Abstract
Correct tumour restaging is pivotal for identifying the most personalised surgical treatment for patients with locally advanced rectal cancer undergoing neoadjuvant therapy, and works to avoid both poor oncological outcome and overtreatment. Digital rectal examination, endoscopy, and pelvic magnetic resonance imaging are the recommended modalities for local tumour restaging, while chest and abdominal computed tomography are utilised for the assessment of distant disease. The optimal length of time between neoadjuvant treatment and restaging, in terms of both oncological safety and clinical effectiveness of treatment, remains unclear, especially for patients receiving prolonged total neoadjuvant therapy. The timely identification of patients who are radioresistant and at risk of disease progression remains challenging.
Keywords: Locally advanced rectal cancer, Restaging, Pelvic magnetic resonance imaging, Endorectal ultrasound, Computed tomography scan, Colonoscopy
Core Tip: Correct tumour restaging is pivotal for identifying the most personalised surgical treatment for patients with locally advanced rectal cancer undergoing neoadjuvant therapy; it allows avoidance of both poor oncological outcomes and overtreatment. However, there are no guidelines regarding the definition, timing, and diagnostic techniques to be carried out. This study provides the most up-to-date evidence on this topic and the outstanding issues worthy of future research.
INTRODUCTION
Treatment of locally advanced rectal cancer (LARC) requires a multidisciplinary approach. In recent decades, the widespread use and optimisation of total mesorectal excision (TME) and the constant use of neoadjuvant chemoradiotherapy (nCRT) have sharply decreased the rate of local recurrence after surgery[1,2]. Two randomised phase 3 trials investigating total neoadjuvant therapy (TNT) have recently resulted in a significant improvement in disease-free survival (DFS) and disease-related treatment failure as compared with standard nCRT, setting a new standard of care[3,4]. Nevertheless, the response to neoadjuvant therapy remains highly divergent. It is well established that, after neoadjuvant therapy, many patients with LARC respond very well to the treatment; indeed, pathological complete response (pCR), defined as the absence of residual tumour cells at the primary tumour site and the mesorectal lymph nodes, is achieved in approximately 20% of patients. This rate may be as high as 28%-38% with the implementation of TNT regimens; as a result, an even larger proportion may have a near-complete response[5-7]. Patients with pCR after TME resection demonstrate excellent survival, with fewer than 1% having local failure and 8% having systemic recurrence[8]. Therefore, the benefit of TME in patients achieving a complete response has been questioned. Organ-preservation strategies are becoming more popular to safely avoid the morbidities associated with radical surgery and to maintain anorectal function in those patients who achieved a clinical complete response (cCR) or a near-cCR (ncCR)[9]. On the other hand, approximately 40% of patients respond poorly or not at all to therapy[5]. This is likely attributable to more aggressive tumour biology. Poor responders and non-responders to neoadjuvant therapy are at risk of both local and distant relapse, which may be higher than that of the average LARC patient[10,11]. In these patients, the possibility of disease progression during neoadjuvant treatment or the waiting period should be taken into account. Its correct identification allows for modification of the treatment plan, intensifying the systemic treatment, or optimising surgical management by extending resection beyond the mesorectal plane or performing multiorgan resection.
Therefore, the ability to accurately assess the response to neoadjuvant therapy is the key to tailored treatment to avoid poor oncological outcomes or overtreatment. The aim of this review is to evaluate the current evidence regarding tumour response assessment in terms of definition, timing, and diagnostic techniques.
DEFINITION OF TUMOUR RESPONSE TO NEOADJUVANT THERAPY
There is no standardisation with respect to tumour response assessment criteria. Originally, Habr-Gama et al[12] dichotomised the categorisation into complete and incomplete. They considered patients to have cCR if there was an absence of any residual ulcer, mass, or stenosis of the rectum by digital rectal exam (DRE) and proctoscopy; whitening of the mucosa, teleangiectasias, and subtle loss of pliability of the rectum were also considered to be consistent with cCR. They did not routinely perform endoscopic biopsies and considered radiological imaging consistent with cCR in the absence of suspicious mesorectal enlarged, irregularly bordered, and heterogeneous nodes, and in the presence of fibrotic changes within the rectum (i.e. low signal intensity areas with or without submucosal hypertrophy)[13]. The guidelines suggested the same criteria for the definition of cCR[14,15]. In the attempt to standardise the definition of a clinical response, Memorial Sloan Kettering graded response as complete, near-complete, or incomplete based on the findings of DRE, endoscopy, and magnetic resonance imaging (MRI) [T2-weighted and diffusion-weighted imaging (DWI) sequences][16]. They classified ncCR as tumours that showed a marked response to neoadjuvant therapy but did not fulfil all the criteria of cCR at the time of response assessment, such as: (1) Smooth induration or minor mucosal abnormalities on DRE; (2) Irregular mucosa, small mucosal nodules, or minor mucosal abnormalities, superficial ulceration or mild persisting erythema of the scar on endoscopy; and (3) Mostly dark T2 signal, some remaining intermediate signal, and/or partial regression of the lymph nodes on MRI. If patients did not meet all these criteria and those for cCR, they were regarded as incomplete responders. This 3-tiered response/regression schema was tested prospectively in the OPRA trial[17]. Maas et al[18] and Martens et al[19] provided a pragmatic definition of cCR, ncCR, and non-complete response. This classification has recently been recommended by a panel of experts for use in the definition of tumour response (Table 1; Figures 1-4)[20].
Table 1.
Recommended tumour response schema for rectal cancer after neoadjuvant chemoradiotherapy
|
|
cCR
|
ncCR
|
Poor response
|
| DRE | No palpable tumour material present | Minor mucosal abnormalities | Palpable tumour mass; Cases who do not fulfill the criteria for either a cCR or ncCR |
| Endoscopy | No residual tumour material or only a small residual erythematous ulcer or scar; Endoscopic biopsy not mandatory to define a cCR, biopsy should not be performed, especially if the DRE, rectoscopy and MRI criteria for a cCR are all fulfilled | Small and smooth regular irregularities including residual ulcer, or small mucosal nodules or minor mucosal abnormalities, with mild persisting erythema of the scar; Endoscopic biopsy not mandatory | Visible macroscopic tumour; Cases who do not fulfill the criteria for either a cCR or ncCR |
| MRI | Substantial downsizing with no observable residual tumour material, or residual fibrosis only (with limited signal on diffusion weighted imaging), sometimes associated with residual wall thickening owing to oedema, no suspicious lymph nodes | Obvious downstaging with residual fibrosis but heterogeneous or irregular aspects and signal or regression of lymph nodes with no malignant enhancement features, but with a size > 5 mm | Visible macroscopic tumour and/or lack of regression of involved lymph nodes; Cases who do not fulfill the criteria for either a cCR or ncCR |
DRE: Digital rectal exam; cCR: Clinical complete response; ncCR: Near clinical complete response; MRI: Magnetic resonance imaging.
