medication adherence score, weight and SBP in the paper. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised. The intervention involves many providers from only one clinic. Unclear whether teams worked with both groups. |
| Other bias | Low risk | No evidence of other bias. |
D'Souza 2019.
| Study characteristics | ||
| Methods |
How do multi‐modality strategies affect outcomes in T2D using a randomized control trial? RCT (NA clusters and NA providers), conducted in 1) Intervention delivered in an outpatient clinic in Sultan Qaboos University Hospital. 2) Multi‐modality strategies (MMS) provided by the diabetes nurse educator (DNE). In Oman 2 arms: 1. Control (structured diabetes booklet) (control arm) and 2. Intervention (MMS: multi‐modality strategies) (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 100, NA, NA Diabetes type: 2 Mean age: NR ± 11 % Male: 62.14 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (structured diabetes booklet) 1) Patient education Intervention arm: (MMS: multi‐modality strategies) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This study was supported by College of Nursing, Sultan Qaboos University grant (IG/SQU/CN/14/2) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A stratified block randomisation system was created using Stata's ralloc procedure to generate the blocks and later a random allocation sequence. After the adults were assigned into blocks, a simple randomisation using a random number table was performed within each block to allocate the adults to the intervention and the control group (2 arms). |
| Allocation concealment (selection bias) | Low risk | A computerized minimisation combined with allocations kept in a locked encrypted computer file sequentially numbered was undertaken by a statistician blind to practice identity to conceal randomisation sequence. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. They do not report data for all the patients randomised (only 140/200). However, all demographic characteristics have P values higher than 0.05. Quote: "The experimental and control groups did not significantly differ in demographic and clinical characteristics (Table 1 and Table 2) before the intervention." |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. They do not report data for all the patients randomised (only 140/200). However, all outcomes have P values higher than 0.05. Quote: "The experimental and control groups did not significantly differ in demographic and clinical characteristics (Table 1 and Table 2) before the intervention." |
| Incomplete outcome data (attrition bias) | High risk | They analysed 70 out of 100 patients randomised in each group (30% lost in each group). High numbers and reasons for loss reported and some are not balanced (discontinued intervention/study). They have randomised non‐eligible patients (n = 22). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c and blood pressure were objectively assessed. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol. They added many statistical analysis in the paper (Table 4: ANOVA between experimental and control groups, Table 5: Multivariate general linear model, Table 6: General linear model). Unclear assessment time in the protocol. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised. All patients were followed at the same outpatient clinic in a selected hospital. Looks like the same diabetes nurse educator (DNE) met with all patients. |
| Other bias | Low risk | No evidence of other bias. |
Dai 2018.
| Study characteristics | ||
| Methods |
Application and effect evaluation on Internet + home care mobile APP in patients with type 2 diabetes in young and middle‐age RCT (NA clusters and NA providers), conducted in 1) Patients recruited at the People's Hospital affiliated to Jiangsu University, Jiangsu, 212002, China. 2) The patients in intervention group received the health management by the Internet + diabetes home care mobile APP developed by the project team. Nurses could monitor and manage the data. In China. 2 arms: 1. Control (routine health management) (control arm) and 2. Intervention (Internet + mobile APP health management) (intervention arm) |
|
| Participants | Control arm N: 64 Intervention arm N: 65, NA, NA Diabetes type: 2 Mean age: 38.90 ± 13.16 % Male: 55.81 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (routine health management) Intervention arm: (Internet + mobile APP health management) 1) Case management 2) Electronic patient registry 3) Clinician reminder 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Chinese Nursing Society Technology Program: ZHKY201511 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They used a random number table to generate the sequence. A total of 129 patients with type 2 diabetes in young and middle‐age were randomly divided into intervention group (65 cases) and control group (64 cases). |
| Allocation concealment (selection bias) | Unclear risk | The method to keep the allocation sequence concealed is not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values are higher than 0.05 including mean age, gender and education level. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. Fasting blood glucose, post‐prandial blood glucose, HbA1c and BMI have P values higher than 0.05 between groups at baseline. Table 3. Quality of life is also not significant as well as the outcomes reported in Table 5 (blood glucose). |
| Incomplete outcome data (attrition bias) | Low risk | A total of 129 patients were randomly divided into intervention group (65 cases) and control group (64 cases). In Tables 2‐3‐4‐5, they report data for 62/64 patients in the control group (3.1% lost) and for 64/65 patients in the intervention group (1.5% lost). Low and balanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c and the number of hypoglycaemia events (from a glucose device) were objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Low risk | Patient randomised. Unlikely that patients in the control group had access to the internet/mobile APP. |
| Other bias | Low risk | No evidence of other risk of bias |
Dale 2009.
| Study characteristics | ||
| Methods |
Telephone peer‐delivered intervention for diabetes motivation and support: the telecare exploratory RCT Patient RCT, conducted in general practice clinics in central England, United Kingdom Three arms: 1. Control group (control arm), 2. Peer support (intervention arm 1) and 3. Diabetes specialist nurse (intervention arm 2) |
|
| Participants | Control arm N: 97 Intervention arm 1 N: 90 Intervention arm 2 N: 44 Diabetes type: type 2 Mean age: NR ± NR % Male: 57.4 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Case management 2) Promotion of self‐management Intervention arm 2: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.7 (1.3), post 7.9 (1.1) Intervention arm 1: pre 8.4 (1.1), post 8.0 (1.5) Intervention arm 2: pre 8.9 (1.5), post 7.9 (0.9) |
|
| Funding source | We are grateful to the BUPA Foundation for its funding of this study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available |
| Other bias | Low risk | Information not available. |
Dario 2017.
| Study characteristics | ||
| Methods |
Telemonitoring of type 2 diabetes mellitus in Italy RCT (NA clusters and NA providers), conducted in 1) This RCT was carried out within the Local Health Authority (LHA) of Alto Vicentino, in the Veneto Region, northern Italy, without major changes to the existing organisation. Participants were enrolled during planned specialist visits. The patients randomised to the TM group received home telehealth service. 2) Regional eHealth Center (ReHC) operators and clinicians delivered the intervention. In Italy 2 arms: 1. Control (UC: usual care) (control arm) and 2. Intervention (TM: telemonitoring) (intervention arm) |
|
| Participants | Control arm N: 91 Intervention arm N: 208, NA, NA Diabetes type: 2 Mean age: 73.05 ± NR % Male: 55.78 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (UC: usual care) Intervention arm: (TM: telemonitoring) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The study is co‐founded by the European Commission and 21 partners from 9 European regions in the context of the REgioNs of Europe WorkINg toGether for HEALTH (RENEWING HEALTH) project | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out following the randomisation table generated by PASS 2008 in a proportion of 2:1. |
| Allocation concealment (selection bias) | Low risk | A centralised online system associated the assigned ID to either the intervention or the control group. |
| Patient's baseline characteristics (selection bias) | Low risk | Tables 1 and 2. All P values above 0.05 except Mental Component Summary (MCS, P = 0.03). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | A total of 299 DM patients were randomised, 208 patients in the TM group and 91 patients in the control group. For HbA1c, they have data for 168 (19% lost) and 78 (14%) patients in the TM group and control group at 12 months follow‐up, respectively. High and quite unbalanced numbers. Reasons not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. HbA1c listed as primary outcome in the protocol, but reported as secondary outcome in the paper. Quote paper: "The primary outcome was HRQoL... The secondary outcomes, measured at the start and at the conclusion of the follow‐up period, included HbA1c." |
| Risk of contamination (other bias) | Low risk | Patient‐RCT but unlikely that control patients used the telemonitoring system and had clinicians checked their data through a Home Care portal and took appropriate action. |
| Other bias | Low risk | No evidence of other bias. |
Davidson 2005.
| Study characteristics | ||
| Methods |
The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial RCT (NA clusters and NA providers), conducted in 1) Study was carried out in a Diabetes Managed Care Program (DMCP) at a community clinic associated with King‐Drew Medical Center. 2) Intervention carried out by trained nurses and dieticians. In United States of America. 2 arms: 1. Control (nurse management) (control arm) and 2. Intervention (SMBG and nurse management) (intervention arm) |
|
| Participants | Control arm N: 45 Intervention arm N: 44, NA, NA Diabetes type: 2 Mean age: 50.34 ± NR % Male: 26.14 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (nurse management) 1) Case management 2) Team change 3) Patient education Intervention arm: (SMBG and nurse management) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by grants from Eli Lilly and Company and the NIH (DK 54047) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. All 89 patients consented and were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. *There are no significant differences between the 2 groups (for baseline characteristics). There were no differences in the baseline characteristics of the patients randomised to the monitoring group and those who were randomised to the control group (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. There are no significant differences between the 2 groups (for baseline characteristics: HbA1c and medications). There were no differences in the baseline characteristics of the patients randomised to the monitoring group and those who were randomised to the control group (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | One patient out of 89 was excluded after randomisation. A final A1C level was measured at the end of the study in all of the 78 patients remaining on oral antidiabetes drugs without insulin. HbA1c data for 87.6% (78/89) of patients at the end of intervention (12.4% loss). Numbers of loss in each arm not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). The nurse was unaware of whether the patient was randomised to the monitoring group or not. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. A1C levels were measured at entry into the study and every 2 months. The authors only report HbA1c data at 6 months. |
| Risk of contamination (other bias) | High risk | Both groups received education and counselling from nurse and dietician. Both groups met the dietitian 5 times. All patients were followed by the same specially trained nurse. All patients were part of the Diabetes Managed Care Program (DMCP) intervention. Quote: "These negative findings occurred in a practice in which intensive follow‐up and treatment are the norm, indicating that monitoring does not improve A1C levels in this setting." |
| Other bias | Low risk | None |
Davis 2003.
| Study characteristics | ||
| Methods |
Telemedicine improves eye examination rates in individuals with diabetes: a model for eye‐care delivery in underserved communities Patient RCT, conducted in a rural, federally funded, primary care practice, USA Two arms: 1. Control (control arm) and 2. Telemedicine retinal screening programme ‐ TRSP (intervention arm) |
|
| Participants | Control arm N: 29 Intervention arm N: 30 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: NR months |
|
| Interventions |
Control arm: None Intervention arm: 1) Facilitated relay of clinical information |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 4 (14) Intervention arm: pre NR (NR), post 23 (77) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Risk of contamination (other bias) | Unclear risk | Not reported. |
| Other bias | Unclear risk | Not reported. |
Davis 2010.
| Study characteristics | ||
| Methods |
Telehealth improves diabetes self‐management in an underserved community Patient RCT, conducted in 3 community health centres in northeast South Carolina (a part of FQHCs, which must serve an underserved area/population), USA Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 80 Intervention arm N: 85 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Team changes 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 37 (46), post 31 (39) Intervention arm: pre 44 (52), post 69 (81) 2) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 8.9 (1.8), post 8.6 (0.3) Intervention arm: pre 9.3 (1.9), post 8.2 (0.4) 3) SBP, mean mmHg (pre: SD, post: SE) Control arm: pre 138.5 (19.9), post 130.9 (3.8) Intervention arm: pre 135.3 (21.2), post 127.6 (4.0) 4) DBP, mean mmHg (pre: SD, post: SE) Control arm: pre 74.8 (10.4), post 71.4 (2.2) Intervention arm: pre 76.2 (12.0), post 70.2 (2.2) 5) LDL, mean mg/dL (pre: SD, post: SE) Control arm: pre 107.1 (33.2), post 103.1 (6.8) Intervention arm: pre 108.6 (36.2), post 89.7 (6.9) |
|
| Funding source | Funded by NIH/NIDDK R18DK067312 to R.M.D. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: HbA1c (P = 0.19); LDL (P = 0.78); SBP (P = 0.33); DBP (P = 0.42). |
| Incomplete outcome data (attrition bias) | Unclear risk | Did not provide reasons for losses to follow‐up, "Retention rates at 6 and 12 months were 90.9% and 82.4%, respectively." Did not provide percentage per arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: laboratory methods described for HbA1c. Outcome assessor would not be blinded as she delivers intervention and collects data. Secondary outcome: blood pressure measured using Detecto balance bean scale. |
| Selective reporting (reporting bias) | Low risk | Matches up with protocol registered on clincialtrials.gov. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
De Greef 2010.
| Study characteristics | ||
| Methods |
A cognitive‐behavioural pedometer‐based group intervention on physical activity and sedentary behaviour in individuals with type 2 diabetes Patient RCT, conducted with patients from the Endocrinology Department at a Belgian Hospital (Saint‐Augustinus Hospital in Veurne), Belgium Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm). |
|
| Participants | Control arm N: 21 Intervention arm N: 20 Diabetes type: type 2 Mean age: 61.3 ± 6.5 % Male: 68.0 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.0 (1.3), post 8.0 (1.3) Intervention arm: pre 7.5 (1.1), post 7.9 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 148.6 (21.0), post 144.2 (22.6) Intervention arm: pre 155.1 (25.3), post 148.8 (18.8) 3) DBP, mean mmHg (SD) Control arm: pre 82.6 (11.0), post 70.0 (23.3) Intervention arm: pre 84.4 (9.9), post 75.9 (9.8) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not discuss method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelopes were used and group allocation was concealed until the point of allocation." Opaque envelopes? |
| Patient's baseline characteristics (selection bias) | Low risk | Perceived health status was different between both groups (P = 0.02), but all other characteristics balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those randomised; provide numbers lost to follow‐up but do not provide reasons. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Secondary outcomes: SBP using Omron, HbA1c not described. Quote: "…acknowledged that the trial is not blinded." |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
De Greef 2011.
| Study characteristics | ||
| Methods |
Increasing physical activity in Belgian type 2 diabetes patients: a three arm randomized controlled trial Patient RCT, conducted in 3 Belgian general practices, Belgium Three arms: 1. Control group (control arm), 2. Group counselling (intervention arm 1) and 3. Individual consultation (intervention arm 2) |
|
| Participants | Control arm N: 24 Intervention arm 1 N: 21 Intervention arm 2 N: 22 Diabetes type: type 2 Mean age: 67.4 ± 9.3 % Male: 70.0 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm 2: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.0 (0.9), post 6.9 (0.8) Intervention arm 1: pre 7.1 (1.4), post 6.9 (1.2) Intervention arm 2: pre 7.2 (0.7), post 6.9 (0.6) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…via computerized random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was concealed in sealed enveloped until the point of allocation…". Opaque envelopes? |
| Patient's baseline characteristics (selection bias) | High risk | BMI (P ≤ 0.05), waist circumference (P ≤ 0.01), DBP (P ≤ 0.05). |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up was < 10%, all for medical reasons. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | General practitioners were the ones who collected outcome data and they were not blinded to allocation of treatment arms. HbA1c: Adams haemoglobin A1c procedure. |
| Selective reporting (reporting bias) | High risk | Did not assess SBP, DBP (as stated in protocol) and various other outcomes including DEXA (Dual‐Energy X‐ray Absorptiometry) scan. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
de Vries McClintock 2016.
| Study characteristics | ||
| Methods |
Diabetes and depression care: a randomized controlled pilot trial RCT (NA clusters and NA providers), conducted in 1) Patients with an upcoming appointment from 3 primary care practices in Philadelphia, Pennsylvania were approached. 2) The basic intervention involved the interventionist collaborating with physicians. Three research co‐ordinators (one Master’s level and 2 bachelor’s level) were trained as interventionists and administered all intervention activities. In United States of America. 2 arms: 1. Control (basic: integrated intervention alone) (control arm) and 2. Intervention (enhanced: integrated intervention and patient prioritised planning) (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 37, NA, NA Diabetes type: 2 Mean age: NR ± 11.7 % Male: 24.33 Longest follow‐up: 2.77 months |
|
| Interventions |
Control arm: (basic: integrated intervention alone) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5)Promotion of self‐management Intervention arm: (enhanced: integrated intervention and patient prioritised planning) 1) Case management 2) Team change 3) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. After completion of the initial 2‐week run‐in phase, patients entered phase 2 of the study in which they were randomised to the basic or enhanced intervention. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. Participants in the 2 groups were well matched with regard to baseline demographic characteristics (Table 1). Characteristics of patients in the basic and enhanced interventions did not differ significantly (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. Participants in the 2 groups were well matched with regard to baseline demographic characteristics (Table 1). Characteristics of patients in the basic and enhanced interventions did not differ significantly (Table 1). |
| Incomplete outcome data (attrition bias) | Low risk | Only 2 losses out of 78 (2.6%). Numbers balanced. Reasons for loss to follow‐up not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Both groups received all QI interventions although intervention patients had more intense education and were more empowered. To enable collaboration with the study team, physicians were told which patients were enrolled in the basic or enhanced intervention. Physicians may have changed their care approach. Three research co‐ordinators (one Master’s level and 2 bachelor’s level) were trained as interventionists and administered all intervention activities. Not clear if the same interventionist managed patients in both groups. |
| Other bias | Low risk | No evidence of other bias. |
de Wit 2018.
| Study characteristics | ||
| Methods |
Cost‐effectiveness of the psycho‐educational blended (group and online) intervention HypoAware compared with usual care for people with type 1 and insulin‐treated type 2 diabetes with problematic hypoglycaemia: analyses of a cluster‐randomized controlled trial Clustered RCT (8 clusters and 2 to 3 diabetes healthcare professionals per cluster providers), conducted in 1) 8 self‐selected outpatient diabetes clinics in the Netherlands, 2) 2 or 3 diabetes healthcare professionals per hospital (18 diabetes nurses, 7 medical psychologists and 2 diabetes dieticians). In The Netherlands. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (HypoAware) (intervention arm) |
|
| Participants | Control arm N: 66 Intervention arm N: 71, NA, NA Diabetes type: 3 Mean age: 52.03 ± 11.84 % Male: 53.93 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (HypoAware) 1) Electronic patient registry 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Harms | |
| Funding source | This study was funded by ZonMw (837001406), the Dutch Organization for Health Research and Development and unrestricted co‐funding from Novo Nordisk, Agis Achmea and VU University Medical Centre. All had no further involvement in the study. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation performed by 2 members of the study team, who randomly select notes from 2 opaque envelopes, one with the 8 names of the clinics and one with 4 notes with ‘intervention’ and 4 notes with ‘control’. |
| Allocation concealment (selection bias) | Low risk | Cluster‐randomisation was carried out prior to recruitment of participants at the level of the participating clinics to avoid contamination between treatment groups within the clinics. randomly select notes from 2 opaque envelopes, one with the 8 names of the clinics and one with 4 notes with ‘intervention’ and 4 notes with ‘control’. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. Education levels look to be different, all other characteristics balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Outcomes look balanced. |
| Incomplete outcome data (attrition bias) | High risk | Not reported. We have no records of the number of participants invited. Baseline measurement was completed by 137 participants (Table 1): 66 participants (48%) in the control group and 71 participants (52%) in the intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Self‐report of harms. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol with same outcomes. |
| Risk of contamination (other bias) | Low risk | Cluster‐randomised. |
| Other bias | Low risk | None identified. |
Debussche 2012.
| Study characteristics | ||
| Methods |
Quarterly individual outpatients lifestyle counseling after initial inpatients education on type 2 diabetes: The REDIA Prev‐2 randomized controlled trial in Reunion Island Patient RCT, conducted in 2 endocrinology departments of the Regional Hospital of Reunion Island, Reunion Island Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 192 Intervention arm N: 206 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.3 (2.2), post 8.3 (1.5) Intervention arm: pre 10.0 (2.2), post 8.2 (1.6) 2) SBP, mean mmHg (SD) Control arm: pre 126.0 (15.0), post 139.0 (NR) Intervention arm: pre 126.0 (16.0), post 140.0 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 71.0 (10.0), post 77.5 (NR) Intervention arm: pre 72.0 (9.0), post 79.0 (NR) |
|
| Funding source | Supported by grants from Inserm, and the Regional Council and General Council of Reunion Island | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for sequence generation not provided. |
| Allocation concealment (selection bias) | Unclear risk | Technical envelopes? |
| Patient's baseline characteristics (selection bias) | High risk | Triglycerides and diabetic treatment. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | ~14% lost to follow‐up in N1 and ~26% lost to follow‐up in N2, reasons for dropouts not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective laboratory methods used for HbA1c; blinding of outcome assessor not described. |
| Selective reporting (reporting bias) | High risk | The following were presented in the manuscript but not listed as secondary outcomes in the protocol: fasting blood glucose, lipids, blood pressure, diet and physical activity. |
| Risk of contamination (other bias) | High risk | Lifestyle intervention, patients not blinded, they can go and seek out additional things. |
| Other bias | Low risk | No evidence of other bias. |
Del Prato 2012.
| Study characteristics | ||
| Methods |
Telecare provides comparable efficacy to conventional self‐monitored blood glucose in patients with type 2 diabetes titrating one injection of insulin glulisine‐the ELEONOR Study Patient RCT, an Italian, multi‐centre, parallel‐group RCT, Italy Two arms: 1. Self‐monitored blood glucose (control arm) and 2. Telecare (intervention arm) |
|
| Participants | Control arm N: 149 Intervention arm N: 142 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 11.5 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.9 (1.0), post 8.2 (0.8) Intervention arm: pre 8.8 (0.9), post 8.1 (0.8) |
|
| Funding source | This study was supported by Sanofi‐Aventis | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Quote: "Patient characteristics at screening were comparable in the telecare and conventional Self‐Monitoring of Blood Glucose (SMBG) groups." but not in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "Patient characteristics at screening were comparable in the telecare and conventional Self‐Monitoring of Blood Glucose (SMBG) groups." |
| Incomplete outcome data (attrition bias) | High risk | Not a true intention‐to‐treat analysis (despite being stated, since they had criteria on what was considered an intention‐to‐treat analysis: i.e. had to have at least one follow‐up value, etc.). Numbers and reasons for loss to follow‐up were provided and seem balanced. Baseline based on those analysed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding of patients or outcome assessors was not described. HbA1c methods not described. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias |
Denver 2003.
| Study characteristics | ||
| Methods |
Management of uncontrolled hypertension in a nurse‐led clinic compared with conventional care for patients with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) The study was organized from Whittington Hospital, which serves an inner‐city community of 154,000 adults in North Islington, London. Outpatient nurse‐led hypertension clinic. 2) Hypertension nurse, attending physicians. In United Kingdom. 2 arms: 1. Control (conventional primary care) (control arm) and 2. Intervention (nurse‐led hypertension clinic) (intervention arm) |
|
| Participants | Control arm N: 60 Intervention arm N: 60, NA, NA Diabetes type: 2 Mean age: 60.25 ± 5.5 % Male: 63.34 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (conventional primary care) 1) Clinician education 2) Facilitated relay of clinical information Intervention arm: (nurse‐led hypertension clinic) 1) Case management 2) Team change 3) Promotion of self‐management |
|
| Outcomes | Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Hypertension control |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each of the 3 investigators independently assessed and randomly referred eligible patients from their clinics. Patients were then allocated to conventional primary care (CPC) or the nurse‐led hypertension clinic group on an alternate basis. This scheme prevented individual physicians from predicting the treatment patients would receive, thereby eliminating referral bias and generating equally sized groups. |
| Allocation concealment (selection bias) | Unclear risk | Each of the 3 investigators independently assessed and randomly referred eligible patients from their clinics. Patients were then allocated to conventional primary care (CPC) or the nurse‐led hypertension clinic group on an alternate basis. This scheme prevented individual physicians from predicting the treatment patients would receive, thereby eliminating referral bias and generating equally sized groups. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided, above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided, above 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | The study was completed by 56 (93%) and 59 (98%) patients in the CPC and NLC groups, respectively. Three patients failed to attend the final visit, and one patient died in the CPC group. One patient from the NLC group refused to continue in the study. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c, BP, Htn‐C, medication prescription. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol, methods match outcomes. |
| Risk of contamination (other bias) | Low risk | This scheme prevented individual physicians from predicting the treatment patients would receive, thereby eliminating referral bias and generating equally sized groups. Unlikely that control patients received intervention from hypertension nurse. |
| Other bias | Low risk | None identified. |
DePue 2013.
| Study characteristics | ||
| Methods |
Nurse‐community health worker team improves diabetes care in American Samoa Cluster‐RCT (12 clusters), conducted in Tafuna Clinic of the AS Community Health Centers, Department of Health, AS Government, USA Two arms: 1. Usual care (control arm) and 2. Community health worker (intervention arm) |
|
| Participants | Control arm N: 164 Intervention arm N: 104 Diabetes type: type 2 Mean age: 55.0 ± 12.7 % Male: 38.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Clinician education 2) Patient education Intervention arm: 1) Case management 2) Team changes 3) Clinician education 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.0 (2.3), post 10.0 (2.3) Intervention arm: pre 9.6 (2.1), post 9.3 (2.0) |
|
| Funding source | This project was funded by the National Institute of Diabetes, Digestive, and Kidney Disorders (R18‐DK075371) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Not reported but since cluster, low risk. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Text: alcohol, physical activity, but not provided in table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | 10% lost to follow‐up in usual care group; 9% lost to follow‐up in control group; reasons seem balanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: HbA1c, DCA 2000 Analyzer, SBP using 3 measurements, standard American Heart Association protocol. |
| Selective reporting (reporting bias) | High risk | Some secondary outcomes listed in protocol, not in paper. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Dickinson 2014.
| Study characteristics | ||
| Methods |
Practice facilitation to improve diabetes care in primary care: a report from the EPIC randomized clinical trial Cluster‐RCT (40 clusters), conducted in small to mid‐sized community health centres and independent mixed payer primary care practices in Colorado, USA Three arms: 1. Self‐directed ‐ SD (control arm), 2. Continuous quality improvement ‐ CQI (intervention arm 1) and 3. Reflective adaptive process ‐ RAP (intervention arm 2) |
|
| Participants | Control arm N: 321 Intervention arm 1 N: 189 Intervention arm 2 N: 312 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 18 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm 1: 1) Clinician education 2) Continuous quality improvement Intervention arm 2: 1) Clinician education 2) Continuous quality improvement |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 19 (6), post 40 (12) Intervention arm 1: pre 16 (8), post 34 (18) Intervention arm 2: pre 50 (16), post 70 (22) 2) Foot screening, N screened (%) Control arm: pre 113 (35), post 168 (52) Intervention arm 1: pre 64 (34), post 130 (69) Intervention arm 2: pre 136 (44), post 188 (60) 3) Renal screening (nephropathy), N screened (%) Control arm: pre 78 (24), post 76 (24) Intervention arm 1: pre 35 (19), post 51 (27) Intervention arm 2: pre 119 (38), post 105 (34) |
|
| Funding source | Funding for this work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DF067083) and the National Institute of Mental Health (MH069809‐04) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Not reported, but since cluster, low risk. |
| Provider's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | HbA1c‐ P ≤ 0.01, SBP P ≤ 0.01; note for results they only provide proportion for HbA1c, so not extracted. |
| Incomplete outcome data (attrition bias) | Unclear risk | Random sample of patients from clusters. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary objectives included a composite of outcomes, for the objective outcomes, methods of measurement not described. For the subjective outcomes, blinding of outcome assessors not described. |
| Selective reporting (reporting bias) | High risk | Secondary outcome listed in protocol, but not listed in paper. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Dijkstra 2005.
| Study characteristics | ||
| Methods |
Introduction of diabetes passports involving both patients and professionals to improve hospital outpatient diabetes care Cluster‐RCT (9 clusters with 42 providers), conducted in 9 Dutch general hospitals, the Netherlands Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 750 Intervention arm N: 600 Diabetes type: type 1 and type 2 Mean age: 58.0 ± 15.5 % Male: 48.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Facilitated relay of clinical information 4) Patient education |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 351 (84), post 370 (89) Intervention arm: pre 308 (88), post 330 (94) 2) Foot screening, N screened (%) Control arm: pre 145 (35), post 171 (41) Intervention arm: pre 123 (35), post 183 (52) 3a) Renal screening (creatinine), N screened (%) Control arm: pre 343 (82), post 363 (87) Intervention arm: pre 280 (80), post 298 (85) 3b) Renal screening (renal), N screened (%) Control arm: pre 329 (79), post 343 (82) Intervention arm: pre 238 (68), post 270 (77) 4) HbA1c, mean % (SD) Control arm: pre 8.0 (1.2), post 8.2 (NR) Intervention arm: pre 8.1 (1.3), post 7.8 (NR) 5) SBP, mean mmHg (SD) Control arm: pre 144.9 (21.4), post 144.7 (NR) Intervention arm: pre 143.7 (22.5), post 144.8 (NR) 6) DBP, mean mmHg (SD) Control arm: pre 78.7 (11.0), post 79.7 (NR) Intervention arm: pre 79.9 (10.4), post 79.2 (NR) |
|
| Funding source | This study was supported by a grant from The Netherlands Ministry of Health, Welfare and Sport (Grant number: 68659754527226605897) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report that it was done by someone outside their department. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | The number of beds was higher at the control hospitals and they also had more DSNs. |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. No P values provided; large difference in patient numbers between groups; numbers otherwise were somewhat consistent |
| Patient's baseline outcomes (selection bias) | Unclear risk | No P values reported |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Dijkstra 2008.
| Study characteristics | ||
| Methods |
Implementing diabetes passports to focus practice reorganization on improving diabetes care Cluster‐RCT (40 clusters with 61 providers), conducted in practices in the middle and south regions of The Netherlands Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 1055 Intervention arm N: 1004 Diabetes type: type 2 Mean age: 63.4 ± 9.6 % Male: 49.8 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Facilitated relay of clinical information |
|
| Outcomes | 1) Retinopathy screening (eye exam) 2) Foot screening 3) Renal screening (creatinine) 4a) Controlled hypertension (DBP < 85 mmHg) 4b) Controlled hypertension (SBP < 150 mmHg) |
|
| Funding source | This study was funded by the Netherlands organisation for health research and development (ZONMW grant number 2300 0018) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Data is provided in Table 1 and P values are reported. No significant differences. No report of rural/urban. |
| Patient's baseline characteristics (selection bias) | High risk | The patients in the intervention group were more often women than in the control group. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 3: no P values; looks balanced. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Dinneen 2013.
| Study characteristics | ||
| Methods |
Group follow‐up compared to individual clinic visits after structured education for type 1 diabetes: a cluster randomised controlled trial Clustered RCT (6 clusters and NR providers), conducted in 1) Study participants were recruited from waiting lists of individuals who had expressed an interest in receiving DAFNE training in participating centres. Trial involving patients attending hospital diabetes clinics in Ireland. Intervention delivered in 6 clinics delivering outpatient diabetes care on the island of Ireland (in the Republic and Northern Ireland). 2) Intervention provided by trained doctors and diabetes educators. In Ireland. 2 arms: 1. Control (individual clinic visits) (control arm) and 2. Intervention (booster group follow‐up) (intervention arm) |
|
| Participants | Control arm N: 221 Intervention arm N: 216, NA, NA Diabetes type: 1 Mean age: 40.8 ± 10.2 % Male: 46.2 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (individual clinic visits) 1) Patient education 2) Promotion of self‐management Intervention arm: (booster group follow‐up) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Health Research Board, Ireland, Health Services Research and Development Award. This study was supported by the Health Research Board through a Health Services Research award (HS‐2005‐25). Meetings of the study steering group and 3 annual meetings of the Irish DAFNE Study team were funded by Novo Nordisk Ireland. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (by a computer‐generated numbers list) was undertaken by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Clustered‐RCT. Randomisation was undertaken by an independent statistician. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Supplementary Table 1 provides baseline characteristics of participating centres, but we do not know to which arm was assigned each centres. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Characteristics look balanced between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Outcomes balanced between groups. |
| Incomplete outcome data (attrition bias) | High risk | At 18 months (figure 1), they have HbA1c data for 150 out of 216 patients randomised in the intervention arm (30.6% lost) and 169/221 in the control arm (23.5% lost). High and unbalanced numbers. Missing data were greater than expected. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective (HbA1c) and subjective outcomes (self‐reported hypoglycaemia, but secondary outcome). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Secondary outcomes included weight, blood pressure, lipid levels and rates of severe hypoglycaemia. Likewise no significant change was seen over time in blood pressure or lipid levels (data not shown). Many secondary outcomes were added to the protocol after the end of patient recruitment. |
| Risk of contamination (other bias) | Low risk | Clustered RCT, but all participants received DAFNE at baseline (involves patient education and promotion of self‐management). |
| Other bias | Low risk | No evidence of other bias. |
Dobson 2018.
| Study characteristics | ||
| Methods |
Effectiveness of text message based, diabetes self management support programme (SMS4BG): two arm, parallel randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Participants were referred to the study by healthcare professionals at their primary and secondary care centres across New Zealand. Additionally, participants could self‐refer to the study. 2) Text message‐based, diabetes self‐management support programme (SMS4BG) delivered by a specifically designed automated content management system. In New Zealand. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (SMS4BG: self‐management support for blood glucose by text message) (intervention arm) |
|
| Participants | Control arm N: 183 Intervention arm N: 183, NA, NA Diabetes type: 3 Mean age: 47 ± NR % Male: 51.5 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (SMS4BG: self‐management support for blood glucose by text message) 1) Electronic patient registry 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The development of SMS4BG was funded by Waitemata District Health Board. The randomised controlled trial was funded by the Health Research Council of New Zealand in partnership with the Waitemata District Health Board and Auckland District Health Board (through the Research Partnerships for New Zealand Health Delivery initiative), and the New Zealand Ministry of Health. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated by computer program using variable block sizes of 2 or 4, and overseen by the study statistician. |
| Allocation concealment (selection bias) | Low risk | The research assistant randomised the participant to intervention or control, using the REDCap randomisation module. The REDCap randomisation module ensured that treatment allocation was concealed until the point of randomisation. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. Baseline characteristics data reported for all patients randomised, but no P values. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. Data reported for HbA1c for 354/366 patients randomised, but no P values. |
| Incomplete outcome data (attrition bias) | Low risk | They included a total of 356/366 patients in the final analysis (2.7% lost), 175/183 in the intervention group (4.4% lost) and 181/183 in the control group (1.1%). Quite unbalance numbers but still very low. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective primary outcome (HbA1c) was measured by blinded assessors throughout the study period. HbA1c blood tests (at baseline, 3, 6 and 9 months) were undertaken through standard care and results obtained through medical records. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. In Table 4, they report all items for the SDSCA = summary of diabetes self‐care activities, except for smoking behaviours. In the protocol, most outcome time points were at baseline and 9 months, but they also reported 3 and 6 months in the paper. Protocol: change in HbA1c as measured by blood test at 2 years (not reported in the paper). |
| Risk of contamination (other bias) | Low risk | Patient randomised. Unlikely that patients in the control group received SMS and had access to the password protected website. |
| Other bias | Low risk | No evidence of other bias. |
Donohoe 2000.
| Study characteristics | ||
| Methods |
Improving foot care for people with diabetes mellitus‐‐a randomized controlled trial of an integrated care approach Clustered RCT (10 clusters and 150 providers), conducted in 1) Practices from 10 towns drawn from mid and east Devon, United Kingdom. 2) A primary care team (general practitioners, practice and district nurses and chiropodists) delivered the intervention. Education of the whole primary care team was provided by one member of the foot care team. In United Kingdom. 2 arms: 1. Control (comparison group) (control arm) and 2. Intervention (integrated foot care approach) (intervention arm) |
|
| Participants | Control arm N: 958 Intervention arm N: 981, NA, NA Diabetes type: 3 Mean age: 65.76 ± 12.50 % Male: 53.51 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (comparison group) 1) Clinician education Intervention arm: (integrated foot care approach) 1) Case management 2) Clinician education 3) Patient education |
|
| Outcomes | Foot screening | |
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. A pragmatic randomised controlled study was undertaken with matched cluster‐randomisation of practices from 10 towns drawn from mid and east Devon. Practices were randomised either to the intervention group for delivery of the integrated care model, or to the comparative control group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Provider's baseline characteristics (selection bias) | Low risk | Table 2. They provided mean list size and mean number of partners in each group. Ten practices were matched on the basis of potential major general confounding variables (practice location, urbanity, distance to district general hospital, list size, number of partners). P values above 0.05. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. P values provided and above 0.05. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. They do not report foot screening data at baseline. HbA1c is not significant. |
| Incomplete outcome data (attrition bias) | High risk | They have foot examination data for 642/958 (33% lost) for the control group and 652/981 (34% lost) for the intervention at 1‐year follow‐up. High attrition rate. One potential problem of the study is the large number of incomplete questionnaires, approximately 40%. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | They asked to patients using a questionnaire: "Were your feet examined at annual review?" (self‐reported, subjective outcome). However, everyone appears to be blinded to the study hypothesis (alternative educational package about diabetic nephropathy was given to control group). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. They do not report foot screening data at baseline (secondary outcome). |
| Risk of contamination (other bias) | Low risk | Clustered RCT. The control group of practices continued with their current foot care arrangements but also received a practice visit where an alternative educational package (diabetic nephropathy) was given. Even if no information was given about foot care to the control group, the health professionals might have changed their approach about all diabetes care aspects. Increase in knowledge was seen in patients from both intervention and control practices. |
| Other bias | Low risk | No evidence of other bias. |
Doucette 2009.
| Study characteristics | ||
| Methods |
Community pharmacist‐provided extended diabetes care Patient RCT, conducted with adults who had completed 2 diabetes education sessions at a local diabetes education centre, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 42 Intervention arm N: 36 Diabetes type: type 2 Mean age: 60.0 ± 12.0 % Male: 57.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Clinician education 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.9 (1.9), post 8.0 (NR) Intervention arm: pre 8.0 (1.5), post 7.7 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 119.8 (17.6), post 124.3 (NR) Intervention arm: pre 118.2 (11.7), post 125.3 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 67.1 (8.3), post 67.4 (NR) Intervention arm: pre 66.6 (8.9), post 67.8 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 105.1 (36.6), post 93.1 (NR) Intervention arm: pre 100.7 (28.0), post 81.1 (NR) |
|
| Funding source | This study was supported through a grant from the Community Pharmacy Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Duran 2008.
| Study characteristics | ||
| Methods |
Family physician and endocrinologist coordination as the basis for diabetes care in clinical practice Patient RCT, conducted in St Carlos Hospital, Spain Two arms: 1. Group A (control arm) and 2. Group B (intervention arm) |
|
| Participants | Control arm N: 63 Intervention arm N: 63 Diabetes type: type 2 Mean age: 70 (range: 57 to 76) % Male: 70.8 Longest follow‐up: 30 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Clinician education |
|
| Outcomes | 1) HbA1c, median % (SD) Control arm: pre 7.2 (1.5), post 7.3 (1.0) Intervention arm: pre 7.2 (2.0), post 7.1 (1.4) 2) SBP, median mmHg (SD) Control arm: pre 140.0 (14.8), post 130.0 (7.4) Intervention arm: pre 145.0 (20.7), post 135.0 (19.3) 3) DBP, median mmHg (SD) Control arm: pre 80.0 (8.9), post 76.0 (6.7) Intervention arm: pre 85.0 (10.4), post 78.0 (8.2) 4) LDL, median mg/dL (SD) Control arm: pre 104.0 (30.4), post 78.0 (12.6) Intervention arm: pre 107.0 (36.3), post 81.0 (20.0) 5a) Controlled hypertension (DBP < 80 mmHg), N under control (%) Control arm: pre 30 (53), post 47 (82) Intervention arm: pre 27 (46), post 51 (86) 5b) Controlled hypertension (SBP < 130 mmHg), N under control (%) Control arm: pre 12 (21), post 29 (51) Intervention arm: pre 14 (24), post 25 (42) 6) Smoking cessation, N smokers (%) Control arm: pre 11 (19), post 7 (12) Intervention arm: pre 11 (19), post 7 (12) |
|
| Funding source | The foot care programme was partially supported by grants from the European Union, Sociedad Española de Endocrinología y Nutrición, Fundación Fernandez Cruz and Fundación del Servicio de Endocrinologia y Nutrición | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1: no P values reported, but looks balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | See Table 2, P values > 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 6 lost in group A (9.5%) and 4 lost in group B (6%), reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measurement of outcomes. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered; apolipoprotein A1, apolipoprotein B and bodyweight not reported. |
| Risk of contamination (other bias) | Low risk | Group A treated at hospital and Group B treated at primary healthcare centre; unlikely to have received the same treatment. |
| Other bias | Low risk | No other evidence of risk of bias. |
Döbler 2018.
| Study characteristics | ||
| Methods |
Telephone‐delivered lifestyle support with action planning and motivational interviewing techniques to improve rehabilitation outcomes RCT (NA clusters and NA providers), conducted in 1) Patients were recruited at the Niederrhein‐Klinik (Bad NeuenahrAhrweiler, Germany), a rehabilitation centre specialising in diabetes care. 2) Telephone follow‐up counselling and support delivered by 2 counsellors (trained non‐medical dieticians). In Germany 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (telephone follow‐up counselling and support) (intervention arm) |
|
| Participants | Control arm N: 126 Intervention arm N: 123, NA, NA Diabetes type: 2 Mean age: 52 ± 15 % Male: 70 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (telephone follow‐up counselling and support) 1) Case management 2) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The study was supported by refonet—the Rehabilitations‐Forschungsnetzwerk of the Deutsche Rentenversicherung Rheinland (FKZ: 05006) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised patients externally using a computer‐generated random number table (Randlist, the Institute for Community Medicine of the Ernst‐Moritz‐Arndt‐University, Greifswald, Germany) and stratified for gender. |
| Allocation concealment (selection bias) | Low risk | Intervention delivery staff requested allocation by fax and were blinded to the allocation procedure. Randomised patients externally using a computer‐generated random number table (Randlist, the Institute for Community Medicine of the Ernst‐Moritz‐Arndt‐University, Greifswald, Germany). |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. They only provide data on 101/126 patients in the control group and 98/123 in the intervention group. All sociodemographic characteristics have P values higher than 0.05. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. They only provide data on 101/126 patients in the control group and 98/123 in the intervention group. All outcomes have P values higher than 0.05, except that marginal significant differences were observed in their general health status and cardiovascular risk at baseline (P = 0.05). |
| Incomplete outcome data (attrition bias) | High risk | They included 101/126 patients in the control group (20% lost) and 98/123 (20% lost) in the intervention group in the analysis. Balanced but high numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | HbA1c was subjectively measured. Quote: "The use of self‐report measures as outcomes can lead to over or underreporting, especially for weight and HbA1c... the timespan between HbA1c testing and self‐report at one of our assessments varied." Quote: "Because of the design of our intervention, participants and study counselors were not blinded as to the randomization status." |
| Selective reporting (reporting bias) | Unclear risk | No published or registered protocol. They report the cardiovascular risk after the intervention but they do not report the SBP used to calculate it. Both outcomes are reported at baseline. They do not report data on smoking behaviour after the intervention (only OR). |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that control patients received calls from the counsellors. |
| Other bias | Low risk | No evidence of other bias. |
Eakin 2013.
| Study characteristics | ||
| Methods |
Six‐month outcomes from Living Well with Diabetes: A randomized trial of a telephone‐delivered weight loss and physical activity intervention to improve glycemic control Patient RCT, conducted with patients recruited from nine general practices in city of Logan, a largely ethnically and socio‐economically diverse community in the state of Queensland, Australia Two arms: 1. Usual care (control arm) and 2. Telephone counselling (intervention arm) |
|
| Participants | Control arm N: 151 Intervention arm N: 151 Diabetes type: type 2 Mean age: 58.0 ± 8.6 % Male: 56.3 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.5 (1.7), post 7.5 (1.6) Intervention arm: pre 7.4 (1.5), post 7.5 (1.7) |
|
| Funding source | This study was supported by a National Health and Medical Research Council (NHMRC) project grant and a Diabetes Australia Research Trust grant. Eakin is supported by a NHMRC Senior Research Fellowship; Reeves is supported by a NHMRC Postdoctoral Training Fellowship; Winkler is supported by Queensland Health core infrastructure funding; Healy is supported by a NHMRC/National Heart Foundation of Australia Postdoctoral Fellowship; Dunstan is supported by a VicHealth Public Health Research Fellowship; Owen is supported by a NHMRC Senior Principal Research Fellowship; Marshall is supported by a NHMRC Career Development Award. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation. |
| Allocation concealment (selection bias) | Unclear risk | Minimisation was used; technically you would know to which arm patient was enrolled if based on prognostic variables. |
| Patient's baseline characteristics (selection bias) | Unclear risk | They compare baseline to another study: the AusDiab study. |
| Patient's baseline outcomes (selection bias) | Unclear risk | They compare baseline to another study: the AusDiab study. |
| Incomplete outcome data (attrition bias) | High risk | ~12.6% lost to follow‐up in intervention group; ~7.3% lost to follow‐up in control group. Unbalanced in numbers per group per reason. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: HbA1c. Whole blood samples by high performance liquid chromatography method. |
| Selective reporting (reporting bias) | High risk | Some items do not match protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Earle 2010.
| Study characteristics | ||
| Methods |
Mobile telemonitoring for achieving tighter targets of blood pressure control in patients with complicated diabetes: a pilot study RCT (NA clusters and NA providers), conducted in 1) The study was based at the Thomas Addison Unit, St. George’s Hospital, South London, UK, which serves an inner‐city population characterised by a diverse ethnic mix ‐ 22% of residents belong to a non‐white minority ethnic group ‐ with a social deprivation score that is higher than the national average (see www.capitalambition.gov.uk/documents). 2) Clinicians received patients' data in real‐time and using a web‐based application provided management advice to the patient and their physicians. In United Kingdom. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (m‐Health group) (intervention arm) |
|
| Participants | Control arm N: 65 Intervention arm N: 72, NA, NA Diabetes type: 3 Mean age: 58.41 ± 9.55 % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (m‐Health group) 1) Case management 2) Electronic patient registry 3) Clinician education 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | The authors would like to acknowledge the financial and technical support from the IDEN Group, Motorola Inc., USA and the Motohealth team in the United Kingdom. This study was funded by Motorola Inc., USA. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to the m‐Health group (MH group) or usual care group (UC group) according to a computer‐generated random number sequence (Stat MateTM version 1.01i, GraphPad, San Diego, CA). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values higher than 0.05. The groups were well matched according to their demographic characteristics (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values higher than 0.05. The groups were well matched according to their baseline blood pressure, diabetes control determined by the HbA1c, renal function and lipid profile (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | 26 out of 72 (36%) patients defaulted in the MH group compared with 16 out of 65 (25%) from the control group at 6 months. High and unbalanced numbers. In each group, 29 patients had a record of diabetic retinopathy. The higher than expected default rate in the intervention arm was largely the result of technological problems with the use of the hardware and the patient’s ability to transmit data. In particular, patients reported unreliability of the equipment and that the troubleshooting support systems were not intuitive. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (blood pressure). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol. Results match protocol for blood pressure outcome, but they added an analysis based on racial heritage. HbA1c was previously published in reference 8. |
| Risk of contamination (other bias) | Low risk | Patients allocated to the UC group did not receive any mHealth equipment. They were not required to report their blood pressure and did not receive any support from the research nurses. All of their management was provided by their local practitioners who were not involved in the study. |
| Other bias | Low risk | No evidence of other risk of bias. |
Eccles 2007.
| Study characteristics | ||
| Methods |
A pragmatic cluster randomised controlled trial of a Diabetes REcall And Management system: the DREAM trial Cluster‐RCT (58 clusters with 58 providers), conducted in three Primary Care Trusts in the northeast of England, United Kingdom Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 1934 Intervention arm N: 1674 Diabetes type: type 2 Mean age: 66.0 ± 11.5 % Male: 53.0 Longest follow‐up: 15 months |
|
| Interventions |
Control arm: 1) Audit and feedback 2) Electronic patient registry Intervention arm: 1) Audit and feedback 2) Electronic patient registry 3) Clinician reminders 4) Patient reminders |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 10 (1), post 164 (8) Intervention arm: pre 34 (2), post 308 (18) 2) Antihypertensives (any), N users (%) Control arm: pre 118 (6), post 274 (14) Intervention arm: pre 131 (8), post 415 (25) 3) Retinopathy screening (fundoscopy), N screened (%) Control arm: pre 957 (49), post 977 (51) Intervention arm: pre 721 (43), post 1014 (61) 4) Foot screening, N screened (%) Control arm: pre 892 (46), post 944 (49) Intervention arm: pre 804 (48), post 1127 (67) 5) Renal screening (creatinine), N screened (%) Control arm: pre 928 (48), post 1168 (60) Intervention arm: pre 887 (53), post 1229 (73) 6) HbA1c, mean % (SD) Control arm: pre 7.6 (NR), post 7.4 (NR) Intervention arm: pre 7.8 (NR), post 7.3 (NR) 7) SBP, mean mmHg (SD) Control arm: pre 144.5 (NR), post 144.6 (NR) Intervention arm: pre 145.8 (NR), post 144.2 (NR) 8) DBP, mean mmHg (SD) Control arm: pre 80.2 (NR), post 78.1 (NR) Intervention arm: pre 79.2 (NR), post 77.8 (NR) 9) Smoking cessation, N smokers (%) Control arm: pre 373 (19), post 379 (20) Intervention arm: pre 347 (21), post 358 (21) |
|
| Funding source | This study was funded by Diabetes UK, and Northern and Yorkshire Regional NHS R&D Office | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Low risk | Table 1 shows the baseline characteristics of control and intervention practices and patients. None of the differences in these variables between the intervention and control group are statistically significant. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 shows the baseline characteristics of control and intervention practices and patients. None of the differences in these variables between the intervention and control group are statistically significant. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. No P values provided; clinical comparisons appear similar. There was text stating that baseline comparisons between groups were in additional file 2 but the file was not accessible. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Process measures. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT. |
| Other bias | Low risk | Information not available. |
Edelman 2015.
| Study characteristics | ||
| Methods |
Nurse‐led behavioral management of diabetes and hypertension in community practices: a randomized trial RCT (NA clusters and NA providers), conducted in 1) Patients receiving care in 9 primary care practices (community fee‐for‐service) in the Duke Clinical Research Institute Primary Care Research Consortium (PCRC). Practices comprised both physician and mid‐level primary care providers, trained in either general internal medicine or family medicine, and located in either urban or rural settings. 2) Intervention provided by nurses. In United States of America. 2 arms: 1. Control (non‐tailored phone calls) (control arm) and 2. Intervention (tailored phone calls from nurses) (intervention arm) |
|
| Participants | Control arm N: 184 Intervention arm N: 193, NA, NA Diabetes type: 2 Mean age: 58.7 ± 9.64 % Male: 45.4 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (non‐tailored phone calls) Intervention arm: (tailored phone calls from nurses) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure |
|
| Funding source | This research was supported by grant number R01DK074672 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated randomisation sequence maintained by the study data manager at a site separate from the site of patient enrollment (stated in reference 12 of the paper). |
| Allocation concealment (selection bias) | Low risk | The randomisation sequence was integrated with the study tracking database; once an eligible patient was enrolled in the study and baseline assessment was completed, the data manager could populate the randomisation field in the tracking database with the patient’s group assignment by clicking a button. The data manager then contacted patients by phone with their randomisation assignment (stated in reference 12 of the paper). |
| Patient's baseline characteristics (selection bias) | Low risk | Patient characteristics in the intervention and control arms were similar at baseline (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Patient characteristics in the intervention and control arms were similar at baseline (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | 78% of patients completed the 12‐month follow‐up, and 263 patients (70 %) reached the primary 24‐month endpoint. 30% lost. Reasons reported and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c and SBP). Study staff responsible for data collection remained blinded to randomisation assignments throughout the study. Primary care providers also remained blinded to patient randomisation status, unless a patient chose to reveal his/her assignment. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted in September 2009 before data analysis, recruitment began on June 2009, 2 years intervention). All outcomes of interest are reported. |
| Risk of contamination (other bias) | Unclear risk | A single nurse with extensive experience in case management delivered both the tailored behavioural intervention and the attention control. Calls content was tightly scripted, designed to limit the potential for productive interaction between nurse and patient, and was informed by standard guidelines as stated on government websites. Used an attention control; most previous studies of disease management for DM and/or HTN have used usual care controls (including their previous studies in HTN). The reason for attention controls is precisely the concern that contact time with the patient, independent of content of intervention, may be a potent intervention, and theoretically this could explain the A1c result (decreased in control). |
| Other bias | Low risk | No evidence of other bias. |
Egede 2017.
| Study characteristics | ||
| Methods |
Randomized controlled trial of technology‐assisted case management in low income adults with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) The participants were recruited from 8 community‐based adult medicine primary care practices within the Franklin C. Fetter Family Health Centers, Inc., South Carolina, USA ‐downtown Charleston, Summerville Health Center, Low Country Pediatrics and Adults, Enterprise Health Center, Cross Health Center, Hollywood, Walterboro, and John’s Island. 2) Intervention delivered by a full‐time registered nurse using the FORA 2‐in‐1 telehealth system for Technology assisted Case Management (TACM). The nurse case manager was supervised by an internist and an endocrinologist. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (TACM: technology‐assisted case management) (intervention arm) |
|
| Participants | Control arm N: 59 Intervention arm N: 54, NA, NA Diabetes type: 2 Mean age: 54.2 ± 10.34 % Male: 18.6 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (TACM: technology‐assisted case management) 1) Case management 2) Team change 3) Electronic patient registry 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This work was supported by Grant No. W81XWH‐10‐2‐0057 from the Department of Defense | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The nurse case manager verified inclusion and exclusion criteria for all participants before randomisation (1:1) to one of the 2 study groups. The randomisation was performed in waves such that about 50 participants were randomised every 6 months. The randomisation sequence was web‐based computer‐generated and was accessible to the nurse case manager, but remained confidential to all study sites. |
| Allocation concealment (selection bias) | Low risk | The randomisation sequence was web‐based computer‐generated and was accessible to the nurse case manager, but remained confidential to all study sites. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. Quote: "There were no significant differences between groups in demographic characteristics." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All outcomes reported have P values above 0.05 including HbA1c, BMI and smoking. |
| Incomplete outcome data (attrition bias) | High risk | There were 113 patients at baseline, 87 (23% lost) at 3 months and 85 (25% lost) at 6 months that had complete HbA1c measurements. Among the 85 participants that had complete HbA1c measurements at 6 months, 41/54 (24% lost) were in TACM and 44/59 (25%) in the usual care group. Balanced but high number of lost. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. This article is focused on reporting the results of the primary outcome analyses (HbA1c) as indicated in our protocol article. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised. It is unlikely that control patients used the FORA system. However, the nurse could have been involved in the care of patients from both groups as clinic nurses were used to follow‐up on any problematic patients. Quote: "We did not control for attention in the intervention group; therefore, it is reasonable to suggest that while diabetes education and skills training were not directly provided to the patients in the control group, support of any kind from the nurse case manager may have influenced behaviors that resulted in improved glycaemic control." |
| Other bias | Low risk | No evidence of other bias. |
Ell 2010.
| Study characteristics | ||
| Methods |
Collaborative care management of major depression among low‐income, predominantly Hispanic subjects with diabetes Patient RCT, conducted in 2 public safety net clinics, USA Two arms: 1. Enhanced usual care (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 194 Intervention arm N: 193 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 18 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.1 (2.2), post 8.5 (2.2) Intervention arm: pre 9.0 (2.2), post 8.3 (2.0) |
|
| Funding source | The study is supported by R01 MH068468 from the National Institute of Mental Health (principal investigator, K.E.) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted via a computer‐generated random number in blocks of 10…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…with random assignment number enclosed in sealed envelopes: patients selected one of five sequential envelopes following baseline interviews." Opaque envelopes? |
| Patient's baseline characteristics (selection bias) | High risk | P ≤ 0.05: living in USA (years), Spanish speaking, socioeconomic stress, Whitty‐9 diabetes symptoms, chronic pain, pain medications, depression, dysthymic disorder, history of major depression |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c: P = 0.98 |
| Incomplete outcome data (attrition bias) | High risk | ~30% lost to follow‐up in control; ~25% lost to follow‐up in intervention, reasons seems balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome was the 20‐item symptom checklist. Unclear if outcome assessor was blinded. Primary outcome was also Hba1c: derived from medical records. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Quote: "Because the same practitioners treated both intervention and Enhanced Usual Care (EUC) patients, there may have also been a spillover effect on quality of depression treatment." |
| Other bias | Low risk | Information not available. |
Emerson 2016.
| Study characteristics | ||
| Methods |
A multidisciplinary intervention utilizing virtual communication tools to reduce health disparities: a pilot randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Two Carolinas Healthcare System (CHS) safety‐net clinics in Mecklenburg County, NC. 2) The intervention involved multidisciplinary primary care (consisting of a primary care physician, social worker, pharmacist and behavioural therapist) utilising health coach‐facilitated virtual visits and cloud‐based glucose monitoring, called Carolinas Partners. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Carolinas Partners with virtual communication tools) (intervention arm) |
|
| Participants | Control arm N: 5 Intervention arm N: 5, NA, NA Diabetes type: 4 Mean age: 48.2 ± 8.4 % Male: 60 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Carolinas Partners with virtual communication tools) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Unfunded pilot study. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. All patients meeting the initial inclusion criteria after a query of the data available through the electronic medical record data warehouse underwent pre‐consent randomisation to either control or intervention groups. These groups were then stratified into 4 geographically‐defined regions within Mecklenburg County. Randomisation was completed by the data analytics department at Carolinas Healthcare System, and study personnel were not involved in the randomisation process. Regions were included in the randomisation to demonstrate the method of tailoring future interventions aimed at health disparities at the neighbourhood level, a component that would be vital in a larger study. |
| Allocation concealment (selection bias) | Low risk | "Randomization was completed by the data analytics department at Carolinas Healthcare System and study personnel were not involved in the randomization process." |
| Patient's baseline characteristics (selection bias) | Low risk | No P value reported. Nothing mentioned in text. Only 5 patients per arm; relatively balanced |
| Patient's baseline outcomes (selection bias) | Low risk | No P value reported. Nothing mentioned in text. Only 5 patients per arm; relatively balanced |
| Incomplete outcome data (attrition bias) | High risk | Four out of 5 patients completed the follow‐up in each arm (20% loss). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | High risk | No registered protocol or previously published protocol. They measured blood pressure but they do not report the results (quote: All participants attended an initial and final in‐person visit to measure haemoglobin A1C, blood pressure and psychosocial parameters through survey. Blood pressures were recorded as the average of the 3 readings on an automated cuff). |
| Risk of contamination (other bias) | Low risk | Patients were followed by a multidisciplinary team only seeing patients in the intervention arm. |
| Other bias | Low risk | No evidence of other bias. |
Esmatjes 2014.
| Study characteristics | ||
| Methods |
The efficiency of telemedicine to optimize metabolic control in patients with type 1 diabetes mellitus: Telemed study RCT (NA clusters and NA providers), conducted in 1) Patients were invited to participate on attending routine clinical outpatient appointments in 5 hospitals in Spain. Intervention was delivered at hospital and using a telemedicine system. 2) Telemedicine follow‐up was delivered by hospital diabetes team and investigators. In Spain 2 arms: 1. Control (face‐to‐face appointments) (control arm) and 2. Intervention (face‐to‐face and telematic appointments) (intervention arm) |
|
| Participants | Control arm N: 76 Intervention arm N: 78, NA, NA Diabetes type: 1 Mean age: 31.85 ± NR % Male: 44.87 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (face‐to‐face appointments) 1) Promotion of self‐management Intervention arm: (face‐to‐face and telematic appointments) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | "This work was supported by the Centro de Investigacion Biomedica en Red de Diabetes y Enfermedades Metabolicas Asociadas (ISCIII, Ministerio de Ciencia e Innovacion). We thank to Ferran Torres for statistical support. Devices, strips, and logbook for self‐monitoring blood glucose were supported by Menarini Diagnostics, Firenze, Italy." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prior to the trial, a blinded computer‐based, non‐clustered (by centre) prespecified randomisation list was created. Patients were electronically randomised. |
| Allocation concealment (selection bias) | Low risk | After inclusion of participants, physicians allocated patients to either group after calling a centralised number. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All outcomes have P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Significant differences at baseline for Insulin dosage (P = 0.001) and adherence to self‐care (P = 0.024) between groups. |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. They lost 12/76 (16%) patients in the control group and 24/78 (31%) in the intervention group. High and unbalanced numbers. Reasons unbalanced as 11 patients had connection problem in the intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c was objectively assessed but hypo‐ and hyper‐ glycaemic events were objectively and subjectively assessed. Quote: "hypoglycemic episodes were obtained from patients' logbooks (self‐reported) and the memory of the glucose meters (objective). Mild events were defined as signs or symptoms associated with hypoglycemia experienced by the patient (self‐reported)." |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. They were supposed to assess HbA1c and hypo/hyperglycaemic events at 12 months, and not at 6 months as reported in the paper. |
| Risk of contamination (other bias) | Unclear risk | Only the patients in the intervention group had access to the telemonitoring system. However, the usual physicians allocated patients to either group after calling a centralised number, and they were involved in delivering the intervention and to follow control patients. |
| Other bias | Low risk | No evidence of other risk of bias. |
Estrada 2011.
| Study characteristics | ||
| Methods |
A web‐based diabetes intervention for physician: a cluster‐randomized effectiveness trial Cluster‐RCT (205 clusters with 205 providers), conducted with family, general and internal medicine physicians in rural areas of 11 southeastern USA (Alabama, Arkansas, Florida, Georgia, Kentucky, Mississippi, Missouri, North Carolina, South Carolina, Tennessee and West Virginia), USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 467 Intervention arm N: 715 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Audit and feedback 2) Clinician education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.3 (2.3), post 7.5 (1.8) Intervention arm: pre 7.4 (2.3), post 7.4 (1.8) 2) SBP, mean mmHg (SD) Control arm: pre 135.8 (21.9), post 134.5 (19.8) Intervention arm: pre 132.1 (17.3), post 132.6 (18.7) 3) DBP, mean mmHg (SD) Control arm: pre 77.6 (13.0), post 77.9 (10.5) Intervention arm: pre 77.9 (NR), post 76.5 (11.3) 4) LDL, mean mg/dL (SD) Control arm: pre 96.7 (46.9), post 93.7 (39.2) Intervention arm: pre 97.5 (50.2), post 94.2 (36.1) 5a) Controlled hypertension (< 140/90 mmHg), N under control (%) Control arm: pre 262 (56), post 275 (59) Intervention arm: pre 450 (63), post 297 (62) 5b) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 121 (26), post 135 (29) Intervention arm: pre 222 (31), post 153 (32) 6) Smoking cessation, N smokers (%) Control arm: pre 55 (12), post 53 (11) Intervention arm: pre 85 (12), post 63 (13) |
|
| Funding source | Awards by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 5R18DK065001 to Dr J.J.A. Drs A.H.S and C.A.E. were supported by the Veterans Affairs National Quality Scholars Program. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how they generated their sequence. |
| Allocation concealment (selection bias) | Low risk | Unit of analysis: cluster. Quote: "..block size of four was concealed to the investigators and statisticians." |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not provided. |
| Patient's baseline characteristics (selection bias) | Low risk | Age (P = 0.02), race (P = 0.001). |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not provided. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "All analyses followed the Intention‐to‐treat principle". Although not sure they followed this correctly, since the numbers analysed does not match those randomised. Also, there was a large numbers for attrition, and number of practices dropping out were not equal, and the reasons for doing so in the intervention group, i.e. too busy, may have been related to outcomes. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: BP control, outcome assessors were blinded. Quote: "Data abstraction was performed by trained personnel on blinded records sent to the study center (or abstracted on site)" Secondary outcome: HbA1c, LDL, smoking; methods not described, however outcome assessor blinded. |
| Selective reporting (reporting bias) | High risk | Checked protocol, and secondary outcomes listed in protocol do not match those listed in the manuscript. And what were listed as primary were actually secondary in the manuscript. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias. |
Fairall 2016.
| Study characteristics | ||
| Methods |
Educational outreach with an integrated clinical tool for nurse‐led non‐communicable chronic disease management in primary care in South Africa: a pragmatic cluster randomised controlled trial Clustered RCT (38 clusters and 90 providers), conducted in 1) 38 public sector primary care clinics in the Western Cape Province (Eden and Overberg districts, socio‐economically deprived areas), South Africa, were randomised. 2) Nurses in the intervention clinics were trained to use the PC101 management tool in South Africa 2 arms: 1. Control (PALSA‐PLUS management tool) (control arm) and 2. Intervention (PC101 management tool) (intervention arm) |
|
| Participants | Control arm N: 991 Intervention arm N: 851, NA, NA Diabetes type: 4 Mean age: 52.01 ± 17.18 % Male: 25 Longest follow‐up: 14 months |
|
| Interventions |
Control arm: (PALSA‐PLUS management tool) Intervention arm: (PC101 management tool) 1) Team change 2) Clinician education 3) Clinician reminder 4) Continuous quality improvement |
|
| Outcomes | Anti‐platelet drugs Glycated haemoglobin |
|
| Funding source | This project has been funded in part with Federal funds by the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900030C (http://www.nhlbi.nih.gov/). Funding was also received from United Health, USA; the Department of Health of the Provincial Government of the Western Cape; the Department of Medicine, University of Cape Town, South Africa; the United Kingdom Department for International Development; and the University of Cape Town Lung Institute, South Africa. Funding was received by NL. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completed by the trial statistician using nQuery Advisor (Sample Size software) after recruitment of clinics, independently of the managers giving permission for the clinics to be included in the trial, and prior to patient recruitment and implementation of the intervention. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | High risk | Baseline clinic characteristics are provided in Table A in S1 Appendix (no P values). Intervention and control clinics had similar numbers of nurses and doctors. Control clinics tended to be larger and, by chance, had more psychiatric services and on‐site pharmacy facilities. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. Baseline patient characteristics were generally well balanced between arms (4 cohorts pooled, but no P values provided). Supplementary tables, Table D, only for diabetes patients, n = 1842, significant P values for Mossel Bay Stratum (P = 0.006) and history of CVD (P = 0.019). |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. Baseline patient characteristics were generally well balanced between arms (4 groups pooled, but no P values provided). Supplementary tables, Table D, only for diabetes patients, n = 1842, more patients with BMI> 30 in the intervention group (P = 0.009). |
| Incomplete outcome data (attrition bias) | High risk | They only analysed a subgroup of diabetes patients for HbA1c: 394/991 (39.8%) and 310/851 (36.4%) for the control and intervention group, respectively. They have data for 333/394 (15.5% lost) for the control group and 285/310 (8.1%) for the intervention group at 14 months follow‐up, respectively. High and unbalanced numbers. Insufficient resources to measure important health outcomes, such as HbA1c, at follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes: HbA1c (pre‐planned blood sampling) and aspirin addition (at follow‐up, prescription data for the period since baseline were extracted, photocopied, analysed and documented in the same way as at baseline). |
| Selective reporting (reporting bias) | High risk | Unclear if protocol was prospectively or retrospectively registered. However, secondary outcomes listed in the protocol for diabetic patients are not reported (proportion reporting dilated eye exam, proportion reporting foot exam). |
| Risk of contamination (other bias) | Unclear risk | Other strengths of the study include the cluster‐randomised design (appropriate to reduce the risk of contamination in an intervention directed at groups of nurses working in clinics). However, the PC101 tool used for the intervention is available online so control group had access to it (Quote: "For examples of updated content, see http://knowledgetranslation.co.za/programmes/pack‐adult/"). |
| Other bias | Unclear risk | Some variation in uptake of the management tool by nurses was reported. A further potential reason for the failure to show differences between groups was the effect of a co‐intervention, the concurrent Chronic Disease Season campaign, instituted by the clinic managers in both control and intervention clinics. The impact of this unforeseen development is seen in the higher rates of treatment intensification for hypertension and diabetes (the focus of the campaign) than for chronic respiratory disease or depressive symptoms in both the intervention and control clinics. Other limitations of the study design include dependence on self‐reported diagnoses for inclusion in the patient cohorts. Follow‐up visits being only every 3 to 6 months limited opportunities for treatment intensification. The expanded prescribing provisions initially resulted in some tensions between nurses, doctors and pharmacists. These were resolved through a facilitated group session and informal communication within clinics, sometimes involving the nurse trainer. This intervention was the only modification to the training during the trial. |
Faridi 2008.
| Study characteristics | ||
| Methods |
Evaluating the impact of mobile telephone technology on type 2 diabetic patients' self‐management: the NICHE pilot study Patient RCT, conducted in 2 community health centres, Connecticut, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 15 Diabetes type: type 2 Mean age: 56.0 ± 9.7 % Male: 36.7 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Clinician reminders 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 6.5 (0.7), post 6.8 (NR) Intervention arm: pre 6.4 (0.6), post 6.3 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 128.6 (22.3), post 139.4 (NR) Intervention arm: pre 134.7 (16.7), post 138.4 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 73.0 (11.5), post 81.1 (NR) Intervention arm: pre 79.6 (9.8), post 81.1 (NR) 4) Smoking cessation, N smokers (%) Control arm: pre 7 (47), post 5 (33) Intervention arm: pre 5 (33), post 5 (33) |
|
| Funding source | This NICHE study was funded by NIH under the Small Business Technology Transfer Research Program, grant number 1R21DKK072321‐01 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Farmer 2005.
| Study characteristics | ||
| Methods |
A randomized controlled trial of the effect of real‐time telemedicine support on glycemic control in young adults with type 1 diabetes Patient RCT, conducted with patients registered with Pediatric Transition Clinic or Young Adult Diabetes Clinic in Oxford, UK. Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 46 Intervention arm N: 47 Diabetes type: type 1 Mean age: 23.9 ± 4.2 % Male: 59.1 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.3 (1.5), post 8.9 (1.4) Intervention arm: pre 9.2 (1.1), post 8.6 (1.4) 2a) Harms (Grade 3 hypoglycaemic events), N (%) Control arm: pre NR (NR), post 1 (2) Intervention arm: pre NR (NR), post 0 (0) 2b) Harms (ketoacidosis), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 2 (4) |
|
| Funding source | The study was funded by an unrestricted grant from the Vodafone Group Foundation. Lifescan supplied the blood glucose meters. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Farmer 2007.
| Study characteristics | ||
| Methods |
Impact of self monitoring of blood glucose in the management of patients with non‐insulin treated diabetes: open parallel group randomised trial RCT (NA clusters and NA providers), conducted in 1) Study delivered in 48 general practices in Oxfordshire and South Yorkshire. The trial was managed from the co‐ordinating centre at the Department of Primary Health Care, University of Oxford. 2) Intervention delivered by researches nurses. In United Kingdom. 3 arms: 1. Control (standardised usual care) (control arm) and 2. Intervention 1 (less intensive self‐monitoring) (intervention arm)3. Intervention 2 (more intensive self‐monitoring) (other arm) |
|
| Participants | Control arm N: 152 Intervention arm N: 150, 151, NA Diabetes type: 2 Mean age: 65.7 ± 7.53 % Male: 57.39 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standardised usual care) 1) Case management 2) Clinician reminder 3) Facilitated relay of clinical information Intervention arm: (less intensive self‐monitoring) 1) Case management 2) Clinician education 3) Clinician reminder 4) Facilitated relay of clinical information 5) Promotion of self‐management Intervention arm: (more intensive self‐monitoring) 1) Case management 2) Clinician education 3) Clinician reminder 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Lipid‐lowering drugs Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Harms |
|
| Funding source | The trial was funded by the NHS and the National Institute for Health Research health technology assessment programme. The opinions expressed in this report are not necessarily those of the Department of Health. Abbott Diabetes Care provided blood glucose meters (Optium). AF was supported by an NHS research and development career development award from 2001‐5. AW was supported by a Rhodes scholarship. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computerised randomisation (Minim, www.sghms.ac.uk/depts/phs/guide/randser.htm) incorporating a partial minimisation procedure. |
| Allocation concealment (selection bias) | Low risk | The minimisation procedure to assign patients to their allocated intervention was conducted independently of the research nurses who managed recruitment and carried out the assessment visit. In this study patients were independently randomised. The allocation was also concealed from laboratory staff. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All baseline characteristics appear similar between arms. Baseline personal and clinical characteristics were well balanced between the groups (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All outcomes appear similar between arms. Baseline personal and clinical characteristics were well balanced between the groups (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | A total of 57 (12.6%) patients were lost to follow‐up (Figure 1). Reasons for loss to follow‐up partly provided. 4 and 5 patients died in the intervention arms compared to 1 in the control arm. Many patients did not persist at monitoring in the intervention arms (123 out of 301, 40.9%), which suggests a problem with intervention feasibility. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The primary outcome is objective (HbA1c). The allocation was concealed from laboratory staff. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol (protocol applied on January 2004, recruitment started on October 2002, 1‐year intervention). They did not report data for smoking status at the end of intervention. Also, they did a lot of subgroup analysis in the paper that were not planned in the protocol. |
| Risk of contamination (other bias) | High risk | All patients received diabetes education. Quote: "At the assessment visit, after obtaining informed consent, beliefs about diabetes were elicited using a standard approach to help patients understand how diabetes might present a threat to their health.The roles of diet, physical activity, and drugs were discussed within the framework of the commonsense model of illness representation". Patients in the control arm were asked not to use a blood glucose meter unless their doctor considered it essential for their clinical management, but 8 started to monitor. A total of 123 patients out of 301 (40.9%) in the 2 intervention arms did not persist monitoring. |
| Other bias | Unclear risk | None identified |
Farsaei 2011.
| Study characteristics | ||
| Methods |
Effect of pharmacist‐led patient education on glycemic control of type 2 diabetics: a randomized controlled trial Patient RCT, conducted in Isfahan Endocrine and Metabolism Research Center (IEMRC) outpatient clinic, Iran Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 87 Intervention arm N: 87 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.9 (1.1), post 9.0 (1.2) Intervention arm: pre 9.3 (1.7), post 7.5 (1.6) |
|
| Funding source | This study was funded from Isfahan University of Medical Sciences (Grant no. 387311) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | No differences based on text and Table 2 P values. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P > 0.05). |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not specified. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Laboratory methods to measure HbA1c not described and blinding of outcome assessor not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Fernandes 2018.
| Study characteristics | ||
| Methods |
A randomized controlled trial of financial incentives for medicaid beneficiaries with diabetes RCT (NA clusters and NA providers), conducted in 1) Kaiser Permanente Hawaii (KPHI) 2) Study co‐ordinator. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Hawaii Patient Reward And Incentives to Support Empowerment (HI‐PRAISE) project) (intervention arm) |
|
| Participants | Control arm N: 161 Intervention arm N: 159, NA, NA Diabetes type: 3 Mean age: 48.15 ± 9.8 % Male: 45.66 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Hawaii Patient Reward And Incentives to Support Empowerment (HI‐PRAISE) project) 1) Financial Incentives |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The project described was supported by Grant Number 1B1CMS330884 from the Department of Health and Human Services, Centers for Medicare & Medicaid Services (CMS). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table generated by the randomisation function in statistics computing and graphics software R. |
| Allocation concealment (selection bias) | Low risk | Random number table generated by the randomisation function in statistics computing and graphics software R. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Relatively balanced. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. P values provided but are not related to baseline values. |
| Incomplete outcome data (attrition bias) | High risk | 19% (131/161) lost in control group, 21% (125/159) lost in intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c, BP, LDL, measured objectively. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unclear whether groups were followed by the same clinicians. Unlikely that control participants received incentives. |
| Other bias | Low risk | None identified. |
Fiscella 2010.
| Study characteristics | ||
| Methods |
A novel approach to quality improvement in a safety‐net practice: concurrent peer review visits Patient RCT, conducted in 2 sites at a federally qualified health centre, USA Two arms: 1. Usual care (control arm) and 2. Peer review visit (intervention arm) |
|
| Participants | Control arm N: 117 Intervention arm N: 169 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Clinician reminders Intervention arm: 1) Team changes 2) Clinician education 3) Clinician reminders |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 8.8 (1.8), post 8.7 (0.2) Intervention arm: pre 9.3 (2.3), post 9.0 (0.2) |
|
| Funding source | Support was provided by The Robert Wood Johnson Foundation, Finding Answers Program | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer‐generated random numbers…" |
| Allocation concealment (selection bias) | Unclear risk | Does not describe process of allocation concealment. |
| Patient's baseline characteristics (selection bias) | Low risk | Age (P = 0.006), clinic site (P = 0.04). |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, did not have outcome data for all participants randomised (not necessarily lost to follow‐up though?). Baseline based on those analysed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding of outcome assessor not discussed; objective outcome methods not described. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | High risk | Quote: "…we cannot exclude the possibility that clinician learning extended to the control group, thus biasing results to the null." |
| Other bias | Low risk | None identified. |
Fischer 2012.
| Study characteristics | ||
| Methods |
Nurse‐run, telephone‐based outreach to improve lipids in people with diabetes Patient RCT, conducted at Denver Health's Westside Family Health Center (Westside Clinic). Serves a large Latino minority population. In USA. Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 381 Intervention arm N: 381 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 20 months |
|
| Interventions |
Control arm: 1) Patient reminders Intervention arm: 1) Case management 2) Promotion of self‐management 3) Patient reminders |
|
| Outcomes | 1) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 168 (44), post 170 (45) Intervention arm: pre 186 (49), post NR (NR) |
|
| Funding source | This study was funded by the American Diabetes Association | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not describe, states that they were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Does not describe, states that they were randomised. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Gender (P = 0.05), creatine levels (P = 0.01), cerebrovascular disease (P = 0.04). |
| Patient's baseline outcomes (selection bias) | Low risk | LDL (P = 0.84); HbA1c (P = 0.13); SBP (P = 0.96); DBP (P = 0.86); statin (P = 0.69); antihypertensive ACE (P = 0.58); beta‐blocker (P = 0.49). |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis done, numbers and reasons for loss to follow‐up not provided; they state that 65 of those in intervention group lost to follow‐up. Baseline based on those randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective laboratory methods not described. Data analysts were not blinded. Nurse who had delivered the intervention was also not blinded. However, it seems like they gathered the laboratory data from the clinic's medical records, which would mean that despite blinding status, there would be little influence for altering these laboratory results. |
| Selective reporting (reporting bias) | Low risk | Protocol matches and the study design was published: Fischer H, Mackenzie T, McCullen K, Everhart R, Estacio RO. Design of a nurse‐run, telephone‐based intervention to improve lipids in diabetics. Contemporary Clinical Trials. 2008; 29: 809‐816. |
| Risk of contamination (other bias) | High risk | Quote: "…participants may have used services in other healthcare centres…given the nurse also interacted with control patients, contamination of the intervention was a possibility." |
| Other bias | Low risk | Information not available. |
Fogelfeld 2017.
| Study characteristics | ||
| Methods |
Combined diabetes‐renal multifactorial intervention in patients with advanced diabetic nephropathy: proof‐of‐concept RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from the existing patient population in the Cook County Health & Hospitals System (CCHHS) general medicine clinic and specialty diabetes and renal clinics. The study site was the Fantus outpatient clinic (large public hospital system), the primary CCHHS outpatient clinic in Chicago, IL, USA. 2) Intervention delivered by a multifactorial‐multidisciplinary team (an endocrinologist, nephrologist, nurse practitioners, registered dietitians, certified diabetes educator/dietitian, and research coordinator). In United States of America 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (multifactorial‐multidisciplinary intervention) (intervention arm) |
|
| Participants | Control arm N: 60 Intervention arm N: 60, NA, NA Diabetes type: 2 Mean age: 57.48 ± 10.6 % Male: 58.35 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (multifactorial‐multidisciplinary intervention) 1) Case management 2) Team change 3) Patient education |
|
| Outcomes | Lipid lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lLipoprotein Hypertension control Harms |
|
| Funding source | The study was supported in part as an investigator‐initiated trial by Sanofi. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Stratified randomised controlled trial. Consented patients were randomised into the multifactorial‐multidisciplinary intervention and control as follows: 20, 20 into CKD 3a, 20, 20 into CKD 3b, and 20, 20 into CKD 4 for a total of 60 in intervention and 60 in control. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The baseline characteristics of both groups are summarised in Table 1. At baseline, there were no significant differences for age, gender, ethnicity and duration of diabetes. |
| Patient's baseline outcomes (selection bias) | Low risk | The baseline characteristics of both groups are summarised in Table 1. At baseline, there were no significant differences for eGFR, albumin creatinine ratio (ACR), SBP, A1C, and BMI. Nothing reported about the other outcomes of interest (DBP, medication usage, LDL). |
| Incomplete outcome data (attrition bias) | High risk | The dropout rate was 17.5%, with 23% (14/60) in the intervention and 12% (7/60) in the control. Numbers unbalanced. 8 dropped‐out in the intervention compare to 3 in control. No reasons reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All objective outcomes (statins and antihypertensives usage, HbA1c, blood pressure, LDL) except for hypoglycaemia that is subjective (but secondary outcome). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol (protocol first posted on July 2008, the study started on May 2007, 2 years intervention). The authors only mention the primary outcome in the protocol: "Delay in development in end‐stage renal failure in subjects with Advanced Diabetic Nephropathy (CKD stages 3 and 4) [ Time Frame: 2 years ]" and did not talk about all the secondary outcomes reported in the paper. |
| Risk of contamination (other bias) | Unclear risk | Both groups had access to the same specialists. Control patients received usual care, which included visits with their primary care physicians and, for most of them, visits with board certified specialists in separate diabetes and renal clinics with visit frequency determined by physicians in the relevant clinics (typically quarterly or 16 visits over two years). Patients in the intervention group had individual visits with the entire study staff (an endocrinologist, nephrologist, nurse practitioners, certified diabetes educator/dietitian and research co‐ordinator).The multifactorial‐multidisciplinary intervention visit frequency was monthly for the first 6 months and bimonthly for the next 18 months for a planned total of 15 clinic visits over two years. Not clear if the same specialists were following patients from the 2 groups. |
| Other bias | Low risk | No evidence of other bias. |
Fornos 2006.
| Study characteristics | ||
| Methods |
A pharmacotherapy follow‐up program in patients with type‐2 diabetes in community pharmacies in Spain Patient RCT, conducted in community pharmacies in Pontevedra, Spain Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 56 Intervention arm N: 58 Diabetes type: type 2 Mean age: 63.7 ± 10.7 % Male: 42.9 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.8 (1.7), post 8.5 (1.9) Intervention arm: pre 8.4 (1.8), post 7.9 (1.7) 2) SBP, mean mmHg (SD) Control arm: pre 148.0 (18.9), post 150.0 (19.9) Intervention arm: pre 143.0 (20.3), post 135.0 (16.4) 3) DBP, mean mmHg (SD) Control arm: pre 82.2 (11.1), post 82.1 (9.3) Intervention arm: pre 80.2 (9.1), post 78.2 (8.4) 4) LDL, mean mg/dL (SD) Control arm: pre 136.0 (40.2), post 133.0 (41.1) Intervention arm: pre 141.0 (43.1), post 126.0 (40.5) |
|
| Funding source | We should also like to thank Bayer Spain, the Official College of Pharmacist of Pontevedra and the Pharmaceutical Northwest Wholesaler Cooperative (Cofano) for their financial support. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Fortmann 2017.
| Study characteristics | ||
| Methods |
Dulce Digital: an mHealth SMS‐based intervention improves glycemic control in Hispanics with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Remote m‐Health intervention. Participants were recruited from clinic sites within Neighborhood Healthcare, a network of federally qualified health centres in San Diego and Riverside, California counties that serves predominantly low‐income individuals of an ethnic/racial minority. 2) Study co‐ordinator. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Dulce Digital mHealth) (intervention arm) |
|
| Participants | Control arm N: 63 Intervention arm N: 63, NA, NA Diabetes type: 2 Mean age: 48.43 ± 2.12 % Male: 25 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education 2) Financial Incentives Intervention arm: (Dulce Digital mHealth) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders 5) Financial incentives |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The current research was supported by McKesson Foundation grant 115M803379 and National Center for Advancing Translational Sciences grant NCATS 1UL1 TR001114‐01. The Investigator‐Initiated Study Program of LifeScan, Inc., provided glucose testing meters and strips for all participants. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked random assignment with equal allocation was used to assign participants to Dulce Digital or usual care (UC), using a randomly generated numbers sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Participants were informed of group assignment after the baseline assessment. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Almost all characteristics are statistically similar, except oral medication and insulin, which have significant P values. |
| Patient's baseline outcomes (selection bias) | Low risk | No between‐group differences were observed in clinical outcomes at baseline (P values 0.10). |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. 57/63 (90%) completed both 3‐ and 6‐month follow‐up, 6 dropouts (10%) in control. 47/63 (75%) completed both 3‐ and 6‐month follow‐up, 16 dropouts (25%) in intervention. Quote: "First, although attrition was comparable to that observed in prior studies (35), attrition was higher in the intervention group relative to the UC group. Thus, it is possible that participants who remained in the study were more engaged. A worst‐case scenario sensitivity analysis is presented in Supplement A." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c, BP, LDL. Outcomes objectively measured (extracted from electronic health records, determined using laboratory tests and standardised protocols). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Only HbA1c was listed as an outcome in the protocol; paper had many more outcomes. Table 2 shows that data were only collected for a subset for several outcomes. |
| Risk of contamination (other bias) | Low risk | Patient randomised, however mHealth remotely ran intervention. Unlikely that the intervention received Dulce Digital. |
| Other bias | Low risk | None identified. |
Foster 2013.
| Study characteristics | ||
| Methods |
A randomized comparison of a commercially available portion‐controlled weight‐loss intervention with a diabetes self‐management education program RCT (NA clusters and NA providers), conducted in 1) Patients recruited and treated at 2 medical centres in Philadelphia. 2) Sessions led by experienced practitioners (that is, lifestyle interventionists or certified diabetes educators, as appropriate). In United States of America. 2 arms: 1. Control (diabetes self‐management education‐DSME) (control arm) and 2. Intervention (group lifestyle intervention with portion‐controlled diet‐PCD) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 55.6 ± 9.53 % Male: 41 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (diabetes self‐management education ‐ DSME) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Continuous quality improvement Intervention arm: (group lifestyle intervention with portion‐controlled diet ‐ PCD) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This trial was supported by research grants from Nutrisystem, Inc. to Temple University and the University of Pennsylvania. This study was also supported by LifeScan, Inc. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned within site (stratified for insulin use), via a random‐number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. The study statistician generated the random allocation sequence, and research co‐ordinators enrolled participants and randomly assigned them to treatment conditions. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Legends: There were no significant differences between the 2 groups. The 2 treatment conditions did not differ significantly on any baseline characteristics (as shown in Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Legends: There were no significant differences between the 2 groups. The 2 treatment conditions did not differ significantly on any baseline characteristics (as shown in Table 1). |
| Incomplete outcome data (attrition bias) | Low risk | Only one lost to follow‐up in the intervention arm out of 100 (1%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All objective outcomes (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol (protocol first posted in December 2010, study started in March 2010, 6 months intervention). The authors only mention weight and HbA1c as outcomes, but the paper also reports blood pressure and LDL. The outcomes should have been reported for 0, 3 and 6 months time frame, but they were only reported at baseline and 6 months. |
| Risk of contamination (other bias) | Unclear risk | Both study conditions offered a stronger behavioural intervention than what most patients with type 2 diabetes are likely to receive in practice. Lifestyle intervention included the use of a prepackaged, portion‐controlled diet (PCD, Nutrisystem D, Fort Washington, PA, USA). Control sessions, led by a certified diabetes educator, were conducted in a format similar to the PCD intervention group, beginning with a review of the prior session’s readings and homework and followed by the introduction of a new topic. Control participants were instructed to consume a balanced deficit diet. Participants in both treatment conditions attended group sessions (at weeks 0, 1, 2, 4, 8, 12, 16, 20 and 24). All participants were instructed to monitor and record their blood glucose. All records were reviewed by study staff at each session. |
| Other bias | Low risk | None identified. |
Fountoulakis 2015.
| Study characteristics | ||
| Methods |
Impact and duration effect of telemonitoring on ΗbA1c, BMI and cost in insulin‐treated diabetes mellitus patients with inadequate glycemic control: a randomized controlled study RCT (NA clusters and NA providers), conducted in 1) Setting: outpatient care at the Department of Endocrinology at Athens General Hospital “G. Gennimatas”. 2) Data of telemonitoring group (TG) were transmitted from the glucose‐meters to our computers via modem. Communication with an endocrinologist was achieved via e‐mails and mobile phone text‐messages through integrated software. In Greece. 2 arms: 1. Control (usual outpatient care alone) (control arm) and 2. Intervention (telemonitoring group) (intervention arm) |
|
| Participants | Control arm N: 39 Intervention arm N: 76, NA, NA Diabetes type: 3 Mean age: 55.27 ± 9.57 % Male: 65.71 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual outpatient care alone) 1) Patient education Intervention arm: (telemonitoring group) 1) Case management 2) Electronic patient registry 3) Clinician reminder 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Not reported. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned (2:1), using random number generator and sealed envelopes, into two groups matched for age, BMI and HbA1c. Protocol: randomisation table created by computer software |
| Allocation concealment (selection bias) | Low risk | Patients were randomly assigned (2:1), using random number generator and sealed envelopes (protocol: sealed opaque envelopes), into 2 groups matched for age, BMI and HbA1c. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, no P values. There were no significant differences regarding age, sex and number of glucose measurements per day between TG and CG at baseline. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1, there were no significant differences regarding BMI between TG and CG at baseline. |
| Incomplete outcome data (attrition bias) | Low risk | Figure 1. They lost 4/39 (10.3% lost) patients in the control group, and 6/76 (7.9% lost) in the telemonitoring group. Low and balanced numbers. No reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c using lab method and data for hyperglycaemias and hypoglycaemias were obtained by patient's glucosemeter measurements). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. In the paper, they added frequency of doctor‐to‐patient communication (secondary outcomes). Sub‐groups analysis were planned in the protocol. |
| Risk of contamination (other bias) | Low risk | Only the intervention patients had access to the telemonitoring system. However, it is unclear if the endocrinologist involved in the intervention group was part of the endocrinologist's team involved in the care of the control group. |
| Other bias | Low risk | No evidence of other risk of bias. |
Franciosi 2011.
| Study characteristics | ||
| Methods |
ROSES: role of self‐monitoring of blood glucose and intensive education in patients with Type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Patient RCT, conducted in 3 diabetes clinics and home self‐monitoring, Italy Two arms: 1. Usual care (control arm) and 2. Self‐monitoring (intervention arm) |
|
| Participants | Control arm N: 16 Intervention arm N: 46 Diabetes type: type 2 Mean age: 48.9 ± 0.5 % Male: 74.2 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Clinician education 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 7.9 (0.6), post 7.2 (0.2) Intervention arm: pre 7.9 (0.6), post 6.7 (0.1) 2) SBP, mean mmHg (SE) Control arm: pre 132.0 (4.0), post 125.0 (4.0) Intervention arm: pre 137.0 (3.0), post 133.0 (2.0) 3) DBP, mean mmHg (SE) Control arm: pre 80.0 (2.0), post 79.0 (2.1) Intervention arm: pre 80.0 (2.0), post 77.0 (1.0) 4) Harms (hypoglycaemic events), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 0 (0) |
|
| Funding source | The study was unconditionally supported by LifeScan Inc. Clinical Research Management and Monitoring; Miriam Valentini, Celeste Pirozzoli and Rosalia Di Lallo, Department of Clinical Pharmacology and Epidemiology, Consorzio Mario Negri Sud, SantaMaria Imbaro, Italy. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization tables". |
| Allocation concealment (selection bias) | Low risk | Quote: "Centrally randomized by telephone". |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (%), P = 0.80; SBP (mmHg), P = 0.32; DBP (mmHg), P = 0.65. |
| Incomplete outcome data (attrition bias) | High risk | Intervention group had greater number of losses. Quote: "All of the efficacy analyses were performed on the intention‐to‐treat population." Number lost to follow‐up and reasons provided. Those lost to follow‐up in the intervention group likely related to outcome, and these numbers are not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: HbA1c is objective measure using laboratory methods, assessors not blinded. Secondary: SBP, measurement not reported, and assessors were unblinded. |
| Selective reporting (reporting bias) | High risk | Checked clinical trials.gov and after proposal also included some more endpoints. |
| Risk of contamination (other bias) | Low risk | Not indication that there was contamination. |
| Other bias | Low risk | Information not available. |
Franz 1995.
| Study characteristics | ||
| Methods |
Effectiveness of medical nutrition therapy provided by dietitians in the management of non‐insulin‐dependent diabetes mellitus: a randomized, controlled clinical trial Patient RCT, conducted in diabetes centres in Minnesota, Florida, and Colorado, USA Two arms: 1. BC Group ‐ basic nutrition care (control arm) and 2. PGC group ‐ practice guidelines nutrition care (intervention arm) |
|
| Participants | Control arm N: 85 Intervention arm N: 94 Diabetes type: type 2 Mean age: 56.4 ± 7.8 % Male: 44.1 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Team changes 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.3 (1.9), post 7.6 (1.7) Intervention arm: pre 8.3 (1.8), post 7.4 (1.3) 2) LDL, mean mg/dL (SD) Control arm: pre 135.4 (38.7), post 135.7 (44.5) Intervention arm: pre 129.2 (38.7), post 125.7 (30.2) |
|
| Funding source | This research was funded by The American Dietetic Association | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | They do not report the numbers of dropouts by group, so unclear risk. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | The doctors were aware of the allocation and could have treated the dietician group differently. Also, the control group did see the dietician once and this could have made a huge impact on the control group. |
| Other bias | Low risk | Information not available. |
Frei 2014.
| Study characteristics | ||
| Methods |
Implementation of the chronic care model in small medical practices improves cardiovascular risk but not glycemic control Cluster‐RCT (30 clusters), conducted in primary care physicians (PCPs) participating in routine primary care of unselected patients, Switzerland Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 164 Intervention arm N: 162 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Clinician education 5) Clinician reminders 6) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.6 (1.1), post 7.3 (1.0) Intervention arm: pre 7.8 (1.5), post 7.6 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 137.8 (16.8), post 137.5 (16.9) Intervention arm: pre 140.3 (18.4), post 136.4 (17.5) 3) DBP, mean mmHg (SD) Control arm: pre 78.7 (10.2), post 79.2 (11.2) Intervention arm: pre 83.1 (10.4), post 79.6 (9.9) 4) LDL, mean mg/dL (SD) Control arm: pre 96.7 (42.5), post 100.5 (38.7) Intervention arm: pre 108.3 (42.5), post 104.4 (38.7) |
|
| Funding source | This study was supported by grants from the Swiss Academy of Medical Sciences (grants RRMA 8‐09 and RRMA 13/10) and from the Margrit und Ruth Stellmacher Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomly generated As and Bs and attached #1 to #30 to them; they then put these tickets into an envelope and tickets for intervention and allocation. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | High risk | Control group had more primary care physicians (PCPs) working in single‐handed practices. |
| Patient's baseline characteristics (selection bias) | Unclear risk | In text, but P values not in table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Baseline differences for outcomes was not reported. |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up ~9% in N1 and ~4% in N2; reasons are imbalanced for "patients nor reliable, almost double in intervention group." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective laboratory methods not described. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes do not match protocol. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Frias 2017.
| Study characteristics | ||
| Methods |
Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open‐label, cluster‐randomized pilot clinical trial Clustered RCT (16 clusters and NR providers), conducted in 1) Primary Care Clinics, California and Colorado, USA; 2) Primary Care Physician in United States of America 3 arms: 1. Control (usual care) (control arm) and 2. Intervention 1 (4‐week digital medicine offering) (intervention arm)3. Intervention 2 (12‐week digital medicine offering) (other arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 41, 41, NA Diabetes type: 2 Mean age: 58.81 ± 10.08 % Male: 49.59 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (4‐week digital medicine offering) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders Intervention arm: (12‐week digital medicine offering) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | This study was funded and supported by the sponsor, Proteus Digital Health | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation methodology provided. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | No statistically different characteristics were reported. |
| Patient's baseline outcomes (selection bias) | High risk | Intervention 2 (12‐week DMO) had significantly lower mean SBP compared to usual care group (P < 0.05). |
| Incomplete outcome data (attrition bias) | High risk | Unbalanced attrition: control (7/36 = 19.4%), intervention 1 (1/41 = 2.4%), intervention 2 (1/41 = 2.4%) |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcomes were objectively measured (HbA1c, BP, LDL, HTN‐c). |
| Selective reporting (reporting bias) | Low risk | Retrospectively registered (July 2016) following completion of enrolment (October 2015). |
| Risk of contamination (other bias) | High risk | One usual care site with 5 participants was not included in the final analysis over concern about violation of the cluster‐randomisation. This usual care site was activated in September and was joined by the lead study co‐ordinator from a 4‐week DMO site previously activated in May; this study co‐ordinator had intervened with both DMO and usual care participants. |
| Other bias | Low risk | No evidence of other bias. |
Frijling 2002.
| Study characteristics | ||
| Methods |
Multifaceted support to improve clinical decision making in diabetes care: a randomized controlled trial in general practice Cluster‐RCT (124 clusters with 185 providers), conducted in the Netherlands Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 707 Intervention arm N: 703 Diabetes type: type 2 Mean age: 65.0 ± 11.5 % Male: 44.6 Longest follow‐up: 21 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Continuous quality improvement |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 474 (67), post 469 (65) Intervention arm: pre 492 (70), post 575 (79) 2) Foot screening, N screened (%) Control arm: pre 276 (39), post 346 (48) Intervention arm: pre 302 (43), post 451 (62) |
|
| Funding source | This study was supported by a research grant from the Netherlands Heart Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ no P values reported, looks balanced, # physicians not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ no education level reported, age and gender looks balanced, no P values. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1 shows the data (for uncontrolled glucose) and the P values are not significant. No other information on other outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Frosch 2011.
| Study characteristics | ||
| Methods |
Evaluation of a behavior support intervention for patients with poorly controlled diabetes Patient RCT, conducted in 3 academic primary care practices (2 internal, 1 family), 1 community‐based safety net clinic (poor/uninsured) in Los Angeles, CA area, USA Two arms: 1. Control group (control arm) and 2. Experimental group (intervention arm) |
|
| Participants | Control arm N: 101 Intervention arm N: 100 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 9.8 (2.1), post 9.2 (0.2) Intervention arm: pre 9.4 (1.9), post 8.9 (0.2) 2) SBP, mean mmHg (pre: SD, post: SE) Control arm: pre 127.7 (17.2), post 128.2 (1.9) Intervention arm: pre 127.6 (17.3), post 129.1 (1.9) 3) DBP, mean mmHg (pre: SD, post: SE) Control arm: pre 74.0 (10.4), post 73.6 (1.0) Intervention arm: pre 73.2 (11.6), post 74.3 (1.0) 4) LDL, mean mg/dL (pre: SD, post: SE) Control arm: pre 99.0 (34.9), post 97.3 (4.0) Intervention arm: pre 102.5 (39.3), post 106.0 (4.0) |
|
| Funding source | This study was supported by grants from the RobertWood Johnson Foundation and the Foundation for Informed Medical Decision Making. Dr Mangione and Ms Ochoa also received support from the University of California, Los Angeles, Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under grant P30‐AG021684 from the National Institute on Aging (NIA) and National Institutes of Health (NIH). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…a predetermined randomization sequence." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…sequence concealed in sealed envelopes." Envelopes opaque? |
| Patient's baseline characteristics (selection bias) | High risk | Quote: "Age (P = 0.05)". |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "HbA1c (P = 0.44); LDL (P = 0.51); SBP (P = 0.96); DBP (P = 0.60)". |
| Incomplete outcome data (attrition bias) | High risk | Although true intention‐to‐treat analysis done, numbers lost to follow‐up in reference group was much more than experimental (unreachable), and vice versa (discontinued intervention). Numbers and reasons for loss to follow‐up provided; baseline based on those randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: HbA1c measured using high performance liquid chromatography. Secondary: LDL, BP: objective methods not described. Outcome assessors were not blinded, however the authors state that "…primary outcome was a biological measure that arguably is not sensitive to unblinding", however we do not know about LDL, SBP and DBP. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias. |
Furler 2017.
| Study characteristics | ||
| Methods |
Supporting insulin initiation in type 2 diabetes in primary care: results of the Stepping Up pragmatic cluster randomised controlled clinical trial Clustered RCT (74 clusters and 162 providers), conducted in 1) General practices in Victoria, Australia. 2) General practitioner, practice nurse, registered nurse with diabetes educator credentials in Australia 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Stepping Up model of care) (intervention arm) |
|
| Participants | Control arm N: 115 Intervention arm N: 151, NA, NA Diabetes type: 2 Mean age: 61.83 ± 9.97 % Male: 61.23 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Stepping Up model of care) 1) Case management 2) Team change 3) Clinician education |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Funding from the Australian National Health and Medical Research Council (project grant application: APP1023738). The study was also supported by an educational/research grant by Roche Diabetes Care, the RACGP Foundation RACGP/Independent Practitioner Network Grant and received in‐kind support from Sanofi. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study statistician computer‐generated stratified block randomisation sequences with varying block sizes (4, 6 and 8) before recruitment. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT with randomisation occurring at one time. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table 1. No indication of statistically significant differences between groups for the patient baseline outcomes. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No P value s provided, yet no indication of statistically significant differences between groups for the patient baseline outcomes. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. Triglycerides were indicated as significantly different between groups in the Table 1 note. |
| Incomplete outcome data (attrition bias) | Low risk | 9/115 lost in control group (7.8%) and 9/151 lost in intervention group (6%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Cluster‐randomised. Quote: "strengths include the cluster randomised design, minimising the risk of contamination" |
| Other bias | Low risk | None identified. |
Gabbay 2006.
| Study characteristics | ||
| Methods |
Nurse case management improves blood pressure, emotional distress and diabetes complication screening Patient RCT, conducted in 2 primary care clinics of Penn State Hershey Medical Centre, USA Two arms: 1. Control (control arm) and 2. NCM ‐ nurse case management ‐ intervention (intervention arm) |
|
| Participants | Control arm N: 182 Intervention arm N: 150 Diabetes type: type 2 Mean age: 64.5 ± 16.4 % Male: 54.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 47 (26) Intervention arm: pre NR (NR), post 102 (68) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 86 (47) Intervention arm: pre NR (NR), post 96 (64) 3) Renal screening (microalbumin), N screened (%) Control arm: pre NR (NR), post 62 (34) Intervention arm: pre NR (NR), post 108 (72) 4) HbA1c, mean % (SD) Control arm: pre 7.4 (1.5), post 7.4 (1.8) Intervention arm: pre 7.5 (1.4), post 7.5 (1.4) 5) SBP, mean mmHg (SD) Control arm: pre 136.0 (17.0), post 138.0 (19.0) Intervention arm: pre 137.0 (19.0), post 129.0 (18.0) 6) DBP, mean mmHg (SD) Control arm: pre 77.0 (10.0), post 78.0 (10.0) Intervention arm: pre 77.0 (10.0), post 72.0 (9.0) 7) LDL, mean mg/dL (SD) Control arm: pre 105.0 (35.0), post 99.0 (32.0) Intervention arm: pre 105.0 (36.0), post 97.5 (32.0) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Gabbay 2013.
| Study characteristics | ||
| Methods |
Diabetes nurse case management and motivational interviewing for change (dynamic): results of a 2‐year randomized controlled pragmatic trial Patient RCT, conducted in 12 primary care clinics within 2 health systems in Central Pennsylvania (Penn State Milton S. Hershey Medical Center and Reading Hospital), USA Two arms: 1. Control (control arm) and 2. Treatment (intervention arm) |
|
| Participants | Control arm N: 271 Intervention arm N: 274 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 56 (24) Intervention arm: pre NR (NR), post 64 (34) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 33 (14) Intervention arm: pre NR (NR), post 41 (22) 3) Renal screening (Nephropathy), N screened (%) Control arm: pre NR (NR), post 198 (85) Intervention arm: pre NR (NR), post 173 (92) 4) HbA1c, mean % (SD) Control arm: pre 9.1 (2.3), post 8.0 (1.8) Intervention arm: pre 8.8 (2.4), post 7.8 (1.7) 5) SBP, mean mmHg (SD) Control arm: pre 142.0 (20.5), post 135.0 (18.2) Intervention arm: pre 145.0 (18.8), post 131.0 (15.9) 6) DBP, mean mmHg (SD) Control arm: pre 78.0 (11.5), post 74.0 (11.0) Intervention arm: pre 80.0 (12.6), post 74.0 (11.4) 7) LDL, mean mg/dL (SD) Control arm: pre 127.0 (45.6), post 100.0 (35.5) Intervention arm: pre 128.0 (39.7), post 102.0 (35.6) |
|
| Funding source | This study was supported by a grant from National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases grant R18‐DK067495 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Low risk | No evidence of other bias |
Gaede 2008.
| Study characteristics | ||
| Methods |
Effect of a multifactorial intervention on mortality in type 2 diabetes Patient RCT, conducted in diabetes clinics in Denmark Two arms: 1. Conventional therapy (control arm) and 2. Intensive therapy (intervention arm) |
|
| Participants | Control arm N: 80 Intervention arm N: 80 Diabetes type: type 2 Mean age: 55.1 ± 7.2 % Male: NR Longest follow‐up: 160 months (mean) |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 10 (13), post 29 (76) Intervention arm: pre 11 (14), post 47 (85) 2) Statins, N users (%) Control arm: pre 2 (3), post 31 (82) Intervention arm: pre 0 (0), post 46 (84) 3) Antihypertensives (any), N users (%) Control arm: pre 33 (41), post 38 (100) Intervention arm: pre 33 (41), post 51 (93) 4) HbA1c, mean % (SD) Control arm: pre 8.8 (1.7), post 8.0 (1.4) Intervention arm: pre 8.4 (1.6), post 7.7 (1.2) 5) SBP, mean mmHg (SD) Control arm: pre 149.0 (19.0), post 146.0 (18.0) Intervention arm: pre 146.0 (11.0), post 140.0 (14.0) 6) DBP, mean mmHg (SD) Control arm: pre 86.0 (11.0), post 73.0 (7.0) Intervention arm: pre 85.0 (10.0), post 74.0 (8.0) 7) LDL, mean mg/dL (SD) Control arm: pre 137.0 (37.0), post 77.0 (28.0) Intervention arm: pre 133.0 (36.0), post 71.0 (29.0) 8) Smoking cessation, N smokers (%) Control arm: pre 28 (35), post 7 (18) Intervention arm: pre 32 (40), post 12 (22) |
|
| Funding source | Supported by the Danish Health Research Council | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | > 30% dropout. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Gagliardino 2013a.
| Study characteristics | ||
| Methods |
Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR) Cluster‐RCT (36 clusters and 36 providers), conducted in 1) Public health, social security or private prepaid primary care clinics in Corrientes, Argentina. 2) Diabetes education course delivered by professional educators (control group) or previously trained peers supervised by Dr Gagliardino (intervention group). In Argentina. 2 arms: 1. Control (education by professional educators) (control arm) and 2. Intervention (education by trained peers) (intervention arm) |
|
| Participants | Control arm N: 117 Intervention arm N: 117, 117, 117 Diabetes type: 2 Mean age: NR % Male: NR Longest follow‐up: 42 months |
|
| Interventions |
Control arm: (no education) Intervention arm: (physician education) 1) Clinician education Intervention arm: (patient education) 1) Patient education Intervention arm: (patient and physician education) 1) Clinician education 2) Patient education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure |
|
| Funding source | This study was funded by the National Research Council of Argentina, La Plata National University, Novo Nordisk DAWN Programme, Health Ministry of Corrientes, IOSCOR, PAMI, OSPLAD, PROFE, Colegio Medico de Corrientes‐Salud, COMECOR‐Salud and SPS‐Salud | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported |
| Patient's baseline characteristics (selection bias) | Low risk | The 2 groups had comparable characteristics at baseline (age, gender and diabetes duration). |
| Patient's baseline outcomes (selection bias) | Low risk | Outcomes relatively balanced between groups. |
| Incomplete outcome data (attrition bias) | High risk | ~28% LFU in N1, 24% LFU in N2, 44% LFU in N3, 28% LFU in N4. Reasons provided overall, but not per arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary ‐ Hba1c‐ HbA1c assay ‐ immunoturbidimetric procedure. |
| Selective reporting (reporting bias) | Low risk | Matched protocol. |
| Risk of contamination (other bias) | Low risk | Unlikely that control group received intervention. |
| Other bias | Low risk | No evidence of other bias. |
Gagliardino 2013b.
| Study characteristics | ||
| Methods |
Type 2 diabetes patients educated by other patients perform at least as well as patients trained by professionals RCT (NA clusters and NA providers), conducted in 1) The Bernardo A. Houssay Centre in the city of La Plata (Argentina) is a non‐profit entity supported by funds from governmental organisations such as the Health Ministry of the province of Buenos Aires, the pharmaceutical industry and private organisations such as Rotary International, the International Diabetes Federation and personal donors. The Houssay Centre is a referral centre for the education of both people with diabetes and health professionals. 2) Diabetes education course delivered by professional educators (control group) or previously trained peers supervised by Dr Gagliardino (intervention group). 2 arms: 1. Control (education by professional educators) and 2. Intervention (education by trained peers) |
|
| Participants | Control arm N: 105 Intervention arm N: 93 Diabetes type: 2 Mean age: 60.94 ± 9.57 % Male: 48.59 Longest follow‐up: 12 months |
|
| Interventions | Interventions Control arm: (education by professional educators) 1) Patient education Intervention arm: (pharmacist management and SMBG) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Statins 2) Antihypertensives 3) Glycated haemoglobin 4) Systolic blood pressure 5) Diastolic blood pressure 6) Hypertension control |
|
| Funding source | This study was partially supported by a grant provided by the American Academy of Family Physicians Foundation for the Peers for Progress Program | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients who voluntarily accepted to participate in the study were randomly assigned either to the standard education or the peer education group, until at least 94 participants in each group were recruited. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The two groups had comparable characteristics at baseline (age, gender and diabetes duration). |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. They do not have HbA1c data for all patients at baseline (control: 78/105 and intervention: 66/93). |
| Incomplete outcome data (attrition bias) | High risk | Table 2. They do not have HbA1c data for all patients at baseline. Control: 78/105 (26% missing data) and intervention: 66/93 (29% missing data). No information on loss to follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c and blood pressure). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. No mention of classical symptoms, FBG, HDL, triglycerides and drug treatments in the methods. |
| Risk of contamination (other bias) | Unclear risk | Both groups had education, but that was planned. Only the intervention group had continuing psychological and behavioural support from peers. Physicians received quarterly reports for their patients managed by peers and the study co‐ordinator (Dr Gagliardino). Not clear if physicians were taking care of patients from both groups. Non‐inferiority study. |
| Other bias | Low risk | No evidence of other bias. |
Gamiochipi 2016.
| Study characteristics | ||
| Methods |
Effect of an intensive metabolic control lifestyle intervention in type‐2 diabetes patients RCT (NA clusters and NA providers), conducted in 1) The trial was carried out in eight Family Medicine Units (UMF) in Mexico City. 2) The IIEV (intervention) curriculum was applied by certified nutritionists. The COED (control) curriculum was applied by diabetes educators in Mexico. 2 arms: 1. Control (collaborative education model‐COED) (control arm) and 2. Intervention (intensive lifestyle intervention‐IIEV) (intervention arm) |
|
| Participants | Control arm N: 104 Intervention arm N: 95, NA, NA Diabetes type: 2 Mean age: 49.48 ± 9.33 % Male: 22.11 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (collaborative education model‐COED) 1) Patient education 2) Promotion of self‐management Intervention arm: (intensive lifestyle intervention‐IIEV) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Continuous quality improvement 5) Financial incentives |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This work was funded by Research Grant 2004/497 from Fondo de Fomento a la Investigación (FOFOI)/IMSS. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A total of 199 patients accepted to participate in the trial (99% response rate), with 95 being assigned to the control group and 104 to the intervention group using the Epistat package (Epistat Services, Richardson, TX, USA). Epistat's 25 programs perform over 40 common statistical tests or functions and provide utilities for data entry, editing, printing, graphing, sorting, selecting, transforming and cross tabs. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. Table 1 indicates the baseline characteristics for the 2 groups, and shows no significant differences. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. Table 1 indicates the baseline characteristics for the 2 groups, and shows no significant differences. |
| Incomplete outcome data (attrition bias) | Low risk | A total of 199 patients were recruited for the trial in 8 independent sites (Family Medicine Clinics). Of these, 17 did not finish the trial (8.5%); 7 had been assigned to the IIEV group, and 10 to the COED group (P > 0.05). Reasons not reported but small numbers and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All objective outcomes (HbA1c, SBP, DBP and LDL). Double‐blind evaluation of results. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Method: measurements were taken at baseline, 3 and 6 months. The authors only report 6‐month results. |
| Risk of contamination (other bias) | Low risk | Each study arm was followed by different case managers. The IIEV (intervention) curriculum was applied by certified nutritionists while the COED (control) curriculum was applied by diabetes educators. Only the intervention arm received prizes, awards, calls and psychologist consultations. The 2 groups were summoned to the UMF on distinct non overlapping dates, and all patients were informed that recommendations would be individual. |
| Other bias | Low risk | None reported. |
Garcia 2015.
| Study characteristics | ||
| Methods |
Home‐based diabetes symptom self‐management education for Mexican Americans with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) The study was set in urban and rural communities in Central Texas where Hispanics comprise 34% of the population. 2) Participants in the experimental condition received 8 weekly in‐home, interactive, one‐on‐one educational and behaviour modification sessions with a bilingual registered nurse (RN) in United States of America 2 arms: 1. Control (wait‐listed control) group (control arm) and 2. Intervention (symptom‐based diabetes self‐management education (DSME) programme) (intervention arm) |
|
| Participants | Control arm N: 37 Intervention arm N: 41, NA, NA Diabetes type: 2 Mean age: 49.6 ± 9.33 % Male: 33.33 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (wait‐listed control) Intervention arm: (symptom‐based diabetes self‐management education (DSME) programme) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | National Institute of Diabetes and Kidney and Digestive Diseases at the National Institutes of Health (R21DK076705) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to the experimental (symptom‐based diabetes self‐management education (DSME) programme) or the wait‐listed control group (WLC). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2 ‐ all P values greater than 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 2 ‐ fasting blood glucose and HbA1c levels were significantly different between control and experimental group at baseline. |
| Incomplete outcome data (attrition bias) | High risk | Large dropout. 27% loss in control group, 28% loss in experimental group. The authors do not provide reasons for the loss (they just mention what they think might have happened). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measures for HbA1c, SBP, DBP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. No discrepancy between methods and outcomes. |
| Risk of contamination (other bias) | Low risk | The participants in the intervention arm were followed by RNs, so contamination seems unlikely. |
| Other bias | Low risk | No evidence of other bias. |
Garg 2017.
| Study characteristics | ||
| Methods |
Effect of follow‐up by a hospital diabetes care team on diabetes control at one year after discharge from the hospital RCT (NA clusters and NA providers), conducted in 1) Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. 2) Nurse practitioner, endocrinologist. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (continued care) (intervention arm) |
|
| Participants | Control arm N: 91 Intervention arm N: 93, NA, NA Diabetes type: 2 Mean age: 64.02 ± 10.72 % Male: 60.39 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (continued care) 1) Case management 2) Team change 3) Facilitated relay of clinical information |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This work was supported by Health Resources and Services Award (HRSA), U.S. Department of Health and Human Services (UD7HP25059) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ all P values > 0.05, "Baseline characteristics of the two groups were similar", but they also say "Patients included in this study had variable geographical location, insurance coverage and primary care coverage". |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. Baseline HbA1c P = 0.04. |
| Incomplete outcome data (attrition bias) | High risk | 17% lost in intervention (16/93) group, 18% lost in control group. Reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Unlikely that control patients were contacted by the study team. |
| Other bias | Low risk | None. |
Gary 2003.
| Study characteristics | ||
| Methods |
Randomized controlled trial of the effects of nurse case manager and community health worker interventions on risk factors for diabetes‐related complications in urban African Americans Patient RCT, conducted in a university ambulatory clinic serving inner‐city population in Baltimore, MD, USA Four arms: 1. Usual care (control arm), 2. NCM ‐ nurse case manager (intervention arm 1), 3. CHW ‐ community health worker (intervention arm 2) and 4. NCM/CHW (intervention arm 3) |
|
| Participants | Control arm N: 34 Intervention arm 1 N: 38 Intervention arm 2 N: 41 Intervention arm 3 N: 36 Diabetes type: type 2 Mean age: 59.0 ± 9.0 % Male: 23.0 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm 1: 1) Case management 2) Patient education Intervention arm 2: 1) Case management 2) Patient education Intervention arm 3: 1) Case management 2) Team changes 3) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (2.0), post 8.5 (NR) Intervention arm 1: pre 8.8 (2.2), post 8.5 (NR) Intervention arm 2: pre 8.4 (2.0), post 8.1 (NR) Intervention arm 3: pre 8.6 (1.9), post 7.8 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 127.0 (20.0), post 127.0 (NR) Intervention arm 1: pre 125.0 (15.0), post 131.3 (NR) Intervention arm 2: pre 129.0 (20.0), post 125.7 (NR) Intervention arm 3: pre 129.0 (14.0), post 127.4 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 78.0 (11.0), post 78.0 (NR) Intervention arm 1: pre 75.0 (12.0), post 75.0 (NR) Intervention arm 2: pre 75.0 (11.0), post 72.0 (NR) Intervention arm 3: pre 76.0 (15.0), post 71.0 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 135.0 (33.0), post 118.3 (NR) Intervention arm 1: pre 138.0 (39.0), post 144.0 (NR) Intervention arm 2: pre 149.0 (43.0), post 155.0 (NR) Intervention arm 3: pre 132.0 (36.0), post 136.0 (NR) |
|
| Funding source | This work was supported by grants from the National Institutes of Health (R01‐DK48117‐04, R01‐DK48117‐03S1, T32‐HL07024) and the Johns Hopkins University Outpatient Department General Clinical Research Center (R00052) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Gary 2009.
| Study characteristics | ||
| Methods |
The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial Patient RCT, conducted in university affiliated managed care organisation with five sites in under‐serviced areas of Baltimore, USA Two arms: 1. Minimal intervention (control arm) and 2. Intensive NCM/CHW intervention (intervention arm) |
|
| Participants | Control arm N: 273 Intervention arm N: 269 Diabetes type: type 2 Mean age: 58.0 ± 11.0 % Male: 27.0 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Patient reminders Intervention arm: 1) Case management 2) Team changes 3) Clinician reminders 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.0 (2.2), post 7.9 (NR) Intervention arm: pre 7.7 (2.1), post 7.5 (NR) |
|
| Funding source | This study was funded by grants from the National Institutes of Health (R01‐DK48117 and R00052). Dr Gary was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U01‐DK57149‐05S1) and National Heart, Lung, and Blood Institute (K01‐HL084700), and Dr Brancati was funded by a grant from the NIDDK (K24‐ DK6222). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | The intensive intervention group was slightly older than the minimal group (59 vs 56 years; P = 0.01), otherwise, there were no statistically significant differences between the intervention groups at baseline. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. The intensive intervention group was slightly older than the minimal group (59 vs 56 years; P = 0.01), otherwise, there were no statistically significant differences between the intervention groups at baseline. |
| Incomplete outcome data (attrition bias) | Low risk | The rate of follow‐up was high, with 92% of participants completing the 24‐month visit (488 of 528 (542 patients −14 deaths)). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The HbA1c level was measured using high‐pressure liquid chromatography. The lipid profile (total and HDL‐C) was measured using standard techniques. Blood pressure was assessed using a random‐zero sphygmomanometer; the mean of 3 readings at 1 visit was used at baseline and again at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No reported protocol. Methods match outcomes reported. |
| Risk of contamination (other bias) | Low risk | Control group was not managed by nurse; contamination unlikely. |
| Other bias | Low risk | None identified |
George 2008.
| Study characteristics | ||
| Methods |
Clinical effectiveness of a brief educational intervention in Type 1 diabetes: results from the BITES (Brief Intervention in Type 1 diabetes, Education for Self‐efficacy) trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from people with diabetes attending our specialist diabetes service in a hospital setting (secondary care setting, York Hospital, UK). 2) A specifically trained diabetes specialist nurse and a specialist diabetes dietician facilitated intervention delivery. In United Kingdom. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (brief 2.5 days psycho‐educational intervention) (intervention arm) |
|
| Participants | Control arm N: 60 Intervention arm N: 54, NA, NA Diabetes type: 1 Mean age: 41 ± 10.06 % Male: 44.74 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (brief 2.5 days psycho‐educational intervention) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | From trial register. Funder type: Government. Funder name: York NHS Trust Research and Development Innovation Fund. (UK) (Ref 01/08/016). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Of the 117 patients attending randomisation, 54 were allocated to the intervention group and 60 to the control group. |
| Allocation concealment (selection bias) | Unclear risk | An independent evaluator then allocated participants using block randomisation (block size = 6) to intervention or control groups using sealed envelopes (opaque?) in strict ascendant order. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 3. Characteristics of participants in the 2 groups were comparable at entry (Table 3). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3. Characteristics of participants in the 2 groups were comparable at entry. Lipids, blood pressure, use of insulin and BMI demonstrated no statistical significance between the 2 groups (Table 3). Unclear about HbA1c. |
| Incomplete outcome data (attrition bias) | High risk | At 12 months, they lost 8/60 patients in the control group (13.3%) and 4/54 in the intervention group (7.4%). Unbalanced numbers. Reasons not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcomes were HbA1c (objectively measured) and severe hypoglycaemia (objectively and subjectively measured). Severe hypoglycaemia was defined as a recorded episode in which the patient required assistance with treatment and either documented blood glucose < 2.7 mmol/L or detected clinical signs that required oral carbohydrate administered by a third party, subcutaneous glucagon or intravenous glucose. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Secondary outcomes were blood pressure, weight, height, lipids and psychometric profile. No post‐intervention data are reported for blood pressure. Also, only the difference between means are reported for the other outcomes. |
| Risk of contamination (other bias) | Low risk | Only the intervention group had the brief (2.5 days) psycho‐educational intervention facilitated by a specifically trained diabetes specialist nurse and a specialist diabetes dietician. We made every effort to ensure that the control group received less input than the intervention group. However, principles of self‐management from the BITES course may have spilled over into the day‐to‐day practice of healthcare providers. |
| Other bias | Low risk | None. |
Gill 2019.
| Study characteristics | ||
| Methods |
Using electronic clinical decision support in patient‐centered medical homes to improve management of diabetes in primary care: The DECIDE Study Clustered RCT (12 clusters and 59 providers), conducted in 1) The main setting was small‐ to medium‐sized independent primary care practices in Delaware that were already participating in statewide PCMH projects, of which there were 39 at the time the study was initiated. In addition, 10 offices in Maryland that were in a joint Delaware‐Maryland Accountable Care Organization (which assisted offices to implement PCMH principles) were also eligible. There was 1 cluster of practices from the state of Maryland and 5 clusters of practices from the state of Delaware. Only offices that already had EHR systems in place were eligible. 2) The CDS system generated reports for the practice staff and clinicians before each appointment in United States of America. 2 arms: 1. Control (no intervention) (control arm) and 2. Intervention (CDS: electronic health record–based clinical decision support tools) (intervention arm) |
|
| Participants | Control arm N: 1902 Intervention arm N: 4484, NA, NA Diabetes type: 3 Mean age: 61.09 ± 14.46 % Male: 50.67 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (no intervention) Intervention arm: (CDS: electronic health record–based clinical decision support tools) 1) Audit and feedback 2) Case management 3) Electronic patient registry 4) Clinician reminder 5) Facilitated relay of clinical information |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | This study was funded by Sanofi US, Inc. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Overall, 41 practices were eligible for inclusion, of which 15 agreed to participate, and 12 were randomised. Of the remaining 10 offices (52 clinicians), 5 (23 clinicians) were randomised as controls. Clustered randomisation was based on practice size (no minimum) to reduce bias by office and by US state. |
| Allocation concealment (selection bias) | Low risk | Clustered trial. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Practice characteristics not reported. Clustered randomisation was based on practice size (no minimum) to reduce bias by office and by US state. This required collection of pre‐enrollment surveys that provided precise information on practice characteristics. They just mention that the control offices had fewer patients per clinician and fewer patients with diabetes. |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. Age group and mean age have significant P values (both < 0.0001). Quote: "Patients in the CDS group were significantly older than those in the control group". The 2 groups were similar in terms of gender and diabetes complications. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. HbA1c and LDL have significant P values (< 0.0001 and 0.0086, respectively). Quote: "Patients in the CDS group had better glycemic control and lower LDL‐C than those in the control group." |
| Incomplete outcome data (attrition bias) | High risk | Of the 15 offices that agreed to participate, 3 were excluded prior to randomisation and 2 were excluded after randomisation. Only 10 offices were analysed. At baseline, they have HbA1c data for 1068/1902 and 2732/4484 for control (44% lost) and intervention (39% lost) patients randomised. At 1 year, they have HbA1c data for 723/1902 and 2041/4484 for control (62% lost) and intervention (55% lost) patients randomised. Quote: "However, the main limitation of this study was the amount of missing A1C and LDL‐C data. Although some of these missing data were due to patients not undergoing the relevant tests at suitable time points (as would be expected in a real‐world study), most were due to problems of interoperability. A lack of interoperability also resulted in the exclusion of 5 offices." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c and LDL were objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Low risk | Clustered trial. Unlikely that control clusters had POC CDS systems implemented as an add‐on product to their EHRs. It was not possible to identify whether patients moved between practices (and therefore potentially between systems). |
| Other bias | Low risk | No evidence of other risk of bias. |
Gillani 2016.
| Study characteristics | ||
| Methods |
Determining effective diabetic care; a multicentre ‐ longitudinal interventional study RCT (NA clusters and NA providers), conducted in 1) outpatient department (OPD) for diabetic treatment, All the type 2 diabetes patients registered more than 6 months with diabetic clinics in Pulau Pinang and general hospital Penang were recruited. 2) Participants (in the telemonitoring intervention) received feedback upon receipt of transmission from the study co‐ordinator; a registered pharmacist provide once weekly visit to participant (in pharmacist intervention arm) home for monitoring and evaluation (also provide counselling). In Malaysia 3 arms: 1. Control (usual care) (control arm) and 2. Intervention (tele‐monitoring) (intervention arm)3. Intervention (pharmacist) (other arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, 50, NA Diabetes type: 2 Mean age: 53 ± 13.86 % Male: 56.67 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (tele‐monitoring) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management Intervention arm: (pharmacist) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | NR | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Systemic random sampling technique is used to distribute the participants to the arms. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Initial baseline comparison showed "No significant difference" between the 2 intervention arms and control group. Table 1 P values support this. |
| Patient's baseline outcomes (selection bias) | Low risk | Initial baseline comparison showed "No significant difference" between the 2 intervention arms and control group. Table 1 P values support this. |
| Incomplete outcome data (attrition bias) | Unclear risk | Sample size was calculated to account for a 20% loss to follow‐up, however no discussion about dropout was made, and no post values were provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure via glucometer for HbA1c, subjective for harms. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Outcomes match methods description. |
| Risk of contamination (other bias) | Low risk | Patient randomised. Unlikely that control group received intervention. |
| Other bias | Low risk | None identified. |
Gillani 2017.
| Study characteristics | ||
| Methods |
A randomised controlled trial in diabetes demonstrating the positive impact of a patient activation strategy on diabetes processes and glycated hemoglobin: the WICKED project Clustered RCT (9 clusters and NR providers), conducted in 1) Patients were recruited from primary care practices in nine subsectors in Wolverhampton, UK. 2) Study staff mailed structured personalised report to patients containing information on nine diabetes care processes. In United Kingdom. 2 arms: 1. Control (SM: single mailing of structured personalised report) (control arm) and 2. Intervention (MM: multiple mailing of structured personalised report) (intervention arm) |
|
| Participants | Control arm N: 6185 Intervention arm N: 8374, NA, NA Diabetes type: 3 Mean age: 63.69 ± 11.7 % Male: 54.42 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (SM: single mailing of structured personalised report) Intervention arm: (MM: multiple mailing of structured personalised report) 1) Case management 2) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | British Medical Association (BMA) Joan Dawkins Grant 2012 partly funded this project | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From reference 13. Method not reported: Utilising pilot processes based on information gathered over the preceding 15 months, the 9 subsectors and clients belonging to primary care practices within each, were cluster‐randomised by subsector into 2 groups intended to be matched for age, gender, ethnicity, type of diabetes and baseline process measures. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT with randomisation performed all at once. |
| Provider's baseline characteristics (selection bias) | Unclear risk | The 9 subsectors were randomised into 2 groups intended to be matched for patient's age, gender, ethnicity, type of diabetes and baseline process measures. No data reported for subsector, clinic and physician characteristics. |
| Patient's baseline characteristics (selection bias) | Low risk | From reference 13. All demographics data were not significant except Deprivation score (P value under 0.05). Quote: "The groups were well matched on all a priori demographic factors although, post hoc, there were very minor differences in the measure of deprivation" (Table 1). |
| Patient's baseline outcomes (selection bias) | High risk | From reference 13: HbA1c is not significant, but smoking status, systolic blood pressure and serum cholesterol have P values below 0.05. Quote: "There were very minor differences in the measure of smoking status outcome, systolic blood pressure and serum cholesterol" (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | Compared with the baseline population, at the end of 12 months 866 people in total were lost to ascertainment (453 deceased, 378 moved away and 35 not traceable), leaving a final cohort of 13,956 out of 14,822 people randomised at baseline (5.8% lost). However, for HbA1c, they only analysed the data from 10,015 patients at 12 months (32.4% lost). After randomisation, because of a mismatch in the baseline level of the full 9‐parameter Failed Process Score (FPS), they removed the 3 lowest scoring primary care practices from the control group, accounting for 1871 control participants. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively collected (HbA1c). Quote: "Data were captured within our local integrated diabetes information system." |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Primary outcome matches. However secondary outcomes do not match. Only HbA1c is reported in the paper, and not the other outcomes that define hard outcomes in diabetes (blood pressure, lipid and coronary heart disease (CHD) risk parameters, smoking cessation, renal markers, and eye and foot outcomes). |
| Risk of contamination (other bias) | Low risk | Clustered trial. All clients belonging to an individual primary care practice and all practices within an individual subsector cluster were randomised into blocks so that no practice had a mixture of mailed and non‐mailed individuals, thus minimising any impact of care‐providing professionals or practice‐specific process on the outcomes. |
| Other bias | Low risk | No evidence of other risk of bias. |
Ginsberg 1996.
| Study characteristics | ||
| Methods |
Preliminary results of a disease management program for diabetes Patient RCT, conducted in a family practice clinic at a major university, USA Two arms: 1. Control (control arm) and 2. Staged diabetes management (SDM) programme (intervention arm) |
|
| Participants | Control arm N: NR Intervention arm N: NR Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Clinician reminders 4) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.3 (2.1), post 10.4 (2.1) Intervention arm: pre 10.2 (2.8), post 8.8 (0.7) |
|
| Funding source | Not reported. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Glasgow 1996.
| Study characteristics | ||
| Methods |
Effects of a brief office‐based intervention to facilitate diabetes dietary self‐management Patient RCT, 2 providers, conducted in the 2 offices; 1 endocrinologist and 1 internist in Oregon, USA Two arms: 1. Usual care (control arm) and 2. Brief intervention (intervention arm) |
|
| Participants | Control arm N: 98 Intervention arm N: 108 Diabetes type: type 1 and type 2 Mean age: 62.4 ± 11.4 % Male: 38.4 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Clinician reminders 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.9 (NR), post 7.7 (NR) Intervention arm: pre 7.8 (NR), post 7.6 (NR) |
|
| Funding source | This research was supported by grant #ROl DK‐35524 from the National Institute of Diabetes, Digestive, and Kidney Diseases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | 12% lost to follow‐up in intervention group and 11% in usual care group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Primary outcome is food diaries. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Glasgow 2002.
| Study characteristics | ||
| Methods |
Implementation, generalization and long‐term results of the "choosing well" diabetes self‐management intervention RCT (NA clusters and NA providers), conducted in 1) Outpatients of primary care physicians at 12 medical offices. These offices were located in one of 6 small‐ to moderate‐sized communities within 30 miles of Eugene, Oregon, USA. The first session was held at the Center for Healthy Living, a centralised location for most participants. 2) Intervention delivered by health counsellors and 4 interventionists (a nurse/certified diabetes educator, a registered dietitian, a doctoral level psychologist and an education major). In United States of America. 4 arms: 1. Control (basic goal setting condition) (control arm) and 2. Intervention 1 (basic and community resources condition) (intervention arm), 3. Intervention 2 (basic and telephone follow‐up condition) (other arm), 4. Intervention 3 (combined condition: telephone and community resources) (other arm) |
|
| Participants | Control arm N: 80 Intervention arm N: 80, 80, 80 Diabetes type: 2 Mean age: 59.375 ± 10.14 % Male: 43.25 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (basic goal‐setting condition) 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management Intervention arm: (basic and community resources condition) 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management Intervention arm: (basic and telephone follow‐up condition) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by Grant R01‐35524 from the National Institute of Diabetes Digestive and Kidney Diseases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. 320 participants were randomised within providers to eliminate confounding of intervention and provider effects. The number of patients randomised per provider ranged from 1 to 29 with a median of 4. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. As can be seen in Table 1, there were no differences between conditions on any of the demographic or medical characteristic variables collected. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No P values reported for HbA1c between arms at baseline (Table 2). HbA1c data are not included in Table 1 (baseline characteristics by treatment conditions). |
| Incomplete outcome data (attrition bias) | High risk | One‐year follow‐up data were collected on 89% of the randomised participants (range of 84% to 93% among conditions, NS). According to the range, they lost 7% in one group and 16% in another one. Unbalanced lost between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol, but we found a previous study (reference 10) with more details about protocol. Results match protocol. |
| Risk of contamination (other bias) | Unclear risk | 320 participants were randomised within providers (same professionals had patients in different arms) to eliminate confounding of intervention and provider effects. However, a parallel form containing the participant’s lab results was sent to each primary care provider, which may have led them to change their clinical care to patients. |
| Other bias | Low risk | None. |
Glasgow 2005.
| Study characteristics | ||
| Methods |
Randomized effectiveness trial of a computer‐assisted intervention to improve diabetes care Cluster‐RCT (52 clusters with 52 providers), conducted by family physicians and general internists insured by Sopic Insurance Co in Colorado, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 417 Intervention arm N: 469 Diabetes type: type 2 Mean age: 63.0 ± NR % Male: 48.9 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 223 (67), post 243 (72) Intervention arm: pre 228 (68), post 259 (77) 2) Foot screening, N screened (%) Control arm: pre 261 (78), post 280 (84) Intervention arm: pre 267 (80), post 314 (94) 3) Renal screening (microalbumin), N screened (%) Control arm: pre 251 (75), post 273 (81) Intervention arm: pre 267 (80), post 306 (91) 4) HbA1c, mean % (SD) Control arm: pre 7.3 (1.2), post 7.2 (NR) Intervention arm: pre 7.3 (1.3), post 7.1 (NR) |
|
| Funding source | This work was supported by the Agency for Health Research and Quality, grant HS‐10123 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and greater than 0.05 |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and greater than 0.05 |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. Baseline comparisons do not seem to have P value associated, but numbers are reasonably balanced. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Glasgow 2012.
| Study characteristics | ||
| Methods |
Twelve‐month outcomes of an internet‐based diabetes self‐management support program Patient RCT, conducted in five primary care clinics within Kaiser Permanente Colorado (KPCO), USA Three arms: 1. Enhanced usual care (control arm), 2. Computer assisted self‐management ‐ CASM (intervention arm 1) and 3. Computer assisted self‐management + / CASM + (intervention arm 2) |
|
| Participants | Control arm N: 132 Intervention arm 1 N: 169 Intervention arm 2 N: 162 Diabetes type: type 2 Mean age: 58.4 ± 9.2 % Male: 50.2 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Electronic patient registry 2) Patient education 3) Promotion of self‐management Intervention arm 2: 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 8.2 (0.2), post 8.0 (0.1) Intervention arm 1: pre 8.0 (0.1), post 8.1 (0.1) Intervention arm 2: pre 8.3 (0.1), post 8.2 (0.1) |
|
| Funding source | This study was supported by grant DK35524 from the National Institute of Diabetes and Digestive and Kidney Diseases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that they…"randomized via a computer program". They do not report how they generated the sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "There were no significant differences among outcomes on baseline characteristics." |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "There were no significant differences among outcomes on baseline characteristics." |
| Incomplete outcome data (attrition bias) | High risk | Attrition rates of 18.2% in enhanced usual care (EUC) group, 31.4% in computer‐assisted self‐management (CASM) group, 25.3% in computer‐assisted self‐management + / CASM + (CASM+SS) group, and reasons for dropouts not provided |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Blinding not described. HbA1c measured using bio‐rad variant II turbo liquid by high‐pressure liquid chromatography. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Goderis 2010.
