Figure 5.
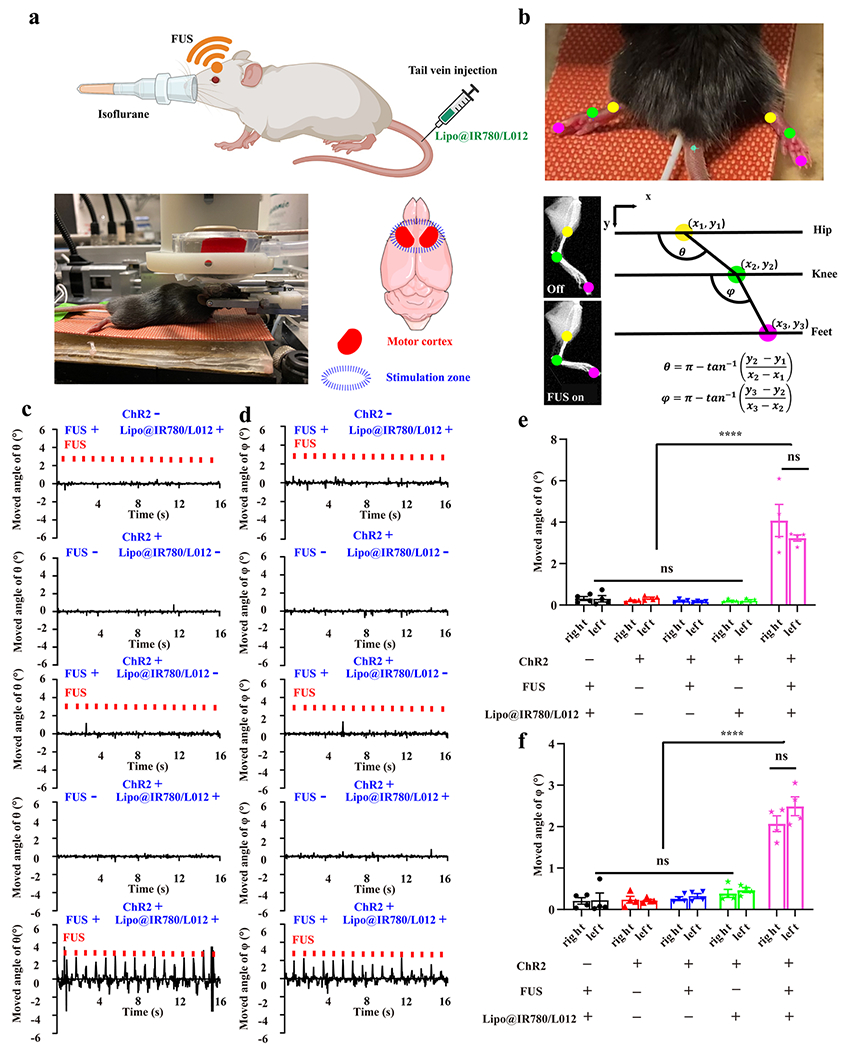
In vivo sono-optogenetic motor cortex stimulation. (a) Schematic of in vivo noninvasive sono-optogenetic brain stimulation, where the mouse was fixed in a stereotaxic frame and deeply anesthetized using 2.5% isoflurane, and then, Lipo@IR780/L012 nanoparticles were injected through the tail vein. The FUS transducer was in direct contact with the scalp of the mouse during brain stimulation, where the photograph of the in vivo sono-optogenetics is given. The motor cortex zone was irradiated via FUS, created using BioRender.com. (b) Limbs’ response to FUS was recorded via a camera and analyzed via DeepLabCut. The photograph of limbs’ response to FUS was used to track the brain activation, where different colored dots were marked on joints to track the movement, and kinematic joint angle changes of hip–knee (θ) and knee–feet (φ) response to FUS were tracked and calculated through DeepLabCut. (c) Time-resolved right limb’s hip–knee response and (d) knee–feet response to FUS, including no FUS and no Lipo@IR780/L012 nanoparticles, with FUS (1.5 MHz, puls 100 ms on, 900 ms off, 1 Hz, 2.2 MPa) but no Lipo@IR780/L012 nanoparticles, no FUS with Lipo@IR780/L012 nanoparticles, and with both FUS (1.5 MHz, puls 100 ms on, 900 ms off, 1 Hz, 2.2 MPa) and Lipo@IR780/L012 nanoparticles in Thy1-ChR2-YFP transgenic mice and C57BL/6J wild type mice with Lipo@IR780/L012 and FUS. (e) Statistical analysis of the hip–knee and (f) knee–feet angle changes in different groups of subjects (n = 4 per group, two-way ANOVA) in response to FUS irradiation. All plots show mean ± SEM unless otherwise mentioned. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant.