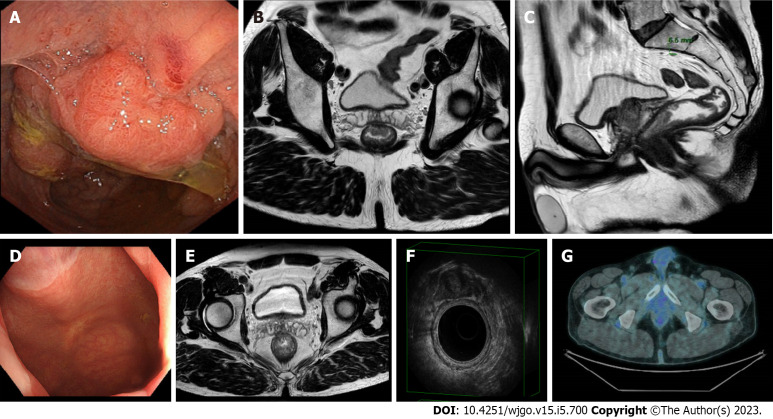
Figure 1.
A case of clinical complete response confirmed at pathology. A-C: A 61-year-old male patient with rectal cancer. Endoscopy (A) and magnetic resonance imaging (MRI) (B and C) findings staged a tumour of the low rectum (cT3aN1, mesorectal fascia negative, extramural venous invasion negative, pelvic nodes negative). The patient underwent neoadjuvant chemoradiotherapy; D-G: Restaging at 15 wk after the beginning of the neoadjuvant chemoradiotherapy showed a clinical complete response at endoscopy (D), MRI (E), endorectal ultrasound (F), and 18-fluorodeoxyglucose-computed tomography/positron emission tomography (G).
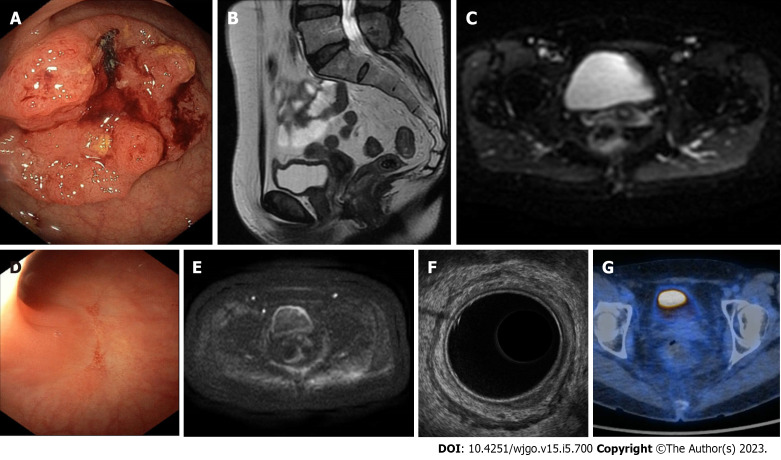
Figure 2.
A case of clinical complete response confirmed at pathology. A-C: A 57-year-old female patient with rectal cancer. Endoscopy (A) and magnetic resonance imaging (MRI) (B and C) findings staged a tumour of the low of rectum (cT3aN0 mesorectal fascia negative, extramural vascular invasion negative, pelvic nodes negative). The patient underwent neoadjuvant chemoradiotherapy; D-G: Restaging at 15 wk after the beginning of therapy showed a clinical complete response at endoscopy (D), MRI (E), endorectal ultrasound (F), and 18-fluorodeoxyglucose-computed tomography/positron emission tomography (G).
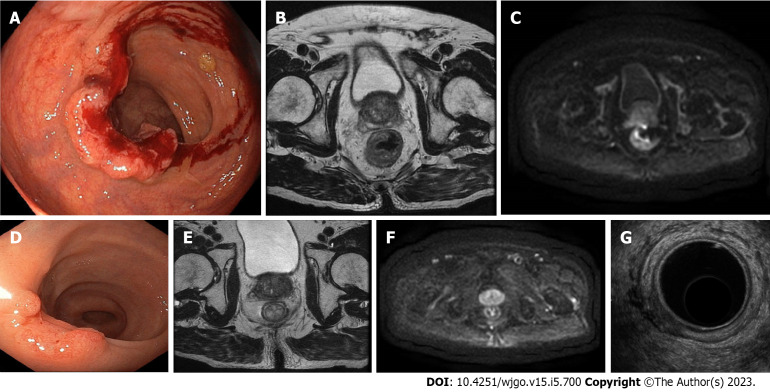
Figure 3.
A case of near clinical complete response confirmed at pathology (ypT1N0). A-C: An 84-year-old male patient with rectal cancer. Endoscopy (A) and magnetic resonance imaging (MRI) (B and C) staged a tumour of the low rectum (cT3aN0M0, mesorectal fascia negative, extramural vascular invasion negative, pelvic nodes negative). The patient underwent short-course radiotherapy; D-G: The restaging at 15 wk after the beginning of neoadjuvant radiotherapy showed a near clinical complete response at endoscopy (D), MRI (E and F), and endorectal ultrasound (G).
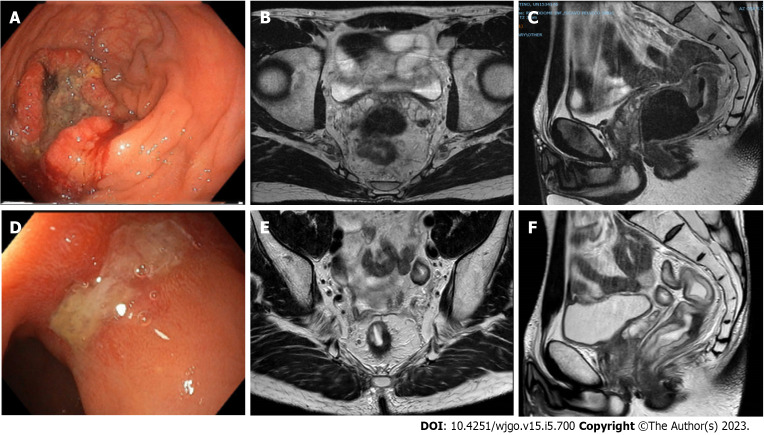
Figure 4.