| Study characteristics | ||
| Methods |
Start improving the quality of care for people with type 2 diabetes through a general practice support program: a cluster randomized trial Cluster‐RCT (90 clusters with 142 providers), conducted in primary care practices in Leuven, Belgium Two arms: 1. UQIP ‐ usual quality improvement programme (control arm) and 2. AQIP ‐ advanced quality improvement programme (intervention arm) |
|
| Participants | Control arm N: 918 Intervention arm N: 1577 Diabetes type: type 2 Mean age: 68.0 ± 12.0 % Male: 48.3 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: 1) Audit and feedback 2) Clinician education 3) Patient education Intervention arm: 1) Audit and feedback 2) Team changes 3) Clinician education 4) Clinician reminders 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) Aspirin (aspirin/clopidogrel), N users (%) Control arm: pre 349 (38), post 459 (50) Intervention arm: pre 647 (41), post 978 (62) 2) Statins, N users (%) Control arm: pre 349 (38), post 450 (49) Intervention arm: pre 647 (41), post 867 (55) 3) HbA1c, mean % (SD) Control arm: pre 7.2 (1.3), post 6.8 (NR) Intervention arm: pre 7.1 (1.3), post 6.7 (NR) 4) SBP, mean mmHg (SD) Control arm: pre 137.0 (18.0), post 134.0 (NR) Intervention arm: pre 136.0 (15.0), post 132.0 (NR) 5) DBP, mean mmHg (SD) Control arm: pre 80.0 (9.0), post 78.0 (NR) Intervention arm: pre 80.0 (9.0), post 78.0 (NR) 6) LDL, mean mg/dL (SD) Control arm: pre 111.0 (34.0), post 98.0 (NR) Intervention arm: pre 107.0 (33.0), post 93.0 (NR) 7) Smoking cessation, N smokers (%) Control arm: pre 147 (16), post 110 (12) Intervention arm: pre 221 (14), post 189 (12) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Gold 2015.
| Study characteristics | ||
| Methods |
Feasibility and impact of implementing a private care system's diabetes quality improvement intervention in the safety net: a cluster‐randomized trial Clustered RCT (11 clusters and NR providers), conducted in 1) The 11 study community health centres (CHCs) are ambulatory primary care clinics managed by 3 Federally Qualified Health Center (FQHC) systems in the Portland, Oregon metropolitan area. All are members of Oregon Community Health Information Network (OCHIN, Inc.), a non‐profit organisation that provides health information technology to safety net clinics. 2) The intervention is a system‐ and clinic‐level QI intervention (iterative, stakeholder‐driven process). In United States of America. 2 arms: 1. Control (late clinics) (control arm) and 2. Intervention (early clinics: cardioprotective prescribing) (intervention arm) |
|
| Participants | Control arm N: 1179 Intervention arm N: 1446, NA, NA Diabetes type: 4 Mean age: NR ± 8.6 % Male: 40.32 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (late clinics) Intervention arm: (early clinics: cardioprotective prescribing) 1) Audit and feedback 2) Clinician education 3) Clinician reminder 4) Patient education |
|
| Outcomes | Lipid‐lowering drugs Antihypertensive drug |
|
| Funding source | National Heart, Lung & Blood Institute, 1R18HL095481‐01A1 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Cluster‐randomisation was used because this is a clinic‐level intervention with clinic‐level outcomes. Randomisation was matched on size of the clinics’ patient population and the FQHC system operating the clinic. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | 11 community health centres (CHCs) were randomised with 6 “early” CHCs implementing the intervention 1 year before 5 “late” CHCs. No data on CHCs' characteristics are reported in each arm at baseline. |
| Patient's baseline characteristics (selection bias) | Low risk | Percentage of CVD, age and gender are similar between control and intervention arms at baseline (June 2011, Table 2). Nothing about possible differences in text are provided. |
| Patient's baseline outcomes (selection bias) | Low risk | Percentage of patients with active prescription for ACE/ARB, statin at baseline (June 2011): intervention: 49.9%, control: 45.4%. Percentage of patients with active prescription for statin at baseline (June 2011): intervention: 51.6%, control: 47.8%. Similar % between control and intervention at baseline, nothing reported in text about possible differences. |
| Incomplete outcome data (attrition bias) | High risk | They have more people at the end of the intervention than at baseline (control: 1179 to 1436 (+21.8%), intervention: 1446 to 1599 (+10.6%). Increases not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes. Data for cardioprotective prescription was extracted from electronic health record (EHR). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol (first posted 24 November 2014 and the study began in May 2010). Protocol does not mention percent appropriately prescribed statins only. |
| Risk of contamination (other bias) | High risk | Clustered RCT. However, some patients and providers likely “crossed over” from early to late clinics during the study year, even though the intervention components were only activated for staff at specified clinics per our randomisation strategy; this is because a small percentage of CHC staff served patients at both early and late clinics, and the point‐of‐care alerts were seen by early CHC providers regardless of where they provided care. |
| Other bias | Low risk | No evidence of other bias. |
Goldberg 2004.
| Study characteristics | ||
| Methods |
Self‐management support in a web‐based medical record: a pilot randomized controlled trial Quasi‐RCT, conducted in 2 primary care clinics at Haborview Medical Centre, University of Washington, USA Two arms: 1. Usual care ‐ UC (control arm) and 2. Self‐management support ‐ SMS (intervention arm) |
|
| Participants | Control arm N: 127 Intervention arm N: 132 Diabetes type: unclear/not reported Mean age: 57.2 ± NR % Male: 56.8 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician reminders 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.1 (NR), post 7.7 (NR) Intervention arm: pre 8.0 (NR), post 7.6 (NR) |
|
| Funding source | The study was supported by a grant (I.D. # 041871) from the Robert Wood Johnson Foundation’s Improving Chronic Illness Care program | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Last digit of their medical record. |
| Allocation concealment (selection bias) | Unclear risk | Patient randomised and allocation concealment not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Lots of dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Process measures. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Goruntla 2019.
| Study characteristics | ||
| Methods |
Impact of pharmacist‐directed counseling and message reminder services on medication adherence and clinical outcomes in type 2 diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) The trial was carried out in outpatient medical department of a secondary care referral hospital, which was located in resource limited settings in Anantapuramu District, Andhra Pradesh, India. 2) Patients received pharmacist counselling and mobile phone daily messages about medication intake in India 2 arms: 1. Control (usual care by physician) (control arm) and 2. Intervention (pharmacist counselling and daily messages) (intervention arm) |
|
| Participants | Control arm N: 165 Intervention arm N: 165, NA, NA Diabetes type: 2 Mean age: 58.8 ± 10.7 % Male: 51.8 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care by physician) Intervention arm: (pharmacist counselling and daily messages) 1) Case management 2) Team change 3) Patient education 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein |
|
| Funding source | Financial support and sponsorship: nil | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. 330 patients who met study criteria were enrolled and randomised into intervention (n = 165) and control (n = 165) group by simple randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. Quote: "Sociodemographic characteristics, such as gender, marital status, education, occupation, comorbidities, and duration of diabetes, were closely matched in intervention and control groups as depicted in Table 1." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1: P value above 0.05 for BMI at baseline. Table 5: P values above 0.05 for HbA1c, SBP, LDL, TG and BMI at baseline. |
| Incomplete outcome data (attrition bias) | Low risk | In intervention group, 14 and in control group, 10 participants failed to show up for follow‐up visits. A total of 151/165 (8.5% lost) in intervention and 155/165 (6.1%) in the control group were subjected to data analysis. The flowchart of participants is shown in Figure 1. Low and balanced numbers of lost. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest were objectively assessed (HbA1c, SBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No published or registered protocol. They reported data on SBP but not on DBP, and they do not explain why. |
| Risk of contamination (other bias) | Low risk | Patient RCT, but it is unlikely that control patients received pharmacist directed counselling or text messages. |
| Other bias | Low risk | No evidence of other risk of bias |
Grant 2008.
| Study characteristics | ||
| Methods |
Practice‐linked online personal health records for type 2 diabetes mellitus: a randomized controlled trial Cluster‐RCT (11 clusters), conducted in primary care practices in hospital and community‐based settings in eastern Massachusetts, USA Two arms: 1. Active control arm (control arm) and 2. Intervention arm (intervention arm) |
|
| Participants | Control arm N: 118 Intervention arm N: 126 Diabetes type: type 2 Mean age: 56.1 ± 11.6 % Male: 51.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c | |
| Funding source | This study was supported in part by a grant from the Agency for Healthcare Research and Quality (AHRQ R01 HS013660‐02: Shared Online Health Records for Patient Safety and Care). Dr Grant is also supported by a National Institute of Diabetes and Digestive and Kidney Diseases Career Development Award (K23DK067452) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported |
| Patient's baseline characteristics (selection bias) | High risk | Table ‐ P values < 0.05 for age, sex, race. |
| Patient's baseline outcomes (selection bias) | Low risk | Table ‐ outcomes P < 0.05 for study participants. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Graumlich 2016.
| Study characteristics | ||
| Methods |
Effects of a patient‐provider, collaborative, medication‐planning tool: a randomized, controlled trial RCT (NA clusters and NA providers), conducted in 1) Recruitment occurred from ambulatory care general internal medicine clinics in Chicago and Peoria, Illinois, which served as the performance sites for this study. 2) The intervention involved nurses using the tool to support patients’ medication planning. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Medtable: medication‐planning tool) (intervention arm) |
|
| Participants | Control arm N: 348 Intervention arm N: 326, NA, NA Diabetes type: 2 Mean age: 63.65 ± 7.49 % Male: 44.80 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Medtable: medication‐planning tool) 1) Electronic patient registry 2) Clinician reminder 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The clinical research was supported by a grant from the National Institutes of Health, 1R01NR01130 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Research personnel at the clinical trial co‐ordination centre generated the random allocation sequence with computer‐generated random numbers. The allocation ratio was 1:1 with stratification by site, Chicago versus Peoria, and random permuted blocks within site. |
| Allocation concealment (selection bias) | Low risk | The co‐ordination centre personnel in Champaign, Illinois, transferred the allocation sequence to sequentially numbered, opaque envelopes and then distributed the sealed envelopes to the clinical sites in Chicago and Peoria. Next, the clinical trial coordinators obtained the concealed allocation to Medtable or usual care by opening the sealed envelope. |
| Patient's baseline characteristics (selection bias) | Low risk | The patient‐participants who received the randomised intervention, Medtable versus usual care, were comparable at baseline (Table 1) except for years with diabetes mellitus. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 7. P value above 0.05 at baseline. |
| Incomplete outcome data (attrition bias) | High risk | Lost 121 patients out of 674 (18.0%) at 6 months, 18.4% in control and 17.5% in intervention. Reasons partially reported. Numbers balanced but high. Number of patients measured at 12 months not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on February 2011, study started on February 2011). Results match protocol, but they added stratified analysis by patients’ literacy status. |
| Risk of contamination (other bias) | Unclear risk | One of the limitations of our trial design was the unmasked intervention. For participants assigned to usual care, their clinic nurses may have changed communication and collaborative planning after observation of colleagues who used the Medtable. This phenomenon is encountered in unmasked trials and is called contamination. Intervention nurses received training whereas usual care providers did not. |
| Other bias | Low risk | None. |
Greenfield 1988.
| Study characteristics | ||
| Methods |
Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes RCT (NA clusters and NA providers), conducted in 1) This study was conducted in 2 university hospital outpatient clinics. The first clinic, devoted exclusively to diabetes treatment, was administered by the Endocrinology Division in the Department of Medicine. The second was a general medical ambulatory care (MAC) clinic. 2) Three clinical research assistants delivered the intervention. In United States of America. 2 arms: 1. Control: standard educational material (control arm) and 2. Intervention: patient involvement (intervention arm) |
|
| Participants | Control arm N: 34 Intervention arm N: 39, NA, NA Diabetes type: 4 Mean age: 49.67 ± 8.2 % Male: 50.24 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: standard educational material 1) Patient education 2) Promotion of self‐management Intervention arm: patient involvement 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Supported by a grant (AM27547) from the National Institute for Arthritis, Diabetes, Digestive and Kidney Diseases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 notes indicate that all measurements are similar. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1 notes indicate that all measurements are similar. Table 2 for HbA1c. |
| Incomplete outcome data (attrition bias) | High risk | Loss of 8/34 in control (24%), loss of 6/39 in intervention (15%). Reasons for dropout not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. No follow‐up on several baseline measurements. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised. The same physicians were taking care of control and intervention patients. Physicians might have changed their approach after seeing intervention patients. Research assistants administering the intervention were not blind to the patient's study group and could have been differentially enthusiastic in delivering one intervention over the other. Care was taken in training the assistants to avoid bias in intervention administration, including use of standardised materials and review of sample tapes of assistant‐patient interactions. Although personal styles of administration varied, there were no discernible biases in intervention delivery. |
| Other bias | Low risk | None identified. |
Greenwood 2015.
| Study characteristics | ||
| Methods |
Overcoming clinical inertia: a randomized clinical trial of a telehealth remote monitoring intervention using paired glucose testing in adults with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Primary care. The study was conducted in a large healthcare system in California with an established diabetes management program with telephonic nurse care co‐ordination for diabetes population health management. 2) Intervention was delivered by certified diabetes educators (CDEs). In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Telehealth Remote Monitoring) (intervention arm) |
|
| Participants | Control arm N: 45 Intervention arm N: 45, NA, NA Diabetes type: 2 Mean age: 58 ± 10.75 % Male: 77 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Case management 2) Team change 3) Electronic patient registry 4) Patient education 5) Promotion of self‐management 6) Patient reminders Intervention arm: (Telehealth Remote Monitoring) 1) Case management 2) Team change 3) Electronic patient registry 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This research project has research support from the Investigator Initiated Studies program of LifeScan Corporation, IntelGE Care Innovations, Sutter Institute for Medical Research, The Betty Irene Moore School of Nursing, The Jonas Center for Nursing Excellence, and the University of California Davis, National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR 000002 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A permuted block, with blocks of 4 and 6, and a computer‐generated random number table were utilised for randomisation. |
| Allocation concealment (selection bias) | Low risk | After participants signed the consent form, the research co‐ordinator assigned sequential study identification (ID) numbers. The investigator matched the ID numbers to the random number table to assign study group. Participants were notified of group assignment by email after completing online baseline self‐assessment questionnaires. |
| Patient's baseline characteristics (selection bias) | Unclear risk | See Table 1. P > 0.06 except more people with a high cholesterol comorbidity (self‐reported hyperlipidaemia) in the intervention arm (n = 36/45, 80%) compared to the control arm (n = 24/45 = 53%), P = 0.006. Unsure if this will bias results. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Means HbA1c are similar between control (8.2±1.1) and intervention (8.5±1.1) at baseline, P above 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | Overall, 9 out of 90 participants were lost to follow‐up (10%). Numbers balanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). Blinding of participants, providers and the research team was not possible. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on October 2012, study was conducted between January and October 2013). All outcomes of interest are reported. |
| Risk of contamination (other bias) | Unclear risk | The same CDEs and nurses managed the care of patients in the intervention and the control arms, possibly contaminating the usual care group. The control arm was exposed to many components of the intervention, but to a lower extent. |
| Other bias | Low risk | No evidence of other bias. |
Griffin 2011.
| Study characteristics | ||
| Methods |
Effect of early intensive multifactorial therapy on 5‐year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION‐Europe): a cluster‐randomised trial Cluster‐RCT (343 clusters and NR providers), conducted in general practices in Denmark, The Netherlands and UK 2 arms: (control arm) (intervention arm) |
|
| Participants | Control arm N: 1379 Intervention arm N: 1678, NA, NA Diabetes type: 2 Mean age: NR ± NR % Male: 57.9 Longest follow‐up: 60 months |
|
| Interventions |
Control arm: (routine care) Intervention arm: 1) Case management 2) Audit and feedback 3) Clinician education 4) Patient education 5) Patient reminder |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Smoking status |
|
| Funding source | National Health Service Denmark, Danish Council for Strategic Research, Danish Research Foundation for General Practice, Danish Centre for Evaluation and Health Technology Assessment, Danish National Board of Health, Danish Medical Research Council, Aarhus University Research Foundation, Wellcome Trust, UK Medical Research Council, UK NIHR Health Technology Assessment Programme, UK National Health Service R&D, UK National Institute for Health Research, Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, Novo Nordisk, Astra, Pfizer, GlaxoSmithKline, Servier, HemoCue, Merck | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation was used. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Low risk | Reported in text, but not in table; no major differences. |
| Patient's baseline characteristics (selection bias) | Low risk | Reported in text, but not in table; no major differences. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No mention in text and no P values in table. |
| Incomplete outcome data (attrition bias) | High risk | ~19% lost to follow‐up in control group; ~27% in intervention group; not balanced. Reasons why 2 patients withdrew consent in the control group not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: microvascular outcomes. Used standardised equipment and outcome assessors were blinded. |
| Selective reporting (reporting bias) | High risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Griffin 2014.
| Study characteristics | ||
| Methods |
Multiple behaviour change intervention and outcomes in recently diagnosed type 2 diabetes: the ADDITION‐Plus randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from 34 general practices in the East of England. The majority of practices (n = 26) were participating in the intensive treatment arm of the ADDITION‐Cambridge trial. A further 8 practices were recruited to increase the participation of recently clinically diagnosed patients. The intervention was delivered over 1 year at the participants’ surgeries. 2) The intervention was delivered by 3 female trained lifestyle facilitators, who were not part of the general practice team. In United Kingdom. 2 arms: 1. Control (comparison group: intensive treatment) (control arm) and 2. Intervention (intervention group: intensive treatment plus facilitator‐led behaviour change intervention) (intervention arm) |
|
| Participants | Control arm N: 239 Intervention arm N: 239, NA, NA Diabetes type: 2 Mean age: 59.65 ± 13.40 % Male: 62.35 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (comparison group: intensive treatment) 1) Audit and feedback 2) Case management 3) Clinician education 4) Patient education 5) Promotion of self‐management Intervention arm: (intervention group: intensive treatment plus facilitator‐led behaviour change intervention) 1) Audit and feedback 2) Case management 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Smoking status |
|
| Funding source | The trial is supported by the Medical Research Council (grant reference no. G0001164), the Wellcome Trust (grant reference no. G061895), National Health Service R&D support funding (including the Primary Care Research and Diabetes Research Networks) and National Institute of Health Research under its Programme Grants for Applied Research scheme (RP‐PG‐0606‐1259). The Primary Care Unit is supported by NIHR Research funds. ATP is supported by the NIHR Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College, London, UK. Bio‐Rad provided equipment for HbA1c testing during the screening phase. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was central and independent using a partial minimisation procedure to balance stratifiers (age, sex and general practice; and within screen‐detected and clinically diagnosed subgroups: BMI, self‐reported smoking and medication adherence) between treatment arms. |
| Allocation concealment (selection bias) | Low risk | Randomisation was central and independent. Randomisation was undertaken independently of study co‐ordination or knowledge of or contact with participants or their data, other than the stratifiers. |
| Patient's baseline characteristics (selection bias) | Low risk | Baseline characteristics were similar in the 2 trial groups (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 4. Outcomes similar between groups. |
| Incomplete outcome data (attrition bias) | Low risk | According to Figure 1, 220 (92%) attended 1 year visit at clinical research facility in the control arm, while 224 (94%) did it in the intervention arm, but it is unclear if data were available for each outcomes (HbA1c, LDL and blood pressure) for all patients. For self‐reported smoking status (table 3), they had data for 222 participants in the control arm (7.1% missing) and 227 in the intervention arm (5.0% missing). See Figure 1 for reasons for loss, balanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All outcomes were objectively measured (HbA1c, SBP, DBP and LDL), even smoking status with cotinine validation (smoking was also subjectively self‐reported). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on May 2001, study started on October 2001). Results match protocol. |
| Risk of contamination (other bias) | Unclear risk | The control arm involved an intensive treatment intervention also included in the intervention arm. Quote: "There may have been limited scope for additional benefit among ADDITION‐Plus patients, who were already receiving intensive treatment, including theory‐based educational materials and lifestyle advice on all the target behaviours by the primary care team." |
| Other bias | Low risk | None identified. |
Grilo 2015.
| Study characteristics | ||
| Methods |
Food insecurity and effectiveness of behavioral interventions to reduce blood pressure, New York City, 2012‐2013 RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from the Ambulatory Care Clinic at Bellevue Hospital in New York City via flyers, face‐to‐face recruitment and physician referral. Intervention delivered at home (telemonitoring). 2) The combined intervention supplements the home blood pressure telemonitoring protocol with patient self‐management support from a nurse case manager. In United States of America. 2 arms: 1. Control (HBPTM: home BP telemonitoring) (control arm) and 2. Intervention (NCM + HBPTM: home BP telemonitoring plus nurse case management) (intervention arm) |
|
| Participants | Control arm N: NR Intervention arm N: NR, NA, NA Diabetes type: 2 Mean age: 60.7 ± 55.7 to 73.6 % Male: 42.9 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (HBPTM: home BP telemonitoring) 1) Electronic patient registry 2) Clinician reminder 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management Intervention arm: (NCM + HBPTM: home BP telemonitoring plus nurse case management) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician reminder 5) Facilitated relay of clinical information 6) Patient education Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Systolic blood pressure | |
| Funding source | Funding for this study was provided by K24HL111315 (Ogedegbe) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Participants were randomly assigned to either 1) home BP telemonitoring (HBPTM) alone; or 2) home BP telemonitoring plus nurse case management (HBPTM+NCM). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | No patient characteristics reported for each arm at baseline. They only report pooled data from both arms (Table 1). |
| Patient's baseline outcomes (selection bias) | Unclear risk | No patient characteristics reported for each arm at baseline. They only report pooled data from both arms (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | Of the 28 enrolled participants, 23 (82%) completed the 6‐month visit (18% lost). The primary reasons for dropout were family or housing issues and leaving the area. Numbers of lost and reasons for each arm are not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (SBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Patients were instructed to take BP readings, but they only report SBP and not DBP. However, the primary outcome was 6‐month change in systolic blood pressure (SBP). |
| Risk of contamination (other bias) | Unclear risk | Only the intervention group was managed by nurses. However, nurses could communicate with patients' physicians, so this may have changed their behaviour with the control patients they are following. |
| Other bias | High risk | No evidence of other bias |
Groeneveld 2001.
| Study characteristics | ||
| Methods |
An assessment of structured care assistance in the management of patients with type 2 diabetes in general practice Cluster‐RCT (15 clusters with 17 providers), conducted in 15 practices in Leiden, The Netherlands Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 155 Intervention arm N: 91 Diabetes type: type 2 Mean age: 62.4 ± 10.4 % Male: 41.9 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre NR (NR), post 7.5 (1.8) Intervention arm: pre NR (NR), post 7.1 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 149.0 (24.0), post 143.0 (21.0) Intervention arm: pre 137.0 (21.0), post 135.0 (18.0) 3) DBP, mean mmHg (SD) Control arm: pre 86.0 (9.7), post 82.0 (9.0) Intervention arm: pre 81.0 (9.0), post 80.0 (8.0) 4) Harms (hypoglycaemic events), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 7 (8) |
|
| Funding source | This study was supported by a grant from NOVO Nordisk Farma BV | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | They assign a 15th practice to the intervention group for no valid reason. |
| Provider's baseline characteristics (selection bias) | High risk | Data not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Table III, data are available for gender and age only. There is a significant difference between groups with regards to age (P < 0.05). |
| Patient's baseline outcomes (selection bias) | High risk | Table III, data are available with P values There is a significant difference in SBP between groups with regards to age (P < 0.05). |
| Incomplete outcome data (attrition bias) | High risk | 3% lost to follow‐up in control group vs 15% in intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Gucciardi 2007.
| Study characteristics | ||
| Methods |
Assessment of two culturally competent diabetes education methods: individual versus individual plus group education in Canadian Portuguese adults with type 2 diabetes Patient RCT, conducted in a Diabetes Education Centre, Toronto, ON, Canada Two arms: 1. Individual only (control arm) and 2. Individual plus group (intervention arm) |
|
| Participants | Control arm N: 46 Intervention arm N: 41 Diabetes type: type 2 Mean age: 59.6 ± 10.4 % Male: 31.3 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Team changes 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 7.4 (0.3), post 6.9 (0.2) Intervention arm: pre 7.4 (0.3), post 6.8 (0.2) |
|
| Funding source | Sources of financial support: The Banting and Best Diabetes Centre for funding this study; Canadian Institute for Health Research (CIHR) for Health Services and Policy Research for a Canadian Graduate Scholarship Doctoral Award to E.G., CIHR Institute of Gender and Health for a postdoctoral award to S.L.G. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Guirguis 2001.
| Study characteristics | ||
| Methods |
A pilot study to evaluate the impact of pharmacists as certified diabetes educators on the clinical and humanistic outcomes of people with diabetes Patient RCT, conducted with residents of the Edmonton area, Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 29 Intervention arm N: 33 Diabetes type: type 2 Mean age: 59.5 ± 10.9 % Male: 53.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.9 (1.3), post 7.1 (NR) Intervention arm: pre 7.9 (2.2), post 6.9 (NR) |
|
| Funding source | Supported by a grant from the Canadian Diabetes Association in honour of Alice Minerva Tufteland, Shoppers Drug Mart, and the Alberta Pharmaceutical Association. In addition, Bayer Inc., LifeScan, Medisense and Roche contributed monitoring devices and test strips provided to study participants. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The intervention and control groups had similar demographic characteristics with the exception of the method of diabetes treatment (Table 1), where participants in the control group were more likely to use medications to treat diabetes. |
| Patient's baseline outcomes (selection bias) | Low risk | The intervention and control groups had similar demographic characteristics. |
| Incomplete outcome data (attrition bias) | Low risk | Over the course of the study 13 participants (21%) dropped out. There was a comparable number of dropouts from the intervention (n = 7) and control (n = 6) groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measurement of outcomes. To avoid inter‐site variation, all HbA1c were analysed at 1 central laboratory. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registry. Outcomes in methods match those reported in results. |
| Risk of contamination (other bias) | Low risk | Unlikely that control group received intervention. |
| Other bias | Low risk | No other evidence of risk of bias. |
Guldberg 2011.
| Study characteristics | ||
| Methods |
Improved quality of type 2 diabetes care following electronic feedback of treatment status to general practitioners: a cluster randomized controlled trial Cluster‐RCT (86 clusters with 86 providers), conducted in 145 eligible General practices in Vejle County, Denmark Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 1263 Intervention arm N: 1453 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 15 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Electronic patient registry |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 516 (45), post 617 (54) Intervention arm: pre 585 (44), post 862 (65) 2) Antihypertensives (any), N users (%) Control arm: pre 448 (39), post 748 (66) Intervention arm: pre 505 (38), post 716 (54) 3) Retinopathy screening (eye exam), N screened (%) Control arm: pre 0 (NR), post 111 (12) Intervention arm: pre 0 (NR), post 145 (13) 4) HbA1c, mean % (SD) Control arm: pre 7.4 (1.2), post 7.5 (1.1) Intervention arm: pre 7.5 (1.2), post 7.5 (0.9) |
|
| Funding source | This study was funded by The Vejle County Quality Committee, The Central Region Denmark Quality Committee and The Danish Council for Independent Research, as well as The Tryg Foundation, Vissings Foundation, Danielsens Foundation and The A. P. Moellers Foundation Promoting Medical Science | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was unrestricted and was done using Stata software." |
| Allocation concealment (selection bias) | Low risk | Since cluster, low risk. Can it be assumed that they used a web‐based central allocation (since they used this for randomisation)? They state that allocation concealment was impossible for this study design, but we would assume that they are referring to post‐randomisation, i.e. blinding. |
| Provider's baseline characteristics (selection bias) | Low risk | Quote: "There were no statistically significant differences between the two groups of practices concerning age or sex of the general practitioners or localization and organization of practices". |
| Patient's baseline characteristics (selection bias) | High risk | They provide a P value for the difference between groups. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not provided. |
| Incomplete outcome data (attrition bias) | Low risk | ~9% lost to follow‐up per arm, with same reasons for losses. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Quote: "..both blinding and allocation concealment were impossible in the study design". HbA1c measurement not explicitly stated. |
| Selective reporting (reporting bias) | High risk | Secondary endpoints different between protocol and manuscript. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None. |
Gunawardena 2019.
| Study characteristics | ||
| Methods |
The influence of the smart glucose manager mobile application on diabetes management RCT (NA clusters and NA providers), conducted in 1) The ABCD trial was a randomised clinical trial conducted in collaboration with the faculty of medicine in Kelaniya University and Sri Jayewardenapura Hospital, Sri Lanka. The study includes patients registered at outpatient diabetes clinics at General Hospital, Sri Jayewardenepura. Intervention delivered using a Smart Glucose Manager (SGM) mobile application. 2) The SMBG summary of the SGM group and control groups were reviewed by usual clinic staff. In Sri Lanka. 2 arms: 1. Control (standard methods of diabetes management) (control arm) and 2. Intervention (SGM: Smart Glucose Manager mobile application) (intervention arm) |
|
| Participants | Control arm N: 32 Intervention arm N: 35, NA, NA Diabetes type: 4 Mean age: 52 ± 9.33 % Male: 60 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard methods of diabetes management) Intervention arm: (SGM: Smart Glucose Manager mobile application) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The author(s) received no financial support for the research, authorship, and/or publication of this article | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Of 300 screened, 67 met eligibility criteria and were randomised, using a computer‐generated random sequence method created by Sealed Envelope Ltd, into either the SGM (n = 35) or control (n = 32) group (Figure 1). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Baseline participants’ characteristics reported in Table 2. Looks similar. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. Baseline data for HbA1c reported, looks similar between groups, |
| Incomplete outcome data (attrition bias) | High risk | 67 patients met eligibility criteria and were randomised into either the SGM (n = 35) or control (n = 32) group. Eight patients from the SGM group (8/35 = 23% lost) and 7 from the control group (7/32 = 22% lost) were not able to finish the study. High % lost in both arms. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. They do not report the second primary outcome listed in the protocol: 1.2. Number of harmful blood glucose fluctuations (hyperglycaemia and hypoglycaemia) according to pre‐determined criteria using a questionnaire. No secondary outcomes are reported in the paper. |
| Risk of contamination (other bias) | Unclear risk | Patients randomised from one clinic. Unlikely that control patients had access to the Smart Glucose Manager mobile application. However, usual clinic staff could have changed their approach with control patients after seeing the data generated by the application from the intervention group. |
| Other bias | High risk | Protocol: the application will be tested for a period of 1 year. But they only report 6 months data in the paper. Inclusion criteria in the protocol: duration of diabetes over 1 year, but in the paper: self‐reported having diabetes for at least 6 months. |
Guo 2014.
| Study characteristics | ||
| Methods |
Efficacy of structured education in patients with type 2 diabetes mellitus receiving insulin treatments Patient RCT, conducted in 48 centres throughout China Two arms: 1. Control (control arm) and 2. Education (intervention arm) |
|
| Participants | Control arm N: NR Intervention arm N: NR Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 4 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.5 (1.9), post 7.4 (1.1) Intervention arm: pre 9.4 (2.0), post 7.2 (1.0) 2a) Harms (hypoglycaemia), N (%) Control arm: pre NR (NR), post 156 (24) Intervention arm: pre NR (NR), post 191 (30) 2b) Harms (nocturnal hypoglycaemia), N (%) Control arm: pre NR (NR), post 30 (5) Intervention arm: pre NR (NR), post 52 (8) 2c) Harms (severe hypoglycaemia), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 2 (0.3) |
|
| Funding source | This study was funded by the Bayer Healthcare Company and by grants from the Chinese Medical Association Foundation and the Chinese Diabetes Society | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated numbers sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Text and table. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Reasons not provided for losses. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective laboratory methods not described for HbA1c. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias |
Gutierrez 2011.
| Study characteristics | ||
| Methods |
Shared medical appointments in a residency clinic: an explanatory study among Hispanics with diabetes Patient RCT, conducted in a family medicine residency clinic (for underserved and uninsured indigent populations), USA Two arms: 1. Control (control arm) and 2. Shared medical appointments (intervention arm) |
|
| Participants | Control arm N: 53 Intervention arm N: 50 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 17 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 37 (70), post 36 (68) Intervention arm: pre 29 (58), post 48 (96) 2) Retinopathy screening (eye exam), N screened (%) Control arm: pre 36 (68), post 33 (62) Intervention arm: pre 32 (64), post 46 (92) 3) Foot screening, N screened (%) Control arm: pre 45 (85), post 32 (60) Intervention arm: pre 34 (68), post 47 (94) |
|
| Funding source | This study was supported by the Department of Family and Community Medicine, University of Texas Southwestern Medical School Parkland Family Medicine Clinic; by a Community Action Research Experience project funded by grant D58HP08301 from the Department of Health and Human Services Health Resources and Services Administration; and by a foundation grant from the Texas Academy of Family Physicians. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Characteristics mentioned in text, but no data were presented. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No baseline outcome measures provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of losses to follow‐up; cannot assume 100% follow‐up unless specifically stated. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | HbA1c measurement not described and outcome assessor was not blinded. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; they report all outcomes provided in methods. |
| Risk of contamination (other bias) | High risk | Quote: "Possibility of a halo effect exists, where providers participating in shared medical appointments (SMAs) could have gained new knowledge and insight that allowed them to better treat patients in the control group." |
| Other bias | Low risk | Information not available. |
Halbert 1999.
| Study characteristics | ||
| Methods |
Effect of multiple patient reminders in improving diabetic retinopathy screening. A randomized trial Patient RCT, conducted in a large network‐based health maintenance organization, USA Two arms: 1. Single reminder (control arm) and 2. Multiple reminders (intervention arm) |
|
| Participants | Control arm N: 11,748 Intervention arm N: 11,992 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: 38.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Audit and feedback 2) Clinician education 3) Patient education 4) Patient reminders Intervention arm: 1) Audit and feedback 2) Clinician education 3) Patient education 4) Patient reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 0 (0), post 3403 (35) Intervention arm: pre 0 (0), post 3666 (37) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | 18% lost to follow‐up in single reminder group and 17% in multiple reminder group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Number of eye exams = objective outcome. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Hansen 2013.
| Study characteristics | ||
| Methods |
Structured personal care of type 2 diabetes: a 19 year follow‐up of the study Diabetes Care in General Practice (DCGP) Clustered RCT (311 clusters and 474 providers), conducted in 1) General practice, Copenhagen, Denmark. 2) General practitioners in Denmark 2 arms: 1. Control: routine care (control arm) and 2. Intervention: structured personal care (intervention arm) |
|
| Participants | Control arm N: 620 Intervention arm N: 761, NA, NA Diabetes type: 2 Mean age: 65.4 ± 11.64 % Male: 53.08 Longest follow‐up: 168 months |
|
| Interventions |
Control arm: (routine care) Intervention arm: (structured personal care) 1) Audit and feedback 2) Clinician education 3) Clinician reminder 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Hypertension control Smoking status Harms |
|
| Funding source | Major funding for this study was received from the Danish Medical Research Council, the Danish Research Foundation for General Practice, the Health Insurance Foundation, the Danish Ministry of Health, Novo Nordisk Farmaka Denmark, the Pharmacy Foundation and the Novo Nordisk Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Their practices were allocated, by random numbers, to give patients either structured personal care or routine care. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT allocated by practice. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Baseline characteristics of volunteering GPs not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. No baseline characteristics of patients provided. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. Data provided but no P values given. |
| Incomplete outcome data (attrition bias) | High risk | 60% lost in intervention group, 63% lost in control group. High numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure for HbA1c, BP, harms, lipid‐lowering drugs, antihypertensive drugs. Subjective for smoking. |
| Selective reporting (reporting bias) | High risk | Do not report these outcomes listed in the protocol: 1) the patients' self‐rated health and motivation, 2) the doctor‐patient relationship, 3) diabetic retinopathy. In addition, they reported outcomes not listed in the protocol: HbA1c, BP, total cholesterol, etc. |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT allocated by practice. |
| Other bias | Unclear risk | 1) Both registries have changed registration and coding practices on several occasions, and the concepts and definitions of diseases have changed; new diagnostic criteria for MI were introduced in 2000, for example. In the nationwide DCGP study these time‐dependent changes in registration are unlikely to cause differential misclassification according to treatment allocation. 2) The fact that general practitioners volunteered for the study may have increased the treatment quality in the routine care group. |
Hansen 2017.
| Study characteristics | ||
| Methods |
Video consultations as add‐on to standard care among patients with type 2 diabetes not responding to standard regimens: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was done in co‐operation between the City of Copenhagen and Bispebjerg University Hospital. Recruited participants from the outpatient department of 3 hospitals in Copenhagen, Denmark and from the local healthcare centre. Telemedicine intervention as add‐on to clinic‐based care. 2) The intervention consisted of monthly video conferences with a healthcare centre nurse via a tablet computer. In Denmark. 2 arms: 1. Control (standard clinic‐based care) (control arm) and 2. Intervention (telemedicine consultations with a nurse) (intervention arm) |
|
| Participants | Control arm N: 82 Intervention arm N: 83, NA, NA Diabetes type: 2 Mean age: 58.05 ± 10.53 % Male: 64.5 Longest follow‐up: 14 months |
|
| Interventions |
Control arm: (standard clinic‐based care) Intervention arm: (telemedicine consultations with a nurse) 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | The study was supported by the Capital Region of Denmark and the City of Copenhagen. Furthermore, the study was supported by and by a grant from ‘Smedemester Niels Hansens og hustru Frederikkes’ Fund. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by drawing a sealed envelope containing a number. Depending on the number, the patients were allocated to either intervention or control. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised by drawing a sealed envelope (opaque?) containing a number. Depending on the number, the patients were allocated to either intervention or control. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values are above 0.05 for baseline characteristics. Quote: "There were no significant differences at baseline between the control and the telemedicine group." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values are above 0.05 for baseline tests. Quote: "There were no significant differences at baseline between the control and the telemedicine group." |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. 68/83 and 71/82 patients randomised completed the 14 months follow‐up in the intervention (18% lost) and the control (13% lost) group, respectively. High number for intervention group and reasons for lost not balanced between groups (14 patients discontinued in the intervention group compared to 5 in the control group). Quote: "We found 16% of the participants dropped out of the study." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol. Protocol: Changes in HbA1c (time frame: baseline, 16 weeks, 32 weeks, 6 months after intervention). They do not report data for HbA1c at 16 weeks in the paper. Most secondary outcomes in the protocol are not reported in the paper. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Usual physicians were not involved in the trial. Unlikely that control patients had telemedicine conferences. |
| Other bias | Low risk | No evidence of other risk of bias. |
Hargraves 2012.
| Study characteristics | ||
| Methods |
Community health workers assisting patients with diabetes in self‐management Clustered RCT (12 clusters and NR providers), conducted in 1) Six pairs of community health centres were randomly assigned to employ community health workers (CHWs) on healthcare teams. 2) The intervention involved 2 phases. The first phase, called the Collaborative, sponsored highly structured improvement interventions that focused on quality improvement activities. The goal of the second phase was to assess the impact of adding specially trained CHWs to a subset of the CHCs’ health care teams to address diabetes disparities. In United States of America. 2 arms: 1. Control (ongoing Collaborative intervention) (control arm) and 2. Intervention (ongoing Collaborative intervention with Community Health Workers) (intervention arm) |
|
| Participants | Control arm N: 921 Intervention arm N: 494, NA, NA Diabetes type: 2 Mean age: 53.34 ± 9.05 % Male: 48.04 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: (ongoing Collaborative intervention) Intervention arm: (ongoing Collaborative intervention with Community Health Workers) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Hypertension control | |
| Funding source | Disclosure of funding: Robert Wood Johnson Foundation (RWJF), Finding Answers: Disparities Research for Change | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Matched 6 pairs of CHCs based on baseline performance in the Collaborative intervention (Phase 1), volume of patients, racial/ethnic diversity, and geographic location, and randomly assigned each health centre to either control or intervention status. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | High risk | No table. In general, the intervention and control health centres were significantly different in terms of the profile of patient demographic variables, except for patients’ sex, and thus our analyses have adjusted for them. Some community health centres are staffed with dieticians while others are not; likewise, some CHCs have pharmacies with federal pricing, thus providing medication at low or no cost for the uninsured. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 shows patient characteristics of patient age, sex and ethnicity, along with insurance status. There were a higher percent of non‐Hispanic white patients (53% vs 35%) enrolled in the intervention CHCs. The average age of our intervention centres was 54.9 (SD = 13.0), an average of about 2 years older than the control group, P < .001. |
| Patient's baseline outcomes (selection bias) | High risk | Table 4. The number of patients with HbA1c below 7, LDL below 100 and blood pressure below 130/80 is significantly different between some ethnic groups at baseline or before intervention (see comments b and c in the legend). |
| Incomplete outcome data (attrition bias) | High risk | In Table 4, they have data for 750 out of 921 in the control arm (18.6% missing) and 445 out of 494 in the intervention arm (9.9% missing). Numbers unbalanced. No flow chart. Reasons for loss not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All outcomes were objectively measured (HbA1c, SBP, DBP and LDL) |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. They only report the number of patients at target and not their mean level for HbA1c, LDL and blood pressure. In the methods, they state: "we examined change in performance for several measures... including ... systolic blood pressure", but they reported the number of patients below a blood pressure of 130/80. |
| Risk of contamination (other bias) | High risk | Clustered RCT but all health centres participating in this CHW demonstration project participated in the first 12 months of a statewide diabetes health disparities collaborative (the Collaborative), which sponsored highly structured improvement interventions that focused on quality improvement activities using PDSA cycles to improve care effectiveness for a cohort of patients with diabetes cared for by a primary care provider and support team. This intervention included pre‐work on leading change and data collection; development of a patient registry; monthly conference calls; three 1‐day team training sessions; monthly progress and data reports; and practice redesign coaching. |
| Other bias | Low risk | None identified. |
Harno 2006.
| Study characteristics | ||
| Methods |
Managing diabetes care using an integrated regional e‐health approach Patient RCT, conducted in primary care and university hospital outpatient clinics, Finland Two arms: 1. Controls (control arm) and 2. Study (intervention arm) |
|
| Participants | Control arm N: 74 Intervention arm N: 101 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 8.2 (0.2), post 7.8 (0.2) Intervention arm: pre 7.8 (0.1), post 7.3 (0.1) 2) SBP, mean mmHg (SE) Control arm: pre 136.0 (1.8), post 137.0 (2.3) Intervention arm: pre 134.0 (1.8), post 135.0 (2.2) 3) DBP, mean mmHg (SE) Control arm: pre 84.0 (1.1), post 82.0 (1.5) Intervention arm: pre 81.0 (1.0), post 79.0 (1.1) 4) LDL, mean mg/dL (SE) Control arm: pre 102.5 (3.5), post 106.7 (3.9) Intervention arm: pre 104.4 (3.1), post 97.5 (3.1) |
|
| Funding source | We thank the Royal Brompton and Harefield NHS Trust and the European Commission (eTen Programme) who co‐funded the IREMMA project | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Control: regular visits. Intervention: home phone and e‐health. |
| Other bias | Low risk | Information not available. |
Harris 2005.
| Study characteristics | ||
| Methods |
Teleconferenced educational detailing: diabetes education for primary care physicians Cluster‐RCT (90 clusters with 90 providers), conducted in family physician clinics from 8 geographic regions in Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 313 Intervention arm N: 347 Diabetes type: type 2 Mean age: NR ± NR % Male: 55.9 Longest follow‐up: 15 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician education |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 16 (5) Intervention arm: pre NR (NR), post 42 (12) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 53 (17) Intervention arm: pre NR (NR), post 89 (26) 3) Renal screening (Renal), N screened (%) Control arm: pre NR (NR), post 65 (21) Intervention arm: pre NR (NR), post 106 (31) 4) HbA1c, mean % (SD) Control arm: pre NR (NR), post 7.6 (NR) Intervention arm: pre NR (NR), post 7.3 (NR) |
|
| Funding source | This investigation was sponsored by GlaxoSmithKline (SmithKline Beecham at the time of study initiation), Oakville, Canada | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Table 1. No P values provided, CFPC certification numbers are not balanced. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values provided, fairly balanced, but missing data for 8 controls and 16 intervention participants |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Harris 2013.
| Study characteristics | ||
| Methods |
Can community retail pharmacist and diabetes expert support facilitate insulin initiation by family physicians? Results of the AIM@GP randomized controlled trial Clustered RCT (15 clusters and 154 providers), conducted in 1) Intervention conducted in family physician clinics and community pharmacies across Canada. The University of Western Ontario Centre for Studies in Family Medicine served as the co‐ordinating centre. 2) 15 diabetes specialist sites and 107 community pharmacists provided the intervention. In Canada. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (family physicians education on insulin initiation) (intervention arm) |
|
| Participants | Control arm N: 2788 Intervention arm N: 2858, NA, NA Diabetes type: 2 Mean age: 49.75 ± 5.9 % Male: 74.15 Longest follow‐up: 15 months |
|
| Interventions |
Control arm: (usual care) 1) Clinician education Intervention arm: (family physicians education on insulin initiation) 1) Audit and feedback 2) Team change 3) Clinician education 4) Clinician reminder |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was sponsored by Sanofi‐Aventis including the provision of LantusW or NPH insulin to all insulinised patients for 6 months. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. All eligible physicians were stratified by the study geographic site and their level of comfort prescribing insulin (determined by questionnaire) and randomly allocated (1:1) in a blocked manner to an insulin initiation strategy (intervention) or usual care (control) by the co‐ordinating centre. Sanofi‐Aventis generated the mechanism used to implement the random allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. Randomly allocated (1:1) in a blocked manner to an insulin initiation strategy (intervention) or usual care (control) by the co‐ordinating centre. |
| Provider's baseline characteristics (selection bias) | High risk | Table 2. Some characteristics have P values at or under 0.05 (mean age, mean years in practice, CME attendance, mean number of patients seen per day per physician). |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3. All P values above 0.05. Patient summary data (Table 3) were computed for consenting patients (49.6% consent rate) and were comparable between study groups. |
| Incomplete outcome data (attrition bias) | Unclear risk | The paper is unclear about the number of patients initially recruited. Out of the 75 physicians randomised to the intervention arm, 2 did not receive the workshop and 3 withdrew (6.7%). In the control arm, out of the 79 physicians, 1 physician did not attend the workshop and 3 withdrew (5.1%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol (protocol first posted on January 2008, study was conducted from July 2006 to April 2010). Not reported in paper: 1) mean A1C of insulin‐eligible patient per family physician post‐workshop (only provided baseline data). 2) Proportion of patients at target (A1C ≤ 6.5%) at time of the workshop and post, 2) mean glycaemic control (A1C, FPG) at insulin initiation, 3 months post initiation and 6 months post initiation (only in text). Also, physician scores for knowledge, attitude and self‐efficacy of glycaemia control insulin initiation and titration not reported. |
| Risk of contamination (other bias) | Unclear risk | Risk of contamination of the control physicians could have occurred if they practiced in the same location as an intervention physician. However, this situation occurred for only 3 control physicians thus we do not believe it affected the overall results. Study sites hosted insulin initiation workshops for all physicians enrolled in the study to ensure comparable knowledge on the appropriate use of insulin therapy in T2DM. All physicians received a complete registry of insulin‐eligible patients in their practice. Pharmacists attended the same programme but were educated separately to avoid contamination. In addition, diabetes specialists and pharmacists did not provide support to the control physicians in the study. |
| Other bias | Unclear risk | Participation bias may have led to the inclusion of physicians with a more active interest in insulin initiation. Pharmacist recruitment challenges delayed the start of the intervention for some physicians, perhaps impacting the intervention and potential outcomes. The estimated sample size of 89 physicians per group was not achieved hence the final results may have been underpowered. |
Hawkins 2010.
| Study characteristics | ||
| Methods |
Improving glycemic control in older adults using a videophone motivational diabetes self‐management intervention Patient RCT, conducted with patients recruited by referral from 3 primary care providers within the same clinical practice, USA Two arms: 1. Control (control arm) and 2. Experimental (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 40 Diabetes type: unclear/not reported Mean age: 64.9 ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.9 (3.1), post 8.3 (NR) Intervention arm: pre 9.0 (2.3), post 7.3 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 160.2 (28.7), post 159.2 (NR) Intervention arm: pre 154.4 (30.4), post 154.0 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 82.3 (12.2), post 81.2 (NR) Intervention arm: pre 79.5 (10.2), post 78.9 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 128.8 (20.7), post 123.9 (NR) Intervention arm: pre 112.2 (24.8), post 105.2 (NR) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized…using a computer‐generated randomized list." |
| Allocation concealment (selection bias) | Unclear risk | Do not describe allocation concealment. They say that patients and nurses were blinded to allocation until after receiving intervention packets but that does not mean that those who were in charge of randomising the groups were blinded. |
| Patient's baseline characteristics (selection bias) | Low risk | Table and text. Quote: "There were no statistically significant differences between the groups in baseline characteristics." |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.76); LDL (P = 0.79); DBP (P = 0.45); SBP (P = 0.68). |
| Incomplete outcome data (attrition bias) | Low risk | Per‐protocol analysis completed, baseline based on those analysed, however numbers and reasons for loss to follow‐up provided, and they seem reasonably balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c: standard laboratory method. Blood pressure using oscillometric technology. LDL measured using blood analyser. Blinding not well described. We do not know if outcome assessors were blinded. They note that investigators were not blinded to outcome measurements, but we also do not know whether they knew the assignment of patients. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Hayashino 2016.
| Study characteristics | ||
| Methods |
A cluster randomized trial on the effect of a multifaceted intervention improved the technical quality of diabetes care by primary care physicians: The Japan Diabetes Outcome Intervention Trial‐2 (J‐DOIT2) Clustered RCT (22 clusters and 192 providers), conducted in 1) 11 district medical associations (DMAs) in Japan were divided into 2 subregions (clusters). 2) Intervention delivered by physicians and certified diabetes educators, registered dieticians or public health nurses. In Japan. 2 arms: 1. Control (ordinary medical treatment) (control arm) and 2. Intervention (Achievable Benchmark of Care‐ABC) (intervention arm) |
|
| Participants | Control arm N: 1265 Intervention arm N: 971, NA, NA Diabetes type: 2 Mean age: 56.5 ± NR % Male: 62.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (ordinary medical treatment) Intervention arm: (Achievable Benchmark of Care‐ABC) 1) Audit and feedback 2) Case management 3) Team change 4) Clinician education 5) Clinician reminder 6) Patient education 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | Funding was received from the Japan Agency for Medical Research and Development, Grant Number: Practical Research Project for Life‐Style related Diseases including CVD and Diabetes; Ministry of Health, Labour and Welfare, Japan, Grant Number: Strategic Outcomes Research Program for Research on Diabetes; Ministry of Health, Labour and Welfare, Japan, Grant Number: Comprehensive Research on Life‐Style Related Diseases including CVD and Diabetes H25‐016 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The statistician, blind to the identities of the clusters, randomly allocated 0 (control) or 1 (intervention) codes generated by statistical software, to 22 clusters stratified by each DMA. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table 3. Similar overall quality‐of‐care score, % point (95% CI), at baseline between intervention and control arms, but no P value. No other information. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. HbA1c ok. No data for SBP and DBP at baseline. Table 3. No P value between arms at baseline for eye, foot and renal screening. |
| Incomplete outcome data (attrition bias) | Low risk | 971 patients were enrolled in the IG and 1265 in the CG. For the final analysis, 954 patients (1.8% missing) were enrolled in the IG and 1245 patients (1.6%) in the CG after excluding patients who proved to be ineligible or who declined to participate at a later stage. Low missing data, numbers balanced, but reasons not quite balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All outcomes were objectively measured: 1) HbA1c, SBP, DBP with laboratory methods, and 2) eye, foot and renal screening data from medical chart. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol (protocol submitted on July 2009, study started at the same time). No information about HbA1c, SBP and DBP in the protocol. Protocol only mentions dropout rate of outpatients as outcome. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. |
| Other bias | Low risk | No evidence of other risk of bias. |
Hayes 1984.
| Study characteristics | ||
| Methods |
Randomised controlled trial of routine hospital clinic care versus routine general practice care for type II diabetics Patient RCT, conducted in diabetic clinics from a hospital in Wales, UK Two arms: 1. General practice (control arm) and 2. Hospital (intervention arm) |
|
| Participants | Control arm N: 103 Intervention arm N: 97 Diabetes type: type 2 Mean age: 59.0 ± 8.2 % Male: 41.0 Longest follow‐up: 60 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Team changes |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre NR (NR), post 10.4 (1.7) Intervention arm: pre NR (NR), post 9.5 (1.8) |
|
| Funding source | The trial was financed by a grant from the Office of the Chief Scientist, Department of Health and Social Security | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
He 2018.
| Study characteristics | ||
| Methods |
Application effect of multidisciplinary nursing model for diabetic nephropathy patients with uremia complicated with cerebral infarction RCT (NA clusters and NA providers), conducted in 1) Intervention delivered at the dialysis clinic, city of Shenzhen, province of Guangdong, China. 2) Intervention involved a multidisciplinary nursing model. Multidisciplinary team led by a nurse: neurologist, endocrinologist, rehabilitation physician, nutritionist and psychological counsellor. In China. 2 arms: 1. Control (routine nursing dialysis) (control arm) and 2. Intervention (multidisciplinary nursing dialysis model) (intervention arm) |
|
| Participants | Control arm N: 33 Intervention arm N: 33, NA, NA Diabetes type: 4 Mean age: 62.84 ± 12.47 % Male: 65.15 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (routine nursing dialysis) Intervention arm: (multidisciplinary nursing dialysis model) 1) Case management 2) Team change 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The trial was financed by a grant from the Office of the Chief Scientist, Department of Health and Social Security | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence generated with a random number table. A total of 66 diabetic nephropathy patients were randomly divided into the observation group and the control group with 33 cases in each. |
| Allocation concealment (selection bias) | Unclear risk | Method to conceal the allocation sequence is not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Mean age in each group is reported in text (62.63 ± 8.45 and 63.04 ± 7.92). Gender is also reported in text in each group (men: 20/33 vs 23/33). |
| Patient's baseline outcomes (selection bias) | Low risk | Tables 1 to 5. All outcomes are not significantly different between groups at baseline (all P values higher than 0.05). |
| Incomplete outcome data (attrition bias) | Unclear risk | No report on the number lost. Looks like they only included the patients who completed the whole study in the paper. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively measured (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Patients randomised and recruited from a single dialysis clinic. Unsure if the same health professionals were taking care of patients in both groups. |
| Other bias | Low risk | No evidence of other risk of bias. |
Heisler 2010.
| Study characteristics | ||
| Methods |
Diabetes control with reciprocal peer support versus nurse care management Patient RCT, conducted in 2 mid‐western U.S. Department of Veterans Affairs (VA) facilities, USA Two arms: 1. Nurse‐Care Management Group (control arm) and 2. Reciprocal Peer‐Support Group (intervention arm) |
|
| Participants | Control arm N: 119 Intervention arm N: 126 Diabetes type: unclear/not reported Mean age: 62.0 ± 6.3 % Male: 100.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.9 (1.4), post 8.2 (1.7) Intervention arm: pre 8.0 (1.3), post 7.7 (1.3) 2) SBP, mean mmHg (SD) Control arm: pre 136.4 (16.9), post 135.0 (17.7) Intervention arm: pre 140.3 (18.6), post 136.9 (16.8) 3) DBP, mean mmHg (SD) Control arm: pre 75.8 (10.7), post 76.1 (10.6) Intervention arm: pre 77.1 (11.5), post 76.8 (11.9) |
|
| Funding source | By the U.S. Department of Veterans Affairs Health Services Research and Development Service (grant IIR 04‐239), the Michigan Diabetes Research and Training Center (National Institutes of Health [NIH] grant 5P60‐DK20572), the Robert Wood Johnson Foundation Clinical Scholars Program, and the Michigan Institute for Clinical and Health Research (NIH grant UL1RR024986). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random sequence generation." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…treatment group assignment was determined centrally.." but did not describe if/how concealment was accomplished. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "The groups did not significantly differ in any measure." |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.70); SBP (P = 0.96); DBP (0.81). |
| Incomplete outcome data (attrition bias) | High risk | ~9% lost to follow‐up for HbA1c analysis in intervention group and ~13% in control group. Reasons for loss to follow‐up not explicitly reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Quote: "Data assessors remained blinded to group assignments throughout the study." HbA1c measured using Bayer DCA2000+ Analyzer, BP using Omron. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes such as medication adherence, emotional distress, etc. were not listed in the protocol. |
| Risk of contamination (other bias) | High risk | Quote: "We also cannot exclude treatment bias, because the same care managers provided care to patients in both groups." |
| Other bias | Low risk | Information not available. |
Heisler 2012.
| Study characteristics | ||
| Methods |
Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high‐performing health systems. The adherence and intensification of medications cluster randomized, controlled pragmatic trial. Cluster RCT (16 clusters, 5 to 28 providers per cluster), conducted in 5 outpatient primary care clinics (3 urban Veteran's Affairs (VA) in the Midwest and 2 (Kaiser Permanente) KP in California, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 2303 Intervention arm N: 2319 Diabetes type: type 2 Mean age: 65.3 ± NR % Male: NR Longest follow‐up: 20 months |
|
| Interventions |
Control arm: 1) Case management Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.4 (1.6), post 7.6 (1.6) Intervention arm: pre 7.4 (1.6), post 7.4 (1.4) 2) SBP, mean mmHg (SD) Control arm: pre 153.0 (12.0), post 144.0 (NR) Intervention arm: pre 154.0 (10.0), post 145.1 (NR) 3) LDL, mean mg/dL (SD) Control arm: pre 95.0 (34.0), post 87.8 (32.9) Intervention arm: pre 94.0 (33.0), post 89.1 (31.1) |
|
| Funding source | This work was supported by Department of Veterans Affairs Health Services Research and Development award SDP 06‐128 and the National Institute of Diabetes and Digestive and Kidney Diseases grant 5 R18 DK076622 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Not described. Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not provided. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Unclear in text but not in table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not described in Table 1, significance values not provided. |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis, baseline based on those randomised. However they do not provide reasons for and numbers of loss to follow‐up in control group, if any had occurred. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary: SBP, objective laboratory methods not described. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Quote: "Team level randomization also minimized cross‐over contamination due to pharmacist contact within teams." |
| Other bias | Low risk | Information not available. |
Heisler 2014.
| Study characteristics | ||
| Methods |
Comparison of community health worker‐led diabetes medication decision‐making support for low‐income Latino and African American adults with diabetes using e‐health tools versus print materials Patient RCT, conducted in a community health centre in Detroit, serving Latino and African American low‐income, USA Two arms: 1. Print materials (control arm) and 2. iDecide (intervention arm) |
|
| Participants | Control arm N: 95 Intervention arm N: 93 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.3 (2.2), post 7.9 (1.9) Intervention arm: pre 8.2 (1.9), post 7.8 (1.7) |
|
| Funding source | Agency for Healthcare Research and Quality and National Institute of Diabetes and Digestive and Kidney Diseases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by a computer program, through use of a random‐sequence algorithm, into 1 of 2 study groups. |
| Allocation concealment (selection bias) | Low risk | Web‐based (central allocation). |
| Patient's baseline characteristics (selection bias) | High risk | Education (P < 0.001), difficulty with written info (P = 0.03), confident completing forms (P = 0.003). |
| Patient's baseline outcomes (selection bias) | Low risk | Secondary outcome: HbA1c (P = 0.58). |
| Incomplete outcome data (attrition bias) | Low risk | ~6% lost in both arms, reasons provided and seem balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Secondary outcome: HbA1c, used objective laboratory methods, measured using Bayer DCA2000+ point of care analyser. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | High risk | Same community centre, with community health workers (CHWs) providing intervention and control, possible contamination? |
| Other bias | Low risk | Information not available. |
Hendricks 2000.
| Study characteristics | ||
| Methods |
The effect of diabetes self‐management education with frequent follow‐up on the health outcomes of African American men RCT (NA clusters and NA providers), conducted in 1) Participants attended diabetes classes at the IHCA Diabetes Self‐Management Skills Training Center (IHCN DSMSTC) in Wheaton, Maryland. 2) Patients were instructed 2 hours each week for 4 weeks by a licensed clinical social worker and a nurse practitioner, both CDEs in United States of America 2 arms: 1. Control: 3 month follow‐up (twice over 6 months) (control arm) and 2. Intervention: monthly follow‐up (6 times over 6 months) (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 15, NA, NA Diabetes type: 2 Mean age: 58.15 ± NR % Male: 100 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (3 month follow‐up (twice over 6 months)) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (monthly follow‐up (6 times over 6 months)) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by a grant from the AADE Education and Research Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | High risk | Convenience sampling. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 5 notes. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 5. Reported only for those who completed the study. |
| Incomplete outcome data (attrition bias) | High risk | 5 men dropped out, all in the 1‐month follow‐up group; 33.3% (5/15). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Not all participants were measured for HbA1c values. |
| Risk of contamination (other bias) | Unclear risk | Both groups received the same intervention with different follow‐up times. |
| Other bias | Low risk | None identified. |
Hendrie 2014.
| Study characteristics | ||
| Methods |
Cost‐effectiveness of reducing glycaemic episodes through community pharmacy management of patients with type 2 diabetes mellitus Clustered RCT (8 clusters and 11 providers), conducted in 1) We recruited patients and delivered the intervention in 8 metropolitan community pharmacies in Perth, Western Australia for the study. Each pharmacy had a project pharmacist. 2) Three education pharmacists delivered the Diabetes Management Education Program (DMEP) intervention to patients. In Australia. 2 arms: 1. Control (standard pharmacy care) (control arm) and 2. Intervention (pharmacist‐led Diabetes Management Education Program (DMEP)) (intervention arm) |
|
| Participants | Control arm N: 126 Intervention arm N: 119, NA, NA Diabetes type: 2 Mean age: 64.17 ± NR % Male: 46.73 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard pharmacy care) 1) Patient education 2) Promotion of self‐management 3) Patient reminders Intervention arm: (pharmacist‐led Diabetes Management Education Program (DMEP)) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Harms | |
| Funding source | "This study would have been impossible without the generous participation of 8 pharmacies and the cooperation of their pharmacists. The Pharmacy Guild of Australia helped fund the study." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Recruited 8 metropolitan community pharmacies in Perth, Western Australia for the study. Paired based on geographical location and the socioeconomic status of the population served, and then one pharmacy in each pair randomly selected to be in the intervention (DMEP protocol) group, with the other assigned to the control (standard care) group. Randomised 245 patients. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Recruited 8 metropolitan community pharmacies in Perth, Western Australia for the study. Paired based on geographical location and the socioeconomic status of the population served. No demographic data reported for each group of clinics. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Characteristics balanced between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Outcomes balanced between groups. |
| Incomplete outcome data (attrition bias) | High risk | The attrition rate was substantial, with 35% of patients randomised into the study deciding not to participate. Among participants, 19% did not complete the 6‐month exit study. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Patients were likely unblinded. Collected data about hypoglycaemia and hyperglycaemia events in patient diaries, at pharmacy visits and from each patient’s general practitioner. No evidence that glucose values were used to identify hypoglycaemia and hyperglycaemia events. Quote: "Furthermore, if DMEP patients kept diaries more diligently than control group patients, their self‐reports may have been more accurate, which could bias comparisons between groups." |
| Selective reporting (reporting bias) | High risk | No registered or published protocol. Obtained available clinical data (e.g. blood pressure, HbA1c, lipids) from the patient’s general practitioner at the same time intervals. No data reported for HbA1c and blood pressure as well as for lipids. |
| Risk of contamination (other bias) | Low risk | Clustered RCT; unlikely that control patients from the control clinics met with the 3 education pharmacists delivering the DMEP intervention only in the intervention clinics. |
| Other bias | Low risk | None identified. |
Hermanns 2017.
| Study characteristics | ||
| Methods |
The effect of an education programme (MEDIAS 2 BSC) of non‐intensive insulin treatment regimens for people with Type 2 diabetes: a randomized, multi‐centre trial RCT (NA clusters and NA providers), conducted in 1) The sample was recruited from 18 outpatient study centres in Germany. A study centre is a medical practice that is operated by a diabetologist and a diabetes educator or nurse (secondary level of diabetes care). 2) Certified diabetes educators conducted MEDIAS 2 BSC and the control group in outpatient settings. In Germany. 2 arms: 1. Control (established group education programme) (control arm) and 2. Intervention (self‐management oriented education programme, MEDIAS 2 BSC) (intervention arm) |
|
| Participants | Control arm N: 90 Intervention arm N: 92, NA, NA Diabetes type: 2 Mean age: 63.75 ± 12.34 % Male: 55.23 Longest follow‐up: 7.2 months |
|
| Interventions |
Control arm: (established group education programme) 1) Patient education 2) Promotion of self‐management Intervention arm: (self‐management oriented education programme, MEDIAS 2 BSC) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Funding sources: None. NH, BK and TH received unrestricted research grants from BerlinChemie, Roche, Dexcom, Abbott Germany and Novo Nordisk. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed at the participant level. Recruited participants from each study centre were randomised to either MEDIAS 2 BSC or the control group. Statistical software (SYSTAT 12.0) was used for block randomisation, with the block size depending on the size of the pool for each study centre (n = 6 to 16). |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred centrally at the study co‐ordinating centre, whose staff were not involved in the recruitment or treatment of study participants. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | 22 patients were lost out of 182 (12.1%): 12 in control group (13.3%) and 10 in the intervention group (10.9%). Numbers balanced but quite high. 8 lost to follow‐up and 4 terminated insulin treatment in control group, 9 lost to follow‐up and 1 terminated insulin treatment in intervention group. Reasons unbalanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | The primary outcome is objective (HbA1c, laboratory personnel were blinded), and hypoglycaemia is subjective (patient‐reported, secondary outcome). Neither the person with diabetes nor the diabetes educator were blinded to treatment allocation. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol (protocol first posted in April 2016, study conducted from February 2013 to May 2016). They used the EuroQol EQ‐5D instead of Short Form Health Survey (SF‐12) to measure quality of life and they do not look at diabetes‐related distress using the Diabetes Distress Scale (DDS). They looked at hypoglycaemia awareness and hypoglycaemia events in the paper, but these outcomes are not listed in the protocol. Protocol does not mention patient and educator evaluation of programme. |
| Risk of contamination (other bias) | Low risk | Certified diabetes educators conducted MEDIAS 2 BSC and the control group in outpatient settings. Not clear if the same educators met both arms. Seems like diabetes educators for the intervention group were not the same for the control group: "Diabetes educators who delivered MEDIAS 2 BSC rated the self management oriented content of the education programme significantly more positive than diabetes educators who delivered the control group." Both education programmes target type 2 diabetes patients who inject insulin, and both programmes provide skills to titrate insulin. |
| Other bias | Low risk | No risk of other bias. |
Hermans 2013.
| Study characteristics | ||
| Methods |
Benchmarking is associated with improved quality of care in type 2 diabetes Cluster‐RCT (477 clusters with 477 providers), conducted in general practitioner or hospital‐based outpatient clinics, Belgium, Greece, Luxembourg, Portugal, Spain and United Kingdom Two arms: 1. Control group (control arm) and 2. Benchmarking group (intervention arm) |
|
| Participants | Control arm N: 1518 Intervention arm N: 2509 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 718 (48), post 778 (57) Intervention arm: pre 1172 (47), post 1192 (56) 2) Statins, N users (%) Control arm: pre 962 (64), post 955 (70) Intervention arm: pre 1698 (68), post 1623 (76) 3) Antihypertensives (any), N users (%) Control arm: pre 1446 (96), post 1325 (97) Intervention arm: pre 2416 (97), post 2067 (97) 4) Retinopathy screening (eye exam), N screened (%) Control arm: pre 797 (53), post 382 (28) Intervention arm: pre 1546 (62), post 765 (36) 5) Foot screening, N screened (%) Control arm: pre 601 (40), post 504 (37) Intervention arm: pre 1296 (52), post 1041 (49) 6) HbA1c, mean % (SD) Control arm: pre 7.1 (1.3), post 6.9 (1.2) Intervention arm: pre 7.2 (1.4), post 6.9 (1.5) 7) SBP, mean mmHg (SD) Control arm: pre 138.0 (17.0), post 135.7 (16.0) Intervention arm: pre 138.0 (16.4), post 133.0 (14.1) 8) LDL, mean mg/dL (SD) Control arm: pre 103.9 (34.1), post 96.9 (32.8) Intervention arm: pre 104.2 (34.2), post 92.2 (32.4) 9) Controlled hypertension (SBP < 130 mmHg), N under control (%) Control arm: pre 323 (27), post 325 (30) Intervention arm: pre 587 (27), post 736 (40) 10) Smoking cessation, N smokers (%) Control arm: pre 225 (15), post 204 (15) Intervention arm: pre 399 (16), post 319 (15) |
|
| Funding source | They reported that different authors received payments, but it is not clear if the study was funded as well or not | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomized by a centralized randomization procedure (What Health, Belgium)". |
| Allocation concealment (selection bias) | Low risk | Quote: "sequence was concealed until the intervention was assigned". |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Not in table, and in text they said there were no differences. |
| Patient's baseline outcomes (selection bias) | Low risk | Numbers look similar (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | Practices were lost after randomisation (reasons not provided per arm). Patient level: 14% lost in intervention group and 9% in control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | For HbA1c and cholesterol levels, blood was sent to laboratory (methods not described). Did not describe what was used to measure BP. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | None identified. |
Herrin 2006.
| Study characteristics | ||
| Methods |
Effectiveness of diabetes resource nurse care management and physician profiling in a fee‐for‐service setting: a cluster randomized trial Cluster‐RCT (22 clusters with 92 providers), conducted in Family Medicine and Internal Medicine practices within the HealthTexas Provider Network (HTPN) ‐ physician component of the Baylor Health Care System ‐ Dallas‐Fort Worth, Texas. HTPN‐ fee for service setting. In USA. Three arms: 1. Claims (control arm), 2. Claims + MR (intervention arm 1) and 3. Claims + MR + DRN (intervention arm 2). |
|
| Participants | Control arm N: 652 Intervention arm 1 N: 849 Intervention arm 2 N: 654 Diabetes type: unclear/not reported Mean age: 72.9 ± NR % Male: 49.8 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Audit and feedback Intervention arm 1: 1) Audit and feedback Intervention arm 2: 1) Audit and feedback 2) Case management 3) Clinician reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 61 (11), post 58 (10) Intervention arm 1: pre 127 (17), post 114 (15) Intervention arm 2: pre 119 (21), post 119 (21) 2) Foot screening, N screened (%) Control arm: pre 229 (41), post 167 (30) Intervention arm 1: pre 409 (54), post 363 (48) Intervention arm 2: pre 270 (48), post 261 (46) 3) Renal screening (renal), N screened (%) Control arm: pre 168 (30), post 193 (34) Intervention arm 1: pre 304 (40), post 357 (47) Intervention arm 2: pre 234 (41), post 279 (49) 4) HbA1c, mean % (SD) Control arm: pre 7.2 (1.5), post 7.2 (1.4) Intervention arm 1: pre 7.2 (1.4), post 7.0 (1.2) Intervention arm 2: pre 7.1 (1.4), post 7.0 (1.2) 5) SBP, mean mmHg (SD) Control arm: pre 139.5 (18.5), post 137.0 (17.9) Intervention arm 1: pre 139.7 (18.6), post 138.4 (18.3) Intervention arm 2: pre 140.2 (18.0), post 138.1 (18.1) 6) DBP, mean mmHg (SD) Control arm: pre 76.0 (9.9), post 74.7 (10.4) Intervention arm 1: pre 77.7 (9.5), post 76.5 (9.7) Intervention arm 2: pre 77.1 (9.8), post 75.6 (10.7) 7) LDL, mean mg/dL (SD) Control arm: pre 106.9 (32.9), post 106.6 (31.7) Intervention arm 1: pre 104.6 (32.6), post 101.3 (30.7) Intervention arm 2: pre 104.3 (33.9), post 104.1 (31.6) 8a) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 110 (20), post 128 (25) Intervention arm 1: pre 136 (18), post 147 (21) Intervention arm 2: pre 99 (18), post 116 (22) 8b) Controlled hypertension (< 140/90 mmHg), N under control (%) Control arm: pre 273 (49), post 283 (55) Intervention arm 1: pre 329 (44), post 336 (49) Intervention arm 2: pre 242 (43), post 266 (51) |
|
| Funding source | Funding was provided by the American Diabetes Association, Pfizer, Inc., and the Baylor Health Care System | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Similar based on text, but no P values provided in Table 1. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Baseline values P values not provided for outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Although only ~9% lost to follow‐up in each arm, there were n = 3 that were excluded from analysis since chart data were not available; they do not provide details from which arm these are missing, so not able to determine the actual sample size for each arm under analysis. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Although HbA1c laboratory methods not described, the outcome assessors were blinded. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | None identified. |
Hetlevik 2000.
| Study characteristics | ||
| Methods |
Evaluation of effort, process, and patient outcome related to implementation of a computer‐based decision support system Cluster‐RCT (29 clusters with 53 providers), conducted in general practices in 2 Norwegian counties, Norway Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 535 Intervention arm N: 499 Diabetes type: type 1 and type 2 Mean age: 66.0 ± 16.3 % Male: NR Longest follow‐up: 21 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Clinician reminders 4) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.2 (1.8), post 7.9 (1.6) Intervention arm: pre 8.2 (1.8), post 7.8 (1.6) 2) SBP, mean mmHg (SD) Control arm: pre 151.7 (21.3), post 152.7 (19.0) Intervention arm: pre 152.5 (21.6), post 151.5 (22.1) 3) DBP, mean mmHg (SD) Control arm: pre 85.3 (9.9), post 85.1 (10.1) Intervention arm: pre 84.5 (10.0), post 82.8 (10.6) 4) Smoking cessation, N smokers (%) Control arm: pre NR (NR), post 33 (16) Intervention arm: pre NR (NR), post 49 (19) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Only number and gender of GPs and number of patients reported in text, no P values. |
| Patient's baseline characteristics (selection bias) | Unclear risk | At baseline registration (Table 5) 53% of the patients in the intervention group and 55% in the control group (p. 214) were female. Data are also available for age in the table. Not on education. |
| Patient's baseline outcomes (selection bias) | Low risk | No baseline differences were discovered for patient outcome measurements (Table 5). CI is reported for difference between the groups and it is not significant. |
| Incomplete outcome data (attrition bias) | Low risk | 26% lost to follow‐up in intervention group and 24% in control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Hiss 2001.
| Study characteristics | ||
| Methods |
Comprehensive evaluation of community‐based diabetic patients: effect of feedback to patients and their physicians: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) We selected 4 Michigan communities, 2 large and 2 small, from a pool of Michigan communities. Patients attend a specially arranged clinic in their community where they could be examined and followed (The Michigan Diabetes Research and Training Center‐MDRTC). 2) The intervention was delivered by the project diabetes nurse specialists. In United States of America. 2 arms: 1. Control (mailed health report) (control arm) and 2. Intervention (health report and counselling) (intervention arm) |
|
| Participants | Control arm N: 190 Intervention arm N: 186, NA, NA Diabetes type: 2 Mean age: 64.05 ± NR % Male: 42.82 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (mailed health report) 1) Electronic patient registry 2) Facilitated relay of clinical information Intervention arm: (health report and counselling) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This study was supported by National Institutes of Health Grant 5‐P60‐DK‐20572, the National Institute of Diabetes and Digestive and Kidney Diseases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As patients entered the study, they were randomly assigned using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The baseline demographics were similar for all 4 high‐risk groups (Table 1) |
| Patient's baseline outcomes (selection bias) | Low risk | The baseline demographics were similar for all 4 high‐risk groups (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | 62 patients missing out of 376 for post‐intervention data (16.5%). 15.1% in the intervention group and 17.9% in the control group. Reasons partly reported and some are unbalanced (death and lost). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All outcomes were objectively measured (HbA1c, SBP and DBP). |
| Selective reporting (reporting bias) | High risk | No registered protocol. In the methods, patients were tested for LDL, HDL, knowledge, height, weight, etc. at baseline, but these outcomes are not reported after intervention. |
| Risk of contamination (other bias) | Unclear risk | Only those in intervention group met with project nurses, but physicians met with patients in both groups; interaction with the nurses and patients in intervention group may have influenced care of those in control group. Furthermore, a comprehensive evaluation report for all patients were sent by email to physicians. Physicians might have changed their regular care with control patients. |
| Other bias | Low risk | No evidence of other bias. |
Hiss 2007.
| Study characteristics | ||
| Methods |
Nurse care manager collaboration with community‐based physicians providing diabetes care: a randomized controlled trial Patient RCT, conducted in the general population of a large metropolitan area, USA Two arms: 1. Basic intervention (control arm) and 2. Individualised intervention (intervention arm) |
|
| Participants | Control arm N: 102 Intervention arm N: 95 Diabetes type: type 2 Mean age: 56.4 ± 12.2 % Male: 33.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 7.4 (0.2), post 7.2 (NR) Intervention arm: pre 7.7 (0.2), post 7.3 (NR) 2) SBP, mean mmHg (SE) Control arm: pre 129.0 (NR), post 133.1 (NR) Intervention arm: pre 136.0 (NR), post 128.7 (NR) 3) DBP, mean mmHg (SE) Control arm: pre 73.0 (1.0), post 73.7 (NR) Intervention arm: pre 76.0 (1.2), post 75.0 (NR) |
|
| Funding source | Financial support was provided by the National Institutes of Health grant 5 P60 DK20572, National Institute of Diabetes and Digestive and Kidney Diseases. The authors appreciate the collaboration with the Packard Community Clinic in Ann Arbor, the Hope Clinic in Ypsilanti, and the Wayne County Department of Public Health headquartered in Wayne, Michigan. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Holbrook 2009.
| Study characteristics | ||
| Methods |
Individualized electronic decision support and reminders to improve diabetes care in the community: COMPETE II randomized trial Patient RCT, conducted with 46 primary care providers across Ontario, Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 258 Intervention arm N: 253 Diabetes type: type 2 Mean age: 60.7 ± 12.5 % Male: 50.7 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient reminders |
|
| Outcomes | 1) Foot screening, N screened (%) Control arm: pre 72 (28), post 93 (36) Intervention arm: pre 71 (28), post 129 (51) 2) Renal screening (microalbumin), N screened (%) Control arm: pre 67 (26), post 101 (39) Intervention arm: pre 63 (25), post 171 (68) 3) HbA1c, mean % (SD) Control arm: pre 7.1 (1.6), post 7.3 (1.6) Intervention arm: pre 7.0 (1.4), post 6.8 (1.2) 4) SBP, mean mmHg (SD) Control arm: pre 134.8 (18.4), post 135.1 (18.4) Intervention arm: pre 135.2 (17.6), post 130.5 (16.4) 5) DBP, mean mmHg (SD) Control arm: pre 74.7 (10.3), post 75.4 (10.5) Intervention arm: pre 76.1 (11.1), post 73.6 (9.9) 6) LDL, mean mg/dL (SD) Control arm: pre 100.2 (33.6), post 98.2 (31.3) Intervention arm: pre 93.2 (25.1), post 94.0 (30.2) 7) Smoking cessation, N smokers (%) Control arm: pre 8 (3), post 79 (31) Intervention arm: pre 1 (0), post 78 (31) |
|
| Funding source | This study was funded by a grant from the Canada Health Infostructure Partnerships Program Project 35b, Health Canada | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Holbrook 2011.
| Study characteristics | ||
| Methods |
Shared electronic vascular risk decision support in primary care Patient RCT, conducted in community‐based primary care practices using an EMR system certified by province (Ontario), Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 105 Intervention arm N: 133 Diabetes type: unclear/not reported Mean age: 69.1 ± 8.7 % Male: 46.6 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Clinician education 3) Clinician reminders 4) Facilitated relay of clinical information 5) Patient education 6) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre NR (NR), post 7.0 (1.0) Intervention arm: pre NR (NR), post 7.0 (1.0) |
|
| Funding source | This study was supported by the Ontario Ministry of Health and Long‐term Care’s Primary Healthcare Transition Fund competition. Role of the Sponsors: the sponsors had no role in the design and conduct of the study; in the collection, management, analysis or interpretation of the data; or in the preparation, review or approval of the manuscript. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomized using an allocation‐concealed online program." Not sure if a random list of numbers was used? |
| Allocation concealment (selection bias) | Low risk | Quote: "…randomized using an allocation‐concealed online program." |
| Patient's baseline characteristics (selection bias) | Low risk | In text but not in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "Blood pressure, low density lipoprotein cholesterol level, aspirin use, and smoking were reasonably well controlled at baseline for many patients." |
| Incomplete outcome data (attrition bias) | Low risk | Not a true intention‐to‐treat analysis. Per‐protocol analysis. Numbers and reasons for loss to follow‐up provided and similar. Baseline based on those randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Outcome assessors blinded. Objective laboratory methods not described. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Holtrop 2017.
| Study characteristics | ||
| Methods |
Diabetic and obese patient clinical outcomes improve during a care management implementation in primary care Clustered RCT (10 clusters and NR providers), conducted in 1) 5 practices implemented care management and were compared with 5 comparison practices within the same practice organisation. The participating practices were part of a physician‐owned medical group in southeast Michigan. Ten total primary care practices were categorised according to size (large being 5 or more providers or small being 4 or less providers), discipline (family or internal medicine) and rural or suburban location and placed in pairs. One practice from each pair was randomly selected using a random number generator for intervention. 2) Addition of new care managers and using new care management software to help patients co‐ordinate their care and self‐manage their conditions. In United States of America. 2 arms: 1. Control (comparison practices) (control arm) and 2. Intervention (practices implemented care management) (intervention arm) |
|
| Participants | Control arm N: 443 Intervention arm N: 444, NA, NA Diabetes type: 2 Mean age: 57.9 ± 17.35 % Male: 53.8 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (comparison practices) Intervention arm: (practices implemented care management) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician reminder 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The project described was supported by Award Number 1 R18 DK082377‐01A2 from the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support was provided by Grant No. P30DK092926 from the National Institute of Diabetes and Digestive, and Kidney Disease. John Piette is a US Department of Veterans Affairs Research Career Scientist. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Low risk for clinic randomisation and high risk for patient selection. Ten total primary care practices were categorised according to size (large being 5 or more providers or small being 4 or less providers), discipline (family or internal medicine) and rural or suburban location and placed in pairs. One practice from each pair was randomly selected using a random number generator for intervention. Intervention patients were randomly selected but each control patients were matched to an intervention patients based on these criteria: same baseline risk score (defined on an 8‐point scale from 0 = no risk factors to 7 = diabetes and LDL > 100 mg/dL and SBP > 140 mm Hg), disease status (diabetes vs obesity without diabetes), and whose first available clinic datum was within ± 3 months of the enrollment time of the intervention patient. |
| Allocation concealment (selection bias) | Low risk | Pair‐matched cluster‐randomised controlled trial. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No data reported. The practice participants included 10 primary care practices within one physician‐owned practice organisation in southeast Michigan. Intervention practices were pair‐wise matched with similar comparison practices on practice discipline, size and geographic location. There were 2 internal medicine (1 large urban and 1 small urban) and 3 family medicine (1 large urban, 1 large rural, and 1 small rural) in each pair. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, left panel (patients with diabetes). Gender and age have P value higher than 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1, left panel (patients with diabetes). BMI, oral diabetic medication (%), insulin (%) and statin (%), weight (pounds) have significant P values. Quote: "Patients in the intervention group at baseline were overall significantly different on several characteristics as compared with matched comparison patients." |
| Incomplete outcome data (attrition bias) | High risk | The number lost is not reported. Quote: "We noted that variability in patient health care participation was a source of missing data. Clearly, all patients did not visit the clinics every quarter and missing data may be more common among patients with poorer health status." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest were objectively measured (HbA1c, SBP and LDL). |
| Selective reporting (reporting bias) | High risk | No registered protocol. They only reported these data at baseline: we also extracted data on prescriptions for metformin, long‐acting insulin, short‐acting insulin, glitazones, DPP‐4 agents, sulfonylureas, beta‐blockers, angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB‐II), calcium channel blockers, centrally acting antihypertensives (e.g. clonidine), statins, orlistat and appetite suppressants. Only report SBP, not DBP. |
| Risk of contamination (other bias) | Low risk | Pair‐matched cluster‐randomised controlled trial. Clustered by practice and no mention of contamination events. |
| Other bias | Low risk | None. |
Hoskins 1993.
| Study characteristics | ||
| Methods |
Sharing the care of diabetic patients between hospital and general practitioners: does it work? Patient RCT, conducted in diabetic clinic at Royal Prince Alfred Hospital in New South Wales, Australia Three arms: 1. GP care (control arm), 2. Clinic care (intervention arm 1) and 3. Shared care (intervention arm 2) |
|
| Participants | Control arm N: 72 Intervention arm 1 N: 65 Intervention arm 2 N: 69 Diabetes type: type I and type 2 Mean age: 53.4 ± 13.3 % Male: 51.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Team changes Intervention arm 2: 1) Team changes 2) Clinician reminders 3) Patient education 4) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.4 (2.6), post 6.9 (1.3) Intervention arm 1: pre 8.9 (2.5), post 7.3 (1.6) Intervention arm 2: pre 8.5 (2.2), post 6.6 (1.6) 2) SBP, mean mmHg (SD) Control arm: pre 148.0 (23.0), post 136.0 (14.0) Intervention arm 1: pre 150.0 (23.0), post 133.0 (19.0) Intervention arm 2: pre 145.0 (24.0), post 130.0 (25.0) 3) DBP, mean mmHg (SD) Control arm: pre 90.0 (15.0), post 81.0 (11.0) Intervention arm 1: pre 90.0 (13.0), post 81.0 (13.0) Intervention arm 2: pre 88.0 (13.0), post 81.0 (11.0) |
|
| Funding source | This study was supported by a Diabetes Australia/APEX Research Grant and an award from the Ames Division of Bayer, Australia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Hotu 2010.
| Study characteristics | ||
| Methods |
A community‐based model of care improves blood pressure control and delays progression of proteinuria, left ventricular hypertrophy and diastolic dysfunction in Maori and Pacific patients with type 2 diabetes and chronic kidney disease: a randomized controlled trial Patient RCT, conducted in hospital diabetes and renal clinics and primary care practices in 2 areas of Auckland, NZ, which provides comprehensive public health care, New Zealand Two arms: 1. Usual care (control arm) and 2. Community/intervention care (intervention arm) |
|
| Participants | Control arm N: 32 Intervention arm N: 33 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Financial incentives |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (1.9), post 7.9 (1.7) Intervention arm: pre 8.3 (1.6), post 8.0 (1.9) 2) SBP, mean mmHg (SD) Control arm: pre 161.0 (20.0), post 149.0 (23.0) Intervention arm: pre 161.0 (20.0), post 140.0 (19.0) 3) DBP, mean mmHg (SD) Control arm: pre 85.0 (12.0), post 77.0 (12.0) Intervention arm: pre 88.0 (9.0), post 78.0 (11.0) |
|
| Funding source | This work was supported by funding from Auckland District Health Board and grants from the Health Research Council of New Zealand and Eli Lilly | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not describe process of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Does not describe process of allocation concealment. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "there were no significant differences in any of the variables between the groups." |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those randomised. They provide the numbers and reasons for loss to follow‐up, but reasons are not entirely clear for the control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: BP, using Omron.
Secondary: HbA1c, objective laboratory methods not described. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Houweling 2009.
| Study characteristics | ||
| Methods |
Diabetes specialist nurse as main care provider for patients with type 2 diabetes Patient RCT, conducted in diabetes outpatient clinics at 2 hospitals in The Netherlands Two arms: 1. Standard care (control arm) and 2. NSD ‐ nurse specialised in diabetes (intervention arm) |
|
| Participants | Control arm N: 43 Intervention arm N: 50 Diabetes type: type 2 Mean age: 61.5 ± 10.6 % Male: 46.4 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Patient education |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 13 (34), post 26 (68) Intervention arm: pre 21 (46), post 25 (54) 2) Antihypertensives (any), N users (%) Control arm: pre 21 (55), post 27 (71) Intervention arm: pre 31 (67), post 39 (85) 3) HbA1c, mean % (SD) Control arm: pre 8.6 (1.3), post 7.7 (NR) Intervention arm: pre 8.9 (1.2), post 7.4 (NR) 4) SBP, mean mmHg (SD) Control arm: pre 156.3 (19.9), post 152.3 (NR) Intervention arm: pre 154.9 (23.3), post 146.3 (NR) 5) DBP, mean mmHg (SD) Control arm: pre 85.6 (9.4), post 83.2 (NR) Intervention arm: pre 86.6 (10.9), post 85.2 (NR) 6) LDL, mean mg/dL (SD) Control arm: pre 104.4 (38.7), post 81.2 (NR) Intervention arm: pre 100.5 (34.8), post 88.9 (NR) 7) Controlled hypertension (< 140/90 mmHg), N under control (%) Control arm: pre 9 (24), post 9 (24) Intervention arm: pre 10 (22), post 12 (26) |
|
| Funding source | Financial sponsors: the Medical Research Fund Zwolle, the Steering Committee Care Renewal from the Isala Clinics, The Dutch Ministry of Health, Welfare and Sport and the Langerhans Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised. |
| Allocation concealment (selection bias) | Low risk | The patient population was randomised using non‐transparent closed envelopes, with sequential numbers enclosed. Participants with even numbers were assigned to the intervention group, and those with odd numbers were assigned to the control group. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values provided, looks balanced. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. No P values provided, looks balanced. |
| Incomplete outcome data (attrition bias) | Low risk | See Figure 1: 4 lost in intervention group (8%) and 5 lost in control group (12%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of all outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, however all outcomes in methods reported in results. |
| Risk of contamination (other bias) | Low risk | Groups were followed by different personnel, contamination unlikely. |
| Other bias | Low risk | None identified. |
Houweling 2011.
| Study characteristics | ||
| Methods |
Can diabetes management be safely transferred to practice nurses in a primary care setting? A randomised controlled trial Patient RCT, conducted in a group practice with 5 GPs in north east region of The Netherlands Two arms: 1. General practitioner ‐ GP (control arm) and 2. Practice nurse ‐ PN (intervention arm) |
|
| Participants | Control arm N: 114 Intervention arm N: 116 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 14 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Clinician education |
|
| Outcomes | 1) Foot screening, N screened (%) Control arm: pre NR (NR), post 13 (27) Intervention arm: pre NR (NR), post 34 (57) 2) HbA1c, mean % (SD) Control arm: pre 7.4 (1.3), post 7.4 (NR) Intervention arm: pre 7.6 (1.3), post 7.5 (NR) 3) SBP, mean mmHg (SD) Control arm: pre 161.3 (24.8), post 155.7 (NR) Intervention arm: pre 157.5 (20.4), post 150.1 (NR) 4) DBP, mean mmHg (SD) Control arm: pre 87.0 (11.2), post 86.0 (NR) Intervention arm: pre 87.2 (10.7), post 84.0 (NR) 5) Controlled hypertension (< 140/90 mmHg), N under control (%) Control arm: pre 19 (18), post 22 (21) Intervention arm: pre 17 (17), post 26 (25) |
|
| Funding source | This study was sponsored by the Medical Research Fund Zwolle, the Steering Committee Care Renewal form the Isala Clinics, and the Dutch Ministry of Health, Welfare and Sport | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "…containing sequential numbers." Even assigned to intervention and odd assigned to control, i.e. not random. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patient population was randomised using non‐transparent, closed envelopes containing sequential numbers. Subjects with even numbers were assigned to the intervention group, and those with odd numbers were assigned to the control group." |
| Patient's baseline characteristics (selection bias) | High risk | Similar for all, except intervention group had more patients with feet at risk vs control group. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | ~13% lost to follow‐up in intervention vs ~8% in control. Also more withdrawals as reasons for lost to follow‐up in intervention group. Did a per‐protocol analysis. Provided reasons for loss to follow‐up in flow diagram, although numbers are not similar, and the number who were lost to follow‐up due to withdrawal was much higher compared to control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Measurement not described and outcome assessors not blinded. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; outcomes match those in methods. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Hsu 2014.
| Study characteristics | ||
| Methods |
Long‐term glycemic control by a diabetes case‐management program and the challenges of diabetes care in Taiwan RCT (NA clusters and NA providers), conducted in 1) Intervention delivered in 27 community clinics during 2003 to 2005, Taiwan. 2) Self‐care and nutrition‐education programme delivered by National Health Research Institutes (NHRI)‐hired case managers (20 clinics) or local case managers hired by individual clinics (7 clinics). All case managers in both groups were qualified by the NHI Administration. Physicians received pay‐for‐performance (P4P) incentive. In Taiwan. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (case management in pay‐for‐performance (P4P) program) (intervention arm) |
|
| Participants | Control arm N: 271 Intervention arm N: 789, NA, NA Diabetes type: 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 42 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (case management in pay‐for‐performance (P4P) programme) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Financial incentives |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This project was supported by grants (96A1‐HDPP08–017) funded by the National Health Research Institutes of Taiwan | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. The participants in each clinic were randomised into 2 groups. |
| Allocation concealment (selection bias) | Low risk | From protocol: project co‐ordinating centres randomise diabetic patients. |
| Patient's baseline characteristics (selection bias) | Unclear risk | No data reported in text or tables. As previously indicated, the baseline characteristics – including demographics and the biochemical profiles of the intervention and control groups – were comparable [7]. However, reference 7 only includes 154 patients, while this study has 1060 patients. |
| Patient's baseline outcomes (selection bias) | Low risk | Figure 1A shows that the HbA1c levels were not significantly different at baseline (no asterisks at 0 months). |
| Incomplete outcome data (attrition bias) | High risk | They have HbA1c data for 85/271 (69% lost) patients in the control group and 252/789 (68% lost) patients in the intervention group at 42 months follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively measured (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | From protocol: periodic measurements on glucose, blood pressures, lipids and the incidence of complications will also be analysed to set up an optimal target for diabetic control in Taiwan. However, they only report HbA1c in the paper. |
| Risk of contamination (other bias) | Unclear risk | Only the intervention patients were followed by hired case managers. However, physicians received pay‐for‐performance (P4P) incentives for both control and intervention patients (higher amount given for intervention patients to promote recruitment of sicker patients). Quote from reference 4: "An enrolled patient can “earn” a provider 4,640 more reimbursement points than a nonenrolled patient in the first year and 3,670 points in each of the subsequent years." |
| Other bias | Low risk | None identified. |
Hsu 2016.
| Study characteristics | ||
| Methods |
Utilization of a cloud‐based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients RCT (NA clusters and NA providers), conducted in 1) The recruitment took place in a tertiary diabetes centre in Massachusetts with care provided by teams of endocrinologists, nurse practitioners, and certified diabetes educators. 2) Intervention group received additional care through the cloud‐based diabetes management program. Intervention facilitates collaborative care between patients and healthcare providers (HCPs). In United States of America. 2 arms: 1. Control (standard tertiary care) (control arm) and 2. Intervention (cloud‐based diabetes management program) (intervention arm) |
|
| Participants | Control arm N: 20 Intervention arm N: 20, NA, NA Diabetes type: 2 Mean age: 53.6 ± 10.76 % Male: NR Longest follow‐up: 3.23 months |
|
| Interventions |
Control arm: (standard tertiary care) Intervention arm: (cloud‐based diabetes management program) 1) Electronic patient registry 2) Clinician education 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Sponsor: Joslin Diabetes Center | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Study participant was randomly assigned to the intervention or the control group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. The baseline characteristics between the control and the intervention groups were comparable in age, weight, height, body mass index, diabetes duration, initial insulin dose, number of non–insulin antidiabetes agents, and DTSQ score (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. No significant differences were observed for baseline HbA1c. |
| Incomplete outcome data (attrition bias) | High risk | 20 participants were randomised to the intervention group versus 20 to the control group. 5 participants (1 from the intervention group (5%) and 4 from the control group (20%) dropped out from the study. Unbalanced numbers. Specifically, 3 failed to show up at the final visit (1 from the intervention group and 2 from the control group), and 2 opted to participate in a medically supervised weight loss programme, which was not part of the study protocol. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The primary outcome is objective: HbA1c (laboratory method). Hypoglycaemia was subjective in the control group (reviewing the participants’ medical records: participants who either called following an episode or reported hypoglycaemia at the end visit) and objective in the intervention group (digitally capturing hypoglycaemic glucose readings). |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol (protocol first posted on October 2012, study started at the same time). In the paper, they added these outcomes: glucose readings, weight, final insulin dose, mean number of text and video messages sent, common themes emerging from the exit interviews. |
| Risk of contamination (other bias) | Low risk | Participants in the control group received standard care at the clinic in initiating and titrating insulin, with interim face‐to‐face visits, as well as telephone/fax communication with educators and physicians as dictated by their HCPs. |
| Other bias | Low risk | None. |
Huang 2010.
| Study characteristics | ||
| Methods |
Prospective randomized controlled trial to evaluate effectiveness of registered dietitian‐led diabetes management on glycemic and diet control in a primary care setting in Taiwan Patient RCT, conducted in public health clinics in Koahsiung, Taiwan Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 93 Diabetes type: type 2 Mean age: 56.8 ± 7.7 % Male: 43.4 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.4 (1.8), post 8.3 (NR) Intervention arm: pre 8.0 (1.5), post 7.5 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 134.9 (17.4), post 140.9 (NR) Intervention arm: pre 131.8 (19.8), post 131.1 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 84.2 (10.3), post 84.8 (NR) Intervention arm: pre 79.7 (10.5), post 79.7 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 118.5 (32.5), post 118.6 (NR) Intervention arm: pre 117.8 (33.4), post 111.8 (NR) |
|
| Funding source | This project was supported by grants funded by the National Health Research Institute (96A1‐HDPP08‐ 017) of Taiwan | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The name, age and sex of the enrollees were entered into a computer, which randomly assigned them in a 1:1 manner to control groups and intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1: P values > 0.05. There were no significant group differences in age, sex, disease duration and education in the 154 participants who remained (Table 1). |
| Patient's baseline outcomes (selection bias) | High risk | Table 1: P values < 0.05 for BMI and DBP. |
| Incomplete outcome data (attrition bias) | High risk | 19 lost in each group (25% in intervention group, 24% in control group). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measurement of outcomes. |
| Selective reporting (reporting bias) | High risk | No protocol or registry. Outcomes not explicitly stated in methods but no mention anywhere of glutamic pyruvic transanimase, which is reported in results Table 1. |
| Risk of contamination (other bias) | Low risk | Dieticians only had access to participants in intervention group. |
| Other bias | Low risk | No other evidence of risk of bias. |
Huizinga 2010.
| Study characteristics | ||
| Methods |
Preventing glycaemic relapse in recently controlled type 2 diabetes patients: a randomised controlled trial Patient RCT, conducted with patients recruited from urban area surrounding academic medical centre in Nashville, Tennessee ‐ recruited from those who completed an induction programme for poor glycaemic control, USA Three arms: 1. Control (control arm), 2. Quarterly follow‐up (intervention arm 1) and 3. Monthly follow‐up (intervention arm 2) |
|
| Participants | Control arm N: 54 Intervention arm 1 N: 55 Intervention arm 2 N: 55 Diabetes type: type 2 Mean age: 55.1 ± 10.7 % Male: 56.0 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Case management 2) Team changes 3) Promotion of self‐management Intervention arm 2: 1) Case management 2) Team changes 3) Promotion of self‐management |
|
| Outcomes | 1a) Harms (hypoglycaemia), N (%) Control arm: pre 36 (67), post 28 (58) Intervention arm 1: pre 30 (55), post 32 (70) Intervention arm 2: pre 31 (56), post 30 (58) 1b) Harms (severe hypoglycaemia), N (%) Control arm: pre 4 (7), post 3 (6) Intervention arm 1: pre 3 (5), post 3 (7) Intervention arm 2: pre 2 (4), post 5 (10) |
|
| Funding source | The research was supported by the National Institute of Diabetes and Digestive and Kidney Disease R18 DK 062258, P60 DK 020593 and K24 DK 077875. M. M. Huizinga was supported by National Institute of Environmental Health Sciences 1 K12 ES 015855 and National Center for Research Resources 5 K12 RR 023266. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using a computerised randomisation process." |
| Allocation concealment (selection bias) | Low risk | Quote: "...and assignments, which were concealed, were obtained sequentially from a computer program." |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | ~12% lost to follow‐up in N1 and N2. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c: laboratory measurement described. Quote: "Blinding to assignment was not possible given the nature of the study." |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything proposed was completed. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None identified. |
Hurwitz 1993.
| Study characteristics | ||
| Methods |
Prompting the clinical care of non‐insulin dependent (type II) diabetic patients in an inner city area: one model of community care Patient RCT, conducted in 2 hospital outpatient clinics, 38 general practices and 11 optometrists in the catchment area of a district general hospital in Islington, UK Two arms: 1. Control (control arm) and 2. Prompted (intervention arm) |
|
| Participants | Control arm N: 92 Intervention arm N: 89 Diabetes type: type 2 Mean age: 62.6 ± 10.0 % Male: 58.0 Longest follow‐up: 30 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Electronic patient registry 2) Clinician education 3) Clinician reminders 4) Patient reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 22 (24), post 58 (83) Intervention arm: pre 15 (17), post 72 (97) 2) HbA1c, mean % (SD) Control arm: pre NR (NR), post 10.6 (2.5) Intervention arm: pre NR (NR), post 10.3 (2.3) |
|
| Funding source | A development project grant from the British Diabetic Association and funds from the Greater London Enterprise Board of the GLC and the London Residuary Body supported this study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | High risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Hwang 2019.
| Study characteristics | ||
| Methods |
An audit and feedback intervention to improve internal medicine residents' performance on ambulatory quality measures: a randomized controlled trial Clustered RCT (96 clusters and 96 providers), conducted in 1) Trial conducted among resident physicians from the Massachusetts General Hospital (MGH) internal medicine residency programme. 2) Resident physicians received audit and feedback reports about individual ambulatory quality measures (AQM) performance. Population health co‐ordinators assisted residents. In United states of America. 3 arms: 1. Control (list and definitions of ambulatory quality measures (AQMs) (control arm) and 2. Intervention 1 (individual resident's ambulatory quality measures (AQM) vs practice target) (intervention arm) and 3. Intervention 2 (individual resident's ambulatory quality measures (AQM) vs peers' performance) (other arm) |
|
| Participants | Control arm N: 269 Intervention arm N: 294, 315, NA Diabetes type: 4 Mean age: 48.70 ± 10.7 % Male: 50.68 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: (list and definitions of ambulatory quality measures (AQMs) 1) Case management 2) Clinician education 3) Clinician reminder 4) Facilitated relay of clinical information Intervention arm: (individual resident's ambulatory quality measures (AQM) vs practice target) 1) Audit and feedback 2) Case management 3) Clinician education 4) Clinician reminder 5) Facilitated relay of clinical information Intervention arm: (individual resident's ambulatory quality measures (AQM) vs peers' performance) 1) Audit and feedback 2) Case management 3) Clinician education 4) Clinician reminder 5) Facilitated relay of clinical information |
|
| Outcomes | Retinopathy screening | |
| Funding source | The authors received no financial support for this study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) RAND function was used to randomise 96 first and second‐year internal medicine residents at MGH (Boston, MA) into 3 groups: (1) control, (2) practice target and (3) peer comparison. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Low risk | Table 2. Resident characteristics reported, all P values above 0.05. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. No data, only for diabetic patients. Two characteristics are significant in the whole sample (number of hospitalisations and number of emergency department visits). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3. Data reported only for diabetes patients, and it is assumed that P values are not significant because only one significant P value is reported for colorectal cancer screening (*P = 0.02). |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on the number of patients lost during the study period. Quote: "The analysis was restricted to patients who remained part of the residents’ panels throughout the study period [we can assume here that some patients were lost to follow‐up] to ensure that residents had adequate time to work with patients on the quality metrics." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All outcomes were objectively assessed. Quote: "Data on residents and their patients were obtained from the electronic health record (EHR)." |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. They merged many different outcomes in each quality measure, which limits the number of outcomes that we can extract. Because the data were obtained from the EHR of one health system, labs, blood pressure measurements and other tests that were performed outside of the network may not have been captured. |
| Risk of contamination (other bias) | Unclear risk | Clustered RCT but the unit of randomisation is residents from 1 internal medicine training programme. Communications between residents might have happened. Residents in each group could potentially discuss their reports with each other. |
| Other bias | Low risk | None. |
Ilag 2003.
| Study characteristics | ||
| Methods |
Improving diabetes processes of care in managed care Cluster‐RCT (9 clusters with 43 providers), conducted in an university‐affiliated primary care internal medicine practices affiliated with a managed care organisation, USA Two arms: 1. Comparison site (control arm) and 2. Intervention site (intervention arm) |
|
| Participants | Control arm N: 111 Intervention arm N: 173 Diabetes type: type 1 and type 2 Mean age: 59.0 ± 13.1 % Male: 47.0 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician reminders 2) Patient education |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre NR (NR), post 37 (52) Intervention arm: pre 46 (55), post 49 (59) 2) Retinopathy screening (eye exam), N screened (%) Control arm: pre 43 (61), post 47 (66) Intervention arm: pre 58 (70), post 70 (84) 3) Foot screening, N screened (%) Control arm: pre 43 (61), post 34 (48) Intervention arm: pre 52 (63), post 83 (100) 4) Renal screening (microalbumin), N screened (%) Control arm: pre 26 (37), post 32 (45) Intervention arm: pre 36 (43), post 83 (100) 5) Controlled hypertension (< 135/80 mmHg), N under control (%) Control arm: pre NR (NR), post 37 (52) Intervention arm: pre 46 (55), post 48 (58) 6) Smoking cessation, N smokers (%) Control arm: pre NR (NR), post 9 (13) Intervention arm: pre 8 (10), post 10 (12) |
|
| Funding source | This work was supported by a grant from the National Institutes of Health to the Michigan Diabetes Research and Training Center (DK‐20572) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Cluster ‐RCT. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. There were no significant demographic differences between the intervention site and comparison site participants. |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. NNo baseline comparisons made between groups, thus no P values. Inspection shows large differences between groups. |
| Incomplete outcome data (attrition bias) | High risk | > 40% dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcomes are process measures and not reported if assessed blindly. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Iljaž 2017.
| Study characteristics | ||
| Methods |
E‐healthcare for diabetes mellitus type 2 patients ‐ a randomised controlled trial in Slovenia RCT (NA clusters and NA providers), conducted in 1) family practices from 6 different regions in Slovenia (Posavje, Zasavje, Štajerska, Gorenjska, Primorska and Ljubljana). To be included, family practices had at least 1000 patients, a nurse with secondary school training, and a qualified nurse with higher education (the diabetes care co‐ordinator). 2) Intervention delivered by nurse practitioners diabetes care co‐ordinator in Slovenia 2 arms: 1. Control (conventional diabetes care) (control arm) and 2. Intervention (eDiabetes‐ remote e‐treatment) (intervention arm) |
|
| Participants | Control arm N: 62 Intervention arm N: 58, NA, NA Diabetes type: 2 Mean age: 55.5 ± NR % Male: 60.8 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (conventional diabetes care) Intervention arm: (eDiabetes ‐ remote e‐treatment) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The research was financed through the Slovenian Research Agency project L7‐3653 (B) ‐ E‐health care process support | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised randomisation programme assigned patients to the interventional or the control group through a balanced randomisation process using the last 4 patients. |
| Allocation concealment (selection bias) | Low risk | A computerised randomisation programme assigned patients to the interventional or the control group through a balanced randomisation process using the last 4 patients. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2 shows P < 0.05 for males and education (college; Master/PhD; unknown). |
| Patient's baseline outcomes (selection bias) | Low risk | Total cholesterol was the only parameter with a significantly lower level in the control group (CHOL1, P = 0.046), compared to the interventional group. |
| Incomplete outcome data (attrition bias) | High risk | Lost 9% vs 13% so somewhat balanced but no reasons given. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes: HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | High risk | Some differences between protocol and paper: paper does not report change in LDL or HbA1c at 6 months, or quality of life; protocol does not list self‐measured BP, weight, fasting blood glucose; cholesterol and HDL. |
| Risk of contamination (other bias) | Low risk | eDiabetes application available only to intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Imai 2008.
| Study characteristics | ||
| Methods |
Intervention with delivery of diabetic meals improves glycemic control in patients with type 2 diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from outpatients attending the Kajiyama Clinic between 2004 and 2005, in Kyoto, Japan. 2) The intervention involved diabetic meals delivered at home, individual diet counselling with dietitians or conventional dietary education by either a doctor or nurse. In Japan. 3 arms: 1. Control (conventional dietary education) (control arm) and 2. Intervention 1 (individual diet counselling) (intervention arm), 3. Intervention 2 (meal delivery and sessions with dietitians) (other arm) |
|
| Participants | Control arm N: 30 Intervention arm N: 30, 30, NA Diabetes type: 2 Mean age: 63.62 ± 14.03 % Male: 45.45 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (conventional dietary education) 1) Patient education Intervention arm: (individual diet counselling) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (meal delivery and sessions with dietitians) 1) Case management 2) Patient education |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. A total of 77 adults with type 2 diabetes who attended the clinic were assigned into 3 dietary groups by the stratified randomisation method that considered age, gender, and duration of diabetes. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. There were no significant differences between the 3 groups with respect to age, sex, diabetes duration and glucose control methods. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. There were no significant differences between the three groups with respect to body mass index (BMI), FBG, HbA1c, T‐Ch, HDL‐Ch, LDLCh and TG. |
| Incomplete outcome data (attrition bias) | High risk | In this study, we first assigned 30 patients in each of the 3 groups. However, 12 patients in the group with meal delivery and sessions with dietitians (40% lost) were not able to complete the intervention. The main reasons were particular food preferences and the cost of the meal delivery. Problems with intervention feasibility. No patient was lost in the control group and 1 was lost in the diet counselling group (3.3%). Numbers unbalanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. They report HbA1c at months 1, 2, 3, 6, 9 and 12 (Figure 1), but not for fasting blood glucose (just at baseline and endpoint, both primary outcomes). They only report blood pressure at baseline. |
| Risk of contamination (other bias) | Unclear risk | The conventional dietary education group (control) involved the patients receiving their usual outpatients management every month. The control group received higher level of attention compared to most studies included in this systematic review (control patients usually visit their caregivers every 3 to 4 months). Dieticians saw participants in both intervention groups. |
| Other bias | Low risk | No evidence of other bias. |
Ishani 2011.
| Study characteristics | ||
| Methods |
Effect of nurse case management compared with usual care on controlling cardiovascular risk factors in patients with diabetes Patient RCT, conducted in Minneapolis VA Health Care System (MVAHCS) in Minneapolis, MN, USA Two arms: 1. Usual care (control arm) and 2. Case management (intervention arm) |
|
| Participants | Control arm N: 278 Intervention arm N: 278 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre NR (NR), post 9.1 (NR) Intervention arm: pre NR (NR), post 8.6 (NR) 2) SBP, mean mmHg (SD) Control arm: pre NR (NR), post 144.4 (NR) Intervention arm: pre NR (NR), post 133.7 (NR) 3) LDL, mean mg/dL (SD) Control arm: pre NR (NR), post 118.4 (NR) Intervention arm: pre NR (NR), post 107.3 (NR) 4) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre NR (NR), post 53 (34) Intervention arm: pre NR (NR), post 100 (64) |
|
| Funding source | This study was funded by a Veterans Integrated Service Network 23 Grant | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Male gender (P = 0.03); congestive heart failure (P < 0.01). |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Do not provide flow diagram, only numbers, however they included n = 19 who were wrongly randomised, and only report number who had face‐to face visits. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: used standardised procedures for the 3 outcomes. |
| Selective reporting (reporting bias) | High risk | Primary outcome is the same; secondary outcome in protocol is safety, whereas secondary outcome in manuscript is percentage achieving goal in composite. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Low risk | Information not available. |
Islam 2018.
| Study characteristics | ||
| Methods |
A culturally tailored community health worker intervention leads to improvement in patient‐centered outcomes for immigrant patients with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) All intervention sessions and materials were delivered in Bengali and held in clinical and community settings, New York City, United States, 2) Community health worker‐led patient‐centred lifestyle on type 2 diabetes management among Bangladeshis in NYC in United States of America 2 arms: 1. Control (usual care + 1 education session) (control arm) and 2. Intervention (community health worker) (intervention arm) |
|
| Participants | Control arm N: 160 Intervention arm N: 176, NA, NA Diabetes type: 2 Mean age: 54.87 ± 9.71 % Male: 59.84 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care + 1 education session) 1) Patient education Intervention arm: (community health worker) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This publication was supported by the National Institutes of Health (NIH) National Institute on Minority Health and Health Disparities (NIMHD) grants P60MD000538 and U54MD000538; NIH National Center for the Advancement of Translational Science (NCATS) Grant UL1TR001445; NIH National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) grant R01DK110048; and Centers for Disease Control and Prevention (CDC) Grant U48DP001904 and U58DP005621 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised to either the intervention or control group using IBM SPSS Statistics for Windows, versions 21.0 and 22.0. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no statistically significant differences in sociodemographic characteristics between the groups at baseline; however, control group participants were significantly more likely than intervention group participants to report more frequent vigorous weekly physical activity and total weekly physical activity (Table 1). |
| Patient's baseline outcomes (selection bias) | High risk | Table 1 ‐ all outcomes of interest P > 0.05, however in limitations authors say that variables were shown to differ between groups at baseline (e.g. A1C, physical activity and years with diabetes). |
| Incomplete outcome data (attrition bias) | High risk | Among intervention group participants 31 (18%) were lost to follow‐up, whereas among control group participants 14 (9%) were lost to follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | High risk | Some differences between protocol and publication: protocol does not mention BP, publication does not report social support, utilisation of healthcare. Published protocol said there would be 12‐month follow‐up, which is not presented in results. |
| Risk of contamination (other bias) | Low risk | Control group had first session delivered as intervention group, but likely no other communication with CHWs delivering intervention. |
| Other bias | Low risk | No evidence of other bias. |
Ismail 2013.
| Study characteristics | ||
| Methods |
Usage of glucometer is associated with improved glycaemic control in type 2 diabetes mellitus patients in Malaysian public primary care clinics: an open‐label randomised controlled trial Patient RCT, conducted in 5 public primary care clinics in Malaysia Two arms: 1. Group 1 (control arm) and 2. Group 2 (intervention arm) |
|
| Participants | Control arm N: 47 Intervention arm N: 58 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.9 (2.0), post 9.3 (NR) Intervention arm: pre 9.2 (2.1), post 8.3 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 131.7 (18.4), post 130.2 (NR) Intervention arm: pre 131.5 (15.2), post 128.2 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 80.2 (6.9), post 79.2 (NR) Intervention arm: pre 79.2 (8.4), post 77.9 (NR) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not sure: nurses put 1 to 10 consecutively in envelopes and gave them to patients, pre‐specified that certain numbers would be in intervention and control. |
| Allocation concealment (selection bias) | High risk | Nurses were not blinded, so they may have known the next consecutive number in the stack. |
| Patient's baseline characteristics (selection bias) | Low risk | In text and in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | ~8.5% lost to follow‐up in control group and ~3% in intervention group; reasons seem balanced and do not seem to be influenced by intervention. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c: no objective laboratory method described. For BP used sphygmomanometer. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Ivers 2013.
| Study characteristics | ||
| Methods |
Feedback GAP: pragmatic, cluster‐randomized trial of goal setting and action plans to increase the effectiveness of audit and feedback interventions in primary care Clustered RCT (14 clusters and 53 providers), conducted in 1) Participants were family physicians working in primary care clinics throughout Ontario, Canada, who signed data‐sharing agreements with the Electronic Medical Record Administrative data Linked Database (EMRALD), held at the Institute for Clinical Evaluative Sciences (ICES). 2) The intervention involves audit and feedback to physicians and tested the effects of a theory‐informed worksheet to facilitate goal‐setting and action‐planning, appended to feedback reports. The research team produced the reports and worksheets. In Canada. 2 arms: 1. Control (audit and feedback alone) (control arm) and 2. Intervention (audit and feedback + worksheet) (intervention arm) |
|
| Participants | Control arm N: 1823 Intervention arm N: 1612, NA, NA Diabetes type: 2 Mean age: 65.93 ± 8.62 % Male: 55.87 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (audit and feedback alone) 1) Audit and feedback 2) Clinician education 3) Clinician reminder Intervention arm: (audit and feedback + worksheet) 1)Audit and feedback 2) Clinician education 3) Clinician reminder |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Renal screening Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | The conduct and analysis of this trial was supported by a grant from the Canadian Institutes of Health Research (CIHR)—funding reference number, 111218. The development of the intervention and the embedded qualitative study was supported by a team grant from CIHR, Knowledge Translation Improved Clinical Effectiveness Behavioral Research. Group (KT‐ICEBeRG). This study is supported by the ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long Term Care (MOHLTC). NMI is supported by fellowship awards from CIHR and from the University of Toronto. REU is supported by a Canada Research Chair in Primary Care Research. JMG is supported by a Canada Research Chair in Health Knowledge Transfer and Uptake. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were allocated using minimisation (conducted by the study analyst using the free software, MINIM) to achieve balance on baseline values of the primary outcomes and on the number of eligible patients in each cluster. |
| Allocation concealment (selection bias) | Low risk | Clustered‐RCT. Minimisation conducted by the study analyst using the free software, MINIM. |
| Provider's baseline characteristics (selection bias) | High risk | Table 3. Intervention physicians were more likely to be male, with more years experience, and located in rural settings. They also tended to have smaller practices overall but with more eligible patients. |
| Patient's baseline characteristics (selection bias) | Low risk | Values for these variables and other process measures were similar across groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 4. Some differences in outcomes that are not of interest to us. Values for these variables and other process measures were similar across groups, except for greater proportion of patients in the feedback plus worksheet arm group than the feedback alone arm with a recent BP test (85% versus 74%) and HbA1c test (79% versus 69%). |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. Total of 562 lost out of 4617 patients (12.2%). Lost of 9.6% patients in the control arm and 15.1% in the intervention arm. Numbers quite unbalanced. 2 physicians were lost to follow‐up in each arm and reasons are provided and balanced. For HbA1c outcome in patients with diabetes only (not ischaemic heart disease‐IHD), 1329 data were available post‐intervention out of 1534 at baseline in the control arm (13.4% missing) and 1049/1329 in the intervention arm (21.1% missing). Numbers unbalanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The patient‐level primary outcomes were objectively measured (LDL and blood pressure). Given the nature of the intervention, blinding of physicians was not possible, but they were not aware of the exact nature of the intervention being tested. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted in October 2009. Study started in July 2010. Two years intervention). All outcomes are reported. |
| Risk of contamination (other bias) | Unclear risk | Clustered RCT. To reduce the risk of contamination, randomisation was at the level of the primary care clinic. Both groups received the same QI interventions, except physicians in the intervention arm received worksheet, but unfortunately passive dissemination of this worksheet led to inadequate engagement with the intervention. |
| Other bias | Low risk | None identified. |
Jaber 1996.
| Study characteristics | ||
| Methods |
Evaluation of a pharmaceutical care model on diabetes management Patient RCT, conducted in a university‐affiliated internal medicine outpatient clinic, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 22 Intervention arm N: 23 Diabetes type: type 2 Mean age: 62.4 ± 12.2 % Male: 30.8 Longest follow‐up: 4 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 12.2 (3.5), post 12.1 (3.7) Intervention arm: pre 11.5 (2.9), post 9.2 (2.1) 2) Harms (hypoglycaemic reactions), N (%) Control arm: pre NR (NR), post 2 (9) Intervention arm: pre NR (NR), post 17 (100) |
|
| Funding source | This work was supported by a grant from the Diabetes Research and Education Foundation and Upjohn | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Jackson 2013.
| Study characteristics | ||
| Methods |
Benefits of participation in diabetes group visits after trial completion RCT (NA clusters and NA providers), conducted in 1) Group medical clinics (GMC) were delivered at 2 Veterans Affairs Medical Centers (VAMCs), one in Durham (North Carolina) and another one in Richmond (Virginia). 2) Group education and structured group interactions were moderated by a registered nurse or certified diabetes educator. Individual medication adjustments were made by a pharmacist and general internist. Some of the nutrition discussions also included a dietitian. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (GMC: Group medical clinics) (intervention arm) |
|
| Participants | Control arm N: 106 Intervention arm N: 133, NA, NA Diabetes type: 3 Mean age: 62.02 ± 10.95 % Male: 95.81 Longest follow‐up: 30 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (GMC: group medical clinics) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Harms |
|
| Funding source | This research was funded by the Quality Enhancement Research Initiative (QUERI) of the Department of Veterans Affairs (VA) Health Services Research & Development (HSR&D) Service (RRP‐09‐ 407). The Group Visits Trial was funded by VA HSR&D (IIR‐03‐084). Dr Maciejewski is supported by a VA HSR&D Research Career Scientist Award (RCS‐10‐391). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Patients were randomly assigned within each centre to either attend a GMC or receive usual care. We stratified randomisation by VAMC, baseline HbA1c level (≥ 9.0% vs < 9.0%), and baseline systolic blood pressure (≥ 150 mm Hg vs < 150 mm Hg). We randomly assigned patients to the GMC and usual care groups in a 5:4 ratio to account for clustering of patients in the group medical visits group; patients in the usual care group received their usual VAMC primary care. We used stratified, blocked randomisation with block sizes of 11. |
| Allocation concealment (selection bias) | Unclear risk | An unblinded person with no responsibility for outcome ascertainment revealed study group allocation to patients. |
| Patient's baseline characteristics (selection bias) | Low risk | From the original study (reference 2): Patients in the GMC and usual care groups were similar at baseline (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | From the original study (reference 2): Patients in the GMC and usual care groups were similar at baseline (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | From the original study (reference 2): At 12 months, they already lost 17/106 patients in the control group (16%) and 11/133 (8%) in the intervention group. Unbalanced numbers and reasons. Numbers of lost between 12 months follow‐up and 30 months are not given. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest were objectively measured (HbA1c, SBP and LDL). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Primary outcomes match manuscript results (HbA1c, SBP). They have looked at DBP in the original study but not in this longest follow‐up. By opposition, they added LDL in this longest follow‐up. Secondary outcome of "Cost‐effectiveness" was reported; however, "Proportion of Patients With LDL < 100, Health Services Utilization, Quality of Life (as Measured by DQoL), Patient Empowerment (as Measured by DES)" were not reported. |
| Risk of contamination (other bias) | Unclear risk | Patient randomised within 2 Veterans Affairs Medical Centers (VAMCs) in Durham, North Carolina, and Richmond, Virginia. The usual primary care providers might have changed their approach with control patients after receiving information from their patients in the intervention group. Quotes: "We informed these patients’ primary care providers of medication changes solely by means of the electronic medical record". "A record of the plan was entered into the electronic medical record and forwarded electronically to the primary care provider". "Limitation: Measurements of effectiveness may have been limited by concomitant improvements in the usual care group that were due to co‐intervention". "The observed differences may have resulted from the extra attention provided to the GMC group rather than its content". |
| Other bias | Low risk | No evidence of other bias. |
Jacobs 2012.
| Study characteristics | ||
| Methods |
Pharmacist assisted medication program enhancing the regulation of diabetes (PAPMERED) study Patient RCT, conducted in Lahey Clinic in Burlington, MA, USA Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 201 Intervention arm N: 195 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Aspirin (antiplatelet agent), N users (%) Control arm: pre 31 (34), post 37 (40) Intervention arm: pre 40 (56), post 56 (78) 2) Statins, N users (%) Control arm: pre 35 (38), post 37 (40) Intervention arm: pre 25 (35), post 49 (68) 3a) Antihypertensives (ACE inhibitor), N users (%) Control arm: pre 29 (32), post 39 (42) Intervention arm: pre 24 (33), post 41 (57) 3b) Antihypertensives (angiotensin II receptor blockers), N users (%) Control arm: pre 4 (4), post 6 (7) Intervention arm: pre 8 (11), post 14 (19) 3c) Antihypertensives (calcium channel blocker), N users (%) Control arm: pre 10 (11), post 9 (10) Intervention arm: pre 13 (18), post 14 (19) 3d) Antihypertensives (diuretic), N users (%) Control arm: pre 22 (24), post 24 (26) Intervention arm: pre 22 (31), post 29 (40) 3e) Antihypertensives (ß‐blocker), N users (%) Control arm: pre 28 (30), post 31 (34) Intervention arm: pre 26 (36), post 26 (36) 4) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 76 (83) Intervention arm: pre NR (NR), post 70 (97) 5) Renal screening (nephropathy), N screened (%) Control arm: pre NR (NR), post 57 (62) Intervention arm: pre NR (NR), post 69 (96) 6) HbA1c, mean % (SD) Control arm: pre 9.2 (1.0), post 8.4 (1.6) Intervention arm: pre 9.5 (1.1), post 7.7 (1.3) 7) SBP, mean mmHg (SD) Control arm: pre 134.8 (16.9), post 135.4 (14.0) Intervention arm: pre 142.5 (15.2), post 132.5 (16.3) 8) DBP, mean mmHg (SD) Control arm: pre 78.3 (10.4), post 77.6 (8.4) Intervention arm: pre 79.4 (9.9), post 72.0 (8.5) 9) LDL, mean mg/dL (SD) Control arm: pre 115.1 (34.8), post 105.1 (34.3) Intervention arm: pre 121.5 (31.8), post 93.7 (21.2) 10a) Controlled hypertension (DBP ≤ 80 mmHg), N under control (%) Control arm: pre 54 (67), post 54 (77) Intervention arm: pre 43 (62), post 48 (84) 10b) Controlled hypertension (SBP ≤ 130 mmHg), N under control (%) Control arm: pre 40 (49), post 30 (43) Intervention arm: pre 19 (28), post 29 (51) |
|
| Funding source | Unrestricted medical grant from Pfizer | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using a computer randomized sequence of ones and zeros." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Although they state that baseline measures were the same, they also note that BMI for intervention was slightly larger vs control (P < 0.05). |
| Patient's baseline outcomes (selection bias) | High risk | SBP (P = 0.003); SBP ≤ 130 (P = 0.008). |
| Incomplete outcome data (attrition bias) | Unclear risk | Based on the nature of the study it is hard to tell. They did not seek consent from the controls, so losses to follow‐up in the intervention group are based on consenting to the trial, whereas controls would not have had the opportunity to have these losses as it would not apply to them. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described. Objective measures not described. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes listed in protocol but not reported in paper. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Based on the nature of consenting for intervention vs control, selection bias could have been at play. Intervention patients may have been more motivated, and control patients may not have participated in the study if presented with the option. |
Jahangard‐Rafsanjani 2015.
| Study characteristics | ||
| Methods |
Effect of a community pharmacist‐delivered diabetes support program for patients receiving specialty medical care: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was carried out at the Nemooneh‐Taleghani Community Pharmacy, affiliated with the College of Pharmacy, Tehran University of Medical Sciences, Iran. The community pharmacist used the manager’s office on predefined days of the week as a private counselling area to deliver diabetes education. 2) Intervention delivered by a community pharmacist in collaboration with endocrinologists. In Iran. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (education by community pharmacist) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 51, NA, NA Diabetes type: 2 Mean age: 56.64 ± 5.25 % Male: 49.59 Longest follow‐up: 5 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (education by community pharmacist) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | The study was funded by the Deputy of Research, Tehran University of Medical Sciences. (Project ID: 90‐04‐156‐16161) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated based on a block randomisation algorithm (1:1 allocation ratio; block size: 4). |
| Allocation concealment (selection bias) | Low risk | Two authors who were not involved in the recruitment process had access to the randomisation list. The community pharmacist requested an allocation order using telephone calls after a patient signed the informed consent form. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. The demographic characteristics were similar between the study groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. There were no significant differences between groups in the number of diabetes medications, baseline A1C and systolic/diastolic blood pressure. |
| Incomplete outcome data (attrition bias) | High risk | Of them, 101 participants were recruited and randomised to either the intervention group (n = 51) or the control group (n = 50). Six patients in the intervention group (11.8%) and 10 patients in the control group (20%) discontinued the study. Numbers unbalanced. Reasons reported and it seems like their is a problem with intervention feasibility as 5 out of 51 patients were not adherent to pharmacy visit protocol (9.8%). Reasons unbalanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were all objectively measured (HbA1c, DPB, SBP). |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol (protocol first posted in February 2012. Study started in March 2012). Protocol: haemoglobin A1c at 6 months. Paper: A1C was measured at baseline and 5‐month follow‐up. Shorter follow‐up, reason not reported. The study follow‐up period was relatively short (5 months). Publication also reports satisfaction and willingness to pay, not mentioned in protocol. |
| Risk of contamination (other bias) | Unclear risk | All patients were receiving specialty medical care from an endocrinologist. The pharmacist met with all patients at the recruitment visit and provided a brief education session to control patients. |
| Other bias | Low risk | None identified. |
Jain 2018.
| Study characteristics | ||
| Methods |
Community health worker interventions in type 2 diabetes mellitus patients: assessing the feasibility and effectiveness in rural central India RCT (NA clusters and NA providers), conducted in 1) conducted in a tertiary care teaching institute (Mahatma Gandhi Institute of Medical Sciences) situated in a rural district in Maharashtra, India. 2) Intervention delivered by community health workers in India 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (community health worker interventions) (intervention arm) |
|
| Participants | Control arm N: 146 Intervention arm N: 153, NA, NA Diabetes type: 2 Mean age: 56.56 ± 8.87 % Male: 56.86 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard care) 1) Patient education Intervention arm: (community health worker interventions) 1) Case management 2) Patient education 3) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | We would like to acknowledge and thank Division of Clinical Trials and Research, St John’s Medical College in collaboration with National Institutes of Health (NIH), USA who gave us the grant for this study under a 2‐year health research methodology fellowship and mentorship programme. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study participants were randomised into 2 groups – the standard care group and the intervention group using block randomisation by a blinded investigator. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values > 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. Baseline LDL higher in intervention group P = 0.01. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome variables could be collected for 139 (5%) patients in the standard care group (n = 146; 1 patient died after baseline visit and 6 patients refused end of study visit). In the intervention group outcome variables were recorded in 151 (1%) patients (n = 153; 1 patient died after baseline visit and 1 patient died after the 18th week visit). Follow‐up data and end of study data were obtained for 96.98% of patients in both the groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, however all outcomes in methods are reported in results. |
| Risk of contamination (other bias) | Low risk | Community health workers met in home only with intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Jakobsson 2015.
| Study characteristics | ||
| Methods |
Cardiovascular secondary prevention in high‐risk patients: a randomized controlled trial sub‐study RCT (NA clusters and NA providers), conducted in 1) Ostersund Hospital, Jamtland, Sweden. At 1 month after discharge, baseline measurements of blood lipids and BP were performed by a healthcare professional at the patients’ closest healthcare facility and reported to the study team. Corresponding follow‐up measurements were performed at 12 months after discharge. Contact via telephone. 2) Shortly after the measurements of blood lipids and BP, a study nurse contacted participants in both study groups by telephone and interviewed them about their well‐being and adherence to medical treatment. Decisions regarding titration and medication were made by a study physician. In Sweden. 2 arms: 1. Control (local standard management) (control arm) and 2. Intervention (nurse‐based telephone follow‐up) (intervention arm) |
|
| Participants | Control arm N: 128 Intervention arm N: 139, NA, NA Diabetes type: 4 Mean age: 75 ± NR % Male: 63.41 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (local standard management) 1) Facilitated relay of clinical information Intervention arm: (nurse‐based telephone follow‐up) 1) Case management |
|
| Outcomes | Lipid‐lowering drugs Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The study was funded by the Unit of Research, Education and Development, Östersund Hospital, Region Jämtland Härjedalen (Dnr‐JLL‐308781) and by the Heart Foundation of Northern Sweden (Dnr‐2014‐05‐06). The funders did not have any role in the design of the study or collection, analysis and interpretation of data or in writing the manuscript. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Those who agreed to participate were randomised to intervention or control in a 1:1 ratio. The random allocation sequence was computer‐generated in blocks of 4 and stratified for sex and type of ACS (unstable angina or myocardial infarction) for ACS patients. For patients with stroke or TIA the random sequence was generated in blocks of four and was stratified for sex and for degree of disability (modified Rankin Scale). |
| Allocation concealment (selection bias) | Low risk | Those who agreed to participate were randomised to intervention or control in a 1:1 ratio. The random allocation sequence was computer‐generated in blocks of four and stratified for sex and type of ACS (unstable angina or myocardial infarction) for ACS patients. For patients with stroke or TIA the random sequence was generated in blocks of 4 and was stratified for sex and for degree of disability (modified Rankin Scale). |
| Patient's baseline characteristics (selection bias) | Unclear risk | No baseline data provided for the diabetic subpopulation. Significantly more patients included in the study due to acute coronary syndrome (ACS), stroke or transient ischaemic attack (TIA) in the intervention group (P = 0.01). |
| Patient's baseline outcomes (selection bias) | High risk | Baseline outcomes provided in supplemental file 2. P values provided and above 0.05. Data for only 80 of 267 diabetic patients given. |
| Incomplete outcome data (attrition bias) | High risk | Table 1 shows that 128 control + 139 intervention patients had DM but BP and LDL in supplemental file 2 only give data for patients in high‐risk group. 32 (12%) and 38 (15%) patients lost in intervention and control groups respectively. 22 died in control group vs 15 in intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measurement of BP and LDL. Another potential weakness was the BP measurement procedure, which was performed by a large number of healthcare professionals at different healthcare centres in the county and using different devices. The letter of referral included simple instructions for what was considered a standardised BP measurement, but the accuracy of the individual BP measurements could have varied. Data collection method for statin use not reported (from medical report? self‐reported by patient?). |
| Selective reporting (reporting bias) | High risk | Data for only high‐risk diabetic patients provided. Many secondary outcomes listed in protocol are not reported (sitting, standing BP, smoking cessation, BMI, physical activity). |
| Risk of contamination (other bias) | Low risk | Patient‐randomised but unlikely that control participants received nurse telephone follow‐up and no indication that GPs overlapped. |
| Other bias | High risk | Sub‐analysis of an ongoing randomised controlled trial and which is, in addition, restricted to diabetic patients. They randomised all patients, but they only kept diabetic patients followed after the new guideline release for the analysis. |
Jameson 2010.
| Study characteristics | ||
| Methods |
Pharmacist collaborative management of poorly controlled diabetes mellitus: a randomized controlled trial Patient RCT, conducted in 13 primary care offices, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 52 Intervention arm N: 52 Diabetes type: type 1 and type 2 Mean age: 49.5 ± 10.9 % Male: 49.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 11.1 (1.6), post 10.7 (NR) Intervention arm: pre 10.4 (1.2), post 8.9 (NR) |
|
| Funding source | Financial support for this study was provided by Advantage Health Physician Network, Doran Foundation, Michigan Pharmacist Foundation, Priority Health, and Western Michigan Society of Health System Pharmacists | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Jansa 2006.
| Study characteristics | ||
| Methods |
Telecare in a structured therapeutic education programme addressed to patients with type 1 diabetes and poor metabolic control Patient RCT, conducted in Diabetes Unit of the Hospital Clinic Barcelona, Spain Two arms: 1. Conventional group ‐ CG (control arm) and 2. Telecare group ‐ TG (intervention arm) |
|
| Participants | Control arm N: 20 Intervention arm N: 20 Diabetes type: type 1 Mean age: 25.2 ± 8.3 % Male: 60.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.9 (1.3), post 7.6 (0.7) Intervention arm: pre 8.4 (1.2), post 7.6 (0.9) 2a) Harms (severe hypoglycaemia), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 1 (5) 2b) Harms (ketosis), N (%) Control arm: pre NR (NR), post 1 (6) Intervention arm: pre NR (NR), post 0 (0) |
|
| Funding source | This research was supported by grants from: Agencia d’Avaluacio de Tecnologia Medica (ATTM), Barcelona, Spain. Ref 002122000. Associacio Catalana de Diabetis (ACD) Barcelona, Spain. Ref Grants for Therapeutic Education 2001 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | 30% dropout but did an intervention‐to‐treat analysis including ~90% of participants. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Jansink 2013.
