Abstract
Objectives
Lipid nanoparticles, as a nucleic acid delivery system, have been used as an alternative to treat ocular diseases, since they can cross the ocular barrier and efficiently transfecting nucleic acids to various cells of the eye. The size influences the transfection of genes, biological distribution, diffusion, and cellular uptake. It is therefore important to establish a relationship between size, formulation, and encapsulation percentage.
Evidence acquisition
In this review, we used a search strategy to compare studies of nanomedicine systems aimed at eye diseases where the size of the nanoparticles and the efficiency of encapsulation of genetic material are reported based on the criteria of Preferred Reporting Items for Systematic Reviews (PRISMA ScR 2020 guidelines).
Results
Out of the initial 5932, 169 studies met the inclusion criteria and were included to form the basis of the analysis. Nanoparticles reported are composed mainly of PEG-modified lipids, cholesterol, and cationic lipids, that in combination with messenger or interference RNA, allow the formulation of a nanoparticle with an encapsulation efficiency greater than 95%. The diseases treated mainly focus on conditions related to the retina and cornea. Certain characteristics of nanoparticles increase encapsulation efficiency, such as the size of the nanoparticle and the charge of the outer layer of the nanoparticle.
Conclusion
It is still unknown what characteristics lipid nanoparticles should have to successfully treat human eye illnesses. The in vitro and in vivo investigations covered in this review, however, present encouraging results. To improve encapsulation effectiveness and disease gene silencing, nanoparticle formulation is essential. The most stable nanoparticles are those made mostly of cationic lipids, PEG lipids, and cholesterol, which also effectively encapsulate RNA. The encapsulation efficiency is not only influenced by size, but also by other factors such as methods of preparation.
Graphical abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s40199-023-00455-1.
Keywords: Nanoparticles, Delivery system, Lipid, Eye, Encapsulation, Nucleic acid
Introduction
Projections of the number of people who will suffer from blindness, severe or moderate visual impairment are worrisome [1, 2]. For example, it is estimated that by the year 2040 there will be 111.8 million people around the world suffering from glaucoma [3]. Similarly, it is predicted that by 2050, there will be 61.0 million blind people, 474 million suffering from moderate and severe visual impairment, about 360 million will have mild visual impairment, while 866 million will have uncorrected presbyopia [4]. In recent years, genetic research has led to recent advances in our understanding of the genetic origin of some eye conditions [5–14]. It is foreseeable that our current understanding of gene expression and its role in ocular conditions will lead to the development of nanomedicine solutions to address these conditions. These solutions could include gene therapy. Nanomedicine could also be used to deliver drugs to the eye more accurately and efficiently, improving treatment outcomes.
Therefore, gene therapy emerges as an alternative to overcome serious current limitations in the treatment of ocular diseases. In recent years, drug delivery systems have been developed to treat diseases associated with the eye [15, 16]. However, one of the biggest challenges is to deal with the issue of the back of the eye, because the routes of administration for this section of the eye, specifically periocular and intravitreal, have the ocular barriers as a major impediment [17, 18]. In the literature, there are interesting reports that provide an overview of the different ocular barriers that limit the penetration and distribution of gene therapy products using different routes of administration [19]. Nanoparticles play a fundamental role, since, due to their size and characteristics, they have a high capacity to transfect genes. The nanoparticles used are mainly classified into three types, metallic nanoparticles, polymeric nanoparticles, and lipid nanoparticles, which in turn can be classified into viral or non-viral systems [20]. Lipid nanoparticles are colloidal drug and gene delivery systems that consider non-viral vectors for transfection of nucleic acids as an effective and safe alternative to treat genetic diseases. This type of nanoparticle is mainly elaborated by physiological lipids that expel the drug or gene at its target site [21]. Lipid nanoparticles as non-viral systems in ocular therapy have demonstrated great superiority due to their characteristics, such as their great stability, the ability to use non-organic solvents, as well as the use of physiological lipids such as triglycerides, diglycerides, monoglycerides, fatty acids, cholesterol, among others. Likewise, nanoparticles can be classified into solid nanoparticles, nanostructured lipid carriers, lipoplexes, or cationic lipids. These types of nanoparticles are highly biocompatible and capable of packaging nucleic acids to direct them to target cells. In this way, they can treat damaged ocular cells and provide an alternative for dealing with related diseases.
