Background:
Alcohol-associated hepatitis (AH) is among the deadliest liver diseases, but its incidence is poorly defined. The aim of our study was to define the incidence of AH meeting the National Institute on Alcohol Abuse and Alcoholism criteria and to identify risk factors for AH.
Methods:
We conducted a retrospective cohort study using the Rochester epidemiology project database on adult patients hospitalized with AH between January 1, 2000 and December 31, 2018. Patients were screened using ICD-9 codes and then included if they met the National Institute on Alcohol Abuse and Alcoholism criteria on manual chart review. Baseline demographics, comorbidities, access to care, liver-related complications, and outcomes were obtained. The HOUsing-based index of SocioEconomic status index was used to measure socioeconomic status. Incidence rates were calculated in cases per 100,000 person-years of follow-up.
Results:
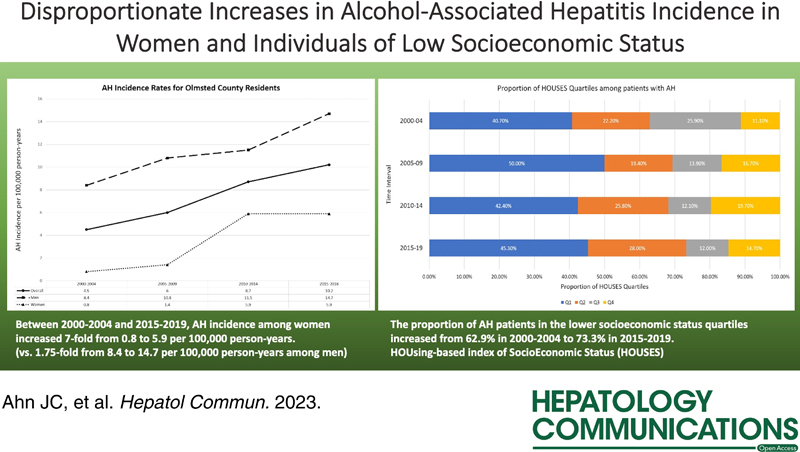
Among 204 patients, the cumulative AH incidence was 6.8 per 100,000 person-years. Between 2000–2004 and 2015–2018, AH incidence among males increased from 8.4 to 14.7 per 100,000 py, whereas AH incidence among females increased by 7-fold from 0.8 to 5.9 per 100,000 py. Such increases among females were accompanied by increases in comorbid depression and anxiety. The proportion of patients with AH in the lower socioeconomic status quartiles increased from 62.9% between 2000 and 2004 to 73.3% between 2015 and 2019.
Conclusions:
The incidence of AH is increasing rapidly, especially among females and individuals of lower socioeconomic status. There are areas of unmet need in preventative measures and treatments for comorbid psychiatric disorders in patients at high risk of AH.
INTRODUCTION
Alcohol-associated liver disease is a major and growing cause of morbidity and mortality worldwide.1 Alcohol-associated liver disease is responsible for ~25% of cirrhosis-related deaths and 30% of liver cancer deaths globally2 and has surpassed hepatitis C to become the leading indication for liver transplantation in the US.3 This trend is only expected to get worse as the burden of alcohol use disorder continues to increase. In the US, the prevalence of alcohol use disorder increased by 50% over the first decade of the 21st century with the highest rates of increase seen among women and individuals of low socioeconomic status (SES).4 Across the spectrum of alcohol-associated liver disease progression from hepatic steatosis to cirrhosis, alcohol-associated hepatitis (AH) is a distinct clinical syndrome that occurs in patients with chronic and active heavy alcohol consumption. Patients with AH present acutely with symptoms and signs of advanced liver disease such as jaundice, right upper quadrant pain, and elevated liver enzymes, as well as systemic inflammatory response syndrome with or without superimposed infections.5 Those with severe AH can develop acute-on-chronic liver failure with multiorgan failures and have over 30% risk of death within 30 days, representing one of the deadliest diseases in clinical hepatology.6 Despite the seriousness of the disease, there has been an unmet need for well-constructed epidemiologic studies defining the incidence of AH in the US.7 This is in part due to the historical lack of a clear consensus definition for AH, with the diagnostic accuracy of administrative coding for AH being highly unreliable.8 In 2016, the National Institute on Alcohol Abuse and Alcoholism (NIAAA)-funded consortia on AH have proposed the following consensus definition for AH: onset of jaundice within 60 days of heavy alcohol consumption (>40 g/d for females; >60 g/d for males) for a minimum of 6 months, serum bilirubin more than 3 mg/dL, AST >50 IU/mL and <400 IU/mL, AST:ALT ratio >1.5, and liver biopsy confirmation in patients with confounding factors, which has been widely adopted in new clinical trials for patients with AH.9 To date, however, there has not been a population-based epidemiologic study to define the incidence of AH according to the NIAAA criteria. The aim of our study was to conduct a population-based study to assess the incidence of AH meeting the NIAAA criteria and its association with their sociodemographic factors such as sex and SES.