A case of poor response confirmed at pathology (ypT2N0). A-C: A 42-year-old male with rectal cancer. Endoscopy (A) and MRI (B and C) staged a tumour of the middle rectum (cT3bN2, mesorectal fascia negative, extramural vascular invasion positive, pelvic nodes negative). The patient underwent total neoadjuvant therapy; D-F: Restaging at 20 wk after the beginning of neoadjuvant radiotherapy showed a poor response at endoscopy (D) and MRI (E and F).
WHEN TO CARRY OUT RESTAGING
Evidence regarding the optimal timing of restaging is not yet available. The ideal interval should allow for the safe identification of responders and non-responders by balancing the time to fully express the maximal effects of the therapy and the time to avoid tumour repopulation or disease progression. In effect, tumour response is a dynamic process associated with tumour-related factors (e.g., size, histology, and molecular profile) and treatment-related factors (e.g., radiotherapy dose and fractionation, chemotherapy, and the time interval between preoperative and/or definitive treatment and the decision to proceed to non-operative management or local excision or TME)[21]. Knowledge of the kinetics of tumour response comes primarily comes from the operative context.
Several trials have shown how lengthening the interval between radiation therapy and surgery and adding systemic therapy leads to higher rates of pCR. In the historic Lyon R90-01 randomised trial, a longer interval (6-8 wk vs 2 wk) between completion of the radiotherapy and surgery led to a significant increase in number of patients having a major pathological response (pCR or few residual cells)[22]. In the phase 3 Stockholm III trial, the rate of complete pathological response in the short course radiation-delay arm (4-8 wk) was 11.8%, as compared to 1.7% for the short course radiation-immediate arm (within 1 wk)[23]. An additional extension beyond 8 wk was subsequently tested in the prospective trials. The GRECCAR-6 trial (7 wk vs 11 wk) showed that the longer interval did not increase the pCR rate (15% vs 17.4%; P = 0.59)[24]. In contrast, a British trial (6 wk vs 12 wk) found a significant increase in the pCR rate (9% vs 20%, P < 0.05)[25]. Similarly, an increased pCR rate (18% vs 10%; P = 0,027) was also reported by a Turkish trial for an interval of more than 8 wk vs less than 8 wk after chemoradiotherapy[26]. A large retrospective series of patients revealed the highest pCR rates in patients operated on 9-13 wk from the end of CRT[27-29]. Analogously, a pooled analysis of international randomised trials (Accord12/0405, EORTC22921, FFCD9203, CAO/ARO/AIO-94, CAO-ARO-AIO-04, INTERACT, and TROG01.04) has also suggested that the best time to achieve pCR is at 10 wk, and the lengthening of the surgical interval was not detrimental with respect to survival outcomes[30]. The Timing of Rectal Cancer Response to Chemoradiation Consortium trial, a prospective phase 2 cohort trial in which preoperative chemoradiotherapy and sequentially increased timing of surgery were evaluated, showed an increase in pCR rates when the average time from radiotherapy to surgery was progressively increased from 6 wk to 11 wk, 15 wk, and 19 wk (18%, 25%, 30%, and 38%, respectively)[6].
Whether these differences can be explained by the use of intensified chemotherapy or by the prolonged interval before surgery remains uncertain, as patients operated on after 11-19 wk received 2 to 6 cycles of FOLFOX during the waiting period before surgery. In any case, consolidation chemotherapy in the TNT approach has recently emerged as the new option for optimizing tumour response; however, it made the detection of the optimal timing of restaging even more complex[31,32].
Moreover, with regard to patients who eventually did not experience a complete or a good response, the benefits related to waiting up to 11-12 wk before proceeding to surgical resection appeared less obvious. Studies evaluating the effects of the delayed time interval did not report a negative impact on long-term cancer outcomes[30,33]. However, not all the studies carried out a sub-analysis by tumour stage; therefore, the favourable long-term outcomes of the responder group may have masked or mitigated the adverse effects occurring in the non-responder group. In the RAPIDO trial, the authors suggested that an early response assessment should be encouraged in order to identify, at an earlier point in time, poor responders and, above all, patients with disease progression during preoperative treatment[3]. A large retrospective series of patients from the population-based Dutch Surgical Colorectal Audit found that the proportion of T4 tumours and metastatic disease increased with a longer time interval to surgery; this was particularly evident in the group resected beyond 10-11 wk from the end of CRT[27]. In a large multicentre retrospective cohort study of 1064 patients with a minor or null tumour response to neoadjuvant chemoradiotherapy, a wait time longer than 8 wk before surgery was associated with significantly worse overall outcome and DFS at 5 y and 10 y (reaching almost a 20% difference at 10 y for the overall survival)[10]. Unfortunately, it is not possible to identify poor responders up-front.
Patient selection based on pre-treatment characteristics is challenging, although some features, including a < 1 mm circumferential margin, extramural venous invasion, and extensive mesorectal and pelvic lymph node involvement, are associated with lower cCR rates[34-36]. Currently, there is insufficient evidence to recommend proper timing for the earlier identification of patients with a poor response before the conventional time. Nevertheless, experts advise caution and selective earlier imaging in patients with tumours featuring certain high-risk characteristics (e.g., advanced clinical T stage)[20]. Moreover, owing to variations in preoperative treatment design and duration across the different trials, they agreed that defining a specific time point for assessing cCR was impossible, and recommended that the response assessment should be determined from the start of treatment[20]. Thus, for patients with early-stage tumours receiving CRT or short-course radiotherapy, they recommended a 2-step approach comprising a response assessment at 12 wk and 16-20 wk after starting treatment; for patients receiving TNT, they recommended that the timing of the cCR assessments should be adapted according to the duration of the treatment (i.e. 20-38 wk after commencing treatment)[20]. In the end, if restaging after preoperative treatment reveals ncCR, taking into account initial tumour stage and treatment approach, the panel supported waiting longer (e.g., 3 mo later as was reported in several case studies) if organ preservation was a priority[20].
HOW TO CARRY OUT RESTAGING
The standard methods of response assessment following preoperative therapy rely on clinical examination using DRE, endoscopy, MRI, endorectal ultrasound (EUS), and CT. However, each of these tools has limitations in predicting pathological findings after a surgical resection. These limitations stem from the inability of these imaging methods to differentiate residual tumour from radiation-induced fibrosis; this leads to erring on the safe side, overestimating the amount of tumour. Nevertheless, the current aim of local response assessment is not to correct T-staging but to differentiate between “good responders” (who are ypT0N0 or ypT1N0) and “poor responders.” In the latter, the risk of incomplete resection [e.g., mesorectal fascia (MRF) positivity, adjacent organ or anal sphincter infiltration, and residual lateral pelvic node involvement] should also be identified.