The general mechanism of nanoparticle uptake into the ocular environment depends on the target cell, the size of the nanoparticle, the type of nanoparticle, and the lipid that was used to characterize it. The various lipid nanoparticles enter the ocular environment by mechanisms such as fusion, adsorption, endocytosis, and lipid exchange. In phagocytosis or endocytosis, nanoparticles can take two routes, both macropinocytosis and clathrin-mediated endocytosis, which typically target Müller cells and photoreceptors. Though, the type and characterization of the nanoparticle are paramount because they are subject to degradation by acid hydrolases found in the environment [22, 23].
The application of lipid nanoparticles for ocular drug delivery dates to 2002, when studies were conducted on solid lipid nanoparticles loaded with tobramycin [24]. One of the advantages of using such nanoparticles, in addition to their formulation with lipids present in the cell membrane, is the broad spectrum of administration, since they can be applied topically, orally, and intravenously (Fig. 1). However, it also has some disadvantages, such as the appearance of unwanted particles during storage, unpredictable gelation, and unsuspected changes in polymorphic transitions. Nevertheless, lipid nanoparticles can improve the therapeutic efficacy, compliance, and safety of ocular drugs administered by different routes. Over the years, lipid nanoparticles have proven to be efficient as delivery systems [16, 18]. Thus, research to determine the size of the nanoparticles that best encapsulates nucleic acid for ocular diseases is of utmost importance, since through these results it is possible to know what the best characteristics of such lipid nanoparticles for future applications in ocular therapy would be. Encapsulation efficiency refers to the amount of nucleic acid encapsulated per unit weight within the nanoparticle. It is the fraction of encapsulated nucleic acid compared to the total nucleic acid added to the formulation at the time of characterizing the lipid nanoparticles. The use of lipid nanoparticles loaded with nucleic acids is an increasingly popular solution for the treatment of ocular diseases. However, to advance in obtaining optimal ophthalmic nanomedicine systems, it is necessary to determine the conditions for these nanoparticles to cross the ocular barriers and reach the target cells for nucleic acid transfection.
Fig. 1.

Lipid nanoparticles administration routes This figure illustrates the different routes of administration for lipid nanoparticles in ocular therapy, including periocular injection, topical administration, and intravitreal injection. Examples of lipid nanoparticles encapsulating nucleic acids for the treatment of ocular diseases are also shown
The importance of this review lies in the analysis of the studies that have been carried out in the last years and to observe how the release of nucleic acids by means of these lipid nanoparticles has been evolving, increasing their encapsulation efficiency by modifying their dimension and some other characteristics. Several of the published investigations on lipid nanoparticle formulation vary in terms of particle size and encapsulation efficacy [24–28]. However, a relationship between these variables specifically related to the treatment of ocular diseases has not been described. Therefore, we propose the research question: What size of lipid nanoparticles targeting eye diseases has a higher encapsulation efficiency depending on the type of encapsulated nucleic acid? The results of this research will be of interest to researchers seeking to develop gene therapy materials or systems for eye treatments.
Methods
The protocol of the present systematic review was recorded elsewhere [29]. The methodology consisted of a search for articles in three databases, namely ScienceDirect, PubMed, and Web of Science with the search strategy (“lipid nanoparticles” OR liposome) AND (Gene) AND (clinical OR therapy) AND (Ocular OR Eye), so that the results were subsequently screened by means of a diagram to help present reports of systematic reviews and meta-analyses. From the results obtained from the PRISMA diagram (Fig. 2), relevant data were extracted in order to analyze and determine the size of the lipid nanoparticle with the highest percentage of nucleic acid encapsulation efficiency. The information gathered from the sources of evidence allowed us to determine the characteristics of the lipid nanoparticles used in each study, such as the type of nucleic acid encapsulated, the type of target cell to which the gene is directed, the disease under study, and the formulation of the LNP. Finally, we identified the predominant characteristics that allow for increased encapsulation efficiency.