| Study characteristics | ||
| Methods |
No identifiable HbA1c or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial Cluster‐RCT (58 clusters with 58 providers), conducted in general practices in South Eastern part of the Netherlands Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 518 Intervention arm N: 422 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 14 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Clinician education |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 106 (40) Intervention arm: pre NR (NR), post 61 (33) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 205 (78) Intervention arm: pre NR (NR), post 131 (70) 3a) Renal screening (creatinine), N screened (%) Control arm: pre NR (NR), post 242 (92) Intervention arm: pre NR (NR), post 174 (94) 3b) Renal screening (microalbumin), N screened (%) Control arm: pre NR (NR), post 223 (85) Intervention arm: pre NR (NR), post 163 (88) 4) HbA1c, mean % (SD) Control arm: pre 7.7 (0.7), post 7.4 (1.0) Intervention arm: pre 7.8 (0.9), post 7.3 (0.7) 5) SBP, mean mmHg (SD) Control arm: pre 140.7 (18.0), post 137.8 (15.8) Intervention arm: pre 144.4 (20.3), post 141.5 (17.0) 6) DBP, mean mmHg (SD) Control arm: pre 79.9 (9.9), post 77.6 (9.2) Intervention arm: pre 81.9 (10.6), post 79.5 (8.4) 7) LDL, mean mg/dL (SD) Control arm: pre 96.7 (30.9), post 92.8 (23.2) Intervention arm: pre 108.3 (38.7), post 100.5 (30.9) |
|
| Funding source | This study was funded by ZonMW – the Netherlands Organization for Health Research and Development, 945‐16‐113 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | P values not in table and no mention in text. |
| Patient's baseline outcomes (selection bias) | Unclear risk | P values not in table and no mention in text. |
| Incomplete outcome data (attrition bias) | High risk | Large losses, reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: HbA1c, objective laboratory methods not described. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes in protocol do not match paper. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Janssen 2009.
| Study characteristics | ||
| Methods |
Randomised controlled trial of intensive multifactorial treatment for cardiovascular risk in patients with screen‐detected type 2 diabetes: 1‐year data from the ADDITION Netherlands study Clustered RCT (79 clusters and NR providers), conducted in 1) 79 general practices in the southwestern region of the Netherlands, all co‐operating with one regional laboratory (SHL Centre for Diagnostic Support in Primary Care, Etten‐Leur, the Netherlands). 2) The intensive treatment protocol was carried out by a diabetes nurse together with a general practitioner (GP). In Netherlands. 2 arms: 1. Control (routine treatment) (control arm) and 2. Intervention (intensified treatment) (intervention arm) |
|
| Participants | Control arm N: 243 Intervention arm N: 255, NA, NA Diabetes type: 2 Mean age: 60.00 ± 13.82 % Male: 53.85 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (routine treatment) 1) Clinician education Intervention arm: (intensified treatment) 1) Case management 2) Team change 3) Clinician education 4) Clinician reminder |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | The ADDITION Netherlands study is made possible by unrestricted grants from NovoNordisk, Glaxo Smith Kline, and Merck | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Randomisation was performed according to stratification of practice organisation (single‐handed, group practices). |
| Allocation concealment (selection bias) | Low risk | Clustered‐RCT. |
| Provider's baseline characteristics (selection bias) | High risk | Table 1. In the routine care group, considerably more urban practices were included (52.4% compared to 29.7% in the intervention arm). However, great similarities were found between baseline cardiovascular risk factor levels in urban and rural practices (data not shown). |
| Patient's baseline characteristics (selection bias) | Low risk | The 2 groups were well matched with respect to clinical, biochemical and behavioural characteristics (Table 1), as well as use of cardiovascular medications and history of cardiovascular events (data not shown). |
| Patient's baseline outcomes (selection bias) | Low risk | The 2 groups were well matched with respect to clinical, biochemical and behavioural characteristics (Table 1), as well as use of cardiovascular medications and history of cardiovascular events (data not shown). |
| Incomplete outcome data (attrition bias) | Low risk | Of all 498 included patients, 7 (1.4%) were lost to follow‐up (2 in the routine care group, 5 in the intensively treated group) for various reasons (relocation, withdrawal of consent, treatment too burdensome). Reasons for study discontinuation were similar between both treatment groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All primary objectives were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol (protocol first posted in October 2005. Study started in January 2001). None of our outcomes of interest are listed in the protocol (HbA1c, SBP, DBP, LDL, hypoglycaemia). |
| Risk of contamination (other bias) | Low risk | Clustered‐RCT but although physicians in the intervention arm used an intensive diabetes treatment algorithm, all physicians received and were trained on a diabetes treatment algorithm. GPs in the routine care practices followed the 1999 Dutch guidelines on type 2 diabetes. These guidelines were updated in 2006 (current paper published in 2009). It should be emphasised that it is not possible to rule out that in advance of the 2006 guidelines these GPs might already have tended towards tighter control for cardiovascular risk factors. |
| Other bias | Low risk | No evidence of other risk of bias. |
Jarab 2012.
| Study characteristics | ||
| Methods |
Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan Patient RCT, conducted in an outpatient diabetes clinic at the Royal Medical Services (RMS) hospital in Jordan Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 86 Intervention arm N: 85 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 55 (64), post 53 (67) Intervention arm: pre 53 (62), post 63 (82) 2) Antihypertensives (any), N users (%) Control arm: pre 69 (80), post 69 (87) Intervention arm: pre 70 (82), post 69 (90) 3) HbA1c, median % (SD) Control arm: pre 8.4 (2.7), post 8.5 (NR) Intervention arm: pre 8.5 (2.5), post 7.7 (NR) 4) SBP, median mmHg (SD) Control arm: pre 134.0 (14.1), post 135.1 (NR) Intervention arm: pre 132.0 (15.6), post 126.2 (NR) 5) DBP, median mmHg (SD) Control arm: pre 85.0 (5.9), post 86.8 (NR) Intervention arm: pre 85.0 (16.3), post 77.9 (NR) 6) LDL, median mg/dL (SD) Control arm: pre 85.1 (63.0), post 85.1 (NR) Intervention arm: pre 81.2 (60.2), post 58.0 (NR) 7) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 42 (49), post 37 (47) Intervention arm: pre 39 (46), post 62 (81) 8) Smoking cessation, N smokers (%) Control arm: pre 39 (45), post 37 (47) Intervention arm: pre 46 (54), post 41 (53) |
|
| Funding source | All authors certify that there was no external funding for this research article and report no financial or other potential conflicts of interest related to the subject of this article | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation technique used. |
| Allocation concealment (selection bias) | High risk | Minimisation technique used, you can predict the next assignment. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Per‐protocol analysis, numbers loss to follow‐up provided with reasons, balanced between both groups. ~ 8% in control arm and 9% in intervention arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: HbA1c, laboratory methods not described and outcome assessor blinding not described. Secondary outcome: blood pressure, medication adherence, etc. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; outcomes match those listed in methods. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Jeong 2018.
| Study characteristics | ||
| Methods |
Smart care based on telemonitoring and telemedicine for type 2 diabetes care: multi‐center randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Volunteers with diabetes were recruited from the outpatient clinic of 4 urban university hospitals in South Korea (Kyungpook National University Hospital, Yeungnam University Medical Center, Yonsei Severance Hospital and Gangnam Severance Hospital). Intervention delivered by telemonitoring or telemedicine. 2) In the 2 intervention groups, the physicians and diabetes specialist nurses at the Smart Care Center were responsible for responses to patients’ telephone calls and management of remote glucose monitoring and feedback. In the telemedicine group, assessment by outpatient visits was replaced by video conferencing with an endocrinologist. In South Korea. 3 arms: 1. Control (conventional face‐to‐face care) (control arm) and 2. Intervention 1 (telemonitoring care) (intervention arm), 3. Intervention 2 (telemedicine care) (other arm) |
|
| Participants | Control arm N: 113 Intervention arm N: 113, 112, NA Diabetes type: 2 Mean age: 53.09 ± 12.29 % Male: 67.46 Longest follow‐up: 5.54 months |
|
| Interventions |
Control arm: (conventional face‐to‐face care) 1) Patient education 2) Promotion of self‐management Intervention arm: (telemonitoring care) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management Intervention arm: (telemedicine care) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI16C1501) and a 2010 consignment research grant from LG Electronics through the Ministry of Trade, Industry and Energy of South Korea (1003518) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Patients were randomised to 3 groups: a conventional care group (n = 113), a telemonitoring group (n = 113) and a telemedicine group (n = 112). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All patient characteristics have P values higher than 0.05. Quote: "These parameters were statistically not different among three groups, as indicated in Table 1 (P > 0.05)." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All baseline outcomes are not significant but no P values reported for the primary outcome (HbA1c, Tables 1 and 2). |
| Incomplete outcome data (attrition bias) | Low risk | Figure 1. 101/113 patients in the control group (11% lost) completed the study while 99/113 and 99/112 did in the telemonitoring (12% lost) and the telemedicine (12% lost) groups, respectively. Reasons reported and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome (HbA1c) was objectively measured as well as blood pressure and LDL. It is assumed that hypoglycaemia events were also objectively collected (auto‐transmitter system for glucose concentration, as determined by a glucometer). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. They added a lot of subgroup analyses for HbA1c based on sex, age, education level, etc. that were not planned in the methods section and protocol. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised. It is likely that most endocrinologists were taking care of patients from all groups. In the 2 interventions groups, the same physicians and nurses at the Smart Care Center were responsible for responses to patients’ telephone calls and management of remote glucose monitoring and feedback. All groups significantly improved for HbA1c level at the end of the intervention (Table 2, P < 0.0001). |
| Other bias | Low risk | No evidence of other risk of bias. |
Ji 2019.
| Study characteristics | ||
| Methods |
Effect of simulation education and case management on glycemic control in type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) The study was conducted in Danyang People's Hospital, Jiangsu province, China 2) Six educators from a health care team, who were either dietitians or registered nurses, delivered the education programme, an experienced nurse case manager was added to the experimental intervention. In China. 2 arms: 1. Control (diabetes self‐management education (DSME)) (control arm) and 2. Intervention (DSME + simulated education + case management) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 54.35 ± 10.05 % Male: 50.52 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (diabetes self‐management education (DSME)) 1) Patient education 2) Promotion of self‐management Intervention arm: (DSME + simulated education + case management) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This research was supported by the Zhenjiang Science and Technology Development Society, Jiangsu Province, China(Grant #FZ2011001) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised 1:1 according to random numbers generated in Excel. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised 1:1 according to random numbers generated in Excel. To ensure that the risk of bias remained low, patients were registered in the database by means of ID codes so that assessors and educators were blinded. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no significant differences between the 2 groups (all P values > 0.05). Table 1. |
| Patient's baseline outcomes (selection bias) | Low risk | There were no significant differences between the 2 groups (all P values > 0.05). |
| Incomplete outcome data (attrition bias) | Low risk | 4 lost (8%) in control group, 5 (10%) lost in intervention group. Reasons not provided. Figure 1. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol available, however outcomes in methods match those in results. |
| Risk of contamination (other bias) | Low risk | Nurse case managers only met with intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Jiang 2019.
| Study characteristics | ||
| Methods |
The effectiveness of a self‐efficacy‐focused structured education programme on adults with type 2 diabetes: A multicentre randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) The trial was conducted in outpatient clinics of 4 hospitals in mainland China, including Yanhua Hospital in Beijing, Jimenli Primary Hospital in Beijing, Wuyishan Municipal Hospital in Fujian province and People's Hospital of Leping City in Jiangxi province. 2) The delivery of the self‐efficacy‐focused structured education programme was mainly completed by a trained education nurse, and a 10‐minute introduction of the programme was finished by a physician in China 2 arms: 1. Control (routine education) (control arm) and 2. Intervention (self‐efficacy‐focused structured education programme) (intervention arm) |
|
| Participants | Control arm N: 132 Intervention arm N: 133, NA, NA Diabetes type: 2 Mean age: 56.91 ± NR % Male: 44.91 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (routine education) Intervention arm: (self‐efficacy‐focused structured education programme) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Project of Prevention Medical Society in Haidian District, Beijing (2016HDPMA08) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number in blocks of 8 was generated from a computer by a researcher in a central randomisation centre. |
| Allocation concealment (selection bias) | Low risk | A multicentre, parallel, randomised, controlled, concealed label trial. A set of sequentially numbered opaque, sealed envelopes was assigned to each centre to ensure the allocation concealment. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. All characteristics have P values higher than 0.05. Quote: "No significant differences among demographic characteristic and diabetes‐related data were found between the intervention and control groups at T0 (Table 2)." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All outcomes have P values higher than 0.05. Quote: "No significant differences among metabolic and psychosocial aspect data were found between the intervention and control groups at T0 (Table 2)." |
| Incomplete outcome data (attrition bias) | Unclear risk | Figure 1. Looks like they only lost 1 patient in the control group. Quote: "The data of one patient that was lost from a follow‐up in the control group were supplemented by the last observation data". Looks like there was no additional data lost for HbA1c and blood pressure outcomes, except for LDL. Quote: "The missing data of TC, LDL‐C, TG and HDL‐C at T2 were 87, 87, 87 and 91 cases that were substituted through multiple imputation methods" (out of 265 patients were randomised, 33%, 33%, 33% and 35% lost, but secondary outcomes). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All outcomes were objectively assessed (HbA1c, blood pressure and LDL). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol (version 1 registered on 29 March 2017 and study data were collected from April 2017 to June 2018). In the protocol, the follow‐up times were supposed to be 0, 3, 6 and 12 months for all outcomes, except for lipid profiles (0, 6 and 12 months). However, the longest follow‐up reported in the paper is 6 months. They did unplanned exploratory subgroup analysis for HbA1c based on education level. |
| Risk of contamination (other bias) | Unclear risk | Patients randomised. The follow‐ups for the patients of the 2 groups were arranged in different time points to avoid the contamination. However, it is unclear if different physicians and nurses were taking care of patients from both groups. |
| Other bias | Low risk | No evidence of other risk of bias. |
Johansen 2007.
| Study characteristics | ||
| Methods |
Effects of structured hospital‐based care compared with standard care for Type 2 diabetes‐The Asker and Baerum Cardiovascular Diabetes Study, a randomized trial Patient RCT, conducted in Asker and Baerum Hospital, Rud, Norway Two arms: 1. Standard care (control arm) and 2. Structured care (intervention arm) |
|
| Participants | Control arm N: 60 Intervention arm N: 60 Diabetes type: type 2 Mean age: 59.0 ± 10.0 % Male: 74.2 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education 3) Financial incentives |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 22 (39), post 33 (58) Intervention arm: pre 24 (49), post 43 (88) 2) Antihypertensives (any), N users (%) Control arm: pre 23 (40), post 27 (47) Intervention arm: pre 34 (69), post 45 (92) 3) HbA1c, mean % (SD) Control arm: pre 7.6 (1.6), post 7.8 (1.5) Intervention arm: pre 7.5 (1.5), post 6.7 (0.8) 4) SBP, mean mmHg (SD) Control arm: pre 130.0 (13.0), post 130.0 (11.0) Intervention arm: pre 136.0 (16.0), post 128.0 (14.0) 5) DBP, mean mmHg (SD) Control arm: pre 77.0 (7.0), post 76.0 (8.0) Intervention arm: pre 80.0 (8.0), post 75.0 (7.0) 6) LDL, mean mg/dL (SD) Control arm: pre 113.1 (35.1), post 101.4 (35.1) Intervention arm: pre 113.1 (31.2), post 85.8 (23.4) 7) Smoking cessation, N smokers (%) Control arm: pre 7 (12), post 8 (14) Intervention arm: pre 3 (6), post 4 (8) |
|
| Funding source | We thank the Eastern Norway Regional Health Authority and Novo Nordisk Scandinavia for financial support | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Johnson 2014.
| Study characteristics | ||
| Methods |
Diabetes control through an educational intervention Clustered RCT (10 clusters and 10 providers), conducted in 1) We conducted this study within the scope of the Baltimore Cardiovascular Partnership, a community‐university collaboration generally aimed at improving communication between research institutions and surrounding communities in Baltimore. We enrolled 10 primary care physicians and patients of these physicians. 2) Physician education intervention was done by members of the study clinical and research team. The patient education intervention was delivered by the study nurse. In United States of America 4 arms: 1. Control (no education) (control arm) and 2. Intervention 1 (patient only education) (intervention arm), 3. Intervention 2 (physician only education) (other arm), 4. Intervention 3 (patient and physician education) (other arm) |
|
| Participants | Control arm N: NR Intervention arm N: NR, NR, NR Diabetes type: 4 Mean age: 64 ± 7 % Male: 35.84 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: (no education) Intervention arm: (patient only education) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (physician only education) 1) Clinician education |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The project described was supported by Grant Number 5U01HL079151 from the National Heart, Lung, and Blood Institute | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. It looks like no randomisation was done at the physician/clinic level; 5 received training and 5 not. Does not report the number of clinics. Within each clinic, patients were randomised to the education group or to the control group. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | No data and no P values. Baseline data are only presented for the entire sample and not for each arm. At baseline, patient characteristics were uniformly distributed across groups and did not differ significantly (data not shown). |
| Patient's baseline outcomes (selection bias) | Unclear risk | No data reported and no P values. Baseline data are only presented for the entire sample and not for each arm. At baseline, patient characteristics were uniformly distributed across groups and did not differ significantly (data not shown). |
| Incomplete outcome data (attrition bias) | High risk | The number of patients in each arm are not reported, both at baseline and at 24 months. They only say that: the study was subject to a great degree of selective attrition after the 24th month. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | Not enough information provided. No registered protocol or previously reported protocol. No raw data for each outcome, except on figure 2 that presents only 2 arms that are not clearly tagged. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. |
| Other bias | Low risk | None identified. |
Joss 2004.
| Study characteristics | ||
| Methods |
Intensified treatment of patients with type 2 diabetes mellitus and overt nephropathy RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from 5 centres in the West of Scotland. 2) The intervention group was seen as often as necessary by a project team (doctor, nurse and dietician), not at their normal clinics. In United Kingdom. 2 arms: 1. Control (standard medical management) (control arm) and 2. Intervention (intensive medical management) (intervention arm) |
|
| Participants | Control arm N: 43 Intervention arm N: 47, NA, NA Diabetes type: 2 Mean age: 63 ± 11.89 % Male: 63 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (standard medical management) 1) Clinician education 2) Patient education Intervention arm: (intensive medical management) 1) Case management 2) Team change 3) Clinician education 4) Patient education |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Hypertension control Smoking status |
|
| Funding source | We also wish to thank the Glasgow Royal Infirmary Renal and Diabetic Units’ funds and Glasgow Royal Infirmary endowment fund | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated to one of 2 groups by random number allocation. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a telephone call to an individual at the Scottish Renal Registry, who then used a prepared set of random numbers. |
| Patient's baseline characteristics (selection bias) | Low risk | Tables 1 and 2. All P values above 0.05. Following randomisation, the 2 groups were well matched at baseline (Table 1). There was no significant difference in gender between the 2 groups: 68% males in the intensive group vs 58% in the control group. |
| Patient's baseline outcomes (selection bias) | Low risk | Tables 1 and 2. All P values above 0.05 except for lipid‐lowering use (P = 0.01, but secondary outcomes). Following randomisation, the 2 groups were well matched at baseline (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | Total of 15 lost out of 90 (16.7%), 8 in the intervention arm (17.0%) and 7 in the control arm (16.3%). Numbers balanced, but high. Reasons reported, but unbalanced. More death in the intervention arm (6 vs 3), and dialysis only in the control arm (3 vs 0). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Some outcomes were objectively measured (HbA1c, blood pressure). Information on smoking habits was obtained at interviews (subjective). They do not report how they measure drug use (ACE inhibitors or ARB, lipid‐lowering and aspirin). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. They collected most data every 3 months but they only report outcomes at 24 months. They do not talk about medication use in the methods. |
| Risk of contamination (other bias) | Unclear risk | Project team only saw those in intervention group but treatment targets were the same for both groups. Assume all physicians had education about it. |
| Other bias | Low risk | No evidence of other risk of bias. |
Judah 2018.
| Study characteristics | ||
| Methods |
Financial disincentives? A three‐armed randomised controlled trial of the effect of financial Incentives in Diabetic Eye Assessment by Screening (IDEAS) trial RCT (NA clusters and NA providers), conducted in 1) General practitioner (GP) practices in Kensington, Chelsea and Westminster. 2) Voucher mail‐out. Statistician in United Kingdom 3 arms: 1. Control (standard letter) (control arm) and 2. Intervention (fixed incentive ) (intervention arm), 3. Intervention (lottery incentive) (other arm) |
|
| Participants | Control arm N: 524 Intervention arm N: 375, 375, NA Diabetes type: 4 Mean age: NR ± 13.1 % Male: 57.9 Longest follow‐up: 1 months |
|
| Interventions |
Control arm: (standard letter) Intervention arm: (fixed incentive) 1) Financial incentives Intervention arm: (lottery incentive) 1) Financial incentives |
|
| Outcomes | Retinopathy screening | |
| Funding source | This project was funded by the National Institute for Health Research (NIHR) Health Services and Delivery Research programme (project number 12/64/112). As per the funder requirements, the report of the study has been published in full in the National Institute for Health ResearchNIHR journals library. https://www.ncbi.nlm.nih.gov/books/NBK425298/. The research was supported by the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | To generate the random allocation sequence, the data manager at 1st Retinal Screen Ltd provided the team statistician with a spreadsheet containing de‐identified/anonymised patient data. For 1274 eligible patients provided in the list, participants were indexed according to numbers generated at random using a standard random number generator, with double precision, to avoid duplicates. Participants were then sorted from smallest to largest according to this random index. |
| Allocation concealment (selection bias) | Low risk | Due to this method of anonymised patient identifiers being allocated to groups by the trial statistician prior to the start of the study, there was no issue resulting from lack of concealment of the intervention sequence. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No P values provided. The sociodemographic characteristics of trial attenders and non‐attenders for each group is shown in Table 3. There were no significant differences in sociodemographic characteristics between attenders and non‐attenders. No significant differences were found among trial attenders between the control and incentive groups. There was no difference between incentive and control groups in terms of outcome recommendation from screening (P = 0.387). Multivariate analysis to determine the covariate‐adjusted differences in attendance rates between groups demonstrated that none of the sociodemographic factors impacted on attendance. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. No P values provided. The sociodemographic characteristics of trial attenders and non‐attenders for each group is shown in Table 3. There were no significant differences in sociodemographic characteristics between attenders and non‐attenders. No significant differences were found among trial attenders between the control and incentive groups. There was no difference between incentive and control groups in terms of outcome recommendation from screening (P = 0.387). Multivariate analysis to determine the covariate‐adjusted differences in attendance rates between groups demonstrated that none of the sociodemographic factors impacted on attendance. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1274 patients were eligible and randomised. Between the randomisation date and patients being sent invitation letters, 223 became ineligible. Therefore, 1051 participants were sent an invitation letter and included in the analysis (n = 435 control; n = 312 fixed; n = 304 lottery). The primary reasons for ineligibility before being sent an appointment invitation letter were: attendance at annual DES appointment (44.4% of ineligible patients) and moving out of the area (22.4%). The loss of patients did not affect the sample size target or predefined ratio. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of screening attendance. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Several secondary outcomes from protocol not reported in paper. Cost‐effectiveness analysis is not reported in manuscript, likely due to the negative nature of the primary result). |
| Risk of contamination (other bias) | Low risk | Patient randomised. Only patients within each group received the mail‐out pertaining to their intervention type. Unlikely that participants received intervention from other group. |
| Other bias | Low risk | Given the designated clinics for each trial condition, it was not possible for the researcher (who was present at intervention clinics to administer the incentive) or the screener to be blinded to group assignment. However, as screening attendance is the primary outcome, the results could not be biased by the lack of blinding at this stage. |
Juul 2014.
| Study characteristics | ||
| Methods |
Effectiveness of a training course for general practice nurses in motivation support in type 2 diabetes care: a cluster‐randomised trial Cluster‐RCT (40 clusters with 50 providers), conducted with patients identified from Central Denmark Region's Chronic Disease Database, Denmark Two arms: 1. Usual practices (control arm) and 2. Intervention practices (intervention arm) |
|
| Participants | Control arm N: 2029 Intervention arm N: 2005 Diabetes type: type 1 and type 2 Mean age: 60.4 ± 8.6 % Male: 56.5 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Clinician education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.1 (1.3), post 7.1 (2.4) Intervention arm: pre 7.1 (1.3), post 7.1 (2.3) |
|
| Funding source | The study was financially supported by The Tryg Foundation (J.no.7597‐08), UCSF Lundbeck Foundation (J.no.FP47/2009), The Health Insurance Foundation (J.no.2009B068), The Danish Nurses’ Organisation (J.no.10/38412) and Aase and Ejnar Danielsens Foundation (J.no.10‐000408) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not provided. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not provided in text or table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | No objective laboratory methods described for HbA1c. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | High risk | Quote: "The allocation of the usual practice group may have tempted nurses in the usual practice to join courses similar to the one offered in the intervention practices." |
| Other bias | Low risk | Information not available. |
Kanadli 2016.
| Study characteristics | ||
| Methods |
Does telephone follow‐up and education affect self‐care and metabolic control in diabetic patients? RCT (NA clusters and NA providers), conducted in 1) Endocrinology outpatient clinic in the internal medicine ward at a Hospital located in the Central Anatolia region of Turkey. Education was given at the education room of the internal medicine ward. 2) Intervention provided by Nurses. In Turkey. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (ETM: telephone follow‐up and education) (intervention arm) |
|
| Participants | Control arm N: 44 Intervention arm N: 47, NA, NA Diabetes type: 3 Mean age: NR ± 11.7 % Male: 36.35 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (ETM: telephone follow‐up and education) 1) Case management 2) Patient education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated into intervention and control groups using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values higher than 0.05 for all characteristics. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 4, P values higher than 0.05 for all outcomes (except waist circumference). |
| Incomplete outcome data (attrition bias) | Low risk | During the study period, 3 (6.4%) patients from the intervention group were excluded because of failure to contact via telephone. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c, LDL, SBP, DBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | Low risk | Only the patients into the intervention group received the education, and patients were called individually by a nurse. |
| Other bias | Low risk | No evidence of other bias. |
Kangovi 2017.
| Study characteristics | ||
| Methods |
Community health worker support for disadvantaged patients with multiple chronic diseases: a randomized clinical trial RCT (NA clusters and NA providers), conducted in 1) Patient enrollment occurred at 2 academic Philadelphia, Pennsylvania, adult internal medicine clinics. Intervention delivered in a single‐centre study. 2) Patients initially selected 1 of their multiple chronic conditions to focus on with their primary care provider. IMPaCT is an intervention in which community health workers (CHWs) provide tailored coaching, social support, advocacy and navigation. In United States of America. 2 arms: 1. Control (chronic disease goal‐setting with primary care provider) (control arm) and 2. Intervention (goal‐setting plus IMPaCT intervention with CHW support) (intervention arm) |
|
| Participants | Control arm N: 83 Intervention arm N: 92, NA, NA Diabetes type: 4 Mean age: 56.3 ± 8.56 % Male: 24.51 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (chronic disease goal‐setting with primary care provider) 1) Promotion of self‐management Intervention arm: (goal‐setting plus IMPaCT intervention with CHW support) 1) Case management 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This work was supported by funding from the University of Pennsylvania School of Medicine Agency for Healthcare Research and Quality Institutional Career Development (K12) grant, a grant from the University of Pennsylvania Institute for Translational Medicine and Therapeutics, and funding from the National Heart, Lung, and Blood Institute (K23 HL128837) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Research assistants then notified a study team member (not involved with outcomes assessment) who performed randomisation by using a computer‐generated algorithm. Randomisation was stratified by the condition patients selected as their focus during goal‐setting. This avoided imbalance between arms in case some conditions were easier to control than others. |
| Allocation concealment (selection bias) | Low risk | They performed randomisation by using a computer‐generated algorithm. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No data only for diabetic patients. Employment status has a significant P value of 0.002. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. Only Hb1Ac is specific to diabetic patients. Data for HbA1c reported, P values higher than 0.05 (based on the legend, only the employment status has a significant P value). However, HbA1c data are only reported for the diabetic patients who selected diabetes condition as their focus (74 out of 175 diabetic patients). |
| Incomplete outcome data (attrition bias) | High risk | 82% (but 83% in figure 1) of patients assigned to a CHW engaged with the programme for the full 6 months (17% to 18% were lost). From Figure 1, it looks like they lost 11% of patients in the control group (89% completed secondary outcomes). High and quite unbalanced numbers. Reason for lost only reported in the intervention group. Finally, HbA1c data are only reported for the diabetic patients who selected diabetes condition as their focus (74 out of 175 diabetic patients). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. They reported all primary and secondary outcomes except this tertiary outcome: patient medical adherence as measured by the Medical Outcomes Study (MOS) measures. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised trial and intervention delivered in a single‐centre study. Primary care providers may have changed their approach with their patients after receiving information from the CHW supporting intervention patients. Quote: "The CHWs sent electronic messages to primary care providers at 0, 3, and 6 months of the intervention, describing the patient action plans and progress. They also sent ad hoc messages or made telephone calls as needed for any clinical matters (e.g., patient running out of medications)." |
| Other bias | Low risk | No evidence of other bias |
Karhula 2015.
| Study characteristics | ||
| Methods |
Telemonitoring and mobile phone‐based health coaching among Finnish diabetic and heart disease patients: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted in the South Karelia Social and Health Care District (Eksote) in Finland. Eksote is responsible for arranging all primary and secondary health care for the inhabitants of 8 municipalities, approximately 100,000 inhabitants. 2) The intervention consisted of health coaching over mobile phones and self‐monitoring of health parameters with the help of a remote patient monitoring (RPM) system. In Finland. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (health coaching and remote monitoring) (intervention arm) |
|
| Participants | Control arm N: 79 Intervention arm N: 208, NA, NA Diabetes type: 2 Mean age: 66.2 ± NR % Male: 51.6 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (health coaching and remote monitoring) 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | A total of 50% of the funding of this study was received from the European Commission Information and Communication Technologies Policy Support Program (ICT PSP) 2009 of the Competitiveness and Innovation framework Programme (CIP), as part of the Renewing Health Project involving nine European countries. The other 50% of the funding was provided by Eksote. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within these subgroups, Excel generated random numbers were produced. The randomisation was conducted by the Technical Research Centre of Finland (VTT). |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed from the research nurse by means of an opaque and sealed envelope until the baseline visit. During the baseline visit the envelope was opened and, according to its content, each patient was assigned to either group. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 displays the baseline characteristics of patients separated according to their primary disease. Overall, patients were similar in the intervention group and in the control group in both disease groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Nothing about possible differences in text. |
| Incomplete outcome data (attrition bias) | High risk | Figure 2. There are 62 lost to follow‐up out of 287 patients randomised (21.6%). 16 out of 79 in the control group (20.2%) and 46 out of 208 in the intervention group (22.1%). Loss to follow‐up balanced between arms. Reasons provided and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest are objective (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol (protocol first posted in March 2011). The study was conducted between February 2011 and December 2012, 1‐year intervention). They added lipid profile in the paper (total cholesterol, HDL, LDL and triglycerides). Also, the protocol mentions these secondary outcomes: medication compliance; activity increase; smoke cessation; alcohol use reduction; cost for the organisation; satisfaction and usability of the technology and equipments, but they are not reported in published study. |
| Risk of contamination (other bias) | Low risk | Only patients in the intervention arm received calls from health coaches and had access to the remote monitoring system. Also, the health coaches only managed patients in the intervention group. |
| Other bias | Low risk | None identified. |
Katalenich 2015.
| Study characteristics | ||
| Methods |
Evaluation of a remote monitoring system for diabetes control RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from the Tulane Medical Center and Southeast Louisiana Veterans Health Care System endocrinology clinics (New Orleans, Louisiana). 2) Intervention provided by The Diabetes Remote Monitoring and Management System (DRMS, mainly an automated system with minimal human interaction). For emergency, patient was immediately and automatically connected to the endocrinologist/physician on call. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (automated Diabetes Remote Monitoring and Management (DRMS) System) (intervention arm) |
|
| Participants | Control arm N: 48 Intervention arm N: 50, NA, NA Diabetes type: 4 Mean age: 59 ± 9.45 % Male: 40 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (automated Diabetes Remote Monitoring and Management (DRMS) System) 1) Electronic patient registry 2) Clinician reminder 3) Facilitated relay of clinical information 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by a grant from Eli Lilly to Tulane University. There was no requirement to use Eli Lilly products in the study, and the sponsor had no role in the conduct of the study or in the analysis and interpretation of the data. This study was also supported in part by grant 1‐U54‐GM104940 from the National Institute of General Medical Sciences, National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Shi has received unrestricted research grants from Bristol‐Myers Squibb, Cepheid, and Genentech. Ms. McDuffie has received consultant’s fees from Amgen and Novo Nordisk. Dr. Fonseca has received consultant’s fees from Abbott, AstraZeneca, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk, Pamlabs, Sano., and Takeda. Tulane University Endocrinology has received grants and research support from Abbott, Asahi, Eli Lilly, EndoBarrier, Gilead, Novo Nordisk and Sanofi. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised, after informed consent was obtained, to either the intervention (DRMS) group or the control group by using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Appears to have more women (68% vs 52%) and black (74% vs 56%) participants in the intervention group. No P value reported in Table 1 or text. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c levels appear similar in both groups at baseline (difference of 0.05%), no P value under 0.05 reported (Table II). |
| Incomplete outcome data (attrition bias) | Low risk | Overall, 87 participants (88.8%) completed follow‐up out of 98 at baseline. The loss to follow‐up percentage was 11.2%; however, there were similar amounts of loss to follow‐up in both the control group (6 participants, 12.0%) and the intervention group (5 participants, 10.4%). Reasons not addressed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | High risk | No registered protocol or previously published protocol. Quote method: "At visit 1, day 0, participants were randomised to their group; had baseline HbA1c, weight, and blood pressure measurements taken... At both 3 and 6 months, each participant returned to the research clinic for a study visit. Each participant had HbA1c, blood pressure, and weight measured." However, no data about blood pressure and weight, both at baseline and after the intervention, are reported in the paper. |
| Risk of contamination (other bias) | Low risk | Unlikely control group received treatment as only the intervention arm had access to the DRMS system. |
| Other bias | Low risk | No evidence of other bias. |
Katon 2004.
| Study characteristics | ||
| Methods |
The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression RCT (NA clusters and NA providers), conducted in 1) 9 GHC (Group Health Cooperative) primary care clinics from a large health maintenance organisation in western Washington were selected for the study. 2) The intervention was provided by a depression clinical specialist nurses in collaboration with psychiatrist and psychologist supervisors and primary care physicians. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (pathways case management) (intervention arm) |
|
| Participants | Control arm N: 165 Intervention arm N: 164, NA, NA Diabetes type: 3 Mean age: 58.35 ± NR % Male: 35.00 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (pathways case management) 1) Case management 2) Team change 3) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by grants MH4‐1739 and MH01643 from the National Institute of Mental Health Services Division, Bethesda, Md (Dr Katon) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computerised algorithm, patients were randomised to the intervention or usual care group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no significant differences between groups in any variable (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | There were no significant differences between groups in any variable (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | The following percentages completed 3‐, 6‐ and 12‐month assessments: 3‐month assessment, 151 (91.5%) intervention patients and 154 (93.3%) usual care patients; 6‐month assessment, 143 (87.8%) intervention patients and 149 (90.9%) usual care patients; and 12‐month assessment, 146 (88.5%) intervention patients and 142 (86.1%) usual care patients. Number balanced but quite high. Reasons not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Participants had enhanced usual care, since routine care patients were encouraged to discuss depression with their primary care physician. Primary care physicians treated both intervention and control patients, leaving room for a spillover effect due to potential physician improvements in knowledge and skills in treating depression. Primary care physicians at the GHC frequently prescribe antidepressant medication and can refer patients to the GHC Mental Health Services. Both intervention and usual care patients could also self‐refer to a GHC mental health care provider. Half of the usual care controls in this study received antidepressant. |
| Other bias | Low risk | None identified. |
Katon 2010.
| Study characteristics | ||
| Methods |
Collaborative care for patients with depression and chronic illnesses Patient RCT, conducted in 14 primary care clinics (within a Group Health Cooperative) in Washington State, USA Two arms: 1. Usual care group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 108 Intervention arm N: 106 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.0 (1.9), post 7.8 (1.9) Intervention arm: pre 8.1 (2.0), post 7.3 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 132.0 (17.2), post 132.3 (17.4) Intervention arm: pre 136.0 (18.4), post 131.0 (18.2) 3) LDL, mean mg/dL (SD) Control arm: pre 109.0 (36.5), post 101.4 (36.6) Intervention arm: pre 106.5 (35.3), post 91.9 (36.7) |
|
| Funding source | Supported by grants (MH041739 and MH069741) from the Services Division of the National Institute of Mental Health (to Dr. Katon) and by institutional support from Group Health Cooperative | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " …use of a permuted‐block design, with randomly selected block sizes of 4, 6, and 8 patients" but how they generated this list is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Mentioned in text, but not in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "The characteristics of the patients in the intervention group and the usual‐care group were similar at baseline." |
| Incomplete outcome data (attrition bias) | High risk | Numbers and reasons for loss to follow‐up not provided; they only provide the percentage who completed 6 months and 12 months follow‐up. Baseline based on those randomised, however seems like a per‐protocol analysis was done. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcomes: laboratory methods not described, however they state that research assistants who carried out the study protocol were blinded; assume they were the outcome assessors. However, physicians were not blinded, had both intervention and comparator as patients, and could have influenced the treatment. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything proposed was completed. |
| Risk of contamination (other bias) | High risk | Quote: "Spillover of the intervention is possible, since primary care physicians cared for patients in both the intervention and control groups." |
| Other bias | Low risk | Information not available. |
Kaur 2015.
| Study characteristics | ||
| Methods |
Telephonic consultation and follow‐up in diabetics: impact on metabolic profile, quality of life, and patient compliance RCT (NA clusters and NA providers), conducted in 1) Guru Gobind Singh Medical College and Hospital, Baba Farid University of Health Sciences, Faridkot, Punjab, India (outpatient department (OPD) of a tertiary care teaching hospital). 2) Physicians in India 3 arms: 1. Group A (Control) rare mode ‐ follow‐up at 3 months (control arm) and 2. Group B (Intervention) moderate mode ‐ monthly follow‐up (intervention arm), 3. Intervention 2 (frequent mode: monthly visits and weekly consultation call) (other arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 40, 40, NA Diabetes type: 3 Mean age: 51.33 ± 10.39 % Male: 50.83 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (rare mode ‐ follow‐up at 3 months) 1) Patient education Intervention arm: (moderate mode ‐ monthly follow‐up) 1) Case management 2) Patient education Intervention arm: (frequent mode: monthly visits and weekly consultation call ) 1) Case management 2) Patient education |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein Harms |
|
| Funding source | Source of support: nil | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Written, informed consent for inclusion into the study was taken from the participants and they were randomly assigned to one of 3 groups, each consisting of 40 participants, using published tables of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Overall, the 3 groups were well matched for the demographic profile and baseline characteristics (shown in Table 1), ruling out any confounding factors that may alter the results of the intervention. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Overall, the 3 groups were well matched for the demographic profile and baseline characteristics (shown in Table 1), ruling out any confounding factors that may alter the results of the intervention. |
| Incomplete outcome data (attrition bias) | Low risk | All of the 120 participants completed the study and there was no case of any dropout, loss to follow‐up or mortality in any of the 3 groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure for HbA1c, LDL. Patient reported harms. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered; methods match objectives. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised. Groups met with the same team. |
| Other bias | Low risk | None. |
Keeratiyutawong 2006.
| Study characteristics | ||
| Methods |
Effectiveness of a self‐management program for Thais with type 2 diabetes: an integrative review RCT (NA clusters and NA providers), conducted in 1) Trial conducted at a community hospital in the eastern part of Thailand. Intervention session was held in a private room in the hospital. 2) A researcher with experience of taking care of diabetes patients conducted the intervention. The research assistants were trained to collect the questionnaires and helped the researcher facilitate the self‐management programme. In Thailand. 2 arms: 1. Control (control group) (control arm) and 2. Intervention (self‐management programme) (intervention arm) |
|
| Participants | Control arm N: 45 Intervention arm N: 45, NA, NA Diabetes type: 2 Mean age: NR ± NR % Male: 26 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Patient reminders Intervention arm: (self‐management programme) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | "Special thanks go to the Commission of Higher Education, Ministry of Education for giving me the financial support throughout my study program. In addition, I would like to thank the Thai Health Promotion Foundation for supporting my research grant." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. The participants who met the study criteria were randomly assigned to either a self‐management or a control group using a sealed envelope technique. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope technique, unclear if opaque envelopes. |
| Patient's baseline characteristics (selection bias) | Unclear risk | No table. No data in text. All variables met every assumption underlying the statistical testing. Not clear which are the variables. |
| Patient's baseline outcomes (selection bias) | High risk | Figure 1. HbA1c, P = 0.02 at baseline between groups. |
| Incomplete outcome data (attrition bias) | Low risk | Of the 90 participants, 45 were in the self‐management group and 45 were in the control group. Nine participants (10%) dropped from the study (5 in the intervention group and 4 in the control group). The reasons for withdrawal were not to do with dissatisfaction with the program. Low numbers, balanced numbers, reasons partly reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | All participants had diabetes education and phone calls at 1, 3 and 5 months by the same researcher. |
| Other bias | Low risk | No evidence of other bias. |
Kempf 2017.
| Study characteristics | ||
| Methods |
Efficacy of the Telemedical Lifestyle intervention Program TeLiPro in advanced stages of type 2 diabetes: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted at the West German Centre of Diabetes and Health in Dusseldorf, Germany, in co‐operation with the German Institute for Telemedicine and Health promotion, 2) Trained diabetes coaches in Germany 2 arms: 1. Control (routine care) (control arm) and 2. Intervention (TeLiPro‐Telemedical Lifestyle intervention Program) (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 102, NA, NA Diabetes type: 2 Mean age: 59.44 ± 10.05 % Male: 54.11 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (routine care) 1) Electronic patient registry 2) Promotion of self‐management Intervention arm: (TeLiPro‐Telemedical Lifestyle intervention Program) 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | "The study was funded by Boehringer Ingelheim International GmbH and by Gesellschaft von Freunden und Forderern der Heinrich‐Heine‐Universitat Dusseldorf e.V. K.K., B.A., J.B., B.G., K.N., and S.M. received research support from Boehringer Ingelheim International GmbH & Co." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1 ratio using an electronically generated random list (created by trial statistician) |
| Allocation concealment (selection bias) | Low risk | For each identification number, there was a closed envelope with the group assignment. The allocation sequence was concealed from the participants, the study nurse and the outcome assessor. |
| Patient's baseline characteristics (selection bias) | Low risk | Baseline characteristics were similar between the 2 study groups (Table 1). |
| Patient's baseline outcomes (selection bias) | High risk | Table 1‐FBG, DBP and body weight different between arms; no P values but looks unbalanced. |
| Incomplete outcome data (attrition bias) | High risk | The dropout rate in the control group was significantly higher than in the TeLiPro group (P = 0.001). 44 lost from control group (44%) and 25 lost from intervention group (25%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes. |
| Selective reporting (reporting bias) | High risk | Physical activity/step count and nutrition not reported in paper. |
| Risk of contamination (other bias) | Low risk | Diabetes coaches assigned to intervention group only. |
| Other bias | Low risk | No evidence of other bias. |
Keogh 2011.
| Study characteristics | ||
| Methods |
Psychological family intervention for poorly controlled type 2 diabetes Patient RCT, conducted in a specialist diabetes clinic at a large suburban hospital, Ireland Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 61 Intervention arm N: 60 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.3 (1.1), post 8.8 (1.4) Intervention arm: pre 9.1 (1.0), post 8.4 (1.0) 2) SBP, mean mmHg (SD) Control arm: pre 138.0 (17.9), post 135.9 (16.5) Intervention arm: pre 139.5 (19.2), post 139.7 (20.8) 3) DBP, mean mmHg (SD) Control arm: pre 77.7 (10.6), post 77.0 (9.9) Intervention arm: pre 76.0 (9.7), post 75.4 (10.3) 4) Smoking cessation, N smokers (%) Control arm: pre 13 (28), post 12 (27) Intervention arm: pre 5 (12), post 3 (7) |
|
| Funding source | This study was funded by the Irish Health Research Board, project grant RP/2005/178 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…remote computer‐generated random number sequence." |
| Allocation concealment (selection bias) | Unclear risk | They talk about concealment but only the fact that the participants did not know their allocation until they began the study. However, the actual process of concealment was not described. |
| Patient's baseline characteristics (selection bias) | Unclear risk | In text not in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those randomised, but numbers and reasons for loss to follow‐up not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Quote: "Outcome assessors were blinded to group allocation." Primary outcome: HbA1c, secondary: SBP. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None identified. |
Keyserling 2002.
| Study characteristics | ||
| Methods |
A randomized trial of an intervention to improve self‐care behaviors of African‐American women with type 2 diabetes: impact on physical activity RCT (NA clusters and NA providers), conducted in 1) This study was conducted at 7 primary care practices in central North Carolina, including 5 community health centres, 1 staff model health maintenance organization and the general medicine clinic at an academic health centre. 2) A single nutritionist provided clinic‐based counselling and the community component of the New Leaf Program was facilitated by a community diabetes advisor (CDA), a nonprofessional peer counsellor. In United States of America. 3 arms: 1. Control (minimal intervention ‐ Group C) (control arm) and 2. Intervention 1 (clinic intervention only ‐ Group B) (intervention arm), 3. Intervention 2 (clinic and community intervention ‐ Group A) (other arm) |
|
| Participants | Control arm N: 67 Intervention arm N: 66, 67, NA Diabetes type: 2 Mean age: 59.16 ± 10.26 % Male: 0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (minimal intervention) 1) Facilitated relay of clinical information 2) Patient education Intervention arm: (clinic intervention only) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: (clinic and community intervention) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | "This study was supported in part by a cooperative agreement (U48/CCU409660) with the Centers for Disease Control and Prevention and through a memorandum of understanding with the National Institutes of Health. Carlos F. Henriquez‐Roldan is a PhD candidate at the University of North Carolina at Chapel Hill and is sponsored by a scholarship from “Beca Presidente de la Republica de Chile.”" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of individuals to treatment groups was stratified by clinic site. A statistical consultant prepared a site‐specific allocation schedule (with randomly permuted blocks of size 3 and 6) from random numbers generated by a personal computer. |
| Allocation concealment (selection bias) | Unclear risk | A statistical consultant prepared a set of sequentially numbered, sealed envelopes (opaque?) containing study group assignments. Participants were assigned to a study group by a research assistant who opened the appropriate envelope. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values are above 0.05 for baseline characteristics except for: living with spouse or someone like a spouse (P = 0.027). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05 for baseline outcomes. |
| Incomplete outcome data (attrition bias) | High risk | For HbA1c, at baseline, they have data for 183 patients (8.5% lost) and for 170 patients (15% lost) at 12 months out of 200 patients randomised. At 12 months, 9/67 patients are missing in the control group (13.4%), 7/66 in the clinic only group (10.6%) and 13/67 in the clinic and community group (19.4%). Numbers unbalanced. More patients did not return for visit in the clinic and community group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol, but the authors refer to reference 16 for detailed protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | All clinicians received patients' clinical data from the research team (HbA1c and blood lipid) and they were all instructed to reinforce educational information provided to participants by the study team, to monitor glycaemic control, and to prescribe medications for diabetes according to their usual practices. The primary care clinicians were informed of participants’ group assignment which may have influenced their care. |
| Other bias | Low risk | None. |
Khan 2018.
| Study characteristics | ||
| Methods |
Effectiveness of an integrated diabetes care package at primary healthcare facilities: a cluster randomised trial in Pakistan Clustered RCT (14 clusters and 28 providers), conducted in 1) 9 rural health centres and 5 sub‐district hospitals in the Sargodha district in Punjab, Pakistan were recruited and randomised into the trial. The study involves only the outpatient department of facilities, which have general practice doctors seeing un‐referred primary care patients. 2) General practice doctors and allied professionals in Pakistan 2 arms: 1. Control (TTR‐testing, treating and recording) (control arm) and 2. Intervention (ACM ‐ additional case management) (intervention arm) |
|
| Participants | Control arm N: 245 Intervention arm N: 250, NA, NA Diabetes type: 2 Mean age: 46.1 ± 9.62 % Male: 38.19 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: (TTR ‐ testing, treating and recording) 1) Case management 2) Clinician education Intervention arm: (ACM ‐ additional case management) 1) Case management 2) Clinician education 3) Patient education 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Hypertension control |
|
| Funding source | The study was funded by COMDIS‐HSD, a research consortium funded by UK aid from the UK government (reference number: COMDIS‐HSD RGNUID 480650) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 14 facilities were randomised to the TTR‐only or the ACM arm on a 1:1 basis using a lottery method with sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | The 14 facilities were randomised to the TTR‐only or the ACM arm on a 1:1 basis using a lottery method with sealed envelopes. In the presence of a 5‐member committee, a staff member from the provincial directorate randomly selected seven envelopes for each arm from among 14 sealed envelopes, each containing a recruited facility name. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table 1, no P values reported and only provider sex reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no substantial differences in patient characteristics between the arms (See Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Outcomes balanced between groups. |
| Incomplete outcome data (attrition bias) | Low risk | 26/245 (10.6%) lost in control group and 12/250 (4.8%) lost in intervention group, 8% lost in total, reasons not provided. No clusters lost. 26/245 (Figure 2). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes: HbA1c, SBP, DBP, hypertension control. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered. Protocol states 18‐month follow‐up, paper reports 9‐month follow‐up; protocol lists cost‐effectiveness as secondary outcome, not mentioned in paper. |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT, randomisation by hospital. |
| Other bias | Low risk | No evidence of other bias. |
Kiefe 2001.
| Study characteristics | ||
| Methods |
Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial Cluster‐RCT (70 clusters with 70 providers), conducted in family medicine, internal medicine and endocrinology from 3 states of Alabama, Iowa and Maryland, USA Two arms: 1. Comparison (control arm) and 2. Experimental (intervention arm) |
|
| Participants | Control arm N: 1648 Intervention arm N: 1643 Diabetes type: unclear/not reported Mean age: 76.0 ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Audit and feedback 2) Clinician reminders 3) Continuous quality improvement Intervention arm: 1) Audit and feedback 2) Clinician reminders 3) Continuous quality improvement |
|
| Outcomes | 1) Foot screening, N screened (%) Control arm: pre 309 (32), post 307 (45) Intervention arm: pre 444 (46), post 414 (61) |
|
| Funding source | This work was supported by grants HS09446, HS11124, and HS/GM 10389 from the Agency for Healthcare Research and Quality and conducted in co‐operation with the Alabama Quality Assurance Foundation and the Healthcare Financing Administration | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Table 2 ‐ no comparisons significant, but do not report age or gender. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Data is provided for age and race, but not age or education. Table 3. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Groups had similar comorbidities, but no baseline outcome data reported. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Kim 2005.
| Study characteristics | ||
| Methods |
Effects of nurse‐coordinated intervention on patients with type 2 diabetes in Korea Patient RCT, conducted in an endocrinology outpatient department of a tertiary care hospital in urban city of South Korea Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 20 Diabetes type: type 2 Mean age: 60.8 ± 6.2 % Male: 36.0 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.2 (0.9), post 8.7 (0.7) Intervention arm: pre 8.9 (1.3), post 7.7 (1.1) |
|
| Funding source | This work was supported by grant R04–2002–000– 20024–0 from the Basic Research Program of the Korea Science & Engineering Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Kim 2009.
| Study characteristics | ||
| Methods |
A community‐based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes Patient RCT (0 clusters and NA providers), conducted in Korean Resource Center in Baltimore in USA 2 arms: (control arm) (intervention arm) |
|
| Participants | Control arm N: 42 Intervention arm N: 41, NA, NA Diabetes type: 2 Mean age: 56.4 ± 8.05 % Male: 55.7 Longest follow‐up: 7 months |
|
| Interventions |
Control arm: (non‐tailored phone calls) Intervention arm: (tailored phone calls from nurses) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This research is supported by a grant from the National Institutes of Health (NIDDK R34 DK071957), LifeScan, Inc (HCC002154), and the Johns Hopkins University School of Medicine General Clinical Research Center (M01‐RR00052), from the National Center for Research Resources/National Institutes of Health (NCT00505960) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to either the SHIP‐DM intervention group (n = 41) or the control (delayed intervention) group (n = 42) by computer‐automated random assignment. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. P values provided and cholesterol levels were significantly different at baseline. |
| Incomplete outcome data (attrition bias) | Low risk | One participant from the intervention group and 3 from the control group withdrew because of a lack of time (retention rate = 95.2%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c, LDL, BP. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol, secondary outcomes between protocol and paper were not consistent. |
| Risk of contamination (other bias) | Low risk | Remotely delivered intervention. Control participants did not have access to testing device. |
| Other bias | Low risk | None identified. |
Kim 2010.
| Study characteristics | ||
| Methods |
Insulin dose titration system in diabetes patients using a short messaging service automatically produced by a knowledge matrix Patient RCT, conducted with patients recruited from an outpatient clinic of Hallym University Sacred Heart Hospital, Republic of Korea Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.8 (1.2), post 7.8 (0.8) Intervention arm: pre 9.8 (1.3), post 7.4 (0.7) 2a) Harms (symptomatic hypoglycaemia episodes), N (%) Control arm: pre NR (NR), post 39 (87) Intervention arm: pre NR (NR), post 42 (89) 2b) Harms (asymptomatic hypoglycaemia), N (%) Control arm: pre NR (NR), post 5 (11) Intervention arm: pre NR (NR), post 5 (11) 2c) Harms (nocturnal hypoglycaemia), N (%) Control arm: pre NR (NR), post 5 (11) Intervention arm: pre NR (NR), post 6 (13) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… by a computer generated allocation sequence using adaptive randomization." Low risk, since minimisation is a type of adaptive randomization. |
| Allocation concealment (selection bias) | High risk | Adaptive: you can predict the next assignment. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: HbA1c (P = 0.759). |
| Incomplete outcome data (attrition bias) | Unclear risk | Although loss to follow‐up per arm was under 10%, numbers who did not have visit at 12 weeks seemed larger for intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described and likely not implemented. HbA1c measurement was not described, no laboratory description. |
| Selective reporting (reporting bias) | Low risk | Found protocol on clinical trials.gov. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Kim 2015.
| Study characteristics | ||
| Methods |
The effect of a community‐based self‐help intervention: Korean Americans with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Naturally occurring community setting. Most research activities took place at a community site, the Korean Resource Center (KRC). 2) The intervention involved a team of bilingual registered nurses and community health workers (CHWs). In United States of America. 2 arms: 1. Control (wait‐list) (control arm) and 2. Intervention (SHIP‐DM: education, counselling and behavioural coaching) (intervention arm) |
|
| Participants | Control arm N: 130 Intervention arm N: 120, NA, NA Diabetes type: 2 Mean age: 58.7 ± 8.38 % Male: 56.9 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (wait‐list) 1) Patient education Intervention arm: (SHIP‐DM: education, counselling and behavioural coaching) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | The study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R18 DK083936), with material support from LifeScan, including devices (OneTouch glucometer, OneTouch UltraSoft test strips, and OneTouch UltraSoft lancets) for study participants. In addition, the Johns Hopkins Institute for Clinical and Translational Research supported the cost of blood serum lab tests. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in text and protocol. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text and protocol |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values higher than 0.05 for all characteristics. |
| Patient's baseline outcomes (selection bias) | High risk | P < 0.05 for LDL and diastolic blood pressure at baseline. More hypertensive patients in control group (P < 0.05) at baseline. |
| Incomplete outcome data (attrition bias) | High risk | Total of 41 out of 250 lost to follow‐up (16.4%). More lost to follow‐up in control group (20.0% vs 12.5% in intervention group). The wait‐list control group was oversampled for its lower retention rate. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c, SBP, DBP, controlled BP and LDL). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted in December 2010, study done between September 2010 and December 2014, 1‐year intervention). Results match protocol. |
| Risk of contamination (other bias) | Low risk | Only patients in the intervention group received education session and calls from case managers. The case managers never called the control group. |
| Other bias | Low risk | No evidence of other bias. |
Kim 2016.
| Study characteristics | ||
| Methods |
Randomized, open‐label, parallel group study to evaluate the effect of internet‐based glucose management system on subjects with diabetes in China RCT (NA clusters and NA providers), conducted in 1) First Bethune Hospital at Jilin University, China. 2) Nurses provided recommendations regarding blood sugar control. Participants who completed less than half of the recommended tests or who exhibited low blood sugar levels were provided with doctor consultations through text messaging or phone calls. In China. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (IBGMS) (intervention arm) |
|
| Participants | Control arm N: 110 Intervention arm N: 110, NA, NA Diabetes type: 2 Mean age: 54.03 ± 8.51 % Male: 48.35 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education 2) Promotion of self‐management Intervention arm: (IBGMS) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This work was supported in part by a research grant from the Investigator Initiated Study Program of UBcare. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A block randomisation design with a block size of 4 and a ratio of 1:1 was used to ensure a balanced distribution. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. Sex looks unbalanced (43% vs 53%). Average age is balanced but age breakdown is not (over 60 = 42% vs 25%). Antidiabetes medication not balanced between arms. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. Triglycerides P = 0.004 but looks balanced. All other outcomes look balanced. |
| Incomplete outcome data (attrition bias) | High risk | A total of 220 participants were assigned to the IBGMS (n = 110) or control (n = 110) groups. During the 6‐month study period, 20 participants were excluded from the control group (18.2%) and 18 participants were excluded from the IBGMS group (16.4%), which left 90 participants in the control group and 92 participants in the IBGMS group for the final analysis. The dropout rate in this study was higher than in other studies. Patients were excluded from the study if they failed to upload data after 3 consecutive warning messages or phone calls. High but balanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Both the IBGMS and the control groups visited the hospital every 3 months for laboratory testing and a clinical examination to evaluate the safety and efficacy of their treatment. Laboratory testing included baseline HbA1c levels, white blood cell counts with the differential counts, red blood cell counts, haemoglobin and hematocrit levels, platelet counts, fasting blood sugar, blood urea nitrogen, creatinine, aspartate transaminase (AST), alanine transaminase (ALT), sodium, and potassium levels. In addition, the patients’ lipid profiles were evaluated, which included total cholesterol (TC), triglycerides (TG), high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C). The participants’ height and weight were evaluated. Many outcomes are not reported (white and red blood cell counts, haemoglobin, hematocrit levels, platelet counts, sodium, and potassium levels). |
| Risk of contamination (other bias) | Low risk | It is unlikely that control group used and received feedback from the Internet‐based glucose monitoring system (IBGMS). |
| Other bias | Unclear risk | "We excluded patients who had not taken their medication regularly in the 3 months before enrollment." Selection bias. |
Kim 2016a.
| Study characteristics | ||
| Methods |
Clinical evaluation of OneTouch diabetes management software system in patients with type 2 diabetes mellitus RCT, 1) This study was conducted in a single centre for diabetes education in Korea (outpatient clinic). 2) Intervention patients were assigned into the OneTouch Diabetes Management Software (OTDMS) group. Nurses provided an OTDMS’s report sheet. Doctors explained and educated patients using OTDMS’s report during consultation hours. Two arms: 1. Control (conventional medical treatment and education) and 2. Intervention (OneTouch Diabetes Management Software‐OTDMS) |
|
| Participants | Participants Control arm N: 58 Intervention arm N: 63 Diabetes type: 2 Mean age: 56.68 ± 10.39 % Male: 45.65 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (conventional medical treatment and education) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: (OneTouch Diabetes Management Software‐OTDMS) 1) Team change 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin 2) Harms (frequency of hypoglycaemia) |
|
| Funding source | This work was supported by the 2011 Inje University research grant | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. A total of 121 patients with type 2 DM were randomly assigned into 2 groups: 63 patients were assigned into the OTDMS group and 58 patients were assigned into the control group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | We compared baseline characteristics of all patients (Table 5). There was no significant difference in age, sex and duration of diabetes. However, they only report data for the patients who completed the study (n = 92) and not for all the patients randomised (n = 121). |
| Patient's baseline outcomes (selection bias) | Unclear risk | We compared baseline characteristics of all patients (Table 5). There was no significant difference in the level of HbA1c, BMI and the type of treatment between the OTDMS and control groups. There was no significant difference in knowledge, compliance, reliability and satisfaction. However, they only report data for the patients who completed the study (n = 92) and not for all the patients randomised (n = 121). |
| Incomplete outcome data (attrition bias) | High risk | 92 patients completed the study out of 121 randomised (24% lost). 50/58 patients in the control group (lost 14%) and 42/63 in the intervention group (lost 33%) completed the study. High and unbalanced numbers. Reasons reported but not for each arm. Quote: "Excluded were patients (total 29) who were under 20 years of age (n = 3), and who had incomplete data in compliance (n = 9), HbA1c (n = 13), and BMI (n = 4)." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. Method to measure hypoglycaemia not reported (but secondary outcome). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match with methods. |
| Risk of contamination (other bias) | Unclear risk | We provided all patients conventional education and a home blood glucose meter that automatically transmits blood glucose data to the hospital. It is unclear if doctors had access to transmitted data from their control patients. Study delivered in a single centre for diabetes. It is possible that doctors changed their approach with their control patients after providing tailored education to their patients in the intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Kinmonth 1998.
| Study characteristics | ||
| Methods |
Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk Cluster‐RCT (41 clusters with 107 providers), conducted in 41 practices in Wessex, UK Two arms: 1. Comparison (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 161 Intervention arm N: 199 Diabetes type: type 2 Mean age: 57.7 ± NR % Male: 59.2 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Clinician education 2) Patient education |
|
| Outcomes | 1) HbA1c 2) SBP 3) DBP 4) Smoking cessation |
|
| Funding source | The study was funded by grants from the Medical Research Council; the South and West region’s research and development committee; and the British Diabetic Association. SG received a Wellcome health services research training fellowship. Novo Nordisk supplied the booklets for patients. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table ‐ all P values > 0.05 but no reporting of urban/rural. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table ‐ P values > 0.05 for age and gender, but no education reported. |
| Patient's baseline outcomes (selection bias) | Low risk | Data are provided in the table with P values. None is significant. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Kirk 2009.
| Study characteristics | ||
| Methods |
A randomized trial investigating the 12‐month changes in physical activity and health outcomes following a physical activity consultation delivered by a person or in written form in Type 2 diabetes: Time2Act RCT (NA clusters and NA providers), conducted in 1) Study took place at the University of Dundee in Scotland sports centre, United Kingdom. 2) Intervention delivered by trained researcher. In United Kingdom. 3 arms: 1. Control (standard care) (control arm) and 2. Intervention 1 (written physical activity consultation, PAC) (intervention arm), 3. Intervention 2 (one‐to‐one physical activity consultation) (other arm) |
|
| Participants | Control arm N: 35 Intervention arm N: 52, 47, NA Diabetes type: 2 Mean age: 61.35 ± 9.2 % Male: 48.51 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard care) 1) Patient education Intervention arm: (written physical activity consultation) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (one‐to‐one physical activity consultation) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | Thank you to Diabetes UK for funding this research (BDA:RD04/0003033) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Participants were randomly assigned on an individual basis using consecutively numbered, sealed envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned on an individual basis using consecutively numbered sealed envelope (opaque?). |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No significant between‐group differences were found at baseline on any measured outcome variable. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. No significant between‐group differences were found at baseline on any measured outcome variable. |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. A total of 18 patients were lost at 12 months out of the 134 randomised (13.4%). 4 were lost in the standard arm (11.4%), 9 in the written PAC (17.3%) and 5 in the person PAC (10.6%). Dropout from the study was around 10% (in‐person delivered and standard care) with slightly higher rates (17%) recorded in the written delivered group. At baseline, those who dropped out (n = 18) were similar in age, employment status and levels of physical activity, but had significantly higher BMI, HbA1c and socio‐economic deprivation (P < 0.05). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, SBP and DBP). Researchers conducting outcome measures were blind to group allocation at baseline and in most cases at 6 and 12 months. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | All patients received follow‐up phone calls and education. Control participants did not have physical education discussed during follow‐up call. Unclear who did the calling. |
| Other bias | Low risk | None. |
Kirkman 1994.
| Study characteristics | ||
| Methods |
A telephone‐delivered intervention for patients with NIDDM. Effect on coronary risk factors RCT (NA clusters and NA providers), conducted in 1) The study was conducted in the General Medical Clinic (GMC) of the Durham (North Carolina, USA) Department of Veterans Affairs Medical Center (VAMC). 2) A research nurse attempted to call TDI group patients at least monthly, with each of 3 nurses following a panel of patients throughout the 12‐month study in United States of America 2 arms: 1. Control group (usual care) (control arm) and 2. Intervention (telephone‐delivered intervention) group (intervention arm) |
|
| Participants | Control arm N: 71 Intervention arm N: 204, NA, NA Diabetes type: 2 Mean age: 63.72 ± 9.48 % Male: 98.89 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (telephone‐delivered intervention) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Lipid‐lowering drugs Low‐density lipoprotein Smoking status |
|
| Funding source | The project was funded through Investigator Initiated Research Grant 89‐079 from the Health Services Research and Development Service, Department of Veterans Affairs | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned to one of 2 study groups, using a permuted blocked randomisation stratified by study nurse and hypoglycaemic regimen |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly assigned to one of 2 study groups, using a permuted blocked randomisation stratified by study nurse and hypoglycaemic regimen |
| Patient's baseline characteristics (selection bias) | Low risk | There were no statistically significant differences in baseline characteristics between the 2 groups at enrollment (Table 1). No P value is reported. No information on income or education is provided. |
| Patient's baseline outcomes (selection bias) | Unclear risk | TDI patients had somewhat lower levels of total cholesterol (199 vs 207 mg/dL) and LDL cholesterol (119 vs 129 mg/dL). |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up for one of the outcomes (LDL) is 52% in each group and for the other outcome (smoking) is even higher. No reason was provided for this loss. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | LDl was measured objectively. Smoking was reported in 2 ways: self‐report and co‐verified. For the other outcomes, patients were interviewed by research assistant, who was unaware of the study hypotheses and study group assignment. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match results. |
| Risk of contamination (other bias) | Low risk | Control patients received usual care, but no telephone calls from the study nurses. There was no systematic provider‐initiated monitoring of health status between visits, and discussions of behavioural changes only occurred if the physician or patient initiated them during GMC visits. |
| Other bias | Low risk | None. |
Kirwan 2013.
| Study characteristics | ||
| Methods |
Diabetes self‐management smartphone application for adults with type 1 diabetes: randomized controlled trial Patient RCT, conducted with patients registered with Diabetes Australia in New South Wales, Queensland, as well as advertisements in a type 1 diabetes national newsletter. In Australia. Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 36 Diabetes type: type I Mean age: 35.2 ± 10.4 % Male: 39.0 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (0.9), post 8.6 (1.2) Intervention arm: pre 9.1 (1.2), post 7.8 (0.8) |
|
| Funding source | This study was funded by Central Queensland University, Australia. C Vandelanotte is supported by a National Health and Medical Research Council of Australia (#519778) and National Heart Foundation of Australia (#PH 07B 3303) postdoctoral research fellowship. M Kirwan is supported by a Queensland Government, Department of Tourism, Regional Development and Industry, SmartFutures PhD Scholarship. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomized patients using a freely available online randomization program." |
| Allocation concealment (selection bias) | Unclear risk | Assuming they are being randomised as they are enrolled. Quote: "…was used during the 3 month rolling recruitment to ensure roughly equal number of patients were allocated to each comparison group." |
| Patient's baseline characteristics (selection bias) | Low risk | More females in control group (P = 0.02). |
| Patient's baseline outcomes (selection bias) | High risk | HbA1c P = 0.02. |
| Incomplete outcome data (attrition bias) | High risk | ~22% lost to follow‐up in control and ~31% in intervention; reasons for loss to follow‐up could not be provided, since study participants could not be re‐contacted. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: HbA1c, collected through a pathology laboratory, need to describe method. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | High risk | Quote: "Although patients in the control group were instructed not to use any mobile applications to self‐manage their diabetes during the study period, it is possible they did." |
| Other bias | Low risk | Information not available. |
Kirwin 2010.
| Study characteristics | ||
| Methods |
Pharmacist recommendations to improve the quality of diabetes care: a randomized controlled trial Cluster‐RCT (8 clusters with 72 providers), conducted in 8 suites within a hospital‐based primary care practice on the main campus of a large academic teaching hospital in Boston, Massachusetts, USA Two arms: 1. Usual care group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 175 Intervention arm N: 171 Diabetes type: type 1 and type 2 Mean age: 63.0 ± NR % Male: 34.2 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Electronic patient registry 3) Clinician reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 56 (37), post 76 (50) Intervention arm: pre 57 (38), post 90 (60) 2) Renal screening (microalbumin), N screened (%) Control arm: pre 71 (47), post 87 (58) Intervention arm: pre 69 (46), post 94 (63) 3) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 68 (45), post 62 (41) Intervention arm: pre 71 (47), post 66 (44) |
|
| Funding source | This project received no external funding | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…random number generator". |
| Allocation concealment (selection bias) | Low risk | Allocation concealment not described. Cluster. |
| Provider's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Annual lipid profiles (P = 0.015). |
| Patient's baseline outcomes (selection bias) | Low risk | Annual eye exam (P = 0.870); annual urine microalbumin exam (P = 0.859); HTN‐C (< 130/80) (P = 0.769). |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those analysed. Numbers provided for loss to follow‐up (balanced), but reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding (and of outcome assessors) not described. Objective outcome methods not described. |
| Selective reporting (reporting bias) | High risk | Does not match protocol for secondary outcomes listed in protocol. |
| Risk of contamination (other bias) | Low risk | Quote: "This randomization unit minimized the potential for contamination of the intervention that might occur if …."cluster"". |
| Other bias | Low risk | Information not available. |
Kjeldsen 2015.
| Study characteristics | ||
| Methods |
Safe and effective use of medicines for patients with type 2 diabetes ‐ a randomized controlled trial of two interventions delivered by local pharmacies RCT (NA clusters and NA providers), conducted in 1) Five community pharmacies in the county of Funen, Denmark 2) Basic intervention provided by a pharmaconomist (BI), extended intervention provided by a pharmacist (EI). Pharmacy staff (pharmacists and pharmaconomists) delivered the interventions in collaboration with the patients, and the patients’ GPs were informed about the programme content and contacted whenever necessary. In Denmark. 3 arms: 1. Control group (control arm) and 2. Basic intervention group (intervention arm), 3. Extended intervention group (other arm) |
|
| Participants | Control arm N: 125 Intervention arm N: 39, 41, NA Diabetes type: 2 Mean age: 62.55 ± 12.98 % Male: 60.96 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: Basic Intervention arm: 1) Case management 2) Team change 3) Electronic patient registry 4) Promotion of self‐management 5) Patient reminders Intervention arm: (extended intervention group) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Systolic blood pressure | |
| Funding source | The project was supported financially by the Danish Health and Medicines Authority, Pharmadanmark, the Danish Association of Pharmaconomists, the Association of Danish Pharmacies and Pharmakon, and the Danish College of Pharmacy Practice | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 205 patients met the inclusion criteria and were randomly allocated into 3 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. P values greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. P values greater than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Loss of 18.4% in control group and 12.5% in intervention group. > 10% lost in each arm (23 from control, 6 from basic intervention and 4 from extended intervention); reasons for not filling questionnaire not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Subjective measure of SBP ‐ self‐report of latest SBP measurement by GP. |
| Selective reporting (reporting bias) | High risk | Also HbA1c, LDL, HDL and triglycerides as reported measured by the GP at the most recent visit were collected, but the response rate for these values was below 50% and consequently considered too low for inclusion in the analyses. |
| Risk of contamination (other bias) | Low risk | Interventions provided by 2 separate groups. BI from pharmaconomists, EI from pharmacists. |
| Other bias | Low risk | No evidence of other bias. |
Kleinman 2016.
| Study characteristics | ||
| Methods |
Impact of the Gather mHealth System on A1C: primary results of a multisite randomized clinical trial among people with type 2 diabetes in India RCT (NA clusters and NA providers), conducted in 1) The study was conducted in 3 diabetes‐focused clinics: DHL Research Centre in Ahmedabad, Diabetes Action Centre in Mumbai, and Prof. M. Viswanathan Diabetes Research Centre in Chennai. 2) All had at least one senior clinician to lead treatment and a health coach to provide regular participant interaction. In India. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Gather mobile health intervention) (intervention arm) |
|
| Participants | Control arm N: 47 Intervention arm N: 44, NA, NA Diabetes type: 2 Mean age: 48.4 ± 2.52 % Male: 70 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Gather mobile health intervention) 1) Electronic patient registry 2) Clinician reminder 3) Facilitated relay of clinical information 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by Gather Health LLC | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was investigator generated, stratified by site, with a 1:1 allocation, in blocks of 6, using the Sealed Envelope Ltd. online system. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed from implementing staff through sequentially numbered, opaque, sealed and stamped envelopes. After a participant’s eligibility was confirmed, research staff opened the next available envelope and assigned the participant to a group. At the time of randomisation, participant ID and randomisation date were written on the envelope and allocation paper to prevent allocation sequence tampering |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 (prior presentation: poster). Text: Demographic characteristics were balanced between study arms. However, there are more females in the control group (41.3% vs 18.2% in the intervention group). Control group also seems less educated. No P values. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | 13/47 (28%) lost in control group, 9/44 (20%) lost in intervention group. 24% lost overall. Reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Blood pressure was listed as an outcome however only baseline data were reported. Blood pressure at 3 and 6 months, Waist circumferences at 3 and 6 months, lipids at 3 and 6 months not reported. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Provider contact with participants outside the system was discouraged, except in cases of high‐risk glycaemic data or technical troubleshooting. Unlikely that the control participants received m‐health intervention contamination |
| Other bias | Low risk | No evidence of other risk of bias. |
Klingeman 2017.
| Study characteristics | ||
| Methods |
Type 2 diabetes specialty clinic model for the accountable care organization era RCT (NA clusters and NA providers), conducted in 1) Intervention conducted at an advanced type 2 specialty clinic model nested in the University of Michigan Endocrinology Clinic. 2) Intervention delivered by the clinical team consisting of an endocrinologist and a nurse educator. In United States of America. 2 arms: 1. Control (standard endocrinology clinics) (control arm) and 2. Intervention (type 2 specialty endocrinology clinic model) (intervention arm) |
|
| Participants | Control arm N: 30 Intervention arm N: 30, NA, NA Diabetes type: 2 Mean age: 54.365 ± 12.5 % Male: 53.35 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard endocrinology clinics) Intervention arm: (type 2 specialty endocrinology clinic model) 1) Case management 2) Clinician reminder 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Lipid‐lowering drugs Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Harms |
|
| Funding source | University of Michigan. Supported by Michigan Center for Diabetes Translational Research (MCDTR). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Patients were randomised 1:1, to the experimental or standard endocrinology clinics. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. Caucasian race and hypertension comorbidity have significant P values. Quote: "More Caucasian individuals were seen in the experimental arm compared to the control (96.6% vs. 76.8%; P < 0.05)." |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. HbA1c has a significant P value. Quote: "Initial A1c in the experimental arm was statistically higher than the control (9.5 ± 0.9% vs. 8.9 ± 0.8%; P < 0.05)." |
| Incomplete outcome data (attrition bias) | High risk | Overall, 60 patients participated in the study (30 in each arm), of whom 44 (73.3%) finished the 1 year follow‐up with the endocrinology clinic (24 in the experimental and 20 in the control arms; P = 0.2). Overall, they lost 27% of patients, with 33% in the control group and 20% in the intervention group. High and quite unbalanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | No clear primary outcome in the paper. HbA1c and BP were objectively measured. Method to collect statin use and severe hypoglycaemic events are not reported. Unmasked randomised controlled clinical trial. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. They reported data for HbA1c at 0, 4, 8 and 12 months, while it was supposed to be only at 12 months in the protocol. No data reported about total cholesterol, LDL, triglycerides, all cause mortality, quality of life, insulin therapy satisfaction in the paper. |
| Risk of contamination (other bias) | Low risk | Patient randomised. Unlikely that control patients met with the endocrinologist meeting only with patients in the intervention group. Quote: "Participants randomized to the control arm (n = 30) were provided a usual endocrine care in the Endocrinology Clinic of the University of Michigan (excluding the experimental model care clinic endocrinologist)". However, endocrinologists were all working in the same endocrinology clinic so communication might have happened. |
| Other bias | Low risk | No evidence of other bias. |
Kobayashi 2019.
| Study characteristics | ||
| Methods |
Automated feedback messages with Shichifukujin characters using IoT system‐improved glycemic control in people with diabetes: a prospective, multicenter randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Two clinics in Nagoya, Japan. 2) Intervention delivered by primary care physicians (local healthcare professionals) and study investigator healthcare professional in Japan 2 arms: 1. Control (usual care ‐ non‐internet of things) (control arm) and 2. Intervention (IoT ‐ Internet of Things) (intervention arm) |
|
| Participants | Control arm N: 51 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 57.1 ± 9.79 % Male: 55.4 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care‐non‐internet of things) 1) Promotion of self‐management Intervention arm: (IoT ‐ Internet of Things) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This work was funded by the Ministry of Economy, Trade and Industry of Japan | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The system automatically determines the eligibility of each patient and randomly assigns him/her in equal numbers to the IoT or non‐IoT group with a dynamic allocation strategy using a minimization method. |
| Allocation concealment (selection bias) | Low risk | The system automatically determines the eligibility of each patient and randomly assigns him/her in equal numbers to the IoT or non‐IoT group with a dynamic allocation strategy using a minimization method. |
| Patient's baseline characteristics (selection bias) | Low risk | Protocol Table 1 ‐ all P < 0.05. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No between‐group differences in baseline characteristics were observed except for triglycerides, which were lower in the IoT group than in the non‐IoT group (P = 0.01). |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported; looks like 1 lost in control group from protocol. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Outcomes measured objectively (Bluetooth‐enabled blood pressure (BP) meter) |
| Selective reporting (reporting bias) | High risk | Major differences from protocol: publication only reports data for HbA1c at baseline and 3 months, does not report BP, LDL, body weight, FBG, HDL, triglycerides, total cholesterol. |
| Risk of contamination (other bias) | Low risk | Control group did not have access to online system to transmit data or receive feedback from physician, only people in the intervention group had cloud access. |
| Other bias | Low risk | None identified. |
Kong 2019.
| Study characteristics | ||
| Methods |
Effectiveness of the chronic care model in type 2 diabetes management in a community health service center in china: a group randomized experimental study Clustered RCT (12 clusters and NR providers), conducted in 1) Zhaohui Community Health Service Center in Hangzhou, Zhejiang province, China. The community health service centre covers 12 communities with a geographic area of 3.03 square kilometres. 2) Each team included a responsible physician, a health manager and a public health assistant. In China. 2 arms: 1. Control (conventional care) (control arm) and 2. Intervention (chronic care model) (intervention arm) |
|
| Participants | Control arm N: 150 Intervention arm N: 150, NA, NA Diabetes type: 2 Mean age: 70.25 ± 11 % Male: 42.62 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: (conventional care) 1) Clinician reminder Intervention arm: (chronic care model) 1) Team change 2) Electronic patient registry 3) Clinician education 4) Clinician reminder 5) Facilitated relay of clinical information 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This study was supported by the National Natural Science Foundation of China (number: 70603024), the Zhejiang Provincial Natural Science Foundation (number: LY16G030005) and the Fundamental Research Funds for the Central Universities of China | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT, community allocation. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. P values for diabetes duration and marital status were less than 0.05. Age had a P value of 0.05 between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | Figure 1. 12/136 lost in control group, 8/142 lost in intervention group. No reasons for loss provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Cluster‐randomised. Community allocated. |
| Other bias | Low risk | None identified. |
Kooiman 2018.
| Study characteristics | ||
| Methods |
Self‐tracking of physical activity in people with type 2 diabetes: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited at the Bethesda General Hospital (Bethesda Diabetes Research Center, Hoogeveen, the Netherlands) and the Martini Hospital (Groningen, the Netherlands). Remotely delivered intervention through FitBit and eHealth programme. 2) Diabetes nurse in Netherlands 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (FitBit + online self‐tracking (eHealth) programme) (intervention arm) |
|
| Participants | Control arm N: 32 Intervention arm N: 40, NA, NA Diabetes type: 2 Mean age: 56 ± 9.93 % Male: 52.8 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (FitBit + online self‐tracking (eHealth) programme) 1) Electronic patient registry |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Funding of the Fitbit devices was received from “FBTO” health insurance company | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to the intervention or control group using block randomisation. A predetermined formula per block (e.g. ICCI) determined to which group a patient was assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values not reported. Self‐perceived health was significantly different between groups at baseline. Outside of LSR scope. "No significant differences existed between the intervention group and the control group at T0. " |
| Patient's baseline outcomes (selection bias) | Low risk | No significant differences existed between the intervention group and the control group at T0. |
| Incomplete outcome data (attrition bias) | Low risk | Figure 1. 2/32 (6%) lost in control group, 4/40 (10%) lost in intervention group. Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c. |
| Selective reporting (reporting bias) | High risk | From registry ‐ trial protocol not prospectively measured and outcomes reported not included in study results (mood, programme compliance). |
| Risk of contamination (other bias) | Low risk | Patient randomised, however unlikely that control group was given access to remotely‐delivered eHealth programme. |
| Other bias | Low risk | None identified. |
Korcegez 2017.
| Study characteristics | ||
| Methods |
Effect of a pharmacist‐led program on improving outcomes in patients with type 2 diabetes mellitus from northern Cyprus: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) a 186‐bed public hospital’s outpatient diabetes clinic in Gazimagusa, Northern Cyprus. 2) There were 2 nurses and 5 physicians (in rotation during week days) providing service to 30 patients a day at the outpatient diabetes clinic. A research clinical pharmacist worked 3 hours per day during weekdays at the outpatient diabetes clinic. In Cyprus. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (pharmacist care) (intervention arm) |
|
| Participants | Control arm N: 80 Intervention arm N: 79, NA, NA Diabetes type: 2 Mean age: 62.01 ± 7.3 % Male: 24.37 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (pharmacist care) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This study was conducted as a PhD thesis by Korcegez under the supervision of Sancar for the clinical pharmacy program at Near East University, Health Sciences Institute, Northern Cyprus, and received no external funding. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Eligible patients were randomised to each group using the registration number. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and greater than 0.05 for characteristics. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Outcomes look balanced between groups. |
| Incomplete outcome data (attrition bias) | Low risk | 3/80 lost in control group (4%), 4/79 lost in intervention group (5%), reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol available, however all outcomes in methods are reported in results. |
| Risk of contamination (other bias) | Low risk | Some cross‐contamination between participants in the usual care and intervention groups might also have occurred because the participants were attending the same outpatient diabetes clinic, which was located in a small community where many residents have close relationships. |
| Other bias | Low risk | No evidence of other bias. |
Korhonen 1987.
| Study characteristics | ||
| Methods |
Efficacy of dietary instructions in newly diagnosed non‐insulin‐dependent diabetic patients. Comparison of two different patient education regimens RCT (NA clusters and NA providers), conducted in 1) Patients recruited in the Department of Internal Medicine, Kuopio University Central Hospital, Kuopio, Finland (primary health care). 2) Specially trained nurses carried out intervention with the help of a doctor. In Finland. 2 arms: 1. Control (dietary leaflet by a doctor ‐ Group A) (control arm) and 2. Intervention (dietary instructions by a nurse ‐ Group B) (intervention arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 40, NA, NA Diabetes type: 2 Mean age: 56.33 ± NR % Male: 50 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (dietary leaflet by a doctor) 1) Patient education Intervention arm: (dietary instructions by a nurse) 1) Case management 2) Team change 3) Patient education |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Thereafter, the patients were randomly allocated (separately for each sex) into 2 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. Data stratified by sex (only age as patients' characteristic). Hard to compare pooled Group A and pooled Group B. No P values. Nothing in text about the homogeneity of groups at baseline. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. Data stratified by sex. Hard to compare pooled Group A and pooled Group B. No P values. Nothing in text about the homogeneity of groups at baseline. |
| Incomplete outcome data (attrition bias) | High risk | During the study, there were no dropouts in group B (nurse), but 3 dropouts in group A (doctor). During the study oral hypoglycaemic drug treatment was necessary in 3 patients in each groups A and B. The data on dropouts and patients treated with oral drugs were excluded from the results. The final number of patients were thus 34 in group A (15% lost) and 37 in group B (7.5%) out of 40 randomised in each group. Numbers and reasons unbalanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c and severe hyperglycaemia with fasting blood glucose ≥12.0 mmol/L). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. The patients were randomly allocated (separately for each sex) into 2 treatment groups. Most studies analyse men and women together and not separately. Reasons for stratification not reported. "Improvements were seen also in corresponding GHbA, values, but the difference between the groups did not achieve statistical significance (data not shown)." |
| Risk of contamination (other bias) | Low risk | Initially the patients were seen by a doctor who described the general outlines of therapy and stressed the importance of diet and weight reduction. Doctor might have changed their approach with their patients about diet and weight loss. Communication between nurses and doctors may have influenced care of patients. |
| Other bias | Low risk | None identified. |
Kotsani 2018.
| Study characteristics | ||
| Methods |
The role of telenursing in the management of diabetes type 1: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Outpatient Department of Diabetes, Endocrinology and Metabolism of a University Hospital in Northern Greece. 2) Intervention delivered by a DM specialised nurse. In Greece. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (telenursing) (intervention arm) |
|
| Participants | Control arm N: 46 Intervention arm N: 48, NA, NA Diabetes type: 1 Mean age: 26.98 ± NR % Male: 50 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (standard care) 1) Patient education Intervention arm: (telenursing) 1) Case management 2) Electronic patient registry 3) Patient education |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They were randomised into two groups by a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, unclear if opaque. |
| Patient's baseline characteristics (selection bias) | Low risk | There is no statistically significant difference regarding age, sex, duration of diabetes and physical activity between the groups. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1, P values for blood glucose < 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcome: HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol available, however outcomes in methods match those in results. |
| Risk of contamination (other bias) | Low risk | Nurses only called those in the intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Kraemer 2012.
| Study characteristics | ||
| Methods |
A randomized study to assess the impact of pharmacist counseling of employer‐based health plan beneficiaries with diabetes: the EMPOWER study Patient RCT (NA clusters and NA providers), conducted in 5 Oregon employers and 2 Oregon‐based health insurance carriers collaborated with the OSU/Oregon Health and Science University College of Pharmacy (CoP). In USA. 2 arms: (control arm) (intervention arm) |
|
| Participants | Control arm N: 32 Intervention arm N: 37, NA, NA Diabetes type: 1 and 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education 2) Financial incentives Intervention arm: 1) Case management 2) Team change 3) Clinician education 4) Patient education 5) Promotion of self‐management 6) Financial incentives |
|
| Outcomes | 1) Lipid‐lowering drugs 2) Antihypertensive drug 3) Glycated haemoglobin 4) Systolic blood pressure 5) Diastolic blood pressure 6) Low‐density lipoprotein |
|
| Funding source | Partial funding for this project was received from the Community Pharmacy Foundation, Sanofi‐Aventis and Lane County Pharmacists Association. The author(s) received no financial support for the authorship and/or publication of this article. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Quote: "The proportion of females was somewhat higher in the control group", no P value. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not described in Table 1, P values not provided. |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those analysed. Withdrawal before study began (numbers were the same in each arm), but numbers and reasons for loss to follow‐up not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary: HbA1c; secondary; LDL, BP: objective laboratory methods not described. Blinding of outcome assessors not described. Blinding of participants was attempted. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias |
Kranker 2018.
| Study characteristics | ||
| Methods |
The efficacy of using financial incentives to change unhealthy behaviors among a rural chronically ill and uninsured population RCT (NA clusters and NA providers), conducted in 1) Primary care clinic in rural Mississippi; the Aaron E. Henry (AEH) Community Health Center. This is a federally qualified health centre (FQHC) based in Clarksdale, Mississippi, that serves over 12,000 patients per year at several sites. Clarksdale is located in the Mississippi Delta region (a rural area of the state). 2) Patients received 0, 1, 2 or 3 financial incentives for weight loss, for medication compliance and/or for physical activity. Care managers who were already part of the clinical team recruited patients, tracked outcomes, and managed the reward payments. In United States of America. 8 arms: 1. Control (usual care, no financial incentives) (control arm) and 2. Intervention 1 (WL: weight loss financial incentives) (intervention arm), 3. Intervention 2 (MC: medication compliance financial incentives) (other arm), 4. Intervention 3 (PA: physical activity financial incentives) (other arm), 5. Intervention 4 (WL + MC: weight loss + medication compliance financial incentives), 6. Intervention 5 (WL + PA: weight loss + physical activity financial incentives), 7. Intervention 6 (MC + PA: medication compliance + physical activity financial incentives), 8. Intervention 7 (WL + MC + PA: weight loss + medication compliance + physical activity financial incentives) |
|
| Participants | Control arm N: 72 Intervention arm N: 52, 77, 6, 51, 9, 3, 8 Diabetes type: 4 Mean age: NR ± 10.44 % Male: 38.4 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (usual care, no financial incentives) 1) Patient education 2) Promotion of self‐management Intervention 1 (WL: weight loss financial incentives) arm 1) Patient education 2) Promotion of self‐management 3) Financial Incentives Intervention 2 (MC: medication compliance financial incentives) arm 1) Patient education 2) Promotion of self‐management 3) Financial incentives |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This work was funded in 2009 by a grant from the Robert Wood Johnson Foundation’s “Finding Answers: Disparities Research for Change” program (Award ID 66711ANRY2, Grant ID AEHCHC). The grantee was the Aaron E. Henry Community Health Services Center (the study site) and Mathematica Policy Research was a subcontractor for the evaluation. Keith Kranker was paid as a consultant for the design prior to joining Mathematica (when he was a PhD candidate at the University of Maryland, Department of Economics). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to randomise patients is never reported. Full factorial (orthogonal) randomised design. Patients randomly received 0, 1, 2 or 3 financial incentives. Incentives for weight loss, medication compliance and physical activity were awarded quarterly over 1 year. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The randomisation procedure described above was largely successful at producing balance between the treatment and comparison groups. Few variables indicated any imbalance using a joint test (Table 3). The group receiving physical activity incentives was more likely to have visited the clinic and have an October start date. |
| Patient's baseline outcomes (selection bias) | High risk | The randomisation procedure described above was largely successful at producing balance between the treatment and comparison groups. Few variables indicated any imbalance using a joint test (Table 3). The group receiving physical activity incentives had lower baseline medication compliance and were heavier than average. |
| Incomplete outcome data (attrition bias) | High risk | From calculations based on Table 1 and Appendix Table 4 (columns 1 and 2) for HbA1c outcome in diabetic patients: 14/72 (20%) lost in control group; 3/52 (6%) from WL; 2/77 (3%) from MC; 1/6 (17%) from PA; 1/51 (2%) from WLxMC; 1/9 (11%) from WLxPA; 0/3 (0%) from MCxPA and 0/8 (0%) from WLxMCxPA. Some high and unbalanced numbers between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. They did a lot of analysis, merging some groups sometimes. They never talk about a method used to counteract the problem of multiple comparisons (such as the Bonferroni correction). Quote: "These tests do not account for multiple comparisons, so a few variables may be unbalanced by chance". Quote: "The follow‐up period was extended to increase the number of patients with follow‐up data; patient outcomes in the sixth quarter after enrollment were used when outcomes in the fifth quarter were not available". Quote: "The original protocol also called for the collection of health‐care utilization data from a nearby hospital, but the hospital declined to participate and the outcome data could not be included in the study". Quote: "Some outcomes could not be analyzed." |
| Risk of contamination (other bias) | Unclear risk | Care managers could not be blinded to patient group assignments, given their active role in the management of the programme. Thus, it is possible that care managers used the incentive payments as a motivational tool or otherwise changed their interactions with the participants. Care managers were managing patients from all groups including the control group. |
| Other bias | Low risk | None. |
Krass 2007.
| Study characteristics | ||
| Methods |
The Pharmacy Diabetes Care Program: assessment of a community pharmacy diabetes service model in Australia Cluster‐RCT (56 clusters), conducted in community pharmacies in Australia (New South Wales, Victoria, Tasmania and Western Australia) Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 159 Intervention arm N: 176 Diabetes type: type 2 Mean age: 62.0 ± 11.0 % Male: 51.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Clinician education 3) Patient education 4) Promotion of self‐management 5) Financial incentives |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.3 (1.3), post 8.0 (1.2) Intervention arm: pre 8.9 (1.4), post 7.9 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 133.0 (12.0), post 135.0 (15.0) Intervention arm: pre 135.0 (14.0), post 133.0 (15.0) 3) DBP, mean mmHg (SD) Control arm: pre 77.0 (9.0), post 76.0 (9.0) Intervention arm: pre 79.0 (8.0), post 77.0 (8.0) |
|
| Funding source | The Pharmacy Diabetes Care Program was funded by the Australian Government Department of Health and Ageing as part of the Third Community Pharmacy Agreement | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No P values or text explanation other than "Overall, the intervention and control pharmacies and pharmacists were well matched in terms of pharmacy and personal demographics". |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. Most patients (79%) reported being treated with oral glucose‐lowering drugs alone; however, the proportion of patients taking a combination of insulin and oral glucose‐lowering drugs was higher in the intervention than the control group (25 vs 13%; P = 0.01). There was also a difference in years since diagnosis of diabetes, with the control group having been diagnosed with diabetes longer than the intervention group (10.4 vs 8.6 years; P = 0.04). |
| Patient's baseline outcomes (selection bias) | High risk | With respect to clinical measures, the control and intervention groups at baseline were similar with the exception of baseline HbA1c (P < 0.01; Table 1). |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Krein 2004.
| Study characteristics | ||
| Methods |
Case management for patients with poorly controlled diabetes: a randomized trial Patient RCT, conducted in Department of Veterans Affairs Medical Centres, USA Two arms: 1. Control (control arm) and 2. Case management (intervention arm) |
|
| Participants | Control arm N: 123 Intervention arm N: 123 Diabetes type: type 2 Mean age: 61.0 ± 10.5 % Male: 96.5 Longest follow‐up: 19 months (mean) |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 68 (55), post 64 (60) Intervention arm: pre 77 (63), post 78 (71) 2) Statins, N users (%) Control arm: pre 29 (24), post 40 (39) Intervention arm: pre 35 (28), post 51 (48) 3) Retinopathy screening (eye exam), N screened (%) Control arm: pre 83 (67), post 84 (79) Intervention arm: pre 94 (76), post 96 (87) 4) HbA1c, mean % (SD) Control arm: pre 9.2 (1.4), post 9.2 (2.1) Intervention arm: pre 9.3 (1.5), post 9.3 (2.1) 5) SBP, mean mmHg (SD) Control arm: pre 145.0 (20.0), post 144.0 (23.3) Intervention arm: pre 145.0 (21.0), post 146.0 (23.6) 6) DBP, mean mmHg (SD) Control arm: pre 86.0 (11.0), post 83.0 (10.4) Intervention arm: pre 86.0 (12.0), post 83.0 (13.1) 7) LDL, mean mg/dL (SD) Control arm: pre 123.0 (38.0), post 109.0 (36.3) Intervention arm: pre 123.0 (37.0), post 106.0 (31.5) |
|
| Funding source | This research was supported by the Office of Research and Development, Health Services Research and Development Service, Department of Veterans Affairs (IIR 970771). This work was also supported in part by the Michigan Diabetes Research and Training Center Grant P60DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, Bethesda, Maryland. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Kulkarni 1998.
| Study characteristics | ||
| Methods |
Nutrition practice guidelines for type 1 diabetes mellitus positively affect dietitian practices and patient outcomes Cluster‐RCT (19 clusters with 19 providers), conducted with dieticians across the USA Two arms: 1. Usual care (control arm) and 2. Practice guidelines (intervention arm) |
|
| Participants | Control arm N: 24 Intervention arm N: 27 Diabetes type: type 1 Mean age: 19.5 ± 10.5 % Male: 46.3 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician education |
|
| Outcomes | 1) HbA1c | |
| Funding source | This project was made possible by an educational grant from PACE, matching funds from the Diabetes Care and Education diabetic practice group | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Practice guidelines dietitians were older than usual care dietitians, but there were no significant differences in their level of education nor in years of experience working with persons with diabetes. Practice guidelines dietitians, however, had a higher caseload of patients with diabetes each month compared with usual care dietitians (Table 1). |
| Patient's baseline characteristics (selection bias) | High risk | Table 4 ‐ age, gender and race look unbalanced, no education reported. No P values given. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 5 ‐ outcome look balanced, no P value reported. |
| Incomplete outcome data (attrition bias) | High risk | Difference in microalbuminuria. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Kulzer 2018.
| Study characteristics | ||
| Methods |
Integrated personalized diabetes management improves glycemic control in patients with insulin‐treated type 2 diabetes: Results of the PDM‐ProValue study program Clustered RCT (101 clusters and NR providers), conducted in 1) General practitioner (GP) practices and diabetes specialist practitioner (DSP) practices across Germany 2) GP and diabetes specialists in Germany 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (iPDM‐Integrated personalised diabetes management) (intervention arm) |
|
| Participants | Control arm N: 494 Intervention arm N: 475, NA, NA Diabetes type: 2 Mean age: 64.71 ± 8.3 % Male: 58.13 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (iPDM ‐ integrated personalised diabetes management) 1) Electronic patient registry 2) Clinician education 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This work was supported by funding from Roche Diabetes Care Deutschland GmbH, Germany | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | GP and DSP practices were randomly assigned (by means of centralised permuted‐block randomisation) to the intervention group (iPDM) or the control group (CNL). |
| Allocation concealment (selection bias) | Low risk | Cluster trial ‐ GP and DSP practices were randomly assigned. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Most characteristics are balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Data looks balanced. |
| Incomplete outcome data (attrition bias) | Low risk | 50/494 (10%) discontinued from control group, 56/475 (12%) discontinued from intervention group; 27/494 (5%) in control group not included in analysis, 35/475 (7%) in intervention group. Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All measurements were performed by a central laboratory. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol, primary outcomes match, however secondary outcomes in protocol are vague ("effects of integrated Personalized Diabetes Management"). |
| Risk of contamination (other bias) | Low risk | Cluster at the clinic level. |
| Other bias | Low risk | No evidence of other risk of bias. |
Kwon 2004.
| Study characteristics | ||
| Methods |
Establishment of blood glucose monitoring system using the internet Patient RCT, conducted in an outpatient clinic at Kangnam St Mary's Hospital, South Korea Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 55 Intervention arm N: 55 Diabetes type: type 2 Mean age: 54.1 ± 9.1 % Male: 60.9 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 7.2 (1.2), post 7.6 (0.1) Intervention arm: pre 7.6 (1.4), post 6.9 (0.1) |
|
| Funding source | This work was supported by the 2001 Korea Health Promotion Research Program and the Korea Health 21 R&D Project, Ministry of Health and Welfare of Republic of Korea Grant 02‐PJ1‐PG3‐21906‐0004 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | < 10% lost to follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Lamers 2011.
| Study characteristics | ||
| Methods |
Treating depression in diabetes patients: dose a nurse‐administered minimal psychological intervention affect diabetes‐specific quality of life and glycaemic control? A randomized controlled trial Patient RCT, conducted in 89 primary care practices in south of the Netherlands Two arms: 1. Usual care (control arm) and 2. Minimal psychological Intervention ‐ MPI (intervention arm) |
|
| Participants | Control arm N: 103 Intervention arm N: 105 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 7.2 (1.4), post 7.8 (0.2) Intervention arm: pre 7.5 (1.2), post 7.3 (0.2) |
|
| Funding source | This study was funded by the Netherlands Organisation for Health Research and Development (ZonMw) programme on Health Care Efficiency Research (grant number 945‐03‐047) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by an external agency using a computerized random number generator". |
| Allocation concealment (selection bias) | High risk | Block size of 2 is too small and you can predict the sequence. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "…shows that groups were comparable at baseline." |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c: P = 0.36. |
| Incomplete outcome data (attrition bias) | High risk | Greater number of losses in intervention group, > 20% during allocation stage after randomisation, however reasons seem balanced thereafter. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding of outcome assessor not described. HbA1c: objective methods not described. |
| Selective reporting (reporting bias) | High risk | Outcomes do not match with protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Larsen 1990.
| Study characteristics | ||
| Methods |
Effect of long‐term monitoring of glycosylated hemoglobin levels in insulin‐dependent diabetes mellitus Patient RCT, conducted in diabetic clinic in Odense University Hospital, Denmark Two arms: 1. Control (control arm) and 2. Monitored (intervention arm) |
|
| Participants | Control arm N: 107 Intervention arm N: 115 Diabetes type: type I Mean age: 36.0 ± NR % Male: 57.5 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Facilitated relay of clinical information |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.0 (1.9), post 10.1 (1.8) Intervention arm: pre 10.1 (1.8), post 9.5 (1.3) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Lauffenburger 2019a.
| Study characteristics | ||
| Methods |
Effectiveness of targeted insulin‐adherence interventions for glycemic control using predictive analytics among patients with type 2 diabetes: a randomized clinical trial RCT (NA clusters and NA providers), conducted in 1) This trial used data from Horizon Blue Cross Blue Shield of New Jersey, Newark, the largest health insurer in New Jersey, United States. 2) Intervention involved tailored telephone by a pharmacist from a pharmacy benefit management company. In United States of America. 3 arms: 1. Control (untargeted, low‐intensity insulin‐adherence Interventions) (control arm) and 2. Intervention 1 (partially targeted, moderate‐intensity insulin‐adherence interventions) (intervention arm), 3. Intervention 2 (highly targeted, high‐intensity insulin‐adherence Interventions) (other arm) |
|
| Participants | Control arm N: 2000 Intervention arm N: 2000, 2000, NA Diabetes type: 2 Mean age: 55.9 ± 7.89 % Male: 59.8 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (untargeted, low‐intensity insulin‐adherence Interventions) 1) Promotion of self‐management Intervention arm: (partially targeted, moderate‐intensity insulin‐adherence Interventions) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders Intervention arm: (highly targeted, high‐intensity insulin‐adherence Interventions) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This research was supported by Sanofi | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by Horizon in a 1:1 ratio to the intervention or usual care group using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, age and gender at baseline are reported. No information on education. Intervention patients were slightly less likely to be female and slightly more likely to have had a prior stroke/transient ischemic attack. |
| Patient's baseline outcomes (selection bias) | Low risk | Baseline HbA1C is reported in Table 1, looks balanced. |
| Incomplete outcome data (attrition bias) | High risk | 71% loss in the control group (684‐196/684), 71% loss in the intervention arm (678‐196/678). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcome HbA1c. |
| Selective reporting (reporting bias) | High risk | There is a published protocol, but they do not talk about the qualitative outcomes in the protocol. However, they report the qualitative outcomes in the study. |
| Risk of contamination (other bias) | Low risk | Control group was not contacted in any way, pharmacists only contacted those in intervention group. |
| Other bias | Low risk | No evidence of other bias. |
Lauffenburger 2019b.
| Study characteristics | ||
| Methods |
Effectiveness of targeted insulin‐adherence interventions for glycemic control using predictive analytics among patients with type 2 diabetes: a randomized clinical trial RCT, 1) This trial used data from Horizon Blue Cross Blue Shield of New Jersey, Newark, the largest health insurer in New Jersey, United States. 2) Intervention involved tailored telephone by a pharmacist from a pharmacy benefit management company Three arms: 1. Control (untargeted, low‐intensity insulin‐adherence interventions), 2. Intervention 1 (partially targeted, moderate‐intensity insulin‐adherence interventions) and 3. Intervention 2 (highly targeted, high‐intensity insulin‐adherence interventions) |
|
| Participants | Control arm N: 2000 Intervention arm N: 2000, 2000, NA Diabetes type: 3 Mean age: 55.9 ± 11 % Male: 59.8 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (untargeted, low‐intensity insulin‐adherence interventions) 1) Promotion of self‐management Intervention arm: (partially targeted, moderate‐intensity insulin‐adherence intervention) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders Intervention arm: (highly targeted, high‐intensity insulin‐adherence interventions) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | 1) Glycated haemoglobin 2) Harms (hypoglycaemia) |
|
| Funding source | This research was supported by unrestricted funding from AstraZeneca to Brigham and Women’s Hospital (awarded to NKC). The BWH had ultimate decision‐making over study design, data collection and analysis, decision to publish, and preparation of the manuscript. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From final protocol: This randomisation will occur using a random number generator at Horizon Analytics. Randomisation codes will be assigned strictly sequentially as patients become eligible. |
| Allocation concealment (selection bias) | Low risk | From final protocol: This randomisation will occur using a random number generator at Horizon Analytics. The randomisation key will be maintained at Horizon Analytics as well as the allocation of patients to the intervention arms. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Age, gender, clinical diagnoses are not significant. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. HbA1c; insulin and hypoglycaemic use; diabetic complications (including hypoglycaemia); and resource utilisation; have P values higher than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | The number of patients with at least 1 HbA1c value are 663/2000 in Arm 1, 640/2000 in Arm 2 and 642/2000 in Arm 3. Overall, about 68% missing data. Our evaluation of glycaemic control was limited to patients for whom Horizon had baseline laboratory data available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Data extracted from Horizon database, the largest health insurer in New Jersey. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol and detailed protocol provided as supplementary material. They added subgroup analysis for insulin persistence in the paper (table 3). |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Number of calls and texts received dependent on arm allocated to. Unlikely to receive wrong amount of communication. |
| Other bias | Low risk | No evidence of other bias. |
Lee 2011.
| Study characteristics | ||
| Methods |
General practice and social service partnership for better clinical outcomes, patient self efficacy and lifestyle behaviours of diabetic care: randomised control trial of a chronic care model RCT (NA clusters and NA providers), conducted in 1) The patients attended general outpatient clinics (GOPCs) in Hospital Authority New Territory East Cluster of Hong Kong, which is a public service with only a nominal charge for consultation and medication. 2) The programme was facilitated by a social worker of the Community Rehabilitation Network (CRN) of the Hong Kong Society of Rehabilitation (HKSR), who had been accredited as a trainer for the self‐management programme. In Hong Kong. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (self‐management programme) (intervention arm) |
|
| Participants | Control arm N: 73 Intervention arm N: 84, NA, NA Diabetes type: 4 Mean age: NR ± 10.56 % Male: 38.08 Longest follow‐up: 6.46 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (self‐management programme) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Other funders: self‐funded. Sponsor type: research organisation. Subvention from the Department of Social Services. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. The participants were then assigned to either the experimental or control groups by simple randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | No statistical significant difference was observed between the experimental and control groups for the main demographic characteristics (Table 1). Mean age is not reported. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. Data reported for HbA1c at baseline looks similar. Nothing in text. Number of people with hypertension and smoking are similar (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | No sample size on the flow chart. Table 2, at 26 weeks, they have data for 51 patients out of 73 in the control arm (30.1% lost) and 66 out of 84 (21.4%) in the intervention arm. Many lost, numbers unbalanced. Some patients did not attend for blood tests. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | High risk | Registered protocol available. Expected outcomes: 2. An improvement in blood pressure, but do not report data. Only report this: no statistically significant results were found regarding changes in blood pressure. Reports results for baseline, week 16 and week 28, instead of baseline, week 8 and week 28 as mentioned in protocol. |
| Risk of contamination (other bias) | Unclear risk | In this study the attending doctors had attended a seminar on patient self‐management so they could emphasise the importance of referral for self‐management for better control. Doctors might have changed their usual care. Social workers only worked with patients in the experimental group. |
| Other bias | Low risk | No evidence of other risk of bias. |
Lee 2015.
| Study characteristics | ||
| Methods |
Diabetes telemonitoring reduces the risk of hypoglycaemia during Ramadan: a pilot randomized controlled study RCT (NA clusters and NA providers), conducted in 1) Five primary care provider practices in Malaysia. 2) Intervention delivered by a telemedicine system periodically reviewed by the physician. In Malaysia. 2 arms: 1. Control (Ramadan‐focused pre‐education) (control arm) and 2. Intervention (telemonitoring group during Ramadan) (intervention arm) |
|
| Participants | Control arm N: 19 Intervention arm N: 18, NA, NA Diabetes type: 2 Mean age: 50.75 ± 9.41 % Male: 43.32 Longest follow‐up: 1.38 months |
|
| Interventions |
Control arm: (Ramadan‐focused pre‐education) 1) Patient education 2) Promotion of self‐management Intervention arm: (telemonitoring group during Ramadan) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Low‐density lipoprotein Harms |
|
| Funding source | This study was supported in part by the Telemedicine Cluster, Tropical Medicine and Biology platform, Monash University Malaysia (52140757‐314‐00) and SEGi University Research Fund (SEGi/2013/SKK/04/1) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Participants were randomly allocated within 5 primary care provider practices to either a usual care group (UC) or telemonitoring group (TG). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Table S1. Many characteristics have P values at or under 0.05 (duration of diabetes, education level, employment status, insulin use, etc). |
| Patient's baseline outcomes (selection bias) | Low risk | Table S1. All clinical measurements have P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Figure S1. They lost 4 patients out of 18 in the intervention arm (22.2%), and only one out of 19 in the control arm (5.3%). Numbers unbalanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | The primary outcome is hypoglycaemia events during Ramadan reported by participants over the study period. Quote: "Records of self‐reported hypoglycaemic symptoms and daily blood glucose levels were by participant declaration, which may not be entirely reliable." |
| Selective reporting (reporting bias) | High risk | Registered protocol available. For the primary outcome, they report the number of hypoglycaemia events in the paper, and not the number of patients having hypoglycaemia symptoms as written in the protocol. Protocol describes treatment time frame of 12 weeks, article describes treatment duration of 6 weeks. Protocol mentions glycaemic control measured with HbA1c, article reports glycaemic control with fasting plasma glucose. |
| Risk of contamination (other bias) | Low risk | Only the intervention group was telemonitored. Physicians may have treated patients in both groups, which may have influenced care. |
| Other bias | Low risk | No evidence of other bias. |
Lee 2017.
| Study characteristics | ||
| Methods |
Effect of a health literacy‐considered diabetes self‐management program for older adults in South Korea RCT (NA clusters and NA providers), conducted in 1) 2 senior centres (D and G) in a single South Korean city. 2) Senior centre nurses; telephone counselling delivered by authors in South Korea 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (health literacy programme) (intervention arm) |
|
| Participants | Control arm N: 28 Intervention arm N: 28, NA, NA Diabetes type: 2 Mean age: 74.5 ± 11.01 % Male: 43.16 Longest follow‐up: 2.76 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (health literacy programme) 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This work was funded by the Sigma Theta Tau International Honor Society of Nursing Lambda Alpha Chapter‐at‐Large 2015 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a randomisation program (access http://www.randomization.com) by an independent researcher who did not have contact with participants. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised using a randomisation program (access http://www.randomization.com) by an independent researcher who did not have contact with participants. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no significant differences in the sociodemographic characteristics (Table 1), P values > 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | The pretest analysis did not reveal any significant inter‐group differences in diabetes biomarkers. |
| Incomplete outcome data (attrition bias) | Low risk | 56 participants were randomly assigned to the groups, but 5 subsequently withdrew because of hospitalisation (2 in the intervention group and 1 in the control group), change of address (1 in the control group) and personal issues (1 in the control group). Therefore, data from 51 participants were analysed (26 in the intervention group and 25 in the control group). 2/28 lost in intervention group (7%) and 3/28 (11%) lost in control group; reasons provided and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, however methods match results. |
| Risk of contamination (other bias) | Low risk | However, to account for any dissemination of the programme, the control group completed the programme before the intervention group began the programme. |
| Other bias | Low risk | No evidence of other bias. |
Lee 2018.
| Study characteristics | ||
| Methods |
The effectiveness, reproducibility, and durability of tailored mobile coaching on diabetes management in policyholders: a randomized, controlled, open‐label study Cross‐over RCT (NA clusters and NA providers), conducted in 1) Kangbuk Samsung Hospital, Seoul, Korea; 2) Health professionals (an endocrinologist, a nurse and a dietitian) in South Korea 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (tailored mobile coaching) (intervention arm) |
|
| Participants | Control arm N: 74 Intervention arm N: 74, NA, NA Diabetes type: 2 Mean age: 51.96 ± 4.8 % Male: 63.24 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (tailored mobile coaching) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | Samsung Fire & Marine Insurance Company funded the study. This funder had no role in the design, conduct or analysis of the trial. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | For allocation of the participants, a computer‐generated list of random numbers produced by statistician with no clinical involvement in the trial was used. |
| Allocation concealment (selection bias) | Unclear risk | Participants were allocated in the order in which they were registered. |
| Patient's baseline characteristics (selection bias) | Low risk | Significantly higher proportion of alcohol drinkers in C‐I (control) group (P = 0.022) Suppl Table 1, but all other characteristics look balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values above 0.05. No statistically significant difference between groups (Suppl Table S1). |
| Incomplete outcome data (attrition bias) | High risk | 51/74 (31% dropout) remained in control group, 54/74 (27% dropout remained in intervention group). Reasons provided in Figure 2. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective primary measures (HbA1c, BP, LDL, harms). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Methods and outcomes match. |
| Risk of contamination (other bias) | Low risk | Only participants in the intervention phase had access to the app and tailored management advice. |
| Other bias | Low risk | No evidence of other risk of bias. |
Leichter 2013.
| Study characteristics | ||
| Methods |
Impact of remote management of diabetes via computer: The 360 Study ‐ A Proof‐of‐Concept Randomized Trial Patient RCT, conducted with patients being currently treated at the Center for Diabetes and Metabolism, Columbus, GA, USA Two arms: 1. Control group (control arm) and 2. Study group (intervention arm) |
|
| Participants | Control arm N: 49 Intervention arm N: 49 Diabetes type: type 1 and type 2 Mean age: 48.2 ± 12.0 % Male: 56.1 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 7.3 (1.2), post 7.1 (0.2) Intervention arm: pre 7.7 (1.5), post 7.4 (0.2) 2) SBP, mean mmHg (pre: SD, post: SE) Control arm: pre 132.4 (17.3), post 133.0 (2.6) Intervention arm: pre 133.2 (14.1), post 134.7 (2.8) 3) DBP, mean mmHg (pre: SD, post: SE) Control arm: pre 76.9 (9.7), post 76.9 (1.1) Intervention arm: pre 79.3 (6.1), post 78.5 (1.2) 4) LDL, mean mg/dL (pre: SD, post: SE) Control arm: pre 99.4 (61.7), post 90.7 (4.5) Intervention arm: pre 92.9 (32.4), post 79.7 (4.8) 5) Harms (hospitalisations due to hypoglycaemia or hyperglycaemia), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 0 (0) |
|
| Funding source | This was an investigator‐initiated study supported by an unrestricted educational grant from Roche Diagnostics, Indianapolis, IN. Support included provision of blood glucose meters and test strips, funding for statistical analysis services (by Z.J., BioStat International, Tampa, FL), and funding for editorial assistance in editing and formatting the manuscript for publication (Christopher G. Parkin, CG Parkin Communications, Inc., Boulder City, NV). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Based on answer for allocation concealment: alternation basis, so we know for sure that an allocation sequence was not adequately generated. |
| Allocation concealment (selection bias) | High risk | Quote: "The investigator then randomized subjects within each treatment subgroup (1:1 ratio) on an alternating basis to control group and study group". Alternating basis: we can predict the next allocation. |
| Patient's baseline characteristics (selection bias) | High risk | Age (P = 0.03). |
| Patient's baseline outcomes (selection bias) | Low risk | In table and text. |
| Incomplete outcome data (attrition bias) | High risk | ~24% losses in N1 and ~32% losses in N2. Reasons for losses not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcomes: HbA1c, LDL, SBP, DBP, etc, methods of ascertainment not described. |
| Selective reporting (reporting bias) | High risk | No outcomes listed in protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None identified. |
Levetan 2002.
| Study characteristics | ||
| Methods |
Impact of computer‐generated personalized goals on HbA(1c) Patient RCT, conducted in Medstar Clinical Research Center, Washington DC, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 75 Intervention arm N: 75 Diabetes type: unclear/not reported Mean age: 58.5 ± NR % Male: 32.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician reminders 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.4 (2.0), post 7.8 (1.9) Intervention arm: pre 8.9 (2.5), post 7.8 (2.2) 2) SBP, median mmHg (SD) Control arm: pre 143.0 (NR), post 139.0 (NR) Intervention arm: pre 142.0 (NR), post 138.0 (NR) 3) DBP, median mmHg (SD) Control arm: pre 83.0 (NR), post 78.0 (NR) Intervention arm: pre 83.0 (NR), post 79.0 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 116.0 (NR), post 109.0 (NR) Intervention arm: pre 115.0 (NR), post 110.0 (NR) |
|
| Funding source | Funding for this study was provided by an unrestricted educational grant from Roche Diagnostics (Indianapolis, IN) and by MedStar Research Institute | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Physicians received training; cannot guarantee that the control group did not benefit from this. |
| Other bias | Unclear risk | Information not available. |
Levy 2015.
| Study characteristics | ||
| Methods |
The Mobile Insulin Titration Intervention (MITI) for insulin adjustment in an urban, low‐income population: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) We recruited patients for this study from Bellevue’s Adult Primary Care Center (APCC) in New York city. Most clinic visits are for patients who are uninsured (31%) or have Medicaid (45%). The majority of patients are non‐white: Hispanic (41%), Black (24%) and Asian (6%). 2) The intervention was provided by clinic’s diabetes nurse educator helped by a physician if not available. In United States of America. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (Mobile Insulin Titration Intervention‐MITI) (intervention arm) |
|
| Participants | Control arm N: 28 Intervention arm N: 33, NA, NA Diabetes type: 2 Mean age: 46.7 ± NR % Male: 49 Longest follow‐up: 2.77 months |
|
| Interventions |
Control arm: (standard care) 1) Promotion of self‐management Intervention arm: (Mobile Insulin Titration Intervention‐MITI) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | "We would like to thank our funders, the New York UniversityHealth and Hospitals Corporation Clinical and Translational Science Institute (NYUHHC CTSI) for the 2013 NYU CTSI Pilot Grant and the 2014 HHC H3 Research Grant award # UL1 TR000038 from the National Center for the Advancement of Translational Science (NCATS), National Institutes of Health. We would also like to thank the HHC Office of Healthcare Improvement for their donation of diabetic testing supplies." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised on the day of enrollment after the informed consent process. The random allocation sequence was computer‐generated by a co‐investigator and concealed in sequentially numbered envelopes. Patients were stratified by whether they were initiating insulin treatment or having their existing insulin dose adjusted. Within each stratification, the allocation sequence used blocks of 4 to help keep the number of patients balanced in each arm. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised on the day of enrollment after the informed consent process. The random allocation sequence was computer‐generated by a co‐investigator and concealed in sequentially numbered envelopes. Patients were stratified by whether they were initiating insulin treatment or having their existing insulin dose adjusted. Within each stratification, the allocation sequence used blocks of 4 to help keep the number of patients balanced in each arm. |
| Patient's baseline characteristics (selection bias) | Low risk | No significant differences in baseline characteristics/demographics were found between the 2 study arms. Demographics of participants are shown in Table 1. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Means HbA1c are similar between control (12.05 ± 1.91) and intervention (11.43 ± 1.75) at baseline. No significant differences in baseline characteristics/demographics were found between the 2 study arms. |
| Incomplete outcome data (attrition bias) | High risk | For HbA1c data, 19 patients were lost to follow‐up out of 61 (31%); 14 in the control arm (50%) and 5 in the intervention arm (15%). The large difference between the raw result (of HbA1c) and the multiple imputation result indicates that the missing mechanism was missing‐not‐at‐random and the missing data problem was a limitation of this study for examining HbA1c change. 6 patients (5 in MITI and 1 in usual care) met inclusion criteria when screened at the time of enrollment, but were discovered to be ineligible to participate soon after they consented and were randomised. These 6 patients did not receive the allocated intervention, but were included in the intention‐to‐treat analysis. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). Patients, clinicians and researchers in this trial were not blinded to arm assignments. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol first posted on June 2013, the study started at the same time). All outcomes of interest are reported. |
| Risk of contamination (other bias) | Low risk | Unlikely control group received treatment as only patients in the intervention arm received SMS and phone call from nurses. |
| Other bias | Low risk | No evidence of other bias. |
Li 2016.
| Study characteristics | ||
| Methods |
Impact of "Conversation Maps" on diabetes distress and self‐efficacy of Chinese adult patients with type 2 diabetes: a pilot study RCT (NA clusters and NA providers), conducted in 1) Department of Health Education, Jiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing, Jiangsu, People’s Republic of China 2) a trained diabetes educator took on the role as the facilitator of Diabetes Conversation Map in China 2 arms: 1. Control (traditional education) (control arm) and 2. Intervention (Diabetes Conversation Maps‐based education) (intervention arm) |
|
| Participants | Control arm N: 26 Intervention arm N: 27, NA, NA Diabetes type: 2 Mean age: 62.4 ± 9.85 % Male: 53.4 Longest follow‐up: 7 months |
|
| Interventions |
Control arm: (traditional education) 1) Patient education Intervention arm: (Diabetes Conversation Maps‐based education) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Group assignments were delivered in sealed, opaque envelopes generated by off‐site study staff. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 ‐ P values provided and all greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1 ‐ P values provided and all greater than 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | Borderline. 4 lost to control (15%), 3 lost in intervention (11%). Reasons for loss provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised. Same educator provided educational sessions for both groups. |
| Other bias | Low risk | No evidence of other bias. |
Li 2017.
| Study characteristics | ||
| Methods |
Diabetes nurse case management in a Canadian tertiary care setting: results of a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted at BCDiabetes.ca, based in the Gordon and Leslie Diamond Health Care Centre. The centre is the main tertiary care centre in Vancouver, British Columbia 2) Nurse case management was conducted by a single individual, GK, a certified diabetes educator with a master’s degree in nursing and 37 years of nursing experience in Canada 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (nurse case management) (intervention arm) |
|
| Participants | Control arm N: 68 Intervention arm N: 72, NA, NA Diabetes type: 2 Mean age: 57.43 ± 9.34 % Male: 58 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard care) 1) Patient education Intervention arm: (nurse case management) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Harms |
|
| Funding source | This study was supported by the British Columbia Endocrine Research Foundation (itself supported by a grant from SanofiAventis Canada) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Randomised assignments were completed in advance and kept in individual, sealed, sequentially labelled envelopes that were opened at the time of the randomisation of each participant. Unclear if envelopes were opaque. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No significant differences between randomised groups in terms of gender, ethnicity, marital status, education level, employment status or household income; all P values > 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | P value not reported, but values look balanced. |
| Incomplete outcome data (attrition bias) | Low risk | 3/72 (4%) in intervention group did not complete HbA1c assessment vs 7/68 (10%) in control group. Loss to follow‐up was not different in the 2 groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol, no differences between protocol and publication. |
| Risk of contamination (other bias) | Low risk | Nurse manager interacted with control group, but only to provide education and schedule follow‐up tests. |
| Other bias | Low risk | No evidence of other bias |
Lian 2013.
| Study characteristics | ||
| Methods |
Screening for diabetic retinopathy with or without copayment in a randomized controlled trial: influence of the inverse care law Patient RCT, conducted in 2 public family medicine clinics operated by the Hong Kong Hospital Authority (HA) West Cluster, China Two arms: 1. Free group (control arm) and 2. Pay group (intervention arm) |
|
| Participants | Control arm N: 2319 Intervention arm N: 2325 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: NR months |
|
| Interventions |
Control arm: 1) Patient reminders Intervention arm: 1) Patient reminders 2) Financial incentives |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 1052 (82) Intervention arm: pre NR (NR), post 1165 (89) |
|
| Funding source | The study received funding from the Health and Health Services Research Fund of the Hong Kong SAR Government (HHSRF: 06071021) and the Azalea (1972) Endowment Fund | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation of digits 0 or 1 by computer…" using a computer random number generator." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | In table and text. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported for screening uptake. |
| Incomplete outcome data (attrition bias) | High risk | They randomised then excluded based on eligibility criteria. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Screening uptake is an objective measure. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Liang 2012.
| Study characteristics | ||
| Methods |
Two‐year foot care program for minority patients with type 2 diabetes mellitus of Zhuang Tribe in Guangxi, China RCT (NA clusters and NA providers), conducted in China 1) Participants were recruited from the ED of an urban tertiary care teaching hospital. Following discharge, intervention participants completed 3 additional on‐site visits within 30 days and 1 phone visit within 90 days. 2) A CDE assisted by a research assistant (RA) under the supervision of an endocrinologist delivered the intervention 2 arms: 1. Control (conventional care) and 2. Intervention (tailored foot care programme) |
|
| Participants | Control arm N: 31 Intervention arm N: 31 Diabetes type: 2 Mean age: 56 % Male: 55.94 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (conventional care) Intervention arm: (foot care management programme) 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Glycated haemoglobin | |
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no significant differences in age, duration of diabetes, diabetic foot risk category and A1C among patients assigned to the control and study groups. |
| Patient's baseline outcomes (selection bias) | Low risk | There were no significant differences in age, duration of diabetes, diabetic foot risk category and A1C among patients assigned to the control and study groups. |
| Incomplete outcome data (attrition bias) | Low risk | Study participants were evaluated for 2 years, with 2 participants from the control group and 1 from the study group dropping out. Reasons for dropout not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure for HbA1c, subjective for foot exam. Knowledge adequately prevented. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. No discrepancy between methods and outcomes. |
| Risk of contamination (other bias) | Unclear risk | Unclear if control group received care from members of the multidisciplinary team caring for the intervention group. If so, it may have influenced care of the control group. |
| Other bias | Low risk | No evidence of other bias. |
Lim 2016.
| Study characteristics | ||
| Methods |
Study investigating the impact of pharmacist involvement on the outcomes of diabetes medication therapy adherence program Malaysia RCT (NA clusters and NA providers), conducted in 1) This study was conducted at the Outpatient Clinic of Hospital Pulau Pinang, Malaysia. 2) Intervention provided by pharmacists. In Malaysia. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention groups (DMTAC: pharmacist care managers) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 56.33 ± 11.9 % Male: 46.05 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (DMTAC: pharmacist care managers) 1) Case management 2) Team change 3) Patient education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. The selected patients were then randomly divided into 2 arms, intervention and non‐intervention groups, according to their most recent HbA1c. Type 2 diabetes patients with HbA1c ≥ 8% were recruited and arbitrarily divided into the intervention group (usual care plus DMTAC) and the non‐intervention group (usual care only). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No significant differences between control and intervention for age, gender and ethnicity. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. No significant differences between control and intervention for HbA1c, FBG, BP and drugs. |
| Incomplete outcome data (attrition bias) | High risk | Total of 22 patients withdrawn or were lost to follow‐up out of 100 people randomised (22%). The changes of lipid profile and adherence were only analysed in intervention group as there were not sufficient data in non‐intervention group (they do not report the number of patients analysed). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c, BP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Finally, limitations exist in this study. Although the participants were randomly divided, this is an open‐labelled study and the physicians were aware of this study. Thus, overall care may be improved during the duration of the study. |
| Other bias | Low risk | No evidence of other bias. |
Lindberg 2017.
| Study characteristics | ||
| Methods |
Telemonitoring and health counseling for self‐management support of patients with type 2 diabetes: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The Swedish part of the project was conducted in 4 healthcare centres situated in the northern part of Sweden during the years 2011 to 2013. 2) Caregiver, health care provider, general practitioner, diabetes nurse, physiotherapists, nutritionists in Sweden 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (prescribed healthcare) (intervention arm) |
|
| Participants | Control arm N: 79 Intervention arm N: 87, NA, NA Diabetes type: 2 Mean age: 67.52 ± 9.27 % Male: 70.52 Longest follow‐up: 19 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (prescribed healthcare) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician reminder 5) Facilitated relay of clinical information 6) Patient education 7) Promotion of self‐management 8) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Smoking status |
|
| Funding source | This project was funded by the EU through the ICT Policy Support Programme, as part of the Competitiveness and Innovation Framework Programme (CIP), and by the Norrbotten County Council and Luleå University of Technology | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | PC‐based generation of random sequences. |
| Allocation concealment (selection bias) | Low risk | Allocation based on consecutive assignment, (however) a statistician performed the randomisation; he or she had no access to the participants’ personal code numbers. The researchers handling the database had no access to the participants’ personal code numbers, when they analysed the data. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No P values provided except for "The difference between randomized participants and responders due to use of PC are statistically significant with P=.001" indicating that significance was assessed and was not assigned to other rows in the table. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. No P values provided but the Table 1 footnote states "The difference between randomized participants and responders due to use of PC are statistically significant with P=.001" indicating that significance was assessed and was not assigned to other rows in the table. |
| Incomplete outcome data (attrition bias) | High risk | Figure 2. Control: 2 died, 5 lost to follow‐up: 7/79. Intervention: 36/86 lost to follow‐up 42%. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure for primary objectives HbA1c, BP, LDL. Self‐report for secondary smoking status. |
| Selective reporting (reporting bias) | High risk | Some differences between protocol and paper: follow‐up time (12 vs 19 months), alcohol consumption, sense of coherence, EQ‐5D not reported in paper. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised; unlikely that the control group received the intervention. |
| Other bias | High risk | The findings indicate that non‐responders had poorer mental health at inclusion than the responders of the intervention. |
Litaker 2003.
| Study characteristics | ||
| Methods |
Physician ‐ nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients' perception of care Patient RCT, conducted in the department of General Internal Medicine at the Cleveland Clinic Foundation, Ohio, USA Two arms: 1. MD only ‐ usual care (control arm) and 2. NP‐MD team ‐ team‐based care ‐ (intervention arm) |
|
| Participants | Control arm N: 78 Intervention arm N: 79 Diabetes type: type 2 Mean age: 60.5 ± 9.0 % Male: 41.0 Longest follow‐up: 24 months (HbA1c) |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 53 (68) Intervention arm: pre NR (NR), post 62 (78) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 28 (36) Intervention arm: pre NR (NR), post 79 (100) 3) HbA1c, mean % (SD) Control arm: pre 8.5 (1.6), post 8.4 (NR) Intervention arm: pre 8.4 (1.4), post 7.8 (NR) 4) Controlled hypertension (< 130/85 mmHg), N under control (%) Control arm: pre 7 (9), post 10 (13) Intervention arm: pre 7 (9), post 11 (14) |
|
| Funding source | This study was generously supported through grants from the Arison Foundation and the I.H. Page Center for Health Outcomes Research at the Cleveland Clinic Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Liu 2012.
| Study characteristics | ||
| Methods |
Effectiveness of using group visit model to support diabetes patient self‐management in rural communities of Shanghai: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The trial was undertaken in 2 rural communities in Songjiang District, Shanghai, China. New community diabetes care service provided by the community health centre. 2) 8 general practice team members (general practitioners, diabetes specialists and community nurses) from the 2 community health centres delivered the intervention. In China. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (group visit programme) (intervention arm) |
|
| Participants | Control arm N: 89 Intervention arm N: 119, NA, NA Diabetes type: 2 Mean age: 62.20 ± NR % Male: 37.97 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (group visit programme) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Systolic blood pressure Diastolic blood pressure |
|
| Funding source | The development of the Chinese diabetes group visit programme was supported by grants from the Initiative for Cardiovascular Health Research in Developing Countries (IC‐HEALTH) (ICH/DIA/PDG/O6/03) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and allocation to study group were carried out by using a random number table. Randomisation was conducted at each of the 2 rural communities separately. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 3. No P values reported, but the only significant difference was for hypertension prevalence (outcome). |
| Patient's baseline outcomes (selection bias) | High risk | Table 4. P values above 0.05 for systolic and diastolic blood pressure, but the prevalence of hypertension was significantly different between those in the intervention and control groups (P = 0.02). |
| Incomplete outcome data (attrition bias) | High risk | They lost 11 out of 89 patients (12.4%) in the control group and 21 out of 119 (17.6%) in the intervention group. High and unbalanced numbers. Reasons reported and some are unbalanced (moved out and unknown). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Not clear if GPs involved in the intervention also followed patients in the control group. |
| Other bias | Low risk | No evidence of other bias. |
Liu 2019.
| Study characteristics | ||
| Methods |
Effect of intensive nursing education on the prevention of diabetic foot ulceration among patients with high‐risk diabetic foot: a follow‐up analysis RCT (NA clusters and NA providers), conducted in 1) Department of Orthopedics, Center of Diabetic Foot, Beijing Shijitan Hospital, Capital Medical University, NO. 10, Tie Yi Road, Yang Fang Dian, Haidian District, Beijing 100038, People’s Republic of China. 2) Not reported but acknowledgments thank doctors and nurses in China 2 arms: 1. Control (conventional care) (control arm) and 2. Intervention (transitional care) (intervention arm) |
|
| Participants | Control arm N: 142 Intervention arm N: 142, NA, NA Diabetes type: 2 Mean age: 58.75 ± 11.3 % Male: 57.4 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (conventional care) 1) Patient education 2) Promotion of self‐management Intervention arm: (transitional care) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and all above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3. P values provided under each pre intervention case/control dimer (not the right column). |
| Incomplete outcome data (attrition bias) | Low risk | All patients enrolled were followed up for 2 years. No mention of loss. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measures for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Patients randomised at a single location. Considerable likelihood of contamination. No mention of who is providing intervention. |
| Other bias | Low risk | None identified. |
Logan 2012.
| Study characteristics | ||
| Methods |
Effect of home blood pressure telemonitoring with self‐care support on uncontrolled systolic hypertension in diabetes Patient RCT, conducted with physicians (offices or clinics) in metropolitan Toronto, Canada Two arms: 1. Control group (control arm) and 2. Self‐care support (intervention arm) |
|
| Participants | Control arm N: 55 Intervention arm N: 55 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | 1) SBP, mean mmHg (SD) Control arm: pre 142.6 (10.2), post 141.1 (NR) Intervention arm: pre 142.7 (10.9), post 133.6 (NR) 2) DBP, mean mmHg (SD) Control arm: pre 77.9 (9.2), post 76.6 (NR) Intervention arm: pre 76.3 (10.5), post 71.7 (NR) 3) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre NR (NR), post 16 (31) Intervention arm: pre NR (NR), post 28 (52) |
|
| Funding source | The Heart and Stroke Foundation of Ontario (ESA 5970) was the sole source of funding for this project and was not involved in any aspect of the study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Quote: " group allocation schedule was based on blocks of 4 and 6 patients randomly arranged and administered by a person not directly involved in the study." |
| Patient's baseline characteristics (selection bias) | High risk | Quote: "There were no significant differences between groups for these features apart from borderline higher percentage of subjects with dyslipidemia in the intervention group." P = 0.037. |
| Patient's baseline outcomes (selection bias) | Low risk | Outcomes were in Table 1. Quote: "There were no significant differences between the groups." |
| Incomplete outcome data (attrition bias) | High risk | Although losses were < 10% per arm, the reasons in the control arms seem quite different from the intervention arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Quote: "...interventions were not masked…", assume outcome assessors not blinded. However, SBP and DBP measured using validated oscillometric recorders. |
| Selective reporting (reporting bias) | Low risk | All endpoints match. |
| Risk of contamination (other bias) | Low risk | Both used home‐based monitoring, but only one had Bluetooth to relay data for self‐care support. |
| Other bias | Low risk | Information not available. |
Long 2012.
| Study characteristics | ||
| Methods |
Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized, controlled trial Patient RCT, conducted in the Philadelphia VA Medical Center, USA Three arms: 1. Control (control arm), 2. Mentors (intervention arm 1) and 3. Incentives (intervention arm 2) |
|
| Participants | Control arm N: 39 Intervention arm 1 N: 39 Intervention arm 2 N: 40 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Case management 2) Promotion of self‐management Intervention arm 2: 1) Financial incentives |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.9 (1.6), post 9.9 (NR) Intervention arm 1: pre 9.8 (1.8), post 8.7 (NR) Intervention arm 2: pre 9.5 (1.2), post 9.0 (NR) 2a) Harms (1 to 3 minor hypoglycaemic symptoms), N (%) Control arm: pre NR (NR), post 38 (97) Intervention arm 1: pre NR (NR), post 52 (137) Intervention arm 2: pre NR (NR), post 51 (128) 2b) Harms (more than 3 minor hypoglycaemic symptoms), N (%) Control arm: pre NR (NR), post 21 (54) Intervention arm 1: pre NR (NR), post 15 (39) Intervention arm 2: pre NR (NR), post 16 (40) |
|
| Funding source | The work was funded by a National Institute of Aging Roybal Center pilot grant. Funders were not involved in the design, conduct or reporting of the study. Funding for this project was supported by the National Institute of Aging Roybal Center 1P30AG034546 Volpp principal investigator. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using the random number generator function." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…we gave each arm assignment a random number and put the ordered numbers in envelopes. Sealed envelopes were shuffled and stacked and the research assistant took the top envelope after consent was obtained to determine arm assignment." Opaque envelopes? |
| Patient's baseline characteristics (selection bias) | High risk | Quote: "Numbers of people with complications from diabetes." |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis (using imputed values). Reasons for and numbers of lost to follow‐up provided but not balanced. Baseline based on those randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c methods not described but outcome assessor (the phlebotomist) was blinded, but the interventionist was not. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Luley 2011.