METHODS
Study setting and population
We conducted a population-based retrospective cohort study using the Rochester epidemiology project (REP) database. The REP is a unique medical records-linkage system that encompasses the care delivered to all eligible residents of Rochester and Olmsted County, Minnesota, enabling epidemiologic studies on the occurrence and natural history of a wide array of diseases in a defined population.10 The REP database includes clinical documentation and test results from Mayo Clinic, Olmsted Medical Center, and their affiliated clinics and hospitals. The REP population has been shown to be representative of Minnesota and the Upper Midwest, and studies have shown that mortality rates of Olmsted County residents are similar to those of Minnesota and the entire US.11 Within the field of hepatology, the REP was used for epidemiologic studies of NAFLD,12 primary sclerosing cholangitis,13 HCC,14 and cholangiocarcinoma.15
Study design
We conducted a population-based, retrospective cohort study that assessed the incidence of all AH cases in adults living in the Olmsted County, MN overall and by sex during the study period (2000–2018). By estimating period incidence, our present study results allow us to assess stability or trends of AH incidence during our study period. We also assessed association of accumulative incidence of AH with individual-level SES as measured by HOUsing-based index of SocioEconomic Status (HOUSES) index.
Study subjects
A search of the REP database was conducted to identify all eligible residents of the Olmsted County above 18 years of age who were hospitalized with a primary diagnosis of AH (ICD-9-CM 571.1; ICD-10-CM K70.1) or alcohol-associated cirrhosis (ICD-9-CM 571.2; ICD-10-CM K70.3) between January 1, 2000, and December 31, 2018. A manual review of every patient’s medical record was performed to determine whether they met the consensus definition of AH according to the NIAAA criteria: onset of jaundice within 60 days of heavy alcohol consumption (>40 g/d for females; > 60 g/d for males) for a minimum of 6 months, serum bilirubin more than 3 mg/dL, AST > 50 IU/mL and < 400 IU/mL, AST:ALT ratio >1.5, and liver biopsy confirmation in patients with confounding factors. Only patients meeting all of the NIAAA criteria were included, and patients not meeting one or more of the NIAAA criteria were excluded. This study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center.
Data collection
We collected information on baseline demographic characteristics (age, sex, and ethnicity), underlying comorbidities (obesity, hypertension, hyperlipidemia, depression, anxiety, tobacco use, i.v. drug use, and hepatitis C infection), access to care (addiction therapy and primary care visits), presence of liver-related complications (ascites, variceal hemorrhage, hepatic encephalopathy, acute kidney injury, sepsis, and cirrhosis), the model for end-stage liver disease (MELD) score on admission, and clinical outcomes [intensive care unit (ICU) admission and 30 and 180-day mortality] for those confirmed AH cases by manual chart review.
Housing-based index of socioeconomic status index as a measure of individual-level socioeconomic status
For assessment of patient’s SES, we used an individual-level measure of SES called the HOUSES index. Developed by the HOUSES Program at Mayo Clinic, the HOUSES index is an innovative individual housing-based measure of SES.16 In capturing one’s SES, many surrogate measures (income, education, occupation, or composite scores) that are commonly used in the literature are self-reported, nonscalable, and often unavailable in commonly used data set for research such as medical records. HOUSES is an objective, scalable, and precise measure not relying on self-report. HOUSES is a composite index (z-score) consisting of publicly available real property data (ie, assessed housing value, square footage, and the numbers of bedrooms and bathrooms), which can be directly linked to patient’s address at the time of index date (eg, first diagnosis date of AH).17 Based on the calculated z-score (the higher HOUSES, the higher SES), subjects can be assigned into categorical variable such as quartiles [Q1 (lowest SES group)–Q4] or even more granular categories as it is a continuous numerical variable. To date, the HOUSES index has been shown to be a reliable measure of SES predicting a broad range of (38 different) health outcomes in adults and including chronic conditions (eg, rheumatoid arthritis,18 glioma,19 and kidney transplant outcomes.20), acute conditions (eg, pneumococcal diseases21 and ICU mortality), and other health outcomes (eg, HPV vaccine update rate). As HOUSES is formulated and standardized among nearly all Olmsted County population, we applied the HOUSES index to all patients in our study to assess disparities in AH incidence according to SES.
Statistical Analysis
Continuous variables such as age, body mass index, and MELD score were summarized in median values with interquartile ranges. Categorical variables were summarized in overall proportions. Age and sex-adjusted incidence rates for AH for Olmsted County residents were calculated in cases per 100,000 person-years of follow-up with the adjusting population based on the U.S. census. We assessed the relationship of crude incidence rates to sex, year of diagnosis, age at diagnosis, and quartile of HOUSE index using generalized linear models assuming a Poisson error structure, and using a log link function and a log (population) offset.