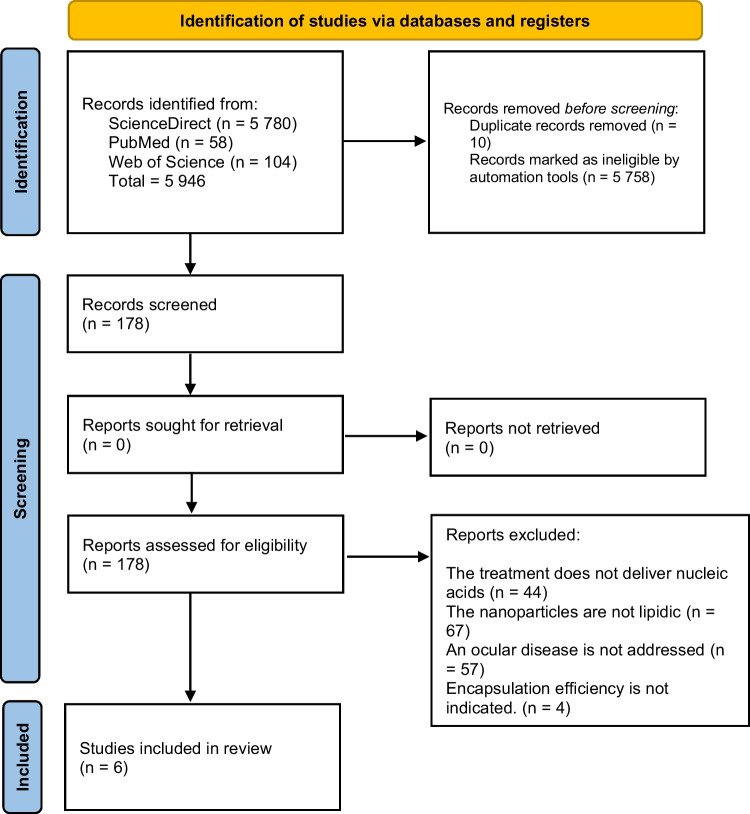
Fig. 2.
PRISMA flowchart of the study selection process. This figure illustrates the flow of articles from the initial search to the final selection of studies included in the review. The flowchart includes the number of studies screened, the number of studies excluded, and the number of studies included for data extraction and analysis. The reasons for exclusion of studies are also highlighted in the flowchart, such as studies not meeting the inclusion criteria, studies not related to the topic, and duplicate studies. The flowchart also indicates the number of studies included from each database searched. This figure serves as a visual representation of the rigorous and systematic approach used in the selection of studies for this review
The eligibility criteria for obtaining the articles were specific to acquire the desired results. Firstly, current articles were selected, between 2015 and 2021, due to the growth that the study of lipid nanoparticles has had in recent years and to understand the line of research that applications in the biomedical area are taking. Due to this, another criterion for the eligibility of the articles was the application in ocular diseases, since it was determined that ocular ailments have great potential and biocompatibility with this type of nanoparticles. In order to answer the research question, it was necessary for the articles to indicate the encapsulation efficiency of the nucleic acid inside the target cell for ocular treatment and, therefore, the type of nucleic acid, type of target cell and ocular disease to be treated. It was also essential to specify in the article the nanoparticle size corresponding to the encapsulation efficiency percentage. Another eligibility criterion was that the selected articles should be research articles to analyze and study the results of scientific research projects and to know the process that was carried out to obtain them, so review articles, conferences, and comments were discarded since they do not have these characteristics. On the other hand, in vitro and/or in vivo experimental studies between the ocular cell and the lipid nanoparticle were considered, as well as articles referring to the synthesis of lipid nanoparticles. In addition, only articles written in the English language were included. Finally, articles that did not have the necessary information to answer the research question or that did not have access to the full text were excluded. Screening of articles and data extraction were independently carried out by two reviewers (J. V. M. S. and C. C. G.), with a third reviewer asked in case of disagreement.
Results and discussion
The process of selecting sources of evidence was summarized in a PRISMA diagram showing the steps of identification, screening, and inclusion of articles. Initially, 5946 records were identified. Full-text screening led to 6 articles being selected for the review [30–35].
In vivo and in vitro studies were carried out in the studies of the selected articles, of which five of six studies worked with groups of animals (mice) that were provided with a pre-treatment preparation to be provided with specific nanoparticles for each ocular disease [30–34]. While one of the studies was carried out in vitro, using human conjunctival fibroblasts, the other one was carried out in vitro, using human conjunctival fibroblasts [35]. In addition, to know and compare the results obtained by this treatment, a control was selected to which a substance other than lipid nanoparticles was administered and to observe the differences. The essential characteristics of the sources of evidence are shown in Table 1. A more detailed description of the sources of evidence can be found in Table 1 of the supplementary material.