| Study characteristics | ||
| Methods |
Weight loss in obese patients with type 2 diabetes: effects of telemonitoring plus a diet combination ‐ the Active Body Control (ABC) Program Patient RCT, conducted with patients recruited through advertisement in regional newspaper. In Germany. Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 35 Intervention arm N: 35 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 7.6 (1.1), post 7.8 (NR) Intervention arm: pre 7.5 (1.1), post 6.7 (NR) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | No report in text or in tables. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No baseline measures of outcomes presented. |
| Incomplete outcome data (attrition bias) | Low risk | ~0% lost to follow‐up in N1 and ~5% in N2. Reasons for N2 not associated with interventions and outcomes. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective methods used to measure HbA1c, although blinding of outcome assessors not addressed. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Lum 2018.
| Study characteristics | ||
| Methods |
Development of a collaborative algorithm for the management of type 2 diabetes during Ramadan: an anchor on empowerment RCT (NA clusters and NA providers), conducted in 1) Conducted at a primary care institution and tertiary hospital in Singapore 2) Intervention delivered by healthcare providers in Singapore 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (FAST‐ Fasting Algorithm for Singaporeans with type 2 diabetes) (intervention arm) |
|
| Participants | Control arm N: 32 Intervention arm N: 30, NA, NA Diabetes type: 2 Mean age: 58.4 ± 8.97 % Male: 32.3 Longest follow‐up: 1 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (FAST‐ Fasting Algorithm for Singaporeans with Type 2 Diabetes) 1) Case management 2) Clinician education 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This research was supported by the Academic Research Fund (AcRF) Tier 1 Grant from the Ministry of Education Singapore | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The differences in other baseline characteristics between both groups were also insignificant. |
| Patient's baseline outcomes (selection bias) | Low risk | "The baseline HbA1c level was not significantly different between both groups (intervention: 7.9% – 0.9% vs control: 7.8% – 1.0%, P = 0.549)" |
| Incomplete outcome data (attrition bias) | Unclear risk | No report of dropout. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c objective outcome, hypoglycaemic events subjective. |
| Selective reporting (reporting bias) | High risk | Incidence of hyperglycaemia, change in blood pressure, lipid panel, general health status, diabetes‐related distress, diabetes‐specific quality of life mentioned in protocol but not reported in paper. |
| Risk of contamination (other bias) | Unclear risk | Patient randomised, one setting, possible that doctors saw both groups and could have contaminated. |
| Other bias | Low risk | None. |
Ma 2009.
| Study characteristics | ||
| Methods |
Case management to reduce risk of cardiovascular disease in a county health care system Patient RCT, conducted in 4 San Mateo Medical Center outpatient clinics, USA Two arms: 1. UC ‐ usual care (control arm) and 2. CM ‐ case management (intervention arm) |
|
| Participants | Control arm N: 207 Intervention arm N: 212 Diabetes type: type 2 Mean age: 55.1 ± 9.6 % Male: 34.4 Longest follow‐up: 16 months (mean) |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.7 (1.7), post 8.0 (NR) Intervention arm: pre 7.6 (1.7), post 7.6 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 135.1 (20.2), post 137.7 (NR) Intervention arm: pre 132.7 (19.4), post 128.5 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 79.6 (10.1), post 76.6 (NR) Intervention arm: pre 79.6 (10.6), post 73.6 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 104.2 (31.8), post 89.3 (NR) Intervention arm: pre 104.2 (33.6), post 93.6 (NR) |
|
| Funding source | This study was primarily supported by research award R01 HL070781 from the National Heart, Lung, and Blood Institute. It was also supported with resources and the use of facilities at the Veterans Affairs Palo Alto Health Care System. Additional resources were received from the SMMC, which provided guidance on the design, implementation, and reporting of the project. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Participants were equally randomised to the CM or the UC group, using the permuted block method (block size = 6) stratified by sex and ethnicity (Hispanic vs non‐Hispanic) within each clinic. |
| Allocation concealment (selection bias) | Low risk | Concealment of treatment allocation was achieved by having study staff who were not involved in the recruitment, intervention, and assessment generate the sequence of treatment allocations and prepare randomisation letters. The letters were sealed in sequentially numbered opaque envelopes and opened firsthand by patients at randomisation, after completion of the baseline assessment. |
| Patient's baseline characteristics (selection bias) | High risk | See Table 1. Patients in the CM group, however, were less likely to have completed eighth grade (P = 0.02). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1 ‐ P values > 0.05. |
| Incomplete outcome data (attrition bias) | High risk | 38 lost in control group (18%) and 40 lost in intervention group (19%), reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measurement of outcomes. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered, does not mention any secondary outcomes. |
| Risk of contamination (other bias) | Low risk | Nurse and dietician case managers had access only to intervention participants, unlikely that control group received case management intervention. |
| Other bias | Low risk | No other evidence of risk of bias. |
Maclean 2009.
| Study characteristics | ||
| Methods |
The Vermont Diabetes Information System: a cluster randomized trial of a population based support system Cluster‐RCT (64 clusters with 132 providers), conducted in a largely rural, community, primary care setting which includes hospital based clinical laboratories in Vermont and adjacent New York State that provide services to community practices, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 3526 Intervention arm N: 3886 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Electronic patient registry 3) Clinician reminders 4) Patient education 5) Patient reminders |
|
| Outcomes | 1) Renal screening (creatinine), N screened (%) Control arm: pre 3032 (86), post 2821 (80) Intervention arm: pre 3303 (85), post 3264 (84) 2) HbA1c, mean % (SD) Control arm: pre 7.0 (1.5), post 7.1 (NR) Intervention arm: pre 7.1 (1.4), post 7.3 (NR) 3) SBP, mean mmHg (SD) Control arm: pre NR (NR), post 138.4 (NR) Intervention arm: pre NR (NR), post 137.4 (NR) 4) DBP, mean mmHg (SD) Control arm: pre NR (NR), post 76.4 (NR) Intervention arm: pre NR (NR), post 76.3 (NR) 5) LDL, mean mg/dL (SD) Control arm: pre 107.0 (34.0), post 95.8 (NR) Intervention arm: pre 106.0 (33.0), post 95.0 (NR) |
|
| Funding source | Funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK61167 and K24 DK068380) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | They randomised practices in blocks. They do not describe how they generated this sequence within the block size. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "No significant differences were observed between the two groups at baseline." |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.46); LDL (P = 0.67). |
| Incomplete outcome data (attrition bias) | High risk | Despite intention‐to‐treat analysis and imputations, there were still ~26% lost to follow‐up in the control group and ~32% in the intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described. Primary outcome: HbA1c, objective methods not described. BP not mentioned as primary or secondary, but sphygomanometer used. |
| Selective reporting (reporting bias) | Low risk | Outcomes match those listed in the protocol. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
MacMahon Tone 2009.
| Study characteristics | ||
| Methods |
An intensive nurse‐led, multi‐interventional clinic is more successful in achieving vascular risk reduction targets than standard diabetes care RCT (NA clusters and NA providers), conducted in 1) Outpatient department and diabetes centre of Beaumont Hospital in Dublin, Ireland, 2) Patients randomised to intensive care were seen by the vascular intervention nurse every 2–3 months and continued to receive annual review in the diabetes clinic in Ireland 2 arms: 1. Control group (standard care) (control arm) and 2. Intervention (intensive nurse‐led care) (intervention arm) |
|
| Participants | Control arm N: 99 Intervention arm N: 101, NA, NA Diabetes type: 2 Mean age: 61.65 ± 10.6 % Male: 54.26 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard care) 1) Case management 2) Team change 3) Patient education Intervention arm: (intensive nurse‐led care) 1) Case management 2) Team change 3) Patient education |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Smoking status |
|
| Funding source | This research was support by a research grant from Bristol Myer Squibb (Ireland) and Pfizer Healthcare Ireland | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to either intensive (n = 101) or standard (n = 99) groups by the use of standard randomisation tables, in order to eliminate bias. Using these tables, patients were randomised on the basis of the date of presentation for their first visit and the last digit of their hospital number. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | P values stated as not significant. |
| Patient's baseline outcomes (selection bias) | Unclear risk | More patients were on diuretics in the standard group (35.9%) at the beginning of the study, as opposed to the intensive group (20.2%) (P = 0.022). |
| Incomplete outcome data (attrition bias) | Low risk | Loss of 5 from standard group (99 to 94) after randomisation (~5%). Loss of 7 from intervention group (101 to 94) after randomisation (~7%). Balanced and less than 10%. Reasons for loss provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure for HbA1c, SBP, DBP, LDL Htn‐C. Subjective for ASA, statin, antihypertensives, smoking. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Both groups seen by vascular intervention nurse. |
| Other bias | Low risk | None identified. |
Magee 2015.
| Study characteristics | ||
| Methods |
The synergy to enable glycemic control following emergency department discharge program for adults with type 2 diabetes: STEP‐DIABETES RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from the ED of an urban tertiary care teaching hospital. Following discharge, intervention participants completed 3 additional on‐site visits within 30 days and 1 phone visit within 90 days. 2) A CDE assisted by a research assistant (RA) under the supervision of an endocrinologist delivered the intervention. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (diabetes self‐management education (DSME)) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 51, NA, NA Diabetes type: 2 Mean age: 50 ± 8.78 % Male: 45.56 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (diabetes self‐management education (DSME)) 1) Case management 2) Team change 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This study was funded by an American Diabetes Association Core Research Award | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 3. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3. All P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Of the 101 participants, 77.2% completed the 4‐week study. 14/51 (27%) in the intervention group and 9/50 (18%) in the control group dropped out. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c and harms measured objectively through BG logs in intervention group. Self‐reported harms in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Patient randomised. Unlikely that the control group received the DMSE intervention. |
| Other bias | Low risk | No evidence of other bias. |
Mahwi 2013.
| Study characteristics | ||
| Methods |
Role of the pharmaceutical care in the management of patients with type 2 diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) Study was conducted at Diabetic Center in Sulaimani, Kurdistan‐Iraq. 2) Intervention provided by pharmacists. In Iraq. 2 arms: 1. Control (usual medical care) (control arm) and 2. Intervention (pharmaceutical care) (intervention arm) |
|
| Participants | Control arm N: 65 Intervention arm N: 65, NA, NA Diabetes type: 2 Mean age: 52.69 ± 10.1 % Male: 30.88 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual medical care) Intervention arm: (pharmaceutical care) 1) Case management 2) Team change |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by an American Diabetes Association Core Research Award | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In this study, patients were divided into 2 groups by simple randomisation technique; the first group is the intervention group, who received pharmaceutical care, while the second one is the control group who only received traditional medical care. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | There are more hyperlipidaemic patients in intervention than control (33% vs 24%). Duration of diabetes seems shorter for the intervention group. No P values calculated for all baseline characteristics. |
| Patient's baseline outcomes (selection bias) | High risk | At baseline, FPG value were 211 ± 70.3 vs 249 ± 88.9 in the control and intervention groups. At baseline, HbA1c value were 9.97 ± 2.75 vs 11.53 ± 1.83 in the control and intervention groups. No P values reported. |
| Incomplete outcome data (attrition bias) | Low risk | 7 lost to follow‐up out of 130 (5.4%). The 7 patients were excluded because they came too late after first or second visits. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | Low risk | Given the intervention is case management, contamination is not a concern. Patients individually met a pharmacist or they were called by him. Patients do not see each other. |
| Other bias | Low risk | No evidence of other bias. |
Maidana 2016.
| Study characteristics | ||
| Methods |
Evaluation of a program of pharmaceutical care to patients with type 2 diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) In the Paraguayan population, it was proposed to develop a program of "Atención Farmacéutica" (AF, Pharmaceutical Care) for diabetic patients under pharmacological treatment at the National Diabetes Program (NDP) Health Center Nº9 (Centro de Salud Nº 9, CSNº9). 2) The medical team involved 3 MDs and a pharmacist. Pharmaceutical care was provided by a single community pharmacist to ensure rigour and consistency. In Republic of Paraguay. 2 arms: 1. Control (traditional pharmaceutical service) (control arm) and 2. Intervention (AF: Atención Farmacéutica, Pharmaceutical Care) (intervention arm) |
|
| Participants | Control arm N: 32 Intervention arm N: 32, NA, NA Diabetes type: 2 Mean age: 55.6 ± 11.16 % Male: 28 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (traditional pharmaceutical service) Intervention arm: (AF: Atención Farmacéutica, Pharmaceutical Care) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin Hypertension control |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method to generate the random list not reported. The 64 patients enrolled to participate in the study were randomly assigned to one of the groups (intervention and control) the size of each group was 32 units of analysis. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | In the results section, they only report patient characteristics for the whole sample (age, gender, diabetes duration, education level, marital status), not per group. However, in the discussion, the authors wrote this: "The comparison of the distribution of patients by assignment groups (intervention and control)... by their sociodemographic characteristics... showed no statistically significant differences at the time of study initiation." From co‐publication: Tables 2 and 3: All the baseline characteristics reported are not significantly different between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Glycaemia and HbA1c have P values higher than 0.05 (C e I (inicio) = control vs intervention at baseline). Discussion: "The comparison of the distribution of patients by assignment groups (intervention and control)... by their ... clinical (glycaemia, glycosylated haemoglobin)... showed no statistically significant differences at the time of study initiation. From co‐publication: Table 5: Blood pressure and IMC not reported for the control group at baseline. |
| Incomplete outcome data (attrition bias) | Low risk | The 64 patients enrolled to participate in the study were randomly assigned to one of the groups (intervention and control); the size of each group was 32 units of analysis. In the intervention group, there was 6% dropout, with 30 patients at the end of the study. Only one patient was lost in the control group out of 32 (3% lost). Low numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and from co‐publication: blood pressure). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Patient‐randomised from one health centre (Centro de Salud Nº 9). A single pharmacist delivered the intervention to ensure rigour and consistency. It is unclear if this pharmacist also saw patients in the control group during traditional pharmaceutical service. |
| Other bias | Low risk | No evidence of other risk of bias. |
Maljanian 2005.
| Study characteristics | ||
| Methods |
Intensive telephone follow‐up to a hospital‐based disease management model for patients with diabetes mellitus Patient RCT, patients referred to hospital‐based disease management program, USA Two arms: 1. Control (control arm) and 2. Telephone (intervention arm) |
|
| Participants | Control arm N: 160 Intervention arm N: 176 Diabetes type: type 1 and type 2 Mean age: 58.0 ± 12.7 % Male: 46.7 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Team changes 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Foot screening, N screened (%) Control arm: pre NR (NR), post 117 (82) Intervention arm: pre NR (NR), post 144 (91) 2) HbA1c, mean % (SD) Control arm: pre 7.7 (1.7), post 6.6 (1.1) Intervention arm: pre 8.1 (1.9), post 6.9 (1.5) |
|
| Funding source | This study was funded by the Aetna Quality of Care Research Foundation through the Academic Medicine and Managed Care Forum | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Mansberger 2015.
| Study characteristics | ||
| Methods |
Long‐term comparative effectiveness of telemedicine in providing diabetic retinopathy screening examinations: a randomized clinical trial RCT (NA clusters and NA providers), conducted in 1) We included patients from two community health clinics (Yellowhawk Tribal Health Center (Pendleton, OR) and Hunter Health Clinic (Wichita, KS)). 2) Clinic technicians performed nonmydriatic testing and transferred the retinal images using a telemedicine system to 2 experienced Devers Eye Institute investigators (S.D. and S.L.M.) for review. In United States of America. 2 arms: 1. Control (traditional surveillance with an eye care provider) (control arm) and 2. Intervention (telemedicine with a nonmydriatic camera) (intervention arm) |
|
| Participants | Control arm N: 271 Intervention arm N: 296, NA, NA Diabetes type: 4 Mean age: 51.1 ± 11.12 % Male: 48.3 Longest follow‐up: 60 months |
|
| Interventions |
Control arm: (traditional surveillance with an eye care provider) 1) Facilitated relay of clinical information 2) Patient education Intervention arm: (telemedicine with a nonmydriatic camera) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education |
|
| Outcomes | Retinopathy screening | |
| Funding source | This research was supported by grant NEI 3 K23 EY0155501‐01 from the National Eye Institute, grants CDC U48DP000024‐01 and 1U48DP002673‐01 from the Centers for Disease Control and Prevention, and the Good Samaritan Foundation at Legacy Health (Dr Mansberger) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We used a random number generator to randomly assign participants to the telemedicine group or the traditional surveillance group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. We used a random number generator to randomly assign participants to the telemedicine group or the traditional surveillance group. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. All P values are above 0.05 for baseline characteristics. There were no differences in demographic and medical characteristics at enrollment between the telemedicine (n = 296) and traditional surveillance (n = 271) groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All P values are above 0.05 for baseline outcomes. There were no differences in demographic and medical characteristics at enrollment between the telemedicine (n = 296) and traditional surveillance (n = 271) groups. |
| Incomplete outcome data (attrition bias) | High risk | No loss to follow‐up at 18 months (before both arms received intervention). But 133 lost to follow‐up at 5 years follow‐up (total of 23.5%, 23% in the control arm and 24% in intervention arm). Numbers balanced. Reasons not reported. The study population included a high percentage of participants who had transient housing and moved in and out of the healthcare system. Consequently, communities that display more stable housing may actually observe higher percentages of patients receiving long‐term follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are objective (research staff reviewed participants’ clinic medical records at regular intervals to identify eye examination visits). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (first posted in June 2011, patients were recruited between August 2006 and September 2011). Protocol only mentions proportion of participants receiving annual eye exam; publication also reports patients requiring referral to eye care professionals and worsening of diabetic retinopathy. |
| Risk of contamination (other bias) | High risk | The project staff encouraged all participants to see an eye care professional once per year for a comprehensive eye examination. This could explains why so many patients had a traditional eye examination in the control group during the first 6 months. Control group received intervention after 2 years. |
| Other bias | Low risk | No evidence of other bias. |
Mazzaglia 2016.
| Study characteristics | ||
| Methods |
Effects of a computerized decision support system in improving pharmacological management in high‐risk cardiovascular patients: a cluster‐randomized open‐label controlled trial Clustered RCT (230 clusters and 230 providers), conducted in 1) General practitioners (GPs) from the Health Search Network research group of nearly 800 GPs representative of each Italian geographic area in terms of patient population, which sent (from 1998) all clinical information from its patient list to Health Search Cegedim Strategic Data Longitudinal Patient Database (HS‐CSD‐LPD). 2) Intervention delivered at the general practitioners level through a computerised decision support system (CDSS). In Italy. 2 arms: 1. Control (standard software, usual care) (control arm) and 2. Intervention (CDSS: computerised decision support system) (intervention arm) |
|
| Participants | Control arm N: 9326 Intervention arm N: 11904, NA, NA Diabetes type: 2 Mean age: 70.44 ± 12.68 % Male: 52.98 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (standard software, usual care) 1) Clinician education Intervention arm: (CDSS: computerised decision support system) 1) Electronic patient registry 2) Clinician education 3) Clinician reminder |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by funds from the Italian Medicines Agency (AIFA). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial allocating participating GPs to one of the 2 groups by a computerised randomisation process. The random allocation of GPs was performed using STATA software, version 10.1 (STATA Corp., College Station, TX, USA). |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Low risk | Randomisation process was stratified by age and geographic location (i.e. north‐east, north‐west, central, southern, major islands); such features are in fact the most relevant predictors of physician prescribing behaviour in Italy. Characteristics of GPs (i.e. age, gender and geographic location) did not significantly differ between intervention and control groups. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. Outcome measurements were not stratified to the diabetes subgroup, but they represented 21,230 out of 25,491 patients randomised (83.3%). Some demographic data have significant P values (congestive heart failure comorbidity, hypertension comorbidity, COPD comorbidity, Charlson index and familiar anamnesis with diabetes). |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. Outcome measurements were not stratified to the diabetes subgroup, but they represented 21,230 out of 25,491 patients randomised (83.3%). Some outcomes have significant P values (DBP, total blood cholesterol levels and mean number of concurrent drug use). |
| Incomplete outcome data (attrition bias) | High risk | 230 GPs have been randomly allocated to receive either the CDSS (intervention group: 115 GPs) or paper‐based information (control group: 115 GPs). Only 197 GPs, 106 in the intervention group (8% lost) vs 91 in the control group (21% lost) sent all baseline information about eligible patients (14% lost overall). High and unbalanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Use of recommended cardiovascular drugs reported by GPs (subjective outcome). GPs are not blinded (cluster‐randomised, open‐label controlled trial). Quote: "GPs voluntarily agreed to collect patient information and attend specified training courses for data entry into a specific designed software used for managing patient information during their routine practice." |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. They added sub‐analysis by baseline exposure (per GPs tertiles) on figures 2 and 3. |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT. Unlikely that control GPs received alerts from the computerised decision support system integrated into standard software. However, since the randomisation was at the clinician level, it is possible that physicians working in the same clinic communicated together. |
| Other bias | Low risk | No evidence of other bias. |
McCarrier 2009.
| Study characteristics | ||
| Methods |
Web‐based collaborative care for type 1 diabetes: a pilot randomized trial Patient RCT, conducted in a diabetes care centre near University of Washington Medical Centre, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 42 Diabetes type: type 1 Mean age: 37.3 ± 8.1 % Male: 67.5 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.1 (1.3), post 8.2 (1.5) Intervention arm: pre 8.0 (1.1), post 7.6 (1.4) |
|
| Funding source | This clinical trial received financial support from Aventis Pharmaceuticals, Inc. through a research grant to H.I.G. and I.B.H. and through funds from grant T32 HS013853 from the Agency for Healthcare Research and Quality to K.P.M. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
McClellan 2003.
| Study characteristics | ||
| Methods |
Improved diabetes care by primary care physicians: results of a group‐randomized evaluation of the Medicare Health Care Quality Improvement Program (HCQIP) Cluster‐RCT (123 clusters with 477 providers), conducted in primary care physicians in a Southern State treating Medicare beneficiaries, USA Two arms: 1. Comparison (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 11,067 Intervention arm N: 11,904 Diabetes type: type 1 and type 2 Mean age: 74.0 ± NR % Male: 43.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Audit and feedback 2) Clinician education 3) Patient education |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 4349 (39), post 4357 (40) Intervention arm: pre 4631 (39), post 4360 (37) |
|
| Funding source | The analyses upon which this publication is based were performed under Contract Number 500‐96‐P704, and sponsored by the Centers for Medicare & Medicaid Services (CMS), Department of Health and Human Services. This article is a direct result of the HCQIP initiated by CMS, which has encouraged identification of quality improvement projects derived from analysis of patterns of care, and therefore required no special funding on the part of this contractor. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No P values reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values provided. The 2 groups were comparable with respect to race, gender and the mean age of the diabetic. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. No P values provided. At baseline, usage of quantitative urine tests, HbA1C tests and retinal examinations were also similar across intervention and comparison groups. |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers dropouts for patients per group not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcomes are process measures and not reported if assessed blindly. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
McDermott 2001.
| Study characteristics | ||
| Methods |
Improving diabetes care in the primary healthcare setting: a randomised cluster trial in remote Indigenous communities Cluster‐RCT (21 clusters and 3 providers), conducted in 21 primary health care centres in Torres Strait and Northern Peninsula Area in Queensland Australia in Australia 2 arms: (control arm) (intervention arm) |
|
| Participants | Control arm N: 305 Intervention arm N: 250, NA, NA Diabetes type: 4 Mean age: 52.3 ± 7.9 % Male: 38 Longest follow‐up: 14 months |
|
| Interventions |
Control arm: (usual care/wait‐list) Intervention arm: (community health workers management) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Anti‐hypertensive drug Retinopathy screening Foot screening Renal screening Hypertension control |
|
| Funding source | This study was supported by National Health and Medical Research Council Grant number 99/3801. The follow‐up workshops were funded by Diabetes Australia. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Picked out of a hat. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | High risk | There is no report. |
| Patient's baseline characteristics (selection bias) | Unclear risk | From text ‐ no significant differences in age, sex ratio or duration of diabetes between groups. No mention of education. No P values. |
| Patient's baseline outcomes (selection bias) | High risk | From text ‐ intervention sites scored higher on blood pressure and HbA1c measurement in the previous 6 months. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
McDermott 2015.
| Study characteristics | ||
| Methods |
Community health workers improve diabetes care in remote Australian Indigenous communities: results of a pragmatic cluster randomized controlled trial Clustered RCT (12 clusters and 12 providers), conducted in 1) The study setting was 12 small remote communities (Indigenous population range 260 to 3000) in far north Queensland where the majority of the population was Aboriginal or Torres Strait Islander, served by a single provider. Primary health care is provided by either a community‐controlled service (n = 4) or the Queensland Government (n = 8). 2) Intervention provided by community‐based Indigenous health worker supported by a clinical outreach team. In Australia 2 arms: 1. Control (usual care/wait‐list) (control arm) and 2. Intervention (community health workers management) (intervention arm) |
|
| Participants | Control arm N: 113 Intervention arm N: 100, NA, NA Diabetes type: 2 Mean age: 47.9 ± 10.9 % Male: 37.6 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (usual care/wait‐list) Intervention arm: (community health workers management) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Lipid‐lowering drugs Antihypertensive drug Retinopathy screening Foot screening Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Smoking status |
|
| Funding source | Support from the peak Aboriginal and Torres Strait Islander Health Councils. RM is supported by NHMRC and QH Practitioner Fellowship and Barbara Schmidt and Sean Taylor are supported in part by a Fellowship funded under the Australian Primary Health Care Research Institute. This study was funded by NHMRC and the Queensland Government, Partnership Grant No. 570149, and the Centre for Research Excellence in Primary Health Care. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 12 services were randomly allocated (names out of a hat) to either the intervention (n = 6) or wait‐list group (n = 6). |
| Allocation concealment (selection bias) | Low risk | Clustered RCT (unit of allocation was community health centre). |
| Provider's baseline characteristics (selection bias) | Unclear risk | 12 remote communities served by a single provider. The unit of randomisation was the community health service. No data on community health services' characteristics are reported in each arm at baseline. |
| Patient's baseline characteristics (selection bias) | Low risk | At baseline, there were no significant differences between allocation groups in age (mean age 47.9 years), sex ratio (62% women), employment status, years of schooling, median household income, self‐reported food insecurity, household size and median AQoL score on the mental health scale (Table 1, P value higher than 0.05). |
| Patient's baseline outcomes (selection bias) | Low risk | At baseline, there were no significant differences between allocation groups in smoking prevalence (Table 1), HbA1c (10.7%, Table 3) and mean BMI (32.5, Table 1). P values higher than 0.05. |
| Incomplete outcome data (attrition bias) | Unclear risk | Over the study period, 22 patients (10.3%) were lost to follow‐up: 6 died, 15 moved away from the community permanently and one withdrew. More patients in the intervention group than the wait‐list group were lost to follow‐up (16.0% vs 5.3%) (Figure 1). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective: HbA1c, blood pressure, statins, LDL, ACEi or ARB drugs, eyes and foot check. Self‐reported outcome: current smoking (subjective but secondary outcome). The study was not blinded as the allocation arm was known following recruitment and baseline data collection and the study was designed as a pragmatic trial reflecting effectiveness in real world practice. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol submitted on September 2010, enrolment occurred between December 2011 and July 2012). All outcomes of interest are reported. |
| Risk of contamination (other bias) | Low risk | Clustered RCT (allocation by health centre). |
| Other bias | Low risk | None identified. |
McKay 2002.
| Study characteristics | ||
| Methods |
Internet‐based diabetes self‐management and support: initial outcomes from the Diabetes Network project RCT (NA clusters and NA providers), conducted in 1) Primary care practices, United States of America. Home‐based study via Internet‐mediated support and feedback. 2) Internet‐mediated access to a professional who had expertise in providing dietary advice to diabetes patients. Participants worked with their coach and interactive resources on the website to reach their dietary goals. Peer‐directed (but professionally monitored) forum for participants to interact with one another. In United States of America. 4 arms: 1. Control (information‐only condition (IOC)) (control arm) and 2. Intervention 1: (personalised self‐management coach condition (PSMCC)) (intervention arm), 3. Intervention 2: (peer support condition (PSC)) (other arm)4. Intervention 3: (combined condition (CC)) (other arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 40, 40, 40 Diabetes type: 2 Mean age: 59.53 ± 11.05 % Male: 46.88 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: (information‐only condition (IOC)) 1) Patient education Intervention arm: (personalised self‐management coach condition (PSMCC)) 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management Intervention arm: (peer support condition (PSC)) 1) Patient education 2) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. There were no statistically significant between‐conditions differences at Time 1 for any of these variables |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. No P values provided. |
| Incomplete outcome data (attrition bias) | High risk | Of the 160 participants randomised (40 to each condition), 16% failed to complete the 3‐month (T2) assessment procedures that measured total cholesterol. The 4 conditions did not significantly differ in their number of missing cases (7 in the IOC, 10 in the PSC, 3 the PSMCC, and 7 in the CC). The number lost is unbalanced between the arms (17.5%, 25%, 7.5%, 17.5%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol; methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Patient randomised. Unclear whether groups may have had access to the resources of other groups. |
| Other bias | Unclear risk | Participants had low HbA1C even at baseline. The follow‐up period was very short. |
McLean 2008.
| Study characteristics | ||
| Methods |
A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: study of cardiovascular risk intervention by pharmacists‐hypertension (SCRIP‐HTN) Patient RCT, conducted in community pharmacies in Edmonton, AB, Canada Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 112 Intervention arm N: 115 Diabetes type: type 1 and type 2 Mean age: 64.9 ± 12.3 % Male: 59.9 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Clinician education 4) Patient education |
|
| Outcomes | 1) Antihypertensives (any), N users (%) Control arm: pre 81 (72), post 81 (72) Intervention arm: pre 85 (74), post 76 (66) 2) SBP, mean mmHg (SD) Control arm: pre 139.9 (11.9), post 134.9 (NR) Intervention arm: pre 142.5 (15.5), post 132.4 (NR) |
|
| Funding source | SCRIP‐HTN was supported by grants from the Canadian Diabetes Association, Heart and Stroke Foundation of Canada, Canadian Council of Cardiovascular Nurses, Alberta Heritage Foundation for Medical Research, and Merck Frosst Canada Ltd. This study was further supported by ManthaMed (in‐kind provision of BpTru devices). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
McMahon 2005.
| Study characteristics | ||
| Methods |
Web‐based care management in patients with poorly controlled diabetes Patient RCT, conducted in 4 hospital‐based and 10 community‐based Veterans Affairs clinics in Boston, Mass, USA Two arms: 1. Education and usual care (control arm) and 2. Web‐based care management (intervention arm) |
|
| Participants | Control arm N: 52 Intervention arm N: 52 Diabetes type: type 1 and type 2 Mean age: 63.5 ± 7.0 % Male: 99.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.9 (0.8), post 8.7 (NR) Intervention arm: pre 10.0 (0.8), post 8.4 (NR) 2) LDL, mean mg/dL (SD) Control arm: pre 97.0 (21.0), post 92.0 (NR) Intervention arm: pre 100.0 (35.0), post 94.0 (NR) 3) Controlled hypertension (< 140/90 mmHg), N under control (%) Control arm: pre 17 (33), post 15 (29) Intervention arm: pre 15 (29), post 24 (46) |
|
| Funding source | This project was supported by grants from the Department of the Army Cooperative Agreement (DAMD 17·98‐ 2‐8017), the Department of Veterans Affairs, Health Services Research and Development Program (TEl‐02‐100) and the National Institutes of Health (K24‐DK06321) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but not opaque. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Figure shows that 5 participants did not receive the intervention. |
| Other bias | Low risk | Information not available. |
McMahon 2012.
| Study characteristics | ||
| Methods |
A randomized comparison of online‐ and telephone‐based care management with internet training alone in adult patients with poorly controlled type 2 diabetes Patient RCT, conducted in the Department of Veterans Affairs (VA) Boston Healthcare System. Four hospital based clinics or 10 community‐based outpatient clinics. In USA. Three arms: 1. Web training (control arm), 2. Telephone care (intervention arm 1) and 3. Online care (intervention arm 2) |
|
| Participants | Control arm N: 50 Intervention arm 1 N: 51 Intervention arm 2 N: 51 Diabetes type: type 2 Mean age: 60.2 ± 10.8 % Male: 94.7 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm 1: 1) Case management 2) Team changes 3) Electronic patient registry 4) Patient education 5) Promotion of self‐management Intervention arm 2: 1) Case management 2) Team changes 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.1 (1.4), post 8.4 (1.7) Intervention arm 1: pre 9.9 (1.2), post 8.5 (1.6) Intervention arm 2: pre 9.6 (1.0), post 8.3 (1.1) 2) SBP, mean mmHg (SD) Control arm: pre 139.8 (19.1), post 136.7 (19.3) Intervention arm 1: pre 139.9 (17.4), post 133.2 (17.1) Intervention arm 2: pre 135.6 (17.4), post 135.2 (19.2) 3) DBP, mean mmHg (SD) Control arm: pre 83.1 (15.8), post 77.3 (11.5) Intervention arm 1: pre 80.8 (13.1), post 74.6 (10.7) Intervention arm 2: pre 75.7 (11.8), post 73.2 (10.7) 4) LDL, mean mg/dL (SD) Control arm: pre 92.5 (32.3), post 86.3 (29.4) Intervention arm 1: pre 91.7 (37.8), post 85.9 (27.1) Intervention arm 2: pre 95.1 (29.4), post 92.4 (27.4) |
|
| Funding source | The study was supported by grants from VA Health Services Research and Development (TEL‐02‐100), National Institutes of Health (K24 DK063214) and the Department of the Army Cooperative Agreement (DAMD 17‐98‐2‐8017) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…through the use of a random number generator…" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…and a series of sealed envelopes." Envelopes opaque? |
| Patient's baseline characteristics (selection bias) | Low risk | P values in table are all not significantly different. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No baseline measures of outcome provided. |
| Incomplete outcome data (attrition bias) | High risk | ~18% lost to follow‐up in N1 and ~13% in N2, ~7% in N3. Reasons not provided; the numbers who completed the study (where we were able to calculate percentages) were in the text and not in the flow diagram (since they did an intention‐to‐treat analysis). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c and SBP measurements described, laboratory methods. Blinding of outcome assessor not described. |
| Selective reporting (reporting bias) | Low risk | All endpoints match. |
| Risk of contamination (other bias) | High risk | Same case managers between telephone and internet group, potential contamination. |
| Other bias | Low risk | No evidence of other bias. |
McMurray 2002.
| Study characteristics | ||
| Methods |
Diabetes education and care management significantly improve patient outcomes in the dialysis unit RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from individuals undergoing haemodialysis (HD) or peritoneal dialysis (PD) at the Northeast Indiana Kidney Centers at Jefferson and Marion dialysis units. 2) Initial nutritional counselling was performed by the renal dietitian and reinforced by the diabetes care manager. A multidisciplinary Diabetes Advisory Committee that met quarterly throughout the study period provided programme oversight. A full‐time diabetes care co‐ordinator was designated to implement the project. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (self‐management education, diabetes care monitoring and management, and motivational coaching) (intervention arm) |
|
| Participants | Control arm N: 38 Intervention arm N: 45, NA, NA Diabetes type: 3 Mean age: 62.04 ± 8.86 % Male: 53.92 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (self‐management education, diabetes care monitoring and management, and motivational coaching) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Retinopathy screening Glycated haemoglobin Harms |
|
| Funding source | Supported in part by the Renal Care Group and a grant from The Kidney Foundation of Indiana | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | At the Jefferson unit, randomisation occurred by assigning patients who underwent HD Monday, Wednesday and Friday to the study group and Tuesday, Thursday and Saturday to the control group. At the Marion unit, the reverse schedule was used for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. P values provided. The control and study groups were similar in all characteristics listed: age, sex, dialysis modality, type of diabetes, duration of diabetes and time on dialysis therapy. No information on education level. |
| Patient's baseline outcomes (selection bias) | Low risk | Figure 1 shows that baseline HbA1c was similar between groups with support of P value. Table 3 and 4 (with the exception of foot checks) have all baseline outcomes similar between groups with P values provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | No discussion of loss/dropout; no indication whether ITT or PP. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, harms, eye. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Outcomes match methods. |
| Risk of contamination (other bias) | Low risk | "Efforts were made to reduce contamination as much as possible. Because the HD treatment environment is a close‐knit one, separation of the control and study groups by treatment days was chosen in hopes of reducing knowledge diffusion and discussion among patients between the two groups. It also was important to remove any physician biases. Physicians in the dialysis facility cared for patients in either the study group or control group. There was no crossover of physician care." |
| Other bias | High risk | "We acknowledge that there could be observer bias in the behaviour change results. Because the care manager educated the study group, as well as administered follow‐up tests to both groups, it is possible that knowledge of the patients in the study was improved instead of actually changing behaviour. That we had an increase in number of patients measuring blood glucose levels and undergoing eye examinations suggests that we influenced some behaviour change." |
Medi‐Cal Group 2004.
| Study characteristics | ||
| Methods |
Closing the gap: effect of diabetes case management on glycemic control among low‐income ethnic minority populations: the California Medi‐Cal type 2 diabetes study Patient RCT, conducted in clinical sites in Santa Barbara, San Diego, Los Angeles serving racial/ethnic minority, low‐income Medi‐Cal populations, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 172 Intervention arm N: 186 Diabetes type: type 2 Mean age: 57.0 ± 0.9 % Male: 28.3 Longest follow‐up: 25.3 months (mean) |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 9.7 (0.1), post 8.5 (0.2) Intervention arm: pre 9.6 (0.1), post 7.7 (0.2) 2) SBP, mean mmHg (SE) Control arm: pre 134.0 (NR), post 134.6 (NR) Intervention arm: pre 136.0 (NR), post 133.4 (NR) 3) DBP, mean mmHg (SE) Control arm: pre 76.0 (1.0), post 75.5 (NR) Intervention arm: pre 81.0 (4.0), post 74.4 (NR) 4) LDL, mean mg/dL (SE) Control arm: pre 130.1 (3.6), post 121.0 (NR) Intervention arm: pre 129.8 (3.2), post 115.6 (NR) 5) Harms (severe hypoglycaemia), N (%) Control arm: pre NR (NR), post 6 (3) Intervention arm: pre NR (NR), post 13 (7) |
|
| Funding source | The State of California Medi‐Cal Managed Care Division and Center for Disease Control and Prevention funded the study. The study also received support from the GCRC at Harbor‐UCLA Medical Center grant number MO1‐RR00425. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but not opaque. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Mehuys 2011.
| Study characteristics | ||
| Methods |
Effectiveness of a community pharmacist intervention in diabetes care: a randomized controlled trial Cluster‐RCT (66 clusters with 66 providers), conducted in 66 community pharmacies in Flanders (Dutch speaking area), Belgium Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 135 Intervention arm N: 153 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.3 (1.2), post 7.2 (1.0) Intervention arm: pre 7.7 (1.7), post 7.1 (1.1) 2) Smoking cessation, N smokers (%) Control arm: pre 29 (21), post 28 (21) Intervention arm: pre 28 (18), post 27 (18) |
|
| Funding source | This study was funded by Ghent University | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequence of allocation to control or intervention group was predetermined by the investigators based on randomization schedule generated using SPSS." |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "Values did not differ significantly among both groups, according to independent sample t‐test for continuous variables and chi‐square test for categorical variables." |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.08). |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those randomised. Although very low attrition. numbers and reasons for loss to follow‐up provided. Numbers provided for each arm, but reasons were provided overall, so we do not know if there were significant differences in reasons for both arms. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c, methods not described. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | No evidence of other bias |
Meigs 2003.
| Study characteristics | ||
| Methods |
A controlled trial of web‐based diabetes disease management: the MGH diabetes primary care improvement project Cluster‐RCT (66 clusters with 66 providers), conducted with 39 staff MD and 104 resident MD in the Adult Medicine Clinic in Harvard Medical School in Boston, Massachusetts, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 291 Intervention arm N: 307 Diabetes type: type 2 Mean age: 67.5 ± 12.0 % Male: 48.1 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam) 2) Foot screening 3) HbA1c 4) SBP 5) DBP 6) LDL 7) Controlled hypertension (< 130/85 mmHg) |
|
| Funding source | This study was supported by a grant from the National Pharmaceutical Council and by the MGH Primary Care Operations Improvement and Clinical Research Programs. Funds for dissemination of results were also provided by Aventis Pharmaceuticals. J.B.M. received support from an American Diabetes Association clinical research grant and a junior faculty development grant from SmithKline Beecham. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ all P value > 0.05, but age not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ race P = 0.04. Data are provided with P values for age and gender in Table 1; none are significantly different between the groups. No information on education. |
| Patient's baseline outcomes (selection bias) | High risk | From text ‐ hypertension more common in intervention group, Table 1 (P value < 0.05). Table 2 ‐ outcomes look unbalanced. |
| Incomplete outcome data (attrition bias) | Unclear risk | Patient exclusions not reported by study group in Figure 1. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Menard 2005.
| Study characteristics | ||
| Methods |
Efficacy of intensive multitherapy for patients with type 2 diabetes mellitus: a randomized controlled trial Patient RCT (NA clusters and NA providers), conducted in 1) Patients in the intensive multi‐therapy group each had monthly visits to the Clinical Research Centre of the Centre Hospitalier Universitaire de Sherbrooke. 2) Clinical team members (2 endocrinologists, 1 nurse, 1 dietician, 1 fitness trainer and study co‐ordinator) in Canada 2 arms: 1. Control group (conventional treatment by physician) (control arm) and 2. Intensive multi‐therapy group (IMTG) (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 36, NA, NA Diabetes type: 2 Mean age: 54.8 ± NR % Male: 68.06 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (conventional treatment by physician) Clinician education Facilitated relay of clinical information Patient education Intervention arm: (intensive multi‐therapy group (IMTG)) Case management Team change Facilitated relay of clinical information Patient education Promotion of self‐management |
|
| Outcomes | Lipid‐lowering drugs Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Harms |
|
| Funding source | This work was supported by the Clinical Research Centre of the Centre Hospitalier Universitaire de Sherbrooke and by grants from Brystol‐Myers Squibb and the Quebec Diabetes Association | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a blocked randomisation (n = 4) stratified by haemoglobin A1c value (< 10% and ≥ 10%), patients were assigned by an independent person using a computer program to receive intensive multi‐therapy or usual care. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There was no significant difference between groups (each n = 36) with respect to age, gender, duration of diabetes, lifestyle habits and arrangements, education or occupation. |
| Patient's baseline outcomes (selection bias) | Low risk | At baseline, no difference was observed between the 2 groups (IMT/control) for clinical or biochemical data. |
| Incomplete outcome data (attrition bias) | Unclear risk | 36 in each group to start. 34 in each group to finish. Loss of 2 for each group, however explanation for dropout was only provided for 3 participants. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes (HbA1c, SBP, hypoglycaemia was defined as any glucose measurement of 3.5 mmol/L or less, and an episode was recorded as “severe” if the assistance of another person was required). |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Control group received usual care from their physicians. |
| Other bias | Low risk | None found. |
Miranda 2019.
| Study characteristics | ||
| Methods |
The effect of individual and mixed rewards on diabetes management: a feasibility randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Patients attending the outpatient clinic of the endocrinology service from Hospital Nacional Arzobispo Loayza were approached and invited into the study from July to October 2016 (15 weeks). This hospital is located in Lima, Peru’s capital, and is one of the national tertiary hospitals from the Ministry of Health. 2) Diabetes educator, nutritionist in Peru 3 arms: 1. Control (individual incentive ‐ no partner) (control arm) and 2. Intervention 1 (mixed altruism ‐ cash given to patient only) (intervention arm), 3. Intervention 2 (mixed co‐operation ‐ cash split with partner) (other arm) |
|
| Participants | Control arm N: 18 Intervention arm N: 18, 18, NA Diabetes type: 2 Mean age: 54.8 ± 0.9 % Male: 33 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (individual incentive ‐ no partner) 1) Patient education 2) Promotion of self‐management 3) Financial incentives Intervention arm: (mixed altruism ‐ cash given to patient only) 1) Patient education 2) Promotion of self‐management 3) Financial incentives Intervention arm: (mixed co‐operation ‐ cash split with partner) 1) Patient education 2) Promotion of self‐management 3) Financial incentives |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by the DFID/MRC/Wellcome Global Health Trials (MR/M007405/1 and 107435/Z/15/Z). AB‐O (103994/Z/14/Z) and JJM (074833/Z/04/Z, 205177/Z/16/Z) are supported by Wellcome Trust. JJM acknowledges receiving additional support from the Alliance for Health Policy and Systems Research (HQHSR1206660), Fogarty International Center (R21TW009982, D71TW010877), Grand Challenges Canada (0335‐04), International Development Research Center Canada (106887, 108167), Inter‐American Institute for Global Change Research (IAI CRN3036), Medical Research Council (MR/P008984/1, MR/P024408/1, MR/P02386X/1), National Cancer Institute (1P20CA217231), National Heart, Lung and Blood Institute (HHSN268200900033C, 5U01HL114180, 1UM1HL134590), National Institute of Mental Health (1U19MH098780), Swiss National Science Foundation (40P740‐160366), and the World Diabetes Foundation (WDF15‐1224) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants were randomly assigned (1:1:1) to receive one of the 3 interventions using a computer‐generated list of numbers. |
| Allocation concealment (selection bias) | Low risk | For allocation concealment, participants were randomised using sequentially numbered, opaque, sealed envelopes. The sealed envelope was assigned after the patient had been recruited and all baseline measurements were completed. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values provided. Education looks to differ between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. P values provided and above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. Group 1 lost 5/18 (28%), group 2 lost 5/18 (28%), group 3 lost 4/18 (22%). Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol; methods match outcomes. In Figure 1 it shows that a number of patients were available for analysis but only a subset was analysed for HbA1c. |
| Risk of contamination (other bias) | Low risk | There really is no way to contaminate this study. Participants either did or did not have a "supportive" partner and financial incentives were provided based on performance. |
| Other bias | Low risk | None identified. |
Moattari 2012.
| Study characteristics | ||
| Methods |
Impact of self management on metabolic control indicators of diabetes patients RCT (NA clusters and NA providers), conducted in 1) The study was carried out in Nader Kazemi Diabetic clinic in Shiraz located in Fars province, southern Iran. This is the main diabetic clinic in Shiraz affiliated with Shiraz University of Medical Sciences. This centre offers services (treatment and follow‐up) to at least 50 patients on a daily basis. 2) Akram Ghobadi and Parvin Beigi run the intervention (authors). Both are affiliated to a School of Nursing & Midwifery. In Iran. 2 arms: 1. Control (control arm) and 2. Intervention (self‐management programme based on 5A) (intervention arm) |
|
| Participants | Control arm N: 35 Intervention arm N: 35, NA, NA Diabetes type: 3 Mean age: 24.13 ± 11.45 % Male: 70.42 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: Intervention arm: (self‐management program based on 5A) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | The authors thank the vice‐chancellery of Shiraz University of Medical Sciences for the financial support provided for this study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The convenience and a purposeful sampling method helped randomly divide the participants into 2 experimental and control groups. |
| Allocation concealment (selection bias) | High risk | The convenience and the purposeful sampling methods do not allow adequate allocation concealment. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Demographic characteristics of patients only reported for all patients and not by study arms. However, there was no significant difference in the demographic variables between the 2 groups with respect to age, marital status and educational level, type of diabetes, job, positive family history and co‐morbidity. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Data reported without P values. However, assessing the results before interventions using independent t‐test showed that the 2 groups were similar in fasting blood sugar, haemoglobin A1c, cholesterol, triglyceride, lipoprotein (HDL and LDL) and body mass index, and there was no statistically significant difference between the 2 groups before the intervention. |
| Incomplete outcome data (attrition bias) | High risk | They lost 12 patients out of 82 randomised (14,6%). Numbers and reasons for lost in each group are not reported. A convenience sample of 82 patients were involved in the study. They were randomly assigned into experimental and control groups. In total, 70 patients completed the study. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | RCT. Intervention delivered in a single clinic. Looks like caregivers were contacted about patients in the intervention (different options/choices were identified based on the input received from the patients, his/her significant other and the caregiver). They could have changed their care approach. Chances are high that there was communication between participants and providers across the groups. |
| Other bias | Low risk | None identified. |
Moattari 2013.
| Study characteristics | ||
| Methods |
The impact of electronic education on metabolic control indicators in patients with diabetes who need insulin: a randomised clinical control trial Patient RCT, conducted with 52 patients being followed at the Nader Kasemi and Moshir Fatemi Diabetes Centers in Iran Two arms: 1. Control group (control arm) and 2. Experimental group (intervention arm) |
|
| Participants | Control arm N: 26 Intervention arm N: 26 Diabetes type: type 1 and type 2 Mean age: 23.4 (range: 18 to 39) % Male: 43.0 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.4 (1.8), post 8.8 (1.3) Intervention arm: pre 9.1 (1.3), post 7.1 (1.2) 2) LDL, mean mg/dL (SD) Control arm: pre 94.8 (22.2), post 99.9 (24.6) Intervention arm: pre 103.0 (25.1), post 94.8 (21.8) |
|
| Funding source | The authors thank the vice‐chancellery of Shiraz University of Medical Sciences, Shiraz, Iran, for the financial support | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | They randomised by odd and even file numbers; however they note that odd and even were determined by a coin toss. |
| Allocation concealment (selection bias) | High risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not in table or text. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Only ~7% lost to follow‐up in both arms, but reasons not provided for N1. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c and LDL part of primary outcomes. HbA1c measured using high performance liquid chromatography technique using variant. LDL measured using glucose oxidase method using a Hitachi 717 analyser. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | High risk | All patients came from the same centre; we do not know if physicians treated people from both arms. |
| Other bias | Low risk | Information not available. |
Mons 2013.
| Study characteristics | ||
| Methods |
Effectiveness of a supportive telephone counseling intervention in type 2 diabetes patients: randomized controlled study Patient RCT, conducted in general practices located in the area of Ludwigsburg/Heibronn, SW Germany Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 101 Intervention arm N: 103 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Clinician reminders 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, median % (SD) Control arm: pre 8.2 (0.8), post 7.7 (NR) Intervention arm: pre 8.0 (0.7), post 7.8 (NR) 2) SBP, median mmHg (IQR) Control arm: pre 135.0 (15.5), post 136.8 (NR) Intervention arm: pre 140.0 (20.0), post 138.2 (NR) 3) DBP, median mmHg (IQR) Control arm: pre 80.0 (14.5), post 79.9 (NR) Intervention arm: pre 80.0 (5.0), post 80.0 (NR) |
|
| Funding source | This study was supported by a grant of the German Federal Ministry of Education and Research (01GX0746). The funding body had no role in the study design, the collection, analysis or interpretation of the data, the writing of the manuscript, or on the decision to submit the paper for publication. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A web‐based randomization service for clinical trials was used…" minimization technique also used. |
| Allocation concealment (selection bias) | Unclear risk | Not really reported, but we can assume randomised once enrolled. |
| Patient's baseline characteristics (selection bias) | Low risk | In text and table. |
| Patient's baseline outcomes (selection bias) | High risk | Primary: HbA1c (P = 0.15). Secondary: DBP (P = 0.02). |
| Incomplete outcome data (attrition bias) | High risk | ~8% lost to follow‐up in control; ~10% in intervention, numbers of declines slightly higher in intervention group, reasons for decline not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: HbA1c, objective laboratory methods contracted central laboratory, using ion exchange high pressure liquid chromatography. |
| Selective reporting (reporting bias) | High risk | Some secondary outcomes listed in protocol not addressed in paper and vice versa. |
| Risk of contamination (other bias) | High risk | Quote: "This may have led to contamination bias, with the practice nurses conducting the telephone‐based counseling or the general practitioners (GPs) unknowingly enhancing care for patients in the usual care group." |
| Other bias | Low risk | Information not available. |
Montori 2004.
| Study characteristics | ||
| Methods |
Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta‐analysis Patient RCT, Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 16 Intervention arm N: 15 Diabetes type: type 1 Mean age: 42.9 (interquartile range: 24.4 to 52.7) % Male: 32.3 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.8 (1.2), post 8.2 (1.2) Intervention arm: pre 9.1 (1.3), post 7.8 (1.3) 2a) Harms (severe hypoglycaemia), N (%) Control arm: pre NR (NR), post 3 (19) Intervention arm: pre NR (NR), post 3 (20) 2b) Harms (ketoacidosis), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 0 (0) |
|
| Funding source | The Mayo Foundation funded this study with a research award to Y.C.K. Roche Diagnostics donated modems and glucometer equipment for both study groups | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Moreira 2015.
| Study characteristics | ||
| Methods |
Nursing case management and glycemic control among Brazilians with type 2 diabetes: pragmatic clinical trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted in 6 primary healthcare centres in the municipality of Bandeirantes. This city is located in the northern region of the state of Paraná, Brazil, and has approximately 32,290 inhabitants, including 979 adults with type 2 diabetes mellitus, registered in 2011. 2) The intervention was delivered by nurses. In Brazil. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (nursing case management) (intervention arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 40, NA, NA Diabetes type: 2 Mean age: 50.14 ± 11.8 % Male: 35 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (nursing case management) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | "The authors would like to express their appreciation to the Araucaria Foundation for the support given to teacher training and to the Health Secretariat of Bandeirantes for their partnership in this study." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Recruited individuals in each health centre were assigned at random by the researcher to one of two groups, using the lottery method. This consisted of each of the sample members being assigned a unique number. The corresponding numbers for participants from each health centre were placed separately in a bowl and mixed. Then, the blindfolded researcher selected the numbers and participants were assigned at a 1:1 ratio to the intervention or usual care. |
| Allocation concealment (selection bias) | Low risk | Blindfolded researcher selected the numbers in a bowl. |
| Patient's baseline characteristics (selection bias) | Low risk | All patient characteristics are similar at baseline (Tables 1 and 2, P above 0.05). |
| Patient's baseline outcomes (selection bias) | High risk | Tables 1 and 2. Weight, BMI, blood pressure (< 140/90 mmHg) and SBP are significantly different between control patients and intervention patients at baseline (P under 0.05). |
| Incomplete outcome data (attrition bias) | Low risk | Only 3 lost to follow‐up out of 80 (3.8%). Reasons reported and numbers balanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol (protocol registered on August 2011, recruitment began in July 2011, 1‐year intervention). In the methods and in the protocol, the authors state they collected data for foot complications and glomerular filtration rates but they do not provide any data in the paper. They reported changes in HbA1c, but no risk factors for chronic complications are reported (secondary outcomes in protocol). |
| Risk of contamination (other bias) | High risk | Both the control arm and the intervention arm received case management by the same nurses. It is not excluded that nurses also provided the intensive case management programme to the control patients. Quote: "Considering the small community in which the study was conducted, it is possible that participants from the intervention and comparison groups interacted." |
| Other bias | Low risk | None. |
Morgan 2013.
| Study characteristics | ||
| Methods |
The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial Clustered RCT (18 clusters and 18 providers), conducted in 1) Setting: 11 Australian general practices (in city and country areas), 5 randomly allocated to the intervention (3 country, 2 city) and 6 to the control (2 country, 4 city). 2) Collaborative care provided by practice nurses (PNs). In Australia. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (collaborative care by practice nurses managers) (intervention arm) |
|
| Participants | Control arm N: 94 Intervention arm N: 93, NA, NA Diabetes type: 2 Mean age: 67.81 ± 8.91 % Male: 53.38 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (collaborative care by practice nurses managers) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Funding was provided by Beyondblue, the National Depression Initiative in Australia (grant 172), but it had no other involvement in any phase of the study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They were allocated by a random number generator to either the intervention or control arm of the study. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No data reported, but they found some discrepancies between clinics: Of the 5 clinics in the intervention (clinics 4, 5, 13, 15 and 17), only clinics 4 and 17 were significantly different from each other (F(1,76) = 9.6, P < 0.001). Of the 6 clinics in the control group (clinics 1–3, 6, 16 and 18), only clinics 6 and 18 were significantly different from each other (F(1,78) = 14.5, P < 0.001). |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Patient characteristics at the baseline visits. There were no significant differences between the intervention and control group at baseline. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Patient characteristics at the baseline visits. There were no significant differences between the intervention and control group at baseline. |
| Incomplete outcome data (attrition bias) | High risk | Not clear, but it looks like they lost 35 patients out of 229 patients in the control group (15.3%) and 94 out of 300 in the intervention group (31.3%) after randomisation (but before data collection). High and unbalanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol. They do not report data for renal function (as stated in methods) and DBP (as stated in methods and protocol). They also added many outcomes in the paper (triglycerides, exercise, smoking, alcohol, 10 years CVD risk, etc.). We were not able to obtain multiple data sets at 3‐monthly intervals over 12 months of ‘usual care’. |
| Risk of contamination (other bias) | Low risk | Unlikely that control group received intervention. |
| Other bias | Low risk | None identified. |
Moriyama 2009.
| Study characteristics | ||
| Methods |
Efficacy of a self‐management education program for people with type 2 diabetes: results of a 12 month trial RCT (NA clusters and NA providers), conducted in 1) The interview was conducted on a hospital visit day (in Japan) at a place with sufficient space to ensure privacy, 2) The same diabetes educator was assigned to implement the programme throughout the education period of 1 year for each participant. In Japan. 2 arms: 1. Control (usual clinical practise) (control arm) and 2. Intervention (self‐management education programme) (intervention arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 65.98 ± 10.02 % Male: 46.15 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual clinical practise) 1) Patient education Intervention arm: (self‐management education programme) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This research was funded by a Grant‐in‐aid for Scientific Research B, No. 15390671, from the Ministry of Education, Science, Sports and Culture, Japan. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomised in referral order and assigned to the intervention group or the control group. |
| Allocation concealment (selection bias) | High risk | Participants were randomised in referral order. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 ‐ P values provided. Employment status P = 0.035. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1 ‐ P values greater than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | 8 lost from intervention group (50 to 42, 16%), 2 lost from control group (25 to 23, 8%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Objective measure for HbA1c, BP in the intervention group… control group self‐reported their values. Risk for response bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered, outcomes match methods. |
| Risk of contamination (other bias) | Low risk | Control group measured their own values, educators measured the intervention group. |
| Other bias | Low risk | None. |
Mourão 2013.
| Study characteristics | ||
| Methods |
Pharmaceutical care program for type 2 diabetes patients in Brazil: a randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) The study settings were 6 primary health care units integrated into the Brazilian public health system in Ouro Preto, Brazil. 2) Two research pharmacists performed the intervention program. In Brazil. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (usual care and pharmaceutical care) (intervention arm) |
|
| Participants | Control arm N: 64 Intervention arm N: 65, NA, NA Diabetes type: 2 Mean age: 60.65 ± 8.35 % Male: 33 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (usual care and pharmaceutical care) 1) Case management 2) Facilitated relay of clinical information 3) Patient education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The study was supported by CAPES (Coordenacao de Aperfeicoamento de Pessoal de Nıvel Superior) and Universidade Federal de Ouro Preto | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to the intervention or control group using a list of random numbers generated by Minitab software, version 15. Randomisation was stratified by each primary health care unit. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Both groups exhibited similar characteristics (P > 0.05). |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. Both groups exhibited similar characteristics (P > 0.05), except for systolic blood pressure. |
| Incomplete outcome data (attrition bias) | High risk | They only report baseline data for the patients who completed the study and not for all the patients randomised. They lost 29 patients out of 129 randomised (22.5%). Numbers and reasons balanced between arms, but high numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol available (protocol posted in February 2011, study started in January 2010). They did not include drug use in the protocol. They do not report data on drug use in the usual way, that is the number of patients prescribed with a drug. |
| Risk of contamination (other bias) | Unclear risk | The participants in the control group received usual health care characterised by appointments with doctors, nurses, nutritionists or physiotherapists. Only the intervention arm had pharmaceutical care. It is not excluded that physicians changed their approach with their control patients after receiving pharmaceutical reports for their intervention patients. Additionally, as the control group participants had access to laboratory results at baseline, they may have improved self‐care and sought medical attention, which could minimise the effect of the intervention provided in this study. |
| Other bias | Low risk | No evidence of other bias. |
Mulrow 1987.
| Study characteristics | ||
| Methods |
Evaluation of an Audiovisual Diabetes Education Program: negative results of a randomized trial of patients with non‐insulin‐dependent diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) St. Thomas' Hospital, London, United Kingdom, 2) Nurse clinician in United Kingdom 3 arms: 1. Control: group 3 educational lecture (control arm) and 2. Intervention 1: group 2 general education programme (intervention arm), 3. Intervention 2: Group 1 tailored education programme (other arm) |
|
| Participants | Control arm N: 40 Intervention arm N: 40, 40, NA Diabetes type: 2 Mean age: 53.27 ± 11.19 % Male: 45 Longest follow‐up: 11 months |
|
| Interventions |
Control arm: (educational lecture) 1) Patient education Intervention arm: (general education programme) 1) Case management 2) Patient education Intervention arm: (tailored education programme) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Supported by a grant from Pfizer Pharmaceuticals | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Baseline characteristics of patients assigned to the different education programmes were not significantly different (Table 2). |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | 16 (13%) dropped out before 7‐month follow‐up data could be obtained. Their baseline characteristics were similar to the average characteristics of study participants presented in Table 2. Dropouts were evenly distributed among groups (group 1 = 6, group 2 = 5, group 3 = 5) and occurred primarily because of transportation problems or difficulty in obtaining time off from work for appointments. After the final 11‐month visit, complete data for 68% of the patients who had started the programmes. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective HbA1c measure. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol, methods match outcomes. |
| Risk of contamination (other bias) | High risk | Random monitoring of monthly group 2 sessions revealed that some of the information presented audiovisually to group 1 was being addressed. |
| Other bias | Low risk | None identified. |
Munch 2019.
| Study characteristics | ||
| Methods |
Management of people with Type 2 diabetes shared between a specialized outpatient clinic and primary health care is noninferior to management in a specialized outpatient clinic: a randomized, noninferiority trial RCT (NA clusters and NA providers), conducted in 1) Annual comprehensive check‐ups were conducted at the specialised diabetes outpatient clinic at Steno Diabetes Center Copenhagen, Gentofte Hospital, University of Copenhagen. Routine check‐ups were conducted at general practice or at the diabetes outpatient clinic, according to randomisation. 2) Healthcare professionals in primary care, diabetes nurse and an endocrinologist, GPs in Denmark 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (shared care programme) (intervention arm) |
|
| Participants | Control arm N: 69 Intervention arm N: 71, NA, NA Diabetes type: 2 Mean age: 64.93 ± 10.1 % Male: 73.55 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) 1) Case management Intervention arm: (shared care programme) 1) Case management 2) Team change 3) Facilitated relay of clinical information |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This study was supported by unrestricted donations from the Jascha Foundation, Lilly and Herbert Hansen’s Foundation, and Capital Region of Denmark | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A secretary in a neighbouring department prepared sealed envelopes and managed the randomisation process. Randomised in a 1:1 ratio and in blocks of 2 (intervention: control) for each GP. Participants, healthcare professionals and researchers were blinded during the initial baseline visit. |
| Allocation concealment (selection bias) | Unclear risk | A secretary in a neighbouring department prepared sealed envelopes and managed the randomisation process. Randomised in a 1:1 ratio and in blocks of 2 (intervention: control) for each GP. Participants, healthcare professionals and researchers were blinded during the initial baseline visit. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 2/67 dropped out in control group, 5/71 dropped out of intervention group. Reasons provided. 2 lost to follow‐up in control group (3%) and 5 lost in intervention group (7%), reasons for dropout varied between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | High risk | Protocol states 1‐, 2‐, 3‐year follow‐up ‐ study only reports 1‐year. Smoking status, foot exam, QOL some differences between protocol and published report: follow‐up at 24 and 36 months, foot examination, smoking status, quality of life. |
| Risk of contamination (other bias) | Low risk | Endocrinologists only shared information on intervention patients with GPs. |
| Other bias | Low risk | None. |
Munshi 2013.
| Study characteristics | ||
| Methods |
Assessment of barriers to improve diabetes management in older adults Patient RCT, conducted with patients recruited from the Joslin Diabetes Center and the Beth Israel Deaconess Medical Center, USA Two arms: 1. Attention control (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 30 Intervention arm N: 70 Diabetes type: type 1 and type 2 Mean age: 75.0 ± 5.0 % Male: 46.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.0 (0.8), post 8.7 (NR) Intervention arm: pre 9.3 (1.2), post 8.6 (NR) |
|
| Funding source | The study was partly supported by a grant from a clinical research award from the American Diabetes Association, 1‐07‐CR‐40 (M.N.M.), and partly from the U.S. Department of Defense Peer Reviewed Medical Research Program of the Office of the Congressionally Directed Medical Research Programs, W81XWH‐07‐1‐0282 (M.N.M.) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not mentioned in text or table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not mentioned in text or table. |
| Incomplete outcome data (attrition bias) | Low risk | Minimal and equal number of losses, reasons similar. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective methods not described and outcome assessor blinding not described. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Musacchio 2018.
| Study characteristics | ||
| Methods |
Efficacy of self‐monitoring blood glucose as a key component of a chronic care model versus usual care in type 2 diabetes patients treated with oral agents: results of a randomized trial RCT (NA clusters and NA providers), conducted in 1) outpatient clinic of Cusano Milanino, Italy 2) Diabetologist, Nurses and dietician in Italy 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (SINERGIA model) (intervention arm) |
|
| Participants | Control arm N: 120 Intervention arm N: 121, NA, NA Diabetes type: 2 Mean age: 61.8 ± 8.15 % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (SINERGIA model) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician education 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The study was promoted by Sanof SpA, Italy | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No P values reported. |
| Incomplete outcome data (attrition bias) | High risk | Number lost unbalanced between arms (22% and 16%) and reason for lost data not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol, but some outcomes not reported in results (percentage of participants with HbA1c ≤ 7.0%. |
| Risk of contamination (other bias) | Unclear risk | Control patients likely seen by same healthcare professionals delivering intervention. Depending on how standard education was delivered to control arm. |
| Other bias | Low risk | No other evidence of risk of bias. |
Nagrebetsky 2013.
| Study characteristics | ||
| Methods |
Stepwise self‐titration of oral glucose‐lowering medication using a mobile telephone‐based telehealth platform in type 2 diabetes: a feasibility trial in primary care Patient RCT, conducted in 7 general practices in Oxfordshire and Buckinghamshire, United Kingdom Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 9 Intervention arm N: 8 Diabetes type: type 2 Mean age: 58.0 ± 11.0 % Male: 71.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Case management Intervention arm: 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.2 (3.4), post 7.5 (3.6) Intervention arm: pre 8.1 (3.1), post 7.0 (2.8) 2) Harms (hypoglycaemia), N (%) Control arm: pre NR (NR), post 0 (0) Intervention arm: pre NR (NR), post 1 (14) |
|
| Funding source | This study was funded by the National Institute for Health Research School for Primary Care Research and the National Institute for Health Research Biomedical Research Centre Programme, Oxford, United Kingdom | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation. |
| Allocation concealment (selection bias) | High risk | We can predict next sequence with minimisation. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported in text or table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not mentioned in text or table. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c objective measures not described and outcome assessor blinding not described. |
| Selective reporting (reporting bias) | High risk | < 2005 approach used since no protocol. They talked about measuring BP but did not provide results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Naik 2011.
| Study characteristics | ||
| Methods |
Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial Patient RCT, conducted in Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, USA Two arms: 1. Traditional education intervention (control arm) and 2. EPIC intervention (intervention arm) |
|
| Participants | Control arm N: 42 Intervention arm N: 45 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.7 (1.2), post 8.6 (1.4) Intervention arm: pre 8.9 (1.3), post 8.1 (1.4) |
|
| Funding source | The EPIC study was supported by a grant from the Agency for Healthcare Research and Quality (AHRQ), Centers for Research and Education on Therapeutics (CERTs) (U18HS016093, PI: Suarez‐Almazor). Additional support for the EPIC study was provided by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (PI: Naik). Dr. Naik received additional supported from the National Institute of Aging (K23AG027144) and the Houston Health Services Research and Development Center of Excellence (HFP90‐020) at the Michael E. DeBakey Veterans Affairs Medical Center | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Says they randomised based on block size of 10. However, do not state if a random number generator was used to generate sequence within blocks. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation of treatment group assignment was blinded using sequentially numbered and sealed envelopes." Opaque envelopes? |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "Participants… were similar at baseline across a range of socio‐demographic and clinical variables …" |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.66). |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 lost to follow‐up in each group and for the same reason. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Blinding not described. HbA1c using ion exchange liquid chromatography. |
| Selective reporting (reporting bias) | High risk | In the protocol, they state they will measure SBP and LDL as primary outcomes as well, but this was not reported in the manuscript. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Naji 1994.
| Study characteristics | ||
| Methods |
Integrated care for diabetes: clinical, psychosocial, and economic evaluation Patient RCT, conducted in a general practice, United Kingdom Two arms: 1. Conventional care (control arm) and 2. Integrated care (intervention arm) |
|
| Participants | Control arm N: 135 Intervention arm N: 139 Diabetes type: unclear/not reported Mean age: 58.8 ± 18.1 % Male: 56.0 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Electronic patient registry 2) Patient reminders Intervention arm: 1) Electronic patient registry 2) Clinician education 3) Clinician reminders 4) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 5.3 (1.4), post 5.3 (1.7) Intervention arm: pre 5.3 (1.4), post 5.3 (1.7) 2) SBP, mean mmHg (SD) Control arm: pre 153.9 (24.8), post 156.4 (25.7) Intervention arm: pre 155.9 (27.1), post 161.5 (25.1) 3) DBP, mean mmHg (SD) Control arm: pre 84.8 (11.5), post 83.5 (9.9) Intervention arm: pre 85.6 (15.6), post 84.3 (11.1) |
|
| Funding source | This research was funded by the Chief Scientist Office, Scottish Office Home and Health Department | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Patient randomised and allocation concealment not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Nesari 2010.
| Study characteristics | ||
| Methods |
Effect of telephone follow‐up on adherence to a diabetes therapeutic regimen Patient RCT, conducted in an Iranian Diabetes Society, Iran Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 31 Intervention arm N: 30 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.1 (1.6), post 8.6 (1.9) Intervention arm: pre 8.9 (1.4), post 7.0 (1.2) |
|
| Funding source | This study was supported by a grant from Tehran University of Medical Sciences, Iran. We are grateful to the Iranian Diabetes Society for its help with the data collection. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…flipping a coin." |
| Allocation concealment (selection bias) | Low risk | Quote: "...flipping a coin and assignment them to one of the two groups." This is acceptable so long as they were flipped when entering the study. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "showed that there were no significant differences in the demographic and disease characteristics between the two groups". Table 1 with P values also provided. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.7). |
| Incomplete outcome data (attrition bias) | Low risk | ~3% lost to follow‐up in N1 and 0% in N2. 1 lost to follow‐up due to refusal of post test in N1. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective laboratory methods used to measure HbA1c. Unsure if outcome assessors were blinded for analysing HbA1c, since it was sent to a laboratory, however all other outcomes were self‐reported and collected from a blinded research assistant. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; method match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Neto 2011.
| Study characteristics | ||
| Methods |
Effect of a 36‐month pharmaceutical care program on coronary heart disease risk in elderly diabetic and hypertensive patients Patient RCT, conducted in Primary Health Care Unit of the Brazilian public health system, Salto Grande, Sao Paulo State, Brazil Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 18 Intervention arm N: 17 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 36 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.7 (0.5), post 7.7 (NR) Intervention arm: pre 7.7 (0.5), post 7.0 (NR) |
|
| Funding source | This study was supported by Fundação de Apoio ao Desenvolvimento Científico (FADEC) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Software which… Quote: "provided computer‐generated random sequences…" |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "…indicated that the baseline characteristics of the patients in the intervention group closely matched those of the patients in the control group." Table with P values also provided. |
| Patient's baseline outcomes (selection bias) | Low risk | SBP (P = 0.79); DBP (P = 0.36); HbA1c (P = 0.69); LDL (P = 0.90). |
| Incomplete outcome data (attrition bias) | High risk | Although only 3% lost to follow‐up in each arm, specific reasons for loss to follow‐up not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Outcome assessor blinded and laboratory methods used to assess outcomes go into detail for SBP and DBP. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
New 2003.
| Study characteristics | ||
| Methods |
Specialist nurse‐led intervention to treat and control hypertension and hyperlipidemia in diabetes (SPLINT): a randomized controlled trial Patient RCT, conducted in Hope Hospital, Salford, United Kingdom Two arms: 1. Usual care (control arm) and 2. Clinic (intervention arm) |
|
| Participants | Control arm N: 508 Intervention arm N: 506 Diabetes type: type 1 and type 2 Mean age: 63.6 (interquartile range: 55.4 to 72.1) % Male: 50.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Case management 2) Team changes 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) SBP, mean mmHg (SD) Control arm: pre 159.0 (13.3), post 149.0 (NR) Intervention arm: pre 159.0 (14.8), post 147.0 (NR) 2) DBP, mean mmHg (SD) Control arm: pre 77.0 (10.4), post 74.0 (NR) Intervention arm: pre 78.0 (11.1), post 74.0 (NR) 3) Controlled hypertension (< 140/80 mmHg), N under control (%) Control arm: pre 0 (0), post 122 (24) Intervention arm: pre 0 (0), post 135 (27) |
|
| Funding source | This study was funded by an unrestricted educational grant from Pfizer | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts > 20%. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
New 2004.
| Study characteristics | ||
| Methods |
Educational outreach in diabetes to encourage practice nurses to use primary care hypertension and hyperlipidaemia guidelines (EDEN): a randomized controlled trial Cluster‐RCT (44 clusters with 44 providers), conducted in 44 primary care practices within Salford, United Kingdom Two arms: 1. Control (control arm) and 2. Educational outreach (intervention arm) |
|
| Participants | Control arm N: 2531 Intervention arm N: 2474 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Patient education |
|
| Outcomes | 1) Controlled hypertension (< 140/80 mmHg), N under control (%) Control arm: pre 0 (0), post 1212 (48) Intervention arm: pre 0 (0), post 1192 (48) |
|
| Funding source | This study was funded by an unrestricted educational grant from Pfizer | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Unclear risk | See Table 1 ‐ no P values provided but looks balanced. |
| Patient's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Newman 2009.
| Study characteristics | ||
| Methods |
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin‐treated diabetes mellitus (MITRE) Patient RCT, conducted in secondary care diabetes clinics in 4 hospitals in England, United Kingdom Four arms: 1. Standard care control (control arm), 2. Attention control (intervention arm 1), 3. CGMS ‐ continuous glucose monitoring system (intervention arm 2), and 4. Glucowatch (intervention arm 3) |
|
| Participants | Control arm N: 102 Intervention arm 1 N: 100 Intervention arm 2 N: 102 Intervention arm 3 N: 100 Diabetes type: type 1 and type 2 Median age: 52 (range: 41 to 63) % Male: 55.0 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm 1: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders Intervention arm 2: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders Intervention arm 3: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.4 (1.3), post 8.9 (1.6) Intervention arm 1: pre 8.9 (1.1), post 8.4 (1.2) Intervention arm 2: pre 9.0 (1.1), post 8.5 (1.2) Intervention arm 3: pre 9.2 (1.5), post 9.1 (1.4) 2a) Harms (hyperglycaemia), N (%) Control arm: pre NR (NR), post 61 (79) Intervention arm 1: pre NR (NR), post 71 (88) Intervention arm 2: pre NR (NR), post 61 (79) Intervention arm 3: pre NR (NR), post 61 (82) 2b) Harms (hypoglycaemic episodes), N (%) Control arm: pre NR (NR), post 49 (64) Intervention arm 1: pre NR (NR), post 58 (72) Intervention arm 2: pre NR (NR), post 50 (65) Intervention arm 3: pre NR (NR), post 50 (68) |
|
| Funding source | This research was funded by the National Institute of Health Research, Health Technology Assessment Programme | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Medical Research Council’s Clinical Trials Unit randomisation line. |
| Allocation concealment (selection bias) | Low risk | Once written consent had been obtained, the research nurse phoned the Medical Research Council’s Clinical Trials Unit randomisation line. Randomisation was site‐specific and ensured balanced allocation in terms of centre, age and type of diabetes by use of the minimisation method. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 6 ‐ no P values reported. Looks balanced. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 7 ‐ no P values reported. Looks balanced. |
| Incomplete outcome data (attrition bias) | High risk | 18 lost in standard care group (18%), 16 lost in attention control group (16%), 21 lost in Glucowatch group (21%) and 17 lost in CGMS group (17%), reasons provided. In the study, a loss of statistical power occurred because of a greater than expected loss to follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered; outcomes not provided at registration. |
| Risk of contamination (other bias) | Low risk | Control group unlikely to have access to intervention monitoring devices. |
| Other bias | Low risk | No evidence of other bias. |
Nicolucci 2015.
| Study characteristics | ||
| Methods |
A randomized trial on home telemonitoring for the management of metabolic and cardiovascular risk in patients with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Multicentre study conducted in the area of general practice (primary care setting). 2) Home telehealth (HT) intervention carried out by general practitioners and nurses in Italy 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (home telehealth) (intervention arm) |
|
| Participants | Control arm N: 149 Intervention arm N: 153, NA, NA Diabetes type: 2 Mean age: 58.46 ± NR % Male: 61.55 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (home telehealth) 1) Case management 2) Electronic patient registry 3) Clinician reminder 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Harms |
|
| Funding source | This study was supported by a research grant to A.N. from MSD Italia (Merck & Co. Inc. ou Merck Sharp and Dohme) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group allocation was based on centralised telephone randomisation, stratified by participating physician and by treatment (oral agents, insulin). Permuted blocks randomisation was used. |
| Allocation concealment (selection bias) | Low risk | Group allocation was based on centralised telephone randomisation, stratified by participating physician and by treatment (oral agents, insulin). Permuted blocks randomisation was used. |
| Patient's baseline characteristics (selection bias) | High risk | Patients in the control group had higher dyslipidaemia percentage (49.7% vs 36.7%, P = 0.02). |
| Patient's baseline outcomes (selection bias) | Low risk | Patients in the control group had slightly higher diastolic blood pressure values (87.2 ± 4.6 vs 86.2 ± 4.0, P = 0.05). More likely to have dyslipidaemia in control group. See Table 1. |
| Incomplete outcome data (attrition bias) | High risk | Overall, 29 general practitioners from 2 health districts enrolled 302 patients, of whom 153 were assigned to the telemedicine group and 149 to the control group. Shortly after randomisation, 39 participants in the telemedicine group and 14 participants in the control group withdrew their consent (unbalanced numbers). Consequently, 82.5% of the total sample completed the 12‐month assessment (17.5% loss). Some of the patients withdrew their consent shortly after randomisation, mainly for the complexity of the study design or the difficulty in using the telemedicine tools. A few patients also abandoned the study because of emotional problems caused by using the system (reasons related to intervention). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcomes. Clinical information was collected at baseline, after 6 months, and after 12 months, using an ad hoc clinical record form. Blood samples were collected on the same occasions, and HbA1c levels and lipid profiles were measured in a centralised laboratory. |
| Selective reporting (reporting bias) | Unclear risk | According to the retrospectively registered protocol (protocol first posted in July 2014, recruitment started in October 2011, 1‐year intervention), the primary outcomes were blood glucose level, blood pressure and BMI. However, they do not report data for blood glucose levels at baseline and after the intervention. They also do not report BMI data after intervention, just the weight. |
| Risk of contamination (other bias) | Low risk | Unlikely control group received treatment as only those randomised to intervention received telemedicine monitoring system. Group allocation was stratified by participating physician. |
| Other bias | Low risk | None identified. |
Nishita 2012.
| Study characteristics | ||
| Methods |
Empowered diabetes management: Life coaching and pharmacist counseling for employed adults with diabetes. Patient RCT, conducted with patients recruited through community means ‐ newspaper, HR departments, health fairs, etc., intervention took place in public places. In USA. Two arms: 1. Control (control arm) and 2. Treatment (intervention arm) |
|
| Participants | Control arm N: 62 Intervention arm N: 128 Diabetes type: type 1 and type 2 Mean age: 48.5 ± 9.8 % Male: 37.4 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management 5) Financial incentives |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 7.7 (0.2), post 7.8 (0.1) Intervention arm: pre 7.8 (0.2), post 7.6 (0.1) |
|
| Funding source | The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Centers for Medicare and Medicaid Services (Grant No. CFDA No. 93.769). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, but opaque? |
| Patient's baseline characteristics (selection bias) | Low risk | Reported in text and table. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c, P = 0.77. |
| Incomplete outcome data (attrition bias) | High risk | ~16% lost to follow‐up in control; ~35% in intervention. Balance seems okay since 3:1 ratio, but reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: HbA1c and others, no objective laboratory methods described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias. |
Noto 2016.
| Study characteristics | ||
| Methods |
Cluster‐randomized trial to improve the quality of diabetes management: The study for the efficacy assessment of the standard diabetes manual (SEAS‐DM) Clustered RCT (42 clusters and 42 providers), conducted in 1) The present study was carried out in eight domestic districts of the Japan Medical Associations. 2) Clinical research co‐ordinators, who were not aware of the allocation of the PCPs, visited each clinic every 3 months and collected the pertinent data by reviewing the medical records. In Japan. 2 arms: 1. Control group (Diabetes Treatment Guide) (control arm) and 2. Intervention group (Diabetes Treatment Guide + The Manual) (intervention arm) |
|
| Participants | Control arm N: 182 Intervention arm N: 234, NA, NA Diabetes type: 2 Mean age: 62.29 ± NR % Male: 58.43 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (Diabetes Treatment Guide) 1) Clinician education Intervention arm: (Diabetes Treatment Guide + The Manual) 1) Clinician education |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by Grants‐in‐Aid from the Japan Agency for Medical Research and Development (Grant: Practical Research Project for Life‐Style related Diseases including CVD and Diabetes), and from the Ministry of Health, Labour and Welfare, Japan (Grant number: Comprehensive Research on Life‐Style Related Diseases including CVD and Diabetes H25‐016). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The PCPs in each district were randomly allocated to either an intervention or a control group, with each group as a cluster and each district as a stratum. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Outcomes look balanced. |
| Incomplete outcome data (attrition bias) | Low risk | Figure 1. 1 control patient and 4 intervention patients lost. Reasons given. During the 1‐year follow‐up period, 5 patients were lost to follow‐up: the follow‐up rate was 99.8% (Figure 1). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | High risk | Protocol does not mention HbA1c measurement or the adherence to the following recommendation‐concordant performances: measurement of HbA1c (every 3 months), blood pressure (every 3 months) and serum lipids (every 3 months). |
| Risk of contamination (other bias) | Low risk | The PCPs were not notified of the study endpoints at any point during the study period. Cluster‐RCT. |
| Other bias | Low risk | None identified. |
O'Connor 2005.
| Study characteristics | ||
| Methods |
Randomized trial of quality improvement intervention to improve diabetes care in primary care settings Cluster‐RCT (12 clusters with 329 providers), conducted in primary care medical practices in Minnesota, USA Two arms: 1. Control clinics (control arm) and 2. Intervention clinics (intervention arm) |
|
| Participants | Control arm N: 326 Intervention arm N: 428 Diabetes type: unclear/not reported Mean age: 57.8 ± NR % Male: 54.3 Longest follow‐up: 30 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Continuous quality improvement |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 68 (21), post 91 (28) Intervention arm: pre 103 (24), post 128 (30) 2) Retinopathy screening (eye exam), N screened (%) Control arm: pre 127 (39), post 104 (32) Intervention arm: pre 150 (35), post 141 (33) 3) Foot screening, N screened (%) Control arm: pre 189 (58), post 176 (54) Intervention arm: pre 244 (57), post 244 (57) 4) Renal screening (microalbumin), N screened (%) Control arm: pre 52 (16), post 72 (22) Intervention arm: pre 94 (22), post 171 (40) 5) HbA1c, mean % (SD) Control arm: pre 8.0 (NR), post 7.8 (NR) Intervention arm: pre 8.1 (NR), post 8.0 (NR) 6) SBP, mean mmHg (SD) Control arm: pre 137.0 (NR), post 136.0 (NR) Intervention arm: pre 136.0 (NR), post 135.0 (NR) 7) LDL, mean mg/dL (SD) Control arm: pre 130.0 (NR), post 109.0 (NR) Intervention arm: pre 133.0 (NR), post 117.0 (NR) |
|
| Funding source | This work was supported by a grant from the Centers for Disease Control and Prevention; co‐operative agreement no. UC32/CCU500347 to the Minnesota Department of Health Diabetes Program, with a subcontract to the HealthPartners Research Foundation; and a grant from the HealthPartners Research Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Table 1. No P values provided, large difference in number of patients evaluated. |
| Patient's baseline characteristics (selection bias) | High risk | See Table 1. Age and patients using insulin P values < 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | See Table 1, P values > 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Lots of dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
O'Connor 2009a.
| Study characteristics | ||
| Methods |
Customized feedback to patients and providers failed to improve safety or quality of diabetes care: a randomized trial Clustered RCT (123 clusters and 123 providers), conducted in 1) The study was conducted at HealthPartners Medical Group, an 18‐clinic multispecialty group that provides care to 8000 adults with diabetes in Minnesota, United States of America 4 arms: 1. Control (no intervention ‐ group A) (control arm) and 2. Intervention 1 (patient) (intervention arm), 3. Intervention 2 (physician) (intervention arm), 4. Intervention 3 (both) (intervention arm) |
|
| Participants | Control arm N: 3703 Intervention arm N: 847, 869, 1041, 946 Diabetes type: not reported Mean age: 56.1 ± 12.1 % Male: 54 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (no intervention) Intervention arm: (patient) 1) Patient education 2) Patient reminders Intervention arm: (physician) 1) Audit and feedback 2) Clinician reminders Intervention arm: (both) 1) Audit and feedback 2) Clinician reminders 3) Patient education 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | This project was supported through funding from the Agency for Healthcare Research and Quality (Grant 5 U 18HS11919‐02) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Before randomisation, 67 consenting physicians were blocked into groups of 3 based on 1) same specialty (family medicine or internal medicine) and 2) whether they provided care to 50 vs 50 or more diabetic patients. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Only number of eligible patients reported, but P > 0.05. |
| Patient's baseline characteristics (selection bias) | High risk | Table 1, P values < 0.05 for age and sex. |
| Patient's baseline outcomes (selection bias) | High risk | See Table 1, P < 0.05 for insulin use, glucose intervention eligible. |
| Incomplete outcome data (attrition bias) | High risk | Large amount of dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | No information. |
| Selective reporting (reporting bias) | Low risk | No information. |
| Risk of contamination (other bias) | Low risk | No information. |
| Other bias | Low risk | None. |
O'Connor 2009b.
| Study characteristics | ||
| Methods |
Simulated physician learning intervention to improve safety and quality of diabetes care: a randomized trial Cluster‐RCT, 1) The study was conducted at HealthPartners Medical Group, an 18‐clinic multispecialty group that provides care to 8000 adults with diabetes. 2) Primary care physicians were randomised to a simulated case‐based physician learning intervention (software developed by the authors) with a) printed feedback comparing actions taken with the ones taken by an expert physician or b) verbal interaction and feedback from a physician opinion leader who observed the physician Three arms: 1. Control (no intervention ‐ group A), 2. Intervention 1 (simulated physician education and printed feedback ‐ group B) and 3. Intervention 2 (simulated physician education and verbal feedback ‐ group C) |
|
| Participants | Control arm N: 691 Intervention arms N: 725, 604, NA Diabetes type: unclear/not reported Mean age: 64.0 ± 13.0 % Male: 58 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (no intervention ‐ group A) Intervention arm: (simulated physician education and printed feedback ‐ group B) 1) Audit and feedback 2) Clinician education Intervention arm: (simulated physician education and verbal feedback ‐ group C) 1) Audit and feedback 2) Clinician education |
|
| Outcomes | 1) Glycated haemoglobin 2) Low‐density lipoprotein |
|
| Funding source | This project was supported by the Agency for Healthcare Research and Quality (grant no. RO1 HS 10639) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Before randomisation, 67 consenting physicians were blocked into groups of 3 based on 1) same specialty (family medicine or internal medicine) and 2) whether they provided care to 50 vs 50 or more diabetic patients. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05 for physicians, but group C seems to have fewer female physicians (16% vs 37% in group A and 26% in group B). |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. Randomisation at the physician level resulted in similar patient samples except that patients of physicians in group B more often had coronary artery disease and higher Charlson scores (Charlson comorbidity index). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values above 0.05 for HbA1c and LDL (pre‐intervention). |
| Incomplete outcome data (attrition bias) | High risk | Table 2. For HbA1c, they have data for 1686 patients out of 2020 at 12 months (16.5% loss) and for LDL, they have data for 1178 patients (41.7% loss). High numbers. Attrition occurred evenly across randomised groups, and final analysis included 19 physicians in each group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and LDL). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol posted on December 2005, study started on December 2001 and was completed on September 2002). The secondary outcomes do not match between protocol (secondary analysis will assess rates of screening for microvascular complications) and paper (pharmacotherapy intensification rates in patients not at clinical goals, and risky prescribing events). |
| Risk of contamination (other bias) | Unclear risk | Clustered‐RCT but randomisation was at the physician level, so communications might have happen between intervention and control physicians working in the same clinic. Groups B and C are pretty similar. Physicians in groups B and C received the same simulated learning intervention, but group B received printed feedback and group C received verbal feedback. The control group received no education. |
| Other bias | Low risk | None identified. |
O'Connor 2011.
| Study characteristics | ||
| Methods |
Impact of electronic health record clinical decision support on diabetes care: a randomized trial Cluster‐RCT (11 clusters with 41 providers), conducted in clinics from the HealthPartners Medical Group (large medical group in Minnesota that provides care to type 2 diabetics), USA Two arms: 1. Control clinic (control arm) and 2. Intervention clinic (intervention arm) |
|
| Participants | Control arm N: 1362 Intervention arm N: 1194 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Clinician reminders 3) Financial incentives |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 8.4 (0.1), post 8.1 (0.1) Intervention arm: pre 8.5 (0.1), post 7.9 (0.1) 2) SBP, mean mmHg (SE) Control arm: pre 141.6 (0.7), post 131.5 (0.7) Intervention arm: pre 141.3 (0.7), post 130.5 (0.7) 3) DBP, mean mmHg (SE) Control arm: pre 84.6 (0.5), post 77.1 (0.5) Intervention arm: pre 85.1 (0.5), post 76.8 (0.5) 4) LDL, mean mg/dL (SE) Control arm: pre 124.1 (1.7), post 98.3 (1.8) Intervention arm: pre 122.3 (1.7), post 97.9 (1.8) |
|
| Funding source | This project was funded by National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) grant # R01 DK068314 to HealthPartners Research Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | P = 0.02 (family physician). |
| Patient's baseline characteristics (selection bias) | Low risk | Female (%) P < 0.001; White race (%) P < 0.001. |
| Patient's baseline outcomes (selection bias) | High risk | DBP (P = 0.023); LDL (P = 0.019). |
| Incomplete outcome data (attrition bias) | High risk | Looks like a per‐protocol analysis was done. Numbers and reasons for loss to follow‐up were provided, but seem disproportionate and may be related to outcome. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: HbA1c, BP, LDL. HbA1c done using liquid chromatography assay. BP using technique. LDL based on standard assays. Blinding not described. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
O'Hare 2004.
| Study characteristics | ||
| Methods |
Evaluation of delivery of enhanced diabetes care to patients of South Asian ethnicity: the United Kingdom Asian Diabetes Study (UKADS) Cluster‐RCT (6 clusters), conducted in West Midlands general practices, United Kingdom Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 181 Intervention arm N: 180 Diabetes type: type 2 Mean age: 58.9 ± 11.7 % Male: 51.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Team changes 2) Clinician education 3) Patient education 4) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.1 (2.1), post 7.9 (NR) Intervention arm: pre 7.8 (1.9), post 7.6 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 143.8 (21.7), post 141.7 (NR) Intervention arm: pre 146.3 (21.7), post 139.6 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 80.7 (11.3), post 81.0 (NR) Intervention arm: pre 82.8 (10.8), post 79.7 (NR) |
|
| Funding source | "We thank the following companies for providing financial support in the form of grants for this study: Pfizer, Aventis UK, Eli Lilly, NovoNordisk, Boehringer Ingleheim, Servier Laboratories UK, Takeda UK." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Comparing baseline data for the intervention and control groups, respectively, there were no significant differences in percentage of males, blood pressure, HbA1c or cholesterol as a risk factor, number of risk factors or diabetes treatment. There is indication of missing data. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. Comparing baseline data for the intervention and control groups, respectively, there were no significant differences in percentage of males, blood pressure, HbA1c or cholesterol as a risk factor, number of risk factors or diabetes treatment. There is indication of missing data. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Obreli‐Neto 2015.
| Study characteristics | ||
| Methods |
Economic evaluation of a pharmaceutical care program for elderly diabetic and hypertensive patients in primary health care: a 36‐month randomized controlled clinical trial RCT (NA clusters and NA providers), conducted in 1) Clinical trial conducted in a Brazilian public Primary Health Care Unit (PHCU) located in the municipality of Salto Grande, Sao Paulo state. Brazil’s Sistema Único de Saúde (SUS) is a universal, publicly funded, rights‐based public healthcare system. 2) Intervention delivered by pharmacists. In Brazil. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (pharmaceutical care) (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 100, NA, NA Diabetes type: 4 Mean age: 65.3 ± 6.48 % Male: 37.65 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (pharmaceutical care) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | No separate funding was obtained for this study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | JMP 8.0.1 software (SAS Institute, Inc., Cary, NC) provided computer‐generated random sequences (100 patients each in the intervention and control groups) according to the medical record numbers of the 200 patients selected. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All characteristics have P values above 0.05. The intervention and control groups were well balanced at baseline with regard to sociodemographic, clinical and drug therapy characteristics. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All outcomes have P values above 0.05. The intervention and control groups were well balanced at baseline with regard to sociodemographic, clinical and drug therapy characteristics. |
| Incomplete outcome data (attrition bias) | Low risk | Only 6 lost to follow‐up out of 200 (3%). Numbers and reasons balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Methods only mention cost‐effectiveness and economic costs. Nothing about HbA1c and blood pressure measures in the methods. They calculated their sample size based on LDL level. |
| Risk of contamination (other bias) | Unclear risk | Only the intervention arm had individual follow‐ups with pharmacists, education and special reminder pill packages. However, following suggestions by pharmacists to change medication for some intervention patients, it is not excluded that physicians changed their approach with patients in the usual care arm. Also, Quote: "The pharmacists also worked in association with other health care professionals for additional interventions, such as the adjustment of drug dosages, modification of drug therapy (addition or withdrawal), modification of diet plans, and practice of physical activities." It is unclear if the other health professionals were also working with the control group and may have changed their treatment based on meetings with pharmacists. |
| Other bias | Low risk | No evidence of other bias. |
Odegard 2005.
| Study characteristics | ||
| Methods |
Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention Patient RCT, conducted in University of Washington Medicine Neighbourhood Clinics, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 34 Intervention arm N: 43 Diabetes type: type 2 Mean age: 51.7 ± 11.1 % Male: 57.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.6 (1.4), post 8.4 (NR) Intervention arm: pre 10.2 (0.8), post 8.2 (NR) |
|
| Funding source | This research was sponsored by a grant from the Academic and Managed Care Forum, Quality Care Research Fund | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Were differences but they adjusted for them in their analysis. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Odnoletkova 2016.
| Study characteristics | ||
| Methods |
Optimizing diabetes control in people with type 2 diabetes through nurse‐led telecoaching RCT (NA clusters and NA providers), conducted in 1) Primary care setting in Belgium. All coaches were employed by a Flemish home care organisation, ‘Solidariteit voor het Gezin’. 2) Telecoaching was delivered by a certified diabetes nurse educator (referred to as the ‘coach’). In Belgium. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (COACH programme by nurses) (intervention arm) |
|
| Participants | Control arm N: 287 Intervention arm N: 287, NA, NA Diabetes type: 2 Mean age: 63.1 ± 0.91 % Male: 61.50 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (usual care) 1) Facilitated relay of clinical information 2) Patient education Intervention arm: (COACH programme by nurses) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Continuous quality improvement |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Retinopathy screening Renal screening Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Smoking status |
|
| Funding source | The present randomised controlled trial was subsidized by the European Regional Development Fund and the Flemish Government. Partena, MSD and Abbott provided a scientific grant for the clinical trial. Mesh and Tabakstop have provided measuring devices for the purposes of the trial. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a random number generator in Excel was performed by a data analyst in the Independent Health Insurance Fund who was not involved in the study. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1: no P values. Do not report data for all patients at baseline. There was no difference in baseline characteristics between the patients with complete follow‐up and those with at least one missing value. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Tables 1 and 2: No P values. Do not report data for all patients at baseline. There was no difference in baseline characteristics between the patients with complete follow‐up and those with at least one missing value. |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up at 6 and 18 months was 12% and 16% in the intervention group and 9% and 14% in the control group, respectively. 12 patients withdrawn after enrolment in the intervention group (4%) and none in the control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The primary outcome of interest was objectively assessed (HbA1c), as well as all other outcomes of interest. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. The protocol include HbA1c and other modifiable risk factors as outcomes, without clearly listing them. Many outcomes are reported in the paper (LDL, blood pressure, smoking status, drug use, etc). |
| Risk of contamination (other bias) | Low risk | The laboratory results of the blood analysis were mailed to all study participants and their GPs. They could have taken action where warranted. |
| Other bias | Low risk | No evidence of other bias. |
Olry de Labry Lima 2017.
| Study characteristics | ||
| Methods |
Effectiveness of an intervention to improve diabetes self‐management on clinical outcomes in patients with low educational level Clustered RCT (9 clusters and 9 providers), conducted in 1) The study was conducted in 2 general practices in the city of Granada (Andalusia, Spain). Those practices were selected because they were located in a highly deprived area. 2) A total of 9 general practitioners (GPs) in the 2 practices participated in this study. A subgroup of patients received telephone reinforcement by a member of the research team. In Spain. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (patient‐practitioner communication tool ‐DSMRS) (intervention arm) |
|
| Participants | Control arm N: 94 Intervention arm N: 90, NA, NA Diabetes type: 2 Mean age: 61.67 ± 12.02 % Male: 44.57 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (patient‐practitioner communication tool‐DSMRS) 1) Clinician education 2) Clinician reminder 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Regional Health Ministry (Andalusia, Spain). The funder of this study had no role in study design, data analysis, data collection, data interpretation or writing of the report. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We used computerised randomisation to allocate the GPs to the intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No data reported. However, results from the multilevel analysis suggested that the variability attributable to the provider level (cluster effect) was not significant. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05 except social support at 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | 184 patients accepted and were recruited (90 in the intervention and 94 in the control group) and 108 participants ended the follow‐up (41.3% lost). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The primary outcome of interest was objectively assessed (HbA1c) as well as all other outcomes of interest (SBP, DBP and LDL). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol. The protocol only includes HbA1c and not SBP, DBP and LDL as reported in the paper. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. Randomisation was conducted at the GP (and not patient) level, which prevented potential contamination bias. Not clear if GPs allocated to different arms work in the same clinic (risk of communication). 9 physicians in 2 clinics participated in the study. |
| Other bias | Low risk | No evidence of other bias. |
Orsama 2013.
| Study characteristics | ||
| Methods |
Active assistance technology reduces glycosylated hemoglobin and weight in individuals with type 2 diabetes: results of theory‐based randomized trial Patient RCT, conducted in Sipoo, Finland, Community Health Centre, Finland Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 29 Intervention arm N: 27 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 10 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.1 (1.5), post 7.1 (NR) Intervention arm: pre 6.9 (1.6), post 6.5 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 146.5 (15.3), post 136.7 (NR) Intervention arm: pre 157.0 (15.6), post 136.5 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 84.7 (9.1), post 78.4 (NR) Intervention arm: pre 88.5 (10.3), post 78.0 (NR) |
|
| Funding source | The Finnish Funding Agency for Technology and Innovation, Technical Research Centre of Finland, and Bayer HealthCare are acknowledged for funding the study. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Tables and text. |
| Patient's baseline outcomes (selection bias) | High risk | Secondary outcome: SBP (P = 0.029). |
| Incomplete outcome data (attrition bias) | High risk | ~17.7% lost to follow‐up in control; ~11% in intervention. N = 3 did not complete baseline in control; imbalanced reasons between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | No objective laboratory methods described for primary (HbA1c); and secondary (SBP, DBP) outcomes. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Oude Wesselink 2015.
| Study characteristics | ||
| Methods |
Effects of government supervision on quality of integrated diabetes care: a cluster randomized controlled trial Clustered RCT (100 clusters and 31 providers), conducted in 1) Care groups are organisations that provide integrated diabetes care to patients in general practices (primary care). 2) The intervention was delivered by a government supervision programme and practice nurses. In Netherlands. 2 arms: 1. Control (no supervision) (control arm) and 2. Intervention (inspection visit and report to care groups) (intervention arm) |
|
| Participants | Control arm N: 88 Intervention arm N: 88, NA, NA Diabetes type: 2 Mean age: 66.5 ± 7 % Male: 51 Longest follow‐up: 3.61 months |
|
| Interventions |
Control arm: (no supervision) Intervention arm: (inspection visit and report to care groups) 1) Audit and feedback |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Smoking status |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. The supervision programme was randomly assigned to 20 of the 100 care groups in the Netherlands, leaving 80 care groups as control groups. "For the study we randomly approached 17 intervention groups and 22 control care groups. After exclusion of noneligible care groups, we were left with 14 intervention care groups and 19 control care groups. Of these, 10 intervention care groups and 8 control care groups participated in this study." Selection of patients within care groups was random and anonymous, resulting in between 5 and 10 patients per practice per measurement. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Low risk | The supervision programme was assigned to randomly selected care groups. Of these, 10 intervention care groups and 8 control care groups participated in this study. Each participating care group identified 1 or 2 practices for this study, resulting in 16 intervention practices and 15 control practices. Quote: "Most practices' characteristics (structures of care and processes of care) were comparable between the intervention and control group at baseline (table 2). No P values." |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. Patient characteristics are similar. Quote: "In general, patient characteristics and health outcomes at baseline were comparable between the intervention group and the control group." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All health outcomes appears similar at baseline, except for the yearly assessment of albumin in urine (0.57 vs 0.38) and GFR (0.71 vs 0.51). Quote: "In general, patient characteristics and health outcomes at baseline were comparable between the intervention group and the control group." |
| Incomplete outcome data (attrition bias) | Unclear risk | The numbers of patients analysed postintervention are not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All outcomes were extracted from medical records by practice nurses and research assistants. However, the practice nurse was asked to answer questions about guideline adherence in a questionnaire (subjective outcome). Nothing reported about blinding. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Clustered RCT. Only the intervention care groups received a visit and a report. However, some managers worked for 2 or more care groups so it is possible that they applied some recommendations made to intervention clinic(s) to the control clinic(s) they were also managing. |
| Other bias | High risk | For baseline and postintervention measurement, we selected different patients using a randomisation procedure. Selecting the same patients was not possible because of privacy and practical reasons. Multiple outcome measures that increase the risk of type I errors (findings of false 'significance'). Also, selection bias: care groups identified participating practices. It is possible that they selected the practices that they thought were providing the best quality of care. Non‐response was more common in the control group; more care groups in the control group did not understand the purpose of the supervision programme and therefore did not want to participate in our study. |
Pacaud 2012.
| Study characteristics | ||
| Methods |
Successful delivery of diabetes self‐care education and follow‐up through eHealth media RCT (NA clusters and NA providers), conducted in 1) Face‐to‐face meetings were held in a Diabetes Education Center. 2) Intervention led by trained physicians and diabetes educators. In Canada. 3 arms: 1. Control (face‐to‐face education) (control arm) and 2. Intervention 1 (web static education) (intervention arm), 3. Intervention 2 (web interactive education) (other arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 24, 30, NA Diabetes type: 2 Mean age: 54.2 ± 11.05 % Male: 47.06 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (face‐to‐face education) 1) Patient education Intervention arm: (web static education) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: (web interactive education) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported through a grant from the Lawson Foundation and the support of the IS Department of the University of Lethbridge | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. After informed consent, participants were randomly assigned to 1 of 3 education/follow‐up models. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There was also no significant difference between groups for age of patient (F[2, 65] = 1.04, P = 0.358), or gender of the patient (X2 [2] = 0.90, P = 0.638) at baseline. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. The baseline A1C values were not statistically different across the 3 experimental groups (A1C mean = 7.11, SD = 1.8; F[2, 65] = 0.307, P = 0.737). |
| Incomplete outcome data (attrition bias) | High risk | There tended to be more dropouts in the Web Static group than the other 2 groups (25% for the Web Static group vs 16% for controls and 3.3% for Web Interactive). Numbers unbalanced. Reasons for loss not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The only outcome of interest to us is objective (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. However, there are no standard error bars on Figure 1. Also, Quote: "Results using an intent‐to‐treat approach showed no overall significant differences between the 3 groups in A1C at the end of the study (Fig. 1). However, a trend for a group by time by gender interaction for change in A1C from baseline to the final visit was found." This stratified analysis by gender was not planned in methods and nothing was stated in the introduction about its importance. |
| Risk of contamination (other bias) | Unclear risk | All individuals who entered the BHL participated in an initial face‐to‐face assessment session (approximately 60 to 90 minutes) with a trained clinician. After this initial education session, potential participants were screened. The same educators were responsible for the follow‐up of the patients in all 3 groups. |
| Other bias | Low risk | None identified. |
Pape 2011.
| Study characteristics | ||
| Methods |
Team‐based care approach to cholesterol management in diabetes mellitus Cluster‐RCT (9 clusters with 68 providers), conducted in Providence Primary Care Research Network (PPCRN) in Oregon (not‐for profit integrated delivery system), USA Two arms: 1. Control arm (control arm) and 2. Intervention arm (intervention arm) |
|
| Participants | Control arm N: 4160 Intervention arm N: 2069 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Audit and feedback 2) Clinician reminders 3) Continuous quality improvement Intervention arm: 1) Audit and feedback 2) Team changes 3) Electronic patient registry 4) Clinician reminders 5) Patient education 6) Continuous quality improvement |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 2080 (50), post 2950 (60) Intervention arm: pre 952 (46), post 1535 (75) 2) HbA1c, mean % (SD) Control arm: pre NR (NR), post 7.1 (NR) Intervention arm: pre NR (NR), post 7.2 (NR) 3) SBP, mean mmHg (SD) Control arm: pre NR (NR), post 127.0 (NR) Intervention arm: pre NR (NR), post 128.0 (NR) 4) DBP, mean mmHg (SD) Control arm: pre NR (NR), post 73.0 (NR) Intervention arm: pre NR (NR), post 73.0 (NR) 5) LDL, mean mg/dL (SD) Control arm: pre 107.0 (33.0), post 95.0 (NR) Intervention arm: pre 104.0 (32.0), post 83.0 (NR) 6) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre NR (NR), post 2409 (49) Intervention arm: pre NR (NR), post 1126 (55) |
|
| Funding source | This study was supported with grants from the Merck Foundation and Providence Health Plan | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | They state they used secure cluster allocation and provide a reference. Upon checking the reference, there was nothing that described this allocation procedure. Cluster. |
| Provider's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Insurance status (P = 0.02) and frequency of LDL testing (P = 0.04). |
| Patient's baseline outcomes (selection bias) | Unclear risk | LDL (0.17) and do not provide baseline values for HbA1c, SBP, etc. |
| Incomplete outcome data (attrition bias) | High risk | State it was an intention‐to‐treat analysis (but more of a per‐protocol analysis since they exclude those due to attrition). Baseline based on those randomised. 'Open' cohort: additional people added after and analysed. Did not provide reasons for attrition. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described. Objective laboratory methods not described |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | They stated that they used clustering, which would limit contamination. Quote: "...there was the limited possibility of contamination bias because the clinics randomized…" Cluster. |
| Other bias | Low risk | Information not available. |
Parsons 2019.
| Study characteristics | ||
| Methods |
Effect of structured self‐monitoring of blood glucose, with and without additional TeleCare support, on overall glycaemic control in non‐insulin treated type 2 diabetes: the SMBG Study, a 12‐month randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted across 16 sites, 9 of which were general practices and 7 of which were based within hospitals across Wales and England. 2) Study nurses and physicians in United Kingdom 3 arms: 1. Control (usual care ‐ No SMBG) (control arm) and 2. Intervention (SMBG alone) (intervention arm), 3. Intervention (SMBG plus TeleCare) (other arm) |
|
| Participants | Control arm N: 151 Intervention arm N: 147, 148, NA Diabetes type: 2 Mean age: 61.72 ± 11 % Male: 58 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care ‐ no SMBG) 1) Patient education 2) Promotion of self‐management Intervention arm: (SMBG alone) 1) Case management 2) Clinician education 3) Patient education 4) Promotion of self‐management Intervention arm: (SMBG + TeleCare) 1) Case management 2) Electronic patient registry 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by the European Foundation for the Study of Diabetes with additional support by way of SMBG monitoring equipment and an unrestricted grant by Roche Diabetes Care GmbH | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed remotely by Swansea Trials Unit via email using a central database. |
| Allocation concealment (selection bias) | Unclear risk | Study site and previous experience of using SMBG (no) were used as stratifying factors for randomisation. The allocation sequence was generated dynamically to maintain an approximate balance of 1:1:1 across the 3 groups overall. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values provided, no information in text. Baseline education level looks to be different. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. No P values provided, no information in text. Baseline outcome values appear similar. |
| Incomplete outcome data (attrition bias) | High risk | Figure 2. 35/151 (23%) lost in control arm, 48/147 (33%) lost in intervention arm 1 and 40/148 (27%) lost in intervention arm 2. Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome objectively measured (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Secondary outcome measures not provided at time of registration |
| Risk of contamination (other bias) | Low risk | Study site and previous experience of using SMBG (no) were used as stratifying factors for randomisation. |
| Other bias | Low risk | No evidence of other risk of bias. |
Patja 2012.
| Study characteristics | ||
| Methods |
Health coaching by telephony to support self‐care in chronic diseases: clinical outcomes from the TERVA randomized controlled trial Patient RCT (7 providers), conducted with patients identified from primary care and hospital registries in the Paijat Hame region in Southern Finland, Finland Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 501 Intervention arm N: 1034 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1a) Controlled hypertension (DBP < 85 mmHg), N under control (%) Control arm: pre NR (NR), post 49 (38) Intervention arm: pre NR (NR), post 120 (45) 1b) Controlled hypertension (SBP < 140 mmHg), N under control (%) Control arm: pre NR (NR), post 53 (36) Intervention arm: pre NR (NR), post 107 (33) |
|
| Funding source | Joint Authority for Päijät‐Häme Social and Health Care Sitra ‐ the Finnish Innovation Fund TEKES ‐ the Finnish Funding Agency for Technology and Innovation Pfizer Oy | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | ~19% lost to follow‐up in N1, ~21% lost in N2, some provided reasons, but also grouped no information available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective laboratory methods to measure primary outcome of SBP/DBP not reported. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes do not match protocol and vice versa. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Unclear risk | Measurement bias (information bias). |
Perez‐Escamilla 2015.
| Study characteristics | ||
| Methods |
Impact of a community health workers‐led structured program on blood glucose control among Latinos with type 2 diabetes: the DIALBEST trial RCT (NA clusters and NA providers), conducted in 1) The community health workers (CHWs) visited the treatment group participants at home. 2) Intervention delivered by 2 well‐trained and supervised bilingual/bicultural CHWs. In United States of America. 2 arms: 1. Control (standard of healthcare) (control arm) and 2. Intervention (CHW home visits: tailored education and counseling) (intervention arm) |
|
| Participants | Control arm N: 106 Intervention arm N: 105, NA, NA Diabetes type: 2 Mean age: 56.3 ± 5.79 % Male: 26.5 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (standard of healthcare) 1) Clinician education 2) Promotion of self‐management Intervention arm: (CHW home visits: tailored education and counseling) 1) Audit and feedback 2) Case management 3) Team change 4) Clinician education 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein |
|
| Funding source | DIALBEST was funded by the NIH Minority Health and Health Disparities Institute (grant number P20‐MD‐001765 to R.P.‐E., principal investigator). R.P.‐E. received funding support for this publication from the Yale Center for Clinical Investigation through Clinical and Translation Science Award grant UL1‐TR‐ 000142 from the National Center for Advancing Translational Sciences, a component of NIH. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation involving randomly selected block sizes of 4 was implemented through computer‐generated binary random group assignment. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Block randomisation involving randomly selected block sizes of 4 was implemented through computer‐generated binary random group assignment. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. There were no significant between‐group differences in any of the demographic and socioeconomic variables compared at baseline (Table 1). |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. All P values are above 0.05 except for systolic blood pressure (P = 0.001). At baseline, blood glycaemic and lipid profiles were not different between groups, but mean systolic blood pressure was significantly higher in the intervention group yet within normal limits. |
| Incomplete outcome data (attrition bias) | High risk | 63 lost to follow‐up out of 211 (29.9%): 34.9% in the control group and 24.8% in the intervention. Numbers not balanced. Reasons not balanced (blood draw refusal). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are objective (HbA1c, SBP and LDL) and authors state blinding of assessors. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (first posted on February 2011, participants were enrolled from December 2006 to February 2009, 18‐month intervention). Only HbA1c and diabetes knowledge were planned as outcomes in the protocol. However, the authors reported data for SBP, LDL, HDL, triglycerides, total cholesterol, BMI, etc. Also, there are no data about diabetes knowledge in the paper. |
| Risk of contamination (other bias) | High risk | Both arms received many QIs. Quote: "Control group participants did not receive the CHW intervention but were also visited at home for data collection, including HbA1c assessment." This may explain why HbA1c also declined in this group, biasing findings toward the null hypothesis. Also, it is not excluded that the providers learned from meetings with CHWs and that they adapted their usual care. Quote: "Because CHWs were integrated as part of the healthcare management team, it is possible that healthcare providers adjusted the treatment of patients accordingly." |
| Other bias | Low risk | No evidence of other bias. |
Perria 2007.
| Study characteristics | ||
| Methods |
Implementing a guideline for the treatment of type 2 diabetics: results of a cluster‐randomized controlled trial (C‐RCT) Cluster‐RCT (252 clusters with 252 providers), conducted in primary care setting of Italian National Health Service in Lazio region of Central Italy, Italy Three arms: 1. Control (control arm), 2. Passive dissemination (intervention arm 1) and 3. Active dissemination (intervention arm 2) |
|
| Participants | Control arm N: 2232 Intervention arm 1 N: 2106 Intervention arm 2 N: 1952 Diabetes type: type 2 Mean age: 65.0 ± 10.0 (estimated based on distribution of data) % Male: 52.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Clinician education Intervention arm 2: 1) Clinician education |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 512 (23), post 507 (23) Intervention arm 1: pre 530 (24), post 523 (24) Intervention arm 2: pre 494 (25), post 525 (27) 2a) Renal screening (creatinine), N screened (%) Control arm: pre 1052 (47), post 1124 (50) Intervention arm 1: pre 1067 (49), post 1129 (52) Intervention arm 2: pre 1078 (55), post 1070 (54) 2b) Renal screening (microalbumin), N screened (%) Control arm: pre 219 (10), post 265 (12) Intervention arm 1: pre 191 (9), post 229 (10) Intervention arm 2: pre 208 (11), post 271 (14) |
|
| Funding source | The study is funded by the Italian Ministry of Health ("Special Programs" art. 12 bis D.lgs 229/99) and the Lazio Region | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | See Table 1. Age P < 0.05. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. No P values provided and some columns appear unbalanced. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 2. No P values provided and some columns appear unbalanced. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Perry 1997.
| Study characteristics | ||
| Methods |
Lifestyle intervention in people with insulin‐dependent diabetes mellitus (IDDM) Cross‐over RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from the Dunedin Public Hospital Diabetes Clinic and from those responding to a request for volunteers in a radio interview, and in local newspaper advertisements. The study was conducted in the Department of Human Nutrition at the University of Otago in Dunedin, New Zealand. Physical fitness appraisal and training programmes were conducted by the School of Physical Education at the University of Otago. 2) Participants met with the research team at least monthly. No details on team members' expertise (from the department of nutrition, medicine and mathematics). In New Zealand. 2 arms: 1. Control (Group 2: standard care) (control arm) and 2. Intervention (Group 1: intensive lifestyle education) (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 34, NA, NA Diabetes type: 1 Mean age: 42.14 ± 10.03 % Male: 57.38 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (intensive lifestyle education) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This study was supported by the Eli Lilly Research Grant (Eli Lilly and Company (New Zealand) Ltd), The Deans Research Grant (Otago Medical School, New Zealand) and The New Zealand Dietetic Association (Abbott Award) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Participants were randomised after stratification for age and gender to either Group 1 or Group 2. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values. Data only for those who completed the study. Looks like there are more males in group 2. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 3. No P values. Data only for those who completed the study. |
| Incomplete outcome data (attrition bias) | High risk | They lost a total of 9 out of 70 patients randomised (12.9%), 3 out of 34 in Group 1 (8.8%) and 6 out of 36 in Group 2 (16.7%). Numbers unbalanced. 61 participants completed the study (31 in Group 1 and 30 in Group 2). 9 participants (3 from Group 1 and 6 from Group 2) did not complete the study. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. No data on BMI. |
| Risk of contamination (other bias) | Low risk | Intervention delivered by research team, unlikely that control group would receive intervention. |
| Other bias | Low risk | No evidence of other bias. |
Persell 2008.
| Study characteristics | ||
| Methods |
Patient‐directed intervention versus clinician reminders alone to improve aspirin use in diabetes: a cluster randomized trial. Cluster‐RCT (19 clusters with 19 providers), conducted in a group practice affiliated with an academic medical centre, USA Two arms: 1. Clinician reminders only (control arm) and 2. Patient intervention plus reminders (intervention arm) |
|
| Participants | Control arm N: 157 Intervention arm N: 177 Diabetes type: unclear/not reported Mean age: 57.9 ± 10.8 % Male: 37.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Electronic patient registry 2) Clinician reminders Intervention arm: 1) Electronic patient registry 2) Clinician reminders 3) Patient education 4) Patient reminders |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre NR (NR), post 44 (39) Intervention arm: pre NR (NR), post 60 (46) |
|
| Funding source | Northwestern University supported the study. It had no role in the design, conduct, interpretation or decision to submit for publication. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Aspirin use similar for 2 groups at baseline. |
| Incomplete outcome data (attrition bias) | High risk | > 30% dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Self‐reported aspirin use, potential for bias. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Peters 1991.
| Study characteristics | ||
| Methods |
Clinical evaluation of decision support system for insulin‐dose adjustment in IDDM RCT (NA clusters and NA providers), conducted in 1) The study was delivered at the Helbachtal Diabetes Education Centre in Germany. 2) Intervention delivered by an educational team (diabetes educators) or using a computerised algorithm. In Germany. 2 arms: 1. Control (education team recommendations) (control arm) and 2. Intervention (learning memory system‐computerized algorithms) (intervention arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 25, NA, NA Diabetes type: 1 Mean age: 33.4 ± 10.83 % Male: 45.24 Longest follow‐up: 1.05 months |
|
| Interventions |
Control arm: (education team recommendations) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: (learning memory system‐computerised algorithms) 1) Patient education 2) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | The design and development of the learning memory system by the Department of Internal Medicine, Medical University of Lübeck, was supported by grants from the Deutsche Forschungsgemeinschaft as a postgraduate scholarship 1986‐1989 (A.P.) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients without exclusion criteria were randomly assigned to an experimental or control group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2 shows the baseline features of the patients at randomisation, and no significant differences existed between the groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2 shows the baseline features of the patients at randomisation, and no significant differences existed between the groups. |
| Incomplete outcome data (attrition bias) | High risk | 50 patients entered the study. 4 patients from the experimental group were excluded according to the criteria (2 patients removed the microchip and 2 patients developed acute infectious disease). To avoid imbalance between the 2 groups, 4 patients from the control groups were excluded randomly. They lost a total of 8 patients out of 50 (16%). Reasons not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and hypoglycaemia: proportion of blood glucose < 3.3 mM). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Results match methods. |
| Risk of contamination (other bias) | Low risk | The experimental group had the opportunity to discuss every problem, except insulin‐dose adjustments, with the physicians and nurses on the team. They had to determine their insulin dosage by themselves, supported only by the (computer) learning memory system. It would have been unethical from the physicians and the nurses to not help a patient with serious insulin titration problem. |
| Other bias | Low risk | None identified. |
Peterson 2008.
| Study characteristics | ||
| Methods |
Improving diabetes care in practice: findings from the TRANSLATE trial Cluster‐RCT (24 clusters with 238 providers), conducted in community care practices, USA Two arms: 1. Control clinics (control arm) and 2. Intervention clinics (intervention arm) |
|
| Participants | Control arm N: 3819 Intervention arm N: 4588 Diabetes type: type 2 Mean age: 62.8 ± 0.9 % Male: 50.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Audit and feedback Intervention arm: 1) Audit and feedback 2) Electronic patient registry 3) Clinician education 4) Clinician reminders 5) Patient reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam) 2) Foot screening 3) Renal screening (microalbumin) 4) HbA1c 5) SBP 6) LDL 7) Controlled hypertension (≤ 130 mmHg baseline and ≤ 140 mmHg final) |
|
| Funding source | This study was funded by the National Institute of Diabetes, Digestive, and Kidney Disorders, National Institutes of Health Grant 1 R18 DK061709‐01A1, and the National Institutes of Health under Contract HHSN268200425212C, “Re‐engineering the Clinical Research Enterprise” | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only says statistician. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2 ‐ no education reported, age and gender P > 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Retinopathy statistics significant. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Philis‐Tsimikas 2011.
| Study characteristics | ||
| Methods |
Peer‐led diabetes education programs in high‐risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial RCT (NA clusters and NA providers), conducted in 1) Intervention delivered in federally funded community health centres (Neighborhood Health Care and San Ysidro Family Health Centers) in San Diego, California, USA. 2) Intervention led by trained peer educators (individuals with diabetes who exemplified the traits of a natural leader were identified from the patient population and trained as promotoras over a 3‐month period). In United States of America. 2 arms: 1. Control (standard diabetes care) (control arm) and 2. Intervention (Project Dulce, peer education) (intervention arm) |
|
| Participants | Control arm N: 103 Intervention arm N: 104, NA, NA Diabetes type: 2 Mean age: 50.71 ± 9.44 % Male: 29.47 Longest follow‐up: 10 months |
|
| Interventions |
Control arm: (standard diabetes care) 1) Promotion of self‐management Intervention arm: (Project Dulce, peer education) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This study was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Grant (no. DK070666‐01A1), a National Center for Research Resources Grant (no. 1U54RR025204‐01‐01), and a grant from LifeScan and Johnson & Johnson. Glucose meters and strips were provided through an in‐kind donation by LifeScan. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked random assignment with equal allocation was used to assign participants to the control or Project Dulce groups using a randomly generated numbers sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05 except mean age with a P value of 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All clinical values have P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Supplementary Figure 1. They lost 16 out of 103 patients (15.5%) in the control group and 35 out of 104 (33.7%) patients in the intervention group. High and unbalanced numbers. It is possible that participants who remained in the study were more engaged in the intervention, therefore leading to overestimation of intervention effects. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Unclear if those in control and intervention group had the same primary care providers; interaction with intervention patients may have influenced care of control patients. |
| Other bias | Low risk | No evidence of other bias. |
Phillips 2005.
| Study characteristics | ||
| Methods |
An endocrinologist‐supported intervention aimed at providers improves diabetes management in a primary care site: improving primary care of African Americans with diabetes (IPCAAD) 7 Cluster‐RCT (345 clusters with 345 providers), conducted in Grady Medical Clinic, Emory University, Atlanta, GA, USA Four arms: 1. Control (control arm), 2. Reminders (intervention arm 1), 3. Feedback (intervention arm 2) and 4. Feedback + reminders (intervention arm 3) |
|
| Participants | Control arm N: 983 Intervention arm 1 N: 1043 Intervention arm 2 N: 1049 Intervention arm 3 N: 1063 Diabetes type: type 2 Mean age: 59.0 ± 13.0 % Male: 33.0 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Clinician education 2) Clinician reminders Intervention arm 2: 1) Audit and feedback 2) Clinician education Intervention arm 3: 1) Audit and feedback 2) Clinician education 3) Clinician reminders |
|
| Outcomes | 1) HbA1c 2) SBP 3) LDL |
|
| Funding source | This work was supported in pan by awards HS‐07922 and DK‐066204 (10 L.S.P.), RR‐00039 (10 L.S.P. and P.K.), DK‐07298 (to C.D.M. and M.K.R.), and DK‐062668 and RR‐017643 (to tvl.K. R.) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Data is provided with P values for age and gender in Table 1; none are significantly different between the groups. No information on education. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1 ‐ P values > 0.05 |
| Incomplete outcome data (attrition bias) | Unclear risk | Do not report numbers of patients who dropped out. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Low risk | Information not available. |
Phumipamorn 2008.
| Study characteristics | ||
| Methods |
Effects of the pharmacist's input on glycaemic control and cardiovascular risks in Muslim diabetes Patient RCT, conducted in a community hospital in Krabi Province, mid‐south Thailand Two arms: 1. Control (control arm) and 2. Study (intervention arm) |
|
| Participants | Control arm N: 68 Intervention arm N: 67 Diabetes type: type 1 and type 2 Mean age: 54.1 ± 12.4 % Male: 15.9 Longest follow‐up: 8 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Patient education 3) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.7 (1.6), post 8.1 (1.9) Intervention arm: pre 8.7 (1.5), post 7.9 (1.4) 2) LDL, mean mg/dL (SD) Control arm: pre 156.6 (32.3), post 165.7 (42.4) Intervention arm: pre 174.4 (48.1), post 159.1 (37.3) |
|
| Funding source | This study was supported by research grants from the Graduate School, Prince of Songkla University, and the Provincial Public Health Department of Krabi Province, Thailand | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Piatt 2010.
| Study characteristics | ||
| Methods |
3‐year follow‐up of clinical and behavioral improvements following a multifaceted diabetes care intervention: results of a randomized controlled trial Clustered RCT (11 clusters and 24 providers), conducted in 1) This study took place in an underserved suburb of Pittsburgh, Pennsylvania. 11 primary care practices, and their patients, were randomised. 2) Intervention delivered by an endocrinologist and certified diabetes educators. In United States of America. 3 arms: 1. Control (usual care ‐ UC group) (control arm) and 2. Intervention 1 (provider intervention ‐ PROV group) (intervention arm), 3. Intervention 2 (chronic care model intervention ‐ CCM group) (other arm) |
|
| Participants | Control arm N: 51 Intervention arm N: 38, 30, NA Diabetes type: 4 Mean age: 67.54 ± 9.39 % Male: 50.42 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: (usual care) 1) Audit and feedback 2) Team change 3) Electronic patient registry 4) Clinician education Intervention arm: (provider intervention ‐ PROV group) 1) Audit and feedback 2) Team change 3) Electronic patient registry 4) Clinician education Intervention arm: (chronic care model intervention ‐ CCM group) 1) Audit and feedback 2) Case management 3) Team change 4) Electronic patient registry 5) Clinician education 6) Patient education Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | "We acknowledge the University of Pittsburgh Diabetes Institute, the University of Michigan DRTC, the Lions District 14B and 14E, the local hospital foundation, and the UPMC Division of Community Health Services. Portions of this research were sponsored by funding from the United States Air Force administered by the US Army Medical Research Acquisition Activity, Fort Detrick, Maryland, Award Number W81XWH‐04‐2‐003. Review of material does not imply Department of the Air Force endorsement of factual accuracy or opinion. This work utilized the Behavioral, Clinical, Health Services (BCHS) Core of the Michigan Diabetes Research and Training Center funded by Grant No. NIH5P60DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Upon completion of the chart audit, practices were randomised into 1 of 3 study groups (Figure 1). A block randomisation procedure was used with practice size (determined by the number of people with diabetes in each practice) as the blocking factor. Three practices received the CCM intervention; 3 practices received only provider education (PROV), and 5 practices received usual care (UC). |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2 in reference 14. Age has a P value of 0.04 (patients are older in the CCM group). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3 in reference 14. Data appear balanced. |
| Incomplete outcome data (attrition bias) | High risk | For HbA1c, SBP and DBP, there are data for 57 patients out of 119 randomised (52% lost). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol, but reference 14 is cited as the protocol. No data about diabetes knowledge and empowerment (secondary outcomes) in the paper. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. |
| Other bias | Low risk | No evidence of other bias. |
Piette 2000.
| Study characteristics | ||
| Methods |
Do automated calls with nurse follow‐up improve self‐care and glycemic control among vulnerable patients with diabetes? Patient RCT, conducted in 2 general medicine clinics of a county health care system, USA Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 140 Intervention arm N: 137 Diabetes type: unclear/not reported Mean age: 55.0 ± 10.0 % Male: 41.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.6 (1.8), post 8.3 (1.9) Intervention arm: pre 8.8 (1.8), post 8.2 (1.9) 2a) Harms (hyperglycaemic symptoms), median (IQR) Control arm: pre 2 (1 to 4), post 2 (1 to 4) Intervention arm: pre 2 (1 to 4), post 1 (0 to 3) 2b) Harms (hypoglycaemic symptoms), median (IQR) Control arm: pre 2 (0 to 3), post 2 (1 to 3) Intervention arm: pre 1 (0 to 3), post 1 (0 to 2) |
|
| Funding source | Supported by the Clinical Research Grants Program of the American Diabetes Association and by the Health Services Research and Development Service and Mental Health Strategic Health Group, Department of Veterans Affairs | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of randomly permuted numbers. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Adjusted for in their analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Only 11 dropouts in each group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Piette 2001.
| Study characteristics | ||
| Methods |
Impact of automated calls with nurse follow‐up on diabetes treatment outcomes in a Department of Veterans Affairs Health Care System: a randomized controlled trial Patient RCT, conducted in 4 university‐affiliated Veterans Affairs clinics in northern California, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 146 Intervention arm N: 146 Diabetes type: type 1 and type 2 Mean age: 60.5 ± 10.0 % Male: 97.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 41 (29), post 53 (38) Intervention arm: pre 69 (52), post 53 (40) 2) Foot screening, N screened (%) Control arm: pre 114 (81), post 101 (72) Intervention arm: pre 115 (87), post 95 (72) 3) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 8.1 (1.7), post 8.2 (0.1) Intervention arm: pre 8.2 (1.7), post 8.1 (0.1) |
|
| Funding source | This research was supported by the Health Services Research and Development Service, Mental Health Strategic Health Care Group, and Quality Enhancement Research Initiative, Department of Veterans Affairs, and by the American Diabetes Association | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Adjusted for in their analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Piette 2011.
| Study characteristics | ||
| Methods |
A randomized trial of telephone counseling plus walking for depressed diabetes patients Patient RCT, conducted in a community‐based non‐profit healthcare system, a university healthcare system and a VA healthcare system. In USA. Two arms: 1. Enhanced usual care (control arm) and 2. Cognitive behavioural therapy ‐ CBT (intervention arm) |
|
| Participants | Control arm N: 167 Intervention arm N: 172 Diabetes type: type 2 Mean age: 56.0 ± 10.1 % Male: 48.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.7 (1.7), post 7.7 (1.7) Intervention arm: pre 7.5 (1.7), post 7.7 (1.8) 2) SBP, mean mmHg (SD) Control arm: pre 133.8 (16.4), post 134.2 (20.6) Intervention arm: pre 136.0 (17.0), post 130.8 (17.7) 3) DBP, mean mmHg (SD) Control arm: pre 79.6 (11.1), post 78.2 (10.6) Intervention arm: pre 79.8 (10.4), post 76.4 (11.4) 4a) Controlled hypertension (DBP < 80 mmHg), N under control (%) Control arm: pre 74 (51), post 91 (62) Intervention arm: pre 71 (49), post 86 (59) 4b) Controlled hypertension (SBP < 130 mmHg), N under control (%) Control arm: pre 64 (44), post 69 (47) Intervention arm: pre 55 (38), post 73 (50) |
|
| Funding source | This study was supported by NIH grant # 5R18DK66166‐3, the Michigan Diabetes Research and Training Center (NIH #DK020572) and the Michigan Institute for Clinical and Health Research (NIH #UL1RR024986). John Piette is a VA Senior Research Career Scientist. At the time of the study, Caroline Richardson was supported by NIH training grant # K23 HL075098. Dana Striplin, M.P.H., managed all data collection and participated in data analysis. Her effort was supported by NIH grant # 5R18DK66166‐3. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | High risk | Information not available. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Low risk | Information not available. |
Pill 1998.
| Study characteristics | ||
| Methods |
A randomized controlled trial of an intervention designed to improve the care given in general practice to Type II diabetic patients: patient outcomes and professional ability to change behaviour Clustered RCT (29 clusters and 49 providers), conducted in 1) The study was carried out in 29 general practices in South Glamorgan who had participated for at least 2 years in a local scheme of audit and CME in relation to type 2 diabetes care. 2) An intervention team (GP, research nurse and clinical psychologist) was responsible for recruitment and training, and an evaluation team (sociologist and psychologist) was responsible for data collection and analysis. In United Kingdom. 2 arms: 1. Control (control group) (control arm) and 2. Intervention (audit and CME) (intervention arm) |
|
| Participants | Control arm N: 95 Intervention arm N: 95, NA, NA Diabetes type: 2 Mean age: 58.1 ± NR % Male: 50 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: Intervention arm: (audit and CME) 1) Audit and feedback 2) Clinician education 3) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | The project was funded by the Medical Research Council and the research group is now part of the MRC Health Services Collaboration | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. After recruitment, each practice was allocated by block randomisation independently to each arm of the trial. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. The unit of randomisation was the practice. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. There were more men in the intervention group (P = 0.011). All other characteristics balanced between groups. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. At Hospital B, HbA1c has a P value of 0.007. Glyco‐Hb readings were lower in intervention patients in one of the 2 hospital laboratories used (P = 0.007), although only a minority of practices sent blood samples to this laboratory. No data for blood pressure at baseline. |
| Incomplete outcome data (attrition bias) | High risk | Figure 2. More lost in the intervention group (18 out of 95, 18.9%) than control (7 out of 95, 7.4%). Reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Results match methods except that there are no data for blood pressure at baseline (secondary outcome). |
| Risk of contamination (other bias) | Low risk | Clustered RCT. The unit of randomisation was the practice. |
| Other bias | Low risk | No evidence of other bias. |
Pimazoni‐Netto 2011.
| Study characteristics | ||
| Methods |
Rapid improvement of glycemic control in type 2 diabetes using weekly intensive multifactorial interventions: structured glucose monitoring, patient education, and adjustment of therapy‐a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted at the Center for Hypertension and Cardiovascular Metabology, Kidney and Hypertension Hospital (outpatient diabetes clinic), Federal University of Sao Paulo, Sao Paulo, Brazil. 2) In the intensive treatment group, the patient would typically meet with the entire multidisciplinary team, involving the physician, diabetes nurse, diabetes educator, nutritionist, psychologist, physical therapists and exercise trainer. In Brazil. 2 arms: 1. Control (control group) (control arm) and 2. Intervention (intensive treatment group) (intervention arm) |
|
| Participants | Control arm N: 31 Intervention arm N: 32, NA, NA Diabetes type: 2 Mean age: 56.42 ± 10.99 % Male: 28.49 Longest follow‐up: 2.77 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: (intensive treatment group) 1) Case management 2) Team change 3) Clinician education 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Roche Diagnostics of Brazil provided Accu‐Chek Performa glucose meters, monitoring supplies, and the Accu‐Chek 360 software. Nothing reported about grant or funding. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. 32 patients were randomised to the intensive treatment group and 31 were assigned to the control group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. Data reported but no P values. Control group appears older. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Patients appear bigger in the intensive treatment group (higher weight and BMI). |
| Incomplete outcome data (attrition bias) | Low risk | 67 patients with T2DM being followed up. 3 patients were excluded because of noncompliance, and 1 patient failed to complete the study. Lost 6.0% of patients (4/67). Numbers in each arm not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The primary outcome was objectively measured (HbA1c) as hypoglycaemia (% of glucose values ≤ 60 mg/dL). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol but they refer to a previous pilot study (reference 19, poster). Results match methods. |
| Risk of contamination (other bias) | Low risk | Unlikely that control group received intervention. |
| Other bias | Unclear risk | Small sample size (n = 63). Short duration of the study (12 weeks). |
Pladevall 2015.