Pelvic MRI
MRI is the modality of choice for local staging of LARC due to its excellent soft-tissue resolution. It also plays an essential role in the evaluation of treatment response[37,38]. In a recent meta-analysis, the reported global sensitivity and specificity for T-staging were 81% and 67%, respectively and, for N-staging, they were both 77%[39]. These results confirmed those of a previous meta-analysis in which the pooled sensitivity and specificity were 50.4% and 91.2%, respectively for the T-stage, and the sensitivity for the prediction of a complete response was even lower (19%)[40]. The addition of diffusion-weighted (DWI) MRI improved the results, increasing the sensitivity and specificity for T-stage to 83.6% and 84.8%, respectively[40,41]. Nevertheless, many complete responses were still missed. The magnetic resonance tumour regression grade (TRG) system and a pattern-based approach have been proposed to improve diagnostic performance[42,43]. In experienced hands, the sensitivity of detecting a complete response was 74% when using the former system and 94% with the latter approach[42,43]. To properly identify “good responders,” accurate nodal restaging is also important. A pooled analysis showed that the incidence of positive lymph nodes in ypT0 patients was approximately 5%[44]. Although nodal restaging remains a challenge, it seems to be more accurate than primary staging[45]. According to Heijnen et al[46], this could be explained by the following: First, after CRT, approximately 40% of lymph nodes decrease in size and approximately 44% disappear on MRI; and second, the prevalence of pathological positive nodes is lower as compared with the initial staging, leading to a higher negative predictive value (95%) and increased accuracy of nodal staging after CRT[46]. However, in cases of ypT0, the sensitivity, specificity, positive predictive value, and negative predictive value for predicting remaining lymph node metastasis with MRI were quite low (37%, 84%, 70%, and 57%, respectively)[47]; this may be attributable to the fact that residual disease occurs within very small nodes. van Heeswijk and colleagues showed that the absence of lymph nodes on restaging DWI MRI was highly predictive of ypN0 status[48]. Nevertheless, the role of DWI in this setting is still under debate[45]; MRI also plays a pivotal role in identifying the risk factors for incomplete resection. The evaluation of MRF status is less accurate than that of the pretreatment assessment (66%)[40,49,50]. In the case of residual involvement of the adjacent organs or mesorectal fascia, radiologists tend to overstage, as fibrotic strands of prior tumour invasion are challenging to differentiate from residual tumour tissue, unless an intact fat plane becomes visible between the tumour and the MRF or adjacent organs. Moreover, in distal tumours, invasion of the internal sphincter, intersphincteric plane, and external sphincter/levator ani has to be assessed to determine the feasibility of sphincter preservation. Furthermore, careful attention should be paid to identifying the lateral nodes, as these nodes, when involved, have an important influence on long-term outcome. A recent large multicentre cohort study evaluating the lateral nodes before and after CRT showed that nodes 7 mm or greater before CRT (short axis) had a higher risk for local recurrence than smaller nodes[51]. Moreover, in the case of shrinkage of the lateral nodes from 7 mm on a primary MRI to a short axis measurement of 4 mm, lateral lymph node dissection can be avoided[52].
EUS
Similar to MRI, the accuracy of EUS is disappointing in restaging. A number of studies on this topic have shown that the overall accuracy of EUS for ypT-stage and ypN-stage was quite variable, ranging from 38% to 75%, and from 59% to 80%, respectively[53-55]. Overstaging was more common in the majority of series, mainly due to the difficulty in differentiating fibrosis from residual cancer; EUS correctly predicted pCR in only approximately 50%-64%of cases[53-55]. These results were confirmed in a meta-analysis in which the sensitivity and specificity for T0-stage were 37% and 94%, respectively[56]. Zhang et al[57] have recently evaluated 3-dimensional EUS (3D-EUS) parameters to improve accuracy in tumour response assessment. They found that a value of 3.55 mm for adjusted thickness (i.e. the difference between the thickness of the muscularis on the residual side and the thickness of contralateral muscularis) correctly detected the TRG 0 cases with a sensitivity of 73%, a specificity of 81%, and an accuracy of 78%. Moreover, they concluded that utilising the 3D-EUS method as a part of the criteria for cCR would significantly improve the accuracy of the evaluation[57]. Some case-series studies have indicated that optimal accuracy of EUS could be obtained when the tumour location was within 6 cm from the anal verge and the examination was carried out by an experienced operator[54,58,59]. Studies comparing the accuracy of MRI and EUS in the same patients at the same time have reported conflicting results regarding T- and N-staging[59-61]. Nevertheless, EUS was more accurate than MRI for predicting pathologic complete response and anal sphincter infiltration[59-61]. Therefore, EUS is simple and inexpensive tool which, together with MRI and other diagnostic methods, can be useful for restaging rectal cancer. However, this modality is highly operator-dependent and limited to proximal and stenotic rectal tumours and close visual fields that only allow for the evaluation of perirectal lymph nodes.
Endoscopy
Endoscopy only allows for the proper evaluation of the mucosa. Although the healing of the mucosa is generally considered to be a sign of cCR, residual tumour remains deeper in the rectal wall and mesorectum in approximately 27% of cases. On the other hand, the presence of an ulcer on endoscopy, although significantly associated with pathological incomplete response, occurs in 66% of cases with complete response on pathology[62-64]. In clinical practice, to facilitate the decision-making process, additional information can be obtained from the MRI. However, studies that have evaluated this issue have produced contradictory results. Some have shown that a combination of multiple examinations did not improve accuracy[65,66]. In contrast to these findings, in a small prospective cohort study, Maas et al[18] showed that when DRE, endoscopy, and MRI together predict CR, this is correct in 98% of cases; when all 3 modalities indicate residual tumour, there still a 15% chance of CR[67]. Advanced endoscopy technologies, such as narrow-spectrum technologies and autofluorescence imaging, may improve the evaluation of the rectal wall mucosa and mucosal vascularity[68]. In the setting of restaging assessment, they may help in differentiating between clinical response and residual tumour.
Biopsies have only a limited clinical value for ruling out residual cancer. They do not provide any additional diagnostic value and could lead to false-negative results as residual cancer cells are often found in the muscularis propia[69]. Therefore, experts did not recommend biopsy as mandatory for diagnosing a complete or a near complete CR[20].