Table 1.
Characteristics of the sources of evidence
| Reference | Preparation of models for experiments | Treatments involving lipid nanoparticles | Controls |
|---|---|---|---|
| [30] | Male and female BALB/c mice (1–4 months) | LNP-Luciferase, LNP-EGFP, LNP-mCherry (subretinal injection) | 11 LNP formulations, divided into 3 groups, 1 μL of PBS in contralateral eye |
| [31] | Male Sprague-Dawley rats (325–350 g) | 5 μL of LNP and conventional treatment | 5 μL of Saline solution in contralateral eye |
| [32] | Albino BALB/c, Ai9, apoE −/−, Mertk −/− and C57BL6 mice (1–6 months) | LNP-Luciferase, LNP-Cre, LNPmCherry (vitreous chamber) | 1.5 μL of PBS in C57BL6 mice as control animals |
| [33] | C57BL/6 wild-type mice | Luciferase-targeted SiLUC complexed with ProSilic formulation (topical application) | Left eye treated with nonspecific NSC4-ProSilic as negative control |
| [34] | Female or male C57BL/6 mice (5–10 weeks) | Enucleated eyeballs treated with different siRNA-loaded LNP samples | Biomarker Brn3a |
| [35] | Human conjunctival fibroblasts | Incubation with four types of LNP in complete media | siRNA sequence |
The control group used in half of the studies was the contralateral eye in which two of them were injected with saline and the other with nonspecific NSC4-ProSilic [30, 31, 33]. The other studies used biomarkers [32, 34, 35]. In these studies, different groups of nanoparticles were formulated in which variations of drug, cargo, lipids, and concentrations were made to determine which formulation was preferable to obtain better results.
The characterization of lipid nanoparticles is fundamental for a good release of nucleic acids for the treatment of various ocular diseases, mainly, the nanoparticle size is one of the characteristics that greatly influence the efficient encapsulation of the nucleic acid with the objective of releasing the greatest possible amount of the treatment to the target cell. Table 2 presents the results obtained in various studies on the development of nucleic acid-loaded lipid nanoparticles to treat ocular diseases, in which characteristics such as encapsulation efficiency, nanoparticle size, target cell, and disease, are presented. A more detailed description of these characteristics can be found in Table 2 of the supplementary material.
Table 2.
Summary of lipid nanoparticle composition, lipid size and encapsulation efficiency from evidence sources
| Ref. | Ocular disease | Target cell type | Formulation of LNP | Size, nm | Type of nucleic acid encapsulated | EE (%) |
|---|---|---|---|---|---|---|
| [30] | Inherited retinal degenerations specific to RPE | Retinal pigment epithelium (RPE) cells | Microfluidic mixing of MC3 | 148 ± 68.4 | mRNA | 97.9 |
| Microfluidic mixing of KC3 4 | 115 ± 33.4 | mRNA | 97.1 | |||
| Microfluidic mixing of DODMA | 82. 8 ± 27.3 | mRNA | 95.2 | |||
| [31] | Retinoblastoma | Retinoblastoma cells, both primary and Y-79 lineage. | pH-sensitive cationic lipids/DSPC/cholesterol/DSPE-PEG2000 | 171 ± 4 | miR-181a | 93.0 ± 0.2 (Melafalan) and 97.5 ± 0.7 (miR-181a) |
| [32] | Retinal degeneration and glaucoma | RPE cells | Microfluidic mixing of MC3, DSPC, DMG-PEG2k and cholesterol in a molar ratio of 50:10:0.5:0.5:39.5 | 150 | mRNA | 97.8 |
| Microfluidic mixing of MC3, DSPC, DMG-PEG2k and cholesterol in a molar ratio of 50:10:1.5:38.5. | 150 | mRNA | 96.2 | |||
| [33] | Corneal dystrophies | Corneal layer cells | DOPE, cationic lipids and cholesterol 60:30:10 | 397 ± 44 | siRNA | 97 |
| [34] | Glaucoma, age-related macular degeneration | Stable cells (HEK293-GFP) or GFP293 cells and primary retinal ganglion cells | DOTAP, DOPE, CHOL and DSPE-PEG2000. Molar ratios 40:23:25:10:2 | 75 | siRNA | 99 |
| [35] | Ocular fibrosis | Human conjunctival fibroblast |
LNP-MRTF-B siRNA CL4H6, DOPE and PEG-DMG |
214.4 ± 2.6 | siRNA | 92.5 ± 0.7 |
|
LNP + cY-MRTF-B siRNA CL4H6, DOPE and PEG |
211.1 ± 1.9 | siRNA | 99.3 ± 0.1 |
In terms of encapsulated nucleic acid, it should be noted that half of the nanoparticles use mRNA which can be observed in [30, 32] as well as the use of siRNA in four of the ten formulations [33–35] and only in [31] miR-181a is used. Two formulations were smaller than 115 nm in diameter and one of the formulations was up to approximately 400 nm in size [30, 33, 34] whose encapsulation efficiency is between 96% and 99%. On the other hand, we can determine that most of the formulations are in a range of nanoparticle size from 115 nm to 214 nm approximately where the encapsulation efficiency is between 93% to 99% [30–32, 35]. Both encapsulation efficiency and loading parameter are important indicators of formulation quality. The loading parameter refers to the amount of active ingredient (such as a drug or biomolecule) that is loaded or encapsulated in a carrier system, such as a lipid nanoparticle. The loading parameter is usually expressed as a percentage or ratio of the total amount of active ingredient to the total amount of carrier material. It is an important indicator of the efficiency of the formulation process and can affect the yield of the final product. However, none of the studies included in the review report it directly.