| Study characteristics | ||
| Methods |
A randomized controlled trial to provide adherence information and motivational interviewing to improve diabetes and lipid control RCT (NA clusters and NA providers), conducted in 1) Study participants were members of a large health system (Henry Ford Health System, primary care) serving southeast Michigan and metropolitan Detroit. 2) Clinicians trained in providing adherence information and nurses and pharmacists trained in providing motivational interviewing (MI). Six coaches (3 pharmacists and 3 registered nurses) passed the training; 4 of these coaches were also certified diabetes educators. In United States of America. 3 arms: 1. Control (UC ‐ usual care) (control arm) and 2. Intervention 1 (AI ‐ medication adherence information) (intervention arm), 3. Intervention 2 (AI + MI ‐ medication adherence information + motivational interviewing) (other arm) |
|
| Participants | Control arm N: 567 Intervention arm N: 569, 556, NA Diabetes type: 4 Mean age: 64.23 ± 9.3 % Male: 50.66 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: (UC ‐ usual care) Intervention arm: (AI ‐ medication adherence information) 1) Facilitated relay of clinical information 2) Patient education Intervention arm: (AI + MI ‐ medication adherence information + motivational interviewing) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | "This project was made possible through funding from the National Institute of Diabetes and Digestive and Kidney (R01DK064695 to Drs Pladevall and Williams), the National Institute of Allergy and Infectious Diseases (R01AI079139 to Dr Williams), and the National Heart Lung and Blood Institute (R01HL079055 and R01HL118267 to Dr Williams), National Institutes of Health and the Fund for Henry Ford Hospital (to Drs Pladevall and Williams)." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator was first used to randomly sort each participating physician’s list of enrolled patients. The order of treatment arm assignment was then randomly selected for each physician’s patient list of participating patients. The team statistician notified the project co‐ordinator, who provided this information on treatment assignment to the clinical team. |
| Allocation concealment (selection bias) | Low risk | A random number generator was first used to randomly sort each participating physician’s list of enrolled patients. The order of treatment arm assignment was then randomly selected for each physician’s patient list of participating patients. The team statistician notified the project co‐ordinator, who provided this information on treatment assignment to the clinical team. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. There were small but statistically significant differences in the mean age (P = 0.029). |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. There were small but statistically significant differences in baseline A1C levels (P = 0.036) and baseline high‐density lipoprotein cholesterol levels (P = 0.033) between study arms. |
| Incomplete outcome data (attrition bias) | Low risk | They lost a total of 180 patients out of 1692 randomised (10.6%). They lost 57/567 patients in the control arm (10.1%), 69/569 in the AI group (12.1%) and 54/556 in the AI + MI group (9.7%). Low and balanced numbers. Reasons partly reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and LDL). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. All outcomes reported. |
| Risk of contamination (other bias) | Low risk | None. |
| Other bias | Unclear risk | In order to meet recruiting goals, 2 different waves of patient recruitment were implemented on 19 June 2008 and 13 October 2008. Patient participation was poor, with slightly less than half (49%) of patients having 1 or more MI sessions at 18 months following randomisation. Moreover, patients who might have benefited the most from the intervention (i.e. individuals with the lowest levels of adherence) were also the ones least likely to participate (selection bias). Unfortunately, it was beyond the scope of the current study to objectively measure whether such discussions (between the provider and the patient) occurred or whether this information was ignored (medication adherence information). |
Planas 2009.
| Study characteristics | ||
| Methods |
Evaluation of a hypertension medication therapy management program in patients with diabetes Patient RCT, conducted in 5 pharmacies in Tulsa, OK, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 20 Intervention arm N: 32 Diabetes type: unclear/not reported Mean age: 64.6 ± 11.9 % Male: 36.5 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) SBP, mean mmHg (SD) Control arm: pre 145.4 (NR), post 148.1 (NR) Intervention arm: pre 141.8 (NR), post 124.4 (NR) |
|
| Funding source | By the American Pharmacists Association Foundation, the American Society of Health System Pharmacists Foundation and USA Drug Stores | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | More obese in intervention group. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Planas 2012.
| Study characteristics | ||
| Methods |
Evaluation of diabetes management program using selected HEDIS measures Patient RCT, conducted in a regional community pharmacy chain in Tulsa, Oklahoma, USA Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 27 Intervention arm N: 38 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 9 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.8 (1.0), post 7.9 (0.9) Intervention arm: pre 7.6 (1.0), post 7.1 (1.0) 2) SBP, mean mmHg (SD) Control arm: pre 141.1 (24.9), post 140.2 (20.0) Intervention arm: pre 139.2 (17.9), post 124.0 (16.9) 3) DBP, mean mmHg (SD) Control arm: pre 75.3 (12.6), post 74.9 (10.3) Intervention arm: pre 78.1 (10.3), post 73.7 (9.9) 4) LDL, mean mg/dL (SD) Control arm: pre 94.4 (38.2), post 90.5 (31.3) Intervention arm: pre 109.3 (36.8), post 97.3 (24.1) 5) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 5 (23), post 5 (23) Intervention arm: pre 6 (20), post 16 (53) |
|
| Funding source | American Society of Health‐System Pharmacists Research and Education Foundation, the American Pharmacists Association Foundation, and USA Drug Stores | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Previously generated random number list. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | BMI (P < 0.05); all other characteristics were similar between groups. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported in text or table. |
| Incomplete outcome data (attrition bias) | High risk | Large losses from both arms, reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: BP, using a aneroid sphygmomanometers at resting for period of 5 minutes. HbA1c measured using fingerstick DCA 2000, LDL using fingerstick Cholestech LDX, point of care after fasting for 9 to 12 hours. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | No evidence of other bias. |
Plaster 2012.
| Study characteristics | ||
| Methods |
Reduction of cardiovascular risk in patients with metabolic syndrome in a community health center after a pharmaceutical care program of pharmacotherapy follow‐up RCT (NA clusters and NA providers), conducted in 1) Community health centres in Vila Velha (ES, Brazil), 2) Initial interview and monitoring by the CHC team (including physician, nurse, nursing assistant, community agents and others), PC intervention via pharmacist in Brazil 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (pharmaceutical care programme) (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 38, NA, NA Diabetes type: 2 Mean age: NR ± 8.27 % Male: 34.28 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Case management 2) Team change Intervention arm: (pharmaceutical care programme) 1) Case management 2) Team change 3) Patient education |
|
| Outcomes | Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | This work was supported with funds from Rede Brasileira de Assistência Farmacêutica e Vigilância de Medicamentos from Instituto Nacional de Ciência e Tecnologia para Inovação Farmacêutica (REBRAFVIME / INCT_if) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation was performed in blocks of 6 patients each and stratified by gender through a computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no significant differences among the variables between the 2 groups (P > 0.05). |
| Patient's baseline outcomes (selection bias) | Low risk | Outcomes similar between groups. |
| Incomplete outcome data (attrition bias) | High risk | 19.5% loss in control group (29/36), 10.5% loss in intervention group (34/38), reasons for loss for all not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | SBP, DBP, LDL objective, antihypertensive and hypolipidaemic drugs subjective (from questionnaire). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol, outcomes match methods. |
| Risk of contamination (other bias) | Low risk | 1) The control group was typically accompanied by members of the CHC team (including physician, nurse, nursing assistant, community agents and others, but not a pharmacist) and only initially interviewed by the pharmaceutical care team to determine socio‐economic and cultural data, update the pharmacotherapeutic history and determine any negative outcomes associated with medication (NOM). 2) In more complex situations, the pharmacist discussed the problem with the consulting physician; this strategy is part of the Dáder method, and because some problems could have serious consequences, it was applied to the control group as well (Castro et al 2006). |
| Other bias | Low risk | None identified. |
Plotnikoff 2010.
| Study characteristics | ||
| Methods |
Multicomponent, home‐based resistance training for obese adults with type 2 diabetes: a randomized controlled trial Patient RCT, conducted with patients recruited from diabetes clinics at the University of Alberta Hospital and the local community, intervention was home‐based, Canada Two arms: 1. Control (control arm) and 2. Resistance‐training group (intervention arm) |
|
| Participants | Control arm N: 21 Intervention arm N: 27 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 4 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 6.8 (0.8), post 6.8 (0.8) Intervention arm: pre 6.9 (1.5), post 7.0 (1.4) 2) SBP, mean mmHg (SD) Control arm: pre 127.0 (12.6), post 126.7 (10.7) Intervention arm: pre 125.1 (12.7), post 122.4 (8.6) 3) DBP, mean mmHg (SD) Control arm: pre 75.0 (8.9), post 75.2 (7.9) Intervention arm: pre 75.3 (8.1), post 73.9 (7.3) 4) LDL, mean mg/dL (SD) Control arm: pre 101.3 (30.9), post 98.2 (30.9) Intervention arm: pre 102.1 (34.8), post 95.9 (34.8) |
|
| Funding source | RCP was supported by Salary Awards from the Canadian Institutes of Health Research (Applied Public Health Chair Program). RJS was supported by a Health Senior Scholar award from the Alberta Heritage Foundation for Medical Research. This study was funded by the Canadian Institutes of Health Research. Strategic Initiative in Excellence, Innovation and Advancement for the Study of Obesity and Healthy Body Weight. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated sequence." |
| Allocation concealment (selection bias) | Low risk | Quote: "Group assignment being placed into opaque, sealed envelopes that were opened by an individual unaware of the study rationale." |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: HbA1c (P = 0.831). |
| Incomplete outcome data (attrition bias) | High risk | ~14% lost to follow‐up in control and ~15% in intervention. Reasons seem balanced though. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Laboratory methods described for all outcomes, and study testers for outcomes were blinded to treatment allocation. |
| Selective reporting (reporting bias) | Low risk | Matches up with protocol. |
| Risk of contamination (other bias) | High risk | Hypothetically, individuals in the control group could have bought their own equipment at home, and also hired a personal trainer. |
| Other bias | Low risk | Information not available. |
Polonsky 2003.
| Study characteristics | ||
| Methods |
Integrating medical management with diabetes self‐management training: a randomized control trial of the Diabetes Outpatient Intensive Treatment program Patient RCT, conducted in a large hospital providing in‐ and outpatient care, USA Two arms: 1. EDUPOST (control arm) and 2. DOIT ‐ Diabetes Outpatient Intensive Treatment ‐ programme (intervention arm) |
|
| Participants | Control arm N: 98 Intervention arm N: 97 Diabetes type: type 1 and type 2 Mean age: 50.9 ± 15.6 % Male: 53.9 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 10.6 (1.9), post 8.7 (NR) Intervention arm: pre 10.2 (1.7), post 7.9 (NR) |
|
| Funding source | This study was funded in part by a grant from the U.S. Department of Defense | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Text and table show no differences. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | 30% dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Pouwer 2001.
| Study characteristics | ||
| Methods |
Monitoring of psychological well‐being in outpatients with diabetes: effects on mood, HbA(1c), and the patient's evaluation of the quality of diabetes care: a randomized controlled trial Patient RCT, conducted in diabetes clinic at university medical centre in the Netherlands Two arms: 1. Standard care (control arm) and 2. Monitoring (intervention arm) |
|
| Participants | Control arm N: 209 Intervention arm N: 191 Diabetes type: type 1 and type 2 Mean age: 53.5 ± 17.0 % Male: 47.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.8 (1.3), post 7.7 (1.1) Intervention arm: pre 7.8 (1.4), post 7.7 (1.1) |
|
| Funding source | This trial was funded by the Dutch Diabetes Research Foundation (grant no. 95.805) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Primary outcome: psychological measures and not assessed blindly. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Nurses delivered treatments to both groups; may have become better at detecting and discussing emotions thus changing characteristics of standard care condition. |
| Other bias | Low risk | Information not available. |
Powers 2009.
| Study characteristics | ||
| Methods |
The effect of a hypertension self‐management intervention on diabetes and cholesterol control Patient RCT (32 providers), conducted in primary care clinics, Durham Medical Centre, Veterans Study, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 114 Intervention arm N: 102 Diabetes type: unclear/not reported Mean age: 64.0 ± 10.8 % Male: 98.5 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 7.2 (0.2), post 7.4 (0.2) Intervention arm: pre 7.5 (0.2), post 7.3 (0.2) |
|
| Funding source | This research is supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service, investigator initiative grant 20‐034. The first author was supported by Grant Number KL2 RR024127 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIH, or Department of Veterans Affairs. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Prabhakaran 2019.
| Study characteristics | ||
| Methods |
Cluster randomised controlled trial of a theory‐based multiple behaviour change intervention aimed at healthcare professionals to improve their management of type 2 diabetes in primary care Clustered RCT (40 clusters and NR providers), conducted in 1) Recruited 20 community health centres (CHCs) each in Haryana (North India) and Karnataka (South India) for the trial. A CHC caters to a rural population of about 80,000 to 120,000 and serves as a referral centre for 4 primary health centres in the public healthcare delivery system. We selected the CHCs from 4 districts in Haryana and 2 districts in Karnataka that were covered under the National Program for the Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke (NPCDCS). Intervention delivered using mWellcare, an mHealth system consisting of electronic health record storage and an electronic decision support. 2) Intervention delivered by usual physicians and non‐communicable diseases (NCD) nurses. In India. 2 arms: 1. Control (EUC: enhanced usual care) (control arm) and 2. Intervention (mWellcare: mHealth‐Based Electronic Decision Support System) (intervention arm) |
|
| Participants | Control arm N: 924 Intervention arm N: 936, NA, NA Diabetes type: 2 Mean age: 55.1 ± NR % Male: 55.2 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (EUC: enhanced usual care) 1) Electronic patient registry 2) Clinician education 3) Patient education Intervention arm: (mWellcare: mHealth‐Based Electronic Decision Support System) 1) Audit and feedback 2) Electronic patient registry 3) Clinician education 4) Clinician reminder 5) Facilitated relay of clinical information 6) Patient education 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This research study was supported by the Wellcome Trust (grant 096735/A/11/Z). Dr Prabhakaran is partially supported through research grants from the National Institutes of Health (grants 5U01TW01009702, 5U2RTW01010804, 5R01HL12544204, 5R21DK10589102, and 5P20CA21029802). Dr Tandon is the principal investigator for an investigator‐initiated research grant from the National Institutes of Health (grant 5U01HL13863502). Dr Goenka was supported by Bernard Lown Scholars in Cardiovascular Health Program, Harvard School of Public Health (2015−2017), and Wellcome Trust (grant 203124/Z/16/Z) 2018. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The CHC served as the unit of randomisation. An independent biostatistician performed central computer‐based randomisation of CHCs stratified by states (Haryana and Karnataka) and within each state by the availability of NCD nurses recruited under NPCDCS. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. An independent biostatistician performed central computer‐based randomisation of CHCs. |
| Provider's baseline characteristics (selection bias) | High risk | The CHC (community health centre) served as the unit of randomisation. CHCs stratified by states (Haryana and Karnataka) and within each state by the availability of NCD nurses recruited under NPCDCS. No data reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No data only for diabetic patients. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Only HbA1c is reported only for the diabetic patients, and has a SMD, standardised mean difference, of 0.049. No P values. |
| Incomplete outcome data (attrition bias) | Unclear risk | No data only for diabetic patients. Overall, participants with baseline and end‐of‐study data: 1687/1856 (9% lost) for the control group and 1637/1842 (11% lost) for the intervention group. Reasons reported and quite balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c was objectively measured. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. Results match methods and protocol, except for cost‐effectiveness analysis. |
| Risk of contamination (other bias) | High risk | Cluster‐RCT, however, the overall null result, likely resulting from benefits achieved in the enhanced usual care arm. First, the presence of NCD nurses in both the EUC and mWellcare arms could have influenced the results. NCD nurses in the EUC arm proactively maintained a separate register for the trial patients and provided special attention to trial participants in terms of scheduling follow‐up visits and counseling on self‐management, adherence to medications, and regular monitoring of blood pressure and glucose levels. This is reflected in the marked improvement in the quality of care reported in the EUC arm as in the mWellcare arm. |
| Other bias | Low risk | No evidence of other risk of bias |
Presseau 2018.
| Study characteristics | ||
| Methods |
Cluster randomised controlled trial of a theory‐based multiple behaviour change intervention aimed at healthcare professionals to improve their management of type 2 diabetes in primary care Clustered RCT (44 clusters and 325 providers), conducted in 1) General practices in North‐East England and the general medical practitioners (GPs), nurses and healthcare assistants within them involved in providing care for type 2 diabetes participated. 2) Theory‐based multiple behaviour change intervention delivered by a content expert (nurse or MD) and a behaviour change expert (PhD health psychologist) to the primary care team: general medical practitioners (GPs), nurses and healthcare assistants (HCAs). The trial RA was also on hand at each session to manage practical arrangements. In United Kingdom. 2 arms: 1. Control (control arm) and 2. Intervention (IDEA: theory‐based behaviour change intervention) (intervention arm) |
|
| Participants | Control arm N: 8198 Intervention arm N: 7579, NA, NA Diabetes type: 2 Mean age: NR ± 9.48 % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: Intervention arm: (IDEA: theory‐based behaviour change intervention) 1) Clinician education |
|
| Outcomes | Foot screening | |
| Funding source | Diabetes UK (11/0004367). The funder did not play any role in the design or data collection, analysis, interpretation, or in writing the report or submitting the manuscript for publication. Researchers are independent of the funder. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were randomised 1:1 to intervention or control by the trial statistician using computer‐generated random permuted blocks with practice roster (list) size as the blocking factor. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. The study research associate assigned a unique study ID to each practice and then the trial statistician undertook the randomisation of these IDs. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table 1. Data reported, look similar between groups, but no P values. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. Only report data about % of patients > 65 years old. Nothing about gender, education, income, etc. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. % of patients examined for circulation and sensation in feet are reported at baseline; data look similar between groups (75% vs 74%), but no P values. |
| Incomplete outcome data (attrition bias) | Unclear risk | No clear data on the number of patients at baseline and on the number of patients lost. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | For the foot examination, outcome data were extracted from anonymised patient electronic records for all patients meeting inclusion criteria in each practice for 12 months before and 12 months after the intervention period (objective outcome). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. Results match protocol. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. The primary care practice was the unit of randomisation. Unlikely that health professionals from the control clinics received the behaviour change intervention. |
| Other bias | Low risk | No evidence of other risk of bias. |
Pressman 2014.
| Study characteristics | ||
| Methods |
A novel telemonitoring device for improving diabetes control: protocol and results from a randomized clinical trial RCT (NA clusters and NA providers), conducted in 1) Patients recruited at the Kaiser Permanente Northern California (KPNC) in the Santa Rosa, CA clinic. KPNC is an integrated healthcare delivery system with > 3.3 million members across a 14‐county region in northern California. Within the San Francisco and Greater Bay Area, approximately one‐third of insured adults receive their care through KPNC. 2) Intervention delivered by diabetes nurse care managers using a Samsung (Seoul, Korea) Health Diary (SHD) telemonitoring device. In United States of America 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (telemonitoring by nurses) (intervention arm) |
|
| Participants | Control arm N: 128 Intervention arm N: 126, NA, NA Diabetes type: 2 Mean age: 55.2 ± 10.87 % Male: 62 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Promotion of self‐management Intervention arm: (telemonitoring by nurses) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein |
|
| Funding source | This project was partially funded by a grant from the Samsung Group, Inc. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Utilising a blocked design with variable block sizes, participants were randomly assigned either to have an SHD installed in their home or to receive usual care. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Baseline data only for those who completed the study (Table 1). There were no significant differences between the 2 treatment arms in terms of age, gender, weight or body mass index at study entry. P values are not significant too. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Baseline data only for those who completed the study (Table 1). There were no baseline differences between groups in outcome measures of systolic BP, LDL‐C, fructosamine, HbA1c, or self‐efficacy score. P values are also not significant. |
| Incomplete outcome data (attrition bias) | High risk | Removed many patients after randomisation (ineligible, 11.4%). Baseline data only for those who completed the study (Table 1). The proportion of participants who were lost to follow‐up differed by treatment arm (9% telemonitoring versus 16% usual care; P < 0.05). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, SBP and LDL). Outcome assessments were made and analysed without knowledge of the randomisation allocation. |
| Selective reporting (reporting bias) | High risk | No registered protocol. Main outcome: BP control, but they only assessed SBP and not DBP. |
| Risk of contamination (other bias) | Low risk | Unlikely that control group received intervention. |
| Other bias | Low risk | None. |
Prestes 2017.
| Study characteristics | ||
| Methods |
Improving diabetes care at primary care level with a multistrategic approach: results of the DIAPREM programme Clustered RCT (30 clusters and 60 (30 physicians and 30 nurses) providers), conducted in 1) Primary care units (PCU) of La Matanza Health Secretariat, province of Buenos Aires, Argentina. 2) Physicians and nurses were randomly selected to be trained in Argentina 2 arms: 1. Control (traditional care) (control arm) and 2. Intervention (DIAPREM: DIAbetes Primary care, Registry, Education and Management) (intervention arm) |
|
| Participants | Control arm N: 157 Intervention arm N: 154, NA, NA Diabetes type: 2 Mean age: 55.2 ± 6.93 % Male: 33.73 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (traditional care) 1) Team change 2) Electronic patient registry Intervention arm: (DIAPREM: DIAbetes Primary care, Registry, Education and Management) 1) Team change 2) Electronic patient registry 3) Clinician education 4) Clinician reminder 5) Patient reminders |
|
| Outcomes | Lipid‐lowering drugs Antihypertensive drug Retinopathy screening Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | DIAPREM implementation was partially supported by a grant provided by the World Diabetes Foundation (WDF12‐761) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Of the 40 primary care units (PCU) of La Matanza Health Secretariat, they randomly selected 30 physicians and 30 nurses. Of those, 15 were randomly selected to be trained (IG group), and another group of 15 physicians and nurses from another 15 PCUs were also randomly selected and used as controls (CG group). |
| Allocation concealment (selection bias) | Unclear risk | Clustered RCT but the authors never use the term cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No provider and clinic characteristics reported. No evidence of block‐randomisation, pair‐matching or stratification to try to homogenise groups. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values between groups at baseline. Data only for the completers. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. No P values between groups at baseline. Data only for the completers. |
| Incomplete outcome data (attrition bias) | High risk | During the 1‐year follow‐up, patients who dropped out were significantly fewer in the intervention than in the control group (28% and 48%, respectively; P < 0.0003). No significant differences were found between clinical and metabolic characteristics of adherent compared to dropout patients in any of the groups (data not shown). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome was objectively measured (HbA1c) as well as BP and LDL. It is assumed that drug prescription were extracted from QUALIDIAB registry (objective). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. They reported many more outcomes than the main outcomes stated in the methods section. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. Each physician‐nurse team working in different clinics; unlikely that they have communicated. Quote: "Of the 40 primary care units (PCU) of La Matanza Health Secretariat, we randomly selected 30 physicians and 30 nurses. Of those, 15 were randomly selected to be trained (IG group), and another group of 15 physicians and nurses from another 15 PCUs were also randomly selected and used as controls (CG group)." |
| Other bias | Low risk | No evidence of other risk of bias. |
Prezio 2013.
| Study characteristics | ||
| Methods |
Community Diabetes Education (CoDE) for uninsured Mexican Americans: a randomized controlled trial of a culturally tailored diabetes education and management program led by a community health worker RCT (NA clusters and NA providers), conducted in 1) The study site was located in an urban faith‐based community health services clinic in Dallas, Texas, which served exclusively uninsured patients of predominately Mexican American origin. 2) Programme led by a community health worker. In United States of America. 2 arms: 1. Control (usual medical care) (control arm) and 2. Intervention (Community Diabetes Education (CoDE) program) (intervention arm) |
|
| Participants | Control arm N: 90 Intervention arm N: 90, NA, NA Diabetes type: 2 Mean age: 46.8 ± NR % Male: 39.45 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual medical care) 1) Patient education 2) Promotion of self‐management Intervention arm: (Community Diabetes Education (CoDE) program) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Anti‐platelet drugs Antihypertensive drug Retinopathy screening Foot screening Renal screening Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | This study was supported by funding from the University of Texas School of Public Health and the Institute for Faith Health Research‐Dallas | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to either the intervention or control groups using a computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. No significant differences in baseline demographic characteristics were found between the intervention and control groups, with the exception that significantly more control group participants were employed at study entry (P = 0.02; Table 2). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All outcomes have P values above 0.05. The 2 groups did not differ with respect to the baseline clinical measures HbA1c, systolic and diastolic blood pressures, lipid levels (LDL, HDL, triglycerides) or BMI. |
| Incomplete outcome data (attrition bias) | High risk | They only analysed the patients eligible to meet the process and outcome measures, thus over‐estimating the percentage of patients reaching each target. For aspirin, they report data for 55 to 59 patients (34% to 39% missing data) out of 90, 51 to 52 (42% to 43% missing data) for antihypertensive, 67 to 79 (12% to 26% missing data) for eye exam, 76 to 80 (11% to 16% missing data) for foot exam, 77 to 79 (12% to 14% missing data) for microalbumin testing and 78 to 79 (12% to 13% missing data) for hypertension under control. Also, 12/90 participants were lost to follow‐up in both groups (13.3%) largely because of becoming eligible for health insurance or moving out of town. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured: the 16 Modified Diabetes SQUID study indicators were compiled from process and outcome measures utilised by 2 practice‐based quality indices. |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol. No mention of the 16 Modified Diabetes SQUID study indicators tool in the protocol. SQUID indicators was calculated at 5 time points: baseline, 3 months, 6 months, 9 months and 12 months, however only baseline and 12 month values are reported. |
| Risk of contamination (other bias) | Unclear risk | Although both study groups received medical care within the same community clinic, the CHW functioned in an isolated, dedicated space that permitted interaction only with the intervention group, thereby minimising contamination between the 2 study groups to the greatest extent possible. All patients had education and they were all provided with blood glucose monitors and testing strips. The study groups could not be blinded to the physicians and this may in some way have influenced follow‐up schedules of the control group and prompted more aggressive treatment of hypertension and lipid abnormalities. |
| Other bias | Low risk | None. |
Pritchard 1999.
| Study characteristics | ||
| Methods |
Nutritional counselling in general practice: a cost effective analysis RCT (NA clusters and NA providers), conducted in 1) The study was conducted in a university group general practice set in a lower socioeconomic outer suburb of Perth, Western Australia. 2) Intervention delivered by a doctor and a dietitian or only by a dietitian. In Australia. 3 arms: 1. Control (usual care) (control arm) and 2. Intervention 1 (dietitian counselling group) (intervention arm), 3. Intervention 2 (doctor/dietitian counselling group) (other arm) |
|
| Participants | Control arm N: 6 Intervention arm N: 5, 6, NA Diabetes type: 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (dietitian counselling group) 1) Case management 2) Promotion of self‐management Intervention arm: (doctor/dietitian counselling group) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The research was funded by a grant from the Western Australian Health Promotion Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study dietitian used a table of random numbers to allocate each consecutive patient with a diagnosis of one or more of overweight, hypertension and type 2 diabetes to one of the 3 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | No data just for patients with diabetes. There was no significant difference between intervention and control groups with respect to sex or age. There was no significant difference between the groups by socioeconomic status quartiles or occupation. No significant differences were found between the 3 groups in the frequency of diagnoses. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Outcomes balanced between groups. |
| Incomplete outcome data (attrition bias) | High risk | Table 2. Among type 2 diabetes patients, 3 patients did not complete the study out of 17 (17.6%), 2 out of 5 in the dietitian group (40%) and 1 out of 6 in the doctor/dietitian group (16.7%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | High risk | No registered protocol. No data on blood pressure only in patients with diabetes. |
| Risk of contamination (other bias) | Unclear risk | The control group received the results of the initial measurements. Since doctors are involved in one of the 2 intervention groups, they may have changed their approach with their patients in the 2 other groups, including the control group. |
| Other bias | Low risk | No evidence of other bias. |
Quinn 2008.
| Study characteristics | ||
| Methods |
WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction Patient RCT, conducted in 3 community physician practices, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 15 Diabetes type: type 2 Mean age: 51.0 ± 11.0 % Male: 35.0 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.1 (NR), post 8.4 (NR) Intervention arm: pre 9.5 (NR), post 7.5 (NR) |
|
| Funding source | This study was supported by LifeScan, Inc. and Nokia, Inc. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Quinn 2011.
| Study characteristics | ||
| Methods |
Cluster‐randomized trial of a mobile phone personalized behavioral intervention for blood glucose control Cluster‐RCT (26 clusters with 39 providers), conducted in primary care practices in 4 distinct Maryland areas, USA Four arms: 1. Usual care (control arm), 2. Coach only (intervention arm 1), 3. Coach‐PCP portal (intervention arm 2) and 4. Coach‐PCP portal with decision support (intervention arm 3) |
|
| Participants | Control arm N: 62 Intervention arm 1 N: 38 Intervention arm 2 N: 33 Intervention arm 3 N: 80 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Clinician education 2) Promotion of self‐management Intervention arm 1: 1) Case management 2) Electronic patient registry 3) Clinician education 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders Intervention arm 2: 1) Case management 2) Electronic patient registry 3) Clinician education 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders Intervention arm 3: 1) Case management 2) Electronic patient registry 3) Clinician education 4) Clinician reminders 5) Facilitated relay of clinical information 6) Patient education 7) Promotion of self‐management 8) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.2 (1.7), post 8.5 (1.8) Intervention arm 1: pre 9.3 (1.8), post 7.7 (1.0) Intervention arm 2: pre 9.0 (1.8), post 7.9 (1.4) Intervention arm 3: pre 9.9 (2.1), post 7.9 (1.7) 2) SBP, mean mmHg (SD) Control arm: pre 130.0 (22.0), post 133.0 (20.0) Intervention arm 1: pre 130.0 (18.0), post 134.0 (25.0) Intervention arm 2: pre 133.0 (14.0), post 134.0 (16.0) Intervention arm 3: pre 130.0 (14.0), post 128.0 (19.0) 3) DBP, mean mmHg (SD) Control arm: pre 78.0 (12.0), post 79.0 (13.0) Intervention arm 1: pre 79.0 (11.0), post 82.0 (11.0) Intervention arm 2: pre 79.0 (9.0), post 78.0 (9.0) Intervention arm 3: pre 79.0 (9.0), post 78.0 (10.0) 4) LDL, mean mg/dL (SD) Control arm: pre 102.0 (36.0), post 91.0 (34.0) Intervention arm 1: pre 103.0 (29.0), post 94.0 (32.0) Intervention arm 2: pre 103.0 (33.0), post 94.0 (47.0) Intervention arm 3: pre 106.0 (33.0), post 102.0 (32.0) 5) Harms (adverse events: hypoglycaemia, hospitalisations, ED visits, deaths), N (%) Control arm: pre 0 (0), post 0 (0) Intervention arm 1: pre 0 (0), post 0 (0) Intervention arm 2: pre 0 (0), post 0 (0) Intervention arm 3: pre 0 (0), post 0 (0) |
|
| Funding source | This research project is funded through a contract between the University of Maryland Baltimore and WellDoc in addition to contributions by WellDoc, CareFirst Blue Cross/Blue Shield of Maryland, LifeScan, and Sprint. Additional funding was provided by the Maryland Industrial Partnerships program through the University of Maryland, an initiative of the A. James Clark School of Engineering’s Maryland Technology Enterprise Institute. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using a computer‐generated list of random numbers". |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not provided. |
| Patient's baseline characteristics (selection bias) | Unclear risk | In text, but not in table. |
| Patient's baseline outcomes (selection bias) | High risk | Quote: HbA1c (n4 ‐ 9.9% vs n1 ‐ 9.2%), P = 0.04. |
| Incomplete outcome data (attrition bias) | High risk | Although the authors state that "participant data were analyzed according to physician practices", original randomisation treatment assignment. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary: HbA1c, using medical charts, using Bayer DCA 200. Secondary: SBP, lipids, obtained from charts. Quote: "Patients and providers were not blinded, but outcome assessors were. HbA1c measured using the same A1c test device by trained staff who are blind to patient group assignment." From protocol. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Ralston 2009.
| Study characteristics | ||
| Methods |
Web‐based collaborative care for type 2 diabetes: a pilot randomized trial Patient RCT, conducted in University of Washing General Internal Medicine Clinic, USA Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 42 Diabetes type: type 2 Mean age: 57.3 ± NR % Male: 50.6 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Clinician reminders 5) Facilitated relay of clinical information 6) Patient education 7) Promotion of self‐management 8) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.9 (NR), post 8.1 (NR) Intervention arm: pre 8.2 (NR), post 7.3 (NR) |
|
| Funding source | This study was supported by a grant from the Center for Health Management Research. The Center for Health Management Research had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Study funded by an industry; author was consultant of the industry. |
Ramli 2016.
| Study characteristics | ||
| Methods |
Effectiveness of the EMPOWER‐PAR Intervention in Improving Clinical Outcomes of Type 2 Diabetes Mellitus in Primary Care: A Pragmatic Cluster Randomised Controlled Trial Clustered RCT (10 clusters and 25 providers), conducted in 1) This trial was conducted in 10 public primary care clinics from 2 states in Malaysia (developing country), which were Wilayah Persekutuan Kuala Lumpur (WPKL) and Selangor (SEL). 2) Intervention delivered by Chronic Disease Management (CDM) team (family medicine specialists, medical officers, medical assistants/nurses, pharmacists and dieticians/nutritionists). In Malaysia. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (EMPOWER‐PAR) (intervention arm) |
|
| Participants | Control arm N: 417 Intervention arm N: 471, NA, NA Diabetes type: 2 Mean age: 57.53 ± NR % Male: 37.03 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (EMPOWER‐PAR) 1) Audit and feedback 2) Case management 3) Team change 4) Clinician education 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Harms |
|
| Funding source | This study was funded by the Ministry of Higher Education (MOHE) Malaysia: Exploratory Research Grant Scheme (ERGS) no: ERGS/PHASE 1 ‐2011/(Health and Clinical Sciences)/(Universiti Teknologi MARA)/(JPT.S (BPKI) 2000/09/01/018 959) or 600‐RMI/ERGS 5/3 (28/2011) and by the Ministry of Health (MOH) Malaysia: Major Research Grant Scheme (NMRR ID ‐11‐250‐8769). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators used computer‐generated tables to randomly select 5 out of the 10 matched pairs to be included in the study. Then, one clinic in each pair was randomly allocated into the intervention or control arms. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Characteristics of the selected EMPOWER‐PAR intervention and control clinics are summarised in Table 2. Distributions of clinics in terms of geographical locations, workload and staffing were similar in both arms. Nothing about the experience of staff, gender, speciality. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 3. All P values above 0.05. The 2 groups were comparable in terms of age, gender distribution, ethnicity, education attainment, smoking status, coexisting hypertension, history of cardiovascular events (myocardial infarction, stroke and peripheral vascular disease), duration of T2DM and duration of hypertension. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 3. BMI and HDL‐c have P values under 0.05. The proportions of patients achieving biochemical targets were also comparable at baseline, except for triglycerides (TG). |
| Incomplete outcome data (attrition bias) | Low risk | 9 lost to follow‐up out of 417 in the control group (2.2%), and 16 out of 471 in the intervention group (3.4%). Low numbers, quite balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, BP, LDL) except hypoglycaemia (secondary outcome). |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol (posted in March 2012, study started in January 2012). All the other secondary outcome measures listed in the protocol are not reported in the paper including prescribing patterns. |
| Risk of contamination (other bias) | Low risk | Clustered‐RCT. |
| Other bias | Low risk | None. |
Rasmussen 2016.
| Study characteristics | ||
| Methods |
Telemedicine compared with standard care in type 2 diabetes mellitus: a randomized trial in an outpatient clinic RCT (NA clusters and NA providers), conducted in 1) Recruitment of patients happened at the first visit to the outpatient clinic of the Endocrinology Department, Kolding Hospital, referred by their general practitioners (GPs) for treatment. 2) Two nurses in the telemedicine group and 3 in the standard care group did most consultations. The doctor (O.W.R.) was sometimes present or asked for advice during consultations. In Denmark. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (telemedicine by nurses) (intervention arm) |
|
| Participants | Control arm N: 22 Intervention arm N: 18, NA, NA Diabetes type: 2 Mean age: 62.85 ± 7.8 % Male: 67.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard care) 1) Patient education Intervention arm: (telemedicine by nurses) 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The study received a grant from the Danish National Health Department of 2.5 million Danish Kroners, ID 211481/14. No other funding was given. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A person outside the Endocrinology Department performed the computer randomisation. |
| Allocation concealment (selection bias) | Low risk | A person outside the Endocrinology Department performed the computer randomisation. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. HbA1c and BMI have P values under 0.05 between arms at baseline. (Contradiction with text: no significant difference was seen in the clinical variables at inclusion). |
| Incomplete outcome data (attrition bias) | Low risk | No losses. All 40 included patients completed the study. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, BP, LDL). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol posted in August 2014, study started in October 2011). The paper did not report HDL and free fatty acids. The protocol did not list weight as outcome. |
| Risk of contamination (other bias) | High risk | Equivalence study. Both groups received the same intervention, except the intervention group met with nurses by telemedicine instead of face‐to‐face. |
| Other bias | Low risk | No evidence of other bias. |
Ratanawongsa 2014.
| Study characteristics | ||
| Methods |
Diabetes health information technology innovation to improve quality of life for health plan members in urban safety net Cross‐over RCT (NA clusters and NA providers), conducted in 1) Telephone call‐based intervention to member of the SFHP in San Francisco, California, USA. 2) “Out‐of‐range” responses triggered callbacks within 3 days from a language‐concordant SFHP lay health coaches. Health coaches – supervised by an SFHP registered nurse care manager – documented in the SFHP care management database system. In United States of America. 2 arms: 1. Control: wait‐list (control arm) and 2. Intervention: automated telephone self‐management support (ATSM)/health coaching (intervention arm) |
|
| Participants | Control arm N: 180 Intervention arm N: 182, NA, NA Diabetes type: 3 Mean age: 55.76 ± 9.8 % Male: 25.78 Longest follow‐up: 6.21 months |
|
| Interventions |
Control arm: (wait‐list) Intervention arm: (automated telephone self‐management support (ATSM)/health coaching) 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Disease Control and Prevention grant 5U58DP002007‐03; Health Delivery Systems Center for Diabetes Translational Research (CDTR) funded through NIDDK grant 1P30‐ DK092924; National Institute on Minority Health and Health Disparities #P60MD006902; and the McKesson Foundation. NIH grant UL1 RR024131 supports the UCSF Collaborative Research Network. No funders had any role in the study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit the manuscript for publication. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values greater than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Loss of 30%. Loss of 55 in wait‐list group, loss of 55 in intervention. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective HbA1c, BP, LDL measure. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. No mention of LDL measurement in protocol. |
| Risk of contamination (other bias) | Low risk | Wait‐list cross‐over RCT. |
| Other bias | Low risk | None. |
Rees 2017.
| Study characteristics | ||
| Methods |
Problem‐solving therapy for adults with diabetic retinopathy and diabetes‐specific distress: a pilot randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) 40 participants were recruited from retinal clinics at the Royal Victoria Eye and Ear Hospital (RVEEH), Melbourne, Australia. 2) The PST‐D intervention was conducted by a research assistant trained in problem‐solving therapy for diabetes (PST‐D) delivery, under the supervision of a clinical psychologist (BS). In Australia. 2 arms: 1. Control (care as usual) (control arm) and 2. Intervention (problem‐solving therapy) (intervention arm) |
|
| Participants | Control arm N: 19 Intervention arm N: 21, NA, NA Diabetes type: 2 Mean age: 59.9 ± 11.1 % Male: 67.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (care as usual) 1) Patient education Intervention arm: (problem‐solving therapy) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Australian Research Council (grant number LP0884108) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following baseline assessment, participants were randomised to the PST‐D intervention (n = 21) or control (n = 19) group using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Random number sequence was concealed using sealed envelopes by a clinical trials expert (external to the study team). |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. At baseline, the PST‐D and control groups were comparable in terms of sociodemographic. P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. At baseline, the PST‐D and control groups were comparable in terms of sociodemographic and clinical characteristics, as well depressive symptoms and diabetes distress scores, except for the DDS ‘diabetes‐related interpersonal distress’ subscale, which was significantly higher in the PST‐D group than the control group. P value HbA1c = 0.964. |
| Incomplete outcome data (attrition bias) | High risk | For HbA1c outcome, they lost 5 out of 21 patients (23.8%) in the intervention group and none in the control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol registered in August 2016, study started in August 2012). Our outcome of interests match between protocol and results. |
| Risk of contamination (other bias) | Low risk | Only the intervention arm had PST‐D intervention and usual physicians are not involved. |
| Other bias | Low risk | None identified. |
Reiber 2004.
| Study characteristics | ||
| Methods |
Diabetes quality improvement in Department of Veterans Affairs Ambulatory Care Clinics: a group‐randomized clinical trial Cluster‐RCT (14 clusters), conducted in general internal medicine clinics at Department of Veterans Affairs Medical Centres, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 607 Intervention arm N: 986 Diabetes type: type 1 and type 2 Mean age: 65.7 ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician education 2) Facilitated relay of clinical information |
|
| Outcomes | 1a) Controlled hypertension (DBP < 90 mmHg), N under control (%) Control arm: pre 249 (41), post 110 (18) Intervention arm: pre 513 (52), post 246 (25) 1b) Controlled hypertension (SBP < 140 mmHg), N under control (%) Control arm: pre 552 (91), post 536 (88) Intervention arm: pre 858 (87), post 806 (82) |
|
| Funding source | Funding for this supplement was provided by The Seattle Epidemiologic Research and Information Center and the VA Cooperative Studies Program. This research was supported by the Department of Veterans Affairs, Health Services Research and Development Service (SDR 96‐002 and IIR 99‐376). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported |
| Patient's baseline characteristics (selection bias) | High risk | Table 1. Significant differences in ethnic background and overall number of participants between groups. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. Significant differences in coronary heart failure and laser treatment between groups at baseline. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Reichard 1994.
| Study characteristics | ||
| Methods |
Changes in conceptions and attitudes during five years of intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study (SDIS) RCT (NA clusters and NA providers), conducted in 1) Telephone contact and office visits with tutor in Stockholm, Sweden. 2) Home blood glucose testing and telephone contacts with the physician/tutor (PR). ICT patients met with their tutor at his office every second month. In Sweden. 2 arms: 1. Control (regular treatment) (control arm) and 2. Intervention (intensified conventional treatment) (intervention arm) |
|
| Participants | Control arm N: 54 Intervention arm N: 48, NA, NA Diabetes type: 1 Mean age: 31.08 ± 9.42 % Male: NR Longest follow‐up: 60 months |
|
| Interventions |
Control arm: (regular treatment) 1) Promotion of self‐management Intervention arm: (intensified conventional treatment) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by grants from the Swedish Division of NOVO‐Nordisk, Boehringer Mannhetm Scand, and the Swedish Medical Research Council (06615) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The authors just mention that the groups were similar. The 2 treatment groups were similar with regard to sex ratio, age, duration, late complications, blood pressure, smoking habits, reported alcohol consumption and educational level. |
| Patient's baseline outcomes (selection bias) | Low risk | The authors just mention that the groups were similar. The 2 treatment groups were similar with regard to sex ratio, age, duration, late complications, blood pressure, smoking habits, reported alcohol consumption and educational level. |
| Incomplete outcome data (attrition bias) | Low risk | After 5 years, 96 patients were still participating in the study (44 patients in the ICT group and 52 patients in the RT group); 5 patients had died, and 1 had moved. Lost to follow‐up were 3.7% in the control group and 8.3 in the intervention group. The reasons for loss are explained. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match description. Comment on similarities between groups in terms of BP ‐ it would have been nice to see the numbers. |
| Risk of contamination (other bias) | High risk | Patient randomised. Both groups met with the same study physician. |
| Other bias | Low risk | None. |
Renner 2017.
| Study characteristics | ||
| Methods |
Pharmacist‐to‐prescriber intervention to close therapeutic gaps for statin use in patients with diabetes: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) community pharmacy in North Carolina 2) intervention delivered by pharmacists. In United States of America. 2 arms: 1. Control (Usual care) (control arm) and 2. Intervention (pharmacist‐to‐prescriber) (intervention arm) |
|
| Participants | Control arm N: 219 Intervention arm N: 257, NA, NA Diabetes type: 4 Mean age: 66.31 ± NR % Male: 49.33 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (pharmacist‐to‐prescriber) 1) Case management 2) Clinician education 3) Patient education |
|
| Outcomes | Lipid‐lowering drugs | |
| Funding source | APhA Foundation Incentive Grant | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We were able to randomise 476 of the 594 during our study period with the use of SAS 9.0. |
| Allocation concealment (selection bias) | Unclear risk | We were able to randomise 476 of the 594 during our study period with the use of SAS 9.0. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values > 0.05 |
| Patient's baseline outcomes (selection bias) | Low risk | All zero to start with. |
| Incomplete outcome data (attrition bias) | Low risk | None lost ‐ prescribing records review. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured ‐ statin prescribed or not. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, however outcomes in methods match results. |
| Risk of contamination (other bias) | High risk | In addition, prescribers could potentially have patients in both intervention and control groups. If a prescriber received the intervention message, they could have become sensitised to the gap in therapy and worked to close it for all their patients. |
| Other bias | Low risk | No evidence of other bias. |
Rickheim 2002.
| Study characteristics | ||
| Methods |
Assessment of group versus individual diabetes education: a randomized study RCT (NA clusters and NA providers), conducted in 1) The programme was delivered in a large outpatient diabetes centre (the International Diabetes Center, Park Nicollet Institute, Minneapolis, Minnesota) using a classroom setting for the groups and individual consult rooms for individual sessions. 2) A diabetes nurse specialist (RN) and diabetes nutrition specialist (RD) presented all 4 sessions in both settings. In United States of America. 2 arms: 1. Control (group educational settings) (control arm) and 2. Intervention (individual educational settings) (intervention arm) |
|
| Participants | Control arm N: 87 Intervention arm N: 83, NA, NA Diabetes type: 2 Mean age: 52.5 ± 9 % Male: 34.1 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (group educational settings) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management Intervention arm: (individual educational settings) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This research was funded by a grant from the Park Nicollet Institute, Park Nicollet Health Services, and an unrestricted educational grant from the American Association of Diabetes Educators, provided by Pfizer. Roche Diagnostics supplied blood glucose meters free of charge to all study participants. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients randomised in the order of scheduling. Participants were then randomly assigned to either a group or individual setting in block sizes of 6. The first 3 consecutive participants were assigned to the group setting, with the next 3 participants assigned to the individual setting. After the first year of recruitment, block size was increased to 10 to increase efficiency in scheduling while maintaining random and equal opportunity for allocation. |
| Allocation concealment (selection bias) | High risk | Allocation is predictable. The first 3 consecutive participants were assigned to the group setting, with the next 3 participants assigned to the individual setting. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All demographic data have P values above 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. HbA1c has a significant P value (< 0.01) as well as weight (0.02). With the exception of weight and HbA1c, baseline data on these participants did not differ significantly by educational setting. |
| Incomplete outcome data (attrition bias) | High risk | They lost 78 patients out of 170 (45.9%). The retention rate for the study participants was 54% at the 6‐month follow‐up visit, suggesting a need to better assess reasons for dropout from the education programme. To quantify these issues, they queried a subset of participants that did not return for the 6‐month follow‐up visit. These participants indicated a number of consistent reasons for withdrawal from the education programme, such as relocation, a schedule that would not permit them to leave work, other family commitments, or the perception that they were doing well and did not see the value in returning. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. However methods mention data were collected at baseline, 2 weeks, 3 months and 6 months for all patients entering the education programme, but only baseline and 6‐month values are reported in results. |
| Risk of contamination (other bias) | Low risk | Equivalence study. Both groups received the same education intervention, but that was planned this way. |
| Other bias | Low risk | None. |
Riddell 2016.
| Study characteristics | ||
| Methods |
Cardiovascular risk outcome and program evaluation of a cluster randomised controlled trial of a community‐based, lay peer led program for people with diabetes Clustered RCT (24 clusters and NR providers), conducted in 1) 24 geographic communities within the state of Victoria in Australia (Local Government Authorities (LGAs)) were selected, from which study participants were recruited. Monthly community‐based group meetings. 2) Intervention programme led by trained peer supporters. In Australia. 2 arms: 1. Control (routine care) (control arm) and 2. Intervention (peer support intervention) (intervention arm) |
|
| Participants | Control arm N: 120 Intervention arm N: 120, NA, NA Diabetes type: 2 Mean age: 60.9 ± NR % Male: 50.8 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (routine care) 1) Case management Intervention arm: (peer support intervention) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Funding and program support for this study was obtained from Peers for Progress, a global initiative funded by the American Academy of Family Physicians Foundation and the Eli Lily Foundation. Diabetes Australia‐Victoria provided funding and in kind program support. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reference 28: Allocation to intervention or usual care was governed by a random number generation process using Stata statistical software, Release 11. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | 24 study locations (“clusters”). No data on each location and peer supporters involved. Locations are suitable if the population was more than 10,000 and the density of NDSS registrants was more than 2.5%. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 and in text: The median duration of diabetes was 2 years longer in intervention participants compared with usual care participants (9 years vs 7 years, P = 0.01). |
| Patient's baseline outcomes (selection bias) | Low risk | Tables 2 and 3. There was higher baseline risk in the intervention arm, but no P values. |
| Incomplete outcome data (attrition bias) | High risk | They lost a total of 33 patients out of 240 (13,8%), 11 (9,2%) in the control group and 22 (18.3%) in intervention group. Unbalanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Primary outcome in the protocol is HbA1c and the predicted 5 year cardiovascular disease risk using the United Kingdom Prospective Diabetes Study (UKPDS), while in the paper it is just CVD risk score. No data reported at 18 months as stated in the protocol. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. |
| Other bias | Low risk | None. |
Ridgeway 1999.
| Study characteristics | ||
| Methods |
Improved control of type 2 diabetes mellitus: a practical education/behavior modification program in a primary care clinic RCT (NA clusters and NA providers), conducted in 1) The study was conducted at the University Physicians Practice Group ambulatory clinic in Kingsport, Tenn (primary care clinic). This is a private faculty clinic staffed by 5 board certified general internists who are full‐time faculty members of the East Tennessee State University James H. Quillen College of Medicine. These physicians provide longitudinal care for typical general internal medicine patients, 45% of whom are insured by Medicare carriers. 2) The education classes were held by a registered nurse with a Bachelor of Science degree and a registered dietitian, both of whom were certified diabetes educators. In United States of America. 2 arms: 1. Control (usual office visits) (control arm) and 2. Intervention (office‐based education/training classes) (intervention arm) |
|
| Participants | Control arm N: 28 Intervention arm N: 28, NA, NA Diabetes type: 2 Mean age: 63.58 ± NR % Male: 28.95 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual office visits) Intervention arm: (office‐based education/training classes) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | Supported by a grant from the Department of Medicine, James H. Quillen College of Medicine, East Tennessee State University, Johnson City | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients were divided randomly into 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values. Data reported only for completers. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. No P values. Triglyceride looks higher in intervention group (634 vs 381 in control group). Data reported only for completers. |
| Incomplete outcome data (attrition bias) | High risk | They lost 18 patients out of 56 randomised (32%). Ten patients dropped out of the intervention group because of intercurrent illness or failure to attend classes or to have adequate laboratory studies (36%). Eight patients were dropped from the control group because of failure to return for their usual appointments or because of significant intercurrent illnesses (29%). Calculation of LDL‐C was limited to 12 patients. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and LDL). |
| Selective reporting (reporting bias) | High risk | No registered or published protocol. At the monthly sessions, patients were informed of their weight, blood pressure and laboratory results. No data reported for blood pressure. |
| Risk of contamination (other bias) | Unclear risk | Only the intervention group had office visits. However, physicians were following patients in both the control and intervention group. Physicians were not "blinded" to the intervention. Physicians may have changed behaviour based on interactions with intervention group. |
| Other bias | Low risk | No evidence of other risk of bias. |
Rodriguez 2018.
| Study characteristics | ||
| Methods |
The impact of integrating medical assistants and community health workers on diabetes care management in community health centers Clustered RCT (16 clusters and NR providers), conducted in 1) Recruited 16 community health centres (CHCs) sites located in 3 counties in Northern California that were all affiliated with the same regional community clinic association. 2) Team‐based diabetes care management provided by community health workers (CHW) or medical assistants (MA). In United States of America. 4 arms: 1. Control 1 (control for Community Health Worker (CHW) group) (control arm) and 2. Control 2 (control for Medical Assistant (MA) group) (intervention arm), 3. Intervention 1 (Community Health Worker (CHW) group) (other arm), 4. Intervention 2 (Medical Assistant (MA) group) (other arm) |
|
| Participants | Control arm N: 2466 Intervention arm N: 2315, 686, 644 Diabetes type: 4 Mean age: NR ± 11.47 % Male: 41.9 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (control for Community Health Worker (CHW) group) Intervention arm: (control for Medical Assistant (MA) group) Intervention arm: (Community Health Worker (CHW) group) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Hypertension control Harms |
|
| Funding source | The Agency for Healthcare Research and Quality (AHRQ) funded the research project (1R18HS02012001) through the American Recovery and Reinvestment Act (ARRA). Publication made possible in part by support from the Berkeley Research Impact Initiative (BRII) sponsored by the UC Berkeley Library. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. Quote: "Using the response of the site director and mean responses to the clinician and staff surveys, we dichotomized practices into “high” (top eight) vs “low” (bottom eight) on each of the following composite measures: 1) diabetes structural capabilities, 2) primary care team functioning, 3) practice size (mean number of clinicians/staff: 18). Using cluster analyses, we grouped practices into three sampling strata based on the most common combinations of these three composite measures and randomly assigned practices within each strata to the MA intervention (n = 3), CHW (n = 3), and control (n = 10) group arms of the cluster‐randomised trial. A total of 167 randomly sampled patients per control practice and up to 400 patients at each of the intervention practices were sampled for the survey." |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT randomised at community health centre (CHC) level. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No data on community health centre (CHC) characteristics reported. The clinic randomisation process attempted to balance diabetes structural capabilities, primary care team functioning and practice size between the 2 intervention arms and control arm. There were substantial pre‐intervention differences in the overall quality of diabetes care between the arms based on the practices’ underlying patient characteristics that were not ascertained prior to site randomisation. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 2. No P values reported. Quote from text: "Patients in the MA study arms were older and more likely to be uninsured and of Asian descent compared to CHW arm patients. Patients of the CHW study arms were more likely to be of Latino descent and were more likely to have diagnosed and documented mental health co‐morbidities, e.g., depression and anxiety, than MA intervention arm patients." |
| Patient's baseline outcomes (selection bias) | Low risk | Tables 2, 3, 4 and 5. No P values reported between groups at baseline. Quote from text: "Patients of the CHW study arms were more likely to have higher BMI levels than MA intervention arm patients." |
| Incomplete outcome data (attrition bias) | Unclear risk | Of the 16 CHC sites that were initially randomised into the intervention and control groups, 2 sites (1 from the control group and 1 from the MA intervention group) dropped out of the research study during the early intervention period (January to February 2012) because of data reporting challenges that prevented them from fully participating. Number and reasons for patient lost not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blood pressure was objectively measured but hypoglycaemic events were self‐reported by patients in the survey. Most likely that patients were not blinded. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Look at many outcomes and no primary outcomes. |
| Risk of contamination (other bias) | Low risk | Clustered RCT at community health centre (CHC) level. Capacity building required of CHC participation may “contaminate” the study by improving control clinic documentation and care processes, but process improvements did not lead to improved intermediate outcomes for patients of control group clinics. |
| Other bias | High risk | Quote abstract: "A three‐arm cluster‐randomized trial of CHC sites integrating MAs (n = 3) or CHWs (n = 3) for diabetes care management compared control CHC sites (n = 10)". However, they reported data for 4 arms (and not 3). They have constructed separate matched control groups. Quote: "To address the potential bias of unbalanced data on estimating intervention effects and modest patient sample size that limited direct comparisons of these groups, separate control groups were constructed for the MA and CHW intervention groups using exact matching on a set of covariates." |
Rodriguez‐Idigoras 2009.
| Study characteristics | ||
| Methods |
Telemedicine influence on the follow‐up of type 2 diabetes patients RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from 35 family practices in the province of Malaga, Spain. 2) Intervention delivered with a Teleassistance system between patients and their physicians. Nurses were also involved. In Spain. 2 arms: 1. Control (regular follow‐up) (control arm) and 2. Intervention (teleassistance system and telephone consultations) (intervention arm) |
|
| Participants | Control arm N: 167 Intervention arm N: 161, NA, NA Diabetes type: 2 Mean age: 63.93 ± 8.6 % Male: 51.52 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (regular follow‐up) 1) Promotion of self‐management Intervention arm: (teleassistance system and telephone consultations) 1) Electronic patient registry 2) Clinician reminder 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Emminens company finances the research. This study has been funded by Roche Diagnostics Spain (Diabetes Care). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In order to ensure that each physician’s patients were randomly allocated in a balanced way, block randomisation was used, with an allocation sequence being generated by means of a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Lack details. Allocation was concealed, but, given the nature of the intervention, it could not be blind to participating physicians. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. Baseline characteristics of the individuals from both groups were similar (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. Baseline characteristics of the individuals from both groups were similar (Table 1). |
| Incomplete outcome data (attrition bias) | Low risk | They lost 16 patients out of 167 in the control group (9.6%) and 15 patients out of 161 in the control group (9.3%). Low numbers and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All our outcomes of interest were objectively measured (HbA1c, BP, LDL). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol posted in September 2007, study started in October 2003). Results match the protocol for our outcomes of interest. |
| Risk of contamination (other bias) | Unclear risk | An ACCU‐Chek Compact glucometer (Roche Diagnostics, Mannheim, Germany) was provided to all patients. Only the patients in the intervention arm had Teleassistance system. Each clinician was following patients in both arms. This information request at 3 months (HbA1c for all patients) may have made patients feel watched and thus led them to improve their care. |
| Other bias | Low risk | No evidence of other risk of bias. |
Rosal 2005.
| Study characteristics | ||
| Methods |
Diabetes self‐management among low‐income Spanish‐speaking patients: a pilot study RCT (NA clusters and NA providers), conducted in 1) The intervention was delivered in a community room well known to community residents and located approximately three blocks from the community health centre (CHC) and two blocks from the elder programme, both located in a large metropolitan area in western Massachusetts. 2) Intervention was delivered by a diabetes nurse, a nutritionist and an assistant (all bilingual). In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (self‐management group sessions) (intervention arm) |
|
| Participants | Control arm N: 10 Intervention arm N: 15, NA, NA Diabetes type: 2 Mean age: 62.6 ± NR % Male: 20 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Facilitated relay of clinical information 2) Patient education Intervention arm: (self‐management group sessions) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This project was supported by an American Diabetes Association Innovation Award supported in part by Novo Nordisk Pharmaceuticals | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Participants were grouped as closely as possible by age, gender and insulin status (whether or not they used insulin), and randomised to intervention or control in a 3:2 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. Data look similar. |
| Patient's baseline outcomes (selection bias) | High risk | Table 3. There is a big difference for HbA1c between control and intervention at baseline (9.3% vs 7.7%, respectively). No P values. |
| Incomplete outcome data (attrition bias) | High risk | They lost 2 patients out of 25 randomised (8%). 0 out of 15 patients in the intervention group (0%) and 2 out of 10 in the control group (20%). Unbalanced numbers. Completion rates for baseline, 3‐month and 6‐month assessments were 100%, 92% (23/25) and 92% (23/25), respectively. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Both groups received an education booklet, and copies of laboratory results following each assessment time point were sent to primary care providers for both groups. |
| Other bias | Low risk | None. |
Rosal 2011.
| Study characteristics | ||
| Methods |
Randomized trial of a literacy‐sensitive, culturally tailored diabetes self‐management intervention for low‐income Latinos: Latinos en control RCT (NA clusters and NA providers), conducted in 1) The first session was conducted as an individual 1‐hour meeting in the participant’s home. We conducted the remaining sessions in groups at centrally located community settings (e.g. a Latino centre, a senior centre, a YMCA site). 2) The intervention was delivered by a trained team of 2 leaders and an assistant (either a nutritionist or health educator and trained lay individuals or 3 lay individuals supervised by 2 investigators). In United States of America. 2 arms: 1. Control (enhanced usual care condition) (control arm) and 2. Intervention (Latinos en Control) (intervention arm) |
|
| Participants | Control arm N: 128 Intervention arm N: 124, NA, NA Diabetes type: 2 Mean age: NR ± 12.14 % Male: 23.4 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (enhanced usual‐care condition) 1) Facilitated relay of clinical information Intervention arm: (Latinos en Control) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant R18‐DK‐65985 and grants from the Robert Wood Johnson Foundation and Novo Nordisk Pharmaceutical (to M.C.R.) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Randomisation was at the individual level and stratified by site, sex, HbA1c level and insurance status. Within each strata, participants were randomised in randomly allocated blocks. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. High waist circumference and diastolic blood pressure have significant P values (P values of 0.031 and 0.011, respectively). |
| Incomplete outcome data (attrition bias) | Low risk | Reference 10: Overall retention, defined as the proportion of participants who completed at least one study assessment at the 12‐month follow‐up, was 93%. The small patient losses were due to refusal to continue to participate (n = 10), loss to follow‐up (n = 7) and death (n = 2). They lost 19 out of 252 patients (7.5%). No information for each arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | High risk | Study protocol previously published. (The primary outcome was HbA1c. Secondary outcomes were self‐management behaviours, weight, lipids and blood pressure. Additional outcomes included diabetes knowledge, self‐efficacy, depression and quality of life). In the paper, they saw no significant intervention effects on lipids, blood pressure, weight or waist circumference, but the data are not shown. They do not talk about depression and quality of life. |
| Risk of contamination (other bias) | Unclear risk | "Given the nature of the study, we could not blind participants’ PCPs; however, providers were not informed of their patients’ study assignments". Providers may have changed their approach. All providers (both conditions) received laboratory results, including HbA1c, fasting blood glucose and lipid profiles at baseline and at 4 and 12 month. |
| Other bias | Low risk | No evidence of other risk of bias. |
Rossi 2010.
| Study characteristics | ||
| Methods |
Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open‐label, international, multicenter, randomized study RCT (NA clusters and NA providers), conducted in 1) The study involved seven Diabetes Outpatient Clinics: 3 in Italy, 2 in England and 2 in Spain. 2) The course was provided as an outpatient programme of 3 encounters with the physician and/or dietitian in Italy 2 arms: 1. Control (standard carbohydrate counting educational programme) (control arm) and 2. Intervention (DID system) (intervention arm) |
|
| Participants | Control arm N: 63 Intervention arm N: 67, NA, NA Diabetes type: 1 Mean age: 35.74 ± NR % Male: 42.96 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (standard carbohydrate counting educational programme) Intervention arm: (DID system) 1) Case management 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | Funding for this study was provided by Me.Te.Da. and Lifescan, Milpitas, CA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed through a telephone call to the co‐ordinating centre. Random lists were stratified by centre. To ensure equal allocation rates within centres, permuted block randomisation was used. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was performed through a telephone call to the co‐ordinating centre. Random lists were stratified by centre. To ensure equal allocation rates within centres, permuted block randomisation was used. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 ‐ P values greater than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1 ‐ P values greater than 0.05 except for triglycerides at 0.03. |
| Incomplete outcome data (attrition bias) | High risk | Loss of 2 in control, loss of 9 in intervention group; 3% vs 13%, respectively. Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measures for HbA1c, cholesterol, BP, harms, subjective measure for hypo/hyperglycaemia |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Outcomes match methods. |
| Risk of contamination (other bias) | Low risk | There does not appear to be any overlap between groups |
| Other bias | Low risk | None identified. |
Rossi 2013.
| Study characteristics | ||
| Methods |
Impact of the "Diabetes Interactive Diary" telemedicine system on metabolic control, risk of hypoglycemia, and quality of life: a randomized clinical trial in Type 1 diabetes Patient RCT, conducted in 12 Italian diabetes outpatient clinics, Italy Two arms: 1. Standard care (control arm) and 2. DID (intervention arm) |
|
| Participants | Control arm N: 64 Intervention arm N: 63 Diabetes type: type 1 Mean age: 36.9 ± 10.5 % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Patient education Intervention arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 8.5 (0.1), post 8.1 (0.1) Intervention arm: pre 8.4 (0.1), post 7.9 (0.1) 2) SBP, mean mmHg (SE) Control arm: pre 120.0 (1.3), post 118.0 (1.6) Intervention arm: pre 119.0 (1.4), post 118.3 (1.6) 3) DBP, mean mmHg (SE) Control arm: pre 71.5 (1.0), post 71.7 (1.0) Intervention arm: pre 72.9 (1.0), post 72.2 (1.0) 4) LDL, mean mg/dL (SE) Control arm: pre 109.1 (3.6), post 114.1 (5.2) Intervention arm: pre 109.4 (3.7), post 117.6 (5.3) |
|
| Funding source | The study was supported by an unconditional grant from Sanofi‐Aventis SpA, Milan, Italy. Materials for SMBG (glucose meters, strips, lancets and control solutions) were supplied by LifeScan Inc., Milpitas, CA. Me.Te.Da. s.r.l., San Benedetto del Tronto, Italy, is the software company that developed the DID system. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | They said they used a random list, but do not describe how they generated it. |
| Allocation concealment (selection bias) | Low risk | Used central allocation through a telephone call to the co‐ordinating centre. |
| Patient's baseline characteristics (selection bias) | High risk | Age (P = 0.04). |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Reasons between arms seem balanced, but ~11% lost to follow‐up in control and ~12.7% in intervention. Authors also state that dropout was not directly related to Diabetes Interactive Diary (DID) intervention. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: HbA1c. Secondary outcomes: BP and LDL. Used standard methods at laboratories. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | High risk | Not cluster, and physicians cared for both intervention and control, sending back SMS. |
| Other bias | Low risk | Information not available. |
Rothman 2005.
| Study characteristics | ||
| Methods |
A randomized trial of a primary care‐based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes RCT (NA clusters and NA providers), conducted in 1) This randomised trial was conducted at the University of North Carolina General Internal Medicine Practice, which serves a wide socioeconomic range of patients. The practice is staffed by more than 20 attending faculty and 70 medical residents who care for more than 2000 patients with diabetes. 2) Intervention patients received intensive management from clinical pharmacists, a diabetes care co‐ordinator and primary care physicians. In United States of America. 2 arms: 1. Control (one management session and usual care) (control arm) and 2. Intervention (intensive management) (intervention arm) |
|
| Participants | Control arm N: 105 Intervention arm N: 112, NA, NA Diabetes type: 2 Mean age: 55.45 ± 12.36 % Male: 43.78 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (one management session and usual care) 1) Clinician education 2) Patient education Intervention arm: (intensive management) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician education 5) Facilitated relay of clinical information 6) Patient education 7) Patient reminders |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Harms |
|
| Funding source | This project was completed with support from the Robert Wood Johnson Clinical Scholars Program, the University of North Carolina Program on Health Outcomes, the University of North Carolina Division of General Internal Medicine, University of North Carolina Hospital Performance Improvement Department, University of North Carolina Pharmacy, the Vanderbilt Center for Health Services Research, and the Vanderbilt Diabetes Research and Training Center. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We randomly assigned patients to the intervention or control group using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Assignment was contained in sealed envelopes (opaque?) that were opened by the study co‐ordinator. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Baseline characteristics were similar between the 2 groups and revealed a sample with poor glycaemic control, multiple comorbid conditions, and low education and income status (Table 1). However, the intervention patients were slightly older than the control patients (P = 0.05) and more likely to be African American (P = 0.10). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All other comparisons P > 0.20. Clinical characteristics, including blood pressure, A1C level, and aspirin use, were also similar. |
| Incomplete outcome data (attrition bias) | Unclear risk | They lost 23 patients out of 217 randomised (10.6%). 10 lost in the control group (9.5%) and 13 in the intervention group (11.6%). Quite low numbers and balanced. Reasons reported and quite similar. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Aspirin use and adverse events were obtained by self‐report. Unclear for statin (likely self‐reported too). HbA1c and pressure were assessed objectively. Not all outcome assessment was blinded and several measures were based on patient self‐report. |
| Selective reporting (reporting bias) | High risk | No registered protocol. Methods: For lipids, we measured total and high‐density lipoprotein cholesterol levels in all patients, and low‐density lipoprotein (LDL) cholesterol levels only in patients who required statin therapy. Results: They reported data for total cholesterol but not for HDL and LDL. |
| Risk of contamination (other bias) | Unclear risk | All patients participated in a 1‐hour management session that was conducted by a clinical pharmacist. This session included diabetes education for the patient, and treatment recommendations were given to the patient’s primary care provider. Improvements in both groups may in part be attributed to regression to the mean or the initial 1‐hour management session. It is possible that communication between pharmacists and physicians for the intervention group have changed physician's approach with their control patients. |
| Other bias | Low risk | No evidence of other risk of bias. |
Rothschild 2014.
| Study characteristics | ||
| Methods |
Mexican American Trial of Community Health Workers: A randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus Patient RCT. Home visits. Recruited from mailings, outreach, churches, primary care clinics, etc. USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 71 Intervention arm N: 73 Diabetes type: type 2 Mean age: 53.7 ± 12.2 % Male: 32.6 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.1 (1.6), post 8.3 (NR) Intervention arm: pre 8.5 (2.2), post 7.6 (NR) 2) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 27 (38), post 41 (58) Intervention arm: pre 34 (47), post 31 (42) |
|
| Funding source | It was funded by the National Institute for Diabetes and Digestive and Kidney Diseases (grant R01‐DK061289) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "generated randomization lists." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | DBP, ACE inhibitor, anxiety. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | They do not provide a flow diagram of study participants, only state that they did an intention‐to‐treat analysis. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c: sent to a laboratory. BP: took average of 2nd and 3rd measurement. Assessor blinded. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Low risk | Information not available. |
Rubak 2011.
| Study characteristics | ||
| Methods |
Effect of "motivational interviewing" on quality of care measures in screen detected type 2 diabetes patients: a one‐year follow‐up of an RCT, ADDITION Denmark Clustered RCT (80 clusters and 140 providers), conducted in 1) This study included practices/GPs from the intensive arm of ADDITION Denmark from 2 counties in DK 2) Intervention delivered to GPs by trained teacher in Denmark 2 arms: 1. Control (C‐group, no training) (control arm) and 2. Intervention (I‐group, MI training) (intervention arm) |
|
| Participants | Control arm N: 321 Intervention arm N: 307, NA, NA Diabetes type: 2 Mean age: 61 ± NR % Male: 58 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (C‐group, no training) 1) Clinician education Intervention arm: (I‐group, MI training) 1) Clinician education |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Smoking status |
|
| Funding source | The ADDITION trial was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkoebing, Ribe and South Jutland in Denmark, Danish Research Foundation for General Practice, Danish Centre for Evaluation and Health Technology Assessment, the Diabetes Fund of the National Board of Health, the Danish Medical Research Council, the Danish Medical Association Research Fund, the Diabetes Association's Foundation for Scientific Research, the Aarhus University Research Foundation, and Novo Nordisk Foundation. Furthermore the trial has been given unrestricted grants from Novo Nordisk AS, Novo Nordisk Scandinavia AB, ASTRA Denmark, Pfizer Denmark, GlaxoSmithKline Pharma Denmark, SERVIER Denmark A/S, and HemoCue Denmark A/S. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by the project manager using the method "drawing lots". |
| Allocation concealment (selection bias) | Low risk | Cluster. Randomisation was stratified by county, size of practices and by numbers of full‐time GPs. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | No significant differences between the groups. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Two practices (6 GPs) and 2 type 2 diabetes patients dropped out after randomisation. 13 participants in the control group (10%) and 18 in the intervention group (13%) did not complete 1‐year follow‐up. The number of GPs and patients that dropped out of the study after randomisation is not expected to bias the results in consideration of the total number of GPs and patients included in this study. 6 GPs lost in control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Mix of subjective and objective measures. Objective for HbA1c, LDL, BP and medications. Subjective for smoking status. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol. Outcomes in protocol do not match outcomes published. |
| Risk of contamination (other bias) | Low risk | Cluster‐randomised. |
| Other bias | Unclear risk | This study may suffer from a limitation because training in MI was performed by only one person. This makes outcome highly dependent on this person's teaching methods and capacity to train the GPs. The study did not include blinding of behavioural changes and may therefore be influenced by the Hawthorne effect. |
Ruggiero 2010.
| Study characteristics | ||
| Methods |
Supporting diabetes self‐care in underserved populations. A randomized pilot study using medical assistant coaches Patient RCT, conducted in a federally qualified health centre (primary care clinic) in Chicago, USA Two arms: 1. Treatment as usual group ‐ TAU (control arm) and 2. Medical Assistant Coaching ‐ MAC (intervention arm). |
|
| Participants | Control arm N: 25 Intervention arm N: 25 Diabetes type: type 2 Mean age: 65.8 ± 9.4 % Male: 34.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Clinician reminders 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (1.7), post 8.5 (2.3) Intervention arm: pre 8.9 (1.6), post 8.7 (1.7) |
|
| Funding source | This study was supported in part by the following grants: the National Institute of Aging, National Institutes of Health (5 P30 AG022849); the National Institute of Digestive and Kidney Disease, National Institutes of Health (5 R01 NR10313); and the Centers for Disease Control and Prevention (co‐operative agreement 1‐U48‐DP‐000048) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, they simply used the word "randomized". |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Quote: "There were no baseline differences between the two randomized groups with respect to gender, age, ethnicity, BMI…". In text but not in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "…no baseline differences…across the 3 groups for A1C values." |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, baseline based on those randomised, numbers provided for loss to follow‐up for each arm, but reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described. Objective laboratory methods not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Ruggiero 2014.
| Study characteristics | ||
| Methods |
Medical assistant coaching to support diabetes self‐care among low‐income racial/ethnic minority populations: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) This study was conducted at 4 primary care clinics that are part of a large ambulatory care network of federally qualified health centres (FQHCs) that serves predominately uninsured and Medicaid patients in Chicago and its surrounding suburbs. 2) Certified medical assistants served as our Medical Assistant Coaches (MACs). In United States of America. 2 arms: 1. Control (TAU ‐ treatment as usual) (control arm) and 2. Intervention (MAC ‐ medical assistant self‐care coaching) (intervention arm) |
|
| Participants | Control arm N: 134 Intervention arm N: 136, NA, NA Diabetes type: 2 Mean age: 53.15 ± 8.77 % Male: 31.2 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (TAU ‐ treatment as usual) 1) Facilitated relay of clinical information 2) Patient education Intervention arm: (MAC‐medical assistant self‐care coaching) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Research reported in this publication was supported by National Institute of Nursing Research of the National Institutes of Health under grant number R01 NR010313. R.H. was supported by the National Institute of Health (NIH) under Award Number 5T32 HL069771‐10 (M. Daviglus, PI). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A participant’s race/ethnicity, gender and medication type were entered into a computer randomisation program that carried out the random assignment to condition on an equiprobability basis. |
| Allocation concealment (selection bias) | Low risk | A participant’s race/ethnicity, gender and medication type were entered into a computer randomisation program that carried out the random assignment to condition on an equiprobability basis. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. HbA1c and BMI have P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | They lost 94 out of 270 patients randomised (34.8%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | High risk | No registered or published protocol. No significant baseline intervention group differences were found for BMI, A1C, smoking, depression, confidence and all self‐care behaviours, with the exception of general diet (F(1,248) = 3.94, P < 0.05). No data reported except for A1C post intervention. Results stratified by ethnicity post intervention, but not at baseline. |
| Risk of contamination (other bias) | High risk | "It is notable that each of the four groups evidenced improvements in self‐care across time. One hypothesis that may help explain our results is the provision of a standard diabetes education booklet (i.e., enhanced TAU) and increased attention related to diabetes self management may have been a useful intervention." Contamination across interventions was possible because blinding of MACs and clinicians was not feasible. Also, clinic staff‐initiated co‐interventions occurred (e.g. support group started in one clinic). |
| Other bias | Unclear risk | No evidence of other bias. |
Russell 2019.
| Study characteristics | ||
| Methods |
Clinical outcomes of an integrated primary‐secondary model of care for individuals with complex type 2 diabetes: a non‐inferiority randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) This trial was conducted across 2 hospitals (control group) and 3 intervention sites (Beacon clinic, intervention group) in Brisbane, Australia. As a pragmatic trial, it was implemented within routine clinical practice. 2) Beacon model of integrated care delivered by a multidisciplinary team including 2 general practitioners (GPs) with special interests, an endocrinologist and a diabetes nurse educator (DNE). In Australia. 2 arms: 1. Control (gold‐standard hospital‐based specialist outpatient clinics) (control arm) and 2. Intervention (Beacon clinic with integrated care and upskilled GP) (intervention arm) |
|
| Participants | Control arm N: 83 Intervention arm N: 269, NA, NA Diabetes type: 2 Mean age: 55.7 ± 9.5 % Male: 61 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (gold‐standard hospital based specialist outpatient clinics) 1) Facilitated relay of clinical information Intervention arm: (Beacon clinic with integrated care and upskilled GP) 1) Case management 2) Team change 3) Clinician education 4) Clinician education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Harms |
|
| Funding source | This research was funded by the Australian National Health and Medical Research Council (NHMRC) under the Centre of Research Excellence in Quality and Safety in Integrated Primary–Secondary Care (Grant ID: GNT1001157) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved through a bespoke secure internet‐based randomisation program, designed by the study statistician. |
| Allocation concealment (selection bias) | Low risk | Researchers were masked only during the allocation process. Randomisation was achieved through a bespoke secure internet‐based randomisation program, designed by the study statistician. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. They only report data for intention‐to‐treat sample. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. They only report data for intention‐to‐treat sample. Data reported and only education has a star symbol (*) with a significant P value (under 0.05). |
| Incomplete outcome data (attrition bias) | High risk | This non‐inferiority randomised controlled trial was conducted across 2 hospitals and 3 intervention sites in Brisbane, Australia. A third hospital withdrew early in recruitment because of low referral rates of eligible participants. The per‐protocol population (main primary outcome analysis) was confined to individuals who completed the 12‐month study protocol or were discharged having met clinical targets, yielding a sample of 55/83 in usual care (34% lost) and 185/269 in the intervention (31% lost). High numbers lost. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c (main outcome), LDL and blood pressure (secondary outcomes) were all objectively assessed. Safety endpoints included hypoglycaemic events collected as self‐reported questionnaire data at 6 and 12 months (but secondary outcomes). Unlikely that patients were blinded to intervention. |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol and a protocol was published in 2013 (reference 10). They only provide data for secondary outcomes at 12 months, and not at 6 months as specified in the registered protocol. They do not report smoking status, retinopathy and foot complications, and other outcomes after the intervention. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Intervention and control delivered in different clinics. Quote: "This trial was conducted across two hospitals (control) and three intervention sites (Beacon clinics) in Brisbane, Australia". Different endocrinologists were involved in control and intervention group. Quote: "The endocrinologist supervising and co‐consulting with GPs from the 3 Beacon clinics previously worked within the hospital outpatient clinics". Diabetes nurse educator likely saw patients in control group. Quote: "The DNE is specifically skilled in case co‐ordination and comfortable working independently between clinics". However unlikely they did specialised screening appointment with control patients. |
| Other bias | Low risk | No evidence of other bias. |
Ryff‐de Lèche 1992.
| Study characteristics | ||
| Methods |
Clinical application of two computerized diabetes management systems: comparison with the log‐book method Cross‐over RCT, conducted in an outpatient clinic at University Hospital of Basel, Switzerland Two arms: 1. Group 2 ‐ Log book control (control arm) and 2. Group 1 ‐ Camit‐S1 analysis program (intervention arm) |
|
| Participants | Control arm N: 9 Intervention arm N: 10 Diabetes type: type 1 Median age: 52.0 (range: 21 to 60) % Male: 68.4 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 7.0 (0.2), post 6.7 (0.3) Intervention arm: pre 6.8 (0.3), post 6.3 (0.3) |
|
| Funding source | This work was supported by a grant of Boehringer Manheim Switzerland | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | This needs discussion. |
| Other bias | Unclear risk | Information not available. |
Sadur 1999.
| Study characteristics | ||
| Methods |
Diabetes management in a health maintenance organization. Efficacy of care management using cluster visits Patient RCT, conducted in primary care clinics from HMO in California, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 88 Intervention arm N: 97 Diabetes type: type 1 and type 2 Mean age: 56.1 ± 9.1 % Male: 57.3 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.6 (1.5), post 8.4 (1.9) Intervention arm: pre 9.7 (1.8), post 8.5 (1.9) |
|
| Funding source | This research was supported by the Innovation Program of the Kaiser Permanente Medical Care Program, Northern California | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | High risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Saenz 2012.
| Study characteristics | ||
| Methods |
Development and validation of a computer application to aid the physician's decision‐making process at the start of and during treatment with insulin in type 2 diabetes: a randomized and controlled trial Cluster‐RCT (14 clusters with 66 providers), conducted in primary care centres in Madrid, Spain Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 332 Intervention arm N: 365 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 18 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Electronic patient registry 2) Clinician education 3) Clinician reminders 4) Facilitated relay of clinical information 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.8 (1.5), post 7.7 (1.4) Intervention arm: pre 7.9 (1.4), post 7.2 (0.9) |
|
| Funding source | This work has been partially financed by the FIS‐071131 research grant from the Fund for Health Research of the Ministry of Health and Consumption, Spain | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | In text and table. |
| Patient's baseline outcomes (selection bias) | Low risk | Hba1c: In text and table. |
| Incomplete outcome data (attrition bias) | Low risk | Assuming no losses? |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c: objective laboratory method not reported. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Safford 2015.
| Study characteristics | ||
| Methods |
Peer coaches to improve diabetes outcomes in rural Alabama: a cluster randomized trial Clustered RCT (12 clusters and NR providers), conducted in 1) Our partnering communities, each served by at least one primary care practice, were located in 8 counties of the Alabama Black Belt, USA. 2) Intervention provided by peer coaches. In United States of America. 2 arms: 1. Control (control arm) and 2. Intervention (peer coaching) (intervention arm) |
|
| Participants | Control arm N: 226 Intervention arm N: 198, NA, NA Diabetes type: 4 Mean age: 60.2 ± NR % Male: 24.7 Longest follow‐up: 15 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: (peer coaching) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein |
|
| Funding source | Funding for this research was provided by the American Academy of Family Physicians Foundation through the Peers for Progress program with support from the Eli Lilly and Company Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The clusters in this cluster‐randomised trial were communities, blocked on smaller vs larger community size, with participants nested within communities. The study statistician used a random number generator to assign clusters to the 2 trial arms. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | 12 partnering communities, located in 8 counties, were the unit of randomisation. Some data are reported for each of the communities (number of participants per cluster, number of practices per cluster, mean number of participants per practice, travel distance for patients to reach the practice), but no P values are reported. Also no communities', practices' or providers' characteristics are reported for each arm at baseline. |
| Patient's baseline characteristics (selection bias) | Low risk | More black patients and fewer educated patients in the intervention arm (P ≤ 0.05). |
| Patient's baseline outcomes (selection bias) | Low risk | All P values for clinical outcomes are higher than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | 64 patients out of 424 withdrew or were lost to follow‐up (15.1%). Reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c, SBP and LDL). |
| Selective reporting (reporting bias) | High risk | No registered protocol. In their previous publication (recruitment protocol, reference 17), they report data for diastolic blood pressure and the number of patients with controlled hypertension, but not in this paper. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. |
| Other bias | Low risk | No evidence of other bias. |
Sajatovic 2017.
| Study characteristics | ||
| Methods |
A 60‐week prospective RCT of a self‐management intervention for individuals with serious mental illness and diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) Safety‐net health system primary care setting, Cleveland, Ohio, USA. Intervention delivered by in‐person group sessions and by phone. TTIM is intended to be delivered in a primary care setting. 2) Intervention co‐delivered by a nurse educator and a peer educator with serious mental illness and DM. In United States of America. 2 arms: 1. Control (treatment as usual) (control arm) and 2. Intervention (TTIM: Targeted Training in Illness Management) (intervention arm) |
|
| Participants | Control arm N: 100 Intervention arm N: 100, NA, NA Diabetes type: 2 Mean age: 52.7 ± NR % Male: 36 Longest follow‐up: 13.85 months |
|
| Interventions |
Control arm: (treatment as usual) Intervention arm: (TTIM: Targeted Training in Illness Management) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure |
|
| Funding source | Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH085665. The project was also supported by Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Lastly, this project received support from NIH/NCRR CTSA grant number KL2TR000440. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individuals were randomised using a computer‐generated list, allocation concealment, a 1:1 allocation ratio and block randomisation using block sizes of 4 to 8 consecutive patients. |
| Allocation concealment (selection bias) | Unclear risk | They do not report the concealment method. Individuals were randomised using a computer‐generated list, allocation concealment, a 1:1 allocation ratio and block randomisation using block sizes of 4 to 8 consecutive patients. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. There were no clinically important differences between TTIM and treatment as usual as assessed by standardised absolute mean differences. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. There were no clinically important differences between TTIM and treatment as usual as assessed by standardised absolute mean differences. |
| Incomplete outcome data (attrition bias) | High risk | They analysed 76/100 control patients (24% lost) and 74/100 intervention patients (26% lost) at 60 weeks. High numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c and SBP were objectively measured. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Time frame in the protocol was only 60 weeks, but they reported data at 13, 30 and 60 weeks in the paper. The paper does not include the secondary outcomes listed in the protocol: Tablets Routine Questionnaire (TRQ), Self‐rated Diabetes Self‐Care Activities (SDSCA) Questionnaire, Comparison of AUDIT (Alcohol Use Disorders Identification Test) Score, Comparison of ISMI (Internalized Stigma of Mental Illness ‐ Alienation) and many others. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that control patients had group sessions and phone calls. However, physicians might have changed their approach with control patients after receiving communications from the nurse taking care of intervention patients. |
| Other bias | Low risk | No evidence of other risk of bias. |
Saleh 2018.
| Study characteristics | ||
| Methods |
Using mobile health to enhance outcomes of noncommunicable diseases care in rural settings and refugee camps: randomized controlled trial Clustered RCT (16 clusters and NR providers), conducted in 1) 16 PHCCs in Lebanon: 10 located in rural areas and belonging to the Lebanese MOPH PHC National Network and 6 UNRWA centres chosen from Palestinian refugee camps in Lebanon. These centres were randomly assigned into intervention and control groups. Five MOPH and 3 UNRWA centres were allocated to each of the intervention and control groups for a total of 8 sites in each of the groups. Mobile mHealth intervention. 2) Trained community health worker, research assistant, family physician, physicians and nurses in Lebanon 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (eSahha mHealth mobile intervention) (intervention arm) |
|
| Participants | Control arm N: 300 Intervention arm N: 512, NA, NA Diabetes type: 4 Mean age: NR ± 10.99 % Male: 43.74 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (eSahha mHealth mobile intervention) 1) Clinician education 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Retinopathy screening Foot screening Glycated haemoglobin Smoking status |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | No significant differences between gender, setting and disease category across the 2 groups were identified using the Chi² test; the difference in age groups between intervention and control at baseline is statistically significant (P = 0.003). |
| Patient's baseline outcomes (selection bias) | Low risk | Data look similar. |
| Incomplete outcome data (attrition bias) | High risk | Some numbers under some categories may not add up to the total because of missing values. Very large losses indicated in Table 3. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure for HbA1c, eye, foot exam, unclear whether subjective measure of smoking status. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively published protocol. Methods match outcomes. Very large losses, which could have been influenced by selective outcome reporting. |
| Risk of contamination (other bias) | Low risk | Remotely delivered intervention. Very unlikely that control participants received mHealth intervention. |
| Other bias | High risk | Intervention bias could have taken place because QI collectors at PHCCs were aware of data collection post intervention. "As a matter of fact, the increased percentage of recorded dates of visits to PHCCs for HbA1c testing in both the control and intervention groups may be the result of improved documentation rather than an actual enhanced access to PHC services. Our results cannot be solely attributed to our intervention; the presence of advanced NCD programs at both the MOPH and UNRWA PHCC networks may have biased the findings, especially in the cases where a control site showed a significant change. Given that in some cases the owners of the phone numbers to which the SMSs were sent were not the patients themselves but rather family members, the interventional SMS messages may have not been transmitted to their final recipients (ie, patients) who are the target population of our study". |
Samtia 2013.
| Study characteristics | ||
| Methods |
A multifactorial intervention to enhance adherence to medications and disease‐related knowledge in type 2 diabetic patients in Southern Punjab, Pakistan RCT (NA clusters and NA providers), conducted in 1) Carried out for a 5‐month period in selected diabetes clinics in southern Punjab (Nishter Hospital Multan and DHQ Hospital Layyah), 2) Five pharmacists were the part of the study team in Pakistan 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (pharmacist led predefined care) (intervention arm) |
|
| Participants | Control arm N: 170 Intervention arm N: 178, NA, NA Diabetes type: 2 Mean age: 44.24 ± NR % Male: 50.55 Longest follow‐up: 5 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (pharmacist‐led predefined care) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Smoking status |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, data looks similar. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1, data looks similar. |
| Incomplete outcome data (attrition bias) | Low risk | Almost all the patients included completed the study (control group: 168/170 and intervention group: 174/178). Reasons for dropout were mainly non‐affordability of medication and travelling costs to keep hospital appointments. Lack of motivation and one patient died during follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Self‐report. Patients included in both control and intervention groups were asked to perform fasting blood sugar tests every 4 weeks at 0, 4, 8, 12, 16 and 20 weeks. Patients were asked to test their HbA1c values at the start and at the end of the study. Self‐reporting approach was used to assess adherence to medications. Knowledge regarding disease, self‐monitoring and lifestyle modifications were assessed on no basis at the start and end of the study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | Unclear whether intervention pharmacists interacted with control patients. |
| Other bias | Low risk | None identified. |
Samuel‐Hodge 2017.
| Study characteristics | ||
| Methods |
Family PArtners in Lifestyle Support (PALS): Family‐based weight loss for African American adults with type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) The study was conducted at both the University of North Carolina at Chapel Hill and Duke University, 2) Sessions were facilitated by trained staff (registered dietitians) in United States of America. 2 arms: 1. Control (delayed intervention/usual care) (control arm) and 2. Intervention (special weight loss intervention) (intervention arm) |
|
| Participants | Control arm N: 18 Intervention arm N: 36, NA, NA Diabetes type: 2 Mean age: 54.33 ± 10.77 % Male: 26 Longest follow‐up: 4.6 months |
|
| Interventions |
Control arm: (delayed intervention/usual care) Intervention arm: (special weight loss intervention) 1) Patient education 2) Promotion of self‐management 3) Financial Incentives |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This research was supported by the National Institutes of Health grant K01DK080079 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Diastolic blood pressure was the only variable significantly different (P < 0.05) between SI and DI. |
| Patient's baseline outcomes (selection bias) | High risk | Diastolic blood pressure was the only variable significantly different (P < 0.05) between SI and DI. |
| Incomplete outcome data (attrition bias) | Unclear risk | After the intervention, they obtained weight data from 89% (96 of 108) of participants overall (90% (65 of 72) among SI and 86% (31 of 36) in the DI group). Reasons for losses balanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | For the DI participants, no educational materials about weight loss were provided during the study period; participants received one newsletter with Family PALS program updates. |
| Other bias | Unclear risk | 1) No way to quantify strength of relationship with family member. A more supportive family member would make the PALS programme seem more effective than a less supportive one. 2) sample sizes not equal between groups. 3) The comparison of the study intervention with a control group receiving no treatment during the RCT period, limits what Family PALS tells us about the added benefit of actively including family members in weight loss among African American patients with diabetes. |
Sarayani 2018.
| Study characteristics | ||
| Methods |
Efficacy of a telephone‐based intervention among patients with type‐2 diabetes; a randomized controlled trial in pharmacy practice RCT (NA clusters and NA providers), conducted in 1) facilities of a referral pharmacy affiliated with the College of Pharmacy, Tehran University of Medical Sciences (conference hall and the drug information call centre). This pharmacy is in the midtown area of Tehran, the capital city of Iran 2) Intervention delivered by pharmacists in Iran 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (pharmacist telephone consultation) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 55.05 ± 8.8 % Male: 58.45 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education 2) Promotion of self‐management Intervention arm: (pharmacist telephone consultation) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein Smoking status |
|
| Funding source | This study was supported by a research grant from Research Deputy of Tehran University of Medical Sciences (ID: 91‐03‐156‐19496) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | One of the authors who was not involved in eligibility confirmation, diabetes education or telephone intervention generated and concealed the allocation sequence. Not clear how allocation was concealed. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values > 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Moreover, there were no significant differences between groups regarding the duration of diabetes, number or type of diabetes medications and the baseline HbA1c. |
| Incomplete outcome data (attrition bias) | High risk | 14 lost in intervention group (28%), 7 lost in control group (14%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective outcomes: HbA1c, LDL; subjective: smoking. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered ("during recruitment") protocol with outcomes that match those reported in manuscript. |
| Risk of contamination (other bias) | High risk | One of the control group participants insisted on receiving tele‐intervention (Figure 1). The risk of contamination between intervention and control group could not be fully ruled out as we did not document patients’ social ties to other participants in the study. However, the risk of contamination must be low because the study participants were mostly recruited by advertisement in the community pharmacies. |
| Other bias | Low risk | No evidence of other bias. |
Sato 2016.
| Study characteristics | ||
| Methods |
Effect of treatment guidance using a retrospective continuous glucose monitoring system on glycaemic control in outpatients with type 2 diabetes mellitus: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Recruitment done at the outpatient clinic at Juntendo University Hospital, Tokyo, Japan between December 2012 and April 2014. 2) Two trained diabetologists from the study team provided guidance during the intervention. In Japan. 2 arms: 1. Control (advice based on blood glucose and glycated haemoglobin) (control arm) and 2. Intervention (continuous glucose monitoring system and treatment guidance) (intervention arm) |
|
| Participants | Control arm N: 17 Intervention arm N: 17, NA, NA Diabetes type: 2 Mean age: 61.5 ± 12 % Male: 58.82 Longest follow‐up: 8 months |
|
| Interventions |
Control arm: (advice based on blood glucose and glycated haemoglobin) 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: (continuous glucose monitoring system and treatment guidance) 1) Case management 2) Clinician education 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This work was supported by a Grant‐in‐Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (Grant Number 25350902) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients meeting the above criteria were assigned randomly by a computer‐generated method to either the intervention (I) group or the nonintervention (N‐I) group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No significant between‐group differences (P = 0.05); Mann–Whitney U‐test. There were no significant differences between the 2 groups at baseline. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. No significant between‐group differences (P = 0.05); Mann–Whitney U‐test. There were no significant differences between the 2 groups at baseline. |
| Incomplete outcome data (attrition bias) | Low risk | A total of 34 patients meeting the inclusion criteria were assigned randomly to either the I group (n = 17) or the N‐I group (n = 17). All of the patients completed the study (Figure 2). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective outcome (HbA1c, primary outcome) and subjective outcomes (perception of frequency of hyperglycaemic/hypoglycaemic events, secondary outcomes). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (posted in September 2012, trial started in October 2012). Other parameters related to diabetes management (such as body mass index, blood pressure, total cholesterol, high‐density lipoprotein cholesterol and triglyceride levels) were not significantly different at the end of the study relative to the baseline (data not shown). |
| Risk of contamination (other bias) | Low risk | The intervention patients had diabetologists' guidance and the control group had advice from their usual care providers. All patients were equipped with a wireless retrospective CGM device and all patients were asked to undertake self‐monitoring of blood glucose (SMBG). |
| Other bias | Low risk | None. |
Schillinger 2009.
| Study characteristics | ||
| Methods |
Effects of self‐management support on structure, process, and outcomes among vulnerable patients with diabetes: a three‐arm practical clinical trial RCT (NA clusters and NA providers), conducted in 1) Eligible patients attended a study enrollment visit at the San Francisco General Hospital Clinical Research Center. 2) Intervention involved nurse follow‐up for automated telephone self‐management support, or monthly group medical visits co‐facilitated by a primary care physician and health educator. In United States of America. 3 arms: 1. Control (usual care) (control arm) and 2. Intervention 1 (ATSM‐telephone support with nurse) (intervention arm), 3. Intervention 2 (GMV‐group medical visits) (other arm) |
|
| Participants | Control arm N: 114 Intervention arm N: 112, 113, NA Diabetes type: 2 Mean age: 56.1 ± 11.6 % Male: 41 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (ATSM‐telephone support with nurse) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management Intervention arm: (GMV‐group medical visits) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This study was supported by The Commonwealth Fund, Agency for Healthcare Research and Quality Grants R21 HS014864 and R18 HS17261, The California Endowment, the San Francisco Department of Public Health, and The California Healthcare Foundation. D.S. was supported by National Institutes of Health Mentored Clinical Scientist Award K‐23 RR16539. Electronic data and resources of the University of California San Francisco—San Francisco General Hospital General Clinical Research Center were made available through National Institutes of Health Grant UL1 RR024131. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients were allocated using stratified (on languages) blocked randomisation. Reference 10: Randomisation was administered using a blocked randomisation strategy stratified to ensure even distribution of languages. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. There were no statistically significant differences in baseline characteristics across arms. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. There were no statistically significant differences in baseline characteristics across arms. |
| Incomplete outcome data (attrition bias) | Unclear risk | Of the participants, 305 (90%) completed follow‐up interviews at 1 year (out of 339 randomised). For HbA1c, ATSM, n = 101/112 (9.8% lost); for GMV, n = 96/113 (15.0% lost), and for usual care, n = 103/114 (9.6%). Paired values for A1C were available for 88.2% of the sample, blood pressure for 94.1% and BMI for 92.3%. Three participants died during the study period in each of the 3 arms. Other reasons not reported. Participants lost to follow‐up were younger (51.7 vs 56.5 years, P = 0.02). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Results match protocol for our outcomes of interest. |
| Risk of contamination (other bias) | Unclear risk | Interventions took place in one safety net health system. Same physicians could have followed patients from all groups. They might have changed their approach with patients in the usual care group after receiving patients' goal‐setting records from intervention arms. |
| Other bias | Low risk | None. |
Schnipper 2010.
| Study characteristics | ||
| Methods |
Effects of documentation‐based decision support on chronic disease management Cluster‐RCT (239 clusters with 239 providers), conducted in primary care practices at Brigham and Women's Hospital and Massachusetts General Hospital, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 2518 Intervention arm N: 2493 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Clinician reminders |
|
| Outcomes | 1) Antihypertensives (ACE inhibitor or angiotensin II receptor blockers), N users (%) Control arm: pre 0 (0), post 143 (5) Intervention arm: pre 0 (0), post 136 (5) 2) Retinopathy screening (eye exam), N screened (%) Control arm: pre 0 (0), post 434 (11) Intervention arm: pre 0 (0), post 424 (12) 3) Foot screening, N screened (%) Control arm: pre 0 (0), post 85 (1) Intervention arm: pre 0 (0), post 147 (2) 4) Renal screening (renal), N screened (%) Control arm: pre 0 (0), post 620 (41) Intervention arm: pre 0 (0), post 617 (42) |
|
| Funding source | This study was supported by a grant from the Agency for Healthcare Research and Quality | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…random number generation". |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Female (< 0.001), number of problems on problem list (< 0.001), race (< 0.001), primary insurance (0.002), median household income (0.01). |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | No mention of blinding of outcome assessor. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Unclear risk | Confounding by indication. |
Schoenberg 2017.
| Study characteristics | ||
| Methods |
Community to clinic navigation to improve diabetes outcomes RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from 2 federally qualified health clinics (FQHC) in rural Appalachian Kentucky from November 2014 to January 2015. Participants met in the field office. For this randomised clinical trial pilot study, participants met with the interviewer at their home, the project office or another community location, depending on the participant's preferences. 2) Trained Community Health Workers (CHWs) conducted the group self‐management education programme (Diabetes Self‐management Program). In United States of America. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (CCN) (intervention arm) |
|
| Participants | Control arm N: 21 Intervention arm N: 20, NA, NA Diabetes type: 2 Mean age: 58.24 ± 13.56 % Male: 34.15 Longest follow‐up: 7 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (CCN) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Support was provided by the Office of the Vice President for Research at the University of Kentucky the Department of Behavioral Science and the College of Medicine at the University of Kentucky | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Within a week of this initial meeting, our project biostatistician randomly assigned participants to the intervention or control arm. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 3. P values for related components above 0.05 as per note below table. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 3. P values for related components above 0.05 as per note below table. |
| Incomplete outcome data (attrition bias) | Unclear risk | No statement of dropout or loss. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol; outcomes match methods. |
| Risk of contamination (other bias) | Low risk | Usual care for control group. No contact with staff during intervention period presumably. |
| Other bias | Low risk | None identified. |
Scott 2006.
| Study characteristics | ||
| Methods |
Outcomes of pharmacist‐managed diabetes care services in a community health center Patient RCT, conducted in Siouxland Community Health Center, Sioux City, Iowa, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 73 Intervention arm N: 76 Diabetes type: type 2 Mean age: NR ± NR % Male: 38.9 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.7 (NR), post 8.0 (NR) Intervention arm: pre 8.8 (NR), post 7.1 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 130.7 (NR), post 132.8 (NR) Intervention arm: pre 130.0 (NR), post 126.6 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 79.6 (NR), post 78.2 (NR) Intervention arm: pre 79.3 (NR), post 75.9 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 120.5 (NR), post 112.3 (NR) Intervention arm: pre 116.1 (NR), post 96.7 (NR) |
|
| Funding source | Supported by a grant from the Health Resources and Services Administration, Bureau of Primary Care, Office of Pharmacy Affairs, Rockville, MD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome HbA1c? |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Seggelke 2014.
| Study characteristics | ||
| Methods |
Transitional care clinic for uninsured and medicaid‐covered patients with diabetes mellitus discharged from the hospital: a pilot quality improvement study RCT (NA clusters and NA providers), conducted in 1) TCC at the University of Colorado Hospital 2) At the TCC, these patients were seen by an endocrinologist, a nurse practitioner or a physician assistant trained and experienced in DM treatment. In United States of America. 2 arms: 1. Control: usual care (control arm) and 2. Intervention TCC (transitional care clinic) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 3 Mean age: 51.05 ± 8.41 % Male: 74 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (transitional care clinic) 1) Case management 2) Team change |
|
| Outcomes | Harms | |
| Funding source | This work was supported by a grant from the University of Colorado Hospital. Drs Rasouli and Wang are also supported by a grant from the Veterans Affairs Research Service | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Enrolled participants were randomised according to the last digit of their medical record number (odd–even). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Participants in both groups were similar in age, gender and type of DM (type 1 DM vs type 2 DM (T2DM)) (Table 1). Table 1 note indicates that no P values were below 0.05. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for harms. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Outcomes match methods. |
| Risk of contamination (other bias) | Low risk | At TCC, patients were seen by an endocrinologist, a nurse practitioner or a physician assistant trained and experienced in DM treatment, who are different from the outpatient physician. |
| Other bias | Low risk | None. |
Sen 2014.
| Study characteristics | ||
| Methods |
Financial incentives for home‐based health monitoring: a randomized controlled trial Patient RCT, conducted in a primary care medical home practice at the University of Pennsylvania Health System, USA Three arms: 1. Control (control arm), 2. Low‐incentive (intervention arm 1) and 3. High‐incentive (intervention arm 2) |
|
| Participants | Control arm N: 28 Intervention arm 1 N: 21 Intervention arm 2 N: 26 Diabetes type: unclear/not reported Mean age: 54.3 ± 9.5 % Male: 36.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm 1: 1) Clinician reminders 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Financial incentives Intervention arm 2: 1) Clinician reminders 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Financial incentives |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 9.3 (0.3), post 8.6 (NR) Intervention arm 1: pre 9.3 (0.4), post 7.8 (NR) Intervention arm 2: pre 9.8 (0.3), post 8.6 (NR) 2) SBP, mean mmHg (SE) Control arm: pre 128.4 (3.0), post 133.6 (NR) Intervention arm 1: pre 136.1 (5.2), post 127.6 (NR) Intervention arm 2: pre 135.1 (3.8), post 139.3 (NR) |
|
| Funding source | This work was supported by grants RC2AG036592 and P30AG034546 from the National Institute on Aging | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence (electronically). |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Mentioned in text, but no P values provided in table. |
| Patient's baseline outcomes (selection bias) | Low risk | In text for HbA1c. |
| Incomplete outcome data (attrition bias) | Low risk | No losses. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Secondary outcome: HbA1c, objective laboratory methods not described. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Sequeira 2013.
| Study characteristics | ||
| Methods |
Continuous glucose monitoring pilot in low‐income type 1 diabetes patients Patient RCT, conducted in an endocrine Fellows Diabetes Clinic at the Roybal Comprehensive Health Center in East Los Angeles, USA Two arms: 1. SMBG (control arm) and 2. CGM (intervention arm) |
|
| Participants | Control arm N: 20 Intervention arm N: 19 Diabetes type: type 1 Mean age: 40.0 ± 13.0 % Male: 52.0 Longest follow‐up: 7 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.3 (NR), post 7.8 (NR) Intervention arm: pre 8.3 (NR), post 8.0 (NR) |
|
| Funding source | This project was funded by JDRF Artificial Pancreas grant 22‐2006‐1119 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported: HbA1c. |
| Incomplete outcome data (attrition bias) | High risk | ~10% lost to follow‐up in N1; ~21% in N2, provide numbers of lost to follow‐up, however, more losses in N2 (double), reasons after 1 week not really provided for all dropouts. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: HbA1c, used a DCA Vantage 2000 Analyzer. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Sequist 2010.
| Study characteristics | ||
| Methods |
Cultural competency training and performance reports to improve diabetes care for Black patients Cluster‐RCT (31 clusters with 124 providers), conducted in Harvard Vanguard Medical Associates (HVMA) ‐ a multispecialty group practice in Eastern Massachusetts, USA Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 3773 Intervention arm N: 3784 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education 3) Clinician reminders |
|
| Outcomes | 1) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre 1181 (31), post 1171 (35) Intervention arm: pre 994 (26), post 1063 (28) |
|
| Funding source | Primary Robert Wood Johnson Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Not described. Cluster. |
| Provider's baseline characteristics (selection bias) | High risk | Not provided. |
| Patient's baseline characteristics (selection bias) | High risk | Chronic kidney disease (P = 0.024). |
| Patient's baseline outcomes (selection bias) | High risk | Hypertension control < 130/80 (P = 0.037). |
| Incomplete outcome data (attrition bias) | High risk | State that they did an intention‐to‐treat analysis, however losses to follow‐up not described. Baseline based on those randomised. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Blinding not described. They mention that ascertainment of outcomes were taken from medical records, but do we know if the outcome assessors were blinded? However, in protocol on clinicaltrials.gov, they mention outcome assessors were blinded. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | Low risk | Cluster. Quote: "Use of the primary care team as the unit of randomization... limited the potential for the intervention effects to be contaminated by patients receiving care from clinicians in both the intervention and control groups." |
| Other bias | Low risk | Information not available. |
Sevick 2012.
| Study characteristics | ||
| Methods |
Biophysiologic outcomes of the enhancing adherence in type 2 diabetes (ENHANCE) trial Patient RCT, patients recruited by self‐referral, advertisements, emails, etc. USA Two arms: 1. Attention control (control arm) and 2. Technology‐supported behavioral intervention (intervention arm) |
|
| Participants | Control arm N: 149 Intervention arm N: 147 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education 2) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.5 (1.7), post 7.3 (1.6) Intervention arm: pre 7.7 (2.2), post 7.1 (1.3) 2) SBP, mean mmHg (SD) Control arm: pre 138.7 (18.9), post 136.8 (20.1) Intervention arm: pre 135.2 (18.7), post 134.2 (19.0) 3) DBP, mean mmHg (SD) Control arm: pre 75.8 (10.0), post 74.5 (10.6) Intervention arm: pre 75.6 (9.8), post 74.0 (10.1) 4) LDL, mean mg/dL (SD) Control arm: pre 108.6 (34.3), post 106.7 (37.4) Intervention arm: pre 107.4 (40.7), post 102.1 (32.1) |
|
| Funding source | This work was supported by the following grants: National Institutes of Health/National Institute of Nursing Research no. NR‐R01008792, National Institutes of Health/National Center for Research Resources no. CTSA‐UL1‐RR024153, and National Institutes of Health/National Center for Research Resources no. GCRC‐M01‐RR000056 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…computer‐generated permuted blocks." |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Patient's baseline characteristics (selection bias) | Low risk | In text and table. Quote: "The intervention and attention control groups did not differ significantly on any of these baseline characteristics." |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: HbA1c (P = 0.49); LDL (P = 0.34); SBP (P = 0.29); DBP (P = 0.73). |
| Incomplete outcome data (attrition bias) | High risk | Included in analysis those who had at least 1 follow‐up, neither an intention‐to‐treat or per‐protocol analysis. Numbers and reasons for loss to follow‐up provided, however in the intervention group 11 vs 6 were lost to follow‐up for unknown reasons after the 3 months follow‐up. Baseline based on those analysed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective laboratory methods not described. Quote: "Because of the nature of behavioural interventions, neither participants nor investigators could be blinded to group assignment." "Data was collected…by a trained research assistant." We do not know if this person was blinded. |
| Selective reporting (reporting bias) | High risk | Some outcomes listed in protocol not in manuscript. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None. |
Shah 2014.
| Study characteristics | ||
| Methods |
Effect of an educational toolkit on quality of care: a pragmatic cluster randomized trial Cluster‐RCT (80 clusters), conducted in family practices in Ontario, Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 797 Intervention arm N: 795 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 10 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician education |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre NR (NR), post 725 (91) Intervention arm: pre NR (NR), post 700 (88) 2) Antihypertensives (ACE inhibitor or angiotensin II receptor blockers), N users (%) Control arm: pre NR (NR), post 689 (86) Intervention arm: pre NR (NR), post 671 (84) 3) Controlled hypertension (≤ 130/80 mmHg), N under control (%) Control arm: pre NR (NR), post 506 (63) Intervention arm: pre NR (NR), post 420 (53) |
|
| Funding source | The study was funded by an operating grant from the Canadian Institutes for Health Research (CIHR) and the Heart and Stroke Foundation of Canada. BRS receives salary support from the CIHR, and previously received support from the Canadian Diabetes Association. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..random number sequences generated by SAS." |
| Allocation concealment (selection bias) | Low risk | Cluster. Centralised allocation, Quote: "..generated the randomized list and provided it to the mailing house distributing the toolkit on behalf of the CDA." |
| Provider's baseline characteristics (selection bias) | Low risk | Data are similar between groups. |
| Patient's baseline characteristics (selection bias) | Low risk | Data are similar between groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Primary outcome: statin. |
| Incomplete outcome data (attrition bias) | High risk | They do not report numbers lost to follow‐up. Report that an intention‐to‐treat analysis was done. It seems like they randomly selected numbers of patients at end of follow‐up to measure clinical outcomes, and then found their baseline data? |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: statin use. Subjective outcome assessors were blinded. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Shahid 2015.
| Study characteristics | ||
| Methods |
Mobile phone intervention to improve diabetes care in rural areas of Pakistan: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Outpatient services of Department of Endocrinology, Liaquat National Hospital (LNH). 2) The intervention involved the principal Investigator (from the Department of Endocrinology) and diabetes educationist. In Pakistan. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (mobile phone) (intervention arm) |
|
| Participants | Control arm N: 220 Intervention arm N: 220, NA, NA Diabetes type: 2 Mean age: 49.08 ± 1.67 % Male: 61.4 Longest follow‐up: 4 months |
|
| Interventions |
Control arm: (usual care) 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management Intervention arm: (mobile phone) 1) Case management 2) Facilitated relay of clinical information 3) Patient education Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Patients were randomly distributed in the intervention and non‐intervention groups based on gender. |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported about concealment. |
| Patient's baseline characteristics (selection bias) | Low risk | Table I, all P values above 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table II: P values under 0.05 for diastolic blood pressure, number of patients with hypertension and medication intake. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not addressed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c, SBP, DBP, patients with hypertension and LDL). Before randomisation into groups, baseline data were taken by the staff of the clinic, however, it was not possible to blind the patients and the clinicians to the allocation groups. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol or previously published protocol. Results match methods. |
| Risk of contamination (other bias) | Low risk | Patients never saw each other. Intervention made individually by phone. |
| Other bias | Low risk | No evidence of other bias. |
Shao 2015.
| Study characteristics | ||
| Methods |
Clinical effects of comprehensive nursing intervention in elderly liver cirrhosis patients with type 2 diabetes Quasi‐RCT (NA clusters and NA providers), conducted in 1) Patients treated at the Second Hospital of Yinzhou, Ningbo 315100, Zhejiang Province, China. 2) Intervention delivered by nurses (comprehensive nursing intervention) with doctors. In China. 2 arms: 1. Control (conventional nursing) (control arm) and 2. Intervention (comprehensive nursing intervention) (intervention arm) |
|
| Participants | Control arm N: 54 Intervention arm N: 54, NA, NA Diabetes type: 2 Mean age: 75.2 ± 7.16 % Male: 61.11 Longest follow‐up: NR months |
|
| Interventions |
Control arm: (conventional nursing) Intervention arm: (comprehensive nursing intervention) 1) Case management 2) Patient education |
|
| Outcomes | Harms | |
| Funding source | No relevant information reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Seems quasi‐RCT, conflicting information about randomisation between abstract (random table) and full text (allocated according to the order of visit). |
| Allocation concealment (selection bias) | High risk | Seems quasi‐RCT, conflicting information about randomisation between abstract (random table) and full text (allocated according to the order of visit). |
| Patient's baseline characteristics (selection bias) | Low risk | The author declared that there are no statistical differences regarding age and gender (P > 0.05) at baseline. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Nothing reported about baseline outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | Looks like they only report the number of patients analysed. No report about any lost. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Hypoglycaemia events were objectively measured (the author measured the fasting blood‐glucose and blood‐glucose 2 hours after meal). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Patients randomised. Nurses were working with usual doctors so the latter might have changed their approach with their control patients. |
| Other bias | Low risk | No evidence of other risk of bias. |
Shea 2009.
| Study characteristics | ||
| Methods |
A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study Patient RCT, conducted in primary care practices, USA Two arms: 1. Usual care (control arm) and 2. Telemedicine case management (intervention arm) |
|
| Participants | Control arm N: 821 Intervention arm N: 844 Diabetes type: unclear/not reported Mean age: 71.0 ± NR % Male: 37.2 Longest follow‐up: 60 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Electronic patient registry 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.4 (1.6), post 7.3 (1.5) Intervention arm: pre 7.4 (1.5), post 7.1 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 142.5 (23.6), post 139.5 (22.2) Intervention arm: pre 142.8 (24.2), post 136.1 (20.4) 3) DBP, mean mmHg (SD) Control arm: pre 71.0 (10.4), post 68.5 (11.1) Intervention arm: pre 71.6 (11.4), post 67.3 (10.2) 4) LDL, mean mg/dL (SD) Control arm: pre 108.2 (35.8), post 94.4 (34.6) Intervention arm: pre 106.8 (35.0), post 92.0 (34.6) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Shi 2014.
| Study characteristics | ||
| Methods |
Comprehensive nursing intervention to improve quality of life in chronic hepatitis C patients with diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) Central Hospital of Yiwu City, Yiwu 322000, Zhejiang Province, China. 2) Intervention delivered by nurses (comprehensive nursing intervention). In China. 2 arms: 1. Control (conventional nursing) (control arm) and 2. Intervention (comprehensive nursing intervention) (intervention arm) |
|
| Participants | Control arm N: 46 Intervention arm N: 46, NA, NA Diabetes type: 4 Mean age: 44.195 ± NR % Male: 62 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (conventional nursing) Intervention arm: (comprehensive nursing intervention) 1) Case management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | No relevant information reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Distribution of 92 patients in accordance with the random number chart method. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Differences in psychometric scores etc. are not statistically significant (P > 0.05 for mean age and gender). No educational level provided. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Differences in psychometric scores etc. are not statistically significant (P > 0.05 for viral count, FBG and HbA1c). |
| Incomplete outcome data (attrition bias) | Unclear risk | Looks like they only report the number of patients analysed. No report about any lost. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively measured (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Patients randomised. Unlikely that control patients met with the nurses. |
| Other bias | Low risk | No evidence of other risk of bias. |
Siaw 2017.
| Study characteristics | ||
| Methods |
Impact of pharmacist‐involved collaborative care on the clinical, humanistic and cost outcomes of high‐risk patients with type 2 diabetes (IMPACT): a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study was conducted at 4 outpatient healthcare institutions located in Singapore. 2) Multidisciplinary collaborative care by pharmacists, diabetes nurse educators and dietitians vs physician‐centred care in diabetes. In Singapore. 2 arms: 1. Control (usual care, physician‐centred care) (control arm) and 2. Intervention (IMPACT: multidisciplinary collaborative care) (intervention arm) |
|
| Participants | Control arm N: 197 Intervention arm N: 214, NA, NA Diabetes type: 2 Mean age: 59.63 ± 10.6 % Male: 56.42 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care, physician‐centred care) Intervention arm: (IMPACT: multidisciplinary collaborative care) 1) Case management 2) Team change 3) Clinician education 4) Clinician education 5) Patient education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | This study was supported by the Health Services Research Competitive Research Grant from the Ministry of Health, Singapore (HSRG/11MAY/016) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clearly reported. The randomisation procedures were conducted by research assistants with an allocation ratio of 1:1 into the intervention or control arms, using a simple unrestricted randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Overall, the 2 arms were comparable in all baseline parameters (Table 1). All P values higher than 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Mean HbA1c at baseline was comparable between the intervention and control arms (P = 0.70) (Figure 3A). Mean SBP at baseline was comparable between both arms (P = 0.43). Mean LDL (P = 0.81) and TG (P = 0.16) at baseline were comparable between both arms. Average PAID scores at baseline were comparable for both arms (P = 0.19). |
| Incomplete outcome data (attrition bias) | High risk | Overall, 19.7% (intervention arm: 17.8% vs control arm: 1.9%) patients dropped out of the study due to inability to take time off work to attend medical appointments (Figure 2). The dropout rate was much higher in the multidisciplinary collaborative care arm than the usual care arm. Unbalanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | The primary outcomes included surrogate endpoints, such as HbA1c, systolic blood pressure (SBP), low‐density lipoprotein (LDL) and triglycerides (TG), and were collected from the electronic databases at baseline, 3 and 6 months (objective outcomes). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Data collection: The primary outcomes included surrogate endpoints, such as HbA1c, systolic blood pressure (SBP), low‐density lipoprotein (LDL) and triglycerides (TG), and were collected from the electronic databases at baseline, 3 and 6 months. They only reported 6 months follow‐up for SBP, LDL and TG (nothing reported at 3 months). |
| Risk of contamination (other bias) | Unclear risk | The intervention group received multidisciplinary collaborative care by pharmacists, diabetes nurse educators and dietitians vs physician‐centred care in diabetes (control group). Patients randomised into the control arm had no regular contact with clinical pharmacists. In the intervention arm, physicians referred their patients to the diabetes nurse educators or dietitians as needed. The control arm received usual care with referrals to nurses and dietitians as needed. Unclear if the same physicians, nurses and dietitians were taking care of patients from both groups. |
| Other bias | Low risk | No evidence of other risk of bias. |
Sieber 2012.
| Study characteristics | ||
| Methods |
Promoting self‐management in diabetes: efficacy of a collaborative care approach Cluster‐RCT (21 clusters with 21 providers), conducted in 3 academic, family medicine clinics located across San Diego, USA Three arms: 1. Usual care (control arm), 2. Intervention‐lite (intervention arm 1) and 3. Full intervention (intervention arm 2) |
|
| Participants | Control arm N: 350 Intervention arm 1 N: 294 Intervention arm 2 N: 270 Diabetes type: type 2 Mean age: NR ± NR % Male: 47.8 Longest follow‐up: 5 months |
|
| Interventions |
Control arm: None Intervention arm 1: 1) Team changes 2) Electronic patient registry 3) Patient education Intervention arm 2: 1) Team changes 2) Electronic patient registry 3) Patient education |
|
| Outcomes | 1) HbA1c, median % (SD) Control arm: pre 6.8 (NR), post 7.0 (NR) Intervention arm 1: pre 6.5 (NR), post 6.9 (NR) Intervention arm 2: pre 7.2 (NR), post 7.3 (NR) 2) LDL, median mg/dL (SD) Control arm: pre 86.0 (NR), post 87.0 (NR) Intervention arm 1: pre 88.0 (NR), post 89.0 (NR) Intervention arm 2: pre 95.0 (NR), post 102.5 (NR) |
|
| Funding source | "This study was partially funded by a generous grant the Informed Medical Decisions Foundation. Boston. Massachusetts and took place at the UCSD Division of Family Medicine outpatient clinic." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported in text or table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss of patients not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Physician ordered laboratory tests; unsure if objective methods used. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Unclear risk | Selection bias. Physicians to choose those who are more severe in their list of patients. |
Sigurdardottir 2009.
| Study characteristics | ||
| Methods |
Instruments to tailor care of people with type 2 diabetes Patient RCT, conducted in five diabetes clinics, Iceland Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 28 Intervention arm N: 30 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.9 (0.9), post 7.8 (0.8) Intervention arm: pre 8.1 (1.0), post 8.0 (1.2) |
|
| Funding source | Research funds from the University of Akureyri, Iceland and from the Icelandic Nursing Association are acknowledged (no grant number provided) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using a computer program….computer generated list." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…and stratified random allocation"... using the software. |
| Patient's baseline characteristics (selection bias) | Low risk | Age: intervention group was younger (P = 0.044). |
| Patient's baseline outcomes (selection bias) | Unclear risk | P values not provided for baseline values for outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis (only after accounting for those who had completed the trial based on their allocated intervention), but included data for analysis for those lost to follow‐up at 3 and 6 months. They do not state if the 5 who did not receive the intervention and who were excluded from analysis have been imputed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding of outcome assessor not described. HbA1c methods not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None identified. |
Siminerio 2013.
| Study characteristics | ||
| Methods |
Who can provide diabetes self‐management support in primary care? Findings from a randomized controlled trial Patient RCT, conducted in primary care practices affiliated with healthcare networks located in 3 Pennsylvania communities, University of Pittsburgh Medical Center Community Medicine, Federally Qualified Health Centers, and Pennsylvania State University Hershey, USA Four arms: 1. Usual education (control arm), 2. Practice staff (intervention arm 1), 3. Peer (intervention arm 2), and 4. Educator (intervention arm 3) |
|
| Participants | Control arm N: 32 Intervention arm 1 N: 35 Intervention arm 2 N: 36 Intervention arm 3 N: 38 Diabetes type: type 2 Mean age: 60.0 ± NR % Male: 50.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm 1: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm 2: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm 3: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.7 (1.9), post 8.9 (NR) Intervention arm 1: pre 9.0 (2.1), post 9.1 (NR) Intervention arm 2: pre 8.6 (2.4), post 8.6 (NR) Intervention arm 3: pre 8.3 (1.8), post 8.2 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 133.0 (14.0), post 137.3 (NR) Intervention arm 1: pre 133.0 (14.0), post 136.3 (NR) Intervention arm 2: pre 129.0 (13.4), post 126.9 (NR) Intervention arm 3: pre 133.0 (14.0), post 136.7 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 78.0 (10.0), post 81.2 (NR) Intervention arm 1: pre 75.0 (9.0), post 76.6 (NR) Intervention arm 2: pre 76.0 (8.0), post 75.8 (NR) Intervention arm 3: pre 79.0 (9.0), post 82.0 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 108.0 (41.0), post 116.0 (NR) Intervention arm 1: pre 97.0 (34.0), post 106.0 (NR) Intervention arm 2: pre 97.0 (34.0), post 111.3 (NR) Intervention arm 3: pre 98.0 (32.0), post 92.9 (NR) |
|
| Funding source | This research study is sponsored by the Air Force Surgeon General’s Office under agreement number FA7014‐10‐2‐0005. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force Surgeon General’s Office or the US Government. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | In text and in table. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.44); LDL (P = 0.62); SBP (P = 0.35); DBP (P = 0.69). |
| Incomplete outcome data (attrition bias) | High risk | ~9% lost to follow‐up in control; ~20% in primary care physician (PCP); ~14% in peer; ~24% in Educational. Provide overall reasons for losses, but do not pinpoint per arm and numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: HbA1c, do not describe objective laboratory methods. |
| Selective reporting (reporting bias) | High risk | Do not match protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Simmons 2004.
| Study characteristics | ||
| Methods |
The New Zealand Diabetes Passport Study: a randomized controlled trial of the impact of a diabetes passport on risk factors for diabetes‐related complications Cluster‐RCT (135 clusters with 135 providers), conducted in practices in Auckland, Hawkes Bay and Ashburton, New Zealand Two arms: 1. Control (control arm) and 2. Passport (intervention arm) |
|
| Participants | Control arm N: 176 Intervention arm N: 222 Diabetes type: type 1 and type 2 Mean age: 51.5 ± 10.0 % Male: 52.6 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.2 (1.6), post 9.3 (NR) Intervention arm: pre 9.4 (1.5), post 9.1 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 143.0 (22.0), post 141.0 (NR) Intervention arm: pre 138.0 (20.0), post 137.0 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 82.0 (11.0), post 82.0 (NR) Intervention arm: pre 83.0 (11.0), post 82.0 (NR) 4) Smoking cessation, N smokers (%) Control arm: pre 23 (13), post 14 (9) Intervention arm: pre 39 (18), post 27 (14) |
|
| Funding source | Roche Diagnostics for funding and support, Eli Lilly and the Hawkes Bay Research Foundation, BioRad Laboratories for providing HbA1c capillary collection kits and supporting HbA1c analyses | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation to Passport or Control practice used random number sheets. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT, unit of allocation by practice. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ no P values reported, looks balanced. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1 ‐ no P values but looks balanced. |
| Incomplete outcome data (attrition bias) | Unclear risk | 29 lost in intervention group (13%) and 26 lost in control group (15%), reasons not provided |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | HbA1c objectively measured, smoking cessation subjective. Patient blinding was partly successful (% thought Passport: Passport 24.9%, Control 16.0%; % thought Control: Passport 12.7%, Control 16.0%). |
| Selective reporting (reporting bias) | High risk | No protocol or registry. Methods section vague about outcomes, but seems like some are missing (insulin use, exercise, smoking). |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT; unlikely that control group received the passport. |
| Other bias | Low risk | No other evidence of risk of bias. |
Simpson 2011.
| Study characteristics | ||
| Methods |
Effect of adding pharmacists to primary care teams on blood pressure control in patients with type 2 diabetes Patient RCT, conducted in 5 primary care clinics (affiliated with the Edmonton South Side Primary Care Network), Canada Two arms: 1. Control patients (control arm) and 2. Intervention patients (intervention arm) |
|
| Participants | Control arm N: 129 Intervention arm N: 131 Diabetes type: type 2 Mean age: 59.1 ± 11.6 % Male: 42.7 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes |
|
| Outcomes | 1) Antihypertensives (ACE inhibitor or angiotensin II receptor blockers), N users (%) Control arm: pre NR (NR), post 6 (5) Intervention arm: pre NR (NR), post 24 (18) 2a) Retinopathy screening (ophthalmologist visit), N screened (%) Control arm: pre NR (NR), post 39 (30) Intervention arm: pre NR (NR), post 31 (24) 2b) Retinopathy screening (optometrist visit), N screened (%) Control arm: pre NR (NR), post 25 (19) Intervention arm: pre NR (NR), post 30 (23) 3) HbA1c, mean % (SD) Control arm: pre 7.3 (1.3), post 7.3 (NR) Intervention arm: pre 7.5 (1.6), post 7.4 (NR) 4) SBP, mean mmHg (SD) Control arm: pre 128.3 (15.7), post 125.8 (NR) Intervention arm: pre 130.4 (14.9), post 123.0 (NR) 5) DBP, mean mmHg (SD) Control arm: pre 73.9 (10.8), post 74.5 (NR) Intervention arm: pre 74.4 (10.0), post 72.1 (NR) 6) LDL, mean mg/dL (SD) Control arm: pre 93.2 (27.8), post 89.3 (NR) Intervention arm: pre 93.6 (30.9), post 84.7 (NR) |
|
| Funding source | Operating grant funding was provided by the Canadian Diabetes Association, the Institute of Health Economics, and the Alberta Heritage Foundation for Medical Research (AHFMR) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A central randomization service provided computer‐generated random sequences…" |
| Allocation concealment (selection bias) | Low risk | Quote: "...Central randomization service….Pharmacists, analysts, and investigators were unaware of block size and allocation sequence to preserve allocation concealment." |
| Patient's baseline characteristics (selection bias) | Unclear risk | No P values in tables. |
| Patient's baseline outcomes (selection bias) | Unclear risk | They state that baseline blood pressure were the same between both groups. Do not provide a table of characteristics. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis was done with last observation carried forward. Baseline values not provided, however can assume its based on those randomised. Numbers and reasons for loss to follow‐up provided and pretty balanced. The authors also note that those who did not complete the study (in both arms) were similar for all comparisons (P > 0.05 for all comparisons), and that baseline characteristics were the same between those who dropped out for intervention and control. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Blood pressure with automated machine. Outcome assessors were blinded. Allocation concealment was maintained from the pharmacists, analysts and investigators. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches. |
| Risk of contamination (other bias) | High risk | Quote: "…there was the possibility of 'contamination' or 'cointervention' because both intervention and control patients were drawn from the same primary care team." |
| Other bias | Low risk | Information not available. |
Sinclair 2012.
| Study characteristics | ||
| Methods |
Diabetes in care homes: a cluster randomised controlled trial of resident education Clustered RCT (51 clusters and NA providers), conducted in 1) residential care homes identified in Coventry and Warwickshire (UK), 2) medical review was undertaken by a diabetologist (physician). Who led education programme not reported. In United Kingdom. 2 arms: 1. Control: usual care (control arm) and 2. Intervention: medical review education programme (intervention arm) |
|
| Participants | Control arm N: 45 Intervention arm N: 57, NA, NA Diabetes type: 4 Mean age: 81.41 ± 13 % Male: 30.44 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Clinician education Intervention arm: (medical review education programme arm) 1) Case management 2) Clinician education 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This research received funding from Servier UK as an unrestricted educational grant | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Homes were allocated to intervention or control group independently of the research team according to a computer‐generated simple randomisation list. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Cluster comparison not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The groups seem similar regarding age, race and gender, but no information is provided on income and education (which do not seem very related to this population). Table 2. P value is > 0.01. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not recorded for all participants for several categories, and hospitalisation status was significantly different. |
| Incomplete outcome data (attrition bias) | High risk | During the study period, 19 participants (8 (14%) intervention, 11 (24%) control) died or moved out of the home and so were unavailable for follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, harms. |
| Selective reporting (reporting bias) | High risk | No protocol registered. No follow‐up of hypertension. |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT. |
| Other bias | Low risk | No evidence of other bias. |
Skeie 2009.
| Study characteristics | ||
| Methods |
Self‐monitoring of blood glucose in type 1 diabetes patients with insufficient metabolic control: focused self‐monitoring of blood glucose intervention can lower glycated hemoglobin A1C Patient RCT, conducted in a diabetes outpatient clinic (Stavanger University Hospital, Stavanger, Norway), Norway Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 65 Intervention arm N: 69 Diabetes type: type 1 Mean age: NR ± NR % Male: NR Longest follow‐up: 9 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.6 (0.1), post 8.8 (NR) Intervention arm: pre 8.7 (0.1), post 8.2 (1.2) |
|
| Funding source | Hemocue AB, Ängelholm, Sweden, provided funds to the Norwegian Quality Improvement of Laboratory Services in Primary Care for performing this study. Hemocue AB did not take part in the design of the protocol, conduct of the study, or interpretation and publication of results. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Unclear in text and P values not provided in table. |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: "The randomization resulted in comparable study groups with no major differences." |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis, but not sure if true. Reasons and numbers for lost to follow‐up provided and disproportionate; may be due to the fact that intervention group consented after trial was complete. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c using high performance liquid chromatography. Primary care physicians were blinded when data was collected (NB: study investigators were not blinded where visits happened for intervention). However, study participants were not blinded; possible Hawthorne effect. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes in protocol not reported. |
| Risk of contamination (other bias) | Low risk | Quote: "The clinical research location and the study nurse were different and physically separated from the outpatient clinic with the outpatient clinic personnel caring for the control group and regular patients otherwise." |
| Other bias | Low risk | None. |
Smith 1987.
| Study characteristics | ||
| Methods |
A controlled trial to increase office visits and reduce hospitalizations of diabetic patients RCT (NA clusters and NA providers), conducted in 1) Regenstrief Health Center, the outpatient facility of Wishard Memorial Hospital, Indianapolis, USA. Home visits. 2) physician and nurse. In United States of America. 2 arms: 1. Control: usual care (control arm) and 2. Intervention: appointment follow‐up (intervention arm) |
|
| Participants | Control arm N: 429 Intervention arm N: 425, NA, NA Diabetes type: 4 Mean age: 59.45 ± 6.9 % Male: 25.55 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education 2) Patient reminders Intervention arm: (appointment follow‐up) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | Supported in part by Public Health Services Research Grant P60 20542 from the National Institutes of Health and by a grant from the Robert Wood Johnson Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After stratification, the patients were randomised to either the control or the intervention group by coin flip using a block size of 2. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | "Intervention and control groups did not differ significantly in these characteristics either", suggesting that characteristics were not significantly different at baseline. |
| Patient's baseline outcomes (selection bias) | Unclear risk | "Intervention and control groups did not differ significantly in these characteristics either", suggesting that outcomes were not significantly different at baseline. |
| Incomplete outcome data (attrition bias) | High risk | The number lost is 26% in each arm. The reasons are explained. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c and harms. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol, but the outcomes in the methods match the ones in the results. |
| Risk of contamination (other bias) | Unclear risk | Intervention did not seem to be available to control group. |
| Other bias | Low risk | None found. |
Smith 2004.
| Study characteristics | ||
| Methods |
The North Dublin randomized controlled trial of structured diabetes shared care Cluster‐RCT (30 clusters with 50 providers), conducted in general practices in Ireland Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 87 Intervention arm N: 96 Diabetes type: type 2 Mean age: 65.1 ± 11.6 % Male: 55.5 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Clinician education |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre 50 (57), post 44 (51) Intervention arm: pre 45 (47), post 60 (63) 2) Statins, N users (%) Control arm: pre 14 (16), post 30 (34) Intervention arm: pre 21 (22), post 44 (46) 3) Retinopathy screening (fundoscopy), N screened (%) Control arm: pre NR (NR), post 34 (39) Intervention arm: pre NR (NR), post 58 (60) 4) Foot screening, N screened (%) Control arm: pre 42 (48), post 45 (52) Intervention arm: pre 44 (46), post 59 (61) 5a) Renal screening (microalbumin), N screened (%) Control arm: pre NR (NR), post 10 (11) Intervention arm: pre NR (NR), post 43 (45) 5b) Renal screening (creatinine), N screened (%) Control arm: pre NR (NR), post 8 (9) Intervention arm: pre NR (NR), post 44 (46) 6) HbA1c, mean % (SD) Control arm: pre 6.6 (1.9), post 6.7 (NR) Intervention arm: pre 6.9 (1.6), post 7.0 (NR) 7) SBP, mean mmHg (SD) Control arm: pre 167.0 (28.0), post 163.4 (NR) Intervention arm: pre 162.0 (26.0), post 157.7 (NR) 8) DBP, mean mmHg (SD) Control arm: pre 92.0 (14.0), post 90.4 (NR) Intervention arm: pre 88.0 (14.0), post 89.1 (NR) |
|
| Funding source | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1, P values > 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | See Table 1, P values > 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Smith 2008.
