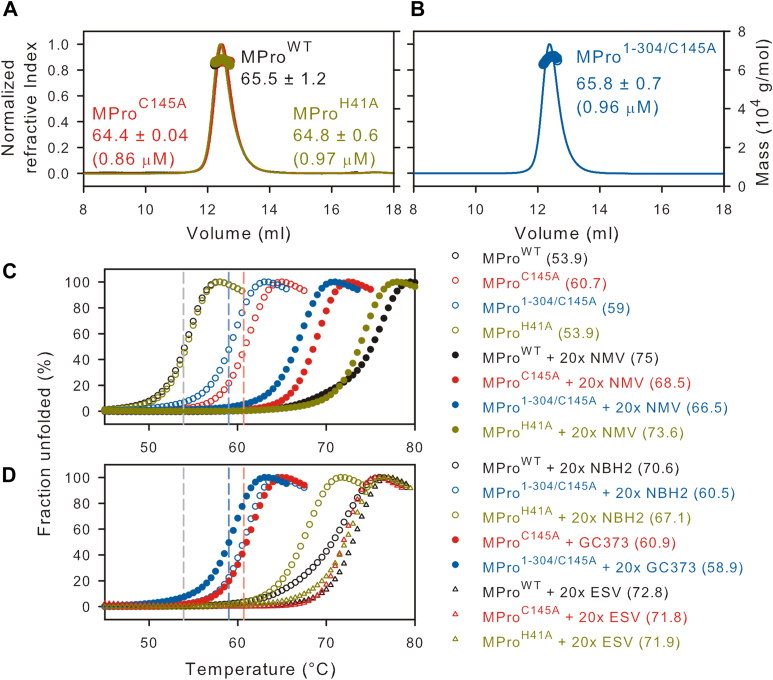
Figure 2.
Characterization of MPro1-304/C145Aand thermal denaturation profiles. A and B, proteins were fractionated on Superose-12 column (1 × 30 cm) in buffer A at 25 °C. Eluting top of peak concentration (in parentheses) and the estimated mass (in kDa) corresponding to the monomer-dimer equilibrium boundary (circles) are indicated. C and D, thermal denaturation DSF traces at a final concentration of 10 μM in buffer B. The vertical dashed lines indicate the midpoint for the transition for MProWT (black trace), MProC145A (red), and MPro1-304/C145A (blue) serving as a reference point to compare traces acquired in the presence of inhibitor. Estimated Tm midpoints are listed in parentheses beside the construct designation, and in Table 1. 20× denotes 20 times (200 μM) the concentration of inhibitor over the protein concentration (10 μM).