Another of the main points evaluated was the formulation of the nanoparticles since the use of certain lipids is essential for the behavior of the nanoparticle in the ocular environment. Among the lipids most used in the studies analyzed were cationic lipids, which are present in [31–33], and cholesterol in eight out of ten nanoparticle formulations [30–34]. Likewise, most of the studies used PEG-lipids, which because it is a well-established strategy to improve target cell specificity, circulation time, and stability of lipid nanoparticles, thus improving their stealth properties, have started to be used in more formulations in recent years. A key aspect that was observed in the results was the target cells to which the LNPs were focused, because the application of these nanoparticles was focused on various diseases, it is not possible to determine in which type of cells the nanoparticle presents a better efficiency. However, the type of lipid nanoparticle with nucleic acid encapsulation was used in half of the studies in retinal pigment epithelium cells, while the others were focused on other types of retinal cells, cornea, fibroblast, and retinoblastoma. Finally, it was observed that the studies had similar forms of application, most of which were performed by subretinal injection while others were intravitreal [30–32, 34]. Likewise, one of the studies used optic drops as the administration medium and another was by means of a culture medium because the study was in vitro [33, 35].
Methods of preparation can greatly affect the encapsulation efficiency of lipid nanoparticles. Each method of preparation in the studies included in the review is unique and has its own set of parameters and variables that can affect the encapsulation efficiency of the LNPs. For example, in [30] the authors used microfluidic mixing of one-part ethanol phase and three parts aqueous phase to form LNPs, and characterized for hydrodynamic radius and polydispersity index (PDI) using dynamic light scattering (DLS) and mRNA encapsulation efficiency using a modified Quant-iT RiboGreen RNA reagent. While in [31] the authors used a combination of CSL3, DSPC, cholesterol, and DSPE-PEG2000 in ethanol to form the LNPs. The final composition of the LNPs was 50:10:37.5:2.5 mol%. Melphalan was added to the lipid mixture to reach 10 mol% of total lipids, and the final LNP was extruded through polycarbonate membranes to get the desired size. In contrast, the authors in [33] used a method of solvent evaporation, hydration with SiNPs aqueous solution and lyophilization to prepare the LNP. While [34] is distinguished by its inclusion of the ethanol-injection method.
In this review, we aimed to identify the size of lipid nanoparticles targeting eye diseases that have a higher encapsulation efficiency depending on the type of encapsulated nucleic acid. Through our analysis of the studies included in our review, we found that there is a wide range of nanoparticle sizes that can be effectively used for the encapsulation of nucleic acids, with encapsulation efficiencies varying depending on the specific type of nucleic acid and the methods used for nanoparticle preparation. One key factor that was identified as impacting encapsulation efficiency is the method of nanoparticle preparation. Different methods of preparation, such as solvent evaporation, microfluidic mixing and hydration of lipid films, can result in variations in nanoparticle size and composition, which in turn can affect encapsulation efficiency. Additionally, the composition of the nanoparticles themselves, including the ratio of different lipids and the inclusion of PEGylation, can also impact encapsulation efficiency. Furthermore, it is important to note that encapsulation efficiency can also be influenced by the type of nucleic acid being encapsulated, with different types having varying levels of stability and susceptibility to degradation. For example, siRNA is more stable than mRNA and therefore can be encapsulated with higher efficiency.