| Study characteristics | ||
| Methods |
Chronic care model and shared care in diabetes: randomized trial of an electronic decision support system Cluster‐RCT (94 clusters with 94 providers), conducted in primary care practices affiliated with Mayo Clinic in Rochester, MN, USA Two arms: 1. No virtual consultation (control arm) and 2. Virtual consultation (intervention arm) |
|
| Participants | Control arm N: 279 Intervention arm N: 360 Diabetes type: type 1 and type 2 Median age: 62.6 (range: 22 to 92) % Male: 47.5 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Electronic patient registry 2) Clinician education 3) Clinician reminders |
|
| Outcomes | 1) Aspirin 2) Statins 3) HbA1c 4) SBP 5) DBP 6) LDL 7) Controlled hypertension (< 130/80 mmHg) |
|
| Funding source | Supported by unrestricted grants from Novo‐Nordisk Copenhagen, American Diabetes Association, and Mayo Clinic. Dr Smith is 1 of 16 inventors of the Diabetes Electronic Management System (DEMS), from which (according to Mayo Clinic policy) all royalties will support education and clinical research in the care of people with diabetes mellitus. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ no P values but looks unbalanced (physician gender, number of patients). |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1 ‐ age and sex P > 0.05 but no education reported. |
| Patient's baseline outcomes (selection bias) | Low risk | Table ‐ P values all > 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Sone 2010.
| Study characteristics | ||
| Methods |
Long‐term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study) RCT (NA clusters and NA providers), conducted in 1) Patients from outpatient clinics in 59 university and general hospitals nationwide that specialise in diabetes care (Japan). 2) Counselling was provided by physicians, nurses, dietitians and other co‐medical staff during each outpatient clinic visit. Telephone counselling done by nurses, dietitians and psychotherapists who were trained in diabetes education. In Japan. 2 arms: 1. Control (CON ‐ conventional treatment) (control arm) and 2. Intervention (INT ‐ lifestyle intervention group) (intervention arm) |
|
| Participants | Control arm N: 1016 Intervention arm N: 1017, NA, NA Diabetes type: 2 Mean age: 58.5 ± NR % Male: 53.47 Longest follow‐up: 96 months |
|
| Interventions |
Control arm: (CON ‐ conventional treatment) Intervention arm: (INT ‐ lifestyle intervention group) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Smoking status |
|
| Funding source | This study was financially supported by the Ministry of Health, Labour and Welfare, Japan. The sponsor had no role in the design and conduct of the study. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and open‐label allocation were done by a central computer system. |
| Allocation concealment (selection bias) | Low risk | Randomisation and open‐label allocation were done by a central computer system. |
| Patient's baseline characteristics (selection bias) | Low risk | Clinical characteristics of the patients at baseline and at the 4th and 8th year after the start of the study are shown in Table 1. There were no differences in all variables between the 2 groups at baseline. |
| Patient's baseline outcomes (selection bias) | Low risk | Clinical characteristics of the patients at baseline and at the 4th and 8th year after the start of the study are shown in Table 1. There were no differences in all variables between the 2 groups at baseline. |
| Incomplete outcome data (attrition bias) | High risk | They lost 729 patients at 8 years follow‐up out of 2033 randomised (35.9% lost overall, 38.7% in the control group and 33.0% in the intervention group). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Many of our outcomes of interest were objectively measured (HbA1c, SBP, DBP) except smoking status (questionnaire filled by patients) and drug use (collected through an annual report from each physician). Nothing about blinding. |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol. Outcomes reported in the protocol are vague (parameters and indices related to glycaemic control, diabetic complications, dyslipidaemia, hypertension, obesity and atherosclerosis were measured several times a year). Data obtained each year for 8 years; only baseline, 4 years and 8 years data reported. Data not shown for the proportion of patients using anti‐platelet agents. |
| Risk of contamination (other bias) | Unclear risk | "As basal therapeutic management of all patients in both the CON and INT groups, regular specialists’ care was provided throughout the study period and patients were treated as they were before the study started. This included dietary advice by an administrative dietitian, using the ‘Food Exchange Lists Dietary Guidance for Persons with Diabetes’. Same physicians followed patients from all groups. They might have changed their approach with patients in the usual care group. Another reason for the limited effects is that, in our study, even patients in the CON group received routine lifestyle education by diabetes specialists, which is an inevitable part of the usual care of persons with diabetes." |
| Other bias | Low risk | None. |
Song 2009.
| Study characteristics | ||
| Methods |
Intensive management program to improve glycosylated hemoglobin levels and adherence to diet in patients with type 2 diabetes Patient RCT, conducted in an university‐affiliated diabetes centre, South Korea Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 24 Intervention arm N: 25 Diabetes type: type 2 Mean age: 50.3 ± 11.0 % Male: 43.0 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.0 (1.2), post 8.6 (1.3) Intervention arm: pre 9.4 (1.8), post 7.1 (1.2) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Not a cluster‐RCT and patients not randomised to equipment. |
| Other bias | Low risk | Information not available. |
Sonnichsen 2010.
| Study characteristics | ||
| Methods |
The effectiveness of the Austrian disease management programme for type 2 diabetes: a cluster‐randomised controlled trial Cluster‐RCT (6 clusters with 92 providers), conducted in 275 eligible primary care physicians with a contract with the public health insurance in Austria (province of Salzburg), Austria Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 840 Intervention arm N: 654 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Clinician education 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 430 (51) Intervention arm: pre NR (NR), post 461 (71) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 379 (45) Intervention arm: pre NR (NR), post 479 (74) 3) HbA1c, mean % (SD) Control arm: pre 7.3 (1.3), post 7.1 (NR) Intervention arm: pre 7.5 (1.5), post 7.1 (NR) 4) SBP, mean mmHg (SD) Control arm: pre 139.0 (17.0), post 138.3 (NR) Intervention arm: pre 141.0 (19.0), post 138.5 (NR) 5) DBP, mean mmHg (SD) Control arm: pre 82.0 (10.0), post 81.4 (NR) Intervention arm: pre 83.0 (11.0), post 81.8 (NR) 6) LDL, mean mg/dL (SD) Control arm: pre 111.0 (35.2), post 109.4 (NR) Intervention arm: pre 111.0 (37.1), post 111.4 (NR) |
|
| Funding source | Paracelsus Medical University, Public Health Insurance of Salzburg, Salzburg Savings Bank, Roche Diagnostics | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…computerised sequence generation." |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not provided. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Quote: BMI (P = 0.01); cholesterol (P = 0.02). |
| Patient's baseline outcomes (selection bias) | Low risk | Quote: HbA1c (P = 0.10); LDL (P = 0.78); SBP (P = 0.12); DBP (P = 0.41). |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat and per‐protocol analysis. For intention‐to‐treat analysis, after randomisation, n = 6 general practitioners (GP) practices withdrew before recruiting patients, and n = 5 in intervention group were excluded since they withdrew consent and did not provide baseline values. They excluded these values and considered it an intention‐to‐treat analysis. Numbers and reasons for lost to follow‐up provided. Percentages are similar. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Primary: HbA1c. Secondary: BP, lipids, eye and foot exams. Objective methods not described. Blinding was not possible in the study (unblinded). |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything matches with manuscript. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | None. |
Spencer 2011.
| Study characteristics | ||
| Methods |
Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) African American and Latino adult participants recruited from 2 health systems in Detroit, Michigan. All participants lived in either southwest Detroit, where residents were predominantly Latino of Mexican origin (70%), or eastside Detroit, which is largely African American (80%). Participants from southwest Detroit received medical care at a federally qualified community health centre, whereas participants from eastside Detroit received medical care at a major local health system. Group education sessions were held at community locations. Home visits and phone calls. 2) Trained a Community Health Worker (CHWs), known in this study as family health advocates, delivered the intervention. In United States of America. 2 arms: 1. Control (delayed control group ) (control arm) and 2. Intervention (community health worker ) (intervention arm) |
|
| Participants | Control arm N: 99 Intervention arm N: 84, NA, NA Diabetes type: 2 Mean age: 52.80 ± 10.7 % Male: 29.27 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (delayed control group) Intervention arm: (community health worker) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This research was supported by the National Institute of Diabetes and Digestive and Kidney Disease (grant R18DK0785501A1), Centers for Disease Control and Prevention (Cooperative Agreement No. U50/CCU417409), the Michigan Diabetes Research and Training Center (NIH grant 5P60‐DK20572), and the Robert Wood Johnson Foundation Clinical Scholars Program. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. We randomised African American and Latino participants with diabetes into a CHW intervention group or a control group in which the CHW intervention was delayed for 6 months. Participants were stratified by race/ethnicity and health care site. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Because age significantly differed by treatment group, it is included as a covariate in outcomes analyses. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. BMI and HbA1c have P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | African American participants were more likely to withdraw from the study and to be missing HbA1c data than were Latino participants. Of 183 randomised participants, 164 completed the baseline interview. At the 6‐month follow‐up, 136 participants completed the study protocols and were analysed for the primary outcome (attrition rate of 17.7%). However, we obtain an attrition rate of 25.7%, with 29.8% lost in the intervention group and 22.2% in the control group. High numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Our outcomes of interest are correctly reported. |
| Risk of contamination (other bias) | High risk | "Because of severe medical conditions, 3 participants assigned to the control group received the CHW intervention; however, following intention‐to‐treat principles, these participants remain assigned to the control group in our analyses. Participants in the control group were contacted once per month to update contact information. All participants in the study, whether in the intervention or control group, received information on, and had access to, REACH Detroit community activities that provided free, publicly available healthy eating demonstrations, physical fitness activity (e.g., dance and exercise classes, walking clubs), and a weekly community farmers’ produce market. All participants also received health care at facilities in which health care providers were trained by REACH Detroit in culturally competent diabetes care through our health systems intervention." |
| Other bias | Low risk | None. |
Spencer 2018.
| Study characteristics | ||
| Methods |
Outcomes at 18 months from a community health worker and peer leader diabetes self‐management program for Latino adults RCT (NA clusters and NA providers), conducted in 1) All work has been conducted with the Community Health and Social Services Center (CHASS), a federally qualified community health centre serving the predominantly Latino community in southwest Detroit, and guided by a steering committee of partnership members. Intervention delivered by in‐person group sessions or telephone outreach. 2) Intervention delivered by community health worker (CHW) alone; or by CHW followed by peer leaders (PLs). In United States of America. 3 arms: 1. Control (EUC: enhanced usual care) (control arm) and 2. Intervention 1 (CHW‐only: CHW‐led DSME plus CHW outreach) (intervention arm), 3. Intervention 2 (CHW‐PL: CHW‐led DSME plus peer leaders outreach) (other arm) |
|
| Participants | Control arm N: 73 Intervention arm N: 89, 60, NA Diabetes type: 2 Mean age: 48.9 ± NR % Male: 39.2 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (EUC: enhanced usual care) 1) Patient education Intervention arm: (CHW‐only: CHW‐led DSME + CHW outreach) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (CHW‐PL: CHW‐led DSME + peer leaders outreach) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This research was supported by a Peers for Progress grant from the American Association of Family Physicians Foundation, by the National Institute of Diabetes and Digestive and Kidney Diseases (grant P30‐DK092926 to the Michigan Center for Diabetes Translational Research and grant R18‐DK‐0785501A1), and by the Centers for Disease Control and Prevention (co‐operative agreement no. U50/CCU417409). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 222 participants were first randomised into the CHW intervention arm (n = 149) or the EUC (n = 73) arm using a computer‐generated process with concealed allocation. At 6 months (immediately after the CHW intervention), CHW intervention participants were further randomised into the CHW‐only intervention (n = 89) or the CHW+PL intervention (n= 60) groups. |
| Allocation concealment (selection bias) | Low risk | The 222 participants were first randomised into the CHW intervention arm (n = 149) or the EUC (n = 73) arm using a computer‐generated process with concealed allocation. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. The number of high school graduates is significantly different between groups (P = 0.008). Quote: "Educational status differed by treatment group and was therefore included as a covariate in outcome analyses, coded as a binary indicator for high school graduation." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All physiological and psychological measures have P values higher than 0.05. Quote: "No physical or psychosocial outcomes significantly differed between groups at baseline." |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. 35/73, 36/89 and 37/60 patients randomised completed the 18 months follow‐up in the control group (EUC, 52% lost), CHW‐only (60% lost) and CHW‐LP (38% lost), respectively. Really high and unbalanced numbers of lost. Quote: "Third, we experienced attrition in our sample through 18 months. Although expected, the reduced sample size may have affected our ability to detect some statistically significant results." |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest were objectively measured (HbA1c, BP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol (the protocol reported in the paper give details on a preliminary study). Quote from methods: "Secondary clinical outcomes included a lipid panel (total cholesterol, LDL cholesterol [LDLc], and HDL cholesterol [HDLc])". However, they only report LDL data. No data for BMI post‐intervention. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that control patients went to weekly group sessions or received telephone outreach both led by community health worker (CHW) or peer leaders (PLs). The control group received a 2‐hour class conducted by a research assistant. |
| Other bias | Low risk | No evidence of other risk of bias. |
Sperl‐Hillen 2010.
| Study characteristics | ||
| Methods |
Simulated physician learning program improves glucose control in adults with diabetes Clustered RCT (11 clusters and 86 providers), conducted in 1) The study was conducted at HealthPartners Medical Group (HPMG), a large medical group in Minnesota that serves about 230,000 patients. Eleven clinics with 41 consenting primary care physicians (PCPs) were randomised. 2) Each intervention primary care physician (PCP) was assigned to 12 simulated type 2 diabetes cases (Simulated Physician Learning Program). A group of PCP experts actively involved in diabetes guideline development designed the cases and the research team assigned tailored cases to PCPs. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Simulated Physician Learning Program) (intervention arm) |
|
| Participants | Control arm N: 2438 Intervention arm N: 2710, NA, NA Diabetes type: 2 Mean age: 56.4 ± NR % Male: 51.1 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Simulated Physician Learning Program) 1) Audit and feedback 2) Clinician education |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | This project was funded by NIDDK Grant #R01 DK068314 to HealthPartners Research Foundation. J.M.S.‐H. received support indirectly through HealthPartners Research Foundation for multisite drug trials funded by Merck, GlaxoSmithKline, and Abbott Pharmaceuticals and from Merck for a randomised trial on educational methods for patients with diabetes. H.L.E. owns stock in Pfizer. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Eleven HPMG clinics were randomly selected and block randomised on the basis of baseline quality of diabetes care and number of consenting primary care physicians (PCPs) to either receive or not receive the intervention. |
| Allocation concealment (selection bias) | Low risk | Clustered‐RCT. |
| Provider's baseline characteristics (selection bias) | Low risk | Table 2. All P values above 0.05. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. Randomisation at the clinic level resulted in an intervention arm with a higher proportion of younger (P = 0.012) and male patients (P < 0.001). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. All P values above 0.05. |
| Incomplete outcome data (attrition bias) | High risk | Randomisation at clinic level. 32 patients died in each arm. They lost 1 physician in the control arm. They analysed 1570/2438 (64%, 36% lost) patients in the control arm, and 1847/2710 (68%, 32% lost) in the intervention arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP, DBP and LDL). |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol. Unsure if protocol mentioned in paper is for the same study? Different study start dates, differences in intervention, arms, outcomes and study design. Outcomes in the protocol do not mention many outcomes reported in the paper (A1C, blood pressure and LDL cholesterol). |
| Risk of contamination (other bias) | Low risk | Clustered RCT. The unit of randomisation was by clinics. |
| Other bias | Low risk | None. |
Sperl‐Hillen 2013.
| Study characteristics | ||
| Methods |
Are benefits from diabetes self‐management education sustained? RCT (NA clusters and NA providers), conducted in 1) ABQ Health Partners in Albuquerque, New Mexico and HP Clinics in Minneapolis, Minnesota. 2) individual sessions and group sessions delivered by either nurse or dietitian certified diabetes educators. In United States of America. 3 arms: 1. Control: usual care (control arm) and 2. Group intervention (intervention arm), 3. Individual intervention (other arm) |
|
| Participants | Control arm N: 134 Intervention arm N: 243, 246, NA Diabetes type: 2 Mean age: 62 ± 10.2 % Male: 51 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (group) 1) Team change 2) Patient education 3) Promotion of self‐management Intervention arm: (individual) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by Merck Sharp and Dohme Corp. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Consented participants were randomly assigned to GE, IE or UC using a random allocation sequence in a 2:2:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Low risk | Looks balanced. |
| Incomplete outcome data (attrition bias) | Low risk | The number of patients lost to long‐term follow‐up in each group is about 6% to 7%, and the reasons are explained. 1 dropout, 8 deaths total. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. They discuss some of the outcomes in other papers. |
| Risk of contamination (other bias) | Unclear risk | Same educators in IE and GE groups. |
| Other bias | Low risk | No evidence of other bias. |
Sriram 2011.
| Study characteristics | ||
| Methods |
Impact of pharmaceutical care on quality of life in patients with type 2 diabetes mellitus Patient RCT, conducted in general medicine department of a multi specialty tertiary care teaching hospital located at Coimbatore, South of India, South India Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 60 Intervention arm N: 60 Diabetes type: type 2 Mean age: NR ± NR % Male: 50.0 Longest follow‐up: 8 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SE) Control arm: pre 9.0 (0.5), post 8.3 (0.2) Intervention arm: pre 8.4 (0.3), post 6.7 (0.2) |
|
| Funding source | This study was identified by the Tamil Nadu Pharmaceutical Sciences Welfare Trust as need of the hour and partial funding was provided to carry out the study. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | In text, data are balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P > 0.05). |
| Incomplete outcome data (attrition bias) | Low risk | No losses indicated. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Blinding not described. HbA1c analysed using an ordinary calibrated biochemical auto analyser. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Steventon 2014.
| Study characteristics | ||
| Methods |
Effect of telehealth on glycaemic control: analysis of patients with type 2 diabetes in the Whole Systems Demonstrator cluster randomised trial Cluster‐RCT (112 clusters), conducted in general practices from the three demonstration WSD sites, Cornwall, Kent and Newham in East London, England Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 213 Intervention arm N: 300 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.3 (1.7), post 8.4 (1.6) Intervention arm: pre 8.5 (1.8), post 8.2 (1.5) |
|
| Funding source | The study was funded by the Department of Health in England. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported in text or table. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Used routine medical records. |
| Selective reporting (reporting bias) | High risk | Does not match protocol. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Steyn 2013.
| Study characteristics | ||
| Methods |
Implementation of national guidelines, incorporated within structured diabetes and hypertension records at primary level care in Cape Town, South Africa: a randomised controlled trial Cluster‐RCT (18 clusters), conducted in public sector primary healthcare clinics also referred to as Community Health Centres (CHCs) in Cape Town, South Africa Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 227 Intervention arm N: 229 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Clinician education 2) Clinician reminders 3) Patient education |
|
| Outcomes | 1a) Retinopathy screening (opthalmoscopy), N screened (%) Control arm: pre 20 (9), post 7 (3) Intervention arm: pre 41 (18), post 31 (14) 1b) Retinopathy screening (visual acuity), N screened (%) Control arm: pre 11 (5), post 25 (12) Intervention arm: pre 14 (6), post 39 (18) 2) Foot screening, N screened (%) Control arm: pre 21 (9), post 31 (15) Intervention arm: pre 30 (13), post 62 (29) 3) HbA1c, mean % (SD) Control arm: pre 8.9 (NR), post 8.8 (NR) Intervention arm: pre 8.8 (NR), post 8.8 (NR) |
|
| Funding source | The development of the protocol of the study was funded as part of the Afro‐implement project funded by the European Union. Financial support for the project was provided by the South African Medical Research Council and an unrestricted grant from the pharmaceutical company, Hoechst, Marion, Roussel. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomly allocated, by stratum, to intervention or control using a computer‐generated list of random numbers." |
| Allocation concealment (selection bias) | Low risk | Not reported. But since cluster, low risk. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Mentioned in text that control group had more schooling, but no P values in table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Do not report on HbA1c baseline differences. |
| Incomplete outcome data (attrition bias) | High risk | Overall, ~11.88% lost to follow‐up in control; ~9.6% in intervention; they provide numbers of lost to follow‐up for a sub‐sample of study participants, and reasons are not provided except that it was incomplete data collection and reason why not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary for diabetics: HbA1c, objective laboratory measure was not reported. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | Information not available. |
Stone 2010.
| Study characteristics | ||
| Methods |
Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial Patient RCT, conducted in VA clinics in Pittsburgh, USA Two arms: 1. CC ‐ care co‐ordination (control arm) and 2. ACM + HT ‐ active care management with home telemonitoring (intervention arm) |
|
| Participants | Control arm N: 77 Intervention arm N: 73 Diabetes type: type 2 Mean age: NR ± NR % Male: 99.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | 1) Antihypertensives (any), N users (%) Control arm: pre 66 (90), post 68 (93) Intervention arm: pre 56 (88), post 58 (91) 2) HbA1c, mean % (SD) Control arm: pre 9.4 (1.4), post 8.6 (1.3) Intervention arm: pre 9.6 (1.6), post 7.9 (1.2) 3) SBP, mean mmHg (SD) Control arm: pre 142.3 (19.0), post 133.0 (19.0) Intervention arm: pre 144.8 (21.7), post 132.0 (24.3) 4) DBP, mean mmHg (SD) Control arm: pre 80.5 (10.1), post 75.9 (13.2) Intervention arm: pre 79.9 (13.3), post 72.4 (14.6) 5) LDL, mean mg/dL (SD) Control arm: pre 101.8 (32.0), post 91.2 (30.6) Intervention arm: pre 98.8 (36.3), post 82.3 (27.9) |
|
| Funding source | This work was supported by award W81XWH‐04‐2‐0030 from the U.S. Air Force, administered by the U.S. Army Medical Research Acquisition Activity, Fort Detrick, Maryland, and by resources and the use of facilities at the VAPHS | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Stone 2012a.
| Study characteristics | ||
| Methods |
The diabetes telemonitoring study extension: an exploratory randomized comparison of alternative interventions to maintain glycemic control after withdrawal of diabetes home telemonitoring Patient RCT, conducted in 3 VA Pittsburgh Healthcare System hospitals and 5 affiliated community‐based outpatient clinics in Pennsylvania and Ohio, USA Two arms: 1. Prev Care co‐ordination ‐ usual care/CC‐UC (control arm) and 2. Prev Care co‐ordination ‐ care co‐ordination/CC‐CC (intervention arm) |
|
| Participants | Control arm N: 29 Intervention arm N: 28 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (1.2), post 8.8 (1.4) Intervention arm: pre 8.6 (1.1), post 8.7 (1.3) |
|
| Funding source | This work was supported by award W81XWH‐04‐2‐0030 from the US Air Force, administered by the US Army Medical Research Acquisition Activity, Fort Detrick, Maryland, and by resources and the use of facilities at the VA Pittsburgh Healthcare System. A portion of the telemonitoring and other equipment costs were supported by Viterion TeleHealthcare, LLC; Tarrytown, New York. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…using a pseudo random binary sequences generated by the study statistician in Stata, with equal allocation within each initial DiaTel group." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…using a pseudo random binary sequences generated by the study statistician in Stata, with equal allocation within each initial DiaTel group." |
| Patient's baseline characteristics (selection bias) | Unclear risk | Do not provide P values for baseline values. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Do not provide P values for baseline outcome values. |
| Incomplete outcome data (attrition bias) | Low risk | Not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Quote: "Staff involved in the collection of laboratory data was blinded to randomization group." HbA1c methods not described. |
| Selective reporting (reporting bias) | High risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Since not cluster, contamination cannot be ruled out. |
| Other bias | Low risk | None. |
Stone 2012b.
| Study characteristics | ||
| Methods |
The diabetes telemonitoring study extension: an exploratory randomized comparison of alternative interventions to maintain glycemic control after withdrawal of diabetes home telemonitoring Patient RCT, conducted in 3 VA Pittsburgh Healthcare System hospitals and 5 affiliated community‐based outpatient clinics in Pennsylvania and Ohio, USA Two arms: 1. Prev Active Care Management ‐ lower‐intensity care co‐ordination/ACM‐CC (control arm) and 2. Prev Active Care Management ‐ care co‐ordination with continued home telemonitoring/ACM‐CCHT (intervention arm) |
|
| Participants | Control arm N: 21 Intervention arm N: 23 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Case management Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.0 (1.4), post 8.2 (1.0) Intervention arm: pre 7.8 (0.8), post 8.0 (1.0) |
|
| Funding source | This work was supported by award W81XWH‐04‐2‐0030 from the US Air Force, administered by the US Army Medical Research Acquisition Activity, Fort Detrick, Maryland, and by resources and the use of facilities at the VA Pittsburgh Healthcare System. A portion of the telemonitoring and other equipment costs were supported by Viterion TeleHealthcare, LLC; Tarrytown, New York. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clearly reported…"reassigned randomly." |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Do not provide P values for baseline values. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Do not provide P values for baseline outcome values. |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis, 2 missing in N1 group to follow‐up, baseline based on those analysed. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Quote: "Staff involved in the collection of laboratory data was blinded to randomization group." HbA1c methods not described. |
| Selective reporting (reporting bias) | High risk | Information not available. |
| Risk of contamination (other bias) | High risk | Quote: "…potential carry‐over effects from the patient education and medication management in the initial DiaTel study…" |
| Other bias | Low risk | None. |
Stroebel 2002.
| Study characteristics | ||
| Methods |
A randomized trial of three diabetes registry implementation strategies in a community internal medicine practice Clustered RCT (29 clusters and 29 providers), conducted in 1) The Mayo Clinic is a large multispecialty group practice in Rochester, Minnesota. The Division of Community Internal Medicine (CIM) has 35 general internists providing primary care for the local adult population. The practice is organised into practice care teams, which are typically composed of 3 or 4 physicians (MDs), 2 licensed practical nurses, 1 registered nurse (RN) and 1 appointment secretary. All physicians are salaried. The majority of patients has either fee‐for‐service or Medicare coverage. Each physician is responsible for a patient age‐ and gender‐adjusted panel of approximately 1600 patients. 2) Intervention delivered by registered nurses and physicians (RN/MD) care teams (1 RN with 3 or 4 MDs). Hot lists were generated by each team’s appointment secretary and distributed to the MD and RN. Patients on the Hot List who needed glycosylated Hgb or low‐density lipoprotein cholesterol determination received a letter from the appointment secretary. In United States of America. 3 arms: 1. Control (Group A: registry + hot lists) (control arm) and 2. Intervention 1 (Group B: registry + hot lists + team time) (intervention arm), 3. Intervention 2 (Group C: registry + hot lists + team time + automatic letters) (other arm) |
|
| Participants | Control arm N: 396 Intervention arm N: 331, 356, NA Diabetes type: 4 Mean age: 65.73 ± 10.68 % Male: 53.20 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (Group A: registery + hot lists) 1) Audit and feedback 2) Electronic patient registry Intervention arm: (Group B: registery + hot lists + team time) 1) Audit and feedback 2) Team changes 3) Electronic patient registry Intervention arm: (Group C: registery + hot lists + team time + automatic letters) 1) Audit and feedback 2) Team changes 3) Case management 4) Electronic patient registry 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein Hypertension control |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. In this randomised, prospective trial, clusters of physicians were assigned to one of 3 intervention arms: A, B or C (Table 2, below). Randomisation was based on the RN/MD care teams (one RN with 3 or 4 MDs) to ensure that the RNs in each team participated in only one care model. The randomisation resulted in a total of 9 or 10 participating physicians, each of whose panel of patients was enrolled in each of the 3 intervention arms. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | No comparison of physicians between groups. All physicians are salaried. The majority of patients has either fee‐for‐service or Medicare coverage. Each physician is responsible for a patient age‐ and gender‐adjusted panel of approximately 1600 patients. |
| Patient's baseline characteristics (selection bias) | Low risk | Top of Table 1. Groups seem similar. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Top of Table 1. Quote: "The baseline mean LDL cholesterol value in Group A was lower than in Group C." |
| Incomplete outcome data (attrition bias) | Low risk | All patients eligible for inclusion in the registry were continued in the study for the duration. New patients were added to the registry during the study but not included in the analysis. Seems like there was no loss to follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively assessed (HbA1c, LDL, blood pressure). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Clustered RCT. In this randomised, prospective trial, clusters of physicians were assigned to one of 3 intervention arms: A, B or C (Table 2, below). Randomisation was based on the RN/MD care teams (one RN with 3 or 4 MDs) to ensure that the RNs in each team participated in only one care model. The randomisation resulted in a total of 9 or 10 participating physicians, each of whose panel of patients was enrolled in each of the 3 intervention arms. However, all physicians and RN were working in the same clinic, communication between them might have happen and/or some physicians/RN might have decided to also do team meeting even if they were not assigned to this group. |
| Other bias | Low risk | None. |
Sugiyama 2015.
| Study characteristics | ||
| Methods |
Effect of a community‐based diabetes self‐management empowerment program on mental health‐related quality of life: a causal mediation analysis from a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Community‐based diabetes self‐management education (DSME) intervention (senior centres, churches, community clinics, and Los Angeles County Community and Senior Service Centers). 2) Intervention provided by health educators. In United States of America. 2 arms: 1. Control (unrelated lectures) (control arm) and 2. Intervention (community‐based DSME) (intervention arm) |
|
| Participants | Control arm N: 258 Intervention arm N: 258, NA, NA Diabetes type: 4 Mean age: 63.5 ± NR % Male: 29.05 Longest follow‐up: 7.2 months |
|
| Interventions |
Control arm: (unrelated lectures) 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: (community‐based DSME) 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | CMM received support in part from the UCLA Robert Wood Johnson Clinical Scholars Program, the U.S. Department of Veterans Affairs (Grant #67799), the UCLA RCMAR/CHIME under NIH/NIA (P30‐AG021684), and the NIH/NCATS UCLA CTSI (UL1TR000124). OKD received support from a Career Development Award from the NIH/NIA (K08‐AG033630) as well as the Harold Amos Medical Faculty Development Award from the Robert Wood Johnson Foundation. TS was funded by National Center for Global Health and Medicine and Honjo International Scholarship Foundation. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants then were randomly assigned to either the intervention or the control group on an individual basis using sealed envelopes with cards marked either “Study” or “Control”. |
| Allocation concealment (selection bias) | Unclear risk | Participants then were randomly assigned to either the intervention or the control group on an individual basis using sealed envelopes (opaque?) with cards marked either “Study” or “Control”. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Baseline characteristics and measurements of participants by group. All patient characteristics have P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Baseline characteristics and measurements of participants by group. HbA1c, P = 0.93. |
| Incomplete outcome data (attrition bias) | High risk | Control group: 257 patients at baseline for HbA1c, 217 at 6 months (15.6% dropouts). Intervention group: 257 patients at baseline for HbA1c, 224 at 6 months (12.8% dropouts). Reasons for dropouts not reported. Approximately 20% of participants in each group dropped out from the study. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol (protocol first posted on December 2005, study started on October 2004). They were supposed to look at the changes in blood pressure, cholesterol and weight (secondary outcomes). |
| Risk of contamination (other bias) | High risk | Both the control and the intervention arms were exposed to most of the intervention strategies (one‐on‐one sessions to review their laboratory and biometric data, the opportunity to have the study team share their results with their physician, all study participants were given glucose meters and testing strips with a training to use it). However, education about diabetes was given only to the intervention group, including a video and a pictorial workbook. |
| Other bias | Low risk | No evidence of other bias. |
Suh 2014.
| Study characteristics | ||
| Methods |
A randomized controlled trial of an internet‐based mentoring program for type 1 diabetes patients with inadequate glycemic control RCT (NA clusters and NA providers), conducted in 1) The participants were patients receiving typical diabetes care in Samsung Medical Center in Seoul, Republic of Korea. 2) counselling with a medical social worker; meetings with the investigators were held 5 times during the study to report progress and discuss any problems during the study. All participants received face‐to‐face diabetes care with physicians at clinic visits every 6 weeks. In South Korea. 2 arms: 1. Control (no feedback) (control arm) and 2. Intervention: (mentor feedback) (intervention arm) |
|
| Participants | Control arm N: 31 Intervention arm N: 26, NA, NA Diabetes type: 1 Mean age: 32.56 ± 10.11 % Male: 36.84 Longest follow‐up: 2.76 months |
|
| Interventions |
Control arm: (no feedback) 1) Team change 2) Electronic patient registry Intervention arm: (mentor feedback) 1) Team change 2) Electronic patient registry 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This study was supported by a grant from i‐SENS Inc. (Seoul, Korea) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study co‐ordinator allocated the participants to different groups using a computerised random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and greater than 0.05, no significant differences. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided and greater than 0.05, no significant differences. |
| Incomplete outcome data (attrition bias) | High risk | 1 lost in mentor group (~4%), 4 lost in control group (~13%). Reasons for loss provided but unbalanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c and harms. |
| Selective reporting (reporting bias) | Low risk | Protocol matches published report. |
| Risk of contamination (other bias) | Unclear risk | All participants received face‐to‐face diabetes care with physicians at clinic visits every 6 weeks. |
| Other bias | Low risk | None. |
Sun 2008.
| Study characteristics | ||
| Methods |
An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes Patient RCT, conducted with employees of Shanghai Turbine Company, Electric Machinery Company, and Huadong Hospital, all in Shanghai, China Two arms: 1. Reference (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 100 Diabetes type: type 2 Mean age: 51.0 ± 1.0 % Male: 71.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Team changes 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 7.0 (1.4), post 7.1 (0.2) Intervention arm: pre 7.1 (1.0), post 6.3 (0.1) 2) SBP, mean mmHg (SE) Control arm: pre 135.0 (2.0), post 133.3 (2.0) Intervention arm: pre 131.0 (1.0), post 123.5 (1.0) 3) DBP, mean mmHg (SE) Control arm: pre 89.0 (2.0), post 88.9 (1.0) Intervention arm: pre 87.0 (2.0), post 83.6 (1.0) 4) LDL, mean mg/dL (SE) Control arm: pre 112.1 (3.9), post 104.4 (NR) Intervention arm: pre 112.1 (3.9), post 100.5 (NR) |
|
| Funding source | This study was funded by Abbott Laboratories. RE Riley and VA Mustad are employees of Abbott Laboratories. J Sun and Y Wang received funding from Abbott Laboratories for conducting this trial. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | High risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Author from industry; industry provided funding and statistical help. |
Sun 2019.
| Study characteristics | ||
| Methods |
Mobile phone‐based telemedicine practice in older Chinese patients with type 2 diabetes mellitus: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) the outpatient endocrinology department of the First Affiliated Hospital of Jilin University, China 2) Intervention delivered by specialist dieticians and endocrinology medical teams in China 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (mHealth management app ‐ telemedicine) (intervention arm) |
|
| Participants | Control arm N: 47 Intervention arm N: 44, NA, NA Diabetes type: 2 Mean age: 68.04 ‐ 67.9 ± 11.88 % Male: 40.66 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Promotion of self‐management Intervention arm: (mHealth management app ‐ telemedicine) 1) Case management 2) Electronic patient registry 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | This study was supported by the Science Technology Department of Jilin Province (20180623006TC) and the Interdisciplinary Project of First Hospital of Jilin University (JDYYJC010) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to the intervention and control groups using the random number sequence generated by SPSS Statistics version 17.0 (IBM Corp). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. No significant between‐group differences were observed with age, physical findings or biochemical indices. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. No significant between‐group differences were observed with age, physical findings or biochemical indices. All P values > 0.05. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if there were any losses, not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered; only HbA1c mentioned as outcome in protocol. |
| Risk of contamination (other bias) | Low risk | mHealth app was only available to intervention patients, dieticians only met with control group at beginning and end of study. |
| Other bias | Low risk | No evidence of other bias. |
Takami 2008.
| Study characteristics | ||
| Methods |
Developmental process of disease management program of type 2 diabetes with a view to acquiring self‐management skills: effects of the trial implementation RCT (NA clusters and NA providers), conducted in 1) Two university hospitals in Hiroshima and Shiga prefectures. 2) A nurse and a registered dietitian conducted interviews once a month and telephone monitoring. In Japan. 2 arms: 1. Control (current treatment) (control arm) and 2. Intervention (structured educational program) (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 21, NA, NA Diabetes type: 2 Mean age: 56.81 ± 6.45 % Male: 46.88 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (current treatment) Intervention arm: (structured educational programme) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Patients randomised to the intervention group and control group in the order of introduction. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | All patient characteristics have P values higher than 0.05 except for insulin drug use. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All outcomes have P values higher than 0.05 except total cholesterol (P = 0.03). |
| Incomplete outcome data (attrition bias) | High risk | They lost 0/15 patient in the control group (0%) and 4/21 in the intervention group (19%). Unbalanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest were objectively measured (HbA1c and BP, from health records). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Patients recruited from 2 hospitals. Unlikely that control group received intervention. |
| Other bias | Low risk | None identified. |
Tang 2013.
| Study characteristics | ||
| Methods |
Online disease management of diabetes: Engaging and Motivating Patients Online with Enhanced Resources‐Diabets (EMPOWER‐D), a randomized controlled trial patient RCT (NA clusters and NA providers), conducted in a not‐for‐profit health organization with 1000 multispecialty physicians. In USA. 2 arms: (control arm) (intervention arm) |
|
| Participants | Control arm N: 213 Intervention arm N: 202, NA, NA Diabetes type: 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminder 8) Financial incentives |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Agency for Healthcare Research and Quality, grant No 1R18HS017179‐01 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation technique. |
| Allocation concealment (selection bias) | High risk | Because of minimisation. |
| Patient's baseline characteristics (selection bias) | High risk | Not in text or in table, but they used minimisation. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | ~9% lost to follow‐up in N1, and ~7% in N2; however, reasons for losses are not described. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcome: HbA1c, objective laboratory methods no described. |
| Selective reporting (reporting bias) | Low risk | Matches protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None identified. |
Tang 2015.
| Study characteristics | ||
| Methods |
Peer‐Led, Empowerment‐Based Approach to Self‐Management Efforts in Diabetes (PLEASED): a randomized controlled trial in an African American Community RCT (NA clusters and NA providers), conducted in 1) This study was conducted in partnership with the Ann Arbor Community Center, Ann Arbor, and Parkside Community Center, Ypsilanti, Michigan, USA. 2) Intervention provided by certified diabetes educator and peer leaders (PLs). In United States of America. 2 arms: 1. Control (DMSE only) (control arm) and 2. Intervention (peer‐led DSMS group) (intervention arm) |
|
| Participants | Control arm N: 52 Intervention arm N: 54, NA, NA Diabetes type: 2 Mean age: 56.3 ± 12.1 % Male: 33 Longest follow‐up: 15 months |
|
| Interventions |
Control arm: (DMSE only) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: (peer‐led DSMS group) 5) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Funding for this research was provided by the American Academy of Family Physicians Foundation through the Peers for Progress program with support from the Eli Lilly and Company Foundation and by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant Number R18DK0785501A1). The funding sources had no role in the study design; data collection; administration of the interventions; analysis, interpretation, or reporting of data; or decision to submit the findings for publication. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Random sequence generation and group assignment were determined centrally just prior to the initial session. |
| Allocation concealment (selection bias) | Low risk | Random sequence generation and group assignment were determined centrally just prior to the initial session |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P value for characteristic measures are higher than 0.05 |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values for physiological measures are higher than 0.05 |
| Incomplete outcome data (attrition bias) | High risk | For lab data collection at 15 months, 42 lost to follow‐up out of 106 at randomisation (39.6%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest are all objective (HbA1c, SBP, DBP and LDL). Data assessors remained blinded to group assignment throughout the study. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol first posted in January 2012, study started in February 2009). Our outcomes of interest are all well reported. However, the results from the "Summary of Diabetes Self‐Care Activities" and from the "Measure of satisfaction with care" are not reported. The authors also reported data from "Diabetes support scale", which was not planned in the protocol. |
| Risk of contamination (other bias) | Unclear risk | Case management by phone. Basically, all participants received a case management intervention for 3 months and the intervention arm received it for an additional 12 months. This could limit the impact of intervention. |
| Other bias | Low risk | No evidence of other bias. |
Taveira 2010.
| Study characteristics | ||
| Methods |
Pharmacist‐led group medical appointment model in type 2 diabetes Patient RCT, conducted in VA (Veterans Affairs) Medical Center’s electronic medical record system, USA Two arms: 1. Usual care (control arm) and 2. VA‐MEDIC ‐ Veterans Affairs Multi‐disciplinary Education and Diabetes Intervention for Cardiac risk reduction (intervention arm) |
|
| Participants | Control arm N: 54 Intervention arm N: 64 Diabetes type: type 2 Mean age: 64.5 ± 10.3 % Male: 95.7 Longest follow‐up: 4 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.9 (1.1), post 7.9 (NR) Intervention arm: pre 8.1 (1.5), post 7.2 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 137.2 (17.5), post 135.5 (NR) Intervention arm: pre 131.1 (18.8), post 123.8 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 74.2 (9.8), post 75.2 (NR) Intervention arm: pre 74.4 (10.8), post 67.9 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 92.8 (34.8), post 85.0 (NR) Intervention arm: pre 92.8 (34.8), post 81.5 (NR) 5a) Controlled hypertension (DBP < 80 mmHg), N under control (%) Control arm: pre 37 (73), post 35 (69) Intervention arm: pre 40 (69), post 51 (88) 5b) Controlled hypertension (SBP < 130 mmHg), N under control (%) Control arm: pre 17 (33), post 20 (39) Intervention arm: pre 35 (60), post 38 (66) 6) Smoking cessation, N smokers (%) Control arm: pre 7 (14), post 7 (14) Intervention arm: pre 20 (34), post 17 (29) |
|
| Funding source | This work was supported in part by Rhode Island Foundation (Dr Wu). Additional supports include American College of Clinical Pharmacy Astra‐Zeneca Health Outcomes Research Award (Dr Taveira), American Society of Health Systems Pharmacists and Education Foundation Federal Services Research Grant Program (Dr Cohen), VA HSR&D Merit Review Award IAB 06‐269 (Dr Taveira, Dr Cohen, Dr Wu), VA HSR&D Career Development Award 04‐123 (Dr Pirraglia), and Targeted Research Enhancement Program grant (TRP 04‐179) from the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs (Dr Friedmann). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to the VA‐MEDIC arm or usual care arm using simple coin toss randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Table 2. Age P value less than 0.05, no education information. |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. Several P values less than 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 3 lost in control, 6 lost in intervention. No reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of all outcomes. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered protocol. Several other outcomes were reported than were listed in protocol. |
| Risk of contamination (other bias) | High risk | Information not available. |
| Other bias | Unclear risk | Participants in both groups saw primary care physicians at the VA Medical Center; unclear if physicians treated patients in both groups ‐ may have influenced care of participants in control group. |
Taveira 2011.
| Study characteristics | ||
| Methods |
Pharmacist‐led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults Patient RCT, conducted with patients from providence VAMC electronic medical record system and referral by primary care providers, USA Two arms: 1. Standard care (control arm) and 2. VA‐MEDIC‐D (intervention arm) |
|
| Participants | Control arm N: 44 Intervention arm N: 44 Diabetes type: type 1 and type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre NR (NR), post 2 (5) Intervention arm: pre NR (NR), post 5 (11) 2) Statins, N users (%) Control arm: pre NR (NR), post 4 (10) Intervention arm: pre NR (NR), post 8 (18) 3a) Antihypertensives (ACE inhibitor or angiotensin II receptor blockers), N users (%) Control arm: pre NR (NR), post 3 (7) Intervention arm: pre NR (NR), post 4 (9) 3b) Antihypertensives (calcium channel blocker), N users (%) Control arm: pre NR (NR), post 1 (2) Intervention arm: pre NR (NR), post 2 (5) 3c) Antihypertensives (diuretic), N users (%) Control arm: pre NR (NR), post 6 (14) Intervention arm: pre NR (NR), post 11 (25) 3d) Antihypertensives (ß‐blocker), N users (%) Control arm: pre NR (NR), post 3 (7) Intervention arm: pre NR (NR), post 3 (7) 4) HbA1c, mean % (SD) Control arm: pre 8.5 (1.9), post 8.4 (2.0) Intervention arm: pre 8.3 (1.7), post 7.4 (1.2) 5) SBP, mean mmHg (SD) Control arm: pre 125.2 (16.5), post 127.0 (17.3) Intervention arm: pre 130.6 (21.9), post 123.4 (12.3) 6) LDL, mean mg/dL (SD) Control arm: pre 101.5 (35.3), post 93.9 (30.6) Intervention arm: pre 101.0 (29.9), post 92.5 (24.3) 7) Controlled hypertension (SBP < 130 mmHg), N under control (%) Control arm: pre 30 (68), post 6 (14) Intervention arm: pre 20 (45), post 14 (32) 8) Smoking cessation, N smokers (%) Control arm: pre 11 (25), post 9 (20) Intervention arm: pre 12 (27), post 11 (25) |
|
| Funding source | This work was supported in part by the American College of Clinical Pharmacy Astra‐Zeneca Health Outcomes Research Award (Dr. Taveira), American Society of Health System Pharmacists and Education Foundation Federal Services Research Grant Program (Dr. Cohen), and VA HSR&D Merit Review Award IAB 06‐269 (Drs. Taveira, Cohen, and Wu) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "simple coin toss randomization". |
| Allocation concealment (selection bias) | Low risk | So long as participants were allocated during the coin toss. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | High risk | SBP < 130 was imbalanced at baseline, but baseline values were adjusted for in analysis P values. |
| Incomplete outcome data (attrition bias) | Low risk | ~4% lost to follow‐up in N1 and ~0% in N2, losses do not seem to be influenced by treatment arms. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective laboratory methods used to measure HbA1c, blinding of outcome assessors not described. |
| Selective reporting (reporting bias) | High risk | < 2005 approach used since no protocol; medication not listed in methods. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Taveira 2014.
| Study characteristics | ||
| Methods |
Interventions to maintain cardiac risk control after discharge from a cardiovascular risk reduction clinic: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Participants had visits at the cardiovascular risk reduction clinic (CRRC). 2) Participants had group medical visits (with family members and supporters) facilitated by a clinical pharmacist or CRRC individual clinic visits with a clinical pharmacist. Clinical pharmacists were diabetes core content experts and certified as diabetes educators. In United States of America. 3 arms: 1. Control (standard care) (control arm) and 2. Intervention 1 (group medical visits, experimental group) (intervention arm) 3. Intervention 2 (CRRC individual clinic sessions, active comparator) (other arm) |
|
| Participants | Control arm N: 53 Intervention arm N: 61, 64, NA Diabetes type: 3 Mean age: 65.16 ± 8.98 % Male: 97.21 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (Standard care) Intervention arm: (group medical visits, experimental group) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management Intervention arm: (CRRC individual clinic sessions, active comparator) 1) Case management 2) Team change |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Hypertension control Smoking status |
|
| Funding source | This trial was sponsored by the Merck and Co. Inc. Disease State Management Grant Program in conjunction with the Providence VA Medical Center. This material is the result of work supported by Merck and Co. Inc. Disease State Management Grant Program and with the resources from the Providence VA Medical Center (PI, Dr. Wu). Dr. Wu’s time was supported by the Providence VA Medical Center and Dr. Taveira’s time was supported by the University of Rhode Island College of Pharmacy. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. 200 patients agreed to participate and were randomly assigned. We performed an open‐label, randomised controlled trial of 200 consecutive CRRC patients. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. However, there was a trend for CRRC individual and group medical visit participants to be diagnosed with a co‐morbid mental health condition (CRRC individual, 50.0% vs group, 54.1% vs standard care, 34.0%, P = 0.08) at baseline; but were otherwise similar in other cardiovascular risk factor values. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. All P values above 0.05. However, there was a trend for CRRC individual and group medical visit participants to be a current smoker (CRRC individual, 17.2% vs group, 18.0% vs standard care, 7.6%, P = 0.19) at baseline; but were otherwise similar in other cardiovascular risk factor values. |
| Incomplete outcome data (attrition bias) | High risk | Of the 200 study participants who were randomised, 89% (n = 178) of them had diabetes and 11% (n = 22) had CVD but no diabetes. Complete 1‐year follow‐up data were available on 181 of participants (90.5%). They lost 7/73 patients in the CRRC intervention (9.6%), 10/72 in the group intervention (13.9%) and 2/55 in the control group (3.6%). Unbalanced numbers. Reasons reported but not balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | All of our outcomes of interest were objectively measured (medication use was obtained from the electronic medical record, and blood pressure) except smoking status (probably self‐reported by patients, but secondary outcomes). Open‐label trial. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. The protocol does not include drug prescription as reported in the paper. |
| Risk of contamination (other bias) | Low risk | Patients in the control group did not see the clinical pharmacists involved in the intervention groups. Unclear if the same primary physicians were taking care of patients from all groups, but the intervention targeted patients. |
| Other bias | Low risk | None identified. |
Taylor 2003.
| Study characteristics | ||
| Methods |
Evaluation of a nurse‐care management system to improve outcomes in patients with complicated diabetes Patient RCT, conducted in a medical centre in Santa Clara, CA, USA Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 85 Intervention arm N: 84 Diabetes type: type 1 and type 2 Mean age: 55.1 ± 10.2 % Male: 53.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 47 (71), post 44 (67) Intervention arm: pre 43 (70), post 49 (80) 2) Foot screening, N screened (%) Control arm: pre 43 (65), post 48 (73) Intervention arm: pre 26 (43), post 45 (74) 3) HbA1c, mean % (SD) Control arm: pre 9.5 (0.3), post 9.2 (NR) Intervention arm: pre 9.5 (0.3), post 8.4 (NR) 4) SBP, mean mmHg (SE) Control arm: pre 128.5 (NR), post 137.1 (NR) Intervention arm: pre 126.5 (NR), post 130.9 (NR) 5) DBP, mean mmHg (SE) Control arm: pre 72.3 (1.5), post 74.2 (NR) Intervention arm: pre 73.3 (1.4), post 75.5 (NR) 6) LDL, mean mg/dL (SE) Control arm: pre 123.9 (4.7), post 117.4 (NR) Intervention arm: pre 124.1 (5.2), post 104.7 (NR) 7a) Controlled hypertension (DBP ≤ 85 mmHg), N under control (%) Control arm: pre 57 (86), post 56 (85) Intervention arm: pre 55 (90), post 51 (84) 7b) Controlled hypertension (SBP ≤ 130 mmHg), N under control (%) Control arm: pre 38 (58), post 28 (42) Intervention arm: pre 42 (69), post 32 (52) |
|
| Funding source | This study was funded by grant no 032643 from The Robert Wood Johnson Foundation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | High risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Taylor 2005.
| Study characteristics | ||
| Methods |
Promoting health in type 2 diabetes: nurse‐physician collaboration in primary care Patient RCT, family practice clinic, Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 19 Intervention arm N: 20 Diabetes type: type 2 Mean age: 62.4 ± NR % Male: 66.7 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.7 (NR), post 8.4 (NR) Intervention arm: pre 7.7 (NR), post 7.4 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 129.0 (NR), post 136.0 (NR) Intervention arm: pre 134.0 (NR), post 132.0 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 70.0 (NR), post 75.0 (NR) Intervention arm: pre 79.0 (NR), post 74.0 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 119.1 (NR), post 120.7 (NR) Intervention arm: pre 116.0 (NR), post 108.3 (NR) |
|
| Funding source | Thanks to the Calgary Health Region for financial support through the Healthy Communities Fund | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Thankappan 2013.
| Study characteristics | ||
| Methods |
Smoking cessation among diabetes patients: results of a pilot randomized controlled trial in Kerala, India Patient RCT, conducted in 2 referral diabetes clinic located in peri‐urban areas of 2 south Indian cities in Kerala state, India Two arms: 1. Intervention Group ‐ 1 (control arm) and 2. Intervention Group ‐ 2 (intervention arm) |
|
| Participants | Control arm N: 112 Intervention arm N: 112 Diabetes type: unclear/not reported Mean age: 53.0 (range: 28 to 75) % Male: 100.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Clinician education 2) Patient education Intervention arm: 1) Case management 2) Clinician education 3) Patient education |
|
| Outcomes | 1) Smoking cessation, N smokers (%) Control arm: pre 112 (100), post 84 (86) Intervention arm: pre 112 (100), post 40 (41) |
|
| Funding source | The Quit Tobacco International Project is supported by a grant from the Fogarty International Centre of the US National Institutes of Health (RO1TW005969‐01) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…computer generated random sequence..." |
| Allocation concealment (selection bias) | Unclear risk | Did not describe. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "Baseline characteristics in both the intervention groups were comparable." |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported for smoking. |
| Incomplete outcome data (attrition bias) | Low risk | Did both an intention‐to‐treat and per‐protocol analysis, baseline based on those randomised. Numbers and reasons for loss to follow‐up the same and balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Outcome assessors not blinded. Only statistician was. |
| Selective reporting (reporting bias) | Low risk | Matches with protocol for primary outcome. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Thomas 2007.
| Study characteristics | ||
| Methods |
Use of a registry‐generated audit, feedback, and patient reminder intervention in an internal medicine resident clinic‐‐a randomized trial Cluster‐RCT (78 clusters with 78 providers), conducted in a resident continuity clinic, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 231 Intervention arm N: 252 Diabetes type: unclear/not reported Mean age: NR ± NR % Male: NR Longest follow‐up: 10 months |
|
| Interventions |
Control arm: 1) Clinician education Intervention arm: 1) Audit and feedback 2) Electronic patient registry 3) Clinician education 4) Patient reminders |
|
| Outcomes | 1) HbA1c 2) SBP 3) DBP 4) LDL |
|
| Funding source | This study was funded by an Education Innovation Award, provided by the Mayo Clinic College of Medicine. The sponsor had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review or approval of the manuscript. The primary author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Resident demographic data (age, sex and year in training) were similar between groups. But no more information or table. |
| Patient's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Low risk | At baseline, there were no significant differences in HgbA1c, LDL cholesterol, blood pressure levels, or adherence to HgbA1c or LDL cholesterol monitoring guidelines between patients cared for by residents in intervention and control groups. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Thompson 1999.
| Study characteristics | ||
| Methods |
Insulin adjustment by a diabetes nurse educator improves glucose control in insulin‐requiring diabetic patients: a randomized trial Patient RCT, conducted in a diabetes hospital clinic in Canada Two arms: 1. Standard care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 23 Intervention arm N: 23 Diabetes type: type 1 and type 2 Mean age: 48.8 ± 13.3 % Male: 48.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.4 (0.8), post 8.9 (1.0) Intervention arm: pre 9.6 (1.0), post 7.8 (0.8) |
|
| Funding source | "We acknowledge the contribution of Eli Lilly and Co. for providing the insulin and funding the salary of Ms. Kozak for the study." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Tildesley 2010.
| Study characteristics | ||
| Methods |
Effect of internet therapeutic intervention on A1c levels in patients with type 2 diabetes treated with insulin Patient RCT. Setting not reported Two arms: 1. Usual care (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 25 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: 1) Facilitated relay of clinical information 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.5 (1.2), post 8.4 (1.4) Intervention arm: pre 8.8 (1.3), post 7.6 (0.7) |
|
| Funding source | This work was supported by the Endocrine Research Society, Vancouver, British Columbia, which received funding from ALR Technologies | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "Baseline demographic and clinical characteristics were similar." |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.425). |
| Incomplete outcome data (attrition bias) | Low risk | Numbers lost to follow‐up were small and comparable between both groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described, HbA1c objective laboratory methods not described. |
| Selective reporting (reporting bias) | Low risk | Outcomes match those listed in the protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Tildesley 2011.
| Study characteristics | ||
| Methods |
Efficacy of A1C reduction using internet intervention in patients with type 2 diabetes treated with insulin RCT (NA clusters and NA providers), conducted in 1) Internet‐based glucose monitoring system (IBGMS) intervention delivered in Vancouver, British Columbia, Canada. 2) Patients received feedback from their endocrinologist. In Canada. 2 arms: 1. Control (conventional care) (control arm) and 2. Intervention (Internet‐based glucose monitoring system‐IBGMS) (intervention arm) |
|
| Participants | Control arm N: 25 Intervention arm N: 25, NA, NA Diabetes type: 2 Mean age: 59.5 ± NR % Male: 63.04 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (conventional care) 1) Facilitated relay of clinical information 2) Promotion of self‐management Intervention arm: (Internet‐based glucose monitoring system‐IBGMS) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This work was supported by Endocrine Research Society (Vancouver, British Columbia), which received funding from ALR Technologies Inc. We extend our appreciation to Abbott Diabetes Care Inc. for their generous gifts of glucose meters and test strips. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to IBGMS or a control group for 6 months using a computer random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. HbA1c has a P value of 0.42. |
| Incomplete outcome data (attrition bias) | Low risk | We enrolled 50 patients. Four patients (2 from each group) were excluded because they were nonadherent. Lost 8% in each group. Low and balanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively measured (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | All patients met with the same endocrinologist and were provided with standard office‐based care. All patients were provided with a blood glucose meter and test strips. Neither group was taught how to interpret SMBG results, although as part of the inclusion criteria, all patients had completed prior training in SMBG. It should be noted that all patients attend a comprehensive 4‐day education course when diagnosed with diabetes. |
| Other bias | Low risk | None identified. |
Tjam 2006.
| Study characteristics | ||
| Methods |
Physiological outcomes of an internet disease management program vs. in‐person counselling: a randomized, controlled trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from the Diabetes Education Centres (DECs) at Cambridge Memorial Hospital and Grand River Hospital in the Waterloo region of Ontario. 2) The intervention group used an interactive internet program instead of in‐clinic follow‐up for disease management. In Canada. 2 arms: 1. Control (Diabetes Education Centres‐DECs) (control arm) and 2. Intervention (interactive diabetes internet program) (intervention arm) |
|
| Participants | Control arm N: 20 Intervention arm N: 37, NA, NA Diabetes type: 3 Mean age: NR ± 12 % Male: 47.14 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (Diabetes Education Centres ‐ DECs) Intervention arm: (interactive diabetes internet program) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | "The authors gratefully acknowledge the financial support of their project through the Change Foundation (Grant – 01018), St. Mary’s General Hospital, Grand River Hospital, and Cambridge Memorial Hospital." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported; 20 were randomised to the control group and 37 to the intervention group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. All P values above 0.05. There were no statistical differences in any of the patient characteristics and factors between the control and intervention groups. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. The baseline laboratory data given in Table 2 showed no significant difference between the 2 groups with respect to A1C, FBG, TC, TG, HDL‐C and LDL‐C. |
| Incomplete outcome data (attrition bias) | High risk | Enrollment was staggered but data collection finished at the same date for all patients. 70 individuals (33 male, 37 female) initially consented to participate in this project; however, 13 dropped out after the education portion and before the 3‐month assessment (18.7% lost). A total of 57 participants completed the 1‐year study or came to their natural censored endpoint at 3 or 6 months because of staggered entry times. An unequal distribution of patients in the control and intervention groups was observed. This was attributed to differential attrition in the control group. Many patients left the study because they were assigned to the control group, and the study design did not allow cross‐over to the intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and LDL). |
| Selective reporting (reporting bias) | High risk | No registered or published protocol. They do not have data at 1‐year follow‐up for many patients due to staggered enrollment. Data collected at baseline, 3, 6 and 12 months, only total cholesterol and HbA1c report values for baseline, 3, 6 and 12 month follow‐up. |
| Risk of contamination (other bias) | Unclear risk | A certified diabetes nurse case manager, working under medical directives, had access to all intervention patient profiles. The nurse was able to monitor data, give feedback and make recommendations to physicians. Physicians might have changed their approach with control patients. |
| Other bias | Low risk | No evidence of other bias |
Tobe 2006.
| Study characteristics | ||
| Methods |
Effect of nurse‐directed hypertension treatment among First Nations people with existing hypertension and diabetes mellitus: the Diabetes Risk Evaluation and Microalbuminuria (DREAM 3) randomized controlled trial Patient RCT, conducted in Battlefords Tribal Council Indian Health Services, Canada Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 49 Intervention arm N: 50 Diabetes type: type 2 Mean age: 55.6 ± 13.0 % Male: 38.5 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Case management 2) Clinician education 3) Patient education Intervention arm: 1) Case management 2) Team changes 3) Clinician education 4) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.7 (1.8), post 7.7 (1.9) Intervention arm: pre 7.9 (1.9), post 7.8 (2.1) 2) SBP, mean mmHg (SD) Control arm: pre 150.5 (19.1), post 133.5 (18.1) Intervention arm: pre 149.7 (10.5), post 125.7 (16.6) 3) DBP, mean mmHg (SD) Control arm: pre 84.2 (11.1), post 77.4 (11.3) Intervention arm: pre 87.1 (8.4), post 75.5 (12.7) |
|
| Funding source | Support for this study was provided by the Canadian Institutes of Health Research (CIHR), in partnership with Pfizer Canada | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Tourkmani 2018.
| Study characteristics | ||
| Methods |
Impact of an integrated care program on glycemic control and cardiovascular risk factors in patients with type 2 diabetes in Saudi Arabia: an interventional parallel‐group controlled study RCT (NA clusters and NA providers), conducted in 1) The study was conducted in Al‐Wazarat Chronic Diseases Center, a division of the Al‐Wazarat Health Care (WHC) Family Medicine Center in Riyadh, Saudi Arabia, 2) The program team included a senior family physician, clinical pharmacy specialist who acted as a case manager, dietician, diabetic educator, health educator and social worker. In Saudi Arabia. 2 arms: 1. Control (standard care) (control arm) and 2. Intervention (integrated care programme) (intervention arm) |
|
| Participants | Control arm N: 72 Intervention arm N: 217, NA, NA Diabetes type: 2 Mean age: 57.11 ± 9 % Male: 35.02 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: (standard care) Intervention arm: (integrated care programme) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This project did not receive any funding | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The biostatistician generated a random sequence of 72 numbers out of 289 using a computer program without knowing the order of the patients. The case manager assigned the patients’ numbers who matched those on the random sequence to the control group. |
| Allocation concealment (selection bias) | Unclear risk | The case manager knew the participant numbers and was the one who allocated them to control based on the random number sequence. |
| Patient's baseline characteristics (selection bias) | Unclear risk | The intervention group had a significantly lower number of comorbidities compared with the control group. |
| Patient's baseline outcomes (selection bias) | High risk | The intervention group had significantly higher body weights and HbA1c compared with the control group. |
| Incomplete outcome data (attrition bias) | Low risk | 22/217 (10%) lost in intervention group, 4/72 (6%) lost in control. Reasons provided, Figure 1. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcomes objectively measured. Blinded the results to the outcomes assessors (i.e. lab workers and nurses). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Outcomes reported in protocol are not all reported in results (BMI, serum cardiac troponin T (cTnT) and creatine kinase MB isoenzyme (CK‐MB)) and total cholesterol reported in results but not listed in protocol. Measurements at 3 and 6 months not reported. |
| Risk of contamination (other bias) | Unclear risk | Control and intervention participants received care at the same centre and by the same clinicians, so control group could have been contaminated. |
| Other bias | Low risk | No other evidence of risk of bias. |
Trento 2008.
| Study characteristics | ||
| Methods |
A randomised controlled clinical trial of nurse‐, dietitian‐ and pedagogist‐led group care for the management of Type 2 diabetes Patient RCT, conducted in Italy Two arms: 1. Controls (control arm) and 2. Group care (intervention arm) |
|
| Participants | Control arm N: 24 Intervention arm N: 25 Diabetes type: type 2 Mean age: 66.3 ± 8.2 % Male: 59.2 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm: 1) Team changes 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) Antihypertensives (any), N users (%) Control arm: pre 18 (75), post 17 (81) Intervention arm: pre 10 (40), post 10 (42) 2) HbA1c, mean % (SD) Control arm: pre 8.0 (1.1), post 8.4 (1.3) Intervention arm: pre 8.0 (1.3), post 7.6 (0.8) 3) SBP, mean mmHg (SD) Control arm: pre 144.8 (23.6), post 150.0 (30.0) Intervention arm: pre 145.0 (22.7), post 135.0 (23.5) 4) DBP, mean mmHg (SD) Control arm: pre 80.4 (10.7), post 76.0 (11.8) Intervention arm: pre 81.0 (11.5), post 76.0 (9.8) |
|
| Funding source | "This article was supported by funds from the University of Turin (fondi 60%)". | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | High risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Tsuyuki 2016.
| Study characteristics | ||
| Methods |
The effectiveness of pharmacist interventions on cardiovascular risk: the multicenter randomized controlled RxEACH trial RCT (NA clusters and NA providers), conducted in 1) The RxEACH study was conducted in 56 community pharmacies in the province of Alberta, Canada. 2) Patients randomised to the intervention group received a Medication Therapy Management consultation from their pharmacist (in Alberta, called a Comprehensive Annual Care Plan or Standard Medication Management Assessment). In Canada. 2 arms: 1. Control (usual pharmacist care) (control arm) and 2. Intervention (medication review and risk assessment by pharmacist) (intervention arm) |
|
| Participants | Control arm N: 287 Intervention arm N: 286, NA, NA Diabetes type: 2 Mean age: 61.15 ± NR % Male: 58 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual pharmacist care) Intervention arm: (medication review and risk assessment by pharmacist) 1) Case management 2) Team change 3) Patient education |
|
| Outcomes | Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin |
|
| Funding source | Funding for the RxEACH study was provided by Alberta Health (Grant no. RES0020309) (Workforce Planning), and the Cardiovascular Health and Stroke Strategic Clinical Network of Alberta Health Services (Grant no. RES0027161). Merck Canada (Grant no. RES0019426) (investigator‐initiated funding for the educational program) provided the funds to develop the educational materials. Dr. Tsuyuki has received investigator‐initiated research grants from Merck, Sanofi, and AstraZeneca. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in a 1:1 ratio to intervention or usual care groups using a centralised secure website at the data management centre (Epidemiology Coordinating and Research (EPICORE) Centre). The randomisation scheme was blocked (random block size) and stratified by pharmacy. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised in a 1:1 ratio to intervention or usual care groups using a centralised secure website at the data management centre (Epidemiology Coordinating and Research (EPICORE) Centre). The randomisation scheme was blocked (random block size) and stratified by pharmacy. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. The 2 treatment groups were well balanced in baseline demographic and clinical parameters. No P values. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. The 2 treatment groups were well balanced in baseline demographic and clinical parameters. No P values. |
| Incomplete outcome data (attrition bias) | Low risk | Lost 29 patients out of 723 randomised (4.0%). 19 out of 370 (5.1%) in the intervention group and 10 out of 353 (2.8%) in the control group. Reasons reported and more patients withdraw their consent in the intervention group (n = 8) compared to usual care (n = 3), but low numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively assessed (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. They do not report data about exercise. |
| Risk of contamination (other bias) | High risk | Physicians might have changed their approach with control patients after receiving recommendations and medication management reports from pharmacists taking care of patients in intervention group. Pharmacists involved in both groups have a broad scope of practice that includes independent prescribing and the ability to order laboratory tests. Further, due to the nature of the intervention, blinding was not possible. Pharmacists who provided the interventions also conducted the assessment and entered the information into the study’s online system where CV risk was calculated. This could have introduced bias. |
| Other bias | Low risk | None identified. |
Tu 1993.
| Study characteristics | ||
| Methods |
Diabetes self‐care knowledge, behaviors, and metabolic control of older adults‐‐the effect of a post educational follow‐up program RCT (NA clusters and NA providers), conducted in 1) Patients were recruited from inpatients of a diabetic hospital in a southeastern health sciences centre, Birmingham, Alabama. Follow‐up intervention via telephone. 2) Telephone made by the primary investigator or a trained research assistant. Instructions on reportable symptoms were emphasised and referrals were made when indicated (e.g. calling the dietitian for additional help with dietary regime). In United States of America. 2 arms: 1. Control (usual care: inpatient education programme) (control arm) and 2. Intervention (inpatient education programme and telephone follow‐up) (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 16, NA, NA Diabetes type: 2 Mean age: 65.44 ± 11.65 % Male: 33.33 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care: in patient education programme) Intervention arm: (in patient education programme and telephone follow‐up) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This study was supported by the Dean’s new investigator research award in geriatric nursing, School of Nursing, University of Alabama at Birmingham, Birmingham, Alabama | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. The participants were randomly assigned to an experimental group (EG) or a control group (CG). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Data reported. No P values but in text: there was no significant difference in demographic and disease‐related variables between the two groups. |
| Patient's baseline outcomes (selection bias) | Unclear risk | No outcome data are reported at baseline. |
| Incomplete outcome data (attrition bias) | High risk | They lost 3/15 (20%) patients in the control group and 1/16 (6%) patients in the intervention group. During the course of the study, 1 participant in the experimental group and 3 participants in the control group developed major medical conditions and were unable to complete the study. Thus, the final sample included 27 participants. Unbalanced numbers of lost and high number in the control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | HbA1c was objectively measured. Hypoglycaemia was self‐reported by patients. Unlikely that patients were blinded. Quote: "If insulin reactions (hypoglycaemia) had occurred, data were obtained regarding the frequency, time, and the relationship of the reaction to meals, snacks, and/or activity level. Measures for preventing hypoglycaemic reactions were reviewed with the participants." |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. No baseline data for HbA1c and hypoglycaemia. Results match methods for other outcomes. |
| Risk of contamination (other bias) | Low risk | Patient RCT. Unlikely that control patients received follow‐up calls. The only phone call made to participants in the control group was to administer the DKN test and to assess behavioural deficits during the week of the posttest. |
| Other bias | Low risk | None identified. |
Tutino 2017.
| Study characteristics | ||
| Methods |
A multicentre demonstration project to evaluate the effectiveness and acceptability of the web‐based Joint Asia Diabetes Evaluation (JADE) programme with or without nurse support in Chinese patients with Type 2 diabetes RCT (NA clusters and NA providers), conducted in 1) Patients recruited from 6 tertiary hospitals in China, namely: 1) Beijing People’s Hospital, Beijing; 2) Peking Union Hospital, Beijing; 3) First Hospital, Peking University Hospital, Beijing; 4) China‐Japan Friendship Hospital, Beijing; 5) Shanghai Sixth People’s Hospital, Shanghai; and 6) Third Affiliated Hospital of Sun Yat‐Sen University, Guangzhou. 2) Integrated care augmented by a web‐based disease management programme and nurse co‐ordinator in China 2 arms: 1. Control (DIAMOND: Diabetes Monitoring Database, comprehensive assessment only) (control arm) and 2. Intervention (JADE: web‐based Joint Asia Diabetes Evaluation, comprehensive assessment plus nurse coordinated structured follow‐up) (intervention arm) |
|
| Participants | Control arm N: 1728 Intervention arm N: 1858, NA, NA Diabetes type: 2 Mean age: 56.44 ± NR % Male: 54.4 Longest follow‐up: 12.5 months |
|
| Interventions |
Control arm: (DIAMOND: Diabetes Monitoring Database, comprehensive assessment only) 1) Electronic patient registry 2) Clinician education 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management Intervention arm: (JADE: web‐based Joint Asia Diabetes Evaluation, comprehensive assessment plus nurse coordinated structured follow‐up) 1) Case management 2) Electronic patient registry 3) Clinician education 4) Clinician reminder 5) Facilitated relay of clinical information 6) Patient education 7) Promotion of self‐management 8) Patient reminders |
|
| Outcomes | Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control Smoking status Harms |
|
| Funding source | This study was supported by an unrestricted educational grant from Merck & Co., Inc. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated codes. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using computer‐generated codes kept in sealed, opaque envelopes, numbered 1 to 600 prefixed by the study site. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. Characteristics relatively balanced between groups. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. Outcomes relatively balanced between groups. |
| Incomplete outcome data (attrition bias) | High risk | 68% retention in DIAMOND, 74% retention in JADE. Thus a 32% loss in DIAMOND and 26% loss in JADE. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcomes were objectively measured (HbA1c, BP and LDL). |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Protocol: Percentage of patients who attain 2 or more of the 3 targets vs Paper: Percentage of patients who attain 1 or more of the 3 targets. In the paper, they added weight, smoking status, drug use and many other outcomes. There is no way to compare baseline data with post‐data as different units, denominators and drug names are used. Pre: statins vs post: lipid‐regulating drugs. Pre: any BP drugs vs post: BP‐lowering drugs. Pre: mean HbA1c/SBP/DBP/LDL vs post: median HbA1c/SBP/DBP/LDL. Pre: number of smokers vs post: number of patients who quit smoking among the baseline smokers. Only the number of patients who reached target blood pressure are comparable. |
| Risk of contamination (other bias) | Unclear risk | Patient randomised. Unclear if the same nurses were involved in both groups, Quote: "Each centre was given a grant to support an additional CUHK project team‐trained nurse to perform the CA, guided by the JADE/DIAMOND portals and supervised by a physician. Treating doctors were not blinded to patient assignment and contamination was possible with participants in both the DIAMOND and JADE groups managed by the same physicians." |
| Other bias | Low risk | None identified. |
Vadstrup 2011.
| Study characteristics | ||
| Methods |
Effect of a group‐based rehabilitation programme on glycaemic control and cardiovascular risk factors in type 2 diabetes patients: The Copenhagen Type 2 Diabetes Rehabilitation Project Patient RCT, conducted in outpatient clinic and general practitioners, Denmark Two arms: 1. Individual group (control arm) and 2. Rehabilitation group (intervention arm) |
|
| Participants | Control arm N: 70 Intervention arm N: 73 Diabetes type: type 2 Mean age: 58.0 ± 10.0 % Male: 59.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.8 (0.9), post 7.2 (NR) Intervention arm: pre 7.9 (0.8), post 7.6 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 145.0 (17.0), post 138.9 (NR) Intervention arm: pre 146.0 (18.0), post 141.2 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 84.0 (9.0), post 81.0 (NR) Intervention arm: pre 85.0 (10.0), post 82.4 (NR) 4) LDL, mean mg/dL (SD) Control arm: pre 100.5 (38.7), post 96.6 (NR) Intervention arm: pre 104.4 (34.8), post 100.5 (NR) |
|
| Funding source | The study was supported by grants from the Jascha Foundation, the Research Foundation of Bispebjerg University Hospital, the Copenhagen Capital Region Research Foundation, National Board of Health, the Ministry of Health and Prevention, GlaxoSmithKline, Servier Denmark, Grosserer Chr. Andersen and Ingeborgs Scholarship, and the Department of Endocrinology at Bispebjerg University Hospital. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Patient's baseline characteristics (selection bias) | High risk | Not mentioned in text or table. |
| Patient's baseline outcomes (selection bias) | Low risk | P values for baseline outcome values not provided. |
| Incomplete outcome data (attrition bias) | High risk | ~17% lost to follow‐up in N1 and ~12% in N2. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Method described for HbA1c, although unsure if outcome assessor was blinded, as they do not explicitly state that. |
| Selective reporting (reporting bias) | High risk | Not all secondary outcomes addressed in protocol are addressed in paper. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None identified. |
van Bruggen 2008.
| Study characteristics | ||
| Methods |
Implementation of locally adapted guidelines on type 2 diabetes Cluster‐RCT (30 clusters), conducted in Apeldoorn, The Netherlands Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 818 Intervention arm N: 822 Diabetes type: type 2 Mean age: 67.1 ± 11.6 % Male: 48.6 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education |
|
| Outcomes | 1) HbA1c 2) SBP 3) DBP 4) Controlled hypertension (< 140/85 mmHg) |
|
| Funding source | AGIS insurance company | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Except for education and the presence of macrovascular complications, patients’ characteristics were highly comparable across study groups. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1 ‐ no P values reported, but outcomes look balanced. Do not use statistical terms in text "Except for education and the presence of macrovascular complications, patients’ characteristics were highly comparable across study groups" |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Unclear risk | Information not available. |
Van Dijk‐de Vries 2015.
| Study characteristics | ||
| Methods |
Lessons learnt from a cluster‐randomised trial evaluating the effectiveness of Self‐Management Support (SMS) delivered by practice nurses in routine diabetes care Clustered RCT (41 clusters and 41 providers), conducted in 1) A regional care group in the South of the Netherlands consisting of 77 family practices. 2) The study involved practice nurses (n = 40) providing care to approximately 4000 patients with diabetes. In Netherlands. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (biopsychosocial Self‐Management Support (SMS) (intervention arm) |
|
| Participants | Control arm N: 147 Intervention arm N: 117, NA, NA Diabetes type: 2 Mean age: 64.56 ± 11 % Male: 53.56 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (biopsychosocial self‐management support (SMS)) 1) Case management 2) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This research was supported by the Dutch Diabetes Research Foundation (Diabetes Fonds) with grant No. 2010.13.1366 (Voice of the Patient programme), and by the ‘Annadal Foundation’ in Maastricht, an independent financial support fund in the field of healthcare. Both the training of practice nurses and operation of the system for registration of SMS were facilitated by the ‘HOZL’ group of collaborating family practices in the eastern part of the Southern Limburg region. During the SMS project, CZ Health Insurance included a fee for SMS in the bundled payment arrangement for diabetes care. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed by an independent research assistant who used a random number seed computer program to assign PNs to study arms, assuming an allocation ratio of 1:1. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT |
| Provider's baseline characteristics (selection bias) | Unclear risk | 77 family practices, involving 40 practice nurses providing care to approximately 4000 patients with diabetes. Practice nurses were cluster‐randomised in 2 arms. No data on nurses' characteristics are reported in each arm at baseline. |
| Patient's baseline characteristics (selection bias) | Low risk | More patients with a paid job in the intervention arm (51% vs 32%, P < 0.05); all other characteristics balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1, P = 0.429 for HbA1c. |
| Incomplete outcome data (attrition bias) | High risk | 41 patients were lost to follow‐up out of 264 at baseline (15.5%). In 10% of the sample, one follow‐up measurement was missing. 3 patients did not complete the baseline measurement and gave informed consent at the 4‐month follow‐up measurement. Another 23 patients completed only the baseline measurement. Reasons for incompleteness not reported, but their numbers are balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol (protocol registered in February 2011, study started in August 2011). All outcomes of interest are reported. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. As some PNs worked together in a team, 15 units of analysis were left in the intervention arm and 19 units of analysis in the control arm. PNs working together in a practice were clustered for being randomised to the same trial arm to avoid the risk of contamination if the SMS and usual care would be delivered in the same family practice. |
| Other bias | Unclear risk | Made few changes to clusters after randomisation (control arm: 21 to 19, and intervention: 20 to 15). Quote: "From the 77 family practices that were approached between April and June 2011, 40 agreed to participate. Their PNs (n=41) were by randomisation assigned to the intervention arm (20 PNs) and the control arm (21 PNs). After randomisation, but before patient recruitment, one family practice in the intervention arm withdrew from study participation due to the heavy workload of the PN. This left 19 PNs who received training in SMS and integrated it into their daily practice, and 21 PNs in the control arm who provided usual care. As some PNs worked together in a team, 15 units of analysis were left in the intervention arm and 19 units of analysis in the control arm". Other Quote: "Also, as PNs’ integration of SMS into consultations could have fluctuated during follow‐up, they may have missed study participants. Registration bias may have also occurred. PNs needed to open an extra data file to record the process and outcomes of SMS. This step might have created a barrier to their compliance." |
Van Dyck 2013.
| Study characteristics | ||
| Methods |
The relationship between changes in steps/day and health outcomes after a pedometer‐based physical activity intervention with telephone support in type 2 diabetes patients Patient RCT, conducted in endocrinology department of the Ghent University Hospital, Belgium Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 32 Intervention arm N: 60 Diabetes type: type 2 Mean age: 62.0 ± 9.0 % Male: 69.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.3 (0.9), post 7.6 (1.5) Intervention arm: pre 7.3 (0.9), post 7.3 (0.9) 2) SBP, mean mmHg (SD) Control arm: pre 129.7 (15.5), post 133.8 (23.6) Intervention arm: pre 133.7 (15.3), post 132.6 (15.7) 3) LDL, mean mg/dL (SD) Control arm: pre 89.7 (28.2), post 89.3 (32.9) Intervention arm: pre 84.7 (24.4), post 81.2 (27.5) |
|
| Funding source | This research was supported by Fund for Scientific Research Flanders (FWO) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table and text. |
| Patient's baseline outcomes (selection bias) | Low risk | In table and text. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 lost to follow‐up in each arm, but reasons not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: SBP using Omron M6 in seated position after 5 minutes of resting, routinely calibrated, HbA1c using the Adams Hemoglobin A1c procedure, LDL using enzymatic colorimetric analysis. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; method match outcomes. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Van Veldhuizen‐Scott 1995.
| Study characteristics | ||
| Methods |
Developing and implementing a pharmaceutical care model in an ambulatory care setting for patients with diabetes RCT (NA clusters and NA providers), conducted in 1) Ambulatory care setting at a Regional Diabetes Center (RDC) in Lafayette, Indiana. 2) Pharmaceutical care model delivered by the study pharmacist. In United States of America. 3 arms: 1. Control (standard pharmacist instruction) (control arm) and 2. Intervention 1 (standard pharmacist instruction plus pharmacist group session) (intervention arm), 3. Intervention 2 (standard pharmacist instruction plus one‐on‐one pharmacist follow‐up) (other arm) |
|
| Participants | Control arm N: 14 Intervention arm N: 13, 14, NA Diabetes type: 3 Mean age: 60 ± 11.07 % Male: 51.22 Longest follow‐up: 2 months |
|
| Interventions |
Control arm: (standard pharmacist instruction) 1) Patient education 2) Promotion of self‐management Intervention arm: (standard pharmacist instruction plus pharmacist group session) 1) Patient education 2) Promotion of self‐management Intervention arm: (standard pharmacist instruction plus one‐on‐one pharmacist follow‐up) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Harms | |
| Funding source | This project was funded in part by the American Foundation for Pharmaceutical Education, National Association of Chain Drug Stores, and the Upjohn Pharmaceutical Company | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. 41 patients (21 males, 20 females) volunteered and were randomly assigned to one of three treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | High risk | Some data reported for each groups, some look unbalanced (age, duration of diabetes), but no P values. The study patients ranged in age from 27 to 87 years, with the overall average age being 60 years (Group 1 = 63, 11 = 56 and III = 60). 39 patients had type 2 diabetes and 2 had type 1. Duration of diabetes ranged from newly diagnosed to 30 years, and the overall average was 7.37 years (Group 1 = 5.45, II = 7.25 and III = 9.36). 2 (5%) patients were on diet therapy, 24 (59%) were on oral sulfonylureas, 10 (24%) were on insulin, and 5 (12%) were on combination insulin and oral sulfonylurea therapy. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. No baseline table. Average weekly blood glucose values appears higher for the control group at 1 week (Figure 1: 190 vs 165 mg/dL for the 2 other groups). Number of weekly hyperglycaemic episodes appear similar between groups at 1 week (Figure 3). |
| Incomplete outcome data (attrition bias) | High risk | Complete documentation of blood glucose values (primary outcome) was not possible for all patients because some of the patients tested their blood glucose infrequently (e.g. once a week). Consequently, data for 32 of the 41 study patients were used for evaluating the blood glucose objective of this study. 22% data lost for this outcome and for hyperglycaemic episodes outcome as well (> 150 mg/dL = hyperglycaemic). Additional data from telephone interventions were obtained for patients in Group III. This information allowed the pharmacist investigator to identify 4 patients who evidenced non‐adherence to the treatment regimen and had markedly higher blood glucose values than other Group III patients. These patients were excluded from several analyses. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective method for hyperglycaemic episodes (blood glucose values > 150 mg/dL). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. For most outcomes, they only report P values, and not the mean values. Also, they look at patient compliance and they added an analysis without non‐adherent patients in group III (Figures 2 and 4). They also did additional analysis by correlating the blood glucose values from the digital memory of the patient’s meter with the values self‐documented by the patient in the self‐care diary. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that patients in the control group met with the pharmacist following the final educational session or had one‐on‐one instruction with follow‐up telephone contact with the study pharmacist. |
| Other bias | Low risk | None identified. |
VanEpps 2018.
| Study characteristics | ||
| Methods |
Financial incentives for chronic disease management: results and limitations of 2 randomized clinical trials with New York Medicaid patients RCT (NA clusters and NA providers), conducted in 1) Medicaid managed care members, New York, United States of America, 2) The programme included primary care visits and prescription medication and the intervention involved financial incentives through Medicaid. In United States of America. 4 arms: 1. Control (usual care) (control arm) and 2. Intervention (process financial incentives‐earned by attending primary care visits and/or receiving prescription medication refills) (intervention arm), 3. Intervention (outcome financial incentives ‐ earned by reducing glycated haemoglobin levels) (other arm), 4. Intervention (combined financial incentives ‐ combined process and outcome financial incentives) (other arm) |
|
| Participants | Control arm N: 256 Intervention arm N: 273, 263, 263 Diabetes type: 2 Mean age: 53 ± NR % Male: 39 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (process financial incentives ‐ earned by attending primary care visits and/or receiving prescription medication refills) 1) Financial incentives Intervention arm: (outcome financial incentives ‐ earned by reducing glycated haemoglobin levels) 1) Financial incentives |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services, 1B1CMS330901 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 2, P value < 0.05 for race, all others balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1C reports are similar between the 4 arms (P value = 0.68), Table 2. |
| Incomplete outcome data (attrition bias) | High risk | The longitudinal design for both studies resulted in substantial numbers of missing outcome measurements at both 3 months (38% in hypertension study and 40% in diabetes study) and 6 months (43% in hypertension study and 39% in diabetes). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1C was objectively measured. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol, but the outcomes in the methods match the results. |
| Risk of contamination (other bias) | Low risk | Because of the nature of the intervention (financial), it is unlikely that the participants in different arms were contaminated. |
| Other bias | High risk | No protocol available, however in text (change in eligibility criteria): Initial recruitment procedures targeted only those with poorly controlled hypertension (systolic blood pressure > 140 mm Hg) or poorly controlled diabetes (HbA1c > 8%), but logistical challenges in identifying participants based on these criteria led to a change in recruitment procedures to allow all diagnosed Medicaid patients of the proper age to enrol. |
Varney 2014.
| Study characteristics | ||
| Methods |
Effect of hospital‐based telephone coaching on glycaemic control and adherence to management guidelines in type 2 diabetes, a randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Diabetes Clinic of St Vincent’s Hospital Melbourne 2) telephone coaching, delivered by a dietitian in Australia 2 arms: 1. Control: (usual care) (control arm) and 2. Intervention: (telephone coaching) (intervention arm) |
|
| Participants | Control arm N: 47 Intervention arm N: 47, NA, NA Diabetes type: 2 Mean age: 61.5 ± 11 % Male: 68 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (telephone coaching) 1) Case management 2) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | "The publication of these results would not be possible without the generous financial support of the St Vincent’s Hospital, Research Endowment Fund." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation was undertaken to obtain a one‐to‐one balanced design. |
| Allocation concealment (selection bias) | Unclear risk | Allocation blinding was maintained until randomisation, after which participants and the principal researcher were informed of randomisation outcome. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1, P values provided. Age and ethnicity < 0.05, all others balanced. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1, P values provided and greater than 0.05. |
| Incomplete outcome data (attrition bias) | High risk | 11 lost in control group (24%), 12 lost in intervention (26%). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP and LDL. |
| Selective reporting (reporting bias) | High risk | Protocol prospectively registered. Results for smoking cessation not reported at 6 or 12 months. Outcomes only provided for 12‐month follow‐up, protocol states 18‐month follow‐up. |
| Risk of contamination (other bias) | High risk | Being unblinded, it is also possible that knowledge of group assignment influenced outcomes through favourable expectations associated with randomisation to the intervention group. |
| Other bias | Low risk | None. |
Vaughan 2017.
| Study characteristics | ||
| Methods |
Integrating CHWs as part of the team leading diabetes group visits: a randomized controlled feasibility study RCT (NA clusters and NA providers), conducted in 1) Investigators recruited participants from a growing, free 501(c) community clinic in southwest Houston with 98% Hispanic patients. 2) Intervention delivered by community health workers (CHW). CHWs were recruited from the host site’s bilingual volunteers who live or work in the vicinity of the clinic. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Community Health Worker led group visits) (intervention arm) |
|
| Participants | Control arm N: 31 Intervention arm N: 31, NA, NA Diabetes type: 2 Mean age: 49.65 ± 11.06 % Male: 35.5 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Community Health Worker led group visits) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | National Institutes of Health. National Institute of Diabetes, Digestive, and Kidney Disorders. Federal Award Identification Number (FAIN) K23DK110341 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | There were no significant baseline differences between groups. This included age (intervention = 51.3 years, control = 48.0 years, P = 0.11), sex (intervention female: n = 21, control female: n = 19, P = 0.48), individuals with prediabetes (intervention: n = 4, control n = 9, P = 0.11), and treatment regimen: lifestyle modifications (intervention: 24.0%, control: 38.5%, P = 0.42), oral agents (intervention: 72.0%, control: 61.5%, P = 0.62), and insulin ± oral agents (intervention: 4%, control: 0.0%, P = 0.98). |
| Patient's baseline outcomes (selection bias) | Low risk | Similarly, there were no significant baseline clinical differences between groups including A1C (P = 0.57), lipids (total cholesterol: P = 0.56, HDL: P = 0.40, LDL: P = 0.13, triglycerides: P = 0.32), blood pressure (systolic: P = 0.42, diastolic: P = 0.57) and BMI (P = 0.47). |
| Incomplete outcome data (attrition bias) | High risk | 6 lost from each group (19%), reasons provided and balanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcome: HbA1c. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, however outcomes in methods match results. |
| Risk of contamination (other bias) | Unclear risk | The same physicians in the clinic followed up on patients from both arms. |
| Other bias | Low risk | No evidence of other bias. |
Vidal‐Pardo 2013.
| Study characteristics | ||
| Methods |
Effect of an educational intervention in primary care physicians on the compliance of indicators of good clinical practice in the treatment of type 2 diabetes mellitus (OBTEDIGA project) Cluster‐RCT (108 clusters with 108 providers), conducted with physicians in Galicia (north‐west Spain), Spain Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 1437 Intervention arm N: 1501 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Audit and feedback 2) Clinician education |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 361 (25), post 395 (28) Intervention arm: pre 531 (35), post 554 (37) 2) Foot screening, N screened (%) Control arm: pre 141 (10), post 200 (14) Intervention arm: pre 293 (20), post 457 (30) 3) Renal screening (microalbumin), N screened (%) Control arm: pre 456 (32), post 481 (34) Intervention arm: pre 648 (43), post 768 (51) 4) Smoking cessation, N smokers (%) Control arm: pre 168 (12), post 159 (11) Intervention arm: pre 137 (9), post 149 (10) |
|
| Funding source | This study was partially financed by an unrestricted grant from Merck Sharp & Dohme (MSD) ‐ Spain and the Fundacion Escola Galega de Administraci on Sanitaria (FEGAS) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Do not describe, only state that they did random sampling. |
| Allocation concealment (selection bias) | Low risk | Not reported, but since cluster, low risk. |
| Provider's baseline characteristics (selection bias) | Low risk | In text and in table. |
| Patient's baseline characteristics (selection bias) | Low risk | In text they say the following differed (but did not provide P values in table): family history of ischaemic heart disease, personal history of prior coronary revascularisation, presence of neuropathy and insulin use. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | Losses per arm with patients seem low, reasons for losses of patients not provided (only for provider). And reason why the intervention group gain n = 16 people is not described in text. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Outcomes: eye, foot and renal exam, objective since they looked at medical records. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | High risk | Quote: "possibility of contamination of the control group and intervention group, as both worked in the same healthcare system". |
| Other bias | Low risk | None. |
Vinicor 1987.
| Study characteristics | ||
| Methods |
DIABEDS: a randomized trial of the effects of physician and/or patient education on diabetes patient outcomes Clustered RCT (12 clusters and 90 providers), conducted in 1) This study, entitled DIABEDS (DIABetes EDucation Study), was conducted in a general medicine clinic at Wishard Memorial Hospital, Indiana University Medical Center. 2) Patient education delivered by a nurse and a dietitian. Resident education provided by diabetes specialists and diabetologists. In United States of America. 4 arms: 1. Control (routine care) (control arm) and 2. Intervention 1 (patient education) (intervention arm), 3. Intervention 2 (physician education) (other arm), 4. Intervention 3 (patient and physician education) (other arm) |
|
| Participants | Control arm N: 135 Intervention arm N: 125, 134, 138 Diabetes type: 3 Mean age: 57 ± 11.49 % Male: 21 Longest follow‐up: 26 months |
|
| Interventions |
Control arm: (routine care) Intervention arm: (patient education) 1) Patient education 2) Promotion of self‐management Intervention arm: (physician education) 1) Audit and feedback 2) Clinician education 3) Clinician reminder |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | This research was supported by a Diabetes Research and Training Center grant from NIADDKD (PHS P60 AM20542) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Based on an “Incomplete Blocks Design” (see Statistics), resident clinic teams (3 or 4 residents/team) and their panel of diabetic patients were randomly assigned to one of 4 study groups. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Nothing reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 2. The percentage of females in the control group was significantly lower than in the physician education group. |
| Patient's baseline outcomes (selection bias) | High risk | Table 2. Analysis of baseline clinical characteristics following randomisation of patients to the 4 study groups indicated a significant difference for post‐prandial plasma glucose (PPG), for which Group 4 was significantly above the other 3 groups (data not shown). Also, the control group had significantly less body fat than patients assigned either to physician education only or patient plus physician education. |
| Incomplete outcome data (attrition bias) | High risk | 257 patients were not reassessed out of 532 (48.3% lost) randomised. Reasons for attrition of these patients included: death (12%); physical/psychological incapacitation, i.e. medical (e.g. severe congestive heart failure) or emotional illnesses resulting in patients being restricted to home (17%); transfer to non‐study faculty physicians (12%); left city (5%); work conflict (9%); unexplained “personal" reasons, i.e. refusal to participate in re‐evaluation despite minimum of 6 phone and/or mail contacts (18%); recurrent failure to keep DRTC appointment (4%); and inability to contact by phone or letter, i.e. phone disconnected, moved without forwarding address, etc. (23%). There were no significant differences in attrition percentages among the four study groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c, SBP and DBP). |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol. Results match methods. |
| Risk of contamination (other bias) | Unclear risk | Clustered RCT. All patients participating in DIABEDS continued to receive their care in the medicine clinic by their own resident physicians regardless of the study group to which they were assigned. All residents work at the same clinic. Communication might have happened between them. |
| Other bias | Low risk | None identified. |
Volpp 2015.
| Study characteristics | ||
| Methods |
A randomized controlled trial of negative co‐payments: the CHORD trial RCT (NA clusters and NA providers), conducted in 1) Study participants were drawn from patients at 3 hospitals in Pennsylvania: the Philadelphia Veterans Affairs Medical Center (PVAMC), the Veterans Administration Pittsburgh Health Care System (VAPitt), and the Pinnacle Health clinic in Harrisburg. 2) Intervention led by study staff and involved financial incentive and/or computerised behavioural intervention (CBI). In United States of America. 2 arms: 1. Control (control group and CBI group) (control arm) and 2. Intervention (financial incentive (FI) group and FI + CBI group) (intervention arm) |
|
| Participants | Control arm N: 92.99 Intervention arm N: 89.10, NA, NA Diabetes type: 4 Mean age: 60.97 ± 9.75 % Male: 80.75 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (control group and CBI group) 1) Patient education Intervention arm: (financial incentive (FI) group and FI + CBI group) 1) Patient education 2) Financial Incentives |
|
| Outcomes | Systolic blood pressure | |
| Funding source | The work in this paper was primarily supported by a grant from the Commonwealth of Pennsylvania, titled Collaboration to Reduce Disparities in Hypertension, grant number ME‐02‐382. Supplemental support was received from Pfizer, Inc. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a random number generator. |
| Allocation concealment (selection bias) | Low risk | Allocation assignments were concealed, with staff unable to access randomisation assignment for each participant until all eligibility criteria were entered in an electronic tracking system and consent forms were completed. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. There were significantly more blacks in the control group (P = 0.01) and age has a P value of 0.05, but no data only for diabetes patients. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1. P values above 0.05 for blood pressure and medication taking, but no data only for diabetes patients. |
| Incomplete outcome data (attrition bias) | High risk | Overall, they lost 20.2% of patients at 12 months, 23.7% in the control group and 16.5% in the intervention group. High and unbalanced numbers. Reasons not reported. No data only for diabetes patients. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest was objectively measured (SBP). |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol. They report DBP data for all patients after the intervention but not for diabetic patients only, as they did for SBP. They did not do cost analysis as stated in the protocol. They also measured adherence using the medication possession ratio in the paper, but this was not included in the protocol. Protocol mentions reporting blood pressure at 6 and 12 months, only reported at 12 months. |
| Risk of contamination (other bias) | Low risk | Both groups had computerised behavioural intervention (CBI, patient education), but that was planned. |
| Other bias | High risk | For the analysis, they merged 2 groups to make the intervention group (financial incentive and financial incentive + CBI) and they merged 2 other groups to make the control group (control and CBI). |
Wagner 2001.
| Study characteristics | ||
| Methods |
Chronic care clinics for diabetes in primary care: a system‐wide randomized trial Cluster‐RCT (35 clusters), conducted in primary care clinics in the Group Health Cooperative of Puget Sound in western Washington, USA Two arms: 1. Control patients (control arm) and 2. Intervention patients (intervention arm) |
|
| Participants | Control arm N: 429 Intervention arm N: 278 Diabetes type: unclear/not reported Mean age: 60.7 ± NR % Male: 53.4 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Team changes 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 267 (62), post 272 (63) Intervention arm: pre 168 (60), post 189 (68) 2) Foot screening, N screened (%) Control arm: pre NR (NR), post 347 (81) Intervention arm: pre NR (NR), post 244 (88) 3) Renal screening (microalbumin), N screened (%) Control arm: pre NR (NR), post 192 (45) Intervention arm: pre NR (NR), post 165 (59) 4) HbA1c, mean % (SD) Control arm: pre 7.4 (NR), post 7.9 (NR) Intervention arm: pre 7.5 (NR), post 7.9 (NR) |
|
| Funding source | This study was supported by Robert Wood Johnson Foundation Grant no. 02479 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Low risk | Information not available. |
| Provider's baseline characteristics (selection bias) | High risk | Not reported |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 all P values > 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1 ‐ outcomes P > 0.05. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Wakefield 2011.
| Study characteristics | ||
| Methods |
Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial Patient RCT, conducted in the Iowa City VA Medical Center. Provides primary, secondary, tertiary medical, surgical, psychiatric and neurological care to veterans residing in eastern Iowa and western Illinois, USA Three arms: 1. Usual care (control arm), 2. Low‐intensity group (intervention arm 1) and 3. High‐intensity group (intervention arm 2) |
|
| Participants | Control arm N: 107 Intervention arm 1 N: 102 Intervention arm 2 N: 93 Diabetes type: type 2 Mean age: 68.0 ± 10.0 % Male: 98.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Case management 2) Promotion of self‐management Intervention arm 1: 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders Intervention arm 2: 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.2 (NR), post 6.9 (NR) Intervention arm 1: pre 7.2 (NR), post 7.0 (NR) Intervention arm 2: pre 7.1 (NR), post 6.9 (NR) 2) SBP, mean mmHg (SD) Control arm: pre 134.0 (NR), post 137.3 (NR) Intervention arm 1: pre 136.0 (NR), post 136.8 (NR) Intervention arm 2: pre 138.0 (NR), post 133.1 (NR) |
|
| Funding source | The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development (VA HSR&D) Service (No. NRI 03‐ 312) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…random number generator." Assume this is used as well for the study. |
| Allocation concealment (selection bias) | Low risk | Quote: "…using sequentially numbered, sealed, opaque envelopes prepared in advance…" |
| Patient's baseline characteristics (selection bias) | Unclear risk | Quote: "There were no statistically significant differences across the three groups for any baseline measures." In text but not in table. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.71); SBP (P = 0.30). |
| Incomplete outcome data (attrition bias) | High risk | In treatment arms (low and high) there were greater number of dropouts than control arms: 6 months approximately 12%, 12 months approximately 22%, also number of deaths was higher in the low‐dose intervention arm. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c measured using ICVAMC laboratory, BP measured using machine. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes do not match. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Wakefield 2014.
| Study characteristics | ||
| Methods |
Effect of home telemonitoring on glycemic and blood pressure control in primary care clinic patients with diabetes RCT (NA clusters and NA providers), conducted in 1) Primary care clinics. The study took place at 6 University of Missouri Family Medicine (FM) and General Internal Medicine (GIM) clinics. 2) Intervention delivered by clinic nurses, advanced practice nurses and providers using an home telemonitoring system. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (home telemonitoring) (intervention arm) |
|
| Participants | Control arm N: 55 Intervention arm N: 53, NA, NA Diabetes type: 2 Mean age: 60.14 ± 9.72 % Male: 44.44 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) 1) Promotion of self‐management Intervention arm: (home telemonitoring) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure |
|
| Funding source | This work was supported in part by grant number R18HS017035 from the Agency for Healthcare Research and Quality | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. After patients signed the study consent form, they were randomised to intervention and control groups by the research assistant using sequentially numbered, sealed, opaque envelopes prepared in advance by the study data manager. |
| Allocation concealment (selection bias) | Low risk | After patients signed the study consent form, they were randomised to intervention and control groups by the research assistant using sequentially numbered, sealed, opaque envelopes prepared in advance by the study data manager. |
| Patient's baseline characteristics (selection bias) | Low risk | The mean age of the sample was 60 years and was significantly different between the 2 groups (control group, mean = 62.5 years, SD = 10.9, range = 32 to 92; intervention group, mean = 57.7 years, SD = 10.8, range = 29 to 82; P = 0.02). There were no significant differences in the remaining demographic variables (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | Table 2. There were no baseline differences for mean A1c or SBP, although SBP differed by gender. |
| Incomplete outcome data (attrition bias) | High risk | They lost 12/55 patients in the control group (22%) and 13/53 in the intervention group (25%). The overall dropout/lost to follow‐up rate was 23% (2 controls and 8 intervention patients dropped out; 10 control and 4 intervention patients were lost to follow‐up). High numbers and unbalanced reasons. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcomes were objectively measured (HbA1c and BP). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. They stratified blood pressure analysis by gender (Quote: "There were no baseline differences for mean A1c or SBP, although SBP differed by gender"). They did not report the secondary outcomes listed in the protocol (Changes in care process and Patient‐entered device data). |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that control patients used the home telemonitoring system. |
| Other bias | Low risk | No evidence of other bias. |
Waki 2014.
| Study characteristics | ||
| Methods |
DialBetics: a novel smartphone‐based self‐management support system for type 2 diabetes patients RCT (NA clusters and NA providers), conducted in 1) University of Tokyo Hospital, Tokyo, Japan. 2) The research team included an endocrinologist as principal investigator, technology implementation and system administration specialists, experts in technical and database application, a diabetes nurse and a dietitian in Japan 2 arms: 1. Control (non‐DialBetics) (control arm) and 2. Intervention (DialBetics) (intervention arm) |
|
| Participants | Control arm N: 27 Intervention arm N: 27, NA, NA Diabetes type: 2 Mean age: 57.25 ± 6.19 % Male: 66 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (non‐DialBetics) Intervention arm: (DialBetics) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NTT DOCOMO and Japan Society for Promotion of Science Grant‐in‐Aid for Young Scientist Research (B) 23790559. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly divided into a DialBetics group and non‐DialBetics group using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Demographic characteristics were similar for both groups except for smoking, which was more prevalent in the non‐DialBetics group (10 vs 4). No P values reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Demographic characteristics were similar for both groups except for smoking, which was more prevalent in the non‐DialBetics group (10 vs 4). No P values reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Three patients from the DialBetics group (11%) and 2 from the non‐DialBetics group (7%) dropped out of the study. One in the DialBetics group (and both in the non‐DialBetics group) dropped out for hospitalisation. The other 2 DialBetics dropouts cited unwillingness to continue constant measurements as their main reason. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Patient randomised, however overlap between groups unlikely due to study design. |
| Other bias | Low risk | None. |
Wallymahmed 2011.
| Study characteristics | ||
| Methods |
Nurse‐led cardiovascular risk factor intervention leads to improvements in cardiovascular risk targets and glycaemic control in people with type 1 diabetes when compared with routine diabetes clinic attendance Patient RCT, conducted in a Diabetes Centre at Aintree University Hospitals, Liverpool, United Kingdom Two arms: 1. Routine group (control arm) and 2. Nurse‐led group (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 40 Diabetes type: type 1 Mean age: 34.6 ± 9.0 % Male: 55.6 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Patient education |
|
| Outcomes | 1) Statins, N users (%) Control arm: pre 9 (22), post 24 (62) Intervention arm: pre 16 (40), post 37 (95) 2) Antihypertensives (any), N users (%) Control arm: pre 9 (22), post 11 (28) Intervention arm: pre 12 (30), post 18 (46) 3) HbA1c, mean % (SD) Control arm: pre 9.9 (1.4), post 9.4 (1.5) Intervention arm: pre 10.1 (1.4), post 9.2 (1.6) 4) SBP, mean mmHg (SD) Control arm: pre 119.0 (17.0), post 124.0 (16.0) Intervention arm: pre 127.0 (22.0), post 120.0 (15.0) 5) DBP, mean mmHg (SD) Control arm: pre 69.0 (10.0), post 70.0 (8.0) Intervention arm: pre 71.0 (13.0), post 68.0 (7.0) 6) LDL, mean mg/dL (SD) Control arm: pre 127.6 (23.2), post 100.5 (30.9) Intervention arm: pre 131.5 (34.8), post 85.1 (34.8) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "blind‐envelope system". Sealed? Opaque? |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "The baseline data showed that the groups were well matched". Also provide non‐significant values for baseline characteristics. |
| Patient's baseline outcomes (selection bias) | Low risk | All outcome assessed were not significant at baseline between both groups. |
| Incomplete outcome data (attrition bias) | High risk | Two were lost to follow‐up in reference group and 1 lost to follow‐up in intervention group. Study states that they failed to attend and were discharged back to general practice, but did not provide reasons for why they were discharged. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding not described. Objective laboratory methods not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Wang 2017.
| Study characteristics | ||
| Methods |
Telemedicine in the management of type 2 diabetes mellitus RCT (NA clusters and NA providers), conducted in 1) Department of Endocrinology and Metabolic Diseases, First Hospital of Jilin University. Information transmitted via glucometer. 2) The medical team at the medical centre logged onto the website every 2 weeks to analyse the patients’ information and to deliver the medical team’s advice to the patient. In China. 2 arms: 1. Control (conventional medical treatment) (control arm) and 2. Intervention (U‐healthcare) (intervention arm) |
|
| Participants | Control arm N: 106 Intervention arm N: 106, NA, NA Diabetes type: 2 Mean age: 53.65 ± 9.8 % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (conventional medical treatment) 1) Promotion of self‐management Intervention arm: (U‐healthcare) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | This study was supported by the Science Technology Department of Jilin Province (20150414054GH) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A total of 212 patients met the inclusion criteria in the screening and were randomised into the intervention group (106 patients) and control group (106 patients). |
| Allocation concealment (selection bias) | Unclear risk | A total of 212 patients met the inclusion criteria in the screening and were randomised into the intervention group (106 patients) and control group (106 patients). |
| Patient's baseline characteristics (selection bias) | Low risk | No statistically significant differences were observed between the intervention group and control group in baseline data including age, diabetes course, physical examination or biochemistry (P > 0.05) except for triglyceride (TG) levels (P = 0.001). |
| Patient's baseline outcomes (selection bias) | Unclear risk | No statistically significant differences were observed between the intervention group and control group in baseline data including age, diabetes course, physical examination or biochemistry (P > 0.05) except for triglyceride (TG) levels (P = 0.001). |
| Incomplete outcome data (attrition bias) | High risk | When the trial ended, 13 (11.8%) and 21 (19%) patients in the intervention and control groups dropped out, respectively. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP and LDL. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Many more outcomes than methods described. |
| Risk of contamination (other bias) | High risk | This study was conducted for a period of 6 months, during which both groups regularly visited the clinic every 3 months for physical and blood biochemical examination and received follow‐up and ambulatory treatment by the same medical team. |
| Other bias | Low risk | NA |
Ward 1996.
| Study characteristics | ||
| Methods |
Educational feedback in the management of type 2 diabetes in general practice Cluster‐RCT (139 clusters with 139 providers), conducted in Western Australia metropolitan general practices, Australia Three arms: 1. No interview (control arm), 2. Doctor interview (intervention arm 1) and 3. Nurse interview (intervention arm 2) |
|
| Participants | Control arm N: 135 Intervention arm 1 N: 130 Intervention arm 2 N: 121 Diabetes type: type 2 Mean age: 61.5 ± 9.7 % Male: 55.2 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Audit and feedback 2) Clinician education 3) Clinician reminders Intervention arm 1: 1) Audit and feedback 2) Clinician education 3) Clinician reminders Intervention arm 2: 1) Audit and feedback 2) Clinician education 3) Clinician reminders |
|
| Outcomes | 1) Retinopathy screening (eye exam), N screened (%) Control arm: pre 40 (30), post 42 (31) Intervention arm 1: pre 30 (23), post 55 (42) Intervention arm 2: pre 24 (20), post 49 (40) 2) Foot screening, N screened (%) Control arm: pre 14 (10), post 16 (12) Intervention arm 1: pre 12 (9), post 34 (26) Intervention arm 2: pre 11 (9), post 25 (21) 3) Renal screening (creatinine), N screened (%) Control arm: pre 29 (21), post 36 (27) Intervention arm 1: pre 24 (18), post 56 (43) Intervention arm 2: pre 13 (11), post 32 (26) |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
| Risk of contamination (other bias) | Unclear risk | Information not available. |
| Other bias | Low risk | Information not available. |
Warren 2018.
| Study characteristics | ||
| Methods |
Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Primary health setting in the Townsville area of Queensland, Australia. The study was conducted with the co‐operation of 25 general practices, which were the usual practices participants attended for their diabetes care. 2) Care co‐ordinators (CC) were nurses with practice nurse experience, and chronic disease management and health coaching training. GPs continued to manage their participants’ care in partnership with CCs in Australia 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (diabetes programme) (intervention arm) |
|
| Participants | Control arm N: 69 Intervention arm N: 88, NA, NA Diabetes type: 2 Mean age: 61.3 ± 10.55 % Male: 54 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (diabetes program) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Townsville Broadband‐Enabled Diabetes Telehealth Trial was supported by funding from the Australian Government under the Digital Regions Initiative Partnership Agreement, and the Queensland Government. The Trial Extension was funded by the Australian Government as represented by the Department of Communications. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Arm allocation was conducted with the participant as the unit of randomisation, using computer‐generated 1:1 simple randomisation. |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was generated by the study research co‐ordinator (KC), held in a secure opaque envelope by the study administrator and concealed from the study care co‐ordinators (CCs). |
| Patient's baseline characteristics (selection bias) | Low risk | Participants in the 2 arms were similar in age distribution and indigenous and marital status, but there were slightly more men in the intervention group compared with the control group (38/63 vs 30/63, respectively). |
| Patient's baseline outcomes (selection bias) | Low risk | Data look balanced. |
| Incomplete outcome data (attrition bias) | High risk | 6 lost in control group (9%), 25 lost in intervention group (28%), 20% overall. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Outcomes measured objectively: HbA1c, BP. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol however time points 3, 9 and 12 months are not reported. Limitations of the present study included the reliance on data from 6 months of participant follow‐up, compared with the planned 12‐month study period. Methods talk about multiple quality of life surveys, but only report SF‐6D index score (with no mention of it earlier). |
| Risk of contamination (other bias) | Low risk | Care co‐ordinators only followed intervention group, different GPs for intervention and control participants. |
| Other bias | Low risk | No evidence of other bias. |
Wayne 2015.
| Study characteristics | ||
| Methods |
Health coaching reduces glycated hemoglobin in type 2 diabetic patients from a lower‐socioeconomic status community: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from 2 primary health clinics in Toronto, Canada. 2) Intervention provided by health coaches. In Canada. 2 arms: 1. Control (health coaches) (control arm) and 2. Intervention (health coaches and mobile phone) (intervention arm) |
|
| Participants | Control arm N: 64 Intervention arm N: 67, NA, NA Diabetes type: 2 Mean age: 53.2 ± 6.55 % Male: 28 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (health coaches) 1) Case management 2) Promotion of self‐management Intervention arm: (health coaches and mobile phone) 1) Case management 2) Electronic patient registry 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Funding was obtained through the Public Health Agency of Canada (Project #0690490). Additional funding was obtained from York University (Connected Health and Wellness Project) through the Federal Development Agency of Southern Ontario. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number sequence was generated using a random number‐generating program without constraints. |
| Allocation concealment (selection bias) | Low risk | After the sequence was generated by the research co‐ordinator, a research assistant with no connection to the trial sealed the sequence in individual, opaque envelopes and numbered each based on sequence generation. Once a candidate participant consented and their HbA1c was verified as meeting the inclusion criteria, the next envelope was opened (in sequence) to ascertain group allocation, and the health coaching intervention commenced. |
| Patient's baseline characteristics (selection bias) | Low risk | No P values, however, Quote: "Of the measures collected, differences at baseline between groups were only detected for the SF‐12 Mental Health Composite Scores." |
| Patient's baseline outcomes (selection bias) | Low risk | Table 6. Time point comparison of HbA1c levels for intervention versus control groups at baseline: P = 0.30. |
| Incomplete outcome data (attrition bias) | High risk | 138 participants were recruited; 67 were randomised to the experimental arm and 64 to the control arm (7 were excluded for substudy analysis, reasons not reported). Of the 131 participants included in the study, there were 34 dropouts (26%), with 19 out of 67 (28%) from the intervention group and 15 out of 64 (23%) from the control group. Reasons for dropouts not reported. Final per‐protocol analysis included 97 participants out of 131 (74%), with 48 in the intervention group and 49 in the control group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcome of interest is objective (HbA1c). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (protocol first posted in January 2014, study started in February 2012). The outcome of interest is reported (HbA1c) but they were supposed to report only at 6 months follow‐up and not at 3 months follow‐up. |
| Risk of contamination (other bias) | Unclear risk | The lack of between‐group differences at 6 months may be due to other, more complex factors. For example, since health coaches were randomly assigned to participants in both arms, it is possible that more effort was expended in coaching the mobile phone‐assisted arm. |
| Other bias | Low risk | No evidence of other bias. |
Webb 2017.
| Study characteristics | ||
| Methods |
A cluster‐randomized trial to estimate the effect of mobile screening and treatment feedback on glycated hemoglobin and diabetes‐related complications in Tshwane primary health care clinics, South Africa Clustered RCT (12 clusters and NR providers), conducted in 1) A cluster‐randomised trial in primary care clinics in the Tshwane district of South Africa, 2) The team consisted of the primary investigator with a trained research assistant and medical students (trained to complete the clinical data forms and examinations as needed). In South Africa. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Mobile Screening for Improving the Management of Diabetes (CCMSD)) (intervention arm) |
|
| Participants | Control arm N: 273 Intervention arm N: 326, NA, NA Diabetes type: 3 Mean age: 58.09 ± 6.2 % Male: 31.5 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (usual care) 1) Clinician education Intervention arm: (Mobile Screening for Improving the Management of Diabetes (CCMSD)) 1) Clinician education 2) Facilitated relay of clinical information 3) Patient education |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by the Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA), the African Population & Health Research Centre (APHRC) and research funds from the University of Pretoria. Novo Nordisk supplied and funded the mobile unit. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Cluster‐RCT, unit of allocation: clinic. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | No P value reported but data are available on age and gender, and seem balanced. Table 1. |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1C levels at baseline are reported in Table 1, no P value is reported. |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up between Phase 1 and 2 and Phase 3 (one year later) was 22.4%. This can be further sub‐divided into 26.1% in the intervention arm and 18.0% in the control arm (Table 2) |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcome: HbA1c. |
| Selective reporting (reporting bias) | High risk | Number of differences between protocol and published report (e.g. does not report BP or lipids or cost of the intervention). |
| Risk of contamination (other bias) | Low risk | Cluster‐RCT: As a mobile unit was used to collect information, all patients at a facility had to be treated the same way to prevent bias, therefore we had to use clinics as the clusters and did not randomise at the individual level. |
| Other bias | Low risk | No evidence of other bias. |
Wei 2017.
| Study characteristics | ||
| Methods |
Implementation of a comprehensive intervention for patients at high risk of cardiovascular disease in rural China: a pragmatic cluster randomized controlled trial Clustered RCT (67 clusters and NR providers), conducted in 1) Primary care in rural setting in China. Three counties located in central Zhejiang province, 67 township hospitals. 2) Family doctors in China 2 arms: 1. Control (conventional care) (control arm) and 2. Intervention (case management) (intervention arm) |
|
| Participants | Control arm N: 5521 Intervention arm N: 4585, NA, NA Diabetes type: 2 Mean age: 64.3 ± 0.6 % Male: 48.9 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (conventional care) Intervention arm: (case management) 1) Audit and feedback 2) Clinician education 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Anti‐platelet drugs Lipid‐lowering drugs Antihypertensive drug Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Smoking status |
|
| Funding source | This study was partly funded by COMDIS‐HSD, a research consortium funded by UK aid from the UK government: however, the views expressed do not necessarily reflect the UK government’s official policies. The study also received funding from Zhejiang Provincial Government, China through the Zhejiang Centre for Disease Control and Prevention (CDC) and the Dalla Lana School of Public Health at the University of Toronto. The funders had no role in research design, data collection, interpretation of the data, writing or submitting the manuscript. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | An independent biostatistician randomly allocated eligible township hospitals to intervention or control arms in a 33:34 ratio using sequential numbers without stratification. |
| Allocation concealment (selection bias) | High risk | An independent biostatistician randomly allocated eligible township hospitals to intervention or control arms in a 33:34 ratio using sequential numbers without stratification. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Demographic, socioeconomic and disease characteristics reported in Tables 1 (whole sample) and 2 (sub‐sample) and but no data are reported only for patients with diabetes. |
| Patient's baseline outcomes (selection bias) | Low risk | The authors did a sub‐sample analysis for these outcomes only in diabetic patients: smoking, SBP, DBP, HbA1c and LDL. Baseline data reported on Tables 3 and 4 but no P values. |
| Incomplete outcome data (attrition bias) | Low risk | At 12 months, all 67 clusters were successfully followed up, with 12,270 (92%) in the intervention arm and 13,118 (90%) in the control arm successfully followed up and included in the analysis. Loss to follow‐up was similar between the intervention and control arms. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective measure of HbA1c, BP, LDL. Subjective measure of drugs and smoking. Patient lifestyle indicators were self‐reported. |
| Selective reporting (reporting bias) | Unclear risk | Clearly state that they are not reporting the primary outcome (CVD events) in this paper. Prospectively registered protocol includes secondary outcomes of appointment adherence, cost‐effectiveness and feasibility which are not reported here. |
| Risk of contamination (other bias) | Low risk | Cluster‐randomised. |
| Other bias | Unclear risk | Used complete case analyses which may introduce bias into the results depending on the missing data mechanism. |
Weinberger 1995.
| Study characteristics | ||
| Methods |
A nurse‐coordinated intervention for primary care patients with non‐insulin‐dependent diabetes mellitus: impact on glycemic control and health‐related quality of life Patient RCT, conducted in general medical clinic at the Department of Veterans Affairs Medical Centre in Durham, North Carolina, USA Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 71 Intervention arm N: 204 Diabetes type: type 2 Mean age: 64.0 ± 8.5 % Male: 99.0 Longest follow‐up: 14 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education |
|
| Outcomes | 1) HbA1c, mean % (pre: SD, post: SE) Control arm: pre 10.7 (3.4), post 11.1 (0.3) Intervention arm: pre 10.7 (3.3), post 10.5 (0.2) |
|
| Funding source | Supported by IIR #89‐079 from the Health Services Research and Development Service, Department of Veterans Affairs | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Weiss 2015.
| Study characteristics | ||
| Methods |
Effect of behavioral intervention on dilated fundus examination rates in older African American individuals with diabetes mellitus: a randomized clinical trial RCT (NA clusters and NA providers), conducted in 1) Participants were recruited from the following 2 sources: 1 of 2 academic medical institutions in Philadelphia, Pennsylvania, and community‐based programmes. Eligible participants had a home‐based visit with a race/ethnicity–concordant community health worker (CHW). Follow‐up assessments were conducted in participants’ homes at 6 months’ follow‐up by CHWs masked to treatment assignment. 2) Community Health Care Workers. In United States of America. 2 arms: 1. Control (supportive therapy) (control arm) and 2. Intervention (behavioral activation for diabetic retinopathy prevention) (intervention arm) |
|
| Participants | Control arm N: 103 Intervention arm N: 103, NA, NA Diabetes type: 2 Mean age: 72.7 ± NR % Male: 34.95 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (supportive therapy) Intervention arm: (behavioral activation for diabetic retinopathy prevention) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Retinopathy screening Glycated haemoglobin |
|
| Funding source | This work was funded by the Pennsylvania Department of Health | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 206 participants who completed the baseline assessment were randomised using random permuted blocks with a 1 to 1 allocation ratio to BADRP or supportive therapy (ST). |
| Allocation concealment (selection bias) | Low risk | Randomisation sheets were stored in sequentially numbered, sealed envelopes that were opened by the project director after each participant completed baseline assessment. The project director notified the appropriate interventionist for the participants’ treatment assignments. |
| Patient's baseline characteristics (selection bias) | Low risk | The 2 arms were balanced with respect to age, education, sex, recruitment site and marital status. No P values provided. |
| Patient's baseline outcomes (selection bias) | High risk | Participants in the BADRP group had lower HbA1c levels and chronic disease scores at baseline. |
| Incomplete outcome data (attrition bias) | Unclear risk | 0 participants lost to follow‐up in behavioural group, 2 lost in control group. Completion rates at 6 months’ follow‐up for BADRP and ST participants were 88% and 85%, respectively. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, retinal screening. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Medical status, literacy, HbA1c, National Eye Institute Visual Function Questionnaire 25 not mentioned in protocol. Cost‐effectiveness and appointment adherence mentioned in protocol but not reported in publication. |
| Risk of contamination (other bias) | Low risk | Supportive therapy is a structured placebo treatment that controls for the nonspecific elements of behavioural activation (e.g. attention). It was administered by a specially trained CHW and was delivered in the home across 4 x 1‐hour sessions. |
| Other bias | Low risk | No evidence of other bias. |
Welch 2011a.
| Study characteristics | ||
| Methods |
Comprehensive diabetes management program for poorly controlled Hispanic type 2 patients at a community health center Patient RCT, conducted in an urban community healthcare centre (CHC) in Springfield, Massachusetts. Underserved/poor population, USA Two arms: 1. Attention control condition (control arm) and 2. Intervention condition (intervention arm) |
|
| Participants | Control arm N: 21 Intervention arm N: 25 Diabetes type: type 2 Mean age: 55.8 ± 10.0 % Male: 33.0 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Case management 2) Team changes 3) Clinician reminders 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management |
|
| Outcomes | 1) Aspirin, N users (%) Control arm: pre NR (NR), post 18 (100) Intervention arm: pre NR (NR), post 21 (100) 2) Retinopathy screening (eye exam), N screened (%) Control arm: pre NR (NR), post 14 (78) Intervention arm: pre NR (NR), post 19 (90) 3) Foot screening, N screened (%) Control arm: pre NR (NR), post 13 (72) Intervention arm: pre NR (NR), post 18 (86) 4) HbA1c, mean % (SD) Control arm: pre 8.5 (1.0), post 7.9 (1.4) Intervention arm: pre 9.0 (1.2), post 7.4 (1.4) 5) SBP, mean mmHg (SD) Control arm: pre 143.0 (28.0), post 134.4 (21.6) Intervention arm: pre 132.0 (17.0), post 124.5 (15.1) 6) DBP, mean mmHg (SD) Control arm: pre 81.0 (14.0), post 82.1 (9.2) Intervention arm: pre 80.0 (12.0), post 77.7 (9.9) 7) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre NR (NR), post 5 (28) Intervention arm: pre NR (NR), post 12 (57) |
|
| Funding source | This study was funded by Baystate Medical Center Academic Affairs Internal Research Grant #AA‐BMC P99 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…by a fair coin toss." |
| Allocation concealment (selection bias) | Low risk | So long as coin was tossed when participants entered the study and was not done a priori to generate a list. |
| Patient's baseline characteristics (selection bias) | High risk | Marital status (P = 0.04). |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.13); SBP (P = 0.10); DBP (P = 0.73). |
| Incomplete outcome data (attrition bias) | High risk | ~14% and ~16% lost to follow‐up in control and intervention arms. Reasons for withdrawal not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Blinding of outcome assessors not described, and how HbA1c was measured was not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | High risk | Quote: "…the diabetes educators in the intervention condition trained and supervised the attention control clinical staff." |
| Other bias | Low risk | Information not available. |
Welch 2011b.
| Study characteristics | ||
| Methods |
Motivational interviewing delivered by diabetes educators: does it improve blood glucose control among poorly controlled type 2 diabetes patients? Patient RCT, conducted in a large hospital medical centre (diabetes clinic), USA Four arms: 1. Standard education ‐ DSME (control arm), 2. Computer alone (intervention arm 1), 3. MI alone (intervention arm 2), and 4. MI with computer (intervention arm 3) |
|
| Participants | Control arm N: 58 Intervention arm 1 N: 58 Intervention arm 2 N: 57 Intervention arm 3 N: 61 Diabetes type: type 2 Mean age: 55.7 ± 10.2 % Male: 41.0 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Patient education Intervention arm 1: 1) Facilitated relay of clinical information Intervention arm 2: 1) Patient education 2) Promotion of self‐management Intervention arm 3: 1) Facilitated relay of clinical information 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.8 (1.3), post 8.2 (NR) Intervention arm 1: pre 8.9 (1.2), post 8.0 (NR) Intervention arm 2: pre 9.1 (1.5), post 8.7 (NR) Intervention arm 3: pre 8.8 (1.0), post 8.5 (NR) |
|
| Funding source | This research was supported by National Institutes of Health grant #1R01DK060076 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process not described. |
| Allocation concealment (selection bias) | Unclear risk | Process not described. |
| Patient's baseline characteristics (selection bias) | High risk | Education status (P = 0.02) and insulin use (P = 0.04). |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.50). |
| Incomplete outcome data (attrition bias) | High risk | Very large attrition rate: 35%. Did not conduct intention‐to‐treat analysis. And those who dropped out and those who stayed differed were significantly in age (P = 0.01) and importance of diabetes self‐management (P < 0.01). Reasons for dropouts not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | HbA1c objective measure using laboratory methods. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach since no protocol. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | None. |
Welch 2015.
| Study characteristics | ||
| Methods |
An internet‐based diabetes management platform improves team care and outcomes in an urban Latino population RCT (NA clusters and NA providers), conducted in 1) The study was conducted at 2 affiliated Federally Qualified Health Centers (FQHCs) located in Western Massachusetts in an area where more than 30% of families locally live below the federal poverty line. The clinics are located in a medically underserved and health professional shortage area. The 29 clinic providers serve a predominantly (about 80%) Latino urban poor community including more than 2400 diabetic patients. 2) Intervention delivered by diabetes nurses, diabetes dietitians and providers. In United States of America. 2 arms: 1. Control (in house usual diabetes care) (control arm) and 2. Intervention (diabetes dashboard condition) (intervention arm) |
|
| Participants | Control arm N: 200 Intervention arm N: 199, NA, NA Diabetes type: 2 Mean age: 55 ± 8,81 % Male: 40 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (in house usual diabetes care) 1) Patient education 2) Promotion of self‐management Intervention arm: (diabetes dashboard condition) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician education 5) Clinician reminder 6) Facilitated relay of clinical information 7) Patient education 8) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Harms |
|
| Funding source | This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, through grant 5R01‐DK‐084325‐04 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. "We used a parallel‐group randomised design for this clinical trial. Eligible patients were randomised either to the diabetes dashboard intervention condition (IC) or to an in‐house UDC program delivered without access to the diabetes dashboard." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. "We used a parallel‐group randomised design for this clinical trial. Eligible patients were randomised either to the diabetes dashboard intervention condition (IC) or to an in‐house UDC program delivered without access to the diabetes dashboard." |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P above 0.05 for age, race and gender. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. BMI is close to being significantly different between control and intervention groups at baseline (P = 0.06). Other variables are similar (P above 0.05). |
| Incomplete outcome data (attrition bias) | High risk | 46 lost to follow‐up out of 399 (11.5%): 9.5% in the control group and 13.6% in the intervention group. Reasons not balanced (6 patients refused the second visit in the intervention, and only 2 in the control group). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objective outcomes (HbA1c, SBP and DPB), but self‐reported hypoglycaemia symptoms (subjective outcome). Blinding not described. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol (first posted in June 2014, patients were recruited from December 2010 to December 2012, 6‐month intervention). In the paper, the authors report data about blood pressure, hypoglycaemia and BMI, which were not planned in the protocol. |
| Risk of contamination (other bias) | Low risk | The interventionists were not the same for the control and the intervention groups. Quote: "The IC was delivered by a team of four bicultural, bilingual diabetes educators (two diabetes nurses and two diabetes dietitians)... The UDC condition was delivered by four additional bicultural, bilingual diabetes nurses and diabetes dietitians." However, the providers might have changed their approaches with the control group as the same providers were involved in both arms. |
| Other bias | Low risk | None identified. |
White 2017.
| Study characteristics | ||
| Methods |
Clinic attendance and disengagement of young adults with type 1 diabetes after transition of care from paediatric to adult services (TrACeD): a randomised, open‐label, controlled trial RCT (NA clusters and NA providers), conducted in 1) Tertiary paediatric diabetes service at the Royal Children’s Hospital, Melbourne, VIC, Australia. 2) The intervention was delivered by appointment manager (MW), medical specialist who undertook the project as part of a research degree. In Australia. 2 arms: 1. Control (current care) (control arm) and 2. Intervention (appointment management) (intervention arm) |
|
| Participants | Control arm N: 60 Intervention arm N: 60, NA, NA Diabetes type: 1 Mean age: 18.8 ± 9.29 % Male: 49 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: (current care) Intervention arm: (appointment management) 1) Case management 2) Patient education 3) Patient reminders |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This study was funded by the Australasian Paediatric Endocrine Group and Lilly (research grant 2012) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A statistician not directly involved in the analysis of study results prepared the randomisation schedule. We randomly assigned participants (1:1), using sequential sealed opaque envelopes, to either appointment management (intervention) or current care (control)." |
| Allocation concealment (selection bias) | Low risk | "We randomly assigned participants (1:1), using sequential sealed opaque envelopes, to either appointment management (intervention) or current care (control)." |
| Patient's baseline characteristics (selection bias) | Low risk | At the time of transition, baseline characteristics including mean age and glycaemic control (HbA1c) were similar between groups (Table 1). |
| Patient's baseline outcomes (selection bias) | Low risk | No P values. At the time of transition, baseline characteristics including mean age and glycaemic control (HbA1c) were similar between groups (Table 1). |
| Incomplete outcome data (attrition bias) | High risk | Figure: Trial profile. By 24 months, 23/60 (38%) lost in control, 28/60 (46%) lost in intervention. Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure HbA1c. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. Unlikely that control group received appointment management. |
| Other bias | Low risk | None identified. |
Whitlock 2000.
| Study characteristics | ||
| Methods |
Telemedicine improved diabetic management RCT (NA clusters and NA providers), conducted in 1) Home telemonitoring, multidisciplinary diabetic education classes at Eisenhower Army Medical Center. 2) The 2 physicians made all diabetic management decisions for the patients in the intervention and control groups. The case manager reinforced care plans with the patients and consulted with the physicians weekly. A team, including the case manager, clinical co‐ordinator, and/or a technician, visited each patient's home to install the unit and train and instruct the patient on the Aviva 20/20 and later the Aviva 10/10 system and how it would be used throughout the study. In United States of America. 2 arms: 1. Control (routine standard of care) (control arm) and 2. Intervention (telemedicine) (intervention arm) |
|
| Participants | Control arm N: 13 Intervention arm N: 15, NA, NA Diabetes type: 2 Mean age: 63 ± 4.45 % Male: 39.28 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (routine standard of care) 1) Patient education Intervention arm: (telemedicine) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | This demonstration project was supported by a 1997 grant from the Office of the Assistant Secretary of Defense, Health Affairs, to evaluate applications of telemedicine technology in the management of the high cost of chronic disease | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No report of dropout. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c. |
| Selective reporting (reporting bias) | High risk | No registered protocol. Lipid results are not explicitly reported, despite being measured. |
| Risk of contamination (other bias) | Unclear risk | "The two physicians made all diabetic management decisions for the patients in the intervention and control groups". |
| Other bias | Low risk | None. |
Wild 2016.
| Study characteristics | ||
| Methods |
Supported telemonitoring and glycemic control in people with type 2 diabetes: the Telescot diabetes pragmatic multicenter randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) We recruited family practices caring for socially diverse populations from Borders, Glasgow, and Lothian in Scotland and Kent in England through primary care research networks, United Kingdom (UK). 2) Telemonitoring intervention reviewed by family practice clinicians or practice nurses. In United Kingdom 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (supported telemonitoring) (intervention arm) |
|
| Participants | Control arm N: 161 Intervention arm N: 160, NA, NA Diabetes type: 2 Mean age: 61 ± NR % Male: 66.7 Longest follow‐up: 9 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education Intervention arm: (supported telemonitoring) 1) Case management 2) Team change 3) Electronic patient registry 4) Clinician education 5) Facilitated relay of clinical information 6) Patient education 7) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Smoking status Harms |
|
| Funding source | The trial was funded by a Chief Scientist Office Applied Research Programme Grant (ARPG/07/3) (http://www.cso.scot.nhs.uk/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. LifeScan (http://www.lifescan.co.uk/) provided the glucometers and test‐strips, but had no other role in the trial. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | People meeting the eligibility criteria were randomised using an allocated treatment code generated by a computer from a minimisation procedure as well as a 1:1 ratio for intervention to control group. |
| Allocation concealment (selection bias) | Low risk | To ensure unpredictability of the minimisation procedure, there was a 1 in 10 chance that the determined treatment allocation was reversed; the corresponding random numbers list was stored securely at the Edinburgh Clinical Trials Unit and concealed from participants and research nurses. Random allocations were obtained by research nurses who enrolled participants through a secure web‐based system prepared and maintained by the Edinburgh Clinical Trials Unit. |
| Patient's baseline characteristics (selection bias) | Low risk | The two groups had similar distributions of relevant characteristics at baseline as shown in Tables 1 and 2 (distribution of minimisation criteria). No P values. |
| Patient's baseline outcomes (selection bias) | Low risk | The two groups had similar distributions of relevant characteristics at baseline as shown in Tables 3 and 4 (summarising clinical characteristics). No P values. |
| Incomplete outcome data (attrition bias) | Unclear risk | HbA1c data at follow‐up were available for 146/160 people in the intervention group (8.8% lost) and 139/161 people in the control group (13.7%). Low and somewhat balanced numbers. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Some of our outcomes of interest were objectively measured (HbA1c, SBP and DBP). Number of hypoglycaemic episodes and smoking status were self‐reported by patients, but are secondary outcomes. |
| Selective reporting (reporting bias) | High risk | Prospectively registered protocol. Many subgroup analysis data reported but were not planned in protocol. |
| Risk of contamination (other bias) | High risk | Usual diabetes care in family practice is financially incentivised in the UK with targets set on a sliding scale of rewards for glycaemic and blood pressure control. All participants were given an information pack containing a range of publicly available leaflets on the management of diabetes and lifestyle modification. Participants followed by the same family practice clinicians who were not blinded to allocation group. |
| Other bias | Low risk | None. |
Williams 2012.
| Study characteristics | ||
| Methods |
A multifactorial intervention to improve blood pressure control in co‐existing diabetes and kidney disease: a feasibility randomized controlled trial Patient RCT, conducted in diabetes, renal, and diabetes and nephrology outpatient clinics at public tertiary metropolitan hospital in Melbourne and regional Victoria, Australia Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 39 Diabetes type: type 1 and type 2 Mean age: 67.0 ± 9.6 % Male: 56.3 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, median % (SD) Control arm: pre 7.5 (1.1), post 8.0 (1.5) Intervention arm: pre 7.5 (1.5), post 7.0 (1.5) 2) SBP, mean mmHg (SD) Control arm: pre 150.2 (NR), post 138.2 (NR) Intervention arm: pre 150.2 (NR), post 133.2 (NR) 3) DBP, mean mmHg (SD) Control arm: pre 77.6 (NR), post 71.1 (NR) Intervention arm: pre 75.7 (NR), post 68.3 (NR) |
|
| Funding source | This research was supported by an Australian Research Council (Linkage) Grant (LP0774989), Sigma Theta Tau International Small Grant, Nurses Memorial Centre Australian Legion of Ex‐ Servicemen and Women Scholarship, and the Mona Menzies Nurses Board of Victoria Grant | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "code numbers assigned prior to randomization by a statistician." How these code numbers were generated is unclear. Then used stratified block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The identity of all participants who were enrolled and randomized to receive the intervention was kept in a locked cabinet". This doesn't mean it was concealed from those conducting the randomisation process. What about block size? if its too small one can predict next assignment. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not provided. |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up reasons seem balanced. Quote: "The analysis was performed on an intention to treat basis." Also, number lost to attrition unlikely to alter results. Numbers lost to follow‐up similar and reasons are balanced. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary outcome: SBP measured using sphygmomanometer, outcome assessors were blinded. Not reported as secondary: HbA1c, no mention of how it was measured, but assessors were blinded. |
| Selective reporting (reporting bias) | Low risk | Checked protocol. |
| Risk of contamination (other bias) | Unclear risk | There were biases of patients showing the research assistant their group allocations; not sure if this constitutes contamination of interventions. |
| Other bias | Low risk | Information not available. |
Wilson 2014.