Contrast-enhanced thoraco-abdominal computed tomography
Although the value of CT in assessing local response is relatively low, this tool plays a role in determining the presence of distant metastases and current guidelines recommend its use in restaging[15]. A recent systematic review showed that restaging identified new metastatic disease in 6% of patients[11]. Although the overall detection rate of disease progression is low, the clinical impact of identifying early disease progression prior to surgical therapy is important to consider. Newly-detected distant disease in such a short period may represent a more biologically aggressive tumour or synchronous distant metastases that were not apparent on initial clinical staging, but that become detectable in the few months of the restaging. In any case, its identification requires modifying the therapeutic programme. Singhal and colleagues found that patients with poorly differentiated tumours had a significantly higher rate of systemic disease progression than those with well- or moderately-differentiated tumours (36% vs 7%, respectively). Nevertheless, more studies are necessary to identify factors that may predict short-interval disease progression.
18F-fluorodeoxyglucose positron emission tomography/CT
According to the guideline, positron emission tomography (PET) should not be routinely used as a tool to determine tumour response[15]. The pooled sensitivity and specificity reported for complete response were 71% and 76%, respectively[70]. Moreover, the metabolic grade [max standardised uptake value (SUVmax)] of the tumour at initial staging did not predict response to chemoradiotherapy; as with pretreatment SUVmax, the arithmetic difference between pre- and post-SUVmax was also not statistically significant[70]. A systematic review showed that PET/CT had higher accuracy in detecting extra-hepatic and hepatic colorectal metastatic disease than CT alone[71].
Future directions and research
Combined 18F-fluorodeoxyglucose (18F-FDG) PET/MRI has recently been proposed as an effective imaging modality for rectal cancer patients, owing to its ability to provide high-resolution anatomical and functional features. Although the role of 18F-FDG PET/MRI in rectal cancer has yet to be established, the evidence in a recent review has suggested that 18F-FDG PET/MRI could be used for rectal cancer restaging due to its better accuracy in T- and N-staging as compared to PET/CT or MRI alone; for M staging, on the other hand, it performed less well than other techniques for lung metastases[72].
Some novel MRI techniques, such as dynamic contrast-enhanced MRI, magnetisation transfer ratio, and textural analysis (e.g., radiomics), have been studied to overcome the limitations of MRI in the restaging of rectal cancer. These tools have been evaluated in promising small retrospective studies; however, they are not currently used in routine clinical practice as they still need large-scale prospective validation.
Circulating biomarkers such as cell-free DNA have been tested to predict cCR and/or tumour regrowth. These have not been incorporated into current practice due to limited data, but represent a promising direction for future investigation and validation.
CONCLUSION
The ultimate goal of restaging is to determine the possibility of changing the planned treatment strategy. DRE, endoscopy, and pelvic MRI are the recommended modalities for local tumour restaging, while chest and abdominal CT are used for assessing distant disease. Nevertheless, the most practical and cost-efficient strategy for assessing tumour response also depends on local logistics and expertise. The optimal length of time between commencing treatment and restaging, in terms of both oncological safety and clinical effectiveness of treatment, remains unclear, especially in patients receiving prolonged TNT. The timely identification of patients who are radioresistant and at risk of disease progression is challenging. Table 2 summarizes the key points discussed in this review.
Table 2.
Take-home message
|
Re-staging
| |
| Why | It allows for the development of a tailored surgical treatment with the goal of avoiding poor oncological outcomes and overtreatment |
| When | It remains unclear. Experts recommend: (1) For patients receiving neoadjuvant chemoradiotherapy or short-course radiotherapy, the 2-step approach, at 12 wk and 16-20 wk after starting treatment if organ preservation is a priority; (2) For patients receiving total neoadjuvant therapy, assessment at 20-38 wk after commencing treatment according to the duration of the treatment; and (3) In case of ncCR, a second assessment 3 mo later taking into account initial tumour stage and treatment approach, if organ preservation is a priority. There is insufficient evidence to recommend proper timing for the earlier identification of patients with a poor response before the conventional time. Nevertheless, experts advise caution and selective earlier imaging in patients with tumours featuring certain high-risk characteristics (such as advanced cT stage) |
| How | Digital rectal examination, endoscopy and pelvic MRI for local tumour restaging; Chest and abdominal CT for distant disease. The current aim of local response assessment is not correct T-staging but the accurate differentiation between “good responders” (who are ypT0N0 or ypT1N0) and “poor responders.” In the latter, the risk of incomplete resection, such as MRF positivity, adjacent organ or anal sphincter infiltration, and residual lateral pelvic node involvement should also be identified |
CT: Computed tomography; ncCR: Near clinical complete response; MRI: Magnetic resonance imaging.
Footnotes
Conflict-of-interest statement: The authors declare having no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 22, 2022
First decision: February 24, 2023
Article in press: March 29, 2023
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gu GL, China; Sun J, China; Tsimogiannis K, Sweden S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang XD
Contributor Information
Dajana Cuicchi, Department of Medical and Surgical Sciences, Surgery of the Alimentary Tract, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy. dajana.cuicchi@aosp.bo.it.
Giovanni Castagna, Department of Medical and Surgical Sciences, Surgery of the Alimentary Tract, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy.
Stefano Cardelli, Department of Medical and Surgical Sciences, Surgery of the Alimentary Tract, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy.
Cristina Larotonda, Department of Medical and Surgical Sciences, Surgery of the Alimentary Tract, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy.
Benedetta Petrello, Department of Medical and Surgical Sciences, Surgery of the Alimentary Tract, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy.
Gilberto Poggioli, Department of Medical and Surgical Sciences, Surgery of the Alimentary Tract, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy.