This review highlighted that encapsulation efficiency can be influenced by a variety of factors, including the method of nanoparticle preparation, the composition of the nanoparticles, and the type of nucleic acid being encapsulated. Further studies are needed to investigate the specific interactions between these factors and encapsulation efficiency. Though, our study has limitations. The comparisons of the properties and formulations of lipid nanoparticles encapsulating genetic material are based on the results of the characterizations. The elicitation methods are variable between studies and the information they provided in the papers had to match the selection criteria. A small number of included papers may be a limitation of our work. However, this was the result after evaluation of the quality and data of all the articles found.
Conclusion
In this review, it was concluded that the type of encapsulated nucleic acid that presents the best behavior and can remain within the LNP efficiently is mRNA and siRNA. Similarly, it can be determined that nanoparticles in the range of 80 nm to 220 nm are able to encapsulate nucleic acid in a percentage greater than 90%, however, an increase in encapsulation efficiency (>97%) is achieved with nanoparticles with a size around 140 nm. Such properties will have an impact on the treatment of ocular diseases, mainly related to the retina, cornea, or optic nerve in cells of the retinal pigment epithelium and corneal layer. In regard to the differences in EE observed among the formulations, it is worth mentioning that variations in EE can be influenced by various factors such as the method of preparation, composition and size of the LNPs. Further studies including statistical analysis are needed to fully understand the impact of these factors on the efficiency of the LNP formulations. Despite this, most of the formulations have an EE range between 93 and 99%, highlighting the potential of LNPs technology as a delivery system for various biomolecules. The optimal properties of LNPs to become an effective treatment for ocular diseases in humans remain unclear. However, the in vitro and in vivo studies addressed in this review paint a promising picture. Nanoparticle formulation plays a key role in increasing encapsulation efficiency and disease gene silencing. Mainly cationic lipids, PEG lipids and cholesterol provide the most stability to the nanoparticle and enable efficient RNA encapsulation.
Supplementary Information
(DOCX 41 kb)
(DOCX 49 kb)
Acknowledgements
The authors thank the NANOMEDICINA-UACJ research group for their constant support and feedback at all stages of this research.
Abbreviations
- °C
Degree Celsius
- 18:0 DAP
18:0-octadecanoic acid-diacylglycerol
- 18:0 EPC
18:0-octadecanoic acid-phosphatidylcholine
- 18:0 TAP
18:0-octadecanoic acid-triacylglycerol
- 18:1 EPC
18:1(9Z,11E)-octadecenoic acid-phosphatidylcholine
- Ai9
A mouse model of retinal degeneration
- apoE −/−
Apolipoprotein E knockout mice
- BALB/c
BALB/c mice (a strain of albino mice)
- Brn3a
Pou4f1, a transcription factor protein
- C57BL6
C57BL/6 mice (a strain of mice)
- Cre
Cre recombinase
- CSL3
Cationic switchable lipid
- DDAB
Dioctadecyldimethylammonium
- DLS
Dynamic light scattering
- DOBAQ
Dioleoyl-beta-alanine
- DODMA
Dioleoyl-3-dimethylammonium-2-hydroxypropane-1-phosphocholine
- DOTAP
N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium
- DOTMA
N,N-dioleyl-N,N-dimethylammonium
- DSPC
Distearoylphosphatidylcholine
- DSPE
Distearoyl phosphatidylethanolamine
- EE
Encapsulation efficiency
- EGFP
Enhanced green fluorescent protein
- h
Hours
- IRR
Irrelevant control
- KC2
DLin-KC2-DMA, an ionizable cationic amino lipid
- LNPs
Lipid nanoparticles
- MC3
DLin-MC3-DMA, an ionizable cationic amino lipid
- mCherry
A red fluorescent protein
- Mertk −/−
Mer receptor tyrosine kinase knockout mice
- mg
Miligram
- miR-181a
MicroRNA 181a
- ml
Mililiter
- mRNA
Messenger RNA
- MRTF
Myocardin related transcription factor B
- ng
Nanogram
- nm
Nanometers
- nM
Nanomolar
- NSC4
Nonspecific silica control
- PBS
Phosphate-buffered saline
- PDI
Polydispersity index
- PEG
Polyethylene glycol
- PRISMA ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ProSilic
A silica-based material
- RPE
Retinal pigment epithelium
- SiLUC
Silicatein-luciferase
- siRNA
Small interfering RNA
- w / w
Weight by weight
- Y-79
A retinoblastoma cell line
- μL
Microliter
Data availability
The data that support the findings of this study are available from the corresponding author, C.C.G., upon reasonable request.
