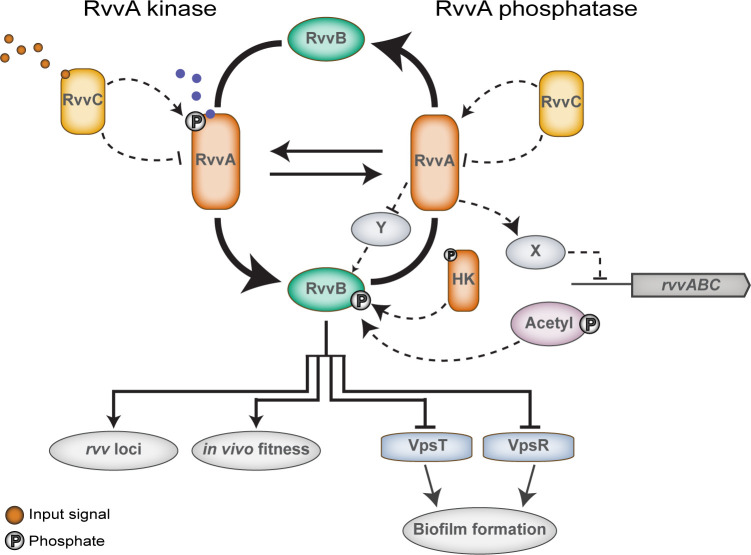
Fig 8. Model of Rvv TCS signal transduction.
In the wild-type background, deletion of the gene encoding the sensor histidine kinase RvvA increases transcription of the rvv loci. Increased rvv transcription is dependent on both the presence and phosphorylation-state of the response regulator RvvB. However, RvvBD57E is not sufficient to increase target gene transcription unless RvvA is lacking. This suggests that rvv transcription might be repressed by an unknown repressor that requires the presence of RvvA, or that RvvA is inhibiting a key protein/signal needed for the function/activation of RvvB and thus presenting RvvB associated phenotypes. A strain harboring RvvAT293A, a mutated form of RvvA that abolishes phosphatase function, phenocopies ΔrvvA, supporting a model where RvvA acts as a phosphatase on RvvB. Under the conditions tested, RvvB phosphorylation may be mediated by acetyl-phosphate or crosstalk with another HK. RvvA’s kinase function may be activated in response to a specific signal, leading to RvvB phosphorylation. The impact of RvvC on RvvAB phosphotransfer and the identity of signals sensed by RvvA or RvvC, have yet to be determined. Increased transcription and phosphorylation of RvvB activates the Rvv TCS, increasing transcription of rvv loci and biofilm formation genes, and reducing in vivo fitness.