| Study characteristics | ||
| Methods |
Evaluation of the clinical and cost effectiveness of intermediate care clinics for diabetes (ICCD): a multicentre cluster randomised controlled trial Clustered RCT (49 clusters and NR providers), conducted in 1) Community‐based, intermediate care clinics for diabetes (ICCDs). Trial involving 3 English primary care trusts (PCT), in the East and West Midlands, with 49 general practices. All were in urban areas with a higher than average prevalence of diabetes and serving ethnically diverse populations. 2) Multidisciplinary team led by specialist nurses. Medical care provided by a diabetologist. The intermediate care clinics (ICC) work closely with hospital‐based specialist teams and community services, including podiatry and dietetic services. Patients were managed by the ICC team until control of risk factors was achieved and then referred back to primary care. In United Kingdom. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (intermediate care clinics) (intervention arm) |
|
| Participants | Control arm N: 940 Intervention arm N: 1057, NA, NA Diabetes type: 2 Mean age: NR ± 10.40 % Male: 58.26 Longest follow‐up: 18 months |
|
| Interventions |
Control arm: (usual care) 1) Clinician education Intervention arm: (intermediate care clinics) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Hypertension control |
|
| Funding source | The study is funded by National Institute for Health Research (NIHR) ‐ Health Services and Delivery Research (HS&DR) stream (previously known as NIHR SDO). This is a UK government funding (publicly funded) body. Project number: SDO/110/2005; Initial service for support costs was provided by Department of health.NHS Leicester City, Thames Valley Diabetes Research Network (TVDRN), West Midlands South Comprehensive Local Research Network (CLRN), Primary Care Research Network (PCRN) and DIERT charity provided additional support for the successful completion of the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript apart from critically reviewing the grant application prior to funding. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices recruited to the study were randomised (in‐house software) by the UK Clinical Research Network (UKCRN) accredited Warwick Clinical Trials Unit to either control (usual care) or intervention. Randomisation was undertaken by an independent clinical trials unit after written agreement had been obtained. Randomisation was stratified by practice size and PCT to achieve balanced intervention and control arms. |
| Allocation concealment (selection bias) | Low risk | Clustered RCT. Randomisation was undertaken by an independent clinical trials unit. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Nothing clearly reported. Table 1. Fewer patients from primary care trusts 1 (PCT1) and more patients from PCT3 in the control group (P < 0.001). Figure 2: intervention, mean (SD) practice size: 48.0 (27.8), range 11 to 126; and control, mean (SD) practice size: 40.9 (22.7), range 16 to 92. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. More White and fewer Asian in the control group (P < 0.001). Variation in baseline characteristics between the groups. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. Controlled blood pressure (< 140/80 mmHg) has a P value of < 0.001 and diastolic BP has a P value of 0.007. Variation in baseline characteristics between the groups. Table 2. |
| Incomplete outcome data (attrition bias) | High risk | 1997 patients were recruited and 1280 followed‐up after 18‐month intervention (35.9% lost). They analysed 644/1057 patients in the intervention arm (39.1% lost) and 636/940 patients in the control ram (32.3% lost). Achieved follow‐up for 64% of patients. High numbers, but quite balanced. The main limitations of the trial were the low number of participants and higher than expected loss to follow‐up. Reduction in size of cluster (lost 5 clinics out of 49 randomised). |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and blood pressure). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. The trial was registered before participants were recruited in the National Research Register (NIHR NRR ID: M0014178167; 29 October 2007) and subsequently in the international trial register (ClinicalTrials.gov: Identifier NCT00945204; 23 July 2009). A detailed protocol has been published elsewhere. They added HbA1c and blood pressure mean and SD in the paper (Table 3). UKPDS risk not assessed in the paper. HRQoL and incremental cost‐effectiveness are not mentioned in protocol. |
| Risk of contamination (other bias) | Low risk | Clustered RCT. Pay for performance, introduced in 2004 in UK. Practices randomised to control were reminded of local diabetes management guidelines and continued to manage their patients, including hospital referrals, in the usual manner. |
| Other bias | Low risk | None. |
Wishah 2015.
| Study characteristics | ||
| Methods |
Impact of pharmaceutical care interventions on glycemic control and other health‐related clinical outcomes in patients with type 2 diabetes: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) The study site was the outpatient diabetes clinic at Jordan University Hospital (JUH), a major teaching hospital in Amman, capital of Jordan. The diabetes clinic at JUH provides usual care services to more than 90 patients daily with regular follow‐up clinic visits every 1 to 3 months, depending on the glycaemic control for each patient. 2) The intervention group patients received pharmaceutical care interventions developed by the clinical pharmacist in collaboration with the physician. In Jordan. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (pharmaceutical care) (intervention arm) |
|
| Participants | Control arm N: 54 Intervention arm N: 52, NA, NA Diabetes type: 2 Mean age: 53.05 ± NR % Male: 43.39 Longest follow‐up: 6 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (pharmaceutical care) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At the time of recruitment, patients were randomised into the intervention group (n = 52) and the control group (n = 54) using a coin‐toss method. |
| Allocation concealment (selection bias) | Low risk | Coin‐toss method. Not predictable. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values above 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | Table 1. HbA1c (P = 0.01) and serum triglycerides (P = 0.02) have significant P values and FBG is close to be significant (P = 0.06). Except for the baseline HbA1c values and serum triglycerides, statistical analyses indicated no significant differences between the 2 groups on the baseline measures. |
| Incomplete outcome data (attrition bias) | Low risk | During the study period, 2/52 (3.8%) patients from the intervention group and 3/54 (5.6%) patients from the control group dropped out from the study. Therefore, a total of 101/106 patients (50 intervention group; 51 control group) completed the 6‐month study period. Low and balanced numbers. Reasons for lost not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and LDL). |
| Selective reporting (reporting bias) | High risk | No registered or published protocol. Other clinical outcomes that were obtained during the course of the study were FBS, serum low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), serum triglycerides, serum total cholesterol, weight, height, and blood pressure. No data reported for blood pressure. |
| Risk of contamination (other bias) | High risk | Trial done in one clinic. Physicians were taking care of both control and intervention patients. They may have changed their care to control patients following pharmacist's recommendations for care of patients in the intervention group. |
| Other bias | Low risk | None. |
Wisse 2010.
| Study characteristics | ||
| Methods |
Prescription of physical activity is not sufficient to change sedentary behavior and improve glycemic control in type 2 diabetes patients Patient RCT, conducted with patients recruited from an outpatient diabetes clinic (Slotervaart Hospital in Amsterdam), The Netherlands Two arms: 1. Control group (control arm) and 2. Intervention group (intervention arm) |
|
| Participants | Control arm N: 36 Intervention arm N: 38 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 24 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Promotion of self‐management |
|
| Outcomes | 1) Aspirin (aspirin/anticoagulants), N users (%) Control arm: pre 5 (17), post 9 (31) Intervention arm: pre 8 (25), post 14 (44) 2) Statins, N users (%) Control arm: pre 20 (69), post 24 (83) Intervention arm: pre 25 (78), post 28 (88) 3) Antihypertensives (any), N users (%) Control arm: pre 26 (90), post 22 (76) Intervention arm: pre 25 (78), post 21 (66) 4) HbA1c, mean % (SE) Control arm: pre 7.6 (0.2), post 7.9 (0.3) Intervention arm: pre 7.6 (0.2), post 7.6 (0.2) 5) SBP, mean mmHg (SE) Control arm: pre 137.0 (4.0), post 137.0 (3.0) Intervention arm: pre 130.0 (3.0), post 132.0 (3.0) 6) DBP, mean mmHg (SE) Control arm: pre 80.0 (2.0), post 82.0 (2.0) Intervention arm: pre 77.0 (1.0), post 76.0 (2.0) 7) LDL, mean mg/dL (SE) Control arm: pre 100.5 (3.9), post 92.8 (7.7) Intervention arm: pre 104.4 (3.9), post 96.7 (9.7) |
|
| Funding source | Research grant: Novo Nordisk Farma B.V., Flemingweg 18, 2408 AV Alphen aan de Rijn, The Netherlands (www.novonordisk.nl) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not describe, just states that they were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Does not describe, just states that they were randomised. |
| Patient's baseline characteristics (selection bias) | High risk | Bodyweight (P = 0.010); body mass index (P = 0.029); waist circumference (P = 0.003). |
| Patient's baseline outcomes (selection bias) | Low risk | SBP (P = 0.165); DBP (P = 0.260); HbA1c (P = 0.999); LDL (P = 0.707). |
| Incomplete outcome data (attrition bias) | High risk | They state that 128 met criteria but only ended up randomising 74, why? Also only 61 completed the trial, but the numbers they provided for loss to follow‐up do not match. There is 1 missing participant from the control group, which they have not accounted for. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary outcomes, objective laboratory methods not described. Physicians and investigators were blinded to participant randomisation. Except for physiotherapist who was administering the intervention. However, there was no mention of who collected the data and whether they were blinded. |
| Selective reporting (reporting bias) | Low risk | Checked protocol and everything proposed was completed. |
| Risk of contamination (other bias) | High risk | Individuals in control group could have sought out an exercise programme. |
| Other bias | Low risk | Information not available. |
Wojcicki 2001.
| Study characteristics | ||
| Methods |
What we can really expect from telemedicine in intensive diabetes treatment: results from 3‐year study on type 1 pregnant diabetic women RCT (NA clusters and NA providers), conducted in 1) 15 pregnant diabetic women treated in the Clinic of Gastroenterology and Metabolic Diseases of the Medical Academy in Warsaw, Poland. 2) All the patients were treated by the same diabetologist in Poland 2 arms: 1. Control (clinical exam only) (control arm) and 2. Intervention (Telematic system) (intervention arm) |
|
| Participants | Control arm N: 15 Intervention arm N: 15, NA, NA Diabetes type: 1 Mean age: 26.05 ± 12.6 % Male: 0 Longest follow‐up: 36 months |
|
| Interventions |
Control arm: (clinical exam only) 1) Patient education Intervention arm: (Telematic system) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This study was supported by grants from the Polish State Committee for Scientific Research, the Bayer Diagnostic Division Warsaw, and the Polish Cellular Telephony Centertel | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1. P values > 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | See Table 1. P values > 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients with comorbidities were not included in intervention group. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, harms. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | High risk | All the patients were treated by the same diabetologist. |
| Other bias | Unclear risk | Control participants were evaluated frequently. So much so that it must have affected PSM. Small sample size. |
Wolf 2013.
| Study characteristics | ||
| Methods |
Clinic‐based versus outsourced implementation of a diabetes health literacy intervention Cluster‐RCT (6 clusters), conducted in 6 community health centres in three cities (representing urban, suburban and rural), USA Two arms: 1. Carve‐out (control arm) and 2. Carve‐in (intervention arm) |
|
| Participants | Control arm N: 272 Intervention arm N: 214 Diabetes type: type 2 Mean age: 54.8 ± 11.0 % Male: 39.1 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management Intervention arm: 1) Case management 2) Electronic patient registry 3) Clinician education 4) Patient education 5) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.7 (1.8), post 7.4 (1.5) Intervention arm: pre 8.3 (1.7), post 8.2 (1.6) 2) SBP, mean mmHg (SD) Control arm: pre 133.1 (15.4), post 132.6 (18.0) Intervention arm: pre 136.7 (16.6), post 136.6 (19.7) 3) DBP, mean mmHg (SD) Control arm: pre 78.8 (9.8), post 78.7 (10.6) Intervention arm: pre 79.5 (8.1), post 78.9 (9.1) 4) LDL, mean mg/dL (SD) Control arm: pre 98.2 (40.5), post 87.0 (31.4) Intervention arm: pre 95.2 (35.1), post 95.3 (37.5) |
|
| Funding source | Funding for this project was supported by Missouri Foundation for Health. Dr. Schillinger was supported by the National Institute of Diabetes and Digestive and Kidney Diseases for Diabetes Translational Research (CDTR) at Kaiser Permanente and University of California, San Francisco (P30 DK092924). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Cluster. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Race (P < 0.001); recent diagnosis of diabetes (P = 0.04); diabetes knowledge (P = 0.02). |
| Patient's baseline outcomes (selection bias) | High risk | HbA1c (P < 0.001); SBP (P = 0.02). |
| Incomplete outcome data (attrition bias) | Unclear risk | Due to routine health data collection, some patients could not have data and were excluded from analysis. Numbers excluded not reported; intention‐to‐treat analysis was not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Primary: fidelity of interventions. Secondary: HbA1c, SBP, DBP, LDL: using existing medical charts, objective methods not described. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | Low risk | Cluster. |
| Other bias | Low risk | None. |
Wu 2018.
| Study characteristics | ||
| Methods |
Costs and effectiveness of pharmacist‐led group medical visits for type‐2 diabetes: A multi‐center randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) 3 Veterans Health Administration Hospitals, West Haven, Connecticut/Providence, Rhode Island/Honolulu, Hawaii, United States of America. 2) Clinical pharmacists, nutritionist, nurse or a physical therapist. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (pharmacist‐led group medical visits) (intervention arm) |
|
| Participants | Control arm N: 133 Intervention arm N: 117, NA, NA Diabetes type: 2 Mean age: 65.37 ± 12.65 % Male: 95.97 Longest follow‐up: 13 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (pharmacist‐led group medical visits) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | This work is supported by the US Department of Veterans Affairs Health Services Research & Development Merit Review Award IAB – 06‐269. Support includes study execution funds and staff salary support. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using urn stratified randomisation generated by an automated computer sequence. |
| Allocation concealment (selection bias) | Low risk | On‐site computer system. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1 of baseline characteristics showed both group visit versus standard care arms to be comparable in demographic characteristics such as mean age (65.8 versus 65 years), gender (95.7 versus 96% male) and race (10 versus 11% African Americans). |
| Patient's baseline outcomes (selection bias) | Unclear risk | Table 1 ‐ groups differed by previous stroke (10.3 versus 3.8%), average hospitalisations 13 months prior (0.4 versus 0.1) and total cholesterol levels (155 versus 165 mg/dL), for group visit and standard care arms, respectively. |
| Incomplete outcome data (attrition bias) | High risk | Figure 1. 20/117 dropped out in intervention group, 16/133 dropped out in control group. Partial reasoning provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c, SBP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol. Outcomes listed match outcomes reported. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised. 3 sites. Unlikely that control group had access to intervention. |
| Other bias | Low risk | None identified. |
Yang 2013.
| Study characteristics | ||
| Methods |
Primary prevention of macroangiopathy in patients with short‐duration of type 2 diabetes by intensified multifactorial intervention Patient RCT, conducted with patients recruited from the First Affiliated Hospital of Dalian Medical University, China Two arms: 1. Conventional group (control arm) and 2. Intensive group (intervention arm) |
|
| Participants | Control arm N: 75 Intervention arm N: 75 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 84 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Patient education |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 8.7 (1.7), post 8.0 (2.0) Intervention arm: pre 8.9 (1.7), post 7.1 (1.2) 2) SBP, mean mmHg (SD) Control arm: pre 128.8 (11.3), post 127.8 (12.1) Intervention arm: pre 129.1 (15.2), post 120.7 (9.6) 3) DBP, mean mmHg (SD) Control arm: pre 76.9 (6.4), post 79.4 (8.5) Intervention arm: pre 79.8 (11.8), post 76.2 (4.4) 4) LDL, mean mg/dL (SD) Control arm: pre 116.0 (20.1), post 117.2 (7.4) Intervention arm: pre 115.6 (23.6), post 106.3 (16.6) |
|
| Funding source | This research was supported by funds from the National Key Research Project for the Tenth Five‐Year Plan (2001BA702B01), the National Key Research Project for the Eleventh Five‐Year Plan (2006BAI02B08), and the Key Research Project of Liaoning Province Bureau of Science and Technology (2002225003‐6) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported in text or table. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | ~9% lost in N1 and ~6% in N2, however reasons for losses not provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Primary and secondary were not any of our outcomes of interest. HbA1c was measured using high performance liquid chromatography. SBP, DBP, LDL: objective measures not reported. |
| Selective reporting (reporting bias) | High risk | Protocol not retrievable. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Yaron 2019.
| Study characteristics | ||
| Methods |
A randomized controlled trial comparing a telemedicine therapeutic intervention with routine care in adults with type 1 diabetes mellitus treated by insulin pumps RCT (NA clusters and NA providers), conducted in 1) Referral centre for T1D in the Maccabi Heath Care Services, Raanana, Israel; 2) Physicians and study co‐ordinators in Israel 2 arms: 1. Control (conventional care) (control arm) and 2. Intervention (telemedicine) (intervention arm) |
|
| Participants | Control arm N: 37 Intervention arm N: 37, NA, NA Diabetes type: 1 Mean age: 44.07 ± 9.48 % Male: 47.76 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (conventional care) 1) Promotion of self‐management Intervention arm: (telemedicine) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | The study was partially supported by Maccabi Health Services Israel | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | The 2 groups did not differ in any of the sociodemographic or clinical characteristics examined. No P values reported. |
| Patient's baseline outcomes (selection bias) | Low risk | Although P values are only reported at baseline for HbA1c (P = 0.07), the rest of the values in Table 1 appear similar. However, a very limited number of outcome measures are reported at baseline. |
| Incomplete outcome data (attrition bias) | High risk | 6 lost in intervention group (16%) and 1 lost in control group (3%), reasons provided in supplementary figure S2. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, hypo/hyperglycaemia recorded when transmitted data met required parameters (< 70 mg/dL or > 300 mg/dL). |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol. Outcomes in protocol match those in published report. |
| Risk of contamination (other bias) | Low risk | Only intervention group had access to CareLink system. |
| Other bias | Low risk | No evidence of other bias. |
Yin 2017.
| Study characteristics | ||
| Methods |
Regular mailing of personalized feedback reports improves glycemic control in diabetes: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Diabetes centre at a university‐affiliated hospital of Hong Kong, China. 2) PhD student arranged reports. In China. 2 arms: 1. Control (R‐: usual comprehensive assessment (CA) service) (control arm) and 2. Intervention (R+: usual comprehensive assessment (CA) service + feedback reports to patients) (intervention arm) |
|
| Participants | Control arm N: 600 Intervention arm N: 600, NA, NA Diabetes type: 3 Mean age: 58.3 ± 11.5 % Male: 55.1 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (R‐: usual comprehensive assessment (CA) service) Intervention arm: (R+: usual comprehensive assessment (CA) service + feedback reports to patients) 1) Case management 2) Electronic patient registry 3) Promotion of self‐management 4) Patient reminders |
|
| Outcomes | Glycated haemoglobin Low‐density lipoprotein |
|
| Funding source | Nothing reported about the current study. The JADE portal was developed and supported by the Asia Diabetes Foundation. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. We consecutively recruited 1200 patients with diabetes who underwent CA and randomised 600 to receive two JADE follow‐up reports by mail (R+) and 600 to go without these reports (R‐). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided and above 0.05. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. Triglycerides have significant P values. |
| Incomplete outcome data (attrition bias) | Unclear risk | Randomised 1200 patients, 600 in each group. Follow‐up of 1 year. Nothing reported about loss to follow‐up. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Our outcomes of interest were objectively measured (HbA1c and LDL). |
| Selective reporting (reporting bias) | High risk | They report a registered protocol but it does not match with the paper. The feedback reports to patients contain information about HbA1c, BP, LDL and body weight, but they do not report post‐data for BP and weight/BMI. |
| Risk of contamination (other bias) | Low risk | Patient‐randomised but unlikely that control patients received mailed periodic follow‐up reports between their CA visits. |
| Other bias | Unclear risk | Research letter, short report. Given a relatively low baseline HbA1c of 7.4%, the effects of providing additional reports was modest, albeit significant. |
Yoo 2009.
| Study characteristics | ||
| Methods |
A ubiquitous chronic disease care system using cellular phones and the internet Patient RCT, conducted in 1 university hospital and 1 public health centre, South Korea Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 61 Intervention arm N: 62 Diabetes type: type 2 Mean age: 58.2 ± 8.8 % Male: 58.5 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.4 (0.9), post 7.6 (1.0) Intervention arm: pre 7.6 (0.9), post 7.1 (0.8) 2) SBP, mean mmHg (SD) Control arm: pre 137.8 (17.8), post 134.0 (13.6) Intervention arm: pre 140.2 (18.8), post 132.7 (16.2) 3) DBP, mean mmHg (SD) Control arm: pre 83.3 (10.0), post 82.2 (7.7) Intervention arm: pre 84.4 (10.0), post 80.3 (9.2) 4) LDL, mean mg/dL (SD) Control arm: pre 92.8 (27.1), post 88.9 (27.1) Intervention arm: pre 100.5 (27.1), post 85.1 (23.2) |
|
| Funding source | This study was funded by a grant from the Seoul R & BD Project. The development of the HSA business model and technology was sponsored by the Ministry of Commerce, Industry and Energy. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. P values provided and greater than 0.05. No education information. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided and greater than 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 5 patients (8.1%) dropped out of the intervention group and 7 (10%) out of the control group. The characteristics of patients who did and did not drop out were similar in both the intervention and control groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure of HbA1c, LDL, BP. |
| Selective reporting (reporting bias) | Unclear risk | No reported protocol. Methods match outcomes reported. |
| Risk of contamination (other bias) | Low risk | Remotely delivered intervention. Control participants did not have access to testing device. |
| Other bias | Low risk | No evidence of other bias. |
Yoon 2008.
| Study characteristics | ||
| Methods |
A short message service by cellular phone in type 2 diabetic patients for 12 months Patient RCT, conducted in an endocrinology outpatient department of a tertiary care hospital in urban city of South Korea Two arms: 1. Control (control arm) and 2. Intervention (intervention arm) |
|
| Participants | Control arm N: 26 Intervention arm N: 24 Diabetes type: type 2 Mean age: 47.2 ± 9.0 % Male: 43.2 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: None Intervention arm: 1) Case management 2) Team changes 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 7.6 (1.1), post 8.4 (1.0) Intervention arm: pre 8.1 (1.7), post 6.8 (0.8) |
|
| Funding source | This work was supported by grants from the Seoul R&BD Program (2006‐10829) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Patient's baseline characteristics (selection bias) | Low risk | Information not available. |
| Patient's baseline outcomes (selection bias) | Low risk | Information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Information not available. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Information not available. |
| Selective reporting (reporting bias) | Low risk | Information not available. |
| Risk of contamination (other bias) | Low risk | Information not available. |
| Other bias | Low risk | Information not available. |
Yu 2019.
| Study characteristics | ||
| Methods |
Effects of mobile phone application combined with or without self‐monitoring of blood glucose on glycemic control in patients with diabetes: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Outpatient Department of Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China 2) Certified clinicians. In China. 4 arms: 1. Control (no mobile app (MPA) or self‐monitored blood glucose (SMBG) (control arm) and 2. Intervention (SMBG only) (intervention arm), 3. Intervention (MPA only) (other arm), 4. Intervention (MPA and SMBG) (other arm) |
|
| Participants | Control arm N: 47 Intervention arm N: 45, 48, 45 Diabetes type: 2 Mean age: 52.53 ± 10.49 % Male: 62.16 Longest follow‐up: 5.52 months |
|
| Interventions |
Control arm: arm (no mobile app (MPA) or self‐monitored blood glucose (SMBG)) Intervention arm: (SMBG only) 1) Promotion of self‐management Intervention arm: (MPA only) 1) Case management 2) Electronic patient registry 3) Clinician reminder 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
|
| Outcomes | Glycated haemoglobin Harms |
|
| Funding source | This study was funded by Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No. PWZz2017‐12) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was created by Stata software version 12.0 (StataCorp, College Station, TX, USA). |
| Allocation concealment (selection bias) | Low risk | One staff not associated with the clinical work of the study generated the randomisation sequence and prepared the sequentially numbered, opaque envelopes independently. |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1. P values < 0.05 for age. |
| Patient's baseline outcomes (selection bias) | Unclear risk | See Table 1. P values < 0.05 for DBP and total cholesterol. |
| Incomplete outcome data (attrition bias) | High risk | 6 lost in control (13%), 7 lost in SMBG only group (16%), 5 lost in MPA only group (10%) and 7 lost in MPA and SMBG group (16%). Reasons reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Unclear risk | Objectively measured outcomes: HbA1c; we think hypoglycaemic events (≤ 3.9 mmol/L) were self‐reported. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. All outcomes reported. |
| Risk of contamination (other bias) | Low risk | Groups may have been followed by same physicians; however, MPA and blood glucose monitors were only offered to the appropriate intervention groups. |
| Other bias | Low risk | No evidence of other bias. |
Yuan 2016.
| Study characteristics | ||
| Methods |
Effect of case management on glycemic control and behavioral outcomes for Chinese people with type 2 diabetes: a 2‐year study RCT (NA clusters and NA providers), conducted in 1) Study recruitment in the area surrounding one hospital in Eastern China. 2) Diabetes educator assessed the participant’s health care needs. If necessary, participants were referred short‐term to a doctor, a nutritionist or psychologist who provided feedback to the diabetes educator through a case report form. In China. 2 arms: 1. Control: usual care (control arm) and 2. Intervention: case management (intervention arm) |
|
| Participants | Control arm N: 60 Intervention arm N: 60, NA, NA Diabetes type: 2 Mean age: 58.69 ± 10.8 % Male: 47.22 Longest follow‐up: 24 months |
|
| Interventions |
Control arm: usual care 1) Patient education 2) Patient reminders Intervention arm: case management 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management 5) Continuous quality improvement |
|
| Outcomes | Glycated haemoglobin | |
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised to the case management (CM) group or the control group based on random numbers generated through Proc Plan (SAS9.2) and block randomisation procedures with a block size of 4. |
| Allocation concealment (selection bias) | Low risk | This process was accomplished by a statistician with no clinical involvement in the trial, and the process was concealed from the researchers. Group assignment for each participant was pulled from a sequentially numbered, opaque, sealed and stapled envelope. |
| Patient's baseline characteristics (selection bias) | Low risk | Table 1. P values provided greater than 0.05. No education or income. |
| Patient's baseline outcomes (selection bias) | Low risk | Table 1. P values provided and > 0.05. |
| Incomplete outcome data (attrition bias) | Low risk | 53/60 followed up in control, 55/60 followed up in intervention. Reasons for dropout provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective HbA1c measure. |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol but all the outcomes in the methods are reported in the results. |
| Risk of contamination (other bias) | Low risk | The nurse educators were different in the arms. |
| Other bias | Unclear risk | The baseline HbA1C in both groups was low. "Finally, just the mere action of asking questions could be interpreted as a mild intervention in itself, as it might have raised patients’ awareness of topics that were important. This could be a factor which might account for changes observed in the control group." |
Zapotoczky 2001.
| Study characteristics | ||
| Methods |
A controlled study of weight reduction in type 2 diabetes treated by two reinforcers RCT (NA clusters and NA providers), conducted in 1) Diabetic outpatient clinic of Internal Medicine, University Hospital Graz, Austria. 2) A clinical dietitian in Austria 2 arms: 1. Control: (standard care) (control arm) and 2. Intervention: (treatment) (intervention arm) |
|
| Participants | Control arm N: 18 Intervention arm N: 18, NA, NA Diabetes type: 2 Mean age: 57.5 ± 12.71 % Male: 36.11 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (standard care) 1) Patient education Intervention arm: (treatment) 1) Case management 2) Patient education 3) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Table 1. No P values reported and numbers look unbalanced. No information on education or income. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP, LDL. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | Unclear risk | They were followed with the same physicians. |
| Other bias | Low risk | No other source of bias. |
Zgibor 2018.
| Study characteristics | ||
| Methods |
Effectiveness of certified diabetes educators following pre‐approved protocols to redesign diabetes care delivery in primary care: Results of the REMEDIES 4D trial Clustered RCT (15 clusters and 59 providers), conducted in 1) Non‐academic primary care practices from the University of Pittsburgh Medical Center (UPMC), 2) Certified diabetes educators (CDE)‐ the 2 CDEs implementing the study were nurses with certification from the National Certification Board for Diabetes Educators. In United States of America. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Redesigning MEDication Intensification protocol) (intervention arm) |
|
| Participants | Control arm N: 65 Intervention arm N: 175, NA, NA Diabetes type: 2 Mean age: 60.9 ± 10.49 % Male: 46.66 Longest follow‐up: 12 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Redesigning MEDication Intensification protocol) 1) Case management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Hypertension control |
|
| Funding source | This study was funded by the American Diabetes Association grant number 1‐12‐SAN‐31 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From protocol ‐ "Practices were then randomly assigned, by flip of a coin, to either intervention or usual care." |
| Allocation concealment (selection bias) | Low risk | Clustered RCT: practices were randomly assigned to the REMEDIES 4D intervention or to usual care. |
| Provider's baseline characteristics (selection bias) | Unclear risk | Not reported in paper however in Protocol ‐ "Fifteen practices agreed to participate in the study. In total, 59 providers were recruited from 15 practices (57 physicians and 2 physician assistants). Three of the practices were in urban settings with the remainder in the suburbs. All but one practice and two or more providers (range 2–11, average 4 per practice). Two‐thirds of the practices had nursing staff, while all practices had medical assistants, clerical support and access to a case manager from a local health plan. Prior to the study, there were no CDEs working in the practice, although certified diabetes education programs were available at local hospitals." |
| Patient's baseline characteristics (selection bias) | Low risk | See Table 1, P values > 0.05. |
| Patient's baseline outcomes (selection bias) | High risk | See Table 1. P values < 0.05 for HbA1c, SBP and DBP. |
| Incomplete outcome data (attrition bias) | Low risk | 18 lost from intervention group (10%); 5 lost from control group (8%). Reasons provided. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objectively measured outcomes: HbA1c, BP, LDL, hypertension. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol, which states "Secondary outcomes include medication adherence, medication intensification, quality of life, and diabetes self‐management behaviors." Only medication intensification was reported in paper. |
| Risk of contamination (other bias) | Unclear risk | One CDE was responsible for 7 intervention practices. The second CDE held monthly support groups in the practices randomised to usual care. Due to the volume of participants in the intervention group, this CDE also implemented the study in one intervention practice. |
| Other bias | Low risk | No evidence of other bias. |
Zhou 2014.
| Study characteristics | ||
| Methods |
Web‐based telemedicine for management of type 2 diabetes through glucose uploads: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) First Affiliated Hospital of Jinan University, China. 2) Not reported ‐ professional staff. In China. 2 arms: 1. Control: usual care (control arm) and 2. Intervention: telemedicine (intervention arm) |
|
| Participants | Control arm N: 57 Intervention arm N: 57, NA, NA Diabetes type: 2 Mean age: NR ± 18.49 % Male: NR Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) 1) Patient education 2) Promotion of self‐management Intervention arm: (telemedicine) 1) Case management 2) Electronic patient registry 3) Patient education 4) Promotion of self‐management |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | This project was supported by Research Fund of the First Clinical Medical College of Jinan University; Medical Science Foundation of Guangdong Province, 20120345 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to time sequence patients enrolled, participants were randomly divided into 2 groups by using random numbering method. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Unclear risk | Not reported. |
| Patient's baseline outcomes (selection bias) | Unclear risk | Reported only for those who completed the study. |
| Incomplete outcome data (attrition bias) | Low risk | Originally 57 in each group ‐ control lost 2, intervention lost 4; < 10%, reasons balanced between groups. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for HbA1c, BP, LDL, harms. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | In control group, without specific intervention, patients freely went to outpatients as usual when researchers collected the clinical information, including blood glucose, HbA1c, blood pressure, etc. All the participants of these 2 groups. |
| Other bias | Low risk | None identified. |
Zhou 2016.
| Study characteristics | ||
| Methods |
A smart phone‐based diabetes management application ‐ improves blood glucose control in Chinese people with diabetes RCT (NA clusters and NA providers), conducted in 1) Department of Endocrinology at The First Affiliated Hospital, College of Medicine, Zhejiang University, China. 2) Welltang smartphone application ‐ virtual educator for diabetes and a virtual endocrinologist for clinicians. Communication between patients and clinicians comprised patients receiving advice from the study team. In China. 2 arms: 1. Control (usual care) (control arm) and 2. Intervention (Welltang application) (intervention arm) |
|
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 3 Mean age: 54.25 ± NR % Male: 57 Longest follow‐up: 3 months |
|
| Interventions |
Control arm: (usual care) Intervention arm: (Welltang application) 1) Case management 2) Clinician reminder 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
|
| Outcomes | Glycated haemoglobin Systolic blood pressure Diastolic blood pressure Low‐density lipoprotein Harms |
|
| Funding source | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a random number table, interested candidates were randomised into 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Patient's baseline characteristics (selection bias) | Low risk | Demographic characteristics, baseline HbA1c and blood pressure were similar for both study groups. No P values recorded. |
| Patient's baseline outcomes (selection bias) | Low risk | Demographic characteristics, baseline HbA1c and blood pressure were similar for both study groups. No P values recorded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Objective measure for all outcomes. The outcomes (HbA1C, LDL, SBP, DBP, hypoglycaemia) were measured objectively. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered protocol. Methods match outcomes. |
| Risk of contamination (other bias) | Low risk | Unlikely that control group received WellTang intervention. Physicians worked with both groups. |
| Other bias | Low risk | None. |
Zolfaghari 2012.
| Study characteristics | ||
| Methods |
The impact of nurse short message services and telephone follow‐ups on diabetic adherence: which one is more effective? Patient RCT, conducted by Iranian Diabetes Association (only those who referred to this association in an outpatient department in the city of Tehran), Iran Two arms: 1. Telephone group (control arm) and 2. Short messaging services ‐ SMS (intervention arm) |
|
| Participants | Control arm N: 41 Intervention arm N: 39 Diabetes type: type 2 Mean age: NR ± NR % Male: NR Longest follow‐up: 6 months |
|
| Interventions |
Control arm: 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: 1) Patient education 2) Promotion of self‐management |
|
| Outcomes | 1) HbA1c, mean % (SD) Control arm: pre 9.4 (1.7), post 8.5 (1.9) Intervention arm: pre 9.0 (1.6), post 8.0 (1.8) |
|
| Funding source | This research was supported by a grant from the Faculty of Nursing and Midwifery of Tehran University of Medical Sciences, Islamic Republic of Iran (project number: 7091‐28‐ 02‐87) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Unsure of block size; if its too small, then one can predict the next assignment. |
| Patient's baseline characteristics (selection bias) | Low risk | Quote: "There were no significant difference in age, gender, BMI, duration of diabetes, insurance support, occupation, financial income and haemoglobin glycosylated levels between the two groups" |
| Patient's baseline outcomes (selection bias) | Low risk | HbA1c (P = 0.227). |
| Incomplete outcome data (attrition bias) | Low risk | There were some losses, but not enough to warrant any attention, also reasons for the 2 groups lost to follow‐up do not really stick out to affect the outcome. |
| Blinding of participants and personnel (performance bias) and of outcome assessors (detection bias) | Low risk | Blinding not described. HbA1c measured using high performance liquid chromatography. |
| Selective reporting (reporting bias) | Low risk | < 2005 approach used since no protocol; methods match results. |
| Risk of contamination (other bias) | High risk | Quote: "…researchers had no control of participants' access to other educational sources." |
| Other bias | Low risk | Information not available. |
ACDC: advanced comprehensive diabetes care; ACE: angiotensin‐converting‐enzyme; ACS: acute coronary syndrome; BMI: body mass index; BP: blood pressure; CBT: cognitive behavioural therapy; CG: control group; CGMS: continuous glucose monitoring system; CHW: community health worker; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CVD: cerebrovascular disease; DBP: diastolic blood pressure; DEP: diabetes education programme; DM: diabetes mellitus; DMO: digital medicine offering; DR: diabetic retinopathy; ED: emergency department; eGFR: estimated glomerular filtration rate; EOS: end‐of‐study; ER: emergency room; FBG: fasting blood glucose; GP: general practitioner; HbA1c: glycated haemoglobin; HDL: high‐density lipoprotein; HTN: hypertension; HTN‐C: hypertension control; HRQOL: health‐related quality of life; IDDM: insulin‐dependent diabetes mellitus; IG: intervention group; ITG: immediate treatment group; ITT: intention‐to‐treat; LDL: low‐density lipoprotein; NA: not applicable; NR: not reported; NS: non‐significant; OAD: oral antihyperglycemic drug; QI: quality improvement; QOL: quality of life; RCT: randomised controlled trial; RN: registered nurse; SBP: systolic blood pressure; SC: Simpler care; SD: standard deviation; SE: standard error; SMBG: self‐monitoring blood glucose; T2DM: type 2 diabetes mellitus; TIA: transient ischaemic attack; TM: telemonitoring; UC: usual care; WLC: wait‐list control
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abkenar 2016 | Patient education only |
| Agewall 2001 | Clinical trial of multi‐risk factor intervention |
| Aguila 2018 | Interventions targeted at patients only |
| Alfadda 2011 | Fewer than 3 clusters per arm |
| Basch 1999 | Intervention targeted towards patients |
| Bhattacharyya 2010 | Fewer than 3 clusters |
| Butt 2016 | Patient education only |
| Campbell 1996 | Intervention targeted towards patients |
| Chen 2008 | Patient education only |
| Chen 2015 | Promotion of self‐management only |
| Chow 2016 | Patient education only |
| Cortez 2017 | Promotion of self‐management |
| Cortés‐Sanabria 2008 | Fewer than 3 clusters per arm |
| Fisher 2011 | Intervention does not meet inclusion criteria |
| Gaillard 2015 | Patient education only |
| Hajbaghery 2012 | Interventions targeted towards patients only |
| Kim 2006 | Participants were not randomly assigned |
| Kushner 2009 | No outcomes of interest |
| Litzelman 1993 | Fewer than 3 clusters per arm |
| Maislos 2004 | Fewer than 3 clusters per arm |
| Mazzuca 1988 | Did not keep randomisation design |
| Mehler 2005 | Focus on improving LDL testing, and report LDL levels only among those tested ‐ varying samples baseline and follow‐up among 3 arms |
| Polonsky 2011 | Intervention does not meet inclusion criteria |
| Saengtipbovorn 2014 | Fewer than 3 clusters |
| Segal 2016 | Secondary analysis |
| Teychenne 2015 | Intervention targeted towards patients |
| Weitzman 2009 | Fewer than 3 clusters per arm |
LDL: low‐density lipoprotein
Characteristics of studies awaiting classification [ordered by study ID]
Al‐Taie 2020.
| Methods |
Impact of clinical pharmacy recommendations and patient counselling program among patients with diabetes and cancer in outpatient oncology setting RCT (NA clusters and NA providers), conducted in 1) Conducted at the outpatient oncology centre of Dr. Lütfi Kırdar Kartal Teaching and Research Hospital located at the Anatolian part of Istanbul, Turkey. 2) Pharmacist 2 arms: 1. Control arm (normal care) and 2. Intervention arm (clinical pharmacy services) |
| Participants | Control arm N: 53 Intervention arm N: 56, NA, NA Diabetes type: 1 and 2 Mean age: 61.82 ± 8.62 % Male: 36 Longest follow‐up: 3 months |
| Interventions |
Control arm: (normal care) Intervention arm: (clinical pharmacy services) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Ali Abdelhamid 2021.
| Methods |
Survivors of intensive care with type 2 diabetes and the effect of shared‐care follow‐up clinics: the SWEET‐AS randomized controlled pilot study RCT (NA clusters and NA providers), conducted in 1) Shared‐care clinic (a mixed medical‐surgical‐trauma quaternary‐referral ICU in Australia), Australia, 2) Research assistant, study doctor, intensivist, endocrinologist 2 arms: 1. Control arm (usual care) and 2. Intervention arm (shared‐care intensivist‐endocrinologist clinic) |
| Participants | Control arm N: 21 Intervention arm N: 21, NA, NA Diabetes type: 2 Mean age: 66 ± 9.71 % Male: 69 Longest follow‐up: 6 months |
| Interventions |
Control arm: (usual care) Intervention arm: (shared‐care intensivist‐endocrinologist clinic) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient reminders |
| Outcomes | 1) Retinopathy screening 2) Foot screening |
| Notes | Extracted |
Alison 2020.
| Methods |
The effectiveness of diabetes medication therapy adherence clinic to improve glycaemic control among patients with type 2 diabetes mellitus: a randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Kota Samarahan Health clinic (treats patients from rural and urban areas), Malaysia, 2) family medicine specialists, medical officers, pharmacists, nutritionists, visiting dieticians, physiotherapists, occupational therapists and diabetic educator nurses, endocrine specialist 2 arms: 1. Control arm (normal clinic visits) and 2. Intervention arm (Diabetes Medication Therapy Adherence Clinic (DMTAC)) |
| Participants | Control arm N: 50 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 52.52 ± 10.37 % Male: 38 Longest follow‐up: 12 months |
| Interventions |
Control arm: (normal clinic visits) 1) Patient reminders Intervention arm: (Diabetes Medication Therapy Adherence Clinic (DMTAC)) 1) Case management 2) Team change 3) Patient education 4) Patient reminders |
| Outcomes | 1) Glycated haemoglobin |
| Notes | — |
Bohingamu Mudiyanselage 2019.
| Methods |
Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Barwon Health, University Hospital Geelong, Geelong, Australia Remotely delivered intervention. 2) Treating medical consultant, registered nurses, GP. In Australia. 2 arms: 1. Control arm (usual care) and 2. Intervention arm (remote patient monitoring) |
| Participants | Control arm N: 69 Intervention arm N: 67, NA, NA Diabetes type: not reported Mean age: 70.42 ± 12.4 % Male: 53.54 Longest follow‐up: 12 months |
| Interventions |
Control arm: (usual care) Intervention arm: (remote patient monitoring) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Promotion of self‐management 5) Continuous quality improvement |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Buysse 2020.
| Methods |
Sustainable improvement of HbA1c and satisfaction with diabetes care after adding telemedicine in patients on adaptable insulin regimens: results of the TeleDiabetes randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) 2 hospitals (Ghent University Hospital and AZ Nikolaas) in Flanders, Belgium, 2) Tele‐education via diabetes educator with supervision of the endocrinologist 2 arms: 1. Control arm (standard care) and 2. Intervention arm (standard care + tele‐education/telemonitoring) |
| Participants | Control arm N: 72 Intervention arm N: 81, NA, NA Diabetes type: 1 and 2 Mean age: 37.47 ± 13.98 % Male: 50.18 Longest follow‐up: 3 months |
| Interventions |
Control arm: (standard care) Intervention arm: (standard care+tele‐education/telemonitoring) 1) Case management 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Chen 2019.
| Methods |
The effect of interactive health education based on the WeChat platform on diabetic outpatients RCT (NA clusters and NA providers), conducted in 1) Remotely delivered intervention, Linyi Central Hospital, Shanghai, China. 2) Intervention delivered by deputy director, head nurse, 1 physician, 3 nurses, information engineer. In China. 2 arms: 1. Control arm (routine nursing) and 2. Intervention arm (interactive health education) |
| Participants | Control arm N: 45 Intervention arm N: 45, NA, NA Diabetes type: 2 Mean age: 45.75 ± 3.15 % Male: 56.7 Longest follow‐up: 6 months |
| Interventions |
Control arm: (routine nursing) Intervention arm: (interactive health education) 1) Case management 2) Team change 3) Electronic patient registry 4) Patient education |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Daud 2020.
| Methods | Clustered RCT (10 Clusters and NR providers), conducted in 1) Primary care clinics led by Family Medicine Specialists (FMS) in Selangor and Kuala Lumpur, Malaysia, 2) Chronic Disease Management (CDM) Team, family medicine specialist (FMS). In Malaysia. 2 arms: 1. Control arm (usual care) and 2. Intervention arm (EMPOWER‐ participatory action research (PAR)) |
| Participants | Control arm N: 417 Intervention arm N: 471, NA, NA Diabetes type: 2 Mean age: 57.42 ± 10.35 % Male: 37.03 Longest follow‐up: 12 months |
| Interventions |
Control arm: (usual care) Intervention arm: (EMPOWER ‐ participatory action research (PAR)) 1) Audit and feedback 2) Clinician education 3) Promotion of self‐management |
| Outcomes | 1) Retinopathy screening 2) Foot screening 3) Renal screening |
| Notes | Extracted |
Egede 2021a.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | — |
Egede 2021b.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | Co‐publication of Egede 2021, new outcome (HbA1c) |
Franc 2019.
| Methods |
Efficacy of two telemonitoring systems to improve glycaemic control during basal insulin initiation in patients with type 2 diabetes: The TeleDiab‐2 randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) French hospitals and primary care, 2) Intervention delivered by specialist physicians in France 3 arms: 1. Control arm (standard care), 2. Intervention arm (IVRS‐Interactive Voice Response System) and 3. Intervention arm (Diabeo‐BI App software) |
| Participants | Control arm N: 63 Intervention arm N: 64, NA, NA Diabetes type: 2 Mean age: 58.7 ± 9.6 % Male: 64.6 Longest follow‐up: 13 months |
| Interventions |
Control arm: (standard care) Intervention arm: (IVRS ‐ Interactive Voice Response System) 1) Electronic patient registry 2) Promotion of self‐management Intervention arm: (Diabeo‐BI App software) 1) Electronic patient registry 2) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin 2) Harms (hypoglycaemic episodes) |
| Notes | Extracted |
Franc 2020.
| Methods |
DIABEO system combining a mobile app software with and without telemonitoring versus standard care: a randomized controlled trial in diabetes patients poorly controlled with a basal‐bolus insulin regimen RCT (NA clusters and NA providers), conducted in 1) Multicentre (95 public and private sites), conducted in real‐life (pragmatic) conditions in France, 2) Physician, nurse 3 arms: 1. Control arm (standard care) and 2. Intervention arm (DIABEO only) and 3. Intervention arm (DIABEO + telemonitoring) |
| Participants | Control arm N: 221 Intervention arm N: 231, 213, NA Diabetes type: 1 and 2 Mean age: 38.5 ± 13.8 % Male: 48.6 Longest follow‐up: 12 months |
| Interventions |
Control arm: (standard care) Intervention arm: (DIABEO only) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Promotion of self‐management Intervention arm: (DIABEO + telemonitoring) 1) Case management 2) Electronic patient registry 3) Clinician reminders 4) Facilitated relay of clinical information 5) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Gimbel 2020.
| Methods |
Enhancing patient activation and self‐management activities in patients with type 2 diabetes using the US department of defense mobile health care environment: feasibility study RCT (NA clusters and NA providers), conducted in 1) Patient‐centred medical home (PCMH). Madigan Army Medical Center, Tacoma, Washington, USA. Mike O’Callaghan Federal Medical Center, Las Vegas, Nevada, USA. 2) Clinicians 2 arms: 1. Control arm (mHealth technology) and 2. Intervention arm (mHealth technology and behavioural messages tailored to Patient Activation Measure (PAM)) |
| Participants | Control arm N: 117 Intervention arm N: 123, NA, NA Diabetes type: 2 Mean age: 62.9 ± 10.3 % Male: 61.6 Longest follow‐up: 12 months |
| Interventions |
Control arm: (usual care) 1) Promotion of self‐management Intervention arm: (pharmacist management and SMBG) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders 7) Continuous quality improvement |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
| Notes | Extracted |
Guo 2019.
| Methods |
Feasibility and efficacy of nurse‐led team management intervention for improving the self‐management of type 2 diabetes patients in a Chinese community: a randomized controlled trial RCT (NA clusters and NA providers), conducted in1) Wangyuehu community health centre in the city of Changsha, Hunan Province, China, 2) The team was composed of community nurses, community doctors, a clinical nursing specialist, 3 diabetes specialists, a nutritionist and nursing post‐graduates. In China. 2 arms: 1. Control arm (routine management) and 2. Intervention arm (nurse‐led team management) |
| Participants | Control arm N: 85 Intervention arm N: 86, NA, NA Diabetes type: 2 Mean age: 63.73 ± 7.57 % Male: 42.1 Longest follow‐up: 12 months |
| Interventions |
Control arm: (routine management) 1) Patient education Intervention arm: (nurse‐led team management) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Heselmans 2020.
| Methods |
Computerized clinical decision support system for diabetes in primary care does not improve quality of care: a cluster‐randomized controlled trial Cluster RCT (51 clusters and 120 providers), conducted in 1) General practice, Belgium, 2) Computerised clinical decision support (CCDS) system, no practitioner delivering 2 arms: 1. Control arm (usual care) and 2. Intervention arm (EBMeDS system) |
| Participants | Control arm N: 2464 Intervention arm N: 1351, NA, NA Diabetes type: 1 and 2 Mean age: 65.53 ± 14.24 % Male: 49.96 Longest follow‐up: 12 months |
| Interventions |
Control arm: (usual care) Intervention arm: (EBMeDS system) 1) Electronic patient registry 2) Clinician reminders |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
| Notes | Extracted |
Hu 2021.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | — |
Javaid 2019.
| Methods | RCT (NA clusters and NA providers), conducted in 1) A primary care facility, Murad Clinic Shalamar link road, Lahore, Pakistan. 2) The clinical setup consisted of 3 physicians, 1 qualified dispenser, 1 co‐ordinator, 1 patient facilitator, 1 lab technician and 1 pathologist. All patients first approached patients’ facilitator and later transferred to co‐ordinator for consulting physician, which after consultation contacted the co‐ordinator again to get medicine from dispenser and later to a pharmacist. The last part is only applicable for the patients of intervention arm for education and counselling. In Pakistan. 2 arms: 1. Control arm (routine care) and 2. Intervention arm (pharmaceutical care plan) |
| Participants | Control arm N: 150 Intervention arm N: 150, NA, NA Diabetes type: 2 Mean age: 50.4 ± 9.2 % Male: 33.6 Longest follow‐up: 9 months |
| Interventions |
Control arm: (routine care) 1) Patient reminders Intervention arm: (pharmaceutical care plan) 1) Case management 2) Team change 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein 5) Hypertension control |
| Notes | Extracted |
Kang 2021.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | — |
Khan 2021.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | — |
Kim 2022.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | — |
Ku 2020.
| Methods |
Clinical efficacy and plausibility of a smartphone‐based integrated online real‐time diabetes care system via glucose and diet data management: a pilot study RCT (NA clusters and NA providers), conducted in 1) Diabetes education centre at Chungbuk National University Hospital, Cheonju, Republic of Korea. 2) Nurse in South Korea 2 arms: 1. Control arm (conventional care) and 2. Intervention arm (smartphone‐based Noom Coach care) |
| Participants | Control arm N: 20 Intervention arm N: 20, NA, NA Diabetes type: 2 Mean age: 50 ± 9.59 % Male: 35 Longest follow‐up: 2.76 months |
| Interventions |
Control arm: (conventional care) 1) Patient education 2) Promotion of self‐management Intervention arm: (smartphone‐based Noom Coach care) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Lee 2020.
| Methods |
Telemonitoring and team‐based management of glycemic control on people with type 2 diabetes: a cluster‐randomized controlled trial Cluster RCT (11 clusters and NR providers), conducted in 1) Primary healthcare centres in the Klang Valley, Malaysia, 2) The intervention was delivered by the research team and doctors at the usual clinics. In Malaysia. 2 arms: 1. Control arm (usual care) and 2. Intervention arm (telemonitoring) |
| Participants | Control arm N: 120 Intervention arm N: 120, NA, NA Diabetes type: 2 Mean age: 56.2 ± 8.89 % Male: 45 Longest follow‐up: 12 months |
| Interventions |
Control arm: (usual care) Intervention arm: (telemonitoring) 1) Electronic patient registry 2) Clinician reminders 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
| Notes | Extracted |
Lee 2020A.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | Not yet extracted |
Lou 2019.
| Methods |
Effectiveness of a clinic‐based randomized controlled intervention for type 2 diabetes management: an innovative model of intensified diabetes management in mainland China (C‐IDM study) RCT (NA clusters and NA providers), conducted in 1) The study was conducted in Dachang district (one of the 11 districts of Nanjing), an urban district, China in local clinics known as community health service centres. 2) GPs, nurses, endocrinologist 2 arms: 1. Control arm (BPHSP Service) and 2. Intervention arm (C‐IDM) |
| Participants | Control arm N: 558 Intervention arm N: 585, NA, NA Diabetes type: 2 Mean age: 66.49 ± 8.69 % Male: 47.28 Longest follow‐up: 24 months |
| Interventions |
Control arm: (BPHSP Service) 1) Patient education Intervention arm: (C‐IDM) 1) Case management 2) Team change 3) Clinician education 4) Patient education |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
| Notes | Extracted |
Millan‐Ferro 2020.
| Methods | RCT (NA clusters and NA providers), conducted in 1) The study took place at the adult medicine department at the South End Community Health Center, a federally funded community health centre affiliated with Boston Medical Center (BMC), and the Joslin Diabetes Center (JDC), a specialised centre for diabetes care. Boston MA, USA. 2) Nurse practitioner, clinical team. In United States of America. 2 arms: 1. Control arm (standard care) and 2. Intervention arm (clinician teleconsult) |
| Participants | Control arm N: 150 Intervention arm N: 157, NA, NA Diabetes type: 2 Mean age: 55.2 ± 11.4 % Male: 44.9 Longest follow‐up: 6 months |
| Interventions |
Control arm: (standard care) 1) Patient education 2) Patient reminders Intervention arm: (clinician teleconsult) 1) Case management 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
| Notes | Extracted |
Murphy 2020.
| Methods |
Supporting care for suboptimally controlled type 2 diabetes mellitus in general practice with a clinical decision support system: a mixed methods pilot cluster randomised trial Cluster RCT (14 Clusters and NR providers), conducted in 1) General Practices in Ireland, Royal College of Surgeons Ireland, 2) General practitioners 2 arms: 1. Control arm (usual care) and 2. Intervention arm (DECIDE: clinical decision support) |
| Participants | Control arm N: 67 Intervention arm N: 67, NA, NA Diabetes type: 2 Mean age: 59.3 ±12.52 % Male: 63.4 Longest follow‐up: 4 months |
| Interventions |
Control arm: (usual care) Intervention arm: (DECIDE: clinical decision support) 1) Electronic patient registry 2) Clinician education |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
| Notes | Extracted |
Noda 2020.
| Methods |
A cluster‐randomized trial of the effectiveness of a triple‐faceted intervention promoting adherence to primary care physician visits by diabetes patients Cluster RCT (22 clusters and 192 providers), conducted in 1) Primary care, Japan, 2) Primary care physicians (PCPs) within each district medical association (DMA). In Japan. 2 arms: 1. Control arm (usual care) and 2. Intervention arm (triple‐faceted intervention programme) |
| Participants | Control arm N: 1265 Intervention arm N: 971, NA, NA Diabetes type: 2 Mean age: 56.5 ± 5.9 % Male: 62.5 Longest follow‐up: 12 months |
| Interventions |
Control arm: (usual care) Intervention arm: (triple‐faceted intervention programme) 1) Audit and feedback 2) Team change 3) Patient education 4) Patient reminders |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
| Notes | Extracted |
Or 2020.
| Methods |
Improving self‐care in patients with coexisting type 2 diabetes and hypertension by technological surrogate nursing: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) 2 diabetes outpatient clinics of 2 public hospitals, Hong Kong, 2) research team, primary care providers 2 arms: 1. Control arm (conventional self‐management) and 2. Intervention arm (TSN: Technological Surrogate Nursing) |
| Participants | Control arm N: 148 Intervention arm N: 151, NA, NA Diabetes type: 2 Mean age: 63.8 ± 9.89 % Male: 64.25 Longest follow‐up: 6 months |
| Interventions |
Control arm: (conventional self‐management) 1) Promotion of self‐management Intervention arm: (TSN: Technological Surrogate Nursing) 1) Electronic patient registry 2) Patient education 3) Promotion of self‐management 4) Patient reminders |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure |
| Notes | Extracted |
Ramallo‐Farina 2020.
| Methods | Clustered RCT (32 Clusters and 211 providers), conducted in 1) Primary healthcare practices, Canary islands (Tenerife, Gran Canaria, Lanzarote and La Palma), Spain, 2) Physicians and nurse. In Spain. 4 arms: 1. Control arm (usual care) and 2. Intervention arm (patient education, monitoring, feedback), 3. Intervention arm (HCP education, decision aid, feedback) and 4. Intervention arm (combined patient and HCP) |
| Participants | Control arm N: 586 Intervention arm N: 537, 654, 557 Diabetes type: Unclear Mean age: 55.7 ± 7.1 % Male: 48.1 Longest follow‐up: 6 months |
| Interventions |
Control arm: (usual care) Intervention arm: (patient education, monitoring, feedback) 1) Patient education 2) Promotion of self‐management Intervention arm: (HCP education, decision aid, feedback) 1) Audit and feedback 2) Clinician education Intervention arm: (combined patient and HCP) 1) Audit and feedback 2) Clinician education 3) Patient education 4) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
| Notes | Extracted |
Reininger 2020.
| Methods |
Improved diabetes control among low‐income Mexican Americans through community‐clinical interventions: results of an RCT RCT (NA clusters and NA providers), conducted in 1) Clinic, community/home, University of Texas Health Science Center at Houston, School of Public Health, The Rio Grande Valley of Texas, United States of America, 2) Healthcare providers, social workers, pharmacist, clinical personnel, nurses, community health workers 2 arms: 1. Control arm (SyV 1.0: Salud y Vida 1.0) and 2. Intervention arm (SyV 2.0: Salud y Vida 2.0) |
| Participants | Control arm N: 177 Intervention arm N: 176, NA, NA Diabetes type: not reported Mean age: 51.58 ± 9.12 % Male: 26.37 Longest follow‐up: 12 months |
| Interventions |
Control arm: (SyV 1.0: Salud y Vida 1.0) 1) Case management 2) Patient education 3) Promotion of self‐management Intervention arm: (SyV 2.0: Salud y Vida 2.0) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Rovner 2020.
| Methods |
Improving glycemic control in African Americans with diabetes and mild cognitive impairment RCT (NA clusters and NA providers), conducted in 1) Primary care practices of Thomas Jefferson University, Philadelphia, PA, USA. 2) Occupational therapists, CHW 2 arms: 1. Control arm (diabetes education) and 2. Intervention arm (occupational therapy) |
| Participants | Control arm N: 51 Intervention arm N: 50, NA, NA Diabetes type: 2 Mean age: 68.4 ± 6.4 % Male: 38 Longest follow‐up: 6 months |
| Interventions |
Control arm: (diabetes education) 1) Patient education Intervention arm: (occupational therapy) 1) Case management 2) Team change 3) Patient education 4) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Ruiz de Adana 2020.
| Methods |
Randomized study to evaluate the impact of telemedicine care in patients with type 1 diabetes with multiple doses of insulin and suboptimal HbA1c in Andalusia (Spain): PLATEDIAN Study RCT (NA clusters and NA providers), conducted in 1) Diabetes units in hospitals in Andalusia, Spain, 2) Endocrinologists and diabetes specialised nurses 2 arms: 1. Control arm (control cohort) and 2. Intervention arm (intervention cohort) |
| Participants | Control arm N: 188 Intervention arm N: 191, NA, NA Diabetes type: 1 Mean age: 35.01 ± 10.35 % Male: 55.76 Longest follow‐up: 6 months |
| Interventions |
Control arm: (control cohort) Intervention arm: (intervention cohort) 1) Facilitated relay of clinical information 2) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin 2) Harms (hypoglycaemic episodes) |
| Notes | Extracted |
Vaccaro 2013.
| Methods |
Feasibility and effectiveness in clinical practice of a multifactorial intervention for the reduction of cardiovascular risk in patients with type 2 diabetes Cluster‐RCT (9 clusters), conducted in 10 large outpatient diabetes clinics, Italy Two arms: 1. Usual care (control arm) and 2. Intensive care (intervention arm) |
| Participants | Control arm N: NR Intervention arm N: NR Diabetes type: type II Mean age: NR ± NR % Male: NR Longest follow‐up: 24 months |
| Interventions |
Control arm: None Intervention arm: 1) Clinician education |
| Outcomes | 1) Aspirin (antiplatelet treatment), N users (%) Control arm: pre 157 (20), post 146 (19) Intervention arm: pre 144 (28), post 380 (73) 2) Statins, N users (%) Control arm: pre 244 (32), post 185 (24) Intervention arm: pre 151 (29), post 333 (64) 3a) Antihypertensives (any), N users (%) Control arm: pre 466 (60), post 671 (87) Intervention arm: pre 352 (68), post 479 (92) 3b) Antihypertensives (a‐blocker), N users (%) Control arm: pre NR (NR), post 62 (8) Intervention arm: pre NR (NR), post 31 (6) 3c) Antihypertensives (ACE inhibitor), N users (%) Control arm: pre NR (NR), post 524 (68) Intervention arm: pre NR (NR), post 396 (76) 3d) Antihypertensives (angiotensin II receptor blockers), N users (%) Control arm: pre NR (NR), post 262 (34) Intervention arm: pre NR (NR), post 135 (26) 3e) Antihypertensives (calcium channel blocker), N users (%) Control arm: pre NR (NR), post 262 (34) Intervention arm: pre NR (NR), post 240 (46) 3f) Antihypertensives (diuretic), N users (%) Control arm: pre NR (NR), post 308 (40) Intervention arm: pre NR (NR), post 281 (54) 3g) Antihypertensives (ß‐blocker), N users (%) Control arm: pre NR (NR), post 131 (17) Intervention arm: pre NR (NR), post 63 (12) 4) HbA1c, mean % (SE) Control arm: pre 7.4 (0.2), post 7.7 (0.1) Intervention arm: pre 7.8 (0.2), post 7.2 (0.1) 5) SBP, mean mmHg (SE) Control arm: pre 145.9 (2.4), post 141.8 (1.7) Intervention arm: pre 146.3 (2.7), post 137.4 (1.9) 6) DBP, mean mmHg (SE) Control arm: pre 86.8 (1.3), post 80.5 (0.5) Intervention arm: pre 86.0 (1.5), post 80.8 (0.6) 7) LDL, mean mg/dL (SE) Control arm: pre 140.7 (2.2), post 123.2 (3.5) Intervention arm: pre 146.5 (2.5), post 112.8 (3.1) 8) Controlled hypertension (< 130/80 mmHg), N under control (%) Control arm: pre NR (NR), post 40 (5) Intervention arm: pre NR (NR), post 70 (13) |
| Notes | — |
von Storch 2019.
| Methods |
Telemedicine‐assisted self‐management program for type 2 diabetes patients RCT (NA clusters and NA providers), conducted in 1) Patients covered by the health insurance Central Krankenversicherung AG (Central). Central is a private insurance company providing telemedical assistance, 2) Telemontoring by a coach and physician. In Germany. 2 arms: 1. Control arm (routine care) and 2. Intervention arm (lifestyle telemedicine‐assisted self‐management programme) |
| Participants | Control arm N: 900 Intervention arm N: 1541, NA, NA Diabetes type: 2 Mean age: 58.92 ± 6.78 % Male: 81.35 Longest follow‐up: 3 months |
| Interventions |
Control arm: (routine care) 1) Promotion of self‐management Intervention arm: (lifestyle telemedicine‐assisted self‐management programme) 1) Case management 2) Electronic patient registry 3) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Wang 2019.
| Methods |
Effects of continuous care for patients with type 2 diabetes using mobile health application: a randomised controlled trial RCT (NA clusters and NA providers), conducted in 1) Remotely delivered mobile intervention, Shanghai, China. 2) Intervention delivered by physician, medical staff, nurse practitioner. In China. 2 arms: 1. Control arm (conventional care) and 2. Intervention arm (continuous care mobile‐based application) |
| Participants | Control arm N: 60 Intervention arm N: 60, NA, NA Diabetes type: 2 Mean age: 45.4 ± 8.08 % Male: 53.3 Longest follow‐up: 6 months |
| Interventions |
Control arm: (conventional care) 1) Patient education Intervention arm: (continuous care mobile‐based application) 1) Case management 2) Electronic patient registry 3) Facilitated relay of clinical information 4) Patient education 5) Promotion of self‐management 6) Patient reminders |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Wang 2020.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | Not yet extracted |
White 2020.
| Methods |
The partnership to improve diabetes education trial: a cluster randomized trial addressing health communication in diabetes care Cluster RCT (10 clusters and 186 providers), conducted in 1) Clinics within the Mid‐Cumberland Region of the Tennessee Department of Health (TDOH). The Mid Cumberland Region has 16 clinics across 12 counties in Tennessee, USA, 2) clinicians, nurse practitioner, registered nurse, dietician 2 arms: 1. Control arm (NDEP) and 2. Intervention arm (PRIDE) |
| Participants | Control arm N: 198 Intervention arm N: 212, NA, NA Diabetes type: 2 Mean age: 51 ± NR % Male: 39 Longest follow‐up: 24 months |
| Interventions |
Control arm: (NDEP) 1) Clinician education Intervention arm: (PRIDE) 1) Clinician education 2) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin 2) Low‐density lipoprotein |
| Notes | Extracted |
Willis 2020.
| Methods |
Effects of an electronic software "prompt" with health care professional training on cardiovascular and renal complications in a multiethnic population with type 2 diabetes and microalbuminuria (the GP‐prompt study): results of a pragmatic cluster‐randomized trial Cluster‐RCT (22 clusters and NR providers), conducted in 1) Primary care practices, Leicestershire, United Kingdom, 2) Educators from the Effective Diabetes Education Now (EDEN) team; health care providers; specialist diabetologist; sought involvement from local clinicians to develop training materials and technical aspects of the intervention 2 arms: 1. Control arm (usual care) and 2. Intervention arm (GP prompt, (HCP)‐focused multifactorial intervention) |
| Participants | Control arm N: 1299 Intervention arm N: 1422, NA, NA Diabetes type: 2 Mean age: 62.9 ± 10 % Male: 58.7 Longest follow‐up: 24 months |
| Interventions |
Control arm: (usual care) Intervention arm: (GP prompt, (HCP)‐focused multifactorial intervention) 1) Audit and feedback 2) Clinician education 3) Clinician reminders 4) Patient education |
| Outcomes | 1) Anti‐platelet drugs 2) Lipid‐lowering drugs 3) Antihypertensive drug 4) Glycated haemoglobin 5) Systolic blood pressure 6) Diastolic blood pressure 7) Hypertension control |
| Notes | Extracted |
Xu 2020.
| Methods |
Improving HbA1c with glucose self‐monitoring in diabetic patients with EpxDiabetes, a phone call and text message‐based telemedicine platform: a randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Remotely delivered telemedicine intervention. Primary care clinic, St. Louis, Missouri. 2) Health care 'provider', pharmacist, EPxDiabetes platform. In the United States of America. 2 arms: 1. Control arm (conventional care) and 2. Intervention arm (EpxDiabetes telemedicine) |
| Participants | Control arm N: 32 Intervention arm N: 33, NA, NA Diabetes type: 2 Mean age: 54.96 ± 10.63 % Male: 31.35 Longest follow‐up: 6 months |
| Interventions |
Control arm: (conventional care) 1) Electronic patient registry Intervention arm: (EpxDiabetes telemedicine) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient reminders |
| Outcomes | 1) Glycated haemoglobin |
| Notes | Extracted |
Xu 2021.
| Methods | Not yet extracted |
| Participants | Not yet extracted |
| Interventions | Not yet extracted |
| Outcomes | Not yet extracted |
| Notes | — |
Yang 2020.
| Methods |
Effect of a mobile phone‐based glucose‐monitoring and feedback system for type 2 diabetes management in multiple primary care clinic settings: cluster randomized controlled trial Cluster‐RCT (13 clusters and NR providers), conducted in 1) Primary care clinics were located in Seoul and other major cities in South Korea, having a patient pool with T2DM, and access to internet services at the clinic, 2) Mobile phone app so no provider giving intervention. Primary care physicians involved. In South Korea. 2 arms: 1. Control arm (usual care) and 2. Intervention arm (mobile phone–based monitoring and feedback system) |
| Participants | Control arm N: 97 Intervention arm N: 150, NA, NA Diabetes type: 2 Mean age: 56.65 ± 10.61 % Male: 50.43 Longest follow‐up: 3 months |
| Interventions |
Control arm: (usual care) Intervention arm: (mobile phone–based monitoring and feedback system) 1) Facilitated relay of clinical information 2) Promotion of self‐management |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein 5) Harms (hypo/hyperglycaemic episodes) |
| Notes | Extracted |
Zhang 2019.
| Methods |
Effectiveness of smartphone app‐based interactive management on glycemic control in Chinese patients with poorly controlled diabetes: randomized controlled trial RCT (NA clusters and NA providers), conducted in 1) Recruited from the outpatient clinic of the Department of Endocrinology and Metabolism of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China. Remotely‐delivered intervention via Welltang application. 2) Clinician, dietician, health manager, 3 arms: 1. Control arm (usual care) and 2. Intervention arm (self‐management with smartphone app) and 3. Intervention arm (interactive management with smartphone app) |
| Participants | Control arm N: 78 Intervention arm N: 78, 78, NA Diabetes type: 1 and 2 Mean age: 53 ± 11 % Male: 62 Longest follow‐up: 6 months |
| Interventions |
Control arm: (usual care) 1) Patient education 2) Promotion of self‐management Intervention arm: (self‐management with smartphone app) 1) Electronic patient registry 2) Facilitated relay of clinical information 3) Patient education 4) Promotion of self‐management Intervention arm: (interactive management with smartphone app) 1) Case management 2) Team change 3) Electronic patient registry 4) Facilitated relay of clinical information 5) Patient education 6) Promotion of self‐management 7) Patient reminders |
| Outcomes | 1) Glycated haemoglobin 2) Low‐density lipoprotein 3) Harms (hypoglycaemic episodes) |
| Notes | Extracted |
Zhao 2020.
| Methods |
Analysis of the effect of nine consecutive years' intensive management and number of times achieving the target control on endpoint events in T2DM patients in Sanlitun Community Health Service Center in Beijing RCT (NA clusters and NA providers), conducted in 1) Sanlitun Community Health Service Center in Beijing, China; 2) Director of endocrinology department, general practitioner. In China. 2 arms: 1. Control arm (standard management) and 2. Intervention arm (intensive management) |
| Participants | Control arm N: 111 Intervention arm N: 113, NA, NA Diabetes type: 2 Mean age: 66.01 ± 9.19 % Male: 34.82 Longest follow‐up: 108 months |
| Interventions |
Control arm: (standard management) 1) Clinician education Intervention arm: (intensive management) 1) Clinician education |
| Outcomes | 1) Glycated haemoglobin 2) Systolic blood pressure 3) Diastolic blood pressure 4) Low‐density lipoprotein |
| Notes | Extracted |
NA: not applicable; NR: not reported; SMBG: self‐monitoring blood glucose
Differences between protocol and review
Eligibility criteria
Minimum number of clusters in cluster trials: In consultation with experts in cluster‐trial design and analysis, we amended the 2014 protocol to exclude cluster studies with three clusters or fewer per arm. This resulted in the exclusion of three studies from this update and five cluster studies from the prior version of the review, leaving a total of 147 reports representing 135 unique studies for the update to be added to the 157 reports representing 137 unique studies included in the previous review.
Data extraction
Contacting authors for additional information: As part of the study protocol (Ivers 2014), we planned to capture additional information about the QI strategies (and the context in which they were evaluated) via a survey of all authors of included studies. We contacted authors of included studies for additional information on the content of the intervention, as well as potential modifiers of intervention effects (population, setting, contextual covariates) using a tailored online web survey. After six contact attempts, the response was 45.2% (126/279) (Danko 2019). Given the low response rate, we have not incorporated data on intervention, population and context obtained from authors in the survey in our present analyses.
Data synthesis
Assessing interactions among QI strategies: We undertook an interim analysis (using 340 RCTs that reported HBA1c) to explore whether there was evidence of synergistic (or antagonistic) interactions of QI strategies using a series of pairwise QI*QI interactions models in which we systematically estimated the magnitude of the interaction of one QI strategy with each of the other QI strategies. However, we did not find any evidence of pairwise interactions among QI strategies (Konnyu (in press)) and so did not repeat.
Contributions of authors
KGS led the first version of this review (Shojania 2006). JMG, NI and ACT led the second version of the review (Tricco 2012). JMG and NI conceived the overall plan for this update. JMG, NI, ACT, KJK and TAT (and Issa Dahabreh) conceived the methodological approach adopted for the review. KJK, JL, KJS and SY provided project management, co‐ordination and quality control for the review. NI and ACT completed recoding of the QI strategies. MA, KK, SK, JL, SL, SM, PMcDR, KJS, SY, BV and MZ screened search results and undertook data abstraction. KJK and SY conducted descriptive analyses of the papers. KJK ran analytic models with feedback from TAT, JMG and NI. MH, JNL, BM, DM, AP, TR, KGS, SES, MT, ACT and CHY were members of the Steering Group and contributed to the framing and interpretation of the review results. KJK, SY, NI and JMG drafted the review. All authors provided feedback on drafts.
Sources of support
Internal sources
-
Ottawa Hospital Research Institute (OHRI), Canada
JMG is employed by OHRI
External sources
-
Canadian Institutes of Health Research, Canada
This project was supported by CIHR Forest and Trees grant (MOP‐123345) and JMG Foundation grant (FDN‐143269)
-
Canada Research Chair, Canada
Canada Research Chair in Health Knowledge Transfer and Uptake (JMG)
-
Canada Research Chair, Canada
Canada Research Chair in Implementation of Evidence‐based Practice (NMI)
-
Canadian Institutes of Health Research, Canada
KJK was supported by a doctoral research fellowship (Frederick Banting and Charles Best Canada Graduate Scholarship; GSD‐134936)
-
Canadian Institutes of Health Research (in partnership with Diabetes Canada), Canada
KJK was supported by a postdoctoral research fellowship (Fellowship ‐ Priority Announcement ‐ Diabetes)
-
David Freeze Chair in Health Research, Canada
David Freeze Chair in Health Research (MT)
-
Canada Research Chair, Canada
Tier 1 Canada Research Chair in Evidence‐Support System (JNL)
-
Canada Research Chair, Canada
Tier 2 Canada Research Chair in Knowledge Synthesis (AT)
-
Canada Research Chair, Canada
Tier 1 Canada Research Chair in Knowledge Translation and Quality of Care (SS)
Declarations of interest
Mostafa Alabousi: none known.
Alun Edwards: none known.
Jeremy Grimshaw: Canada Research Chairs (Grant/Contract); Canadian Institutes of Health Research (Grant/Contract); Co‐ordinating Editor of the Cochrane Effective Practice and Organisation of Care group (1997‐2015) and currently an Emeritus Editor; involved with Diabetes UK, Northern and Yorkshire Regional NHS R&D Office, CIHR ‐ Knowledge Translation Improved Clinical Effectiveness Behavioral Research Group (KT‐ICEBeRG), see Eccles 2007, Ivers 2013, Presseau 2018.
Michael Hillmer: Ontario Ministry of Health and Long‐Term Care (Employment).
Noah Ivers: Merck (Consultant); Novo Nordisk (Consultant); works as a health professional at Women’s College Hospital, Toronto, Ontario, Canada; affiliated to Diabetes Canada; editorial role with Cochrane EPOC; involved with CIHR as principal investigator.
Sathya Karunananthan: none known.
Kristin Konnyu: none known.
John Lavis: none known.
Johanie Lepine: none known.
Stefanie Linklater: none known.
Braden Manns: no relevant interests; works as a physician who sees patients with diabetes and kidney disease (salaried physician).
David Moher: none known.
Sameh Mortazhejri: none known.
Samir Nazarali: none known.
P. Alison Paprica: none known.
Timothy Ramsay: none known.
Paul MacDaragh Ryan: no relevant interests; Paediatrics Resident, University of Toronto/The Hospital for Sick Children.
Peter Sargious: Alberta Health Services (Senior Medical Director); published op‐ep in CMAJ related to impact of Strategic Clinical Network on improving diabetes care; Physician (General Internist) with academic outpatient practice in diabetes.
Kaveh Shojania: none known.
Sharon Straus: no relevant interests; Physician‐in‐Chief, Department of Medicine, St. Michael's Hospital, Unity Health Toronto 30 Bond Street Toronto, Ontario, Canada M5B 1W8.
Katrina Sullivan: none known.
Marcell Tonelli: AstraZeneca (lectures); Section Editor, Uptodate (Wolters Klewer Health, Inc.); works as a nephrologist.
Andrea Tricco: none known.
Thomas Trikalinos: Pacira Pharmaceuticals Incorporated (Expert Witness).
Brigitte Vachon: none known.
Sharlini Yogasingam: none known.
Catherine Yu: no relevant interests; Endocrinologist, St. Michael's Hospital.
Michael Zahradnik: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abuloha 2016 {published data only}
- Abuloha S, Alabbadi I, Albsoul-Younes A, Younes N, Zayed A. The role of clinical pharmacist in initiation and/or dose adjustment of insulin therapy in diabetic patients in outpatient clinic in Jordan. Jordan Journal of Pharmaceutical Sciences 2016;9(1):33. [Google Scholar]
Adachi 2013 {published data only}
- Adachi M, Yamaoka K, Watanabe M, Nishikawa M, Kobayashi I, Hida E, et al. Effects of lifestyle education program for type 2 diabetes patients in clinics: a cluster randomized controlled trial. BMC Public Health 2013;13:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adair 2013 {published data only}
- Adair R, Wholey D R, Christianson J, White K M, Britt H, Lee S. Improving chronic disease care by adding laypersons to the primary care team: a parallel randomized trial. Ann Intern Med 2013;159(3):176-84. [DOI] [PubMed] [Google Scholar]
Adams 2015 {published data only}
- Adams R P, Barton G, Bhattacharya D, Grassby P F, Holland R, Howe A, et al. Supervised pharmacy student-led medication review in primary care for patients with type 2 diabetes: a randomised controlled pilot study. BMJ Open 2015;5(11):e009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adjei 2015 {published data only}
- Adjei D N, Agyemang C, Dasah J B, Kuranchie P, Amoah A G B. The effect of electronic reminders on risk management among diabetic patients in low resourced settings. Journal of Diabetic Complications 2015;29(6):818-21. [DOI] [PubMed] [Google Scholar]
Agarwal 2019 {published data only}
- Agarwal P, Mukerji G, Desveaux L, Ivers N M, Bhattacharyya O, Hensel J M, et al. Mobile app for improved self-management of type 2 diabetes: multicenter pragmatic randomized controlled trial. JMIR MHealth and UHealth 2019;7(1):e10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aguiar 2018 {published data only}
- Aguiar P M, Silva C H P, Chiann C, Dorea E L, Lyra D P Jr Storpirtis. Pharmacist-physician collaborative care model for patients with uncontrolled type 2 diabetes in Brazil: results from a randomized controlled trial. Journal of Evaluation in Clinical Practice 2018;24(1):22-30. [DOI] [PubMed] [Google Scholar]
Ahring 1992 {published data only}
- Ahring K K, Ahring J P, Joyce C, Farid N R. Telephone modem access improves diabetes control in those with insulin-requiring diabetes. Diabetes Care 1992;15(8):971-5. [DOI] [PubMed] [Google Scholar]
Aiello 2015 (annual follow‐ups) {published data only}
- Aiello LP, Ayala AR, Antoszyk AN, Arnold-Bush B, Baker C, Bressler NM, et al. Assessing the effect of personalized diabetes risk assessments during ophthalmologic visits on glycemic control: a randomized clinical trial. JAMA Ophthalmology 2015;133(8):888-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aiello 2015 (more‐frequent‐than‐annual follow‐ups) {published data only}
- Aiello LP, Ayala AR, Antoszyk AN, Arnold-Bush B, Baker C, Bressler NM, et al, Diabetic Retinopathy Clinical Research Network. Assessing the effect of personalized diabetes risk assessments during ophthalmologic visits on glycemic control: a randomized clinical trial. JAMA Ophthalmology 2015;133:888-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alanzi 2018 {published data only}
- Alanzi T, Alanazi N R, Istepanian R, Philip N. Evaluation of the effectiveness of mobile diabetes management system with social networking and cognitive behavioural therapy (CBT) for T2D. mHealth 2018;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albisser 2007 {published data only}
- Albisser A M, Wright C E, Sakkal S. Averting iatrogenic hypoglycemia through glucose prediction in clinical practice: progress towards a new procedure in diabetes. Diabetes Research and Clinical Practice 2007;76(2):207-14. [DOI] [PubMed] [Google Scholar]
Aleo 2015 {published data only}
- Aleo C L, Murchison A P, Dai Y, Hark L A, Mayro E L, Collymore B, Haller J A. Improving eye care follow-up adherence in diabetic patients with ocular abnormalities: The effectiveness of patient contracts in a free, pharmacy-based eye screening. Public Health 2015;129:996-9. [DOI] [PubMed] [Google Scholar]
Ali 2012 {published data only}
- Ali M, Schifano F, Robinson P, Phillips G, Doherty L, Melnick P, et al. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomized controlled study. Diabet Med 2012;29(9):e326-33. [DOI] [PubMed] [Google Scholar]
Ali 2016 {published data only}
- Ali M K, Singh K, Kondal D, Devarajan R, Patel S A, Shivashankar R, et al, Group Carrs Trial. Effectiveness of a multicomponent quality improvement strategy to improve achievement of diabetes care goals: a randomized, controlled trial. Annals of Internal Medicine 2016;165:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2011 {published data only}
- Allen J K, Dennison-Himmelfarb C R, Szanton S L, Bone L, Hill M N, Levine D M, et al. Community Outreach and Cardiovascular Health (COACH) Trial: a randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circulation. Cardiovascular Quality and Outcomes 2011;4(6):595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Mazroui 2009 {published data only}
- Al Mazroui N R, Kamal M M, Ghabash N M, Yacout T A, Kole P L, McElnay J C. Influence of pharmaceutical care on health outcomes in patients with Type 2 diabetes mellitus. British Journal of Clinical Pharmacology 2009;67(5):547-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alotaibi 2016 {published data only}
- Alotaibi M M, Istepanian R, Philip N. A mobile diabetes management and educational system for type-2 diabetics in Saudi Arabia (SAED). mHealth 2016;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Shookri 2012 {published data only}
- Al-Shookri A, Khor G L, Chan Y M, Loke S C, Al-Maskari M. Effectiveness of medical nutrition treatment delivered by dietitians on glycaemic outcomes and lipid profiles of Arab, Omani patients with Type 2 diabetes. Diabetic Medicine 2012;29(2):236-44. [DOI] [PubMed] [Google Scholar]
Amendezo 2017 {published data only}
- Amendezo E, Walker Timothy D, Karamuka V, Robinson B, Kavabushi P, Ntirenganya C, et al. Effects of a lifestyle education program on glycemic control among patients with diabetes at Kigali University Hospital, Rwanda: a randomized controlled trial. Diabetes research and clinical practice 2017;126:129-37. [DOI] [PubMed] [Google Scholar]
Amsberg 2009 {published data only}
- Amsberg S, Anderbro T, Wredling R, Lisspers J, Lins P E, Adamson U, et al. A cognitive behavior therapy-based intervention among poorly controlled adult type 1 diabetes patients--a randomized controlled trial. Patient Education and Counseling 2009;77:72-80. [DOI] [PubMed] [Google Scholar]
Anderson 2005 {published data only}
- Anderson R M, Funnell M M, Nwankwo R, Gillard M L, Oh M, Fitzgerald J T. Evaluating a problem-based empowerment program for African Americans with diabetes: results of a randomized controlled trial. Ethnicity & Disease 2005;15:671-8. [PubMed] [Google Scholar]
Anderson 2010 {published data only}
- Anderson D R, Christison-Lagay J, Villagra V, Liu H, Dziura J. Managing the space between visits: a randomized trial of disease management for diabetes in a community health center. Journal of General Internal Medicine 2010;25(10):1116-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson‐Loftin 2005 {published data only}
- Anderson-Loftin W, Barnett S, Bunn P, Sullivan P, Hussey J, Tavakoli A. Soul food light: culturally competent diabetes education. The Diabetes Educator 2005;31:555-63. [DOI] [PubMed] [Google Scholar]
Andrews 2011 {published data only}
- Andrews R C, Cooper A R, Montgomery A A, Norcross A J, Peters T J, Sharp D J, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011;378(9786):129-39. [DOI] [PubMed] [Google Scholar]
Anzaldo‐Campos 2016 {published data only}
- Anzaldo-Campos M C, Contreras S, Vargas-Ojeda A, Menchaca-Diaz R, Fortmann A, Philis-Tsimikas A. Dulce Wireless Tijuana: a randomized control trial evaluating the impact of project Dulce and short-term mobile technology on glycemic control in a family medicine clinic in northern Mexico. Diabetes Technology & Therapeutics 2016;18:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aubert 1998 {published data only}
- Aubert R E, Herman W H, Waters J, Moore W, Sutton D, Peterson B L, et al. Nurse case management to improve glycemic control in diabetic patients in a health maintenance organization. A randomized, controlled trial. Annals of Internal Medicine 1998;129(8):605-12. [DOI] [PubMed] [Google Scholar]
- Sikka R, Waters J, Moore W, Sutton DR, Herman WH, Aubert RE. Renal assessment practices and the effect of nurse case management of health maintenance organization patients with diabetes. Diabetes Care 1999;22(1):1-6. [DOI] [PubMed] [Google Scholar]
Augstein 2007 {published data only}
- Augstein P, Vogt L, Kohnert K D, Freyse E J, Heinke P, Salzsieder E. Outpatient assessment of Karlsburg Diabetes Management System-based decision support. Diabetes Care 2007;30(7):1704-8. [DOI] [PubMed] [Google Scholar]
Avdal 2011 {published data only}
- Avdal E U, Kizilci S, Demirel N. The effects of web-based diabetes education on diabetes care results: a randomized control study. Computers, Informatics, Nursing 2011;29(2):101-6. [DOI] [PubMed] [Google Scholar]
Ayadurai 2018 {published data only}
- Ayadurai S, Sunderland V B, Tee L B G, Md Said S N, Hattingh H L. Structured tool to improve clinical outcomes of type 2 diabetes mellitus patients: a randomized controlled trial. Journal Of Diabetes 2018;10(12):965-76. [DOI] [PubMed] [Google Scholar]
Ayala 2015 {published data only}
- Ayala GX, Ibarra L, Cherrington AL, Parada H, Horton L, Ji M, Elder JP. Puentes hacia una mejor vida (Bridges to a Better Life): Outcome of a Diabetes Control Peer Support Intervention. Annals of Family Medicine 2015;13 Suppl 1:S9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azizi 2016 {published data only}
- Azizi A, Aboutorabi R, Mazloum-Khorasani Z, fzal-Aghaea M, Tabesh H, Tara M. Evaluating the effect of web-based Iranian diabetic personal health record app on self-care status and clinical indicators: randomized controlled trial. JMIR Medical Informatics 2016;4:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babamoto 2009 {published data only}
- Babamoto K S, Sey K A, Camilleri A J, Karlan V J, Catalasan J, Morisky D E. Improving diabetes care and health measures among Hispanics using community health workers: results from a randomized controlled trial. Health Education & Behavior 2009;36:113-26. [DOI] [PubMed] [Google Scholar]
Barcelo 2010 {published data only}
- Barcelo A, Cafiero E, Boer M, Mesa A E, Lopez M G, Jimenez R A, et al. Using collaborative learning to improve diabetes care and outcomes: the VIDA project. Prim Care Diabetes 2010;4(3):145-53. [DOI] [PubMed] [Google Scholar]
Baron 2017 {published data only}
- Baron J S, Hirani S, Newman S P. A randomised, controlled trial of the effects of a mobile telehealth intervention on clinical and patient-reported outcomes in people with poorly controlled diabetes. Journal of Telemedicine and Telecare 2017;23:207-16. [DOI] [PubMed] [Google Scholar]
Basak 2014 {published data only}
- Basak Cinar A, Schou L. Health promotion for patients with diabetes: health coaching or formal health education? International Dental Journal 2014;64(1):20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basudev 2016 {published data only}
- Basudev N, Crosby-Nwaobi R, Thomas S, Chamley M, Murrells T, Forbes A. A prospective randomized controlled study of a virtual clinic integrating primary and specialist care for patients with Type 2 diabetes mellitus. Diabetic Medicine 2016;33(6):768-76. [DOI] [PubMed] [Google Scholar]
Bebb 2007 {published data only}
- Bebb C, Kendrick D, Coupland C, Madeley R, Stewart J, Brown K, et al. A cluster randomised controlled trial of the effect of a treatment algorithm for hypertension in patients with type 2 diabetes. British Journal of General Practice 2007;57(535):136-43. [PMC free article] [PubMed] [Google Scholar]
Bellary 2008 {published data only}
- Bellary S, O'Hare J P, Raymond N T, Gumber A, Mughal S, Szczepura A, et al. Enhanced diabetes care to patients of south Asian ethnic origin (the United Kingdom Asian Diabetes Study): a cluster randomised controlled trial. Lancet 2008;371(9626):1769-76. [DOI] [PubMed] [Google Scholar]
Benhamou 2007 {published data only}
- Benhamou P Y, Melki V, Boizel R, Perreal F, Quesada J L, Bessieres-Lacombe S, et al. One-year efficacy and safety of Web-based follow-up using cellular phone in type 1 diabetic patients under insulin pump therapy: the PumpNet study. Diabetes & Metabolism 2007;33(3):220-6. [DOI] [PubMed] [Google Scholar]
Benson 2019 {published data only}
- Benson G A, Sidebottom A, Hayes J, Miedema M D, Boucher J, Vacquier M, et al. Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) telemedicine randomized controlled trial on diabetes optimal care outcomes in patients with type 2 diabetes. Journal of the Academy of Nutrition & Dietetics 2019;119(4):585-98. [DOI] [PubMed] [Google Scholar]
Bergenstal 2005 {published data only}
- Bergenstal R M, Anderson R L, Bina D M, Johnson M L, Davidson J L, Solarz-Johnson B, et al. Impact of modem-transferred blood glucose data on clinician work efficiency and patient glycemic control. Diabetes Technology & Therapeutics 2005;7(2):241-7. [DOI] [PubMed] [Google Scholar]
Bertuzzi 2018 {published data only}
- Bertuzzi F, Stefani I, Rivolta B, Pintaudi B, Meneghini E, Luzi L, et al. Teleconsultation in type 1 diabetes mellitus (TELEDIABE). Acta Diabetologica 2018;55(2):185-92. [DOI] [PubMed] [Google Scholar]
Bian 2012 {published data only}
- Bian Lixiang, Sun Qiuying, Tao Ying. Influence of multi-disciplinary team management on psychological health and quality of life of patients with diabetic foot. Chinese Nursing Research 2012;26:2118-20. [Google Scholar]
Biermann 2002 {published data only}
- Biermann E, Dietrich W, Rihl J, Standl E. Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomised, controlled trial. Computer Methods and Programs in Biomedicine 2002;69(2):137-46. [DOI] [PubMed] [Google Scholar]
- Biermann E, Dietrich W, Standl E. Telecare of diabetic patients with intensified insulin therapy. A randomized clinical trial. Studies in Health Technology and Informatics 2000;77:327-32. [PubMed] [Google Scholar]
Bieszk 2016 {published data only}
- Bieszk N, Reynolds S L, Wei W, Davis C, Kamble P, Uribe C. "Act on threes" paradigm for treatment intensification of type 2 diabetes in managed care: results of a randomized controlled study with an educational intervention targeting improved glycemic control. Journal of Managed Care & Specialty Pharmacy 2016;22:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bieszk 2017 {published data only}
- Bieszk N, Grabner M, Wei W, Barron J, Quimbo R, Yan T, et al. Personalized care and the role of insulin as a vehicle to optimizing treatments in diabetes care. Journal of Managed Care & Specialty Pharmacy 2017;23(11):1160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Billiard 1991 {published data only}
- Billiard A, Rohmer V, Roques M A, Joseph M G, Suraniti S, Giraud P, et al. Telematic transmission of computerized blood glucose profiles for IDDM patients. Diabetes Care 1991;14(2):130-4. [DOI] [PubMed] [Google Scholar]
Blackberry 2013 {published data only}
- Blackberry I D, Furler J S, Best J D, Chondros P, Vale M, Walker C, et al. Effectiveness of general practice based, practice nurse led telephone coaching on glycaemic control of type 2 diabetes: the Patient Engagement and Coaching for Health (PEACH) pragmatic cluster randomised controlled trial. BMJ 2013;347:f5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boaz 2009 {published data only}
- Boaz M, Hellman K, Wainstein J. An automated telemedicine system improves patient-reported well-being. Diabetes Technology & Therapeutics 2009;11(3):181-6. [DOI] [PubMed] [Google Scholar]
Bogner 2010 {published data only}
- Bogner H R, Vries H F. Integrating type 2 diabetes mellitus and depression treatment among African Americans: a randomized controlled pilot trial. Diabetes Educator 2010;36(2):284-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bogner 2012 {published data only}
- Bogner H R, Morales K H, Vries H F, Cappola A R. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Annals of Family Medicine 2012;10(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohingamu 2019 {published data only}
- Bohingamu Mudiyanselage S, Stevens J, Watts J J, Toscano J, Kotowicz M A, et al. Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial. Journal of Telemedicine & Telecare 2019;25(6):343-52. [DOI] [PubMed] [Google Scholar]
Bollyky 2018 {published data only}
- Bollyky J B, Bravata D, Yang J, Williamson M, Schneider J. Remote lifestyle coaching plus a connected glucose meter with certified diabetes educator support improves glucose and weight loss for people with type 2 diabetes. Journal of Diabetes Research 2018;2018:3961730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 2007 {published data only}
- Bond G E, Burr R, Wolf F M, Price M, McCurry S M, Teri L. The effects of a web-based intervention on the physical outcomes associated with diabetes among adults age 60 and older: a randomized trial. Diabetes Technology & Therapeutics 2007;9(1):52-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonner 2018 {published data only}
- Bonner L M, Hanson A, Robinson G, Lowy E, Craft S. Care management to promote treatment adherence in patients with cognitive impairment and vascular risk factors: a demonstration project. Journal of Prevention of Alzheimer's Disease 2018;5(1):36-41. [DOI] [PubMed] [Google Scholar]
Bonney 2017 {published data only}
- Bonney A, Dijkmans-Hadley B, Seidel B, MacKinnon D, Phillipson L. A feasibility study of team-based primary care for chronic disease management training in rural Australia. Australian Journal of Rural Health 2017;25(1):66-7. [DOI] [PubMed] [Google Scholar]
Bosi 2013 {published data only}
- Bosi E, Scavini M, Ceriello A, Cucinotta D, Tiengo A, Marino R, et al. Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care 2013;36:2887-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bove 2013 {published data only}
- Bove A A, Homko C J, Santamore W P, Kashem M, Kerper M, Elliott D J. Managing hypertension in urban underserved subjects using telemedicine--a clinical trial. American Heart Journal 2013;165:615-21. [DOI] [PubMed] [Google Scholar]
Brown 2011 {published data only}
- Brown S A, Garcia A A, Winter M, Silva L, Brown A, Hanis C L. Integrating education, group support, and case management for diabetic Hispanics. Ethnicity & Disease 2011;21(1):20-6. [PMC free article] [PubMed] [Google Scholar]
Browne 2016 {published data only}
- Browne J L, Speight J, Martin C, Gilfillan C. Building the evidence for integrated care for type 2 diabetes: a pilot study. Australian Journal of Primary Health 2016;22(5):409‐15. [DOI] [PubMed] [Google Scholar]
Browning 2016 {published data only}
- Browning C, Chapman A, Yang H, Liu S, Zhang T, Enticott J C, Thomas S A. Management of type 2 diabetes in China: the Happy Life Club, a pragmatic cluster randomised controlled trial using health coaches. BMJ Open 2016;6:e009319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bujnowska‐Fedak 2011 {published data only}
- Bujnowska-Fedak M M, Puchala E, Steciwko A. The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland. Telemedicine Journal and E-health 2011;17(3):153-63. [DOI] [PubMed] [Google Scholar]
Buysse 2019 {published data only}
- Buysse H, Coremans P, Pouwer F, Ruige J. Sustainable improvement of HbA(1c) and satisfaction with diabetes care after adding telemedicine in patients on adaptable insulin regimens: Results of the TeleDiabetes randomized controlled trial. Health Informatics Journal 2019;3:1460458219844369. [DOI] [PubMed] [Google Scholar]
Cagliero 1999 {published data only}
- Cagliero E, Levina E V, Nathan D M. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care 1999;22(11):1785-9. [DOI] [PubMed] [Google Scholar]
Cani 2015 {published data only}
- Cani CG, Lopes Lda S, Queiroz M, Nery M. Improvement in medication adherence and self-management of diabetes with a clinical pharmacy program: a randomized controlled trial in patients with type 2 diabetes undergoing insulin therapy at a teaching hospital. Clinics (Sao Paulo) 2015;70(2):102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlson 1991 {published data only}
- Carlson A, Rosenqvist U. Diabetes care organization, process, and patient outcomes: effects of a diabetes control program. The Diabetes Educator 1991;17:42-8. [DOI] [PubMed] [Google Scholar]
Carter 2009 {published data only}
- Carter B L, Ardery G, Dawson J D, James P A, Bergus G R, Doucette W R, et al. Physician and pharmacist collaboration to improve blood pressure control. Archives of Internal Medicine 2009;169(21):1996-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2011 {published data only}
- Carter E L, Nunlee-Bland G, Callender C. A patient-centric, provider-assisted diabetes telehealth self-management intervention for urban minorities. Perspectives in Health Information Management 2011;8:1b. [PMC free article] [PubMed] [Google Scholar]
Carter 2018 {published data only}
- Carter B L, Levy B, Gryzlak B, Xu Y, Chrischilles E, Dawson J, et al. Cluster-randomized trial to evaluate a centralized clinical pharmacy service in private family medicine offices. Circulation. Cardiovascular Quality & Outcomes 2018;11(6):e004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castejon 2013 {published data only}
- Castejon A M, Calderon J L, Perez A, Millar C, McLaughlin-Middlekauff J, Sangasubana N, et al. A community-based pilot study of a diabetes pharmacist intervention in Latinos: impact on weight and hemoglobin A1c. Journal of Health Care for the Poor and Underserved 2013;24(4 Suppl):48-60. [DOI] [PubMed] [Google Scholar]
Chamany 2015 {published data only}
- Chamany S, Walker EA, Schechter CB, Gonzalez JS, Davis NJ, Ortega FM, et al. Telephone intervention to improve diabetes control: a randomized trial in the New York City A1c registry. American Journal of Preventive Medicine 2015;49(6):832-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2009 {published data only}
- Chan J C, So W Y, Yeung C Y, Ko G T, Lau I T, Tsang M W, et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care 2009;32(6):977-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan C W, Siu S C, Wong C K, Lee V W. A pharmacist care program: positive impact on cardiac risk in patients with type 2 diabetes. Journal of Cardiovascular Pharmacology and Therapeutics 2012;17(1):57-64. [DOI] [PubMed] [Google Scholar]
Chan 2014 {published data only}
- Chan J C, Sui Y, Oldenburg B, Zhang Y, Chung H H, Goggins W, et al. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Internal Medicine 2014;174(6):972-81. [DOI] [PubMed] [Google Scholar]
Chao 2015 {published data only}
- Chao J, Yang L, Xu H, Yu Q, Jiang L, Zong M. The effect of integrated health management model on the health of older adults with diabetes in a randomized controlled trial. Archives of Gerontology and Geriatrics 2015;60(1):82-8. [DOI] [PubMed] [Google Scholar]
Chao 2019 {published data only}
- Chao D Y, Lin T M, Ma W Y. Enhanced self-efficacy and behavioral changes among patients with diabetes: cloud-based mobile health platform and mobile app service. JMIR Diabetes 2019;4(2):e11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charpentier 2011 {published data only}
- Charpentier G, Benhamou P Y, Dardari D, Clergeot A, Franc S, Schaepelynck-Belicar P, et al. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care 2011;34(3):533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen J H, Ou H T, Lin T C, Lai E C, Kao Y H. Pharmaceutical care of elderly patients with poorly controlled type 2 diabetes mellitus: a randomized controlled trial. International Journal of Clinical Pharmacy 2016;38:88-95. [DOI] [PubMed] [Google Scholar]
Cho 2006 {published data only}
- Cho J H, Chang S A, Kwon H S, Choi Y H, Ko S H, Moon S D, et al. Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability: a 30-month follow-up study for diabetes management with a ubiquitous medical care system. Diabetes Care 2006;29(12):2625-31. [DOI] [PubMed] [Google Scholar]
Cho 2009 {published data only}
- Cho J H, Lee H C, Lim D J, Kwon H S, Yoon K H. Mobile communication using a mobile phone with a glucometer for glucose control in type 2 patients with diabetes: as effective as an Internet-based glucose monitoring system. Journal of Telemedicine and Telecare 2009;15(2):77-82. [DOI] [PubMed] [Google Scholar]
Cho 2011a {published data only}
- Cho J H, Choi Y H, Kim H S, Lee J H, Yoon K H. Effectiveness and safety of a glucose data-filtering system with automatic response software to reduce the physician workload in managing type 2 diabetes. Journal of Telemedicine and Telecare 2011;17(5):257-62. [DOI] [PubMed] [Google Scholar]
Cho 2011b {published data only}
- Cho J H, Kwon H S, Kim H S, Oh J A, Yoon K H. Effects on diabetes management of a health-care provider mediated, remote coaching system via a PDA-type glucometer and the Internet. Journal of Telemedicine and Telecare 2011;17(7):365-70. [DOI] [PubMed] [Google Scholar]
Cho 2017 {published data only}
- Cho J H, Kim H S, Yoo S H, Jung C H, Lee W J, Park C Y, et al. An Internet-based health gateway device for interactive communication and automatic data uploading: Clinical efficacy for type 2 diabetes in a multi-centre trial. Journal of Telemedicine & Telecare 2017;23(6):595-604. [DOI] [PubMed] [Google Scholar]
Choe 2005 {published data only}
- Choe H M, Mitrovich S, Dubay D, Hayward R A, Krein S L, Vijan S. Proactive case management of high-risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial. American Journal of Managed Care 2005;11(4):253-60. [PubMed] [Google Scholar]
Choudhry 2018 {published data only}
- Choudhry N K, Isaac T, Lauffenburger J C, Gopalakrishnan C, Lee M, Vachon A, et al. Effect of a remotely delivered tailored multicomponent approach to enhance medication taking for patients with hyperlipidemia, hypertension, and diabetes: The STIC2IT Cluster Randomized Clinical Trial. JAMA Internal Medicine 2018;178(9):1182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christian 2008 {published data only}
- Christian J G, Bessesen D H, Byers T E, Christian K K, Goldstein M G, Bock B C. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Archives of Internal Medicine 2008;168(2):141-6. [DOI] [PubMed] [Google Scholar]
Chung 2014 {published data only}
- Chung W W, Chua S S, Lai P S M, Chan S P. Effects of a pharmaceutical care model on medication adherence and glycemic control of people with type 2 diabetes. Patient Preference and Adherence 2014;8:1185-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chwastiak 2018 {published data only}
- Chwastiak L A, Luongo M, Russo J, Johnson L, Lowe J M, Hoffman G, et al. Use of a mental health center collaborative care team to improve diabetes care and outcomes for patients with psychosis. Psychiatric Services 2018;69(3):349-52. [DOI] [PubMed] [Google Scholar]
Ciria de Pablo 2008 {published data only}
- Ciria de Pablo C, Moreno Palanco MA, Ibáñez Sanz P, Sánchez Luis C, Pizarro Portillo A, Suárez Fernández C. Control of risk factor in diabetic patients in secondary prevention. MIRVAS Study. Revista Clinica Espanola 2008;208(3):118-23. [DOI] [PubMed] [Google Scholar]
Clancy 2003 {published data only}
- Clancy D E, Brown S B, Magruder K M, Huang P. Group visits in medically and economically disadvantaged patients with type 2 diabetes and their relationships to clinical outcomes. Topics in Health Information Management 2003;24(1):8-14. [PubMed] [Google Scholar]
Clancy 2007 {published data only}
- Clancy D E, Huang P, Okonofua E, Yeager D, Magruder K M. Group visits: promoting adherence to diabetes guidelines. Journal of General Internal Medicine 2007;22(5):620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleveringa 2008 {published data only}
- Cleveringa F G, Gorter K J, den Donk M, Rutten G E. Combined task delegation, computerized decision support, and feedback improve cardiovascular risk for type 2 diabetic patients: a cluster randomized trial in primary care. Diabetes Care 2008;31(12):2273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clifford 2002 {published data only}
- Clifford R M, Batty K T, Davis T M, Davis W, Stein G, Stewart G, et al. A randomised controlled trial of a pharmaceutical care programme in high-risk diabetic patients in an outpatient clinic. International Journal of Pharmacy Practice 2002;10:85-9. [Google Scholar]
Clifford 2005 {published data only}
- Clifford R M, Davis W A, Batty K T, Davis T M. Effect of a pharmaceutical care program on vascular risk factors in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 2005;28(4):771-6. [DOI] [PubMed] [Google Scholar]
Cohen 2011 {published data only}
- Cohen L B, Taveira T H, Khatana S A, Dooley A G, Pirraglia P A, Wu W C. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educator 2011;37(6):801-12. [DOI] [PubMed] [Google Scholar]
Cohen 2019 {published data only}
- Cohen L B, Taveira T H, Wu W C, Pirraglia P A. Pharmacist-led telehealth disease management for patients with diabetes and depression. Journal of Telemedicine & Telecare 2019;26(5):294-302. [DOI] [PubMed] [Google Scholar]
Crasto 2011 {published data only}
- Crasto W, Jarvis J, Khunti K, Skinner T C, Gray L J, Brela J, et al. Multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: the Microalbuminuria Education and Medication Optimisation (MEMO) study. Diabetes Research and Clinical Practice 2011;93(3):328-36. [DOI] [PubMed] [Google Scholar]
Crowley 2013 {published data only}
- Crowley M J, Powers B J, Olsen M K, Grubber J M, Koropchak C, Rose C M, et al. The Cholesterol, Hypertension, And Glucose Education (CHANGE) study: results from a randomized controlled trial in African Americans with diabetes. American Heart Journal 2013;166(1):179-86. [DOI] [PubMed] [Google Scholar]
Crowley 2016 {published data only}
- Crowley M J, Edelman D, McAndrew A T, Kistler S, Danus S, Webb J A, et al. Practical telemedicine for veterans with persistently poor diabetes control: a randomized pilot trial. Telemedicine and e-Health 2016;22(5):376-84. [DOI] [PubMed] [Google Scholar]
Cummings 2019 {published data only}
- Cummings D M, Lutes L D, Littlewood K, Solar C, Carraway M, Kirian K, et al. Randomized trial of a tailored cognitive behavioral intervention in type 2 diabetes with comorbid depressive and/or regimen-related distress symptoms: 12-month outcomes from COMRADE. Diabetes Care 2019;42(5):841-8. [DOI] [PubMed] [Google Scholar]
D'Souza 2019 {published data only}
- D'Souza M S, Karkada S N, Labrague L J, Ali Ammouri A A. How do multi-modality strategies affect outcomes in T2D using a randomized control trial? Clinical Epidemiology and Global Health 2019;7(4):578-85. [Google Scholar]
Dai 2018 {published data only}
- Dai Limin, Huo Xiaorong, Mo Yongzhen. Application and effect evaluation on Internet + home care mobile APP in patients with type 2 diabetes in young and middle-age. Chinese Nursing Research 2018;32(20):3207-12. [Google Scholar]
Dale 2009 {published data only}
- Dale J, Caramlau I, Sturt J, Friede T, Walker R. Telephone peer-delivered intervention for diabetes motivation and support: the telecare exploratory RCT. Patient Education and Counseling 2009;75(1):91-8. [DOI] [PubMed] [Google Scholar]
Dario 2017 {published data only}
- Dario C, Toffanin R, Calcaterra F, Saccavini C, Stafylas P, Mancin S, et al. Telemonitoring of type 2 diabetes mellitus in Italy. Telemedicine and e-Health 2017;23(2):143-52. [DOI] [PubMed] [Google Scholar]
Davidson 2005 {published data only}
- Davidson M B, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. American Journal of Medicine 2005;118(4):422-5. [DOI] [PubMed] [Google Scholar]
Davis 2003 {published data only}
- Davis R M, Fowler S, Bellis K, Pockl J, Al Pakalnis V, Woldorf A. Telemedicine improves eye examination rates in individuals with diabetes: a model for eye-care delivery in underserved communities. Diabetes Care 2003;26(8):2476. [DOI] [PubMed] [Google Scholar]
Davis 2010 {published data only}
- Davis R M, Hitch A D, Salaam M M, Herman W H, Zimmer-Galler I E, Mayer-Davis E J. TeleHealth improves diabetes self-management in an underserved community: diabetes TeleCare. Diabetes Care 2010;33(8):1712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Debussche 2012 {published data only}
- Debussche X, Rollot O, Le Pommelet C, Fianu A, Le Moullec N, Regnier C, et al. Quarterly individual outpatients lifestyle counseling after initial inpatients education on type 2 diabetes: the REDIA Prev-2 randomized controlled trial in Reunion Island. Diabetes & Metabolism 2012;38(1):46-53. [DOI] [PubMed] [Google Scholar]
De Greef 2010 {published data only}
- De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. A cognitive-behavioural pedometer-based group intervention on physical activity and sedentary behaviour in individuals with type 2 diabetes. Health Education Research 2010;25(5):724-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Greef 2011 {published data only}
- De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. Increasing physical activity in Belgian type 2 diabetes patients: a three-arm randomized controlled trial. International Journal of Behavioral Medicine 2011;18(3):188-98. [DOI] [PubMed] [Google Scholar]
Del Prato 2012 {published data only}
- Del Prato S, Nicolucci A, Lovagnini-Scher A C, Turco S, Leotta S, Vespasiani G. Telecare Provides comparable efficacy to conventional self-monitored blood glucose in patients with type 2 diabetes titrating one injection of insulin glulisine-the ELEONOR study. Diabetes Technology & Therapeutics 2012;14(2):175-82. [DOI] [PubMed] [Google Scholar]
Denver 2003 {published data only}
- Denver E A, Barnard M, Woolfson R G, Earle K A. Management of uncontrolled hypertension in a nurse-led clinic compared with conventional care for patients with type 2 diabetes. Diabetes Care 2003;26:2256‐60. [DOI] [PubMed] [Google Scholar]
DePue 2013 {published data only}
- DePue J D, Dunsiger S, Seiden A D, Blume J, Rosen R K, Goldstein M G, et al. Nurse-community health worker team improves diabetes care in American Samoa: results of a randomized controlled trial. Diabetes Care 2013;36(7):1947-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vries McClintock 2016 {published data only}
- de Vries McClintock, H F, Boyle K B, Rooney K, Bogner H R. Diabetes and depression care: a randomized controlled pilot trial. American Journal of Health Behavior 2016;40(4):503-13. [DOI] [PubMed] [Google Scholar]
de Wit 2018 {published data only}
- Wit M, Rondags Smpa, Tulder M W, Snoek F J, Bosmans J E. Cost-effectiveness of the psycho-educational blended (group and online) intervention HypoAware compared with usual care for people with type 1 and insulin-treated type 2 diabetes with problematic hypoglycaemia: analyses of a cluster-randomized controlled trial. Diabetic Medicine 2018;35(2):214-22. [DOI] [PubMed] [Google Scholar]
Dickinson 2014 {published data only}
- Dickinson W P, Dickinson L M, Nutting P A, Emsermann C B, Tutt B, Crabtree B F, et al. Practice facilitation to improve diabetes care in primary care: a report from the EPIC randomized clinical trial. Annals of Family Medicine 2014;12(1):8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dijkstra 2005 {published data only}
- Dijkstra R F, Braspenning J C, Huijsmans Z, Akkermans R P, Ballegooie E, ten Have P, et al. Introduction of diabetes passports involving both patients and professionals to improve hospital outpatient diabetes care. Diabetes Research and Clinical Practice 2005;68(2):126-34. [DOI] [PubMed] [Google Scholar]
Dijkstra 2008 {published data only}
- Dijkstra R, Braspenning J, Grol R. Implementing diabetes passports to focus practice reorganization on improving diabetes care. International Journal for Quality in Health Care 2008;20(1):72-7. [DOI] [PubMed] [Google Scholar]
Dinneen 2013 {published data only}
- Dinneen S F, O'Hara M C, Byrne M, Smith D, Courtney C H, McGurk C, et al. Group follow-up compared to individual clinic visits after structured education for type 1 diabetes: a cluster randomised controlled trial. Diabetes Research and Clinical Practice 2013;100(1):29-38. [DOI] [PubMed] [Google Scholar]
Döbler 2018 {published data only}
- Döbler A, Herbeck Belnap B, Pollmann H, Farin E, Raspe H, Mittag O. Telephone-delivered lifestyle support with action planning and motivational interviewing techniques to improve rehabilitation outcomes. Rehabilitation Psychology 2018;63(2):170-81. [DOI] [PubMed] [Google Scholar]
Dobson 2018 {published data only}
- Dobson R, Whittaker R, Jiang Y, Maddison R, Shepherd M, McNamara C, et al. Effectiveness of text message based, diabetes self management support programme (SMS4BG): two arm, parallel randomised controlled trial. BMJ 2018;361:k1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donohoe 2000 {published data only}
- Donohoe M E, Fletton J A, Hook A, Powell R, Robinson I, Stead J W, et al. Improving foot care for people with diabetes mellitus--a randomized controlled trial of an integrated care approach. Diabetic Medicine 2000;17(8):581-7. [DOI] [PubMed] [Google Scholar]
Doucette 2009 {published data only}
- Doucette W R, Witry M J, Farris K B, McDonough R P. Community pharmacist-provided extended diabetes care. Annals of Pharmacotherapy 2009;43(5):882-9. [DOI] [PubMed] [Google Scholar]
Duran 2008 {published data only}
- Duran A, Runkle I, Matia P, Miguel M P, Garrido S, Cervera E, et al. Family physician and endocrinologist coordination as the basis for diabetes care in clinical practice. BMC Endocrine Disorders 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eakin 2013 {published data only}
- Eakin E G, Reeves M M, Winkler E, Healy G N, Dunstan D W, Owen N, et al. Six-month outcomes from living well with diabetes: a randomized trial of a telephone-delivered weight loss and physical activity intervention to improve glycemic control. Annals of Behavioral Medicine 2013;46(2):193-203. [DOI] [PubMed] [Google Scholar]
Earle 2010 {published data only}
- Earle K A, Istepanian R S, Zitouni K, Sungoor A, Tang B. Mobile telemonitoring for achieving tighter targets of blood pressure control in patients with complicated diabetes: a pilot study. Diabetes Technology & Therapeutics 2010;12(7):575-9. [DOI] [PubMed] [Google Scholar]
- Istepanian R S, Zitouni K, Harry D, Moutosammy N, Sungoor A, Tang B, et al. Evaluation of a mobile phone telemonitoring system for glycaemic control in patients with diabetes. Journal of Telemedicine and Telecare 2009;15(3):125-8. [DOI] [PubMed] [Google Scholar]
Eccles 2007 {published data only}
- Eccles MP, Whitty PM, Speed C, Steen IN, Vanoli A, Hawthorne GC, et al. A pragmatic cluster randomised controlled trial of a Diabetes REcall And Management system: the DREAM trial. Implementation Science 2007;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edelman 2015 {published data only}
- Edelman D, Dolor RJ, Coffman CJ, Pereira KC, Granger BB, Lindquist JH, et al. Nurse-led behavioral management of diabetes and hypertension in community practices: a randomized trial. Journal of General Internal Medicine 2015;30(5):626-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egede 2017 {published data only}
- Egede L E, Williams J S, Voronca D C, Knapp R G, Fernandes J K. Randomized controlled trial of technology-assisted case management in low income adults with type 2 diabetes. Diabetes Technology & Therapeutics 2017;19(8):476-82. [DOI] [PubMed] [Google Scholar]
Ell 2010 {published data only}
- Ell K, Katon W, Xie B, Lee P J, Kapetanovic S, Guterman J, et al. Collaborative care management of major depression among low-income, predominantly Hispanic subjects with diabetes: a randomized controlled trial. Diabetes Care 2010;33(4):706-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emerson 2016 {published data only}
- Emerson JF, Welch M, Rossman WE, Carek S, Ludden T, Templin M, Moore CG, et al. A multidisciplinary intervention utilizing virtual communication tools to reduce health disparities: a pilot randomized controlled trial. International Journal of Environmental Research and Public Health 2016;13(1):ijerph13010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esmatjes 2014 {published data only}
- Esmatjes E, Jansà M, Roca D, Pérez-Ferre N, Valle L, Martínez-Hervás S, Ruiz de Adana M, et al, Sola-Morales O Telemed-Diabetes Group. The efficiency of telemedicine to optimize metabolic control in patients with type 1 diabetes mellitus: Telemed study. Diabetes Technology & Therapeutics 2014;16:435. [DOI] [PubMed] [Google Scholar]
Estrada 2011 {published data only}
- Billue KL, Safford MM, Salanitro AH, Houston TK, Curry W, Kim Y, et al. Medication intensification in diabetes in rural primary care: a cluster-randomised effectiveness trial. BMJ Open 2012;2(5):e000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada C A, Safford M M, Salanitro A H, Houston T K, Curry W, Williams J H, et al. A web-based diabetes intervention for physician: a cluster-randomized effectiveness trial. International Journal for Quality in Health Care 2011;23(6):682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fairall 2016 {published data only}
- Fairall L R, Folb N, Timmerman V, Lombard C, Steyn K, Bachmann M O, et al. Educational outreach with an integrated clinical tool for nurse-led non-communicable chronic disease management in primary care in South Africa: a pragmatic cluster randomised controlled trial. PLoS Medicine 2016;13(11):e1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faridi 2008 {published data only}
- Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz D L. Evaluating the impact of mobile telephone technology on type 2 diabetic patients' self-management: the NICHE pilot study. Journal of Evaluation in Clinical Practice 2008;14(3):465-9. [DOI] [PubMed] [Google Scholar]
Farmer 2005 {published data only}
- Farmer A J, Gibson O J, Dudley C, Bryden K, Hayton P M, Tarassenko L, et al. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes (ISRCTN 46889446). Diabetes Care 2005;28(11):2697-702. [DOI] [PubMed] [Google Scholar]
Farmer 2007 {published data only}
- Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ 2007;335(7611):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farsaei 2011 {published data only}
- Farsaei S, Sabzghabaee A M, Zargarzadeh A H, Amini M. Effect of pharmacist-led patient education on glycemic control of type 2 diabetics: a randomized controlled trial. Journal of Research in Medical Sciences 2011;16(1):43-9. [PMC free article] [PubMed] [Google Scholar]
Fernandes 2018 {published data only}
- Fernandes R, Chinn C C, Li D, Frankland T B, Wang C M, Smith M D, et al. A randomized controlled trial of financial incentives for medicaid beneficiaries with diabetes. The Permanente Journal 2018;22:17–080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiscella 2010 {published data only}
- Fiscella K, Volpe E, Winters P, Brown M, Idris A, Harren T. A novel approach to quality improvement in a safety-net practice: concurrent peer review visits. Journal of the National Medical Association 2010;102(12):1231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 2012 {published data only}
- Fischer H H, Eisert S L, Everhart R M, Durfee M J, Moore S L, Soria S, et al. Nurse-run, telephone-based outreach to improve lipids in people with diabetes. American Journal of Managed Care 2012;18(2):77-84. [PubMed] [Google Scholar]
Fogelfeld 2017 {published data only}
- Fogelfeld L, Hart P, Miernik J, Ko J, Calvin D, Tahsin B, et al. Combined diabetes-renal multifactorial intervention in patients with advanced diabetic nephropathy: Proof-of-concept. Journal of Diabetes and its Complications 2017;31(3):624-30. [DOI] [PubMed] [Google Scholar]
Fornos 2006 {published data only}
- Fornos J A, Andres N F, Andres J C, Guerra M M, Egea B. A pharmacotherapy follow-up program in patients with type-2 diabetes in community pharmacies in Spain. Pharmacy World & Science 2006;28(2):65-72. [DOI] [PubMed] [Google Scholar]
Fortmann 2017 {published data only}
- Fortmann A L, Gallo L C, Garcia M I, Taleb M, Euyoque J A, Clark T, et al. Dulce Digital: an mHealth SMS- based intervention improves glycemic control in hispanics with type 2 diabetes. Diabetes Care 2017;40(10):1349-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2013 {published data only}
- Foster G D, Wadden T A, LaGrotte C A, Vander Veur S S, Hesson L A, Homko C J, et al. A randomized comparison of a commercially available portion-controlled weight-loss intervention with a diabetes self-management education program. Nutrition and Diabetes 2013;3:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fountoulakis 2015 {published data only}
- Fountoulakis S, Papanastasiou L, Gryparis A, Markou A, Piaditis G. Impact and duration effect of telemonitoring on HbA1c, BMI and cost in insulin-treated Diabetes Mellitus patients with inadequate glycemic control: A randomized controlled study. Hormones (Athens) 2015;14(4):632-43. [DOI] [PubMed] [Google Scholar]
Franciosi 2011 {published data only}
- Franciosi M, Lucisano G, Pellegrini F, Cantarello A, Consoli A, Cucco L, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabetic Medicine 2011;28(7):789-96. [DOI] [PubMed] [Google Scholar]
Franz 1995 {published data only}
- Franz M J, Monk A, Barry B, McClain K, Weaver T, Cooper N, et al. Effectiveness of medical nutrition therapy provided by dietitians in the management of non-insulin-dependent diabetes mellitus: a randomized, controlled clinical trial. Journal of the American Dietetic Association 1995;95(9):1009-17. [DOI] [PubMed] [Google Scholar]
Frei 2014 {published data only}
- Frei A, Senn O, Chmiel C, Reissner J, Held U, Rosemann T. Implementation of the chronic care model in small medical practices improves cardiovascular risk but not glycemic control. Diabetes Care 2014;37(4):1039-47. [DOI] [PubMed] [Google Scholar]
Frias 2017 {published data only}
- Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. Journal of Medical Internet Research 2017;19(7):e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frijling 2002 {published data only}
- Frijling B D, Lobo C M, Hulscher M E, Akkermans R P, Braspenning J C, Prins A, et al. Multifaceted support to improve clinical decision making in diabetes care: a randomized controlled trial in general practice. Diabetic Medicine 2002;19(10):836-42. [DOI] [PubMed] [Google Scholar]
Frosch 2011 {published data only}
- Frosch D L, Uy V, Ochoa S, Mangione C M. Evaluation of a behavior support intervention for patients with poorly controlled diabetes. Archives of Internal Medicine 2011;171(22):2011-7. [DOI] [PubMed] [Google Scholar]
Furler 2017 {published data only}
- Furler J, O'Neal D, Speight J, Manski-Nankervis J A, Gorelik A, Holmes-Truscott E, et al. Supporting insulin initiation in type 2 diabetes in primary care: results of the Stepping Up pragmatic cluster randomised controlled clinical trial. BMJ 2017;356:j783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabbay 2006 {published data only}
- Gabbay R A, Lendel I, Saleem T M, Shaeffer G, Adelman A M, Mauger D T, et al. Nurse case management improves blood pressure, emotional distress and diabetes complication screening. Diabetes Research and Clinical Practice 2006;71(1):28-35. [DOI] [PubMed] [Google Scholar]
Gabbay 2013 {published data only}
- Gabbay R A, Anel-Tiangco R M, Dellasega C, Mauger D T, Adelman A, Van Horn D H. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): results of a 2-year randomized controlled pragmatic trial. Journal of Diabetes 2013;5(3):349-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaede 2008 {published data only}
- Gaede P, Lund-Andersen H, Parving H H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New England Journal of Medicine 2008;358(6):580-91. [DOI] [PubMed] [Google Scholar]
Gagliardino 2013a {published data only}
- Gagliardino J J, Lapertosa S, Pfirter G, Villagra M, Caporale J E, Gonzalez C D, et al. Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR). Diabetic Medicine 2013;30(9):1102-11. [DOI] [PubMed] [Google Scholar]
Gagliardino 2013b {published data only}
- Gagliardino J J Arrechea V, Assad D Gagliardino G G, lez L Lucero S, Rizzuti L Zufriategui Z. Type 2 diabetes patients educated by other patients perform at least as well as patients trained by professionals. Diabetes/Metabolism Research and Reviews 2013;29:152. [DOI] [PubMed] [Google Scholar]
Gamiochipi 2016 {published data only}
- Gamiochipi M, Cruz M, Kumate J, Wacher N H, Group Dimss Study. Effect of an intensive metabolic control lifestyle intervention in type-2 diabetes patients. Patient Education and Counseling 2016;99(7):1184-89. [DOI] [PubMed] [Google Scholar]
Garcia 2015 {published data only}
- Garcia A A, Brown S A, Horner S D, Zuniga J, Arheart K L. Home-based diabetes symptom self-management education for Mexican Americans with type 2 diabetes. Health Education Research 2015;30(3):484-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 2017 {published data only}
- Garg R, Hurwitz S, Rein R, Schuman B, Underwood P, Bhandari S. Effect of follow-up by a hospital diabetes care team on diabetes control at one year after discharge from the hospital. Diabetes Research & Clinical Practice 2017;133:78-84. [DOI] [PubMed] [Google Scholar]
Gary 2003 {published data only}
- Gary T L, Bone L R, Hill M N, Levine D M, McGuire M, Saudek C, et al. Randomized controlled trial of the effects of nurse case manager and community health worker interventions on risk factors for diabetes-related complications in urban African Americans. Preventive Medicine 2003;37(1):23-32. [DOI] [PubMed] [Google Scholar]
Gary 2009 {published data only}
- Gary T L, Batts-Turner M, Yeh H C, Hill-Briggs F, Bone L R, Wang N Y, et al. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Archives of Internal Medicine 2009;169(19):1788-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2008 {published data only}
- George J T, Valdovinos A P, Russell I, Dromgoole P, Lomax S, Torgerson D J, et al. Clinical effectiveness of a brief educational intervention in type 1 diabetes: results from the BITES (Brief Intervention in Type 1 diabetes, Education for Self-efficacy) trial. Diabetic Medicine 2008;25(12):1447-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gill 2019 {published data only}
- Gill J, Kucharski K, Turk B, Pan C, Wei W. Using electronic clinical decision support in patient-centered medical homes to improve management of diabetes in primary care: The DECIDE Study. Journal of Ambulatory Care Management 2019;42(2):105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillani 2016 {published data only}
- Gillani S W. Determining effective diabetic care; a multicentre - Longitudinal interventional study. Current Pharmaceutical Design 2016;22(42):6469-76. [DOI] [PubMed] [Google Scholar]
Gillani 2017 {published data only}
- Gillani S M R, Nevill A, Singh B M. A randomised controlled trial in diabetes demonstrating the positive impact of a patient activation strategy on diabetes processes and HbA1c: The WICKED project. British Journal of Diabetes and Vascular Disease 2017;17(2):58-63. [Google Scholar]
Ginsberg 1996 {published data only}
- Ginsberg BH. Preliminary results of a disease management program for diabetes. COM 1996;3(4):45-51. [Google Scholar]
Glasgow 1996 {published data only}
- Glasgow R E, Toobert D J, Hampson S E. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care 1996;19(8):835-42. [DOI] [PubMed] [Google Scholar]
Glasgow 2002 {published data only}
- Glasgow R E, Toobert D J, Hampson S E, Strycker L A. Implementation, generalization and long-term results of the "choosing well" diabetes self-management intervention. Patient Education and Counseling 2002;48(2):115-22. [DOI] [PubMed] [Google Scholar]
Glasgow 2005 {published data only}
- Glasgow R E, Nutting P A, King D K, Nelson C C, Cutter G, Gaglio B, et al. Randomized effectiveness trial of a computer-assisted intervention to improve diabetes care. Diabetes Care 2005;28(1):33-9. [DOI] [PubMed] [Google Scholar]
Glasgow 2012 {published data only}
- Glasgow R E, Kurz D, King D, Dickman J M, Faber A J, Halterman E, et al. Twelve-month outcomes of an Internet-based diabetes self-management support program. Patient Education and Counseling 2012;87(1):81-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Kurz D, King D, Dickman JM, Faber AJ, Halterman E, et al. Outcomes of minimal and moderate support versions of an internet-based diabetes self-management support program. Journal of General Internal Medicine 2010;25(12):1315-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goderis 2010 {published data only}
- Goderis G, Borgermans L, Grol R, Van Den Broeke C, Boland B, Verbeke G, et al. Start improving the quality of care for people with type 2 diabetes through a general practice support program: a cluster randomized trial. Diabetes Research and Clinical Practice 2010;88(1):56-64. [DOI] [PubMed] [Google Scholar]
Gold 2015 {published data only}
- Gold R, Nelson C, Cowburn S, Bunce A, Hollombe C, Davis J, et al. Feasibility and impact of implementing a private care system's diabetes quality improvement intervention in the safety net: a cluster-randomized trial. Implementation Science 2015;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2004 {published data only}
- Goldberg H I, Lessler D S, Mertens K, Eytan T A, Cheadle A D. Self-management support in a web-based medical record: a pilot randomized controlled trial. Joint Commission Journal on Quality and Safety 2004;30(11):629-35, 589. [DOI] [PubMed] [Google Scholar]
Goruntla 2019 {published data only}
- Goruntla N Mallela V Nayakanti D. Impact of pharmacist-directed counseling and message reminder services on medication adherence and clinical outcomes in type 2 diabetes mellitus. Journal of Pharmacy & Bioallied Sciences 2019;11(1):77‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2008 {published data only}
- Grant R W, Wald J S, Schnipper J L, Gandhi T K, Poon E G, Orav E J, et al. Practice-linked online personal health records for type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2008;168(16):1776-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graumlich 2016 {published data only}
- Graumlich J F, Wang H, Madison A, Wolf M S, Kaiser D, Dahal K, Morrow D G. Effects of a patient-provider, collaborative, medication-planning tool: a randomized, controlled trial. Journal of Diabetes Research 2016;2016:2129838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenfield 1988 {published data only}
- Greenfield S, Kaplan S H, Ware J E, Yano E M, Frank H J. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. Journal of General Internal Medicine 1988;3(5):448-57. [DOI] [PubMed] [Google Scholar]
Greenwood 2015 {published data only}
- Greenwood DA, Blozis SA, Young HM, Nesbitt TS, Quinn C. Overcoming clinical inertia: a randomized clinical trial of a telehealth remote monitoring intervention using paired glucose testing in adults with type 2 diabetes. Journal of Medical Internet Research 2015;17(7):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2011 {published data only}
- Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care 2011;34(10):2244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin S J, Borch-Johnsen K, Davies M J, Khunti K, Rutten G E, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 2011;378(9786):156-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbæk A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GEHM, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care 2014;37(7):2015-23. [DOI] [PubMed] [Google Scholar]
Griffin 2014 {published data only}
- Griffin S J, Simmons R K, Prevost A T, Williams K M, Hardeman W, Sutton S, et al. Multiple behaviour change intervention and outcomes in recently diagnosed type 2 diabetes: the ADDITION-Plus randomised controlled trial. Diabetologia 2014;57(7):1308-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grilo 2015 {published data only}
- Grilo S A, Shallcross A J, Ogedegbe G, Odedosu T, Levy N, Lehrer S, et al. Food insecurity and effectiveness of behavioral interventions to reduce blood pressure, New York City, 2012-2013. Preventing Chronic Disease 2015;12:E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groeneveld 2001 {published data only}
- Groeneveld Y, Petri H, Hermans J, Springer M. An assessment of structured care assistance in the management of patients with type 2 diabetes in general practice. Scandinavian Journal of Primary Health Care 2001;19(1):25-30. [DOI] [PubMed] [Google Scholar]
Gucciardi 2007 {published data only}
- Gucciardi E, Demelo M, Lee R N, Grace S L. Assessment of two culturally competent diabetes education methods: individual versus individual plus group education in Canadian Portuguese adults with type 2 diabetes. Ethnicity & Health 2007;12(2):163-87. [DOI] [PubMed] [Google Scholar]
Guirguis 2001 {published data only}
- Guirguis L, Johnson J, Farris K, Tsuyuki R, Toth E. A pilot study to evaluate the impact of pharmacists as certified diabetes educators on the clinical and humanistic outcomes of people with diabetes. Can J Diabetes Care 2001;25(4):266-76. [Google Scholar]
Guldberg 2011 {published data only}
- Guldberg T L, Vedsted P, Kristensen J K, Lauritzen T. Improved quality of Type 2 diabetes care following electronic feedback of treatment status to general practitioners: a cluster randomized controlled trial. Diabetic Medicine 2011;28(3):325-32. [DOI] [PubMed] [Google Scholar]
Gunawardena 2019 {published data only}
- Gunawardena K C, Jackson R, Robinett I, Dhaniska L, Jayamanne S, Kalpani S, et al. The influence of the smart glucose manager mobile application on diabetes management. Journal of Diabetes Science & Technology 2019;13(1):75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2014 {published data only}
- Guo X H, Ji L N, Lu J M, Liu J, Lou Q Q, Liu J, et al. Efficacy of structured education in patients with type 2 diabetes mellitus receiving insulin treatment. Journal of Diabetes 2014;6(4):290-7. [DOI] [PubMed] [Google Scholar]
Gutierrez 2011 {published data only}
- Gutierrez N, Gimple N E, Dallo F J, Foster B M, Ohagi E J. Shared medical appointments in a residency clinic: an exploratory study among Hispanics with diabetes. American Journal of Managed Care 2011;17(6 Spec No.):e212-4. [PubMed] [Google Scholar]
Halbert 1999 {published data only}
- Halbert R J, Leung K M, Nichol J M, Legorreta A P. Effect of multiple patient reminders in improving diabetic retinopathy screening. A randomized trial. Diabetes Care 1999;22(5):752-5. [DOI] [PubMed] [Google Scholar]
Hansen 2013 {published data only}
- Hansen L J, Siersma V, Beck-Nielsen H, Fine Olivarius N. Structured personal care of type 2 diabetes: a 19 year follow-up of the study Diabetes Care in General Practice (DCGP). Diabetologia 2013;56(6):1243-53. [DOI] [PubMed] [Google Scholar]
- Olivarius N F, Beck-Nielsen H, Andreasen A H, Horder M, Pedersen P A. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ 2001;323(7319):970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2017 {published data only}
- Hansen C R, Perrild H, Koefoed B G, Zander M. Video consultations as add-on to standard care among patients with type 2 diabetes not responding to standard regimens: a randomized controlled trial. European Journal of Endocrinology 2017;176(6):727-36. [DOI] [PubMed] [Google Scholar]