References
- 1.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med . 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med . 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 3.Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol . 2021;22:29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol . 2021;22:702–715. doi: 10.1016/S1470-2045(21)00079-6. [DOI] [PubMed] [Google Scholar]
- 5.Capelli G, De Simone I, Spolverato G, Cinquini M, Moschetti I, Lonardi S, Masi G, Carlomagno C, Corsi D, Luppi G, Gambacorta MA, Valvo F, Cannizzaro R, Grillo F, Barbaro B, Restivo A, Messina M, Pastorino A, Aschele C, Pucciarelli S. Non-Operative Management Versus Total Mesorectal Excision for Locally Advanced Rectal Cancer with Clinical Complete Response After Neoadjuvant Chemoradiotherapy: a GRADE Approach by the Rectal Cancer Guidelines Writing Group of the Italian Association of Medical Oncology (AIOM) J Gastrointest Surg . 2020;24:2150–2159. doi: 10.1007/s11605-020-04635-1. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol . 2015;16:957–966. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giunta EF, Bregni G, Pretta A, Deleporte A, Liberale G, Bali AM, Moretti L, Troiani T, Ciardiello F, Hendlisz A, Sclafani F. Total neoadjuvant therapy for rectal cancer: Making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat Rev . 2021;96:102177. doi: 10.1016/j.ctrv.2021.102177. [DOI] [PubMed] [Google Scholar]
- 8.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg . 2012;99:918–928. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 9.van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJH IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–2545. doi: 10.1016/S0140-6736(18)31078-X. [DOI] [PubMed] [Google Scholar]
- 10.Deidda S, Elmore U, Rosati R, De Nardi P, Vignali A, Puccetti F, Spolverato G, Capelli G, Zuin M, Muratore A, Danna R, Calabrò M, Guerrieri M, Ortenzi M, Ghiselli R, Scabini S, Aprile A, Pertile D, Sammarco G, Gallo G, Sena G, Coco C, Rizzo G, Pafundi DP, Belluco C, Innocente R, Degiuli M, Reddavid R, Puca L, Delrio P, Rega D, Conti P, Pastorino A, Zorcolo L, Pucciarelli S, Aschele C, Restivo A. Association of Delayed Surgery With Oncologic Long-term Outcomes in Patients With Locally Advanced Rectal Cancer Not Responding to Preoperative Chemoradiation. JAMA Surg . 2021;156:1141–1149. doi: 10.1001/jamasurg.2021.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrick LE, Levesque RL, Hinkle NM, Monroe JJ, Glazer ES, Deneve JL, Yakoub D, Shibata D, Dickson PV. Restaging Patients with Rectal Cancer Following Neoadjuvant Chemoradiation: A Systematic Review. World J Surg . 2020;44:973–979. doi: 10.1007/s00268-019-05309-z. [DOI] [PubMed] [Google Scholar]
- 12.Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum . 2010;53:1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 13.Habr-Gama A, São Julião GP, Vailati BB, Sabbaga J, Aguilar PB, Fernandez LM, Araújo SEA, Perez RO. Organ Preservation in cT2N0 Rectal Cancer After Neoadjuvant Chemoradiation Therapy: The Impact of Radiation Therapy Dose-escalation and Consolidation Chemotherapy. Ann Surg . 2019;269:102–107. doi: 10.1097/SLA.0000000000002447. [DOI] [PubMed] [Google Scholar]
- 14.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol . 2017;28:iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 15.Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw . 2022;20:1139–1167. doi: 10.6004/jnccn.2022.0051. [DOI] [PubMed] [Google Scholar]
- 16.Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, Guillem JG, Paty PB, Avila K, Garcia-Aguilar J Rectal Cancer Consortium. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer . 2015;15:767. doi: 10.1186/s12885-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, Verheij FS, Omer DM, Lee M, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Paty PB, Weiser MR, Nash GM, Pappou E, Guillem JG, Temple L, Wei IH, Widmar M, Lin S, Segal NH, Cercek A, Yaeger R, Smith JJ, Goodman KA, Wu AJ, Saltz LB. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol. 2022;40:2546–2556. doi: 10.1200/JCO.22.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewé KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol . 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 19.Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP, Breukink SO, Hoff C, Belgers EJ, Melenhorst J, Jansen R, Buijsen J, Hoofwijk TG, Beets-Tan RG, Beets GL. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst . 2016;108 doi: 10.1093/jnci/djw171. [DOI] [PubMed] [Google Scholar]
- 20.Fokas E, Appelt A, Glynne-Jones R, Beets G, Perez R, Garcia-Aguilar J, Rullier E, Smith JJ, Marijnen C, Peters FP, van der Valk M, Beets-Tan R, Myint AS, Gerard JP, Bach SP, Ghadimi M, Hofheinz RD, Bujko K, Gani C, Haustermans K, Minsky BD, Ludmir E, West NP, Gambacorta MA, Valentini V, Buyse M, Renehan AG, Gilbert A, Sebag-Montefiore D, Rödel C. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat Rev Clin Oncol . 2021;18:805–816. doi: 10.1038/s41571-021-00538-5. [DOI] [PubMed] [Google Scholar]
- 21.Fokas E, Glynne-Jones R, Appelt A, Beets-Tan R, Beets G, Haustermans K, Marijnen C, Minsky BD, Ludmir E, Quirke P, Sebag-Montefiore D, Garcia-Aguilar J, Gambacorta MA, Valentini V, Buyse M, Rödel C. Outcome measures in multimodal rectal cancer trials. Lancet Oncol . 2020;21:e252–e264. doi: 10.1016/S1470-2045(20)30024-3. [DOI] [PubMed] [Google Scholar]
- 22.Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol . 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 23.Pettersson D, Lörinc E, Holm T, Iversen H, Cedermark B, Glimelius B, Martling A. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg . 2015;102:972–8; discussion 978. doi: 10.1002/bjs.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasgen N, Parc Y, Simon T, Tiret E. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6) J Clin Oncol . 2016;34:3773–3780. doi: 10.1200/JCO.2016.67.6049. [DOI] [PubMed] [Google Scholar]
- 25.Evans J, Bhoday B, Sizer B, Tekkis P, Swift R, Perez R, Tait D and Brown G. Results of a prospective randomised control 6 vs 12 trial: is greater tumour downstaging observed on post treatment MRI if surgery is delayed to 12-weeks vs 6-weeks after completion of neoadjuvant chemoradiotherapy? Ann Oncol . 27:4520. [Google Scholar]
- 26.Akgun E, Caliskan C, Bozbiyik O, Yoldas T, Sezak M, Ozkok S, Kose T, Karabulut B, Harman M, Ozutemiz O. Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg . 2018;105:1417–1425. doi: 10.1002/bjs.10984. [DOI] [PubMed] [Google Scholar]
