Abstract
The current study aims to examine the use of horse gram flour and its extracts as a low-cost source of mineral content with anti-urolithiatic, anti-diabetic properties and to compare the biological activities with its biosynthesized AgNPs. The secondary metabolites and the macro and micronutrients present in the selected herbal product, reinforces the utility of the horse gram as a promising herbal. Present investigation reveals that the biomass chosen for the study as a cheap natural source with valuable mineral content of calcium (43.06 mg/L), followed by potassium (13.78 mg/L) and sodium (6.94 mg/L). The grain's water extracts found to contain carbohydrate as dominating component with the value of (65.10 ± 0.95 mg g−1 equivalent). Whereas both water and ethanol extract contain TPC (phenol) viz; 60.13 ± 2.45 mg g−1, 68.24 ± 1.33 mg g−1, TTC (terpenoids)53.21 ± 1.4 mg g−1,51.27 ± 2.65 mg g−1, followed by TSC (sterol)as 45.58 ± 1.7 mg g−1, 57.27 ± 1.65 mg g−1 in moderate amounts respectively. The aqueous extract of Horse gram was used for the synthesis the AgNPs through a straightforward green approach and characterized by FESEM, TEM, zeta potential, X-ray diffraction, UV spectroscopy and particle size measurement. These studies demonstrate the production of AgNPs with an average particle size of 30 nm–60 nm. Investigation on anti-urolithiatic property with aqueous extract, (HGW), ethanol extract (HGE) and its biosynthesized Ag nanoparticles (HG-Ag) reveal that, among the three samples chosen, the biosynthesized Ag nanoparticles possess the best inhibiting activity. The disintegration of crystals in gel medium further validated the extracts and Ag nanoparticles crystal growth inhibitory activity, at concentrations of 2% for extracts and 200 μg/ml for AgNPs. Further, it is also found that the HG-Ag nanoparticle exhibit good anti-diabetic activity (75.36%) than the other two extracts (HGW Extract-67.18% & HGE Extract-44.29%). Thus, the seed flour extracts and its AgNps demonstrated to be a promising natural herbal product with potential mineral content, antidiabetic and anti-urolithiatic activities which could be a used as a value-added product in the dietary formulations.
Keywords: Horse gram flour, Invitro anti-urolithiatic and anti-diabetic, Silver nanoparticles
1. Introduction
The naturally available pulse, horse gram (Macrotyloma Uniflorum) is one of the best pulses for growing in arid climates which is the less known grain legume species. It is mostly used as animal feed and also a good source of minerals and proteins [1]. Horse gram was reported to contain epicatechin and daidzein and is a potent cyclooxygenase inhibitor and thus helpful in the management of inflammation and pain [2]. It is an edible grain which is generally consumed by Indians as a whole seed or as sprouts. The seed's high fiber content proved to help in reducing body fat, and it is believed that consuming them in the diet makes the body stronger and more flexible [3].
Urolithiasis is one of the most common problems which are associated with a condition of stones formation in the kidneys. Majority of the people are found to suffer from the kidney and related problems due to the poor diet dominated by junk food and fast food. Urolithiasis involves a complex process of multiple physiochemical events which consists of different stages namely; (1) Super saturation, (2) Nucleation, (3) Growth, (4) Aggregation, and (5) Retention [4]. When a urease-positive microbe infects the urinary tract, struvite stones found to occur. Because of the problems associated with pyelonephritis episode, struvite is difficult to treat, and hence it is treated with cutting-edge technology. However, acute tubular necrosis (ATN), fibrosis which leads to injuring the kidney cell and ureteral blockage were discovered to be the unfavorable side effects of these procedures [5]. The clinically used pharmacological agents, such as alkaline citrates, allopurinol, l-methionine, and anti-inflammatory drugs like ibuprofen and naproxen, were found to be ineffective despite their significant advancements in the medical treatment of urolithiasis. This was because the primary processes relating to crystal formation and aggregation were not the target, and/or because complications arising from long-term therapy [6].