RESULTS
Cohort selection
The initial search of the REP database between years 2000 and 2018 using the ICD codes identified 254 first-time hospital admissions with diagnosis of “AH” and 365 first-time hospital admissions with diagnosis of “alcohol-associated cirrhosis” among the residents of Olmsted County. After manual chart review, only 156 of 456 (34.2%) patients hospitalized with ICD codes for AH were found to meet the NIAAA criteria for diagnosis of AH. In many cases, the patients had abnormal transaminases in the context of significant drinking history but did not have elevated bilirubin levels. In addition, it was found that 48 of 823 (5.8%) of patients hospitalized with ICD codes for alcohol-associated cirrhosis in fact met the NIAAA criteria for AH but were never diagnosed with AH and instead were documented as having “decompensated alcohol-associated cirrhosis”. This resulted in a total of 204 patients with incident AH meeting the NIAAA criteria (Figure 1).
FIGURE 1.
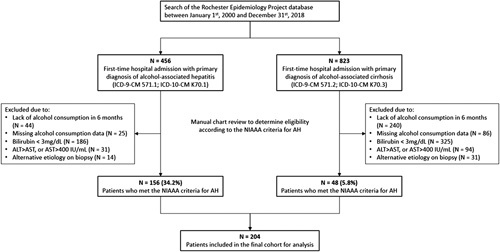
A flowchart of study cohort selection. Abbreviations: AH, alcohol-associated hepatitis; NIAAA, National Institute on Alcohol Abuse and Alcoholism.
Among the 300 patients with ICD codes for alcohol-associated cirrhosis who were excluded for not meeting the NIAAA criteria for AH, 44 (14.6%) had no alcohol consumption in the preceding 6 months, 25 (8.3%) had missing data on alcohol consumption, 186 (62%) had bilirubin <3 mg/dL, 31 (10.3%) had ALT greater than AST or AST over 400 IU/mL, and 14 (4.7%) had alternative etiology identified on liver biopsy. In contrast, among the 775 patients with ICD codes for alcohol-associated cirrhosis who were excluded for not meeting the NIAAA criteria for AH, 240 (30.9%) had no alcohol consumption in the preceding 6 months, 86 (11.1%) had missing data on alcohol consumption, 325 (41.9%) had bilirubin <3 mg/dL, 94 (12.1%) had ALT greater than AST or AST over 400 IU/mL, and 31 (4.0%) had alternative etiology identified on liver biopsy (Figure 1).
Patient characteristics
Table 1 provides the overall baseline characteristics of the study cohort as well as liver-related complications and outcomes. The patient’s median age at the time of presentation was 50 years. There were more males than females, and the vast majority of the patients were White (93.9%). Almost half of the study subjects were from the lowest SES category defined by HOUSES (Q1: 49.3%). Median body mass index was 27.0 kg/m2. Comorbid tobacco use, i.v. drug use, depression, and anxiety were documented in 74.5%, 7.8%, 46.1%, and 27.9% of patients, respectively. Overall, 44.3% of the patients had previously received therapy for alcohol addiction, and 56.9% of patients were seen by a primary care provider within 1 year preceding their AH admission. Patients had a median MELD score of 21 at the time of admission, and 65.7% of patients had either imaging or histologic evidence of cirrhosis. Complications of AH such as ascites, variceal hemorrhage, hepatic encephalopathy, acute kidney injury, and sepsis were documented in 55.9%, 9.3%, 33.3%, 22.1%, and 18.1% of patients during their hospitalization, respectively, and 34.3% of patients were admitted to the ICU. Mortality at 30 days and 180 days was 12.7% and 22.1%, respectively.
TABLE 1.
Baseline characteristics and outcomes of patients with AH
| Total (N = 204); n (%) | Year of diagnosis | No. patients | |
|---|---|---|---|
| Sex | — | 2000 | 4 |
| Female | 72 (35.3) | 2001 | 4 |
| Male | 132 (64.7) | 2002 | 5 |
| Age | — | 2003 | 6 |
| Median | 50.0 | 2004 | 5 |
| Q1, Q3 | 43.5, 58.0 | 2005 | 3 |
| Race | — | 2006 | 4 |
| White | 185 (93.9) | 2007 | 10 |
| Black | 2 (1.0) | 2008 | 8 |
| Asian | 4 (2.0) | 2009 | 11 |
| Am Indian | 1 (0.5) | 2010 | 13 |
| Other | 5 (2.5) | 2011 | 13 |
| BMI | — | 2012 | 10 |
| Median | 27.0 | 2013 | 12 |
| Q1, Q3 | 23.5, 32.0 | 2014 | 20 |
| HTN | 104 (51.0) | 2015 | 13 |
| Hyperlipidemia | 58 (28.4) | 2016 | 21 |
| DM | 31 (15.2) | 2017 | 18 |
| HCV | 23 (11.3) | 2018 | 24 |
| Tobacco use | 152 (74.5) | — | — |
| Intravenous drug use | 16 (7.8) | — | — |
| Depression | 94 (46.1) | — | — |
| Anxiety | 57 (27.9) | — | — |
| Addiction therapy | 90 (44.3) | — | — |
| Primary care | 116 (56.9) | — | — |
| Marital status | |||
| Single | 60 (29.4) | — | — |
| Married | 65 (31.9) | — | — |
| Divorced | 75 (36.8) | — | — |
| Widowed | 4 (2.0) | — | — |
| HOUSES quartile | — | — | — |
| Q1 | 72 (49.3) | — | — |
| Q2 | 31 (21.2) | — | — |
| Q3 | 18 (12.3) | — | — |
| Q4 | 25 (17.1) | — | — |
| Cirrhosis present | 134 (65.7) | — | — |
| MELD admit | |||
| Median | 21.0 | — | — |
| Q1, Q3 | 17.0, 26.0 | — | — |
| Ascites | 114 (55.9) | — | — |
| Variceal hemorrhage | 19 (9.3) | — | — |
| HE | 68 (33.3) | — | — |
| Acute kidney injury | 45 (22.1) | — | — |
| Sepsis | 37 (18.1) | — | — |
| ICU admission | 70 (34.3) | — | — |
| Death within 30 d | 26 (12.7) | — | — |
| Death within 180 d | 45 (22.1) | — | — |
Abbreviations: AH, alcohol-associated hepatitis; BMI, body mass index; HOUSES, HOUsing-based index of SocioEconomic Status; ICU, intensive care unit; MELD, model for end-stage liver disease.