Declarations
Statement of human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8:337–347. doi: 10.1016/S2213-8587(19)30411-5. [DOI] [PubMed] [Google Scholar]
- 2.Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi: 10.1016/J.AJO.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Allison K, Patel D, Alabi O. Epidemiology of Glaucoma: the past, present, and predictions for the future. Cureus. 2020;12. 10.7759/CUREUS.11686. [DOI] [PMC free article] [PubMed]
- 4.Bourne RRA, Steinmetz JD, Flaxman S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9:e130–e143. doi: 10.1016/S2214-109X(20)30425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas T, Krishnan J, Rohner N. Poor eyesight reveals a new vision gene. Elife 2022;11:. 10.7554/ELIFE.81520. [DOI] [PMC free article] [PubMed]
- 6.Ganapathi M, Thomas-Wilson A, Buchovecky C, et al. Clinical exome sequencing for inherited retinal degenerations at a tertiary care center. Sci Rep. 2022;12:9358. doi: 10.1038/s41598-022-13026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J-K, Li W, Gao F-J, et al. Mutation screening of mtDNA combined targeted exon sequencing in a cohort with suspected hereditary optic neuropathy. Transl Vis Sci Technol. 2020;9:11. doi: 10.1167/tvst.9.8.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhang Z, Huang L, et al. Update on the phenotypic and genotypic Spectrum of KIF11-related retinopathy. Genes (Basel). 2022;13. 10.3390/genes13040713. [DOI] [PMC free article] [PubMed]
- 9.Wang P, Li S, Sun W, et al. An ophthalmic targeted exome sequencing panel as a powerful tool to identify causative mutations in patients suspected of hereditary eye diseases. Transl Vis Sci Technol. 2019;8. 10.1167/TVST.8.2.21. [DOI] [PMC free article] [PubMed]
- 10.Hysi PG, Choquet H, Khawaja AP, et al. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat Genet. 2020;52:401–407. doi: 10.1038/S41588-020-0599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Chen Y, Chen H, et al. Systematic evaluation of a targeted gene capture sequencing panel for molecular diagnosis of retinitis pigmentosa. PLoS One. 2018;13. 10.1371/JOURNAL.PONE.0185237. [DOI] [PMC free article] [PubMed]
- 12.Orozco LD, Chen HH, Cox C, et al. Integration of eQTL and a single-cell atlas in the human eye identifies causal genes for age-related macular degeneration. Cell Rep. 2020;30:1246–1259.e6. doi: 10.1016/J.CELREP.2019.12.082. [DOI] [PubMed] [Google Scholar]
- 13.Harb EN, Wildsoet CF. Origins of refractive errors: environmental and genetic factors. Annu Rev Vis Sci. 2019;5:47–72. doi: 10.1146/ANNUREV-VISION-091718-015027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haarman AEG, Thiadens AAHJ, van Tienhoven M, et al. Whole exome sequencing of known eye genes reveals genetic causes for high myopia. Hum Mol Genet. 2022. 10.1093/HMG/DDAC113. [DOI] [PMC free article] [PubMed]
- 15.Ghoraba HH, Akhavanrezayat A, Karaca I, et al. Ocular gene therapy: a literature review with special focus on immune and inflammatory responses. Clin Ophthalmol. 2022;16:1753–1771. doi: 10.2147/OPTH.S364200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukai H, Ogawa K, Kato N, Kawakami S. Recent advances in lipid nanoparticles for delivery of nucleic acid, mRNA, and gene editing-based therapeutics. Drug Metab Pharmacokinet. 2022;44:100450. doi: 10.1016/J.DMPK.2022.100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Rajala A, Rajala RVS. Lipid nanoparticles for ocular gene delivery. J Function Biomater. 2015;6:379–394. doi: 10.3390/JFB6020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battaglia L, Serpe L, Foglietta F, et al. Application of lipid nanoparticles to ocular drug delivery. 2016;13:1743–1757. 10.1080/17425247.2016.1201059. [DOI] [PubMed]