Antiurolithiatic properties, which involve inhibiting the kidney stone formation, are exhibited by various medicinal plants and since long it is being treated with herbal plants traditionally. For example, extracts from citrus medium linn [7], Phyllanthus nirruri [8] and Nurvala bark [9] were found to inhibit the crystal growth. The most promising lead for the calcium oxalate kidney stone degrading biocatalyst and its in-silico studies were determined to for the water extract of Macrotyloma uniflorum seeds (Horse gram) [10,11].
In the current scenario nanotechnology is proved to be a promising approach with potential medical uses in phytomedicine. Especially biosynthesized nanomaterials are gaining its importance owing to their production through greener and environmentally friendly methods. Thus obtained M-Nps are non-toxic; possess high catalytic activity, chemical stability, and antibacterial characteristics etc., which were found to be the contributing factors for their outstanding therapeutic applications [12]. Many works are carried out with green synthesized metal nanomaterial like Ag, Cu, Ni, Cd and Zn and they were tested for the biological applications [13]. Silver nanoparticles are one of the significant metal nanoparticles tried by various researchers and demonstrated its’ medicinal applications. Silver nanoparticles synthesized with Allium fistulosum demonstrated a good zone of inhibition against both bacterial and fungal strains [14]. Synthesized pure and 20% Ag-doped CuO Nps produced from Moringa Oleifera leaf extracts are found to induce the production of ammonium magnesium phosphate hexahydrate crystals which comes a decrease in the nucleation rate of urinary tract infections that cause struvite stones [15]. Silver nanoparticles derived from Hybanthus enneaspermus stem bark extract had the greatest in vitro antiurolithiatic activities [16].
It is evident from the literature that, there is worldwide increase in the incidence of diabetes and many studies have also reported that, with an estimated 8.7% of adults in India between the ages of 20 and 70 have diabetes and this disease is becoming more and more of a problem. This can be due to the rapid urbanisation, sedentary lives, poor diets, cigarette use, and rising life expectancy which could be reasons of the factors contributing to the rising prevalence of diabetes and other noncommunicable diseases. A large portion of the burden associated with diabetes can be avoided or postponed by altering one's behaviour to favour a balanced diet and frequent exercise [17]. It is evident in the literature that green synthesized nanomaterial open up a new avenue for its applications in phytotheraphy. Recently its application expands to treat the metabolic diseases also. The green synthesized silver nano particles from calophyllum tomentosum leaves extract is found to strongly inhibit the alpha glucosidase compared to alpha amylase [18].
Thus based on the literature we can conclude that few studies have been carried out with plant extracts to treat urolithiasis. However the detailed investigation involving the comparison of the extracts and its biosynthesized nanoparticle-Ag is not reported in the literature to our knowledge. Hence in this study we made an attempt to screen the horse gram extracts and its biosynthesized nano particle with reference to its struvite crystal inhibiting property. In addition to this, comparisons also has been discussed in the above property with biosynthesized AgNPs. Further, the antidiabetic property of horse gram extracts and its bio synthesized AgNPs are also examined in this research.
2. Materials and methods
2.1. Authentication and preparation of horse gram seed (HG) flour extract
The horse gram plant was authenticated by Dr. M.U. Sharief, Scientist ‘F’ & Head of office, Botanical survey of India, Southern Regional Centre, Coimbatore. A voucher specimen was deposited (BSI/SRC/5/23/2022/Tech/610) for further reference. The raw material for the experiment was obtained from village at Vellore district nearer to the institute and it was further processed by drying at the controlled temperature and were stored for further use.
2.2. Chemicals
The chemicals used to carry out the experiments were procured from Himedia, Sigma Aldrich and Spectrochem with the purity grade as silver nitrate (99%), Sodium metasilicate (98%), Ammonium dihydrogen phosphate (98%), Magnesium acetate (97%) and 3,5-dinitrosalicilic acid (98%) etc.