Supplemental Table S1 (http://links.lww.com/HC9/A313) provides characteristics of 111 patients (25 with ICD codes for AH + 86 with ICD codes for alcohol-associated cirrhosis) who met the laboratory criteria for AH but were excluded due to lack of data on alcohol consumption. Compared with the 204 patients included in the final study cohort, some differences in baseline demographics and comorbidities were observed. Notably, the proportions of ethnic minorities were higher in this group with Whites accounting for 81.1% and minorities making up the remaining 18.9% (7.2% Black; 5.4% Asian; 2.7% American Indian; and 3.6% others).
AH incidence in Olmsted County between years 2000 and 2018
In Olmsted County, the cumulative incidence rate for AH over the study period was 6.8 per 100,000 person-years (95% CI: 5.7–7.9). Males had a cumulative incidence rate of 10.4 per 100,000 person-years (95% CI: 8.4–12.3), while females had a cumulative incidence rate of 3.3 per 100,000 person-years (95% CI: 2.3–4.4). When the study period was divided into 5-year time intervals, the overall incidence rate for AH in Olmsted County was noted to have increased from 4.5 per 100,000 person-years (95% CI: 2.4–6.2) between 2000 and 2004 to 10.2 per 100,000 person-years (95% CI: 7.3–13.1) between 2015 and 2018 (Figure 2). Among males, the incidence rate for AH increased by 75% from 8.4 per 100,000 person-years (95% CI: 4.3–12.5) between 2000 and 2004 to 14.7 per 100,000 person-years (95% CI: 9.7–19.7) between 2015 and 2018. Among females, there was a striking >7-fold increase in the incidence rate for AH from 0.8 per 100,000 person-years (95% CI: 0.0–2.0) between 2000 and 2004 to 5.9 per 100,000 person-years between 2015 and 2019 (95% CI: 2.9–8.9) (Figure 2). Such discrepant increases in AH incidence among females were accompanied by significant increases in the prevalence of comorbid depression and anxiety. While the prevalence of depression among male patients with AH remained stable around 36%–40% between 2000 and 2014 and even decreased to 30.2% between 2015 and 2018, the prevalence of depression among female patients with AH markedly increased from 28.6% between 2000 and 2004 to 71.4% between 2010 and 2014 and 69.7% between 2015 and 2018 (Figure 3A). As for anxiety, the prevalence of anxiety among male patients with AH increased from 9.1% between 2000 and 2004 to 27.9% between 2015 and 2018, but the prevalence of anxiety among female patients with AH was overall much higher, increasing from 28.6% between 2000 and 2004 to 57.6% between 2015 and 2018 (Figure 3B).
FIGURE 2.
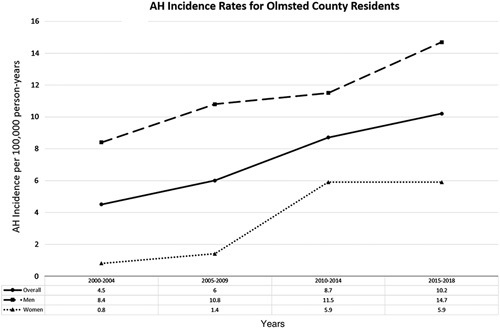
AH incidence rates or Olmsted County residents between years 2000 and 2018. Abbreviation: AH, alcohol-associated hepatitis.
FIGURE 3.
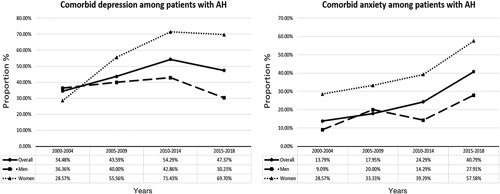
The prevalence of comorbid depression and anxiety among patients with AH between years 2000 and 2018. Abbreviation: AH, alcohol-associated hepatitis.