- 27.Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, Tanis PJ Dutch Surgical Colorectal Audit. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg . 2013;100:933–939. doi: 10.1002/bjs.9112. [DOI] [PubMed] [Google Scholar]
- 28.Macchia G, Gambacorta MA, Masciocchi C, Chiloiro G, Mantello G, di Benedetto M, Lupattelli M, Palazzari E, Belgioia L, Bacigalupo A, Sainato A, Montrone S, Turri L, Caroli A, De Paoli A, Matrone F, Capirci C, Montesi G, Niespolo RM, Osti MF, Caravatta L, Galardi A, Genovesi D, Rosetto ME, Boso C, Sciacero P, Giaccherini L, Parisi S, Fontana A, Filippone FR, Picardi V, Morganti AG, Valentini V. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin Transl Radiat Oncol . 2017;4:8–14. doi: 10.1016/j.ctro.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Probst CP, Becerra AZ, Aquina CT, Tejani MA, Wexner SD, Garcia-Aguilar J, Remzi FH, Dietz DW, Monson JR, Fleming FJ Consortium for Optimizing the Surgical Treatment of Rectal Cancer (OSTRiCh) Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg . 2015;221:430–440. doi: 10.1016/j.jamcollsurg.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gambacorta MA, Masciocchi C, Chiloiro G, Meldolesi E, Macchia G, van Soest J, Peters F, Collette L, Gérard JP, Ngan S, Rödel CC, Damiani A, Dekker A, Valentini V. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials. Radiother Oncol . 2021;154:154–160. doi: 10.1016/j.radonc.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Schmiegel W, Jacobasch L, Weitz J, Folprecht G, Schlenska-Lange A, Flentje M, Germer CT, Grützmann R, Schwarzbach M, Paolucci V, Bechstein WO, Friede T, Ghadimi M, Hofheinz RD, Rödel C German Rectal Cancer Study Group. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol . 2019;37:3212–3222. doi: 10.1200/JCO.19.00308. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Aguilar JPS, Patil S, Kim JK, Yuval JB, Thompson H, Verheij F, Lee M, Saltz L. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol . 2020;38:4008. [Google Scholar]
- 33.Ryan ÉJ, O'Sullivan DP, Kelly ME, Syed AZ, Neary PC, O'Connell PR, Kavanagh DO, Winter DC, O'Riordan JM. Meta-analysis of the effect of extending the interval after long-course chemoradiotherapy before surgery in locally advanced rectal cancer. Br J Surg . 2019;106:1298–1310. doi: 10.1002/bjs.11220. [DOI] [PubMed] [Google Scholar]
- 34.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol . 2008;47:20–31. doi: 10.1080/02841860701697720. [DOI] [PubMed] [Google Scholar]
- 35.Kim YI, Jang JK, Park IJ, Park SH, Kim JB, Park JH, Kim TW, Ro JS, Lim SB, Yu CS, Kim JC. Lateral lymph node and its association with distant recurrence in rectal cancer: A clue of systemic disease. Surg Oncol . 2020;35:174–181. doi: 10.1016/j.suronc.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Chadi SA, Malcomson L, Ensor J, Riley RD, Vaccaro CA, Rossi GL, Daniels IR, Smart NJ, Osborne ME, Beets GL, Maas M, Bitterman DS, Du K, Gollins S, Sun Myint A, Smith FM, Saunders MP, Scott N, O'Dwyer ST, de Castro Araujo RO, Valadao M, Lopes A, Hsiao CW, Lai CL, Smith RK, Paulson EC, Appelt A, Jakobsen A, Wexner SD, Habr-Gama A, Sao Julião G, Perez R, Renehan AG. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol . 2018;3:825–836. doi: 10.1016/S2468-1253(18)30301-7. [DOI] [PubMed] [Google Scholar]
- 37.Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol . 2018;28:1465–1475. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gollub MJ, Arya S, Beets-Tan RG, dePrisco G, Gonen M, Jhaveri K, Kassam Z, Kaur H, Kim D, Knezevic A, Korngold E, Lall C, Lalwani N, Blair Macdonald D, Moreno C, Nougaret S, Pickhardt P, Sheedy S, Harisinghani M. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY) . 2018;43:2893–2902. doi: 10.1007/s00261-018-1642-9. [DOI] [PubMed] [Google Scholar]
- 39.Wei MZ, Zhao ZH, Wang JY. The Diagnostic Accuracy of Magnetic Resonance Imaging in Restaging of Rectal Cancer After Preoperative Chemoradiotherapy: A Meta-Analysis and Systematic Review. J Comput Assist Tomogr . 2020;44:102–110. doi: 10.1097/RCT.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 40.van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology . 2013;269:101–112. doi: 10.1148/radiol.13122833. [DOI] [PubMed] [Google Scholar]
- 41.Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets GL, Beets-Tan RG. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol . 2011;18:2224–2231. doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sclafani F, Brown G, Cunningham D, Wotherspoon A, Mendes LST, Balyasnikova S, Evans J, Peckitt C, Begum R, Tait D, Tabernero J, Glimelius B, Roselló S, Thomas J, Oates J, Chau I. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer . 2017;117:1478–1485. doi: 10.1038/bjc.2017.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambregts DMJ, Delli Pizzi A, Lahaye MJ, van Griethuysen JJM, Maas M, Beets GL, Bakers FCH, Beets-Tan RGH. A Pattern-Based Approach Combining Tumor Morphology on MRI With Distinct Signal Patterns on Diffusion-Weighted Imaging to Assess Response of Rectal Tumors After Chemoradiotherapy. Dis Colon Rectum. 2018;61:328–337. doi: 10.1097/DCR.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 44.Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol . 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol . 2022;43:101739. doi: 10.1016/j.suronc.2022.101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heijnen LA, Maas M, Beets-Tan RG, Berkhof M, Lambregts DM, Nelemans PJ, Riedl R, Beets GL. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis . 2016;31:1157–1162. doi: 10.1007/s00384-016-2576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loftås P, Sturludóttir M, Hallböök O, Almlöv K, Arbman G, Blomqvist L. Assessment of remaining tumour involved lymph nodes with MRI in patients with complete luminal response after neoadjuvant treatment of rectal cancer. Br J Radiol . 2018;91:20170938. doi: 10.1259/bjr.20170938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Heeswijk MM, Lambregts DM, Palm WM, Hendriks BM, Maas M, Beets GL, Beets-Tan RG. DWI for Assessment of Rectal Cancer Nodes After Chemoradiotherapy: Is the Absence of Nodes at DWI Proof of a Negative Nodal Status? AJR Am J Roentgenol . 2017;208:W79–W84. doi: 10.2214/AJR.16.17117. [DOI] [PubMed] [Google Scholar]
- 49.Vliegen RF, Beets GL, Lammering G, Dresen RC, Rutten HJ, Kessels AG, Oei TK, de Bruïne AP, van Engelshoven JM, Beets-Tan RG. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology . 2008;246:454–462. doi: 10.1148/radiol.2462070042. [DOI] [PubMed] [Google Scholar]
