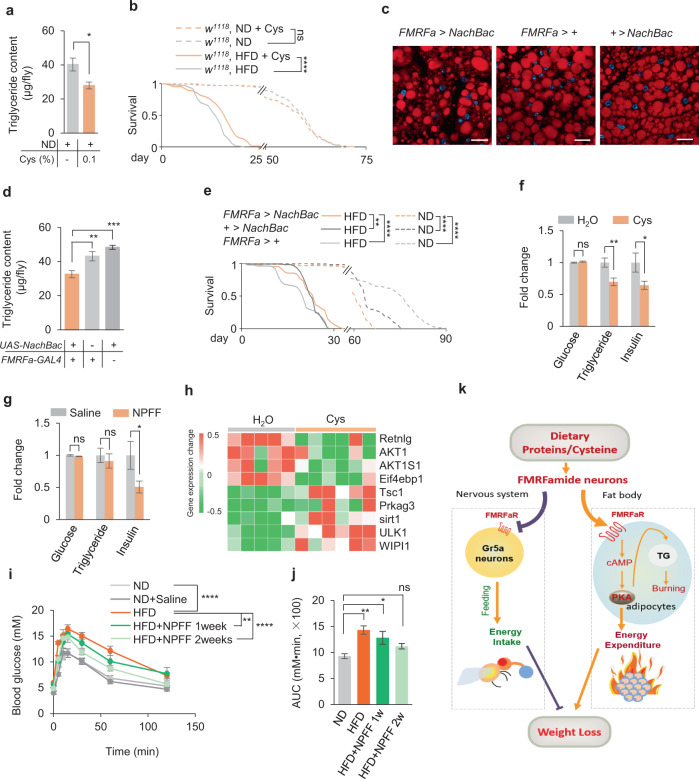
Fig. 7. Dietary cysteine and FMRFa/NPFF provided partial protection against metabolic stress.
a, b Triglyceride content (a, n = 8–9) and survival curve (b, n = 127–130) of flies fed with ND or HFD in the presence or absence of 0.1% cysteine. c Nile Red staining of the fat bodies. Scale bars, 20 μm. d Triglyceride content of flies of the indicated genotypes (n = 8). e Survival curve of flies of the indicated genotypes fed with ND or HFD (n = 101–121). f Blood glucose, triglyceride, and insulin levels of C57BL/6 mice orally gavaged with H2O or cysteine (4 mmol/kg per day) for 40 days (n = 10). g Blood glucose, triglyceride, and insulin levels of C57BL/6 mice with IV administration of NPFF (3.2 mg/kg per 12 h) for 40 days (n = 10). h RNA-seq analysis of the adipose tissue of mice orally gavaged with H2O or cysteine (n = 5–6). A list of differentially expressed insulin signaling-related genes was shown. i, j Oral glucose tolerance assay of mice fed with ND or HFD with IV administration of NPFF (n = 5). The Area Under the Curve (AUC) was shown in j. k A working model. Upon dietary protein intake (cysteine being the key ingredient), the secretion of neuropeptide FMRFa is increased. FMRFa exerts dual effects via its cognate receptor FMRFaR in two different organs: in the nervous system it reduces the sweet sensitivity of Gr5a+ gustatory neurons, leading to a suppression of energy intake; in the fat body, it triggers lipid degradation and increases energy expenditure via activating PKA signaling pathway. Overall, dietary cysteine intake represents a special satiety state in flies and mice and leads to body fat loss. ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Student’s t-test, one-way and two-way ANOVA followed by post hoc test with Bonferroni correction were used for multiple comparisons when applicable.