Socioeconomic disparities in risk of alcohol-associated hepatitis
When stratified by SES using HOUSES quartiles, an inverse relationship between SES and risk of incident AH was observed, with the majority of patients with AH falling into lower SES quartiles (Q1 and Q2). The overall proportions of SES quartiles Q1, Q2, Q3, and Q4 among patients with AH were 44.6%, 25%, 14.2%, and 16.2%, respectively. The proportion of patients with incident AH who belonged to lower SES quartiles (Q1 and Q2) increased from 62.9% (Q1: 40.7%, Q2 :22.2%) between 2000 and 2004 to 73.3% (Q1: 45.3%, Q2: 28.0%) between 2015 and 2019 (Figure 4). In contrast, the proportion of patients with incident AH who belonged to higher SES quartiles (Q3 and Q4) decreased from 37.0% (Q3: 25.9%, Q4: 11.1%) between 2000 and 2004 to 26.7% (Q3: 12.0%, Q4: 14.7%) between 2015 and 2019 (Figure 4).
FIGURE 4.
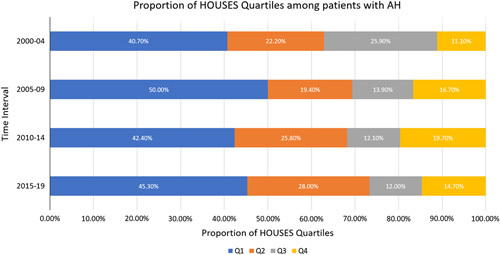
The changes in the distribution of SES represented by HOUSES quartiles among patients with AH between years 2000 and 2018. Q1 represents the lowest socioeconomic status quartile and Q4 represents the highest socioeconomic status quartile. Abbreviations: AH, alcohol-associated hepatitis; HOUSES, HOUsing-based index of SocioEconomic Status; SES, socioeconomic status.
Table 2 shows the baseline demographic characteristics and clinical outcomes of the patients with AH according to the SES quartiles. For the most part, no significant differences in the distributions of baseline demographic characteristics and comorbidities were seen across the SES quartiles. Significant differences were observed in the proportion of comorbid HCV infection (Q1: 17.6%, Q2: 9.8%, Q3: 3.4%, Q4: 3.0%, p = 0.049), i.v. drug use (Q1: 14.3%, Q2: 5.9%, Q3: 0%, Q4: 0%, p = 0.022), and tobacco use (Q1: 86.8%, Q2: 66.7%, Q3: 69.0%, Q4: 57.6%). In addition, patients in the lower SES quartiles were significantly more likely to be divorced compared with patients in the higher SES quartiles (Q1: 47.3%, Q2: 37.3%, Q3: 24.1%, Q4: 18.2%, p = 0.001). No significant differences were observed in terms of underlying cirrhosis, admission MELD score, ascites, variceal hemorrhage, hepatic encephalopathy, acute kidney injury, sepsis, ICU admission, and death within 30 days or 180 days.
TABLE 2.
Baseline characteristics and outcomes of patients with AH according to HOUSES quartiles
| Q1 (N = 91); n (%) | Q2 (N = 51); n (%) | Q3 (N = 29); n (%) | Q4 (N = 33); n (%) | p | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 26 (28.6) | 19 (37.3) | 13 (44.8) | 14 (42.4) | 0.339 |
| Male | 65 (71.4) | 32 (62.7) | 16 (55.2) | 19 (57.6) | — |
| Age | |||||
| Median | 49.0 | 50.0 | 55.0 | 50.0 | 0.053 |
| Q1, Q3 | 42.0, 57.0 | 44.0, 58.0 | 48.0, 62.0 | 42.0, 58.0 | — |
| Race | |||||
| White | 82 (94.3) | 46 (92.0) | 27 (96.4) | 30 (93.8) | 0.807 |
| Black | 2 (2.3) | 0 | 0 | 0 | — |
| Asian | 1 (1.1) | 1 (2.0) | 1 (3.6) | 1 (3.1) | — |
| Am Indian | 0 | 1 (2.0) | 0 | 0 | — |
| Other | 2 (2.3) | 2 (4.0) | 0 | 1 (3.1) | — |
| BMI | |||||
| Median | 26.8 | 28.7 | 28.0 | 25.5 | 0.234 |
| Q1, Q3 | 23.5, 30.9 | 23.6, 33.0 | 24.8, 32.0 | 23.1, 32.8 | — |
| HTN | 43 (47.3) | 26 (51.0) | 18 (62.1) | 17 (51.5) | 0.525 |
| Hyperlipidemia | 23 (25.3) | 14 (27.5) | 9 (31.0) | 12 (36.4) | 0.676 |
| DM | 12 (13.2) | 9 (17.6) | 6 (20.7) | 4 (12.1) | 0.77 |
| HCV | 16 (17.6) | 5 (9.8) | 1 (3.4) | 1 (3.0) | 0.049 |
| Tobacco use | 79 (86.8) | 34 (66.7) | 20 (69.0) | 19 (57.6) | 0.005 |
| Intravenous drug use | 13 (14.3) | 3 (5.9) | 0 | 0 | 0.022 |
| Depression | 42 (46.2) | 25 (49.0) | 10 (34.5) | 17 (51.5) | 0.487 |
| Anxiety | 23 (25.3) | 13 (25.5) | 7 (24.1) | 14 (42.4) | 0.075 |
| Addiction therapy | 44 (48.4) | 23 (45.1) | 8 (27.6) | 15 (46.9) | 0.223 |
| Primary care | 51 (56.0) | 29 (56.9) | 16 (55.2) | 20 (60.6) | 0.938 |
| Marital status | |||||
| Single | 31 (34.1) | 9 (17.6) | 5 (17.2) | 15 (45.5) | 0.001 |
| Married | 16 (17.6) | 22 (43.1) | 16 (55.2) | 11 (33.3) | — |
