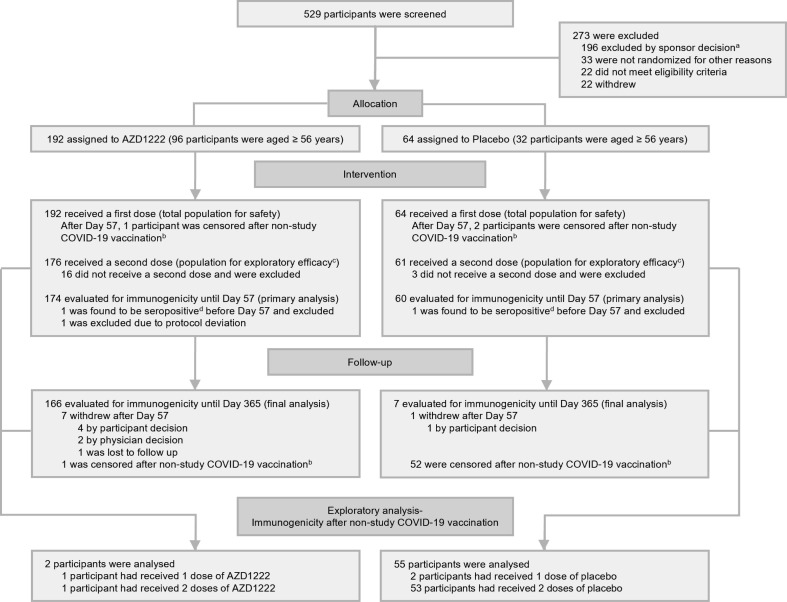
Fig. 1.
Participant disposition.aAll screen failures categorized as ‘excluded by sponsor decision’ were associated with a temporary interruption in the study, and consequent pause in enrollment following a reported serious adverse event of transverse myelitis in another clinical trial [18]. bData collected from participants who reported receiving a non-study COVID-19 vaccination were censored from safety, AZD1222 immunogenicity, and efficacy outcomes from the date of the first dose of non-study COVID-19 vaccination. cData from all participants who received two doses of study intervention and had not discontinued from the study by Day 43 were included in exploratory efficacy analyses. dHad seroresponse (a ≥ 4-fold rise in titer compared to recorded Day 1 baseline values) to nucleocapsid antibodies as quantified by MSD serology assay up to Day 57. Abbreviation: MSD = Meso Scale Discovery.