- 50.Oberholzer K, Junginger T, Heintz A, Kreft A, Hansen T, Lollert A, Ebert M, Düber C. Rectal Cancer: MR imaging of the mesorectal fascia and effect of chemoradiation on assessment of tumor involvement. J Magn Reson Imaging . 2012;36:658–663. doi: 10.1002/jmri.23687. [DOI] [PubMed] [Google Scholar]
- 51.Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Beets GL, Rutten HJT, Kusters M Lateral Node Study Consortium. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol . 2019;37:33–43. doi: 10.1200/JCO.18.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogura A, Konishi T, Beets GL, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Rutten HJT, Tuynman JB, Kusters M Lateral Node Study Consortium. Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surg . 2019;154:e192172. doi: 10.1001/jamasurg.2019.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastor C, Subtil JC, Sola J, Baixauli J, Beorlegui C, Arbea L, Aristu J, Hernandez-Lizoain JL. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum . 2011;54:1141–1146. doi: 10.1097/DCR.0b013e31821c4a60. [DOI] [PubMed] [Google Scholar]
- 54.Huh JW, Park YA, Jung EJ, Lee KY, Sohn SK. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J Am Coll Surg . 2008;207:7–12. doi: 10.1016/j.jamcollsurg.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Marone P, de Bellis M, D'Angelo V, Delrio P, Passananti V, Di Girolamo E, Rossi GB, Rega D, Tracey MC, Tempesta AM. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J Gastrointest Endosc . 2015;7:688–701. doi: 10.4253/wjge.v7.i7.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao RS, Wang H, Zhou ZY, Zhou Q, Mulholland MW. Restaging of locally advanced rectal cancer with magnetic resonance imaging and endoluminal ultrasound after preoperative chemoradiotherapy: a systemic review and meta-analysis. Dis Colon Rectum . 2014;57:388–395. doi: 10.1097/DCR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Fan J, Zhang L, Wang J, Wang M, Zhu J. Association Between Three-Dimensional Transrectal Ultrasound Findings and Tumor Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer: An Observational Study. Front Oncol . 2021;11:648839. doi: 10.3389/fonc.2021.648839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren Y, Ye J, Wang Y, Xiong W, Xu J, He Y, Cai S, Tan M, Yuan Y. The Optimal Application of Transrectal Ultrasound in Staging of Rectal Cancer Following Neoadjuvant Therapy: A Pragmatic Study for Accuracy Investigation. J Cancer . 2018;9:784–791. doi: 10.7150/jca.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martellucci J, Scheiterle M, Lorenzi B, Roviello F, Cetta F, Pinto E, Tanzini G. Accuracy of transrectal ultrasound after preoperative radiochemotherapy compared to computed tomography and magnetic resonance in locally advanced rectal cancer. Int J Colorectal Dis . 2012;27:967–973. doi: 10.1007/s00384-012-1419-5. [DOI] [PubMed] [Google Scholar]
- 60.Pomerri F, Pucciarelli S, Maretto I, Zandonà M, Del Bianco P, Amadio L, Rugge M, Nitti D, Muzzio PC. Prospective assessment of imaging after preoperative chemoradiotherapy for rectal cancer. Surgery . 2011;149:56–64. doi: 10.1016/j.surg.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 61.Kye BH, Kim HJ, Kim G, Kim JG, Cho HM. Multimodal Assessments Are Needed for Restaging after Neoadjunvant Chemoradiation Therapy in Rectal Cancer Patients. Cancer Res Treat . 2016;48:561–566. doi: 10.4143/crt.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duldulao MP, Lee W, Streja L, Chu P, Li W, Chen Z, Kim J, Garcia-Aguilar J. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum . 2013;56:142–149. doi: 10.1097/DCR.0b013e31827541e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith FM, Wiland H, Mace A, Pai RK, Kalady MF. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum . 2014;57:311–315. doi: 10.1097/DCR.0b013e3182a84eba. [DOI] [PubMed] [Google Scholar]
- 64.van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive Value of Endoscopic Features for a Complete Response After Chemoradiotherapy for Rectal Cancer. Ann Surg . 2021;274:e541–e547. doi: 10.1097/SLA.0000000000003718. [DOI] [PubMed] [Google Scholar]
- 65.Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF, Ribeiro U Jr, Cotti GC, Imperiale AR, Capareli FC, Chih Chen AT, Hoff PM, Cecconello I. Pathologic Complete Response in Rectal Cancer: Can We Detect It? Dis Colon Rectum . 2016;59:255–263. doi: 10.1097/DCR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 66.Liu S, Zhong GX, Zhou WX, Xue HD, Pan WD, Xu L, Lu JY, Wu B, Lin GL, Qiu HZ, Xiao Y. Can Endorectal Ultrasound, MRI, and Mucosa Integrity Accurately Predict the Complete Response for Mid-Low Rectal Cancer After Preoperative Chemoradiation? Dis Colon Rectum . 2018;61:903–910. doi: 10.1097/DCR.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 67.Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, Sosef M, Hulsewé KW, Hoff C, Breukink SO, Stassen L, Beets-Tan RG, Beets GL. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol . 2015;22:3873–3880. doi: 10.1245/s10434-015-4687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, Hassan C, Horgan G, Kiesslich R, Longcroft-Wheaton G, Wilson A, Dumonceau JM. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy . 2016;48:1029–1045. doi: 10.1055/s-0042-118087. [DOI] [PubMed] [Google Scholar]
- 69.Perez RO, Habr-Gama A, Pereira GV, Lynn PB, Alves PA, Proscurshim I, Rawet V, Gama-Rodrigues J. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis . 2012;14:714–720. doi: 10.1111/j.1463-1318.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 70.Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of (18)F-FDG PET for Predicting Response to Neoadjuvant Therapy in Rectal Cancer: Systematic Review and Meta-Analysis. AJR Am J Roentgenol . 2015;204:1261–1268. doi: 10.2214/AJR.14.13210. [DOI] [PubMed] [Google Scholar]
- 71.Patel S, McCall M, Ohinmaa A, Bigam D, Dryden DM. Positron emission tomography/computed tomographic scans compared to computed tomographic scans for detecting colorectal liver metastases: a systematic review. Ann Surg . 2011;253:666–671. doi: 10.1097/SLA.0b013e31821110c9. [DOI] [PubMed] [Google Scholar]
- 72.Crimì F, Valeggia S, Baffoni L, Stramare R, Lacognata C, Spolverato G, Albertoni L, Spimpolo A, Evangelista L, Zucchetta P, Cecchin D, Pucciarelli S. [18F]FDG PET/MRI in rectal cancer. Ann Nucl Med . 2021;35:281–290. doi: 10.1007/s12149-021-01580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]