Hargraves 2012 {published data only}
- Hargraves J L, Ferguson W J, Lemay C A, Pernice J. Community health workers assisting patients with diabetes in self-management. Journal of Ambulatory Care Management 2012;35(1):15-26. [DOI] [PubMed] [Google Scholar]
Harno 2006 {published data only}
- Harno K, Kauppinen-Makelin R, Syrjalainen J. Managing diabetes care using an integrated regional e-health approach. Journal of Telemedicine and Telecare 2006;12 Suppl 1:13-5. [DOI] [PubMed] [Google Scholar]
Harris 2005 {published data only}
- Harris S B, Leiter L A, Webster-Bogaert S, Van D M, O'Neill C. Teleconferenced educational detailing: diabetes education for primary care physicians. Journal of Continuing Education in the Health Professions 2005;25(2):87-97. [DOI] [PubMed] [Google Scholar]
Harris 2013 {published data only}
- Harris S B, Gerstein H C, Yale J F, Berard L, Stewart J, Webster-Bogaert S, et al. Can community retail pharmacist and diabetes expert support facilitate insulin initiation by family physicians? Results of the AIM@GP randomized controlled trial. BMC Health Services Research 2013;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkins 2010 {published data only}
- Hawkins S Y. Improving glycemic control in older adults using a videophone motivational diabetes self-management intervention. Research and Theory for Nursing Practice 2010;24(4):217-32. [DOI] [PubMed] [Google Scholar]
Hayashino 2016 {published data only}
- Hayashino Y, Suzuki H, Yamazaki K, Goto A, Izumi K, Noda M. A cluster randomized trial on the effect of a multifaceted intervention improved the technical quality of diabetes care by primary care physicians: The Japan Diabetes Outcome Intervention Trial-2 (J-DOIT2). Diabetic Medicine 2016;33(5):599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 1984 {published data only}
- Hayes T M, Harries J. Randomised controlled trial of routine hospital clinic care versus routine general practice care for type II diabetics. British Medical Journal (Clin Res Ed) 1984;289(6447):728-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2018 {published data only}
- He Zhongyun. Application effect of multidisciplinary nursing model for diabetic nephropathy patients with uremia complicated with cerebral infarction. Chinese Nursing Research 2018;32(21):3406-9. [Google Scholar]
Heisler 2010 {published data only}
- Heisler M, Vijan S, Makki F, Piette J D. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Annals of Internal Medicine 2010;153(8):507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heisler 2012 {published data only}
- Heisler M, Hofer T P, Schmittdiel J A, Selby J V, Klamerus M L, Bosworth H B, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: the adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation 2012;125(23):2863-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heisler 2014 {published data only}
- Heisler M, Choi H, Palmisano G, Mase R, Richardson C, Fagerlin A, et al. Comparison of community health worker-led diabetes medication decision-making support for low-income Latino and African American adults with diabetes using e-health tools versus print materials: a randomized, controlled trial. Annals of Internal Medicine 2014;161(10 Suppl):S13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendricks 2000 {published data only}
- Hendricks L E, Hendricks R T. The effect of diabetes self-management education with frequent follow-up on the health outcomes of African American men. Diabetes Education 2000;26(6):995-1002. [DOI] [PubMed] [Google Scholar]
Hendrie 2014 {published data only}
- Hendrie D, Miller T R, Woodman R J, Hoti K, Hughes J. Cost-effectiveness of reducing glycaemic episodes through community pharmacy management of patients with type 2 diabetes mellitus. Journal of Primary Prevention 2014;35(6):439-49. [DOI] [PubMed] [Google Scholar]
Hermanns 2017 {published data only}
- Hermanns N, Ehrmann D, Schall S, Maier B, Haak T, Kulzer B. The effect of an education programme (MEDIAS 2 BSC) of non-intensive insulin treatment regimens for people with Type 2 diabetes: a randomized, multi-centre trial. Diabetic Medicine 2017;34(8):1084-91. [DOI] [PubMed] [Google Scholar]
Hermans 2013 {published data only}
- Hermans M P, Elisaf M, Michel G, Muls E, Nobels F, Vandenberghe H, et al. Benchmarking is associated with improved quality of care in type 2 diabetes: the OPTIMISE randomized, controlled trial. Diabetes Care 2013;36(11):3388-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrin 2006 {published data only}
- Herrin J, Nicewander D A, Hollander P A, Couch C E, Winter F D, Haydar Z R, et al. Effectiveness of diabetes resource nurse case management and physician profiling in a fee-for-service setting: a cluster randomized trial. Proceedings / Baylor University Medical Center 2006;19(2):95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hetlevik 2000 {published data only}
- Hetlevik I, Holmen J, Kruger O, Kristensen P, Iversen H, Furuseth K. Implementing clinical guidelines in the treatment of diabetes mellitus in general practice. Evaluation of effort, process, and patient outcome related to implementation of a computer-based decision support system. International Journal of Technology Assessment in Health Care 2000;16(1):210-27. [DOI] [PubMed] [Google Scholar]
Hiss 2001 {published data only}
- Hiss R G, Gillard M L, Armbruster B A, McClure L A. Comprehensive evaluation of community-based diabetic patients: effect of feedback to patients and their physicians: a randomized controlled trial. Diabetes Care 2001;24(4):690-4. [DOI] [PubMed] [Google Scholar]
Hiss 2007 {published data only}
- Hiss R G, Armbruster B A, Gillard M L, McClure L A. Nurse care manager collaboration with community-based physicians providing diabetes care: a randomized controlled trial. Diabetes Educator 2007;33(3):493-502. [DOI] [PubMed] [Google Scholar]
Holbrook 2009 {published data only}
- Holbrook A, Thabane L, Keshavjee K, Dolovich L, Bernstein B, Chan D, et al. Individualized electronic decision support and reminders to improve diabetes care in the community: COMPETE II randomized trial. CMAJ 2009;181(1-2):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holbrook 2011 {published data only}
- Holbrook A, Pullenayegum E, Thabane L, Troyan S, Foster G, Keshavjee K, et al. Shared electronic vascular risk decision support in primary care: Computerization of Medical Practices for the Enhancement of Therapeutic Effectiveness (COMPETE III) randomized trial. Archives of Internal Medicine 2011;171(19):1736-44. [DOI] [PubMed] [Google Scholar]
Holtrop 2017 {published data only}
- Holtrop J S, Luo Z, Piatt G, Green L A, Chen Q, Piette J. Diabetic and obese patient clinical outcomes improve during a care management implementation in primary care. Journal of Primary Care & Community Health 2017;8(4):312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoskins 1993 {published data only}
- Hoskins P L, Fowler P M, Constantino M, Forrest J, Yue D K, Turtle J R. Sharing the care of diabetic patients between hospital and general practitioners: does it work? Diabetic Medicine 1993;10(1):81-6. [DOI] [PubMed] [Google Scholar]
Hotu 2010 {published data only}
- Hotu C, Bagg W, Collins J, Harwood L, Whalley G, Doughty R, et al. A community-based model of care improves blood pressure control and delays progression of proteinuria, left ventricular hypertrophy and diastolic dysfunction in Maori and Pacific patients with type 2 diabetes and chronic kidney disease: a randomized controlled trial. Nephrology, Dialysis, Transplantation 2010;25(10):3260-6. [DOI] [PubMed] [Google Scholar]
Houweling 2009 {published data only}
- Houweling S T, Kleefstra N, Hateren K J, Kooy A, Groenier K H, Ten Vergert E, et al. Diabetes specialist nurse as main care provider for patients with type 2 diabetes. Netherlands Journal of Medicine 2009;67(7):279-84. [PubMed] [Google Scholar]
Houweling 2011 {published data only}
- Houweling S T, Kleefstra N, Hateren K J, Groenier K H, Meyboom-de Jong B, Bilo H J. Can diabetes management be safely transferred to practice nurses in a primary care setting? A randomised controlled trial. Journal of Clinical Nursing 2011;20(9-10):1264-72. [DOI] [PubMed] [Google Scholar]
Hsu 2014 {published data only}
- Hsu C C, Tai T Y. Long-term glycemic control by a diabetes case-management program and the challenges of diabetes care in Taiwan. Diabetes Research and Clinical Practice 2014;106 Suppl 2:S328. [DOI] [PubMed] [Google Scholar]
Hsu 2016 {published data only}
- Hsu W C, Lau K H, Huang R, Ghiloni S, Le H, Gilroy S, Abrahamson M, Moore J. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technology & Therapeutics 2016;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2010 {published data only}
- Huang M C, Hsu C C, Wang H S, Shin S J. Prospective randomized controlled trial to evaluate effectiveness of registered dietitian-led diabetes management on glycemic and diet control in a primary care setting in Taiwan. Diabetes Care 2010;33(2):233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huizinga 2010 {published data only}
- Huizinga M M, Gebretsadik T, Garcia Ulen C, Shintani A K, Michon S R, Shackleford L O, et al. Preventing glycaemic relapse in recently controlled type 2 diabetes patients: a randomised controlled trial. Diabetologia 2010;53(5):832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurwitz 1993 {published data only}
- Hurwitz B, Goodman C, Yudkin J. Prompting the clinical care of non-insulin dependent (type II) diabetic patients in an inner city area: one model of community care. BMJ 1993;306(6878):624-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwang 2019 {published data only}
- Hwang A S, Harding A S, Chang Y, O'Keefe S M, Horn D M, Clark A L. An audit and feedback intervention to improve internal medicine residents' performance on ambulatory quality measures: a randomized controlled trial. Population Health Management 2019;22(6):529-35. [DOI] [PubMed] [Google Scholar]
Ilag 2003 {published data only}
- Ilag L L, Martin C L, Tabaei B P, Isaman D J, Burke R, Greene D A, et al. Improving diabetes processes of care in managed care. Diabetes Care 2003;26(10):2722-7. [DOI] [PubMed] [Google Scholar]
Iljaž 2017 {published data only}
- Iljaz R, Brodnik A, Zrimec T, Cukjati I. E-healthcare for diabetes mellitus type 2 patients - a randomised controlled trial in Slovenia. Zdravstveno Varstvo 2017;56(3):150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imai 2008 {published data only}
- Imai S, Kozai H, Matsuda M, Hasegawa G, Obayashi H, Togawa C, et al. Intervention with delivery of diabetic meals improves glycemic control in patients with type 2 diabetes mellitus. Journal of Clinical Biochemistry and Nutrition 2008;42:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishani 2011 {published data only}
- Ishani A, Greer N, Taylor B C, Kubes L, Cole P, Atwood M, et al. Effect of nurse case management compared with usual care on controlling cardiovascular risk factors in patients with diabetes: a randomized controlled trial. Diabetes Care 2011;34(8):1689-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2018 {published data only}
- Islam N S, Wyatt L C, Taher M D, Riley L, Tandon S D, Tanner M, et al. A culturally tailored community health worker intervention leads to improvement in patient-centered outcomes for immigrant patients with type 2 diabetes. CD (Clinical Diabetes) 2018;36(2):100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 2013 {published data only}
- Ismail M, Teng C L, Omar M, Ho B K, Kusiar Z, Hasim R. Usage of glucometer is associated with improved glycaemic control in type 2 diabetes mellitus patients in Malaysian public primary care clinics: an open-label, randomised controlled trial. Singapore Medical Journal 2013;54(7):391-5. [DOI] [PubMed] [Google Scholar]
Ivers 2013 {published data only}
- Ivers N M, Tu K, Young J, Francis J J, Barnsley J, Shah B R, et al. Feedback GAP: pragmatic, cluster-randomized trial of goal setting and action plans to increase the effectiveness of audit and feedback interventions in primary care. Implementation Science 2013;8:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaber 1996 {published data only}
- Jaber L A, Halapy H, Fernet M, Tummalapalli S, Diwakaran H. Evaluation of a pharmaceutical care model on diabetes management. Annals of Pharmacotherapy 1996;30(3):238-43. [DOI] [PubMed] [Google Scholar]
Jackson 2013 {published data only}
- Edelman D, Fredrickson S K, Melnyk S D, Coffman C J, Jeffreys A S, Datta S, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: a randomized trial. Ann Intern Med 2010;152(11):689-96. [DOI] [PubMed] [Google Scholar]
- Jackson GL, Edelman D, Olsen MK, Smith VA, Maciejewski ML. Benefits of participation in diabetes group visits after trial completion. JAMA Internal Medicine 2013;173(7):590‐2. [DOI] [PubMed] [Google Scholar]
Jacobs 2012 {published data only}
- Jacobs M, Sherry P S, Taylor L M, Amato M, Tataronis G R, Cushing G. Pharmacist Assisted Medication Program Enhancing the Regulation of Diabetes (PAMPERED) study. Journal of the American Pharmacists Association 2012;52(5):613-21. [DOI] [PubMed] [Google Scholar]
Jahangard‐Rafsanjani 2015 {published data only}
- Jahangard-Rafsanjani Z, Sarayani A, Nosrati M, Saadat N, Rashidian A, Hadjibabaie M, et al. Effect of a community pharmacist-delivered diabetes support program for patients receiving specialty medical care: a randomized controlled trial. Diabetes Educator 2015;41(1):127-35. [DOI] [PubMed] [Google Scholar]
Jain 2018 {published data only}
- Jain V, Joshi R, Idiculla J, Xavier D. Community health worker interventions in type 2 diabetes mellitus patients: assessing the feasibility and effectiveness in Rural Central India. Journal of Cardiovascular Disease Research 2018;9(3):127-33. [Google Scholar]
Jakobsson 2015 {published data only}
- Jakobsson S, Huber D, Björklund F, Mooe T. Implementation of a new guideline in cardiovascular secondary preventive care: subanalysis of a randomized controlled trial. BMC Cardiovascular Disorders 2016;16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson S, Irewall A L, Bjorklund F, Mooe T. Cardiovascular secondary prevention in high-risk patients: a randomized controlled trial sub-study. BMC Cardiovascular Disorders 2015;15:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jameson 2010 {published data only}
- Jameson J P, Baty P J. Pharmacist collaborative management of poorly controlled diabetes mellitus: a randomized controlled trial. American Journal of Managed Care 2010;16(4):250-5. [PubMed] [Google Scholar]
Jansa 2006 {published data only}
- Jansa M, Vidal M, Viaplana J, Levy I, Conget I, Gomis R, et al. Telecare in a structured therapeutic education programme addressed to patients with type 1 diabetes and poor metabolic control. Diabetes Research and Clinical Practice 2006;74(1):26-32. [DOI] [PubMed] [Google Scholar]
Jansink 2013 {published data only}
- Jansink R, Braspenning J, Keizer E, Weijden T, Elwyn G, Grol R. No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial. Scandinavian Journal of Primary Health Care 2013;31(2):119-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janssen 2009 {published data only}
- Janssen P G, Gorter K J, Stolk R P, Rutten G E. Randomised controlled trial of intensive multifactorial treatment for cardiovascular risk in patients with screen-detected type 2 diabetes: 1-year data from the ADDITION Netherlands study. British Journal of General Practice 2009;59(558):43-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarab 2012 {published data only}
- Jarab A S, Alqudah S G, Mukattash T L, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. Journal of Managed Care Pharmacy 2012;18(7):516-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeong 2018 {published data only}
- Jeong J Y, Jeon J H, Bae K H, Choi Y K, Park K G, Kim J G, et al. Smart care based on telemonitoring and telemedicine for type 2 diabetes care: multi-center randomized controlled trial. Telemedicine Journal & E-Health 2018;24(8):604-13. [DOI] [PubMed] [Google Scholar]
Ji 2019 {published data only}
- Ji H, Chen R, Huang Y, Li W, Shi C, Zhou J. Effect of simulation education and case management on glycemic control in type 2 diabetes. Diabetes/Metabolism Research and Reviews 2019;35(3):e3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2019 {published data only}
- Jiang X J, Jiang H, Lu Y H, Liu S L, Wang J P, Tang R S, Li M Z. The effectiveness of a self-efficacy-focused structured education programme on adults with type 2 diabetes: a multicentre randomized controlled trial. Journal of Clinical Nursing 2019;28(17-18):3299-309. [DOI] [PubMed] [Google Scholar]
Johansen 2007 {published data only}
- Johansen O E, Gullestad L, Blaasaas K G, Orvik E, Birkeland K I. Effects of structured hospital-based care compared with standard care for Type 2 diabetes - The Asker and Baerum Cardiovascular Diabetes Study, a randomized trial. Diabetic Medicine 2007;24(9):1019-27. [DOI] [PubMed] [Google Scholar]
Johnson 2014 {published data only}
- Johnson W, Shaya F T, Winston R, Laird A, Mullins C D, Chirikov V V, et al. Diabetes control through an educational intervention. Ethnicity & Disease 2014;24(2):182-8. [PubMed] [Google Scholar]
Joss 2004 {published data only}
- Joss N, Ferguson C, Brown C, Deighan C J, Paterson K R, Boulton-Jones J M. Intensified treatment of patients with type 2 diabetes mellitus and overt nephropathy. QJM: An International Journal of Medicine 2004;97(4):219-27. [DOI] [PubMed] [Google Scholar]
Judah 2018 {published data only}
- Judah G, Darzi A, Vlaev I, Gunn L, King D, King D, et al. Financial disincentives? A three-armed randomised controlled trial of the effect of financial Incentives in Diabetic Eye Assessment by Screening (IDEAS) trial. British Journal of Ophthalmology 2018;102(8):1014-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juul 2014 {published data only}
- Juul L, Maindal H T, Zoffmann V, Frydenberg M, Sandbaek A. Effectiveness of a training course for general practice nurses in motivation support in type 2 diabetes care: a cluster-randomised trial. PLOS One 2014;9(5):e96683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanadli 2016 {published data only}
- Aytekin Kanadli K, Ovayolu N, Ovayolu Ö. Does telephone follow-up and education affect self-care and metabolic control in diabetic patients? Holistic Nursing Practice 2016;30(2):70-7. [DOI] [PubMed] [Google Scholar]
Kangovi 2017 {published data only}
- Kangovi S, Mitra N, Grande D, Huo H, Smith R A, Long J A. Community health worker support for disadvantaged patients with multiple chronic diseases: a randomized clinical trial. American Journal of Public Health 2017;107(10):1660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karhula 2015 {published data only}
- Karhula T, Vuorinen A L, Raapysjarvi K, Pakanen M, Itkonen P, Tepponen M, et al. Telemonitoring and mobile phone-based health coaching among Finnish diabetic and heart disease patients: randomized controlled trial. Journal of Medical Internet Research 2015;17(6):e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katalenich 2015 {published data only}
- Katalenich B, Shi L, Liu S, Shao H, McDuffie R, Carpio G, et al. Evaluation of a remote monitoring system for diabetes control. Clinical Therapeutics 2015;37(6):1216-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katon 2004 {published data only}
- Katon W, Korff M, Lin E H B, Simon G, Ludman E, Russo J. The Pathways Study: a randomized controlled trial of collaborative care in patients with diabetes and depression. Archives of General Psychiatry 2004;61(10):1042-9. [DOI] [PubMed] [Google Scholar]
Katon 2010 {published data only}
- Katon W J, Lin E H, Von Korff M, Ciechanowski P, Ludman E J, Young B, et al. Collaborative care for patients with depression and chronic illnesses. New England Journal of Medicine 2010;363(27):2611-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EH, Von Korff M, Ciechanowski P, Peterson D, Ludman EJ, Rutter CM, et al. Treatment adjustment and medication adherence for complex patients with diabetes, heart disease, and depression: a randomized controlled trial. Annals of Family Medicine 2012;10(1):6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Katon WJ, Lin EH, Ciechanowski P, Peterson D, Ludman EJ, et al. Functional outcomes of multi-condition collaborative care and successful ageing: results of randomised trial. BMJ 2011;343:d6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 2015 {published data only}
- Kaur R, Kajal K S, Kaur A, Singh P. Telephonic consultation and follow-up in diabetics: impact on metabolic profile, quality of life, and patient compliance. North American Journal of Medicine and Science 2015;7(5):199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keeratiyutawong 2006 {published data only}
- Keeratiyutawong P, Hanucharurnkul S, Melkus G D E, Panpakdee O, Vorapongsathorn T. Effectiveness of a self-management program for Thais with type 2 diabetes. Thai Journal of Nursing Research 2006;10(2):85-97. [Google Scholar]
Kempf 2017 {published data only}
- Kempf K, Altpeter B, Berger J, Reus O, Fuchs M, Schneider M, et al. Efficacy of the telemedical lifestyle intervention program Telipro in advanced stages of type 2 diabetes: a randomized controlled trial. Diabetes Care 2017;40(7):863-71. [DOI] [PubMed] [Google Scholar]
Keogh 2011 {published data only}
- Keogh K M, Smith S M, White P, McGilloway S, Kelly A, Gibney J, et al. Psychological family intervention for poorly controlled type 2 diabetes. American Journal of Managed Care 2011;17(2):105-13. [PubMed] [Google Scholar]
Keyserling 2002 {published data only}
- Keyserling TC, Samuel-Hodge CD, Ammerman AS, Ainsworth BE, Henríquez-Roldán CF, Elasy TA, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabetes Care 2002;25(9):1576-83. [DOI] [PubMed] [Google Scholar]
Khan 2018 {published data only}
- Khan M A, Walley J D, Khan N, Hicks J, Ahmed M, Khan S E, et al. Effectiveness of an integrated diabetes care package at primary healthcare facilities: a cluster randomised trial in Pakistan. BJGP Open 2018;2(4):bjgpopen18X101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiefe 2001 {published data only}
- Kiefe C I, Allison J J, Williams O D, Person S D, Weaver M T, Weissman N W. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA 2001;285(22):2871-9. [DOI] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim H S, Oh J A, Lee H O. Effects of nurse-coordinated intervention on patients with type 2 diabetes in Korea. Journal of Nursing Care Quality 2005;20(2):154-60. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim M T, Han H R, Song H J, Lee J E, Kim J, Ryu J P, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educator 2009;35(6):986-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim C S, Park S Y, Kang J G, Lee S J, Ihm S H, Choi M G, et al. Insulin dose titration system in diabetes patients using a short messaging service automatically produced by a knowledge matrix. Diabetes Technology & Therapeutics 2010;12(8):663-9. [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim M T, Kim K B, Huh B, Nguyen T, Han H R, Bone L R, Levine D. The effect of a community-based self-help intervention: Korean Americans with type 2 diabetes. American Journal of Preventive Medicine 2015;49(5):726-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim H S, Sun C, Yang S J, Sun L, Li F, Choi I Y, et al. Randomized, open-label, parallel group study to evaluate the effect of internet-based glucose management system on subjects with diabetes in China. Telemedicine and e-Health 2016;22(8):666-74. [DOI] [PubMed] [Google Scholar]
Kim 2016a {published data only}
- Kim J M, Lee H J, Kim K O, Won J C, Ko K S, Rhee B D. Clinical evaluation of OneTouch Diabetes Management Software system in patients with type 2 diabetes mellitus. Diabetes & Metabolism Journal 2016;40:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinmonth 1998 {published data only}
- Kinmonth A L, Woodcock A, Griffin S, Spiegal N, Campbell M J. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes Care From Diagnosis Research Team. BMJ 1998;317(7167):1202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirk 2009 {published data only}
- Kirk A, Barnett J, Leese G, Mutrie N. A randomized trial investigating the 12-month changes in physical activity and health outcomes following a physical activity consultation delivered by a person or in written form in Type 2 diabetes: Time2Act. Diabetic Medicine 2009;26(3):293-301. [DOI] [PubMed] [Google Scholar]
Kirkman 1994 {published data only}
- Kirkman M S, Weinberger M, Landsman P B, Samsa G P, Shortliffe E A, Simel D L, et al. A telephone-delivered intervention for patients with NIDDM. Effect on coronary risk factors. Diabetes Care 1994;17(8):840-6. [DOI] [PubMed] [Google Scholar]
Kirwan 2013 {published data only}
- Kirwan M, Vandelanotte C, Fenning A, Duncan M J. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. Journal of Medical Internet Research 2013;15(11):e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirwin 2010 {published data only}
- Kirwin J L, Cunningham R J, Sequist T D. Pharmacist recommendations to improve the quality of diabetes care: a randomized controlled trial. Journal of Managed Care Pharmacy 2010;16(2):104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjeldsen 2015 {published data only}
- Kjeldsen L J, Bjerrum L, Dam P, Larsen B O, Rossing C, Sondergaard B, et al. Safe and effective use of medicines for patients with type 2 diabetes - a randomized controlled trial of two interventions delivered by local pharmacies. Research in Social & Administrative Pharmacy: RSAP 2015;11:47. [DOI] [PubMed] [Google Scholar]
Kleinman 2016 {published data only}
- Kleinman N J, Shah A, Shah S, Phatak S, Viswanathan V. Impact of the gather mHealth system on A1C: Primary results of a multisite randomized clinical trial among people with type 2 diabetes in India. Diabetes Care 2016;39(10):e169-70. [DOI] [PubMed] [Google Scholar]
- Kleinman NJ, Shah A, Shah S, Phatak S, Viswanathan V. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemedicine Journal and E-health 2017 ;23(9):733-40. [DOI] [PubMed] [Google Scholar]
Klingeman 2017 {published data only}
- Klingeman H, Funnell M, Jhand A, Lathkar-Pradhan S, Hodish I. Type 2 diabetes specialty clinic model for the accountable care organization era. Journal of Diabetes & its Complications 2017;31(10):1521-6. [DOI] [PubMed] [Google Scholar]
Kobayashi 2019 {published data only}
- Kobayashi T, Tsushita K, Nomura E, Muramoto A, Kato A, Eguchi Y, et al. Automated feedback messages with Shichifukujin characters using IOT system-improved glycemic control in people with diabetes: a prospective, multicenter randomized controlled trial. Journal of Diabetes Science & Technology 2019;13(4):796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2019 {published data only}
- Kong J X, Zhu L, Wang H M, Li Y, Guo A Y, Gao C, et al. Effectiveness of the chronic care model in type 2 diabetes management in a community health service center in china: a group randomized experimental study. Journal of Diabetes Research 2019;2019:6516581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kooiman 2018 {published data only}
- Kooiman T J M, e Groot M, Hoogenberg K, Krijnen W P, Schans C P, Kooy A. Self-tracking of physical activity in people with type 2 diabetes: a randomized controlled trial. CIN: Computers, Informatics, Nursing 2018;36(7):340-9. [DOI] [PubMed] [Google Scholar]
Korcegez 2017 {published data only}
- Korcegez E I, Sancar M, Demirkan K. Effect of a pharmacist-led program on improving outcomes in patients with type 2 diabetes mellitus from Northern Cyprus: a randomized controlled trial. Journal of Managed Care & Specialty Pharmacy 2017;23(5):573-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korhonen 1987 {published data only}
- Korhonen T, Uusitupa M, Aro A, Kumpulainen T, Siitonen O, Voutilainen E, et al. Efficacy of dietary instructions in newly diagnosed non-insulin-dependent diabetic patients. Comparison of two different patient education regimens. Acta Medica Scandinavica 1987;222(4):323-31. [DOI] [PubMed] [Google Scholar]
Kotsani 2018 {published data only}
- Kotsani K, Antonopoulou V, Kountouri A, Grammatiki M, Rapti E, Karras S, et al. The role of telenursing in the management of diabetes type 1: a randomized controlled trial. International Journal of Nursing Studies 2018;80:29-35. [DOI] [PubMed] [Google Scholar]
Kraemer 2012 {published data only}
- Kraemer D F, Kradjan W A, Bianco T M, Low J A. A randomized study to assess the impact of pharmacist counseling of employer-based health plan beneficiaries with diabetes: the EMPOWER study. Journal of Pharmacy Practice 2012;25(2):169-79. [DOI] [PubMed] [Google Scholar]
Kranker 2018 {published data only}
- Kranker K. The efficacy of using financial incentives to change unhealthy behaviors among a rural chronically ill and uninsured population. American Journal of Health Promotion 2018;32(2):301-11. [DOI] [PubMed] [Google Scholar]
Krass 2007 {published data only}
- Krass I, Armour C L, Mitchell B, Brillant M, Dienaar R, Hughes J, et al. The Pharmacy Diabetes Care Program: assessment of a community pharmacy diabetes service model in Australia. Diabetic Medicine 2007;24(6):677-83. [DOI] [PubMed] [Google Scholar]
Krein 2004 {published data only}
- Krein S L, Klamerus M L, Vijan S, Lee J L, Fitzgerald J T, Pawlow A, et al. Case management for patients with poorly controlled diabetes: a randomized trial. American Journal of Medicine 2004;116(11):732-9. [DOI] [PubMed] [Google Scholar]
Kulkarni 1998 {published data only}
- Kulkarni K, Castle G, Gregory R, Holmes A, Leontos C, Powers M, et al. Nutrition Practice Guidelines for Type 1 Diabetes Mellitus positively affect dietitian practices and patient outcomes. The Diabetes Care and Education Dietetic Practice Group. Journal of the American Dietetic Association 1998;98(1):62-70; quiz 71-2. [DOI] [PubMed] [Google Scholar]
Kulzer 2018 {published data only}
- Kulzer B, Daenschel W, Daenschel I, Schramm W, Messinger D, Weissmann J, et al. Integrated personalized diabetes management improves glycemic control in patients with insulin-treated type 2 diabetes: Results of the PDM-ProValue study program. Diabetes Research & Clinical Practice 2018;144:200-12. [DOI] [PubMed] [Google Scholar]
Kwon 2004 {published data only}
- Kwon H S, Cho J H, Kim H S, Song B R, Ko S H, Lee J M, et al. Establishment of blood glucose monitoring system using the internet. Diabetes Care 2004;27(2):478-83. [DOI] [PubMed] [Google Scholar]
Lamers 2011 {published data only}
- Lamers F, Jonkers C C, Bosma H, Knottnerus J A, Eijk J T. Treating depression in diabetes patients: does a nurse-administered minimal psychological intervention affect diabetes-specific quality of life and glycaemic control? A randomized controlled trial. Journal of Advanced Nursing 2011;67(4):788-99. [DOI] [PubMed] [Google Scholar]
Larsen 1990 {published data only}
- Larsen M L, Horder M, Mogensen E F. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. New England Journal of Medicine 1990;323(15):1021-5. [DOI] [PubMed] [Google Scholar]
Lauffenburger 2019a {published data only}
- Lauffenburger J C, Ghazinouri R, Jan S, Makanji S, Ferro C A, Lewey J, et al. Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: The ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial. PLOS One 2019;14(4):e0214754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauffenburger 2019b {published data only}
- Lauffenburger J C, Lewey J, Jan S, Makanji S, Ferro C A, Krumme A A, et al. Effectiveness of targeted insulin-adherence interventions for glycemic control using predictive analytics among patients with type 2 diabetes: a randomized clinical trial. JAMA Network Open 2019;2(3):e190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee A, Siu C F, Leung K T, Lau L C, Chan C C, Wong K K. General practice and social service partnership for better clinical outcomes, patient self efficacy and lifestyle behaviours of diabetic care: randomised control trial of a chronic care model. Postgraduate Medical Journal 2011;87(1032):688-93. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee J Y, Lee S W, Nasir N H, How S, Tan C S, Wong C P. Diabetes telemonitoring reduces the risk of hypoglycaemia during Ramadan: a pilot randomized controlled study. Diabetic Medicine 2015;32(12):1658-61. [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee S J, Song M, Im E O. Effect of a health literacy-considered diabetes self-management program for older adults in South Korea. Research in Gerontological Nursing 2017;10(5):215-25. [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee D Y, Park J, Choi D, Ahn H Y, Park S W, Park C Y. The effectiveness, reproducibility, and durability of tailored mobile coaching on diabetes management in policyholders: a randomized, controlled, open-label study. Scientific Reports 2018;8(1):3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leichter 2013 {published data only}
- Leichter S B, Bowman K, Adkins R A, Jelsovsky Z. Impact of remote management of diabetes via computer: the 360 study--a proof-of-concept randomized trial. Diabetes Technology & Therapeutics 2013;15(5):434-8. [DOI] [PubMed] [Google Scholar]
Levetan 2002 {published data only}
- Levetan C S, Dawn K R, Robbins D C, Ratner R E. Impact of computer-generated personalized goals on HbA(1c). Diabetes Care 2002;25(1):2-8. [DOI] [PubMed] [Google Scholar]
Levy 2015 {published data only}
- Levy N, Moynihan V, Nilo A, Singer K, Bernik L S, Etiebet M, et al. The Mobile Insulin Titration Intervention (MITI) for insulin adjustment in an urban, low-income population: randomized controlled trial. Journal of Medical Internet Research 2015;17(7):e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li F, Yao P, Hsue C, Xu J, Lou Q. Impact of "Conversation Maps" on diabetes distress and self-efficacy of Chinese adult patients with type 2 diabetes: a pilot study. Patient Preference and Adherence 2016;10:901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li D, Elliott T, Klein G, Ur E, Tang T S. Diabetes nurse case management in a Canadian tertiary care setting: results of a randomized controlled trial. Canadian Journal of Diabetes 2017;41(3):297-304. [DOI] [PubMed] [Google Scholar]
Lian 2013 {published data only}
- Lian J X, McGhee S M, Gangwani R A, Hedley A J, Lam C L, Yap M K, et al. Screening for diabetic retinopathy with or without a copayment in a randomized controlled trial: influence of the inverse care law. Ophthalmology 2013;120(6):1247-53. [DOI] [PubMed] [Google Scholar]
Liang 2012 {published data only}
- Liang R, Dai X, Zuojie L, Zhou A, Meijuan C. Two-year foot care program for minority patients with type 2 diabetes mellitus of Zhuang Tribe in Guangxi, China. Canadian Journal of Diabetes 2012;36:15-8. [Google Scholar]
Lim 2016 {published data only}
- Lim P C, Lim K, Embee Z C, Hassali M A, Thiagarajan A, Khan T M. Study investigating the impact of pharmacist involvement on the outcomes of diabetes medication therapy adherence program Malaysia. Pakistan Journal of Pharmaceutical Sciences 2016;29(2):595-601. [PubMed] [Google Scholar]
Lindberg 2017 {published data only}
- Lindberg I, Torbjornsen A, Soderberg S, Ribu L. Telemonitoring and health counseling for self-management support of patients with type 2 diabetes: a randomized controlled trial. JMIR Diabetes 2017;2(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litaker 2003 {published data only}
- Litaker D, Mion L, Planavsky L, Kippes C, Mehta N, Frolkis J. Physician - nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients' perception of care. Journal of Interprofessional Care 2003;17(3):223-37. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu S, Bi A, Fu D, Fu H, Luo W, Ma X, et al. Effectiveness of using group visit model to support diabetes patient self-management in rural communities of Shanghai: a randomized controlled trial. BMC Public Health 2012;12:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2019 {published data only}
- Liu J, Chen T, Wang S, Liu H. The effect of transitional care on the prevention of diabetic foot ulcers in patients at high risk for diabetic foot. International Journal of Diabetes in Developing Countries 2019;16(9):576-81. [Google Scholar]
Logan 2012 {published data only}
- Logan A G, Irvine M J, McIsaac W J, Tisler A, Rossos P G, Easty A, et al. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension 2012;60(1):51-7. [DOI] [PubMed] [Google Scholar]
Long 2012 {published data only}
- Long J A, Jahnle E C, Richardson D M, Loewenstein G, Volpp K G. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Annals of Internal Medicine 2012;156(6):416-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luley 2011 {published data only}
- Luley C, Blaik A, Reschke K, Klose S, Westphal S. Weight loss in obese patients with type 2 diabetes: effects of telemonitoring plus a diet combination - the Active Body Control (ABC) Program. Diabetes Research and Clinical Practice 2011;91(3):286-92. [DOI] [PubMed] [Google Scholar]
Lum 2018 {published data only}
- Lum Z K, See Toh W Y, Lim S M, Rusli K D B, Abdul Shakoor Sakk, Tsou K Y K, et al. Development of a collaborative algorithm for the management of type 2 diabetes during Ramadan: an anchor on empowerment. Diabetes Technology & Therapeutics 2018;20(10):698-703. [DOI] [PubMed] [Google Scholar]
Ma 2009 {published data only}
- Ma J, Berra K, Haskell W L, Klieman L, Hyde S, Smith M W, et al. Case management to reduce risk of cardiovascular disease in a county health care system. Archives of Internal Medicine 2009;169(21):1988-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maclean 2009 {published data only}
- Maclean C D, Gagnon M, Callas P, Littenberg B. The Vermont diabetes information system: a cluster randomized trial of a population based decision support system. Journal of General Internal Medicine 2009;24(12):1303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacMahon Tone 2009 {published data only}
- MacMahon Tone J, Agha A, Sherlock M, Finucane F, Tormey W, Thompson C J. An intensive nurse-led, multi-interventional clinic is more successful in achieving vascular risk reduction targets than standard diabetes care. Irish Journal of Medical Science 2009;178(2):179-86. [DOI] [PubMed] [Google Scholar]
Magee 2015 {published data only}
- Magee M F, Nassar C M, Mete M, White K, Youssef G A, Dubin J S. The synergy to enable glycemic control following emergency department discharge program for adults with type 2 diabetes: step-diabetes. Endocrine Practice 2015;21(11):1227-39. [DOI] [PubMed] [Google Scholar]
Mahwi 2013 {published data only}
- Mahwi T O Obied K A. Role of the pharmaceutical care in the management of patients with type 2 diabetes mellitus. International Journal of Pharmaceutical Sciences and Research 2013;4(4):1363-9. [Google Scholar]
Maidana 2016 {published data only}
- Maidana G M, Lugo G B, Vera Z, Perez S, Mastroianni P C. Evaluation of a program of pharmaceutical care to patients with type 2 diabetes Mellitus. [Spanish]. Pharmaceutical Care España 2016;18(1):3-15. [Google Scholar]
Maljanian 2005 {published data only}
- Maljanian R, Grey N, Staff I, Conroy L. Intensive telephone follow-up to a hospital-based disease management model for patients with diabetes mellitus. Disease Management 2005;8(1):15-25. [DOI] [PubMed] [Google Scholar]
Mansberger 2015 {published data only}
- Mansberger S L, Sheppler C, Barker G, Gardiner S K, Demirel S, Wooten K, et al. Long-term comparative effectiveness of telemedicine in providing diabetic retinopathy screening examinations: a randomized clinical trial. JAMA Ophthalmology 2015;133(5):518-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansberger SL, Gleitsmann K, Gardiner S, Sheppler C, Demirel S, Wooten K, Becker TM. Comparing the effectiveness of telemedicine and traditional surveillance in providing diabetic retinopathy screening examinations: a randomized controlled trial. Telemedicine Journal and E-health 2013;19(12):942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzaglia 2016 {published data only}
- Mazzaglia G, Piccinni C, Filippi A, Sini G, Lapi F, Sessa E, et al. Effects of a computerized decision support system in improving pharmacological management in high-risk cardiovascular patients: a cluster-randomized open-label controlled trial. Health Informatics Journal 2016;22(2):232-47. [DOI] [PubMed] [Google Scholar]
McCarrier 2009 {published data only}
- McCarrier K P, Ralston J D, Hirsch I B, Lewis G, Martin D P, Zimmerman F J, et al. Web-based collaborative care for type 1 diabetes: a pilot randomized trial. Diabetes Technology & Therapeutics 2009;11(4):211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McClellan 2003 {published data only}
- McClellan W M, Millman L, Presley R, Couzins J, Flanders W D. Improved diabetes care by primary care physicians: results of a group-randomized evaluation of the Medicare Health Care Quality Improvement Program (HCQIP). Journal of Clinical Epidemiology 2003;56(12):1210-7. [DOI] [PubMed] [Google Scholar]
McDermott 2001 {published data only}
- McDermott R A, Schmidt B A, Sinha A, Mills P. Improving diabetes care in the primary healthcare setting: a randomised cluster trial in remote Indigenous communities. Medical Journal of Australia 2001;174(10):497-502. [DOI] [PubMed] [Google Scholar]
McDermott 2015 {published data only}
- McDermott R A, Schmidt B, Preece C, Owens V, Taylor S, Li M, Esterman A. Community health workers improve diabetes care in remote Australian Indigenous communities: results of a pragmatic cluster randomized controlled trial. BMC Health Services Research 2015;15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2002 {published data only}
- McKay H, Glasgow R E, Feil E G, Boles S M, Barrera Jr M. Internet-based diabetes self-management and support: initial outcomes from the diabetes network project. Rehabilitation Psychology 2002;47(1):31-48. [Google Scholar]
McLean 2008 {published data only}
- McLean D L, McAlister F A, Johnson J A, King K M, Makowsky M J, Jones C A, et al. A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: study of cardiovascular risk intervention by pharmacists-hypertension (SCRIP-HTN). Archives of Internal Medicine 2008;168(21):2355-61. [DOI] [PubMed] [Google Scholar]
McMahon 2005 {published data only}
- McMahon G T, Gomes H E, Hickson Hohne S, Hu T M, Levine B A, Conlin P R. Web-based care management in patients with poorly controlled diabetes. Diabetes Care 2005;28(7):1624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMahon 2012 {published data only}
- McMahon G T, Fonda S J, Gomes H E, Alexis G, Conlin P R. A randomized comparison of online- and telephone-based care management with internet training alone in adult patients with poorly controlled type 2 diabetes. Diabetes Technology & Therapeutics 2012;14(11):1060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMurray 2002 {published data only}
- McMurray S D, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. American Journal of Kidney Diseases 2002;40(3):566-75. [DOI] [PubMed] [Google Scholar]
Medi‐Cal Group 2004 {published data only}
- Group California Medi-Cal Type 2 Diabetes Study. Closing the gap: effect of diabetes case management on glycemic control among low-income ethnic minority populations: the California Medi-Cal type 2 diabetes study. Diabetes Care 2004;27(1):95-103. [DOI] [PubMed] [Google Scholar]
Mehuys 2011 {published data only}
- Mehuys E, Van Bortel L, De Bolle L, Van Tongelen I, Annemans L, Remon J P, et al. Effectiveness of a community pharmacist intervention in diabetes care: a randomized controlled trial. Journal of Clinical Pharmacy and Therapeutics 2011;36(5):602-13. [DOI] [PubMed] [Google Scholar]
Meigs 2003 {published data only}
- Meigs J B, Cagliero E, Dubey A, Murphy-Sheehy P, Gildesgame C, Chueh H, et al. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care 2003;26(3):750-7. [DOI] [PubMed] [Google Scholar]
Menard 2005 {published data only}
- Menard J, Payette H, Baillargeon J P, Maheux P, Lepage S, Tessier D, et al. Efficacy of intensive multitherapy for patients with type 2 diabetes mellitus: a randomized controlled trial. CMAJ 2005;173(12):1457-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard J, Payette H, Dubuc N, Baillargeon JP, Maheux P, Ardilouze JL. Quality of life in type 2 diabetes patients under intensive multitherapy. Diabetes & Metabolism 2007;33(1):54-60. [DOI] [PubMed] [Google Scholar]
Miranda 2019 {published data only}
- Miranda J J, Lazo-Porras M, Bernabe-Ortiz A, Pesantes M A, Diez-Canseco F, Cornejo S D P, et al. The effect of individual and mixed rewards on diabetes management: a feasibility randomized controlled trial. Wellcome Open Research 2019;3:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moattari 2012 {published data only}
- Moattari M, Ghobadi A, Beigi P, Pishdad G. Impact of self management on metabolic control indicators of diabetes patients. Journal of Diabetes & Metabolic Disorders 2012;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moattari 2013 {published data only}
- Moattari M, Hashemi M, Dabbaghmanesh M H. The impact of electronic education on metabolic control indicators in patients with diabetes who need insulin: a randomised clinical control trial. Journal of Clinical Nursing 2013;22(1-2):32-8. [DOI] [PubMed] [Google Scholar]
Mons 2013 {published data only}
- Mons U, Raum E, Kramer H U, Ruter G, Rothenbacher D, Rosemann T, et al. Effectiveness of a supportive telephone counseling intervention in type 2 diabetes patients: randomized controlled study. PLOS One 2013;8(10):e77954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montori 2004 {published data only}
- Montori V M, Helgemoe P K, Guyatt G H, Dean D S, Leung T W, Smith S A, et al. Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care 2004;27(5):1088-94. [DOI] [PubMed] [Google Scholar]
Moreira 2015 {published data only}
- Moreira R C, Mantovani M F, Soriano J V. Nursing case management and glycemic control among Brazilians with type 2 diabetes: pragmatic clinical trial. Nursing Research 2015;64(4):272-8. [DOI] [PubMed] [Google Scholar]
Morgan 2013 {published data only}
- Morgan M A J, Coates M J, Dunbar J A, Reddy P, Schlicht K, Fuller J. The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial. BMJ Open 2013;3(1): e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moriyama 2009 {published data only}
- Moriyama M, Nakano M, Kuroe Y, Nin K, Niitani M, Nakaya T. Efficacy of a self-management education program for people with type 2 diabetes: results of a 12 month trial. Japan Journal of Nursing Science 2009;6(1):51-63. [DOI] [PubMed] [Google Scholar]
Mourão 2013 {published data only}
- Mourão AO, Ferreira WR, Martins MA, Reis AM, Carrillo MR, Guimarães AG, et al. Pharmaceutical care program for type 2 diabetes patients in Brazil: a randomised controlled trial. International Journal of Clinical Pharmacy 2013;35(1):79-86. [DOI] [PubMed] [Google Scholar]
Mulrow 1987 {published data only}
- Mulrow C, Bailey S, Sönksen PH, Slavin B. Evaluation of an Audiovisual Diabetes Education Program: negative results of a randomized trial of patients with non-insulin-dependent diabetes mellitus. Journal of General Internal Medicine 1987;2(4):215-9. [DOI] [PubMed] [Google Scholar]
Munch 2019 {published data only}
- Munch L, Bennich B B, Overgaard D, Konradsen H, Middelfart H, Kaarsberg N, et al. Management of people with Type 2 diabetes shared between a specialized outpatient clinic and primary health care is noninferior to management in a specialized outpatient clinic: a randomized, noninferiority trial. Diabetic Medicine 2019;36(7):854-61. [DOI] [PubMed] [Google Scholar]
Munshi 2013 {published data only}
- Munshi M N, Segal A R, Suhl E, Ryan C, Sternthal A, Giusti J, et al. Assessment of barriers to improve diabetes management in older adults: a randomized controlled study. Diabetes Care 2013;36(3):543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musacchio 2018 {published data only}
- Musacchio N, Ciullo I, Scardapane M, Giancaterini A, Pessina L, Maino S, et al. Efficacy of self-monitoring blood glucose as a key component of a chronic care model versus usual care in type 2 diabetes patients treated with oral agents: results of a randomized trial. Acta Diabetologica 2018;55(3):295-9. [DOI] [PubMed] [Google Scholar]
Nagrebetsky 2013 {published data only}
- Nagrebetsky A, Larsen M, Craven A, Turner J, McRobert N, Murray E, et al. Stepwise self-titration of oral glucose-lowering medication using a mobile telephone-based telehealth platform in type 2 diabetes: a feasibility trial in primary care. Journal of Diabetes Science and Technology 2013;7(1):123-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naik 2011 {published data only}
- Naik A D, Palmer N, Petersen N J, Street R L Jr, Rao R, Suarez-Almazor M, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Archives of Internal Medicine 2011;171(5):453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naji 1994 {published data only}
- Naji S, Team Diabetes Integrated Care Evaluation. Integrated care for diabetes: clinical, psychosocial, and economic evaluation. BMJ 1994;308(6938):1208-12. [PMC free article] [PubMed] [Google Scholar]
Nesari 2010 {published data only}
- Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Japan Journal of Nursing Science 2010;7(2):121-8. [DOI] [PubMed] [Google Scholar]
Neto 2011 {published data only}
- Neto P R, Marusic S, Lyra Junior D P, Pilger D, Cruciol-Souza J M, Gaeti W P, et al. Effect of a 36-month pharmaceutical care program on the coronary heart disease risk in elderly diabetic and hypertensive patients. Journal of Pharmacy & Pharmaceutical Sciences 2011;14(2):249-63. [DOI] [PubMed] [Google Scholar]
New 2003 {published data only}
- New J P, Mason J M, Freemantle N, Teasdale S, Wong L M, Bruce N J, et al. Specialist nurse-led intervention to treat and control hypertension and hyperlipidemia in diabetes (SPLINT): a randomized controlled trial. Diabetes Care 2003;26(8):2250-5. [DOI] [PubMed] [Google Scholar]
New 2004 {published data only}
- New J P, Mason J M, Freemantle N, Teasdale S, Wong L, Bruce N J, et al. Educational outreach in diabetes to encourage practice nurses to use primary care hypertension and hyperlipidaemia guidelines (EDEN): a randomized controlled trial. Diabetic Medicine 2004;21(6):599-603. [DOI] [PubMed] [Google Scholar]
Newman 2009 {published data only}
- Newman S P, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al. A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE). Health Technology Assessment 2009;13(28):iii-iv, ix-xi, 1-194. [DOI] [PubMed] [Google Scholar]
Nicolucci 2015 {published data only}
- Nicolucci A, Cercone S, Chiriatti A, Muscas F, Gensini G. A randomized trial on home telemonitoring for the management of metabolic and cardiovascular risk in patients with type 2 diabetes. Diabetes Technology & Therapeutics 2015;17(8):563-70. [DOI] [PubMed] [Google Scholar]
Nishita 2012 {published data only}
- Nishita C, Cardazone G, Uehara D L, Tom T. Empowered diabetes management: life coaching and pharmacist counseling for employed adults with diabetes. Health Education & Behavior 2012;40(5):581-91. [DOI] [PubMed] [Google Scholar]
Noto 2016 {published data only}
- Noto H, Tanizawa Y, Aizawa T, Sone H, Yoshioka N, Terauchi Y, et al. Cluster-randomized trial to improve the quality of diabetes management: the study for the efficacy assessment of the standard diabetes manual (SEAS-DM). Journal of Diabetes Investigation 2016;7(4):539-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2005 {published data only}
- O'Connor P J, Desai J, Solberg L I, Reger L A, Crain A L, Asche S E, et al. Randomized trial of quality improvement intervention to improve diabetes care in primary care settings. Diabetes Care 2005;28(8):1890-7. [DOI] [PubMed] [Google Scholar]
O'Connor 2009a {published data only}
- O'Connor P J, Sperl-Hillen J, Johnson P E, Rush W A, Crain A L. Customized feedback to patients and providers failed to improve safety or quality of diabetes care: a randomized trial. Diabetes Care 2009;32(7):1158-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2009b {published data only}
- O'Connor P J, Sperl-Hillen J M, Johnson P E, Rush W A, Asche S E, Dutta P, Biltz G R. Simulated physician learning intervention to improve safety and quality of diabetes care: a randomized trial. Diabetes Care 2009;32:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2011 {published data only}
- O'Connor P J, Sperl-Hillen J M, Rush W A, Johnson P E, Amundson G H, Asche S E, et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Annals of Family Medicine 2011;9(1):12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Hare 2004 {published data only}
- O'Hare J P, Raymond N T, Mughal S, Dodd L, Hanif W, Ahmad Y, et al. Evaluation of delivery of enhanced diabetes care to patients of South Asian ethnicity: the United Kingdom Asian Diabetes Study (UKADS). Diabetic Medicine 2004;21(12):1357-65. [DOI] [PubMed] [Google Scholar]
Obreli‐Neto 2015 {published data only}
- Obreli-Neto P R, Marusic S, Guidoni C M, Baldoni A O, Renovato R D, Pilger D, et al. Economic evaluation of a pharmaceutical care program for elderly diabetic and hypertensive patients in primary health care: a 36-month randomized controlled clinical trial. Journal of Managed Care & Specialty Pharmacy 2015;21(1):66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Odegard 2005 {published data only}
- Odegard P S, Goo A, Hummel J, Williams K L, Gray S L. Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention. Annals of Pharmacotherapy 2005;39(3):433-40. [DOI] [PubMed] [Google Scholar]
Odnoletkova 2016 {published data only}
- Odnoletkova I, Goderis G, Nobels F, Fieuws S, Aertgeerts B, Annemans L, et al. Optimizing diabetes control in people with Type 2 diabetes through nurse-led telecoaching. Diabetic Medicine 2016;33(6):777-85. [DOI] [PubMed] [Google Scholar]
Olry de Labry Lima 2017 {published data only}
- Olry de Labry Lima A, Bermúdez Tamayo C, Pastor Moreno G, Bolívar Muñoz J, Ruiz Pérez I, Johri M, et al. Effectiveness of an intervention to improve diabetes self-management on clinical outcomes in patients with low educational level. Gaceta Sanitaria 2017;31(1):40-7. [DOI] [PubMed] [Google Scholar]
Orsama 2013 {published data only}
- Orsama A L, Lahteenmaki J, Harno K, Kulju M, Wintergerst E, Schachner H, et al. Active assistance technology reduces glycosylated hemoglobin and weight in individuals with type 2 diabetes: results of a theory-based randomized trial. Diabetes Technology & Therapeutics 2013;15(8):662-9. [DOI] [PubMed] [Google Scholar]
Oude Wesselink 2015 {published data only}
- Oude Wesselink S F, Lingsma H F, Ketelaars C A J, Mackenbach J P, Robben P B M. Effects of government supervision on quality of integrated diabetes care: a cluster randomized controlled trial. Medical Care 2015;53(9):784-91. [DOI] [PubMed] [Google Scholar]
Pacaud 2012 {published data only}
- Pacaud D, Kelley H, Downey AM, Chiasson M. Successful delivery of diabetes self-care education and follow-up through eHealth media. Canadian Journal of Diabetes 2012;36:257-62. [Google Scholar]
Pape 2011 {published data only}
- Pape G A, Hunt J S, Butler K L, Siemienczuk J, LeBlanc B H, Gillanders W, et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Archives of Internal Medicine 2011;171(16):1480-6. [DOI] [PubMed] [Google Scholar]
Parsons 2019 {published data only}
- Parsons S N, Luzio S D, Harvey J N, Bain S C, Cheung W Y, Watkins A, et al. Effect of structured self-monitoring of blood glucose, with and without additional TeleCare support, on overall glycaemic control in non-insulin treated Type 2 diabetes: the SMBG Study, a 12-month randomized controlled trial. Diabetic Medicine 2019;36(5):578-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patja 2012 {published data only}
- Patja K, Absetz P, Auvinen A, Tokola K, Kyto J, Oksman E, et al. Health coaching by telephony to support self-care in chronic diseases: clinical outcomes from The TERVA randomized controlled trial. BMC Health Services Research 2012;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Escamilla 2015 {published data only}
- Perez-Escamilla R, Damio G, Chhabra J, Fernandez M L, Segura-Perez S, Vega-Lopez S, et al. Impact of a community health workers-led structured program on blood glucose control among latinos with type 2 diabetes: the DIALBEST trial. Diabetes Care 2015;38(2):197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perria 2007 {published data only}
- Perria C, Mandolini D, Guerrera C, Jefferson T, Billi P, Calzini V, et al. Implementing a guideline for the treatment of type 2 diabetics: results of a cluster-randomized controlled trial (C-RCT). BMC Health Services Research 2007;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 1997 {published data only}
- Perry T L, Mann J I, Lewis-Barned N J, Duncan A W, Waldron M A, Thompson C. Lifestyle intervention in people with insulin-dependent diabetes mellitus (IDDM). European Journal of Clinical Nutrition 1997;51(11):757-63. [DOI] [PubMed] [Google Scholar]
Persell 2008 {published data only}
- Persell S D, Denecke-Dattalo T A, Dunham D P, Baker D W. Patient-directed intervention versus clinician reminders alone to improve aspirin use in diabetes: a cluster randomized trial. Joint Commission Journal on Quality and Patient Safety 2008;34(2):98-105. [DOI] [PubMed] [Google Scholar]
Peters 1991 {published data only}
- Peters A R A, Rübsamen M, Jacob U, Look D, Scriba P C. Clinical evaluation of decision support system for insulin-dose adjustment in IDDM. Diabetes Care 1991;14(10):875-80. [DOI] [PubMed] [Google Scholar]
Peterson 2008 {published data only}
- Peterson K A, Radosevich D M, O'Connor P J, Nyman J A, Prineas R J, Smith S A, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care 2008;31(12):2238-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philis‐Tsimikas 2011 {published data only}
- Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, Walker C, Gallo L C. Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial. Diabetes Care 2011;34(9):1926-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2005 {published data only}
- Phillips L S, Ziemer D C, Doyle J P, Barnes C S, Kolm P, Branch W T, et al. An endocrinologist-supported intervention aimed at providers improves diabetes management in a primary care site: improving primary care of African Americans with diabetes (IPCAAD) 7. Diabetes Care 2005;28(10):2352-60. [DOI] [PubMed] [Google Scholar]
Phumipamorn 2008 {published data only}
- Phumipamorn S, Pongwecharak J, Soorapan S, Pattharachayakul S. Effects of the pharmacist's input on glycaemic control and cardiovascular risks in Muslim diabetes. Primary Care Diabetes 2008;2(1):31-7. [DOI] [PubMed] [Google Scholar]
Piatt 2010 {published data only}
- Piatt G A, Anderson R M, Brooks M M, Songer T, Siminerio L M, Korytkowski M M, et al. 3-year follow-up of clinical and behavioral improvements following a multifaceted diabetes care intervention: results of a randomized controlled trial. Diabetes Educator 2010;36(2):301-9. [DOI] [PubMed] [Google Scholar]
- Piatt G A, Orchard T J, Emerson S, Simmons D, Songer T J, Brooks M M, et al. Translating the chronic care model into the community: results from a randomized controlled trial of a multifaceted diabetes care intervention. Diabetes Care 2006;29(4):811-7. [DOI] [PubMed] [Google Scholar]
- Piatt GA, Songer TJ, Brooks MM, Anderson RM, Simmons D, Orchard TJ, et al. Impact of patient level factors on the improvement of the ABCs of diabetes. Patient Education and Counseling 2011;82(2):266-70. [DOI] [PubMed] [Google Scholar]
Piette 2000 {published data only}
- Piette J D, Weinberger M, McPhee S J, Mah C A, Kraemer F B, Crapo L M. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? American Journal of Medicine 2000;108(1):20-7. [DOI] [PubMed] [Google Scholar]
Piette 2001 {published data only}
- Piette J D, Weinberger M, Kraemer F B, McPhee S J. Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs Health Care System: a randomized controlled trial. Diabetes Care 2001;24(2):202-8. [DOI] [PubMed] [Google Scholar]
Piette 2011 {published data only}
- Piette J D, Richardson C, Himle J, Duffy S, Torres T, Vogel M, et al. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Medical Care 2011;49(7):641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pill 1998 {published data only}
- Pill R, Stott N C, Rollnick S R, Rees M. A randomized controlled trial of an intervention designed to improve the care given in general practice to Type II diabetic patients: patient outcomes and professional ability to change behaviour. Family Practice 1998;15(3):229-35. [DOI] [PubMed] [Google Scholar]
Pimazoni‐Netto 2011 {published data only}
- Pimazoni-Netto A, Rodbard D, Zanella M T. Rapid improvement of glycemic control in type 2 diabetes using weekly intensive multifactorial interventions: structured glucose monitoring, patient education, and adjustment of therapy-a randomized controlled trial. Diabetes Technology & Therapeutics 2011;13(10):997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pladevall 2015 {published data only}
- Pladevall M, Divine G, Wells K E, Resnicow K, Williams L K. A randomized controlled trial to provide adherence information and motivational interviewing to improve diabetes and lipid control. Diabetes Educator 2015;41(1):136-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Planas 2009 {published data only}
- Planas L G, Crosby K M, Mitchell K D, Farmer K C. Evaluation of a hypertension medication therapy management program in patients with diabetes. Journal of the American Pharmacists Association 2009;49(2):164-70. [DOI] [PubMed] [Google Scholar]
Planas 2012 {published data only}
- Planas L G, Crosby K M, Farmer K C, Harrison D L. Evaluation of a diabetes management program using selected HEDIS measures. Journal of the American Pharmacists Association 2012;52(6):e130-8. [DOI] [PubMed] [Google Scholar]
Plaster 2012 {published data only}
- Plaster C P, Melo D T, Boldt V, Cassaro K O S, Lessa F C R, Boechat G A P, et al. Reduction of cardiovascular risk in patients with metabolic syndrome in a community health center after a pharmaceutical care program of pharmacotherapy follow-up. Brazilian Journal of Pharmaceutical Sciences 2012;48(3):435-46. [Google Scholar]
Plotnikoff 2010 {published data only}
- Plotnikoff R C, Eves N, Jung M, Sigal R J, Padwal R, Karunamuni N. Multicomponent, home-based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. International Journal of Obesity 2010;34(12):1733-41. [DOI] [PubMed] [Google Scholar]
Polonsky 2003 {published data only}
- Polonsky W H, Earles J, Smith S, Pease D J, Macmillan M, Christensen R, et al. Integrating medical management with diabetes self-management training: a randomized control trial of the Diabetes Outpatient Intensive Treatment program. Diabetes Care 2003;26(11):3048-53. [DOI] [PubMed] [Google Scholar]
Pouwer 2001 {published data only}
- Pouwer F, Snoek F J, Ploeg H M, Ader H J, Heine R J. Monitoring of psychological well-being in outpatients with diabetes: effects on mood, HbA(1c), and the patient's evaluation of the quality of diabetes care: a randomized controlled trial. Diabetes Care 2001;24(11):1929-35. [DOI] [PubMed] [Google Scholar]
Powers 2009 {published data only}
- Powers B J, Olsen M K, Oddone E Z, Bosworth H B. The effect of a hypertension self-management intervention on diabetes and cholesterol control. American Journal of Medicine 2009;122(7):639-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prabhakaran 2019 {published data only}
- Prabhakaran D, Jha D, Prieto-Merino D, Roy A, Singh K, Ajay V S, et al. Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare cluster-randomized controlled trial. Circulation 2019;139:380-91. [DOI] [PubMed] [Google Scholar]
Presseau 2018 {published data only}
- Presseau J, Mackintosh J, Hawthorne G, Francis J J, Johnston M, Grimshaw J M, et al. Cluster randomised controlled trial of a theory-based multiple behaviour change intervention aimed at healthcare professionals to improve their management of type 2 diabetes in primary care. Implementation Science 2018;13(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pressman 2014 {published data only}
- Pressman A R, Kinoshita L, Kirk S, Barbosa G M, Chou C, Minkoff J. A novel telemonitoring device for improving diabetes control: protocol and results from a randomized clinical trial. Telemedicine and e-Health 2014;20(2):109-14. [DOI] [PubMed] [Google Scholar]
Prestes 2017 {published data only}
- Prestes M, Gayarre M A, Elgart J F, Gonzalez L, Rucci E, Paganini J M, et al. Improving diabetes care at primary care level with a multistrategic approach: results of the DIAPREM programme. Acta Diabetologica 2017;54(9):853-61. [DOI] [PubMed] [Google Scholar]
Prezio 2013 {published data only}
- Prezio E A, Cheng D, Balasubramanian B A, Shuval K, Kendzor D E, Culica D. Community Diabetes Education (CoDE) for uninsured Mexican Americans: a randomized controlled trial of a culturally tailored diabetes education and management program led by a community health worker. Diabetes Research and Clinical Practice 2013;100(1):19-28. [DOI] [PubMed] [Google Scholar]
- Prezio EA, Balasubramanian BA, Shuval K, Cheng D, Kendzor DE, Culica D. Evaluation of quality improvement performance in the Community Diabetes Education (CoDE) program for uninsured Mexican Americans: results of a randomized controlled trial. American Journal of Medical Quality 2014;29(2):124-34. [DOI] [PubMed] [Google Scholar]
Pritchard 1999 {published data only}
- Pritchard D A, Hyndman J, Taba F. Nutritional counselling in general practice: a cost effective analysis. Journal of Epidemiology and Community Health 1999;53(5):311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 2008 {published data only}
- Quinn C C, Clough S S, Minor J M, Lender D, Okafor M C, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technology & Therapeutics 2008;10(3):160-8. [DOI] [PubMed] [Google Scholar]
Quinn 2011 {published data only}
- Quinn C C, Shardell M D, Terrin M L, Barr E A, Ballew S H, Gruber-Baldini A L. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011;34(9):1934-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ralston 2009 {published data only}
- Ralston J D, Hirsch I B, Hoath J, Mullen M, Cheadle A, Goldberg H I. Web-based collaborative care for type 2 diabetes: a pilot randomized trial. Diabetes Care 2009;32(2):234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramli 2016 {published data only}
- Ramli A S, Selvarajah S, Daud M H, Haniff J, bdul-Razak S, Tg-Abu-Bakar-Sidik T M, et al, Investigators Empower Par. Effectiveness of the EMPOWER-PAR intervention in improving clinical outcomes of type 2 diabetes mellitus in primary care: a pragmatic cluster randomised controlled trial. BMC Family Practice 2016;17(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasmussen 2016 {published data only}
- Rasmussen O W, Lauszus F F, Loekke M. Telemedicine compared with standard care in type 2 diabetes mellitus: a randomized trial in an outpatient clinic. Journal of Telemedicine and Telecare 2016;22(6):363-8. [DOI] [PubMed] [Google Scholar]
Ratanawongsa 2014 {published data only}
- Ratanawongsa N, Handley M A, Sarkar U, Quan J, Pfeifer K, Soria C, Schillinger D. Diabetes health information technology innovation to improve quality of life for health plan members in urban safety net. Journal of Ambulatory Care Management 2014;37(2):127-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rees 2017 {published data only}
- Rees G, O'Hare F, Saeed M, Sudholz B, Sturrock B A, Xie J, et al. Problem-solving therapy for adults with diabetic retinopathy and diabetes-specific distress: a pilot randomized controlled trial. BMJ Open Diabetes Research & Care 2017;5(1):e000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reiber 2004 {published data only}
- Reiber G E, Au D, McDonell M, Fihn S D. Diabetes quality improvement in Department of Veterans Affairs Ambulatory Care Clinics: a group-randomized clinical trial. Diabetes Care 2004;27 Suppl 2:B61-8. [DOI] [PubMed] [Google Scholar]
Reichard 1994 {published data only}
- Reichard P, Toomingas B, Rosenqvist U. Changes in conceptions and attitudes during five years of intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study (SDIS). Diabetes Education 1994;20(6):503-8. [DOI] [PubMed] [Google Scholar]
Renner 2017 {published data only}
- Renner H M, Hollar A, Stolpe S F, Marciniak M W. Pharmacist-to-prescriber intervention to close therapeutic gaps for statin use in patients with diabetes: A randomized controlled trial. Journal of the American Pharmacists Association: JAPhA 2017;57(3S):S236-S242.e1. [DOI] [PubMed] [Google Scholar]
Rickheim 2002 {published data only}
- Rickheim P L, Weaver T W, Flader J L, Kendall D M. Assessment of group versus individual diabetes education: a randomized study. Diabetes Care 2002;25(2):269-74. [DOI] [PubMed] [Google Scholar]
Riddell 2016 {published data only}
- Riddell M A, Dunbar J A, Absetz P, Wolfe R, Li H, Brand M, et al, Australasian Peers for Progress Diabetes Project Investigators. Cardiovascular risk outcome and program evaluation of a cluster randomised controlled trial of a community-based, lay peer led program for people with diabetes. BMC Public Health 2016;16(1):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ridgeway 1999 {published data only}
- Ridgeway N A, Harvill D R, Harvill L M, Falin T M, Forester G M, Gose O D. Improved control of type 2 diabetes mellitus: a practical education/behavior modification program in a primary care clinic. Southern Medical Journal 1999;92(7):667-72. [DOI] [PubMed] [Google Scholar]
Rodriguez 2018 {published data only}
- Rodriguez H P, Friedberg M W, Vargas-Bustamante A, Chen X, Martinez A E, Roby D H. The impact of integrating medical assistants and community health workers on diabetes care management in community health centers. BMC Health Services Research 2018;18(1):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez‐Idigoras 2009 {published data only}
- Rodríguez-Idígoras MI, Sepúlveda-Muñoz J, Sánchez-Garrido-Escudero R, Martínez-González JL, Escolar-Castelló JL, Paniagua-Gómez IM, et al. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technology & Therapeutics 2009;11(7):431-7. [DOI] [PubMed] [Google Scholar]
Rosal 2005 {published data only}
- Rosal M C, Olendzki B, Reed G W, Gumieniak O, Scavron J, Ockene I. Diabetes self-management among low-income Spanish-speaking patients: a pilot study. Annals of Behavioral Medicine 2005;29(3):225-35. [DOI] [PubMed] [Google Scholar]
Rosal 2011 {published data only}
- Rosal M C, Ockene I S, Restrepo A, White M J, Borg A, Olendzki B, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income Latinos: Latinos en control. Diabetes Care 2011;34(4):838-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2010 {published data only}
- Rossi M C, Nicolucci A, Bartolo P, Bruttomesso D, Girelli A, Ampudia F J, et al. Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes Care 2010;33(1):109-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2013 {published data only}
- Rossi M C, Nicolucci A, Lucisano G, Pellegrini F, Di Bartolo P, Miselli V, et al. Impact of the "Diabetes Interactive Diary" telemedicine system on metabolic control, risk of hypoglycemia, and quality of life: a randomized clinical trial in type 1 diabetes. Diabetes Technology & Therapeutics 2013;15(8):670-9. [DOI] [PubMed] [Google Scholar]
Rothman 2005 {published data only}
- Rothman R, Malone R, Bryant B, Shintani A. A randomised trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin level in patients with diabetes. American Journal of Medicine 2005;118(3):279-84. [DOI] [PubMed] [Google Scholar]
Rothschild 2014 {published data only}
- Rothschild S K, Martin M A, Swider S M, Tumialan Lynas C M, Janssen I, Avery E F, et al. Mexican American trial of community health workers: a randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus. American Journal of Public Health 2014;104(8):1540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubak 2011 {published data only}
- Rubak S, Sandbaek A, Lauritzen T, Borch-Johnsen K, Christensen B. General practitioners trained in motivational interviewing can positively affect the attitude to behaviour change in people with type 2 diabetes. One year follow-up of an RCT, ADDITION Denmark. Scandinavian Journal of Primary Health Care 2009;27(3):172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubak S, Sandbæk A, Lauritzen T, Borch-Johnsen K, Christensen B. Effect of "motivational interviewing" on quality of care measures in screen detected type 2 diabetes patients: a one-year follow-up of an RCT, ADDITION Denmark. Scandinavian Journal of Primary Health Care 2011;29(2):92-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruggiero 2010 {published data only}
- Ruggiero L, Moadsiri A, Butler P, Oros S M, Berbaum M L, Whitman S, et al. Supporting diabetes self-care in underserved populations: a randomized pilot study using medical assistant coaches. Diabetes Educator 2010;36(1):127-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruggiero 2014 {published data only}
- Ruggiero L, Riley B B, Hernandez R, Quinn L T, Gerber B S, Castillo A, et al. Medical assistant coaching to support diabetes self-care among low-income racial/ethnic minority populations: randomized controlled trial. Western Journal of Nursing Research 2014;36(9):1052-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 2019 {published data only}
- Russell A W, Donald M, Borg S J, Zhang J, Burridge L H, Ware R S, et al. Clinical outcomes of an integrated primary-secondary model of care for individuals with complex type 2 diabetes: a non-inferiority randomised controlled trial. Diabetologia 2019;62(1):41-52. [DOI] [PubMed] [Google Scholar]
Ryff‐de Lèche 1992 {published data only}
- Ryff-de Leche A, Engler H, Nutzi E, Berger M, Berger W. Clinical application of two computerized diabetes management systems: comparison with the log-book method. Diabetes Research 1992;19(3):97-105. [PubMed] [Google Scholar]
Sadur 1999 {published data only}
- Sadur C N, Moline N, Costa M, Michalik D, Mendlowitz D, Roller S, et al. Diabetes management in a health maintenance organization. Efficacy of care management using cluster visits. Diabetes Care 1999;22(12):2011-7. [DOI] [PubMed] [Google Scholar]
Saenz 2012 {published data only}
- Saenz A, Brito M, Moron I, Torralba A, Garcia-Sanz E, Redondo J. Development and validation of a computer application to aid the physician's decision-making process at the start of and during treatment with insulin in type 2 diabetes: a randomized and controlled trial. Journal of Diabetes Science and Technology 2012;6(3):581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Safford 2015 {published data only}
- Safford M M, Andreae S, Cherrington A L, Martin M Y, Halanych J, Lewis M, et al. Peer coaches to improve diabetes outcomes in rural Alabama: a cluster randomized trial. Annals Of Family Medicine 2015;13 (Suppl 1):S18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sajatovic 2017 {published data only}
- Sajatovic M, Gunzler D D, Kanuch S W, Cassidy K A, Tatsuoka C, McCormick R, et al. A 60- week prospective RCT of a self-management intervention for individuals with serious mental illness and diabetes mellitus. Psychiatric Services 2017;68(9):883-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saleh 2018 {published data only}
- Saleh S, Farah A, Dimassi H, El Arnaout N, Constantin J, Osman M, et al. Using mobile health to enhance outcomes of noncommunicable diseases care in rural settings and refugee camps: randomized controlled trial. JMIR MHealth and UHealth 2018;6(7):e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samtia 2013 {published data only}
- Samtia A M, Rasool M F, Ranjha N M, Usman F, Javed I. A multifactorial intervention to enhance adherence to medications and disease-related knowledge in type 2 diabetic patients in Southern Punjab, Pakistan. Tropical Journal of Pharmaceutical Research 2013;12(5):851-6. [Google Scholar]
Samuel‐Hodge 2017 {published data only}
- Samuel-Hodge C D, Holder-Cooper J C, Gizlice Z, Davis G, Steele S P, Keyserling T C, et al. Family PArtners in Lifestyle Support (PALS): family-based weight loss for African American adults with type 2 diabetes. Obesity (Silver Spring, Md.) 2017;25:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarayani 2018 {published data only}
- Sarayani A, Mashayekhi M, Nosrati M, Jahangard-Rafsanjani Z, Javadi M, Saadat N, et al. Efficacy of a telephone-based intervention among patients with type-2 diabetes; a randomized controlled trial in pharmacy practice. International Journal of Clinical Pharmacy 2018;40(2):345-53. [DOI] [PubMed] [Google Scholar]
Sato 2016 {published data only}
- Sato J, Kanazawa A, Ikeda F, Shigihara N, Kawaguchi M, Komiya K, et al. Effect of treatment guidance using a retrospective continuous glucose monitoring system on glycaemic control in outpatients with type 2 diabetes mellitus: a randomized controlled trial. Journal of International Medical Research 2016;44(1):109-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schillinger 2009 {published data only}
- Schillinger D, Handley M, Wang F, Hammer H. Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: a three-arm practical clinical trial. Diabetes Care 2009;32(4):559-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnipper 2010 {published data only}
- Schnipper J L, Linder J A, Palchuk M B, Yu D T, McColgan K E, Volk L A, et al. Effects of documentation-based decision support on chronic disease management. American Journal of Managed Care 2010;16(12 Suppl HIT):Sp72-81. [PubMed] [Google Scholar]
Schoenberg 2017 {published data only}
- Schoenberg N E, Ciciurkaite G, Greenwood M K. Community to clinic navigation to improve diabetes outcomes. Preventive Medicine Reports 2017;5:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2006 {published data only}
- Scott D M, Boyd S T, Stephan M, Augustine S C, Reardon T P. Outcomes of pharmacist-managed diabetes care services in a community health center. American Journal of Health-system Pharmacy 2006;63(21):2116-22. [DOI] [PubMed] [Google Scholar]
Seggelke 2014 {published data only}
- Seggelke S A, Hawkins R M, Gibbs J, Rasouli N, Wang C, Draznin B. Transitional care clinic for uninsured and medicaid-covered patients with diabetes mellitus discharged from the hospital: a pilot quality improvement study. Hospital Practice 2014;42(1):46-51. [DOI] [PubMed] [Google Scholar]
Sen 2014 {published data only}
- Sen A P, Sewell T B, Riley E B, Stearman B, Bellamy S L, Hu M F, et al. Financial incentives for home-based health monitoring: a randomized controlled trial. Journal of General Internal Medicine 2014;29(5):770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sequeira 2013 {published data only}
- Sequeira P A, Montoya L, Ruelas V, Xing D, Chen V, Beck R, et al. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technology & Therapeutics 2013;15(10):855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sequist 2010 {published data only}
- Sequist T D, Fitzmaurice G M, Marshall R, Shaykevich S, Marston A, Safran D G, et al. Cultural competency training and performance reports to improve diabetes care for black patients: a cluster randomized, controlled trial. Annals of Internal Medicine 2010;152(1):40-6. [DOI] [PubMed] [Google Scholar]
Sevick 2012 {published data only}
- Sevick M A, Korytkowski M, Stone R A, Piraino B, Ren D, Sereika S, et al. Biophysiologic outcomes of the Enhancing Adherence in Type 2 Diabetes (ENHANCE) trial. Journal of the Academy of Nutrition and Dietetics 2012;112(8):1147-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2014 {published data only}
- Shah B R, Bhattacharyya O, Yu C H, Mamdani M M, Parsons J A, Straus S E, et al. Effect of an educational toolkit on quality of care: a pragmatic cluster randomized trial. PLOS Medicine 2014;11(2):e1001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahid 2015 {published data only}
- Shahid M, Mahar S A, Shaikh S, Shaikh Z. Mobile phone intervention to improve diabetes care in rural areas of Pakistan: a randomized controlled trial. Journal of The College of Physicians and Surgeons-Pakistan 2015;25(3):166-71. [PubMed] [Google Scholar]
Shao 2015 {published data only}
- Shao J H, Wu Y Q, Zhang P F. Clinical effects of comprehensive nursing intervention in elderly liver cirrhosis patients with type 2 diabetes. [Chinese]. World Chinese Journal of Digestology 2015;23(23):3771-4. [Google Scholar]
Shea 2009 {published data only}
- Luchsinger JA, Palmas W, Teresi JA, Silver S, Kong J, Eimicke JP, et al. Improved diabetes control in the elderly delays global cognitive decline. Journal of Nutrition, Health & Aging 2011;15(6):445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea S, Weinstock R S, Teresi J A, Palmas W, Starren J, Cimino J J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. Journal of the American Medical Informatics Association: JAMIA 2009;16(4):446-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock RS, Brooks G, Palmas W, Morin PC, Teresi JA, Eimicke JP, et al. Lessened decline in physical activity and impairment of older adults with diabetes with telemedicine and pedometer use: results from the IDEATel study. Age and Ageing 2011;40(1):98-105. [DOI] [PubMed] [Google Scholar]
Shi 2014 {published data only}
- Shi H F, Fu X D. Comprehensive nursing intervention to improve quality of life in chronic hepatitis C patients with diabetes mellitus. [Chinese]. World Chinese Journal of Digestology 2014;22(18):2617-21. [Google Scholar]
Siaw 2017 {published data only}
- Siaw M Y L, Ko Y, Malone D C, Tsou K Y K, Lew Y J, Foo D, et al. Impact of pharmacist-involved collaborative care on the clinical, humanistic and cost outcomes of high-risk patients with type 2 diabetes (IMPACT): a randomized controlled trial. Journal of Clinical Pharmacy & Therapeutics 2017;42(4):475-82. [DOI] [PubMed] [Google Scholar]
Sieber 2012 {published data only}
- Sieber W, Newsome A, Lillie D. Promoting self-management in diabetes: efficacy of a collaborative care approach. Families, Systems & Health 2012;30(4):322-9. [DOI] [PubMed] [Google Scholar]
Sigurdardottir 2009 {published data only}
- Sigurdardottir A K, Benediktsson R, Jonsdottir H. Instruments to tailor care of people with type 2 diabetes. Journal of Advanced Nursing 2009;65(10):2118-30. [DOI] [PubMed] [Google Scholar]
Siminerio 2013 {published data only}
- Siminerio L, Ruppert K M, Gabbay R A. Who can provide diabetes self-management support in primary care? Findings from a randomized controlled trial. Diabetes Educator 2013;39(5):705-13. [DOI] [PubMed] [Google Scholar]
Simmons 2004 {published data only}
- Simmons D, Gamble G D, Foote S, Cole D R, Coster G. The New Zealand Diabetes Passport Study: a randomized controlled trial of the impact of a diabetes passport on risk factors for diabetes-related complications. Diabetic Medicine 2004;21(3):214-7. [DOI] [PubMed] [Google Scholar]
Simpson 2011 {published data only}
- Gilani F, Majumdar SR, Johnson JA, Tsuyuki RT, Lewanczuk RZ, Spooner R, et al. Adding pharmacists to primary care teams increases guideline-concordant antiplatelet use in patients with type 2 diabetes: results from a randomized trial. Annals of Pharmacotherapy 2013;47(1):43-8. [DOI] [PubMed] [Google Scholar]
- Simpson S H, Majumdar S R, Tsuyuki R T, Lewanczuk R Z, Spooner R, Johnson J A. Effect of adding pharmacists to primary care teams on blood pressure control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2011;34(1):20-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinclair 2012 {published data only}
- Sinclair A J, Girling A J, Gadsby R, Bourdel-Marchasson I, Bayer A J. Diabetes in care homes: a cluster randomised controlled trial of resident education. British Journal of Diabetes & Vascular Disease 2012;12:238. [Google Scholar]
Skeie 2009 {published data only}
- Skeie S, Kristensen G B, Carlsen S, Sandberg S. Self-monitoring of blood glucose in type 1 diabetes patients with insufficient metabolic control: focused self-monitoring of blood glucose intervention can lower glycated hemoglobin A1C. Journal of Diabetes Science and Technology 2009;3(1):83-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1987 {published data only}
- Smith D M, Weinberger M, Katz B P. A controlled trial to increase office visits and reduce hospitalizations of diabetic patients. Journal of General Internal Medicine 1987;2(4):232-8. [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith S, Bury G, O'Leary M, Shannon W, Tynan A, Staines A, et al. The North Dublin randomized controlled trial of structured diabetes shared care. Family Practice 2004;21(1):39-45. [DOI] [PubMed] [Google Scholar]
Smith 2008 {published data only}
- Smith S A, Shah N D, Bryant S C, Christianson T J, Bjornsen S S, Giesler P D, et al. Chronic care model and shared care in diabetes: randomized trial of an electronic decision support system. Mayo Clinic Proceedings 2008;83(7):747-57. [DOI] [PubMed] [Google Scholar]
Sone 2010 {published data only}
- Sone H, Tanaka S, Iimuro S, Tanaka S, Oida K, Yamasaki Y, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010;53(3):419-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song M S, Kim H S. Intensive management program to improve glycosylated hemoglobin levels and adherence to diet in patients with type 2 diabetes. Applied Nursing Research 2009;22(1):42-7. [DOI] [PubMed] [Google Scholar]
Sonnichsen 2010 {published data only}
- Sonnichsen A C, Winkler H, Flamm M, Panisch S, Kowatsch P, Klima G, et al. The effectiveness of the Austrian disease management programme for type 2 diabetes: a cluster-randomised controlled trial. BMC Family Practice 2010;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2011 {published data only}
- Spencer M S, Rosland A M, Kieffer E C, Sinco B R, Valerio M, Palmisano G, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. American Journal of Public Health 2011;101(12):2253-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2018 {published data only}
- Spencer M S, Kieffer E C, Sinco B, Piatt G, Palmisano G, Hawkins J, et al. Outcomes at 18 months from a community health worker and peer leader diabetes self-management program for Latino adults. Diabetes Care 2018;41(7):1414-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sperl‐Hillen 2010 {published data only}
- Sperl-Hillen J M, O'Connor P J, Rush W A, Johnson P E, Gilmer T, Biltz G, et al. Simulated physician learning program improves glucose control in adults with diabetes. Diabetes Care 2010;33(8):1727-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sperl‐Hillen 2013 {published data only}
- Sperl-Hillen J, Beaton S, Fernandes O, Worley A, Vazquez-Benitez G, Hanson A, et al. Are benefits from diabetes self-management education sustained? American Journal of Managed Care 2013;19(2):104-12. [PubMed] [Google Scholar]
Sriram 2011 {published data only}
- Sriram S, Chack L E, Ramasamy R, Ghasemi A, Ravi T K, Sabzghabaee A M. Impact of pharmaceutical care on quality of life in patients with type 2 diabetes mellitus. Journal of Research in Medical Sciences 2011;16 Suppl 1:S412-8. [PMC free article] [PubMed] [Google Scholar]
Steventon 2014 {published data only}
- Steventon A, Bardsley M, Doll H, Tuckey E, Newman S P. Effect of telehealth on glycaemic control: analysis of patients with type 2 diabetes in the Whole Systems Demonstrator cluster randomised trial. BMC Health Services Research 2014;14:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steyn 2013 {published data only}
- Steyn K, Lombard C, Gwebushe N, Fourie J M, Everett-Murphy K, Zwarenstein M, et al. Implementation of national guidelines, incorporated within structured diabetes and hypertension records at primary level care in Cape Town, South Africa: a randomised controlled trial. Glob Health Action 2013;6:20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2010 {published data only}
- Stone R A, Rao R H, Sevick M A, Cheng C, Hough L J, Macpherson D S, et al. Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care 2010;33(3):478-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2012a {published data only}
- Stone R A, Sevick M A, Rao R H, Macpherson D S, Cheng C, Kim S, et al. The Diabetes Telemonitoring Study Extension: an exploratory randomized comparison of alternative interventions to maintain glycemic control after withdrawal of diabetes home telemonitoring (RCT #1: n=57, CC to CC or UC). Journal of the American Medical Informatics Association: JAMIA 2012;19(6):973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2012b {published data only}
- Stone R A, Sevick M A, Rao R H, Macpherson D S, Cheng C, Kim S, et al. The Diabetes Telemonitoring Study Extension: an exploratory randomized comparison of alternative interventions to maintain glycemic control after withdrawal of diabetes home telemonitoring (RCT #2: n=44, ACM to CCHT or CC). Journal of the American Medical Informatics Association: JAMIA 2012;19(6):973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stroebel 2002 {published data only}
- Stroebel R J, Scheitel S M, Fitz J S, Herman R A, Naessens J M, Scott C G, et al. A randomized trial of three diabetes registry implementation strategies in a community internal medicine practice. Joint Commission Journal on Quality Improvement 2002;28(8):441-50. [DOI] [PubMed] [Google Scholar]
Sugiyama 2015 {published data only}
- Sugiyama T, Steers W N, Wenger N S, Duru O K, Mangione C M. Effect of a community-based diabetes self-management empowerment program on mental health-related quality of life: a causal mediation analysis from a randomized controlled trial. BMC Health Services Research 2015;15:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suh 2014 {published data only}
- Suh S, Jean C, Koo M, Lee S Y, Cho M J, Sim K H, et al. A Randomized controlled trial of an internet-based mentoring program for type 1 diabetes patients with inadequate glycemic control. Diabetes and Metabolism Journal 2014;38(2):134-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2008 {published data only}
- Sun J, Wang Y, Chen X, Chen Y, Feng Y, Zhang X, et al. An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes. Asia Pacific Journal of Clinical Nutrition 2008;17(3):514-24. [PubMed] [Google Scholar]
Sun 2019 {published data only}
- Sun C, Sun L, Xi S, Zhang H, Wang H, Feng Y, et al. Mobile phone-based telemedicine practice in older Chinese patients with type 2 diabetes mellitus: randomized controlled trial. JMIR MHealth and UHealth 2019;7(1):e10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takami 2008 {published data only}
- Takami C, Moriyama M, Nakano M, Kuroe Y, Nin K, Morikawa H, et al. Developmental process of disease management program of type 2 diabetes with a view to acquiring self-management skills: effects of the trial implementation. Japan Journal of Nursing Science 2008;28(3):59-68. [Google Scholar]
Tang 2013 {published data only}
- Tang P C, Overhage J M, Chan A S, Brown N L, Aghighi B, Entwistle M P, et al. Online disease management of diabetes: engaging and motivating patients online with enhanced resources-diabetes (EMPOWER-D), a randomized controlled trial. Journal of the American Medical Informatics Association: JAMIA 2013;20(3):526-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2015 {published data only}
- Tang T S, Funnell M M, Sinco B, Spencer M S, Heisler M. Peer-Led, Empowerment-Based Approach to Self-Management Efforts in Diabetes (PLEASED): a randomized controlled trial in an African American community. Annals of Family Medicine 2015;13(Suppl 1):S27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taveira 2010 {published data only}
- Taveira T H, Friedmann P D, Cohen L B, Dooley A G, Khatana S A, Pirraglia P A, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educator 2010;36(1):109-17. [DOI] [PubMed] [Google Scholar]
Taveira 2011 {published data only}
- Taveira T H, Dooley A G, Cohen L B, Khatana S A, Wu W C. Pharmacist-led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. Annals of Pharmacotherapy 2011;45(11):1346-55. [DOI] [PubMed] [Google Scholar]
Taveira 2014 {published data only}
- Taveira T H, Wu W C. Interventions to maintain cardiac risk control after discharge from a cardiovascular risk reduction clinic: a randomized controlled trial. Diabetes Research and Clinical Practice 2014;105(3):327-35. [DOI] [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor C B, Miller N H, Reilly K R, Greenwald G, Cunning D, Deeter A, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care 2003;26(4):1058-63. [DOI] [PubMed] [Google Scholar]
Taylor 2005 {published data only}
- Taylor K I, Oberle K M, Crutcher R A, Norton P G. Promoting health in type 2 diabetes: nurse-physician collaboration in primary care. Biological Research for Nursing 2005;6(3):207-15. [DOI] [PubMed] [Google Scholar]
Thankappan 2013 {published data only}
- Thankappan K R, Mini G K, Daivadanam M, Vijayakumar G, Sarma P S, Nichter M. Smoking cessation among diabetes patients: results of a pilot randomized controlled trial in Kerala, India. BMC Public Health 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2007 {published data only}
- Thomas K G, Thomas M R, Stroebel R J, McDonald F S, Hanson G J, Naessens J M, et al. Use of a registry-generated audit, feedback, and patient reminder intervention in an internal medicine resident clinic--a randomized trial. Journal of General Internal Medicine 2007;22(12):1740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 1999 {published data only}
- Thompson D M, Kozak S E, Sheps S. Insulin adjustment by a diabetes nurse educator improves glucose control in insulin-requiring diabetic patients: a randomized trial. CMAJ 1999;161(8):959-62. [PMC free article] [PubMed] [Google Scholar]
Tildesley 2010 {published data only}
- Tildesley H D, Mazanderani A B, Ross S A. Effect of Internet therapeutic intervention on A1C levels in patients with type 2 diabetes treated with insulin. Diabetes Care 2010;33(8):1738-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tildesley 2011 {published data only}
- Tildesley H D, Mazanderani A B, Chan J H M, Ross S A. Efficacy of A1C reduction using internet intervention in patients with type 2 diabetes treated with insulin. Canadian Journal of Diabetes 2011;35(3):250-3. [Google Scholar]
Tjam 2006 {published data only}
- Tjam E Y, Sherifali D, Steinacher N, Hett S. Physiological outcomes of an internet disease management program vs. in-person counselling: a randomized, controlled trial. Canadian Journal of Diabetes 2006;30(4):397-405. [Google Scholar]
Tobe 2006 {published data only}
- Tobe S W, Pylypchuk G, Wentworth J, Kiss A, Szalai J P, Perkins N, et al. Effect of nurse-directed hypertension treatment among First Nations people with existing hypertension and diabetes mellitus: the Diabetes Risk Evaluation and Microalbuminuria (DREAM 3) randomized controlled trial. CMAJ 2006;174(9):1267-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tourkmani 2018 {published data only}
- Tourkmani A M, Abdelhay O, Alkhashan H I, Alaboud A F, Bakhit A, Elsaid T, et al. Impact of an integrated care program on glycemic control and cardiovascular risk factors in patients with type 2 diabetes in Saudi Arabia: an interventional parallel-group controlled study. BMC Family Practice 2018;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trento 2008 {published data only}
- Trento M, Basile M, Borgo E, Grassi G, Scuntero P, Trinetta A, et al. A randomised controlled clinical trial of nurse-, dietitian- and pedagogist-led group care for the management of Type 2 diabetes. Journal of Endocrinological Investigation 2008;31(11):1038-42. [DOI] [PubMed] [Google Scholar]
Tsuyuki 2016 {published data only}
- Al Hamarneh YN, Hemmelgarn BR, Hassan I, Jones CA, Tsuyuki RT. The effectiveness of pharmacist interventions on cardiovascular risk in adult patients with type 2 diabetes: the multicentre randomized controlled R(x)EACHTrial. Canadian Journal of Diabetes 2017;41(6):580-6. [DOI] [PubMed] [Google Scholar]
- Tsuyuki R T, Al Hamarneh Y N, Jones C A, Hemmelgarn B R. The effectiveness of pharmacist interventions on cardiovascular risk: the multicenter randomized controlled RxEACH Trial. Journal of the American College of Cardiology 2016;67(24):2846-54. [DOI] [PubMed] [Google Scholar]
Tu 1993 {published data only}
- Tu K S, McDaniel G, Gay J T. Diabetes self-care knowledge, behaviors, and metabolic control of older adults--the effect of a posteducational follow-up program. Diabetes Education 1993;19:25-30. [DOI] [PubMed] [Google Scholar]
Tutino 2017 {published data only}
- Tutino G E, Yang W Y, Li X, Li W H, Zhang Y Y, Guo X H, et al, China Jade Study Group. A multicentre demonstration project to evaluate the effectiveness and acceptability of the web-based Joint Asia Diabetes Evaluation (JADE) programme with or without nurse support in Chinese patients with type 2 diabetes. Diabetic Medicine 2017;34(3):440-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vadstrup 2011 {published data only}
- Vadstrup E S, Frolich A, Perrild H, Borg E, Roder M. Effect of a group-based rehabilitation programme on glycaemic control and cardiovascular risk factors in type 2 diabetes patients: the Copenhagen Type 2 Diabetes Rehabilitation Project. Patient Education and Counseling 2011;84(2):185-90. [DOI] [PubMed] [Google Scholar]
van Bruggen 2008 {published data only}
- Bruggen R, Gorter K J, Stolk R P, Verhoeven R P, Rutten G E. Implementation of locally adapted guidelines on type 2 diabetes. Family Practice 2008;25(6):430-7. [DOI] [PubMed] [Google Scholar]
Van Dijk‐de Vries 2015 {published data only}
- Dijk-de Vries A, Bokhoven M A, Winkens B, Terluin B, Knottnerus J A, Weijden T, et al. Lessons learnt from a cluster-randomised trial evaluating the effectiveness of Self-Management Support (SMS) delivered by practice nurses in routine diabetes care. BMJ Open 2015;5(6):e007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dyck 2013 {published data only}
- Van Dyck D, De Greef K, Deforche B, Ruige J, Bouckaert J, Tudor-Locke C E, et al. The relationship between changes in steps/day and health outcomes after a pedometer-based physical activity intervention with telephone support in type 2 diabetes patients. Health Education Research 2013;28(3):539-45. [DOI] [PubMed] [Google Scholar]
VanEpps 2018 {published data only}
- VanEpps E M, Troxel A B, Villamil E, Saulsgiver K A, Zhu J, Chin J Y, et al. Financial incentives for chronic disease management: results and limitations of 2 randomized clinical trials with New York Medicaid patients. American Journal of Health Promotion 2018;32(7):1537-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Veldhuizen‐Scott 1995 {published data only}
- Van Veldhuizen-Scott M K, Widmer L B, Stacey S A, Popovich N G. Developing and implementing a pharmaceutical care model in an ambulatory care setting for patients with diabetes. Diabetes Education 1995;21(2):117-23. [DOI] [PubMed] [Google Scholar]
Varney 2014 {published data only}
- Varney J E, Weiland T J, Inder W J, Jelinek G A. Effect of hospital-based telephone coaching on glycaemic control and adherence to management guidelines in type 2 diabetes, a randomised controlled trial. Internal Medicine Journal 2014;44:890. [DOI] [PubMed] [Google Scholar]
Vaughan 2017 {published data only}
- Vaughan E M, Johnston C A, Cardenas V J, Moreno J P, Foreyt J P. Integrating CHWs as part of the team leading diabetes group visits: a randomized controlled feasibility study. Diabetes Educator 2017;43(6):589-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidal‐Pardo 2013 {published data only}
- Vidal-Pardo J I, Perez-Castro T R, Lopez-Alvarez X L, Santiago-Perez M I, Garcia-Soidan F J, Muniz J. Effect of an educational intervention in primary care physicians on the compliance of indicators of good clinical practice in the treatment of type 2 diabetes mellitus [OBTEDIGA project]. International Journal of Clinical Practice 2013;67(8):750-8. [DOI] [PubMed] [Google Scholar]
Vinicor 1987 {published data only}
- Mazzuca S A, Moorman N H, Wheeler M L, Norton J A, Fineberg N S, Vinicor F, et al. The diabetes education study: a controlled trial of the effects of diabetes patient education. Diabetes Care 1986;9(1):1-10. [DOI] [PubMed] [Google Scholar]
- Vinicor F, Cohen S J, Mazzuca S A, Moorman N, Wheeler M, Kuebler T, et al. DIABEDS: a randomized trial of the effects of physician and/or patient education on diabetes patient outcomes. Journal of Chronic Diseases 1987;40(4):345-56. [DOI] [PubMed] [Google Scholar]
Volpp 2015 {published data only}
- Volpp K G, Troxel A B, Long J A, Ibrahim S A, Appleby D, Smith J O, et al. A randomized controlled trial of negative co-payments: the CHORD trial. American Journal of Managed Care 2015;21(8):e465-73. [PMC free article] [PubMed] [Google Scholar]
Wagner 2001 {published data only}
- Wagner E H, Grothaus L C, Sandhu N, Galvin M S, McGregor M, Artz K, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care 2001;24(4):695-700. [DOI] [PubMed] [Google Scholar]
Wakefield 2011 {published data only}
- Wakefield B J, Holman J E, Ray A, Scherubel M, Adams M R, Hillis S L, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemedicine Journal and E-health 2011;17(4):254-61. [DOI] [PubMed] [Google Scholar]
Wakefield 2014 {published data only}
- Wakefield B J, Koopman R J, Keplinger L E, Bomar M, Bernt B, Johanning J L, et al. Effect of home telemonitoring on glycemic and blood pressure control in primary care clinic patients with diabetes. Telemedicine and e-Health 2014;20(4):199-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waki 2014 {published data only}
- Waki K, Fujita H, Uchimura Y, Omae K, Aramaki E, Kato S, et al. DialBetics: A novel smartphone-based self-management support system for type 2 diabetes patients. Journal of Diabetes Science and Technology 2014;8(2):209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallymahmed 2011 {published data only}
- Wallymahmed M E, Morgan C, Gill G V, Macfarlane I A. Nurse-led cardiovascular risk factor intervention leads to improvements in cardiovascular risk targets and glycaemic control in people with Type 1 diabetes when compared with routine diabetes clinic attendance. Diabetic Medicine 2011;28(3):373-9. [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang G, Zhang Z, Feng Y, Sun L, Xiao X, Wang G, et al. Telemedicine in the management of type 2 diabetes mellitus. American Journal of the Medical Sciences 2017;353(1):1-5. [DOI] [PubMed] [Google Scholar]
Ward 1996 {published data only}
- Ward A, Kamien M, Mansfield F, Fatovich B. Educational feedback in the management of type 2 diabetes in general practice. Education for General Practice 1996;7:142-50. [Google Scholar]
Warren 2018 {published data only}
- Warren R, Carlisle K, Mihala G, Scuffham P A. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial. Journal of Telemedicine & Telecare 2018;24(9):586-95. [DOI] [PubMed] [Google Scholar]
Wayne 2015 {published data only}
- Wayne N, Perez D F, Kaplan D M, Ritvo P. Health coaching reduces Hba1c in type 2 diabetic patients from a lower-socioeconomic status community: a randomized controlled trial. Journal of Medical Internet Research 2015;17(10):e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webb 2017 {published data only}
- Webb E M, Rheeder P. A cluster-randomized trial to estimate the effect of mobile screening and treatment feedback on HbA1c and diabetes-related complications in Tshwane primary health care clinics, South Africa. Primary Care Diabetes 2017;11(6):546-54. [DOI] [PubMed] [Google Scholar]
Wei 2017 {published data only}
- Wei X, Walley J D, Zhang Z, Zou G, Gong W, Deng S, et al. Implementation of a comprehensive intervention for patients at high risk of cardiovascular disease in rural China: a pragmatic cluster randomized controlled trial. PLOS One 2017;12(8):e0183169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 1995 {published data only}
- Weinberger M, Kirkman M S, Samsa G P, Shortliffe E A, Landsman P B, Cowper P A, et al. A nurse-coordinated intervention for primary care patients with non-insulin-dependent diabetes mellitus: impact on glycemic control and health-related quality of life. Journal of General Internal Medicine 1995;10(2):59-66. [DOI] [PubMed] [Google Scholar]
Weiss 2015 {published data only}
- Weiss D M, Casten R J, Leiby B E, Hark L A, Murchison A P, Johnson D, et al. Effect of behavioral intervention on dilated fundus examination rates in older African American individuals with diabetes mellitus: a randomized clinical trial. JAMA Ophthalmology 2015;133(9):1005-12. [DOI] [PubMed] [Google Scholar]
Welch 2011a {published data only}
- Welch G, Allen N A, Zagarins S E, Stamp K D, Bursell S E, Kedziora R J. Comprehensive diabetes management program for poorly controlled Hispanic type 2 patients at a community health center. Diabetes Educator 2011;37(5):680-8. [DOI] [PubMed] [Google Scholar]
Welch 2011b {published data only}
- Welch G, Zagarins S E, Feinberg R G, Garb J L. Motivational interviewing delivered by diabetes educators: does it improve blood glucose control among poorly controlled type 2 diabetes patients? Diabetes Research and Clinical Practice 2011;91(1):54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2015 {published data only}
- Welch G, Zagarins S E, Santiago-Kelly P, Rodriguez Z, Bursell S E, Rosal M C, et al. An internet-based diabetes management platform improves team care and outcomes in an urban Latino population. Diabetes Care 2015;38(4):561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2017 {published data only}
- White M, O'Connell M A, Cameron F J. Clinic attendance and disengagement of young adults with type 1 diabetes after transition of care from paediatric to adult services (TrACeD): a randomised, open-label, controlled trial. Lancet Child & Adolescent Health 2017;1(4):274-83. [DOI] [PubMed] [Google Scholar]
Whitlock 2000 {published data only}
- Whitlock W L, Brown A, Moore K, Pavliscsak H, Dingbaum A, Lacefield D, et al. Telemedicine improved diabetic management. Military Medicine 2000;165(8):579. [PubMed] [Google Scholar]
Wild 2016 {published data only}
- Wild S H, Hanley J, Lewis S C, McKnight J A, McCloughan L B, Padfield P L, et al. Supported telemonitoring and glycemic control in people with type 2 diabetes: the Telescot diabetes pragmatic multicenter randomized controlled trial. PLOS Medicine 2016;13(7):e1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2012 {published data only}
- Williams A, Manias E, Walker R, Gorelik A. A multifactorial intervention to improve blood pressure control in co-existing diabetes and kidney disease: a feasibility randomized controlled trial. Journal of Advanced Nursing 2012;68(11):2515-25. [DOI] [PubMed] [Google Scholar]
Wilson 2014 {published data only}
- Wilson A, O'Hare J P, Hardy A, Raymond N, Szczepura A, Crossman R, et al. Evaluation of the clinical and cost effectiveness of intermediate care clinics for diabetes (ICCD): a multicentre cluster randomised controlled trial. PLOS One 2014;9:e93964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wishah 2015 {published data only}
- Wishah R A, Al-Khawaldeh O A, Albsoul A M. Impact of pharmaceutical care interventions on glycemic control and other health-related clinical outcomes in patients with type 2 diabetes: Randomized controlled trial. Diabetology & Metabolic Syndrome 2015;9(4):271-6. [DOI] [PubMed] [Google Scholar]
Wisse 2010 {published data only}
- Wisse W, Boer Rookhuizen M, Kruif M D, Rossum J, Jordans I, ten Cate H, et al. Prescription of physical activity is not sufficient to change sedentary behavior and improve glycemic control in type 2 diabetes patients. Diabetes Research and Clinical Practice 2010;88(2):e10-3. [DOI] [PubMed] [Google Scholar]
Wojcicki 2001 {published data only}
- Wojcicki J M, Ladyzynski P, Krzymien J, Jozwicka E, Blachowicz J, Janczewska E, et al. What we can really expect from telemedicine in intensive diabetes treatment: results from 3-year study on type 1 pregnant diabetic women. Diabetes Technology & Therapeutics 2001;3(4):581-9. [DOI] [PubMed] [Google Scholar]
Wolf 2013 {published data only}
- Wolf M S, Seligman H, Davis T C, Fleming D A, Curtis L M, Pandit A U, et al. Clinic-based versus outsourced implementation of a diabetes health literacy intervention. Journal of General Internal Medicine 2013;29(1):59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2018 {published data only}
- Wu W C, Taveira T H, Jeffery S, Jiang L, Tokuda L, Musial J, et al. Costs and effectiveness of pharmacist-led group medical visits for type-2 diabetes: a multi-center randomized controlled trial. PLOS One 2018;13(4):e0195898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang Y, Yao J J, Du J L, Bai R, Sun L P, Sun G H, et al. Primary prevention of macroangiopathy in patients with short-duration type 2 diabetes by intensified multifactorial intervention: seven-year follow-up of diabetes complications in Chinese. Diabetes Care 2013;36(4):978-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaron 2019 {published data only}
- Yaron M, Sher B, Sorek D, Shomer M, Levek N, Schiller T, et al. A randomized controlled trial comparing a telemedicine therapeutic intervention with routine care in adults with type 1 diabetes mellitus treated by insulin pumps. Acta Diabetologica 2019;56(6):667-73. [DOI] [PubMed] [Google Scholar]
Yin 2017 {published data only}
- Yin J, Luk A, Wong R, Chung H, Kong A, Ozaki R, et al. Regular mailing of personalized feedback reports improved glycemic control in diabetes - a randomized controlled trial. Journal of Diabetes 2017;9(5):536-8. [DOI] [PubMed] [Google Scholar]
Yoo 2009 {published data only}
- Yoo H J, Park M S, Kim T N, Yang S J, Cho G J, Hwang T G, et al. A ubiquitous chronic disease care system using cellular phones and the internet. Diabetic Medicine 2009;26(6):628-35. [DOI] [PubMed] [Google Scholar]
Yoon 2008 {published data only}
- Yoon K H, Kim H S. A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Research and Clinical Practice 2008;79(2):256-61. [DOI] [PubMed] [Google Scholar]
Yu 2019 {published data only}
- Yu Y, Yan Q, Li H, Li H, Wang L, Wang H, et al. Effects of mobile phone application combined with or without self-monitoring of blood glucose on glycemic control in patients with diabetes: a randomized controlled trial. Journal of Diabetes Investigation 2019;10(5):1365-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuan 2016 {published data only}
- Yuan X, Wang F, Fish A F, Xue C, Chen T, Liu C, Lou Q. Effect of case management on glycemic control and behavioral outcomes for Chinese people with type 2 diabetes: a 2-year study. Patient Education and Counseling 2016;99(8):1382-8. [DOI] [PubMed] [Google Scholar]
Zapotoczky 2001 {published data only}
- Zapotoczky H, Semlitsch B, Herzog G, Bahadori B, Siebenhofer A, Pieber T R, et al. A controlled study of weight reduction in type 2 diabetics treated by two reinforcers. International Journal of Behavioral Medicine 2001;8(1):42-9. [Google Scholar]
Zgibor 2018 {published data only}
- Zgibor JC, Maloney MA, Malmi M Jr, Fabio A, Kuo S, Solano FX, et al. Effectiveness of certified diabetes educators following pre-approved protocols to redesign diabetes care delivery in primary care: Results of the REMEDIES 4D trial. Contemporary Clinical Trials 2018;64:201-9. [DOI] [PubMed] [Google Scholar]
Zhou 2014 {published data only}
- Zhou P, Xu L, Liu X, Huang J, Xu W, Chen W. Web-based telemedicine for management of type 2 diabetes through glucose uploads: a randomized controlled trial. International Journal of Clinical and Experimental Pathology 2014;7(12):8848-54. [PMC free article] [PubMed] [Google Scholar]
Zhou 2016 {published data only}
- Zhou W, Chen M, Yuan J, Sun Y. Welltang - a smart phone-based diabetes management application - improves blood glucose control in Chinese people with diabetes. Diabetes Research and Clinical Practice 2016;116:105. [DOI] [PubMed] [Google Scholar]
Zolfaghari 2012 {published data only}
- Zolfaghari M, Mousavifar S A, Pedram S, Haghani H. The impact of nurse short message services and telephone follow-ups on diabetic adherence: which one is more effective? Journal of Clinical Nursing 2012;21(13-4):1922-31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abkenar 2016 {published data only}
- Abkenar MZ, Ghofranipour F, Maghrebi HF, Kashi Z, Shokravi FA. The effect of Islamic care method on nutritional self-care, anthropometric indices and blood pressure in diabetic patients. Journal of Mazandaran University of Medical Sciences 2016;26:36-53. [Google Scholar]
Agewall 2001 {published data only}
- Agewall S, Fagerberg B, Berglund G, Schmidt C, Wendelhag I, Wikstrand J, Risk Factor Intervention Study Group, Sweden. Multiple risk intervention trial in high risk hypertensive men: comparison of ultrasound intima-media thickness and clinical outcome during 6 years of follow-up. Journal of Internal Medicine 2001;249(4):305-14. [DOI] [PubMed] [Google Scholar]
Aguila 2018 {published data only}
- Aguila IP, Velázquez-López L, Goycochea-Robles MAV, Angulo-Angulo F, Peña JE. Multimedia education to support management of type 2 diabetes patients. A quasi-experimental study [La educación multimedia como apoyo en el manejo de pacientes con diabetes tipo 2. Estudio cuasi experimental]. Cirugía y Cirujanos 2018;86(5):404-11. [DOI] [PubMed] [Google Scholar]
Alfadda 2011 {published data only}
- Alfadda AA, Bin-Abdulrahman KA, Saad HA, Mendoza CDO, Angkaya-Bagayawa FF, Yale JF. Effect of an intervention to improve the management of patients with diabetes in primary care practice. Saudi Medical Journal 2011;32(1):36-40. [PubMed] [Google Scholar]
Basch 1999 {published data only}
- Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P. The effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. American Journal of Public Health 1999;89(12):1878-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharyya 2010 {published data only}
- Bhattacharya O, Harris S, Zwarenstein M, Barnsley J. Controlled trial of an intervention to improve cholesterol management in diabetes patients in remote Aboriginal communities. International Journal of Circumpolar Health 2010;69(4):333-43. [DOI] [PubMed] [Google Scholar]
Butt 2016 {published data only}
- Butt M, Mhd Ali A, Bakry MM, Mustafa N. Impact of a pharmacist led diabetes mellitus intervention on HbA1c, medication adherence and quality of life: A randomised controlled study. Saudi Pharmaceutical Journal 2016;24(1):40-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1996 {published data only}
- Campbell EM, Redman S, Moffitt PS, Sanson-Fisher RW. The relative effectiveness of educational and behavioral instruction programs for patients with NIDDM: a randomized trial. Diabetes Educator 1996;22(4):379-86. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen HS, Wu TE, Jap TS, Chen RL, Lin HD. Effects of health education on glycemic control during holiday time in patients with type 2 diabetes mellitus. American Journal of Managed Care 2008;14(1):45-51. [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen MF, Wang RH, Lin KC, Hsu HY, Chen SW. Efficacy of an empowerment program for Taiwanese patients with type 2 diabetes: A randomized controlled trial. Applied Nursing Research 2015;28(4):366-73. [DOI] [PubMed] [Google Scholar]
Chow 2016 {published data only}
- Chow EP, Hassali MA, Saleem F, Aljadhey H. Effects of pharmacist-led patient education on diabetes-related knowledge and medication adherence: a home-based study. Health Education Journal 2016;75(4):421-33. [Google Scholar]
Cortés‐Sanabria 2008 {published data only}
- Cortés-Sanabria L, Cabrera-Pivaral CE, Cueto-Manzano AM, Rojas-Campos E, Barragán G, Hernández-Anaya M, et al. Improving care of patients with diabetes and CKD: a pilot study for a cluster-randomized trial. American Journal of Kidney Diseases 2008;51(5):777-88. [DOI] [PubMed] [Google Scholar]
Cortez 2017 {published data only}
- Cortez DN, Macedo MM, Souza DA, Dos Santos JC, Afonso GS, Reis IA, Torres HC. Evaluating the effectiveness of an empowerment program for self-care in type 2 diabetes: a cluster randomized trial. BMC Public Health 2017;17(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2011 {published data only}
- Fisher L, Polonsky W, Parkin CG, Jelsovsky Z, Amstutz L, Wagner RS. The impact of blood glucose monitoring on depression and distress in insulin-naïve patients with type 2 diabetes. Current Medical Research and Opinion 2011;27(Suppl 3):39-46. [DOI] [PubMed] [Google Scholar]
Gaillard 2015 {published data only}
- Gaillard T, Amponsah G, Osei K. Patient-centered community diabetes education program improves glycemic control in African-American patients with poorly controlled type 2 diabetes: importance of point of care metabolic measurements. Journal of National Black Nurses' Association 2015;26(1):50-7. [PubMed] [Google Scholar]
Hajbaghery 2012 {published data only}
- Hajbaghery MA, Alinaghipoor T. The effects of lecture and multimodal methods of teaching on healing rate of diabetic foot ulcer and patients' compliance with care recommendations. Iran Journal of Nursing 2012;25:1-11. [Google Scholar]
Kim 2006 {published data only}
- Kim HS, Park HJ. Effects of a nurse short-message service via cellular phones for people with diabetes. Journal of Korean Academic Society of Nursing Education 2006;13(2):235-41. [Google Scholar]
Kushner 2009 {published data only}
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2011;17(5):1017-22. [DOI] [PubMed] [Google Scholar]
Litzelman 1993 {published data only}
- Litzelman DK, Slemenda CW, Langefeld CD, Hays LM, Welch MA, Bild DE, et al. Reduction of lower extremity clinical abnormalities in patients with non-insulin-dependent diabetes mellitus. A randomized, controlled trial. Annals of Internal Medicine 1993;119(1):36-41. [DOI] [PubMed] [Google Scholar]
Maislos 2004 {published data only}
- Maislos M, Weisman D. Multidisciplinary approach to patients with poorly controlled type 2 diabetes mellitus: a prospective, randomized study. Acta Diabetologica 2004;41(2):44-8. [DOI] [PubMed] [Google Scholar]
Mazzuca 1988 {published data only}
- Mazzuca SA, Vinicor F, Cohen SJ, Norton JA, Fineberg NS, Fineberg SE, et al. The Diabetes Education Study: a controlled trial of the effects of intensive instruction of internal medicine residents on the management of diabetes mellitus. Journal of General Internal Medicine 1988;3(1):1-8. [DOI] [PubMed] [Google Scholar]
Mehler 2005 {published data only}
- Mehler PS, Krantz MJ, Lundgren RA, Estacio RO, MacKenzie TD, Petralia L, et al. Bridging the quality gap in diabetic hyperlipidemia: a practice-based intervention. American Journal of Medicine 2005;118(12):1414. [DOI] [PubMed] [Google Scholar]
Polonsky 2011 {published data only}
- Polonsky W H, Fisher L, Schikman C H, Hinnen D A, Parkin C G, Jelsovsky Z, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care 2011;34(2):262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saengtipbovorn 2014 {published data only}
- Saengtipbovorn S, Taneepanichuskul S. Effectiveness of lifestyle change plus dental care (LCDC) program on improving glycemic and periodontal status in the elderly with type 2 diabetes. BMC Oral Health 2014;14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segal 2016 {published data only}
- Segal L, Nguyen H, Schmidt B, Wenitong M, McDermott RA. Economic evaluation of Indigenous health worker management of poorly controlled type 2 diabetes in north Queensland. Medical Journal of Australia 2016;204(5):1961e-9. [DOI] [PubMed] [Google Scholar]
Teychenne 2015 {published data only}
- Teychenne M, Ball K, Salmon J, Daly RM, Crawford DA, Sethi P, et al. Adoption and maintenance of gym-based strength training in the community setting in adults with excess weight or type 2 diabetes: a randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2015;12:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weitzman 2009 {published data only}
- Weitzman S, Greenfield S, Billimek J, Hava T, Schvartzman P, Yehiel E, et al. Improving combined diabetes outcomes by adding a simple patient intervention to physician feedback: a cluster randomized trial. Israel Medical Association Journal 2009;11(12):719-24. [PubMed] [Google Scholar]
References to studies awaiting assessment
Ali Abdelhamid 2021 {published data only}
- Ali Abdelhamid Y, Phillips LK, White MG, Presneill J, Horowitz M, Deane AM. Survivors of intensive care with type 2 diabetes and the effect of shared-care follow-up clinics: the SWEET-AS randomized controlled pilot study. Chest 2021;159(1):174-85. [DOI] [PubMed] [Google Scholar]
Alison 2020 {published data only}
- Alison C, Anselm S. The effectiveness of diabetes medication therapy adherence clinic to improve glycaemic control among patients with type 2 diabetes mellitus: a randomised controlled trial. Medical Journal of Malaysia 2020;75(3):246-53. [PubMed] [Google Scholar]
Al‐Taie 2020 {published data only}
- Al-Taie A, Izzettin FV, Sancar M, Köseoğlu A. Impact of clinical pharmacy recommendations and patient counselling program among patients with diabetes and cancer in outpatient oncology setting. European Journal of Cancer Care 2020;29(5):e13261. [DOI] [PubMed] [Google Scholar]
Bohingamu Mudiyanselage 2019 {published data only}
- Bohingamu Mudiyanselage S, Stevens J, Watts JJ, Toscano J, Kotowicz MA, Steinfort CL, et al. Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial. Journal of Telemedicine and Telecare 2019;25(6):343-52. [DOI] [PubMed] [Google Scholar]
Buysse 2020 {published data only}
- Buysse H, Coremans P, Pouwer F, Ruige J. Sustainable improvement of HbA1c and satisfaction with diabetes care after adding telemedicine in patients on adaptable insulin regimens: Results of the TeleDiabetes randomized controlled trial. Health Informatics Journal 2020;26(1):628-41. [DOI] [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen X, Jiang S, Xu Y, Tian Y. The effect of interactive health education based on the WeChat platform on diabetic outpatients. International Journal of Clinical and Experimental Medicine 2019;12(11):13154-62. [Google Scholar]
Daud 2020 {published data only}
- Daud MH, Ramli AS, Abdul-Razak S, Haniff J, Tg-Abu-Bakar-Sidik TMI, Mohd-Hatta NKB, et al. Effectiveness of the EMPOWER-PAR intervention on primary care providers’ adherence to clinical practice guidelines on the management of type 2 diabetes mellitus: a pragmatic cluster randomized controlled trial. Open Access Macedonian Journal of Medical Sciences 2020;8(B):470-9. [Google Scholar]
Egede 2021a {published data only}
- Egede LE, Dawson AZ, Walker RJ, Garraci E, Knapp RG. Randomized controlled trial of technology-assisted case management in low-income adults with type 2 diabetes: Effect on quality of life and blood pressure. Journal of Telemedicine and Telecare 2021;12:1357633X211028491. [DOI] [PubMed] [Google Scholar]
Egede 2021b {published data only}
- Egede LE, Dismuke CE, Walker RJ, Williams JS, Eiler C. Cost-effectiveness of technology-assisted case management in low-income, rural adults with type 2 diabetes. Health Equity 2021;5(1):503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franc 2019 {published data only}
- Franc S, Joubert M, Daoudi A, Fagour C, Benhamou PY, Rodier M, et al, TeleDiab study group. Efficacy of two telemonitoring systems to improve glycaemic control during basal insulin initiation in patients with type 2 diabetes: The TeleDiab-2 randomized controlled trial. Diabetes, Obesity and Metabolism 2019;21(10):2327-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franc 2020 {published data only}
- Franc S, Hanaire H, Benhamou PY, Schaepelynck P, Catargi B, Farret A, et al. DIABEO system combining a mobile app software with and without telemonitoring versus standard care: a randomized controlled trial in diabetes patients poorly controlled with a basal-bolus insulin regimen. Diabetes Technology & Therapeutics 2020;22(12):904-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gimbel 2020 {published data only}
- Gimbel RW, Rennert LM, Crawford P, Little JR, Truong K, Williams JE, et al. Enhancing patient activation and self-management activities in patients with type 2 diabetes using the US Department of Defense mobile health care environment: feasibility study. Journal of Medical Internet Research 2020;22(5):e17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2019 {published data only}
- Guo Z, Liu J, Zeng H, He G, Ren X, Guo J. Feasibility and efficacy of nurse-led team management intervention for improving the self-management of type 2 diabetes patients in a Chinese community: a randomized controlled trial. Patient Prefer Adherence 2019;13:1353-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heselmans 2020 {published data only}
- Heselmans A, Delvaux N, Laenen A, Van de Velde S, Ramaekers D, Kunnamo I, et al. Computerized clinical decision support system for diabetes in primary care does not improve quality of care: a cluster-randomized controlled trial. Implementation Science 2020;15(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2021 {published data only}
- Hu X, Deng H, Zhang Y, Guo X, Cai M, Ling C, Li K. Efficacy and safety of a decision support intervention for basal insulin self-titration assisted by the nurse in outpatients with T2DM: a randomized controlled trial. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2021;14:1315-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Javaid 2019 {published data only}
- Javaid Z, Imtiaz U, Khalid I, Saeed H, Khan RQ, Islam M, et al. A randomized control trial of primary care-based management of type 2 diabetes by a pharmacist in Pakistan. BMC Health Services Research 2019;19(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2021 {published data only}
- Kang J, Chen Y, Zhao Y, Zhang C. Effect of remote management on comprehensive management of diabetes mellitus during the COVID-19 epidemic. Primary Care Diabetes 2021;15(3):417-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2021 {published data only}
- Khan AH, Iqbal MZ, Syed Sulaiman SA, Ibrahim A, Azmi NSBY, Iqbal MS, et al. Impact of pharmacist-led educational intervention on predictors of diabetic foot at two different hospitals of Malaysia. Journal of Pharmacy & Bioallied Sciences 2021;13(1):108-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2022 {published data only}
- Kim Y, Lee H, Seo JM. Integrated diabetes self-management program using smartphone application: a randomized controlled trial. Western Journal of Nursing Research 2022;44(4):383-94. [DOI] [PubMed] [Google Scholar]
Ku 2020 {published data only}
- Ku EJ, Park JI, Jeon HJ, Oh TK, Choi HJ. Clinical efficacy and plausibility of a smartphone-based integrated online real-time diabetes care system via glucose and diet data management: a pilot study. Internal Medicine Journal 2020;50(12):1524-32. [DOI] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee JY, Chan CKY, Chua SS, Ng CJ, Paraidathathu T, Lee KKC, Lee SWH. Telemonitoring and team-based management of glycemic control on people with type 2 diabetes: a cluster-randomized controlled trial. Journal of General Internal Medicine 2020;35(1):87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2020A {published data only}
- Lee DY, Yoo SH, Min KP, Park CY. Effect of voluntary participation on mobile health care in diabetes management: randomized controlled open-label trial. JMIR Mhealth and Uhealth 2020;8(9):e19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lou 2019 {published data only}
- Lou Q, Ye Q, Wu H, Wang Z, Ware RS, Xiong Y, et al. Effectiveness of a clinic-based randomized controlled intervention for type 2 diabetes management: an innovative model of intensified diabetes management in Mainland China (C-IDM study). BMJ Open Diabetes Research & Care 2019;8(1):e001030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Millan‐Ferro 2020 {published data only}
- Millan-Ferro A, Garcia-Dolagaray G, Gautam S, Caballero AE, Mitri J. Impact of monthly A1C values obtained at home on glycemic control in patients with type 2 diabetes: a randomized clinical trial. Clinical Diabetes 2020;38(3):230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2020 {published data only}
- Murphy ME, McSharry J, Byrne M, Boland F, Corrigan D, Gillespie P, et al. Supporting care for suboptimally controlled type 2 diabetes mellitus in general practice with a clinical decision support system: a mixed methods pilot cluster randomised trial. BMJ Open 2020;10(2):e032594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noda 2020 {published data only}
- Noda M, Hayashino Y, Yamazaki K, Suzuki H, Goto A, Kato M, et al. A cluster-randomized trial of the effectiveness of a triple-faceted intervention promoting adherence to primary care physician visits by diabetes patients. Scientific Reports 2020;10(1):2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Or 2020 {published data only}
- Or CK, Liu K, So MKP, Cheung B, Yam LYC, Tiwari A, et al. Improving self-care in patients with coexisting type 2 diabetes and hypertension by technological surrogate nursing: randomized controlled trial. Journal of Medical Internet Research 2020;22(3):e16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramallo‐Farina 2020 {published data only}
- Ramallo-Fariña Y, García-Bello MA, García-Pérez L, Boronat M, Wägner AM, Rodríguez-Rodríguez L, et al, INDICA Team. Effectiveness of internet-based multicomponent interventions for patients and health care professionals to improve clinical outcomes in type 2 diabetes evaluated through the indica study: multiarm cluster randomized controlled trial. JMIR Mhealth and Uhealth 2020;8(11):e18922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reininger 2020 {published data only}
- Reininger BM, Lee M, Hessabi M, Mitchell-Bennett LA, Sifuentes MR, Guerra JA, et al. Improved diabetes control among low-income Mexican Americans through community-clinical interventions: results of an RCT. BMJ Open Diabetes Research & Care 2020;8(1):e000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovner 2020 {published data only}
- Rovner BW, Casten RJ, Piersol CV, White N, Kelley M, Leiby BE. Improving glycemic control in African Americans with diabetes and mild cognitive impairment. Journal of the American Geriatrics Society 2020;68(5):1015-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz de Adana 2020 {published data only}
- Ruiz de Adana MS, Alhambra-Expósito MR, Muñoz-Garach A, Gonzalez-Molero I, Colomo N, Torres-Barea I, et al, Diabetes Group of SAEDYN (Andalusian Society of Endocrinology, Diabetes, and Nutrition). Randomized study to evaluate the impact of telemedicine care in patients with type 1 diabetes with multiple doses of insulin and suboptimal HbA1c in Andalusia (Spain): PLATEDIAN Study. Diabetes Care 2020;43(2):337-42. [DOI] [PubMed] [Google Scholar]
Vaccaro 2013 {published data only}
- Vaccaro O, Franzini L, Miccoli R, Cavalot F, Ardigo D, Boemi M, et al. Feasibility and effectiveness in clinical practice of a multifactorial intervention for the reduction of cardiovascular risk in patients with type 2 diabetes: the 2-year interim analysis of the MIND.IT study: a cluster randomized trial. Diabetes Care 2013;36(9):2566-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Storch 2019 {published data only}
- Storch K, Graaf E, Wunderlich M, Rietz C, Polidori MC, Woopen C. Telemedicine-assisted self-management program for type 2 diabetes patients. Diabetes Technology & Therapeutics 2019;21(9):514-21. [DOI] [PubMed] [Google Scholar]
Wang 2019 {published data only}
- Wang Y, Li M, Zhao X, Pan X, Lu M, Lu J, Hu Y. Effects of continuous care for patients with type 2 diabetes using mobile health application: a randomised controlled trial. International Journal of Health Planning and Management 2019;34(3):1025-35. [DOI] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang W, Cheng MTM, Leong FL, Goh AWL, Lim ST, Jiang Y. The development and testing of a nurse-led smartphone-based self-management programme for diabetes patients with poor glycaemic control. Journal of Advanced Nursing 2020;76(11):3179-89. [DOI] [PubMed] [Google Scholar]
White 2020 {published data only}
- White RO, Chakkalakal RJ, Wallston KA, Wolff K, Gregory B, Davis D, et al. The partnership to improve diabetes education trial: a cluster randomized trial addressing health communication in diabetes care. Journal of General Internal Medicine 2020;35(4):1052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willis 2020 {published data only}
- Willis A, Crasto W, Gray LJ, Dallosso H, Waheed G, Davies M, et al. Effects of an electronic software "prompt" with health care professional training on cardiovascular and renal complications in a multiethnic population with type 2 diabetes and microalbuminuria (the GP-Prompt Study): results of a pragmatic cluster-randomized trial. Diabetes Care 2020;43(8):1893-901. [DOI] [PubMed] [Google Scholar]
Xu 2020 {published data only}
- Xu R, Xing M, Javaherian K, Peters R, Ross W, Bernal-Mizrachi C. Improving HbA1c with glucose self-monitoring in diabetic patients with EpxDiabetes, a phone call and text message-based telemedicine platform: a randomized controlled trial. Telemedicine and e-Health 2020;26(6):784-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2021 {published data only}
- Xu Y, Gallagher PJ, Tan CW, Tsou KY, Tan DH, Ramaya H, Lee JY. Impact of team‐based pharmaceutical care on the humanistic outcomes among patients with long‐standing diabetes: an interim analysis of a randomized, controlled, multicenter study. Journal of the American College of Clinical Pharmacy 2021;4:680-8. [Google Scholar]
Yang 2020 {published data only}
- Yang Y, Lee EY, Kim HS, Lee SH, Yoon KH, Cho JH. Effect of a mobile phone-based glucose-monitoring and feedback system for type 2 diabetes management in multiple primary care clinic settings: cluster randomized controlled trial. JMIR mHealth and uHealth 2020;8(2):e16266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang L, He X, Shen Y, Yu H, Pan J, Zhu W, et al. Effectiveness of smartphone app-based interactive management on glycemic control in Chinese patients with poorly controlled diabetes: randomized controlled trial. Journal of Medical Internet Research 2019;21(12):e15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2020 {published data only}
- Zhao CM, Cui XL, Wan G, Lu YZ, Niu YQ, Su CY, et al. Analysis of the effect of nine consecutive years' intensive management and number of times achieving the target control on endpoint events in T2DM patients in Sanlitun community health service center in Beijing. International Journal of Endocrinology 2020;2020:3646342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
American Diabetes Association 2022
- American Diabetes Association Professional Practice Committee. Standards of Medical Care in Diabetes - 2022. Diabetes Care 2022;45(Suppl 1):S1-S264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arditi 2017
- Arditi C, Rège‐Walther M, Durieux P, Burnand B. Computer‐generated reminders delivered on paper to healthcare professionals: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD001175. [DOI: 10.1002/14651858.CD001175.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 1998
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics 1998;7(4):434–55. [Google Scholar]
Captieux 2018
- Captieux M, Pearce G, Parke HL, Epiphaniou E, Wild S, Taylor SJC, et al. Supported self-management for people with type 2 diabetes: a meta-review of quantitative systematic reviews. BMJ Open 2018;8(12):e024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chambers 2013
- Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Science 2013;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemens 2021
- Clemens KK, Ouédraogo AM, Garg AX, Silver SA, Nash DM. Opportunities to improve diabetes care in the hemodialysis unit: a cohort study in Ontario, Canada. Kidney 360 2021;2(4):653-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danko 2019
- Danko KJ, Dahabreh IJ, Ivers NM, Moher D, Grimshaw JM. Contacting authors by telephone increased response proportions compared with emailing: results of a randomized study. Journal of Clinical Epidemiology 2019;115:150-9. [DOI] [PubMed] [Google Scholar]
DistillerSR 2021 [Computer program]
- DistillerSR. Version 2.35. Evidence Partners, 2021. Accessed March 2017-June 2019. https://www.evidencepartners.com.
Duke 2009
- Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD005268. [DOI: 10.1002/14651858.CD005268.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fang 2021
- Fang W, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999–2018. New England Journal of Medicine 2021;384:2219-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsetlund 2021
- Forsetlund L, O'Brien MA, Forsén L, Mwai L, Reinar LM, Okwen MP, et al. Continuing education meetings and workshops: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2021, Issue 9. Art. No: CD003030. [DOI: 10.1002/14651858.CD003030.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
French 2012
- French SD, Green SE, O’Connor DA, McKenzie JE, Francis JJ, Michie S, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implementation Science 2012;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2010
- Gardner B, Whittington C, McAteer J, Eccles MP, Michie S. Using theory to synthesise evidence from behaviour change interventions: the example of audit and feedback. Social Science and Medicine 2010;70(10):1618-25. [DOI] [PubMed] [Google Scholar]
Gelman 1992
- Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical Science 1992;7(4):457-72. [Google Scholar]
Gelman 2002
- Gelman A, Stevens M, Chan V. Regression modeling and meta-analysis for decision making: a cost-benefit analysis of incentives in telephone surveys. Journal of Business and Economic Statistics 2002;21:213-25. [Google Scholar]
Gæde 2008
- Gæde P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New England Journal of Medicine 2008;358:580-91. [DOI] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JPT, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivers 2012
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard‐Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD000259. [DOI: 10.1002/14651858.CD000259.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivers 2016
- Ivers NM, Grimshaw JM. Reducing research waste with implementation laboratories. Lancet 2016;388(10044):547-8. [DOI] [PubMed] [Google Scholar]
Konnyu (in press)
- Konnyu KJ, Grimshaw JM, Trikalinos TA, Ivers NM, Moher D, Dahabreh IJ. Evidence synthesis for complex interventions using meta-regression models. American Journal Of Epidemiology (in press). [DOI] [PMC free article] [PubMed]
Konnyu 2020
- Konnyu KJ, McCleary N, Presseau J, Ivers NM, Grimshaw JM. Behavior change techniques in continuing professional development. Journal of Continuing Education in the Health Professions 2020;40(4):268-73. [DOI] [PubMed] [Google Scholar]
Konnyu 2021
- Konnyu KJ, Taljaar M, Ivers NM, Moher D, Grimshaw JM. Imputing intracluster correlation coefficients from a posterior predictive distribution is a feasible method of dealing with unit of analysis errors in a meta-analysis of cluster RCTs. Journal of Clinical Epidemiology 2021;S0895-4356(21):00189-X. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials published world-wide – the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerging Themes in Epidemiology 2008;5:13. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021:Available from: www.training.cochrane.org/handbook. [Google Scholar]
Leiter 2019
- Leiter LA, Cheng AYY, Ekoé JM, Goldenberg RM, Harris SB, Hramiak IM, et al. Glycated hemoglobin level goal achievement in adults with type 2 diabetes in Canada: still room for improvement. Canadian Journal of Diabetes 2019;43(6):384-91. [DOI] [PubMed] [Google Scholar]
Liu 2017
- Liu XL, Shi Y, Willis K, Wu CJ, Johnson M. Health education for patients with acute coronary syndrome and type 2 diabetes mellitus: an umbrella review of systematic reviews and meta-analyses. BMJ Open 2017;7(10):e016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2011
- Michie S, Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Science 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Johnston M. Behavioural change techniques. In: Gellman MD, Turner JR, editors(s). Encyclopedia of Behavioral Medicine. New York: Springer New York, 2013:182–7. [Google Scholar]
Mosenzon 2021
- Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovascular Diabetology 2021;20(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2022a
- National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. https://www.nice.org.uk/guidance/ng28 2022. [PubMed]
NICE 2022b
- National Institute for Health and Care Excellence . Type 1 diabetes in adults: diagnosis and management. https://www.nice.org.uk/guidance/ng17 2022. [PubMed]
Oh 2021
- Oh H, Nguyen HD, Yoon IM, Ahn BR, Kim MS. Antidiabetic effect of gemigliptin: a systematic review and meta-analysis of randomized controlled trials with Bayesian inference through a quality management system. Scientific Reports 2021;11(1):20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pantoja 2019
- Pantoja T, Grimshaw JM, Colomer N, Castañon C, Leniz Martelli J. Manually‐generated reminders delivered on paper: effects on professional practice and patient outcomes. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD001174. [DOI: 10.1002/14651858.CD001174.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plummer 2003
- Plummer, M. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In: 3rd International Workshop on Distributed Statistical Computing (DSC 2003). Vienna, Austria, 2003.
Presseau 2015
- Presseau J, Ivers NM, Newham JJ, Knittle K, Danko KJ, Grimshaw JM. Using a behaviour change techniques taxonomy to identify active ingredients within trials of implementation interventions for diabetes care. Implementation Science 2015;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, 2020.
Rose 1981
- Rose G. Strategy of prevention: lessons from cardiovascular disease. British Medical Journal (Clinical research ed.) 1981;282(6279):1847-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubin 1992
- Rubin DB. Meta-analysis: literature synthesis or effect-size surface estimation? Journal of Educational Statistics 1992;17(4):363-74. [Google Scholar]
Rushforth 2016
- Rushforth B, McCrorie C, Glidewell L, Midgley E, Foy R. Barriers to effective management of type 2 diabetes in primary care: qualitative systematic review. British Journal of General Practice 2016;66(643):e114-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmid 2020
- Schmid CH, Stijnen T, White IR. Handbook of Meta-Analysis. Chapman and Hall/CRC, 2020. [Google Scholar]
Schünemann 2013
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Shojania 2009
- Shojania KG, Jennings A, Ramsay CR, Grimshaw JM, Kwan JL, Lo L. The effects of on‐screen, point of care computer reminders on processes and outcomes of care. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD001096. [DOI: 10.1002/14651858.CD001096.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
StataCorp 2021 [Computer program]
- Stata Statistical Software. Version 17. College Station, TX: StataCorp LLC, 2021.
Stratton 2000
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes(UKPDS 35): prospective observational study. BMJ 2000;321:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Worswick 2013
- Worswick J, Wayne SC, Bennett R, Fiander M, Mayhew A, Weir MC, et al. Improving quality of care for persons with diabetes: an overview of systematic reviews - what does the evidence tell us? Systematic Reviews 2013;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ivers 2014
- Ivers N, Tricco AC, Trikalinos TA, Dahabreh IJ, Danko KJ, Moher D, et al. Seeing the forests and the trees--innovative approaches to exploring heterogeneity in systematic reviews of complex interventions to enhance health system decision-making: a protocol. Systematic Reviews 2014;3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shojania 2006
- Shojania K G, Ranji S R, McDonald K M, Grimshaw J M, Sundaram V, Rushakoff R J, Owens D K. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–40. [DOI] [PubMed] [Google Scholar]
Tricco 2012
- Tricco A C, Ivers N M, Grimshaw J M, Moher D, Turner L, Galipeau J, Halperin I, Vachon B, Ramsay T, Manns B, Tonelli M, Shojania K. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–61. [DOI] [PubMed] [Google Scholar]