2.3. Analysis of macro, micro nutrients and heavy metals in HG
HG flour is subjected to the analysis of macro, micronutrients and heavy metals by using various spectroscopic techniques (VARIAN SpectrAA 280 AAS and flame emission spectroscopy (OPTRONIC FP8500)). Acid digestion method was followed to extract the metal ions and further diluted to obtain the known volume of the sample solution. The standard metal ions solutions of Cd, Co, Cr, Cu, Mn, Na, Ca, K, Li were prepared as per the standard procedures [19]. The prepared sample and standard solutions were used to test the presence of mineral content by AAS and Flame Emission Spectroscopy respectively.
Shortly the procedure involves using 2 g of HG powder with 10 ml of Concentrated HNO3, which was cold soaked for 30 min and then raised the temperature to 120 °C for about 2 h. It is then cooled to room temperature. The solution is diluted with 25 ml of distilled water and filtered. The filtrate is transferred into a 100 ml volumetric flask and diluted to the mark and used for both the spectral studies [19].
2.4. Pharmacognostical evaluation
A systematic pharmacognostical analysis was carried out for its organoleptic characteristics, swelling power, and foaming Index. The flour is also subjected to physicochemical analysis for loss on drying, pH, total ash, acid insoluble ash value, water-soluble ash value, water-soluble and alcohol-soluble extractives as described below [20].
2.4.1. Organoleptic property
The organoleptic character of the sample was evaluated for its color, taste, texture as per the standard procedure.
2.4.2. Physiochemical parameters
The physiochemical parameters of the crude HG flour are evaluated for loss on Drying, pH, total ash, acid insoluble ash value, water-soluble ash value, water-soluble and alcohol-soluble extractives as per the standard protocol.
2.4.3. Determination of swelling power
About 1 g of HG flour was taken in a 25 ml stoppered cylinder and 25 ml of water was added to it. It is shaken occasionally for 23 h and kept aside for 1 h. The volume occupied by the powder was measured.
2.4.4. Determination of foaming index
About 1 g of HG flour was taken in a 500 ml conical flask and boiled for 30 min after adding 100 ml of water. The content was cooled and filtered. The filtrate was added to a 100 ml standard flask and adjusted the volume by the addition of water. A measured volume of the filtrate 1 ml, 2 ml, and 3 ml was pipetted out into a different stoppered test tube and adjusted the volume to 10 ml by addition of water and shaken the test tubes for 15 s. The height of the foam formed was measured after 15 min.
2.5. Ultra sonic assisted preparation of extracts
About 5 g of HG flour was taken in a two different conical flask and 100 ml of extracting medium water, ethanol was added respectively each conical flask. This was subjected to ultra-sonic agitation for 5 h at room temperature. This resulted in the two different extracts of horse gram which was obtained by filtering through Whatman filter paper and drying and the yield was calculated. It was stored at −4 °C for further study. The extracts are identified as HGW – (Horse Gram Seed Water Extract) and HGE – (Horse Gram Seed Ethanol Extract) in the further discussions.
2.6. Identification of secondary metabolites of HG
The nature of secondary metabolites presents in the both extracts of HG was identified using the reported procedure [21]. The Mayer's test and the Wagner's test were used to determine the alkaloids. The presence of flavonoids and phenols were determined using the ferric chloride test, lead acetate test, and alkaline reagent test. To check for the presence of carbohydrates, three tests were performed namely; the Molish test, Benidict's test, and the Fehling test. Biuret and Ninhydrin tests were carried out for proteins and copper lead acetate test for terpenoids. Presence of glycosides was identified by Bontrager and saponin by Legal tests. Salkowski's test was performed for the detection of phytosterols present in the HG extracts. Based on the qualitative findings, the extract's total phenol (TPC), total flavonoid (TFC), total terpenoid, (TTC) total carbohydrate (TCC) and total sterol (TSC) contents were determined through quantitative analysis of the extracts [23,28].
