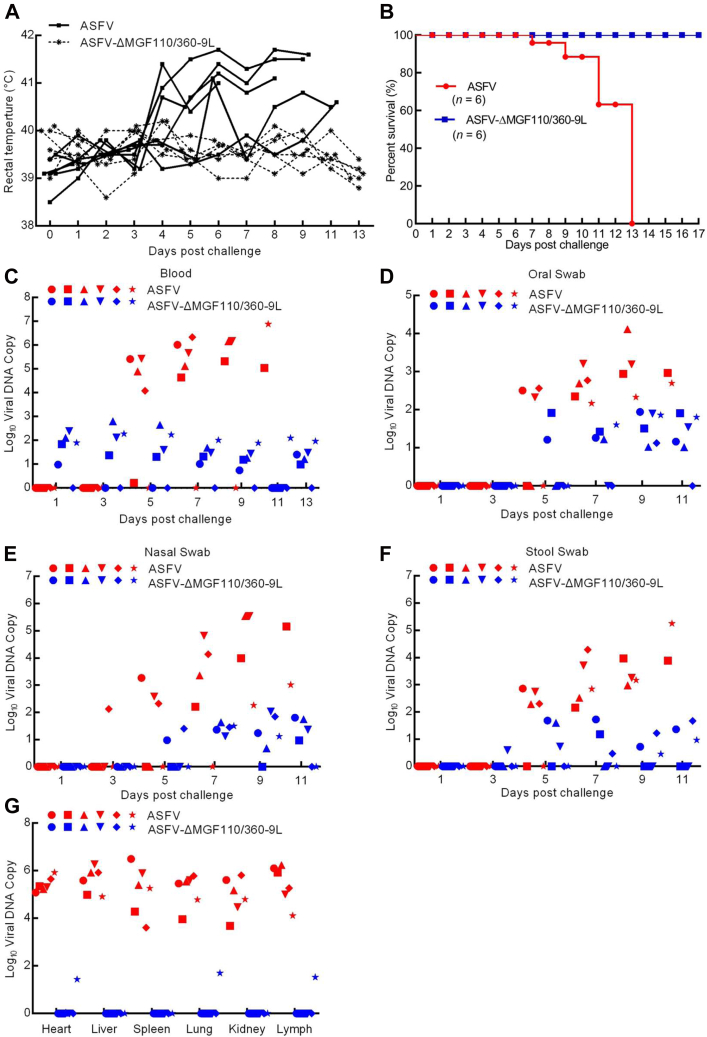
Figure 3.
Protective efficacy of pigs induced by ASFV-ΔMGF110/360-9L. Groups of pigs inoculated with 104 median hemadsorption units (HAD50) of the indicated gene-deleted ASFV were challenged intramuscularly with parental ASFV CN/GS/2018. Samples were collected from dead pigs or surviving pigs that were euthanized on day 13 postchallenge for virus DNA detection. Days postchallenge refers to untreated pigs inoculated with either 104 HAD50 of ASFV CN/GS/2018 or the time after a secondary challenge with 102 HAD50 ASFV CN/GS/2018 from pigs initially challenged with ASFV-ΔMGF110/360-9L for 17 days (shown in Fig. 2). A, body temperature of pigs. B, survival rates of pigs. C–G, viral DNA copies in (C) blood, (D) oral swab, (E) nasal swab, (F) stool swab, or (G) tissues of the pigs, with each shape representing an individual animal from each group. ASFV, African swine fever virus.