| Divorced | 43 (47.3) | 19 (37.3) | 7 (24.1) | 6 (18.2) | — |
| Widowed | 1 (1.1) | 1 (2.0) | 1 (3.4) | 1 (3.0) | — |
| Cirrhosis present | 54 (59.3) | 35 (68.6) | 22 (75.9) | 23 (69.7) | 0.213 |
| MELD | |||||
| Median | 20.0 | 22.0 | 23.0 | 20.0 | 0.212 |
| Q1, Q3 | 17.0, 25.0 | 18.0, 29.0 | 19.0, 26.0 | 18.0, 25.0 | — |
| Ascites | 46 (50.5) | 26 (51.0) | 21 (72.4) | 21 (63.6) | 0.082 |
| Variceal hemorrhage | 7 (7.7) | 3 (5.9) | 3 (10.3) | 6 (18.2) | 0.344 |
| HE | 28 (30.8) | 17 (33.3) | 13 (44.8) | 10 (30.3) | 0.491 |
| Acute kidney injury | 21 (23.1) | 11 (21.6) | 9 (31.0) | 4 (12.1) | 0.290 |
| Sepsis | 16 (17.6) | 13 (25.5) | 7 (24.1) | 1 (3.0) | 0.055 |
| ICU admission | 34 (37.4) | 18 (35.3) | 10 (34.5) | 8 (24.2) | 0.453 |
| Death within 30 d | 10 (11.0) | 8 (15.7) | 5 (17.2) | 3 (9.1) | 0.412 |
| Death within 180 d | 19 (20.8) | 14 (27.4) | 8 (27.6) | 4 (12.1) | 0.334 |
Abbreviations: AH, alcohol-associated hepatitis; BMI, body mass index; HOUSES, HOUsing-based index of SocioEconomic Status; ICU, intensive care unit; MELD, model for end-stage liver disease.
DISCUSSION
Our study provides a longitudinal, population-based incidence study of AH cases meeting the NIAAA criteria, with several important findings to emphasize. First of all, our study confirmed that AH is not an exception to the alarming rise in the overall health care burden of alcohol-associated liver disease and its complications in the US. Over the past 2 decades, the incidence rate for AH in Olmsted County more than doubled from 4.5 per 100,000 person-years to 10.2 per 100,000 person-years. This incidence rate is higher when compared with population-based studies from Europe, which reported AH incidence rates around 2.7 to 6.5 per 100,000 person-years, especially considering that the AH incidence in these studies was likely overestimated based on their use of diagnostic coding for defining AH.22,23 With dramatic increases in alcohol consumption24 and reduced access to addiction therapy or social support system during the severe acute respiratory syndrome coronavirus 2 pandemic, the incidence of AH will likely continue to increase through the pandemic and beyond.25 Our study showed that the incidence for AH in females has significantly increased by more than 7-fold over the past 2 decades, compared with a 75% increase in the incidence for AH in males. Moreover, we also found that the proportion of patients with lower SES increased among incident cases of AH, with patients in the bottom HOUSES quartile making up close to half of all cases of AH. These findings are again consistent with the recent studies that reported worrisome increases in the prevalence of alcohol use disorder and alcohol-associated liver disease among women and individuals of lower SES.4,26
Patients with AH had high prevalence of comorbid conditions closely associated with alcohol use disorder including tobacco use (74.5%), depression (46.1%), and anxiety (27.9%). The prevalence of depression and anxiety increased to much greater extents among female patients with AH than among male patients with AH, explaining in part the disproportionate increases in AH incidence among females in our study. In addition, the prevalence of tobacco use, i.v. drug use, and hepatitis C infection was significantly higher among individuals of lower SES compared with individuals of higher SES. These findings demonstrate areas of unmet need for preventative interventions and psychiatric treatments in such vulnerable groups of patients at risk of developing AH. Indeed, in our cohort of patients with AH, only 44.3% had previously received therapy for alcohol addiction and only 56.9% of patients saw their primary care provider within a year preceding their AH admission. A multidisciplinary approach involving primary care providers, social workers, mental health experts, hepatologists, and community support groups would be essential in prevention, diagnosis, and treatment of AH.