Compositional Analysis of HG Extracts: The compositional analysis of HG extracts was carried out with reference to the most contributing secondary metabolites such as phenol, terpenoid, steroid, carbohydrate and protein content viz by following procedures reported earlier with slight modification [[22], [23], [24], [25], [26], [27]]. In general, spectroscopic quantification method was followed by recording the absorbance at 765 nm, 538 nm, 650 nm, 490 nm and 750 nm for TPC, TTC, TSC, TCC and total protein content (TPrC) respectively.
2.7. Green synthesis of silver nanoparticles (HG-AgNp)
The experiment was carried out at room temperature by adding 10 ml of HG water extract to 90 ml of 1 mM silver nitrate solution and stirring for 5 h at room temperature. The completion of reaction was identified by observing the change in the color from light brown to dark brown which indicates the formation of silver nanoparticles. The bio reduction process was followed by measuring the absorption in the UV–Visible spectral range from 200 nm to 800 nm (Make: JASCO). Repeated centrifugation of the silver nanoparticles formed resulted in the pure biosynthesized silver nanoparticles. Further, the precipitate of AgNp's was washed thrice to obtain pure nanoparticles. The individual HG-AgNP and HGW (blank) were produced in KBr pellets containing 1% (w/w) of the samples in order to determine the functional groups through FT-IR spectroscopy and was scanned in the frequency range; 400–4000 cm−1 (Shimadzu, Japan), HG-AgNPs were subjected to X-Ray Diffractometer (Bruker, D8-Advance) study to determine the samples' crystallinity and phase structure. The surface morphology and elemental makeup of the HG-AgNPs were examined using an EDX and a scanning electron microscope (Carl Zeiss). The particle size of the formed nanoparticle was first measured using ImageJ's “Analyze Particles” approach by converting the photographs to black and white. Each element was quantified using EDX area analysis. Dynamic light scattering study (DLS) and Zeta potential measurements were done on a HORIBA Scientific and Horiba SZ 100 model. For the measurement, the sample was prepared by dissolving 10 mg of the sample followed by sonication in distilled water to dissolve all the particles. For TEM (JEOL JEM 2100 model) analysis the sample was prepared by coating with carbon on a copper grid and then blown with a hand drier to remove any extra particles. The substance was then formed into thin films for imaging.
2.8. In vitro anti-urolithiatic activity
The anti urolithiatic property of the plant extract and its biosynthesized nano material were conducted using the conventional process involving nucleation and aggregation in the hydrogel medium. The inhibition rate of struvite crystals was monitored first by growing the crystal using sodium metasilicate (SMS) (Na2SiO3·9H2O) with specific gravity 1.3 which is obtained by dropwise addition of 0.5 M ammonium dihydrogen phosphate (ADP) (NH4H2PO4·2H2O). The entire gel medium was adjusted to pH 6. The study was performed by using HGW extract (0.5, 1, 1.5 and 2%), HGE extract (0.5, 1, 1.5 and 2%) and Green synthesized HG-AgNp (100, 150, 200, 250 μg/mL) in 1 M magnesium acetate (C4H6MgO4·4H2O) which is used as the control solution. Once the gel medium is firmly set, the tubes were incubated in aseptic condition at 37 °C till the completion of the study. Using a travelling microscope, the average crystal length was measured every 24 h and statistical analysis was carried out using the one way-ANOVA test. The grown struvite crystals were characterized by powdered XRD analysis [5].