- 19.Leclercq B, Mejlachowicz D, Behar-Cohen F. Ocular barriers and their influence on gene therapy products delivery. Pharmaceutics. 2022;14:998. doi: 10.3390/PHARMACEUTICS14050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajala A, Wang Y, Zhu Y, et al. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014;14:5257–5263. doi: 10.1021/nl502275s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Pozo-Rodríguez A, Solinís MÁ, Rodríguez-Gascón A. Applications of lipid nanoparticles in gene therapy. Eur J Pharm Biopharm. 2016;109:184–193. doi: 10.1016/J.EJPB.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Adijanto J, Naash MI. Nanoparticle-based technologies for retinal gene therapy. Eur J Pharm Biopharm. 2015;95:353–367. doi: 10.1016/J.EJPB.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalu L, Tambe V, Pradhan D, et al. Novel nanosystems for the treatment of ocular inflammation: current paradigms and future research directions. J Control Release. 2017;268:19–39. doi: 10.1016/J.JCONREL.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Blakney AK, McKay PF, Yus BI, et al. Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Therapy. 2019;26:363–372. doi: 10.1038/s41434-019-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripoll M, Martin E, Enot M, et al. Optimal self-assembly of lipid nanoparticles (LNP) in a ring micromixer. Sci Rep. 2022;12. 10.1038/S41598-022-13112-5. [DOI] [PMC free article] [PubMed]
- 26.Musielak E, Feliczak-Guzik A, Nowak I. Optimization of the conditions of solid lipid nanoparticles (SLN) synthesis. Molecules. 2022;27. 10.3390/MOLECULES27072202/S1. [DOI] [PMC free article] [PubMed]
- 27.Onugwu AL, Attama AA, Nnamani PO, et al. Development and optimization of solid lipid nanoparticles coated with chitosan and poly(2-ethyl-2-oxazoline) for ocular drug delivery of ciprofloxacin. J Drug Deliv Sci Technol. 2022;74:103527. doi: 10.1016/J.JDDST.2022.103527. [DOI] [Google Scholar]
- 28.Gupta B, Poudel BK, Pathak S, et al. Effects of formulation variables on the particle size and drug encapsulation of Imatinib-loaded solid lipid nanoparticles. AAPS PharmSciTech. 2016;17:652–662. doi: 10.1208/s12249-015-0384-z. [DOI] [PubMed] [Google Scholar]
- 29.Martínez Saraóz JV, Chapa González C. Review research protocol. Analysis of encapsulation efficiency in gene delivery using lipid nanoparticles in ocular diseases. 2021. 10.5281/ZENODO.4741321.
- 30.Patel S, Ryals RC, Weller KK, et al. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release. 2019;303:91–100. doi: 10.1016/J.JCONREL.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabatabaei SN, Derbali RM, Yang C, et al. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J Control Release. 2019;298:177–185. doi: 10.1016/J.JCONREL.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Ryals RC, Patel S, Acosta C, et al. The effects of PEGylation on LNP based mRNA delivery to the eye. PLoS One. 2020;15:e0241006. doi: 10.1371/JOURNAL.PONE.0241006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baran-Rachwalska P, Torabi-Pour N, Sutera FM, et al. Topical siRNA delivery to the cornea and anterior eye by hybrid silicon-lipid nanoparticles. J Control Release. 2020;326:192–202. doi: 10.1016/J.JCONREL.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Chau Y. Enhanced delivery of siRNA to retinal ganglion cells by intravitreal lipid nanoparticles of positive charge. Mol Pharm. 2021;18:377–385. doi: 10.1021/acs.molpharmaceut.0c00992. [DOI] [PubMed] [Google Scholar]
- 35.Sanghani A, Kafetzis KN, Sato Y, et al. Novel PEGylated lipid nanoparticles have a high encapsulation efficiency and effectively deliver MRTF-B siRNA in conjunctival fibroblasts. Pharmaceutics. 2021;(13):382. 10.3390/PHARMACEUTICS13030382. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 41 kb)
(DOCX 49 kb)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, C.C.G., upon reasonable request.