In the process of cohort selection, we confirmed the unreliability of ICD-based administrative coding system for the diagnosis of AH. The positive predictive value of ICD codes for AH in our study was only 34.2%, even lower than the positive predictive value of 54% reported by the single-center study from Pang et al8 in 2015. There seems to be a problem with both over-diagnosis and under-diagnosis of AH by the frontline providers, as some of the patients were labeled as having AH on the basis of drinking history and minor liver enzyme abnormalities, while other patients were not even labeled as having AH when they met all of the clinical and laboratory criteria for severe AH. This finding likely represents the lack of recognition of AH as a distinct clinical syndrome with deadly consequences outside of the gastroenterology and hepatology community. In some cases, AH may be mistaken for acute biliary obstruction due to the presence of jaundice, right upper quadrant pain, and systemic inflammatory response syndrome. Indeed, there are anecdotal reports of gastroenterologists receiving calls for urgent biliary intervention in patients with hyperbilirubinemia and systemic inflammatory response syndrome who turned out to have AH on careful clinical assessments. Therefore, the gastroenterology and hepatology communities need to make an effort to communicate with and update the frontline medical providers about how to correctly recognize and diagnose AH based on the latest consensus guidelines.
Clinical and research implications
The results of our study must be interpreted in the context of its limitations as a retrospective analysis of a large medical records-linkage system. Despite a thorough and structured manual review on all of the patient’s medical records, some patients did not have sufficient clinical documentation on the duration and amount of their alcohol use, and other patients had missing laboratory or imaging information. There is a possibility that we missed patients with AH who were diagnosed and managed as outpatients without hospital admissions, although most cases of AH result in hospitalizations. The AH incidence rate within Olmsted County may not be generalizable to the entire U.S. population, as the REP database represents a suburban/rural population of predominantly White racial background. Indeed, the vast majority of our study population were White. It is possible that minority patients with AH were less likely to seek care due to lack of access or mistrust of the health care system or did not meet the NIAAA criteria due to reluctance to provide a detailed alcohol consumption history. The proportions of non-White patients were indeed higher among the patients who were excluded due to the lack of missing information on alcohol consumption. As there is a growing concern for racial/ethnic disparities in many domains of liver disease, racial/ethnic disparities in AH will need to be further investigated in additional studies.
Nevertheless, the REP database historically has been representative of the age, sex, and ethnic characteristics of the state of Minnesota and the Upper Midwest, and many meaningful epidemiological studies have been conducted using the REP database.11 Furthermore, our study has unique strengths in that it is the first epidemiologic study to define incident AH according to the NIAAA criteria. Complete enumeration of the denominator population within the REP database enabled us to calculate the incidence rates of AH, which is often not feasible in studies of other databases.
CONCLUSION
This is the first population-based epidemiologic study documenting the incidence of AH defined by the NIAAA in a geographically well-defined population in the Midwest region. There are rising trends in the incidence of AH, which disproportionately affect women and individuals of low SES, suggesting significant disparities by sex and SES. The disparities in AH incidence at a population level may indicate major unmet need in access and implementation of preventative measures and treatments for comorbid psychiatric disorders in vulnerable patients at high risk of AH. It will require a multidisciplinary approach through the partnership involving primary care providers, social workers, mental health experts, hepatologists, and community support groups. Future studies need to identify the risk factors underlying the development of AH to formulate strategies to mitigate the risk of AH.
Supplementary Material
AUTHOR CONTRIBUTIONS
Vijay H. Shah: devised the project and the main conceptual ideas for the study. Joseph C. Ahn, Seth Buryska, and Priyadharshini Sivasubramanium: performed manual data extraction. Chung-Il Wi: formulated the HOUsing-based index of SocioEconomic Status (HOUSES) index for all of the study cohort. William S. Harmsen: performed statistical analysis. Joseph C. Ahn, Seth Buryska, Priyadharshini Sivasubramanium, Chung-Il Wi, William S. Harmsen, Patrick S. Kamath, Douglas A. Simonetto, Young Juhn, and Vijay H. Shah: interpreted the results. Joseph C. Ahn: drafted the manuscript. Chung-Il Wi, Patrick S. Kamath, Douglas A. Simonetto, Young Juhn, and Vijay H. Shah: revised the manuscript critically for important intellectual content.
CONFLICTS OF INTEREST
Douglas A. Simonetto and Vijay H. Shah are funded by National Institute of Health U01AA026886–03. The remaining authors have no conflicts to report.
Footnotes
Funding information This study used the resources of the HOUSES Program, which is supported by the National Institute on Aging (AG 65639-02).
Abbreviations: AH, alcohol-associated hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DM, Diabetes mellitus; HOUSES, HOUsing-based index of SocioEconomic Status; HTN, hypertension; ICU, intensive care unit; MELD, model for end-stage liver disease; NIAAA, National Institute on Alcohol Abuse and Alcoholism; REP, Rochester epidemiology project; SES, socioeconomic status.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Joseph C. Ahn, Email: ahn.joseph@mayo.edu.
Chung-Il Wi, Email: Wi.Chung@mayo.edu.
Seth Buryska, Email: sethburyska@gmail.com.
Priyadharshini Sivasubramaniam, Email: sivasubramaniam.priyadharshini@mayo.edu.
William S. Harmsen, Email: harmsen.william@mayo.edu.
Patrick S. Kamath, Email: kamath.patrick@mayo.edu.
Douglas A. Simonetto, Email: simonetto.douglas@mayo.edu.
Young Juhn, Email: juhn.young@mayo.edu.
Vijay H. Shah, Email: shah.vijay@mayo.edu.
REFERENCES
- 1.Asrani SK, Mellinger J, Arab JP, Shah VH. Reducing the global burden of alcohol-associated liver disease: a blueprint for action. Hepatology. 2021;73:2039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatr. 2017;74:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AK, Louvet A, Shah VH, Kamath PS. Grand rounds: alcohol-associated hepatitis. J Hepatol. 2018;69:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandrekar P, Bataller R, Tsukamoto H, Gao B. Alcohol-associated hepatitis: translational approaches to develop targeted therapies. Hepatology (Baltimore, Md). 2016;64:1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–33. [DOI] [PubMed] [Google Scholar]
- 8.Pang JX, Ross E, Borman MA, Zimmer S, Kaplan GG, Heitman SJ, et al. Validation of coding algorithms for the identification of patients hospitalized for alcohol-associated hepatitis using administrative data. BMC Gastroenterol. 2015;15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard definitions and common data elements for clinical trials in patients with alcohol-associated hepatitis: recommendation from the NIAAA alcohol-associated hepatitis consortia. Gastroenterology. 2016;150:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton LJ. History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:266–74. [DOI] [PubMed] [Google Scholar]
- 11.Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakhshi Z, Hilscher MB, Gores GJ, Harmsen WS, Viehman JK, LaRusso NF, et al. An update on primary sclerosing cholangitis epidemiology, outcomes and quantification of alkaline phosphatase variability in a population-based cohort. J Gastroenterol. 2020;55:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JD, Ahmed Mohammed H, Harmsen WS, Enders F, Gores GJ, Roberts LR. Recent trends in the epidemiology of hepatocellular carcinoma in Olmsted County, Minnesota: a US population-based study. J Clin Gastroenterol. 2017;51:742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JD, Kim B, Sanderson SO, Sauver JS, Yawn BP, Larson JJ, et al. Biliary tract cancers in Olmsted County, Minnesota, 1976-2008. Am J Gastroenterol. 2012;107:1256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butterfield MC, Williams AR, Beebe T, Finnie D, Liu H, Liesinger J, et al. A two-county comparison of the HOUSES index on predicting self-rated health. J Epidemiol Community Health. 2011;65:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu E, Wi C-I, Crow SS, Armasu SM, Wheeler PH, Sloan JA, et al. Assessing health disparities in children using a modified housing-related socioeconomic status measure: a cross-sectional study. BMJ Open. 2016;6:e011564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghawi H, Crowson CS, Rand-Weaver J, Krusemark E, Gabriel SE, Juhn YJA. A novel measure of socioeconomic status using individual housing data to assess the association of SES with rheumatoid arthritis and its mortality: a population-based case-control study. BMJ Open. 2015;5:e006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan CS, Juhn YJ, Kaur H, Wi C-I, Ryu E, King KS, et al. Long-term incidence of glioma in Olmsted County, Minnesota, and disparities in postglioma survival rate: a population-based study. Neurooncol Pract. 2019;7:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens MA, Beebe TJ, Wi CI, Taler SJ, St Sauver JL, Juhn YJ. HOUSES index as an innovative socioeconomic measure predicts graft failure among kidney transplant recipients. Transplantation. 2020;104:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson MD, Urm SH, Jung JA, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect. 2013;141:880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcohol-associated hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol. 2011;54:760–4. [DOI] [PubMed] [Google Scholar]
- 23.Sahlman P, Nissinen M, Pukkala E, Färkkilä M. Incidence, survival and cause-specific mortality in alcoholic liver disease: a population-based cohort study. Scand J Gastroenterol. 2016;51:961–6. [DOI] [PubMed] [Google Scholar]
- 24.Lee BP, Dodge JL, Leventhal A, Terrault NA. Retail Alcohol and Tobacco Sales During COVID-19. Ann Intern Med. 2021;174:1027–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology. 2020;72:1102–8. [DOI] [PubMed] [Google Scholar]
- 26.Dang K, Hirode G, Singal AK, Sundaram V, Wong RJ. Alcoholic Liver Disease Epidemiology in the United States: A Retrospective Analysis of 3 US Databases. Official journal of the American College of Gastroenterology | ACG. 2020;115:96–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