2.9. Invitro anti-diabetic activity
Invitro anti-diabetic activity was performed by α-amylase inhibition model by dissolving α-amylase in sodium phosphate buffer (0.5 ml, 0.02 M, pH 6.9). It was then added to different concentration of horse gram extracts and Hg-AgNPs of concentrations varying from 20 to 100 μg/ml 1 ml of starch solution (1%) was added to each the tubes, which were further incubated at 37 °C for 15 min. The reaction was then terminated by adding 1 ml of dinitrosalicylic acid and heating it in a boiling water bath for 10 min [14]. The tubes were then cooled and the absorbance was recorded at 540 nm.
| %inhibition = (Abs control − Abs sample/Abs control) × | 100 |
2.10. Statistical analysis
All the studies were done in triplicate and values are expressed as the mean ± standard error of the mean. The result is also expressed as IC50 value. IC50 value was calculated using regression analysis. Statistical analysis was performed using one-way ANOVA test.
3. Results and discussion
3.1. Pharmacognostical evaluation
The physicochemical characteristics are the salient features in judging the quality of an herbal drug which gives information on its purity. The studies reveal the suitability of horse gram powder as the herb and also helpful in setting the quality parameters for its identification as a drug. The pharmacognostical studies carried out includes the parameters such as loss on drying, pH, total ash value, acid insoluble ash, water soluble ash, water soluble extractives, alcohol soluble extractives along with organoleptic properties which are required to assess the nature and taste of the drug.
The physicochemical properties observed for the HG flour is given in Table 1. HG flour is observed to be dull brown in color, astringent taste and powdery in nature. The ash content represents the inorganic composition of salts, which occur in the flour and generally used to identify the purity of the crude drugs. The total ash value includes the contents of carbonates, phosphates, silicates, and silica which could be the less significant composition of the drug. In the present study the total ash content is found to be 10.4% which is within the permissible limit as per European pharmacopeia (not more than 14%) indicating the suitability of the drug in the acceptable range with less contaminants. The higher ash value signifies the adulteration by minerals and represents both physiological and non-physiological ash. The extractives obtained by exhausting the crude drugs in different solvents are indicative of the approximate measurement of their chemical constituents. The extractives presented in Table 1 shows that the crude extract contains more water-soluble compounds than the alcohol soluble. This suggests that the aqueous extract can be a potential source of phytoconstituents which could be used for detailed analysis of its constituents.
Table 1.
Physiochemical analysis of horse gram powder.
| S. No | Parameters | Percentage |
|---|---|---|
| 1 | Loss On Drying | 6.85% |
| 2 | pH | Neutral |
| 3 | Total ash value | 10.4% |
| 4 | Acid insoluble ash | 1.8% |
| 5 | Water-soluble ash | 5.8% |
| 6 | Water-soluble Extractives | 79.6% |
| 7 | Alcohol soluble Extractives | 12.47% |
3.2. Metal analysis
Plant sources are rich in mineral contents basically. Horse Gram seeds are found to be an important source of minerals and in the present study it is observed that it is enriched with calcium (43.06 mg/L), followed by potassium (13.78 mg/L), sodium (6.94 mg/L) and manganese (4.22 mg/L). Further it also contains copper (2.54 mg/L), cobalt (1.38 mg/L), cadmium (0.41 mg/L), and lithium (0.64 mg/L) in a minimum amount. Trace amount of chromium (0.28 mg/L) is also detected to the HG flour. The studies on mineral content reveal that, the horse gram is rich in minerals and other nutrients which are essential for human health, especially it is found be rich in calcium which good be a natural supplement of calcium.
3.3. Qualitative and quantitative analysis of phytoconstituents
The preliminary phytochemical analysis of HG extracts reveals that carbohydrates, phenols, proteins, saponins, terpenoids, and sterols are present in HGW extract. Whereas HGE extract is found to contain significant amount of phenol, terpenoids, proteins, and sterols and the findings are given in Table 2. The quantitative phytochemical analysis revealed that the horse gram seed extracts have a high TCC of 65.10 ± 0.95 mg g−1 equivalent, in water extract. TPC of 60.13 ± 2.45 (HGW) mg g−1, 68.24 ± 1.33 (HGE) mg g−1 gallic acid equivalent, and TTC of 53.21 ± 1.4 mg g−1 (HGW), 51.27 ± 2.65 mg g−1 (HGE) linalool equivalent, both of which were found in moderate amounts. The extract has a low sterol concentration of 45.58 ± 1.7 mg g−1, which is roughly similar to 57.27 ± 1.65 mg g−1 cholesterol. These finding suggest that water extract contains carbohydrate as the major constituent and ethanol extract contains phenol as the major constituent. Thus, the analysis reveals HGW extract as a potential source of carbohydrates. The presence of valuable secondary metabolites such as carbohydrates, phenols, terpenoids present in the extracts can contribute to the health benefits while consuming the seed flour regularly in the diet. Exceptionally, the higher content of carbohydrates present in the water extract can contribute to the antidiabetic property as reported in the literature [37].
