Fig. 3. Targeting of the purinergic system to treat epilepsy.
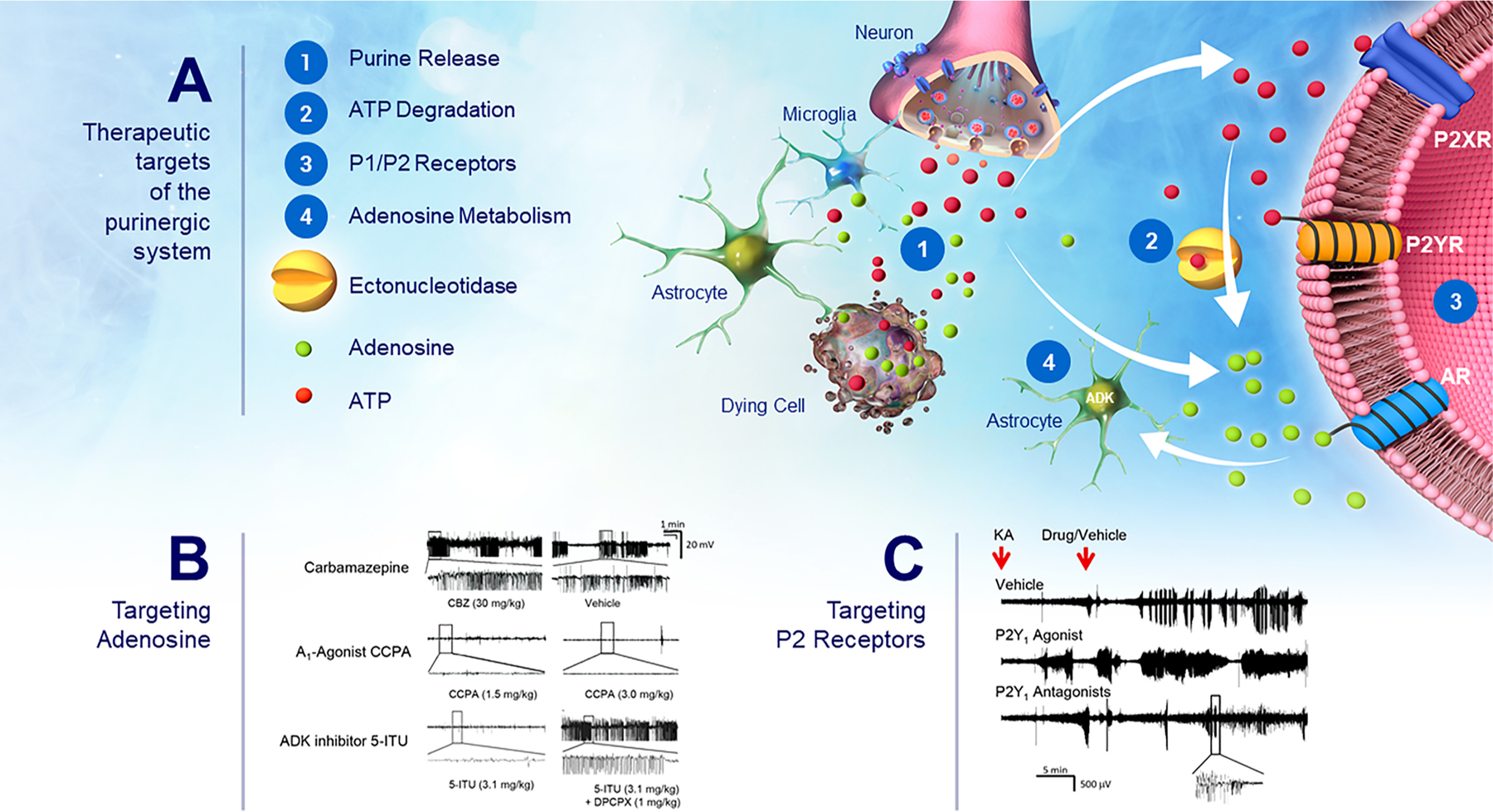
(A) Schematic illustrating possible intervention points targeting different components of the purinergic system to suppress seizures and to treat epilepsy. This includes: (1) Purine release mechanisms: Purines including ATP and adenosine are released in the brain from neurons and glial cells via active release mechanisms (e.g., via synaptic vesicles, pannexin-1 and connexin-43 hemichannels) or from dying cells. (2) ATP-metabolizing ectonucleotidases: Once release into the extracellular space, ATP is rapidly degraded into different breakdown products such as ADP, AMP and adenosine which often mediate opposing effects via their different receptors including P1 and P2 receptors. (3) Purinergic receptors: This includes P1 receptors (A1, A2A, A2B, A3) activated by extracellular adenosine and P2 receptors which are activated by extracellular adenine and uridine nucleotides (e.g., ATP) and are further subdivided into the ionotropic P2X receptor family and the metabotropic P2Y receptor family. (4) Adenosine kinase (ADK): ADK which is predominantly expressed in astrocytes catalyzes the phosphorylation of adenosine to AMP thereby removing extracellular adenosine. (B) Representative EEG traces depicting high amplitude high frequency spiking during intra-amygdala KA-induced status epilepticus. While treatment with the anti-seizure drug Carbamazepine had no effect on seizure severity during status epilepticus, the A1R agonist CCPA and the ADK inhibitor 5-ITU suppressed seizure activity. The seizure-suppressive effects provided via 5-ITU were reversed by treatment with DPCPX (A1R antagonists). (C) EEG traces during intra-amygdala KA-induced status epilepticus showing increased seizure severity when mice were treated with the P2Y1 agonist MRS2500 and seizure suppression when treated with the P2Y1 antagonists MRS2365. Each trace is from a different animal. Drugs/Vehicle was administered 15 min post-intra-amygdala KA injection.