Table 2.
Phytochemical screening of aqueous and ethanol extracts of horsegram.
| S. No | Phytochemicals | Aqueous extract | Ethanol extract |
|---|---|---|---|
| 1 | Carbohydrate | + | − |
| 2 | Steroids | + | + |
| 3 | Saponins | + | + |
| 4 | Terpenoids | + | + |
| 5 | Phenolic compound | + | + |
| 6 | Proteins | + | + |
3.4. Synthesis and characterization of (HG-AgNp)
3.4.1. UV–Vis spectroscopy
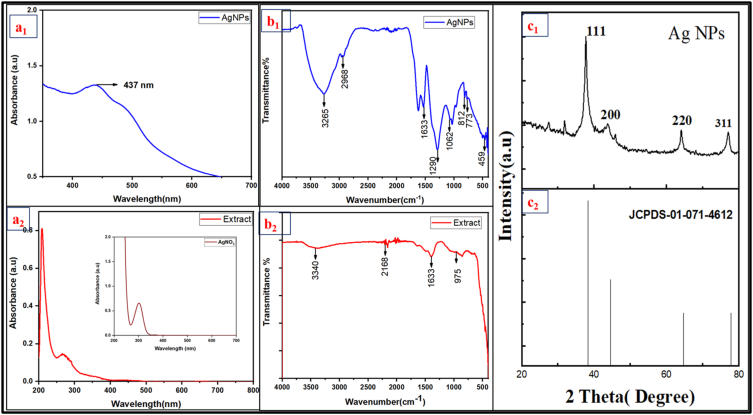
Hg-AgNp's were biosynthesized as per the procedure explained in the experimental section were characterized by using various spectral techniques. The ratio of AgNO3 to plant extracts was optimized as 1:9. The color change of mixture of solution containing the metal nitrate and the biomass was noted and found to be characteristic which indicate the development of AgNps. This is owing to the major composition of phenol in the extract and conversion of silver ion solution to silver metal (Ag+ − Ag0) [26]. This is further supported by the OH group which are available in the polyphenols in the HG extracts. In the control the absorption due to Ag+ ions were not observed. The HG-AgNp exhibited UV–Visible maxima in the range of 400–500 nm Fig. 1a1 which is due to the surface plasmon resonance developed due to the secondary metabolites of HG. UV–Vis spectra of extract showed in Fig. 1a2.
Fig. 1.
(a1, a2) UV–vis spectra of HG-AgNPs, Extract. (b1, b2) FTIR spectra of HG-AgNPs, Extract. (c1, c2) XRD pattern of HG-AgNPs, JCPDS.
3.4.2. FT-IR analysis
FT-IR was used to identify the plant biomolecules that are involved in bio reduction of Ag+ and nanoparticle capping and it is given in Fig. 1b1. The IR absorbance frequencies were found at 3265.49, 2958.80, 1621.13, 1533.41, 1290.38, 1062.07, 773.46, and 459.06 cm−1 in the FTIR spectrum. The stretching frequencies at 2958.80 and 1621.13 cm−1 can be related to C–H and C]O groups, respectively, and the absorption band at 3439.08 cm−1 corresponded to –OH of phenolic compounds. The C]C, C–O–C, and C–H stretching caused the bands at 1533.41, 1062.07, and 773.46 cm−1, respectively, the phenolic –OH is expected to can contribute for the reduction of silver ion [12]. For the seed extract, the FT-IR spectra shows major peaks at 3340.01, 2168.24, 1633.22, and 1213.71 cm−1 (Fig. 1b2). The presence of peak at 3340.01 cm−1 could be due to O–H group in polyphenols. 1633.22 cm−1 related to carbonyl group stretching vibrations. The peaks at 1213.71 cm−1 may be described to C–O–C [25].
3.4.3. Powder- XRD analysis
The biosynthesis of AgNps were confirmed using p-XRD, which is in Fig. 1c1. The diffraction pattern of biosynthesized AgNPs with marked plane indices of 111, 200, 220, and 311 is consistent with JCPDS No. 01-071-4612 (Fig. 1c2) which is characteristic of nano silver.
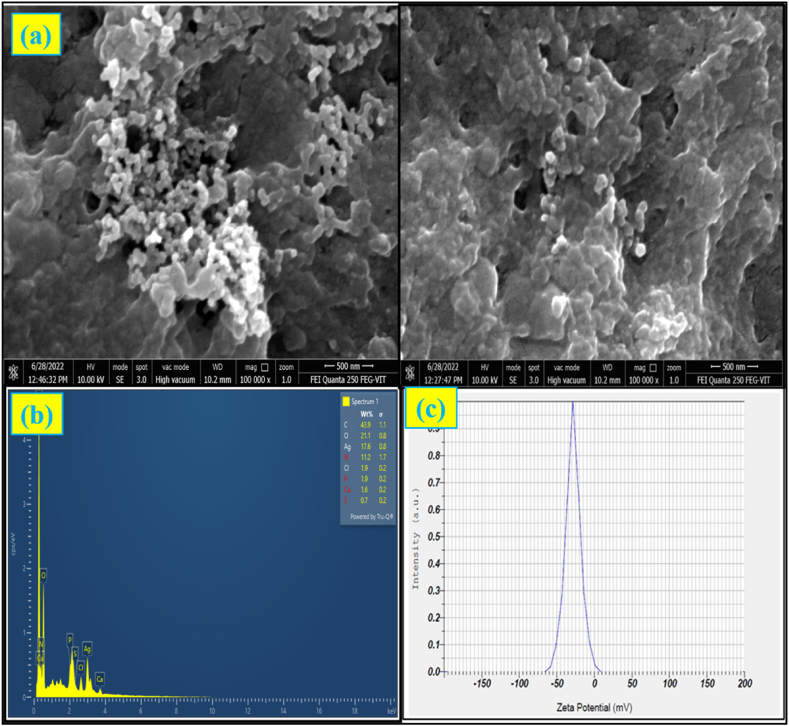
3.4.4. SEM and EDAX analysis
SEM studies confirm the morphology of bio synthesized AgNps Fig. 2a. The bio formation of silver nanoparticles obtained from Horse gram water extract was also confirmed by EDAX analysis Fig. 2b. This reveals the composition of elements presents as 43.9% of carbon, 21.1% of oxygen, and 17.6% of Ag, 11.2% of Nitrogen and minimum percentage of other elements (Cl, P, Ca, S).
Fig. 2.
(a) SEM images, (b) EDAX spectra of synthesized HG-AgNps and (c) Zeta potential of HG-AgNPs.
3.4.5. Zeta Potential and Particle Size analysis
Zeta Potential and Particle Size of biosynthesized AgNps were calculated using the zeta potential graph. The steady dispersion of the particles is indicated by the zeta potential of 27.6 mV Fig. 2c. The presence of surface-active chemicals such as phenols, flavonoids, and terpenoids in HG may contribute to the production and stability of nanoparticles [5].
3.4.6. TEM analysis
The AgNPs' dimensions, morphology, and other characteristics were examined using transmission electron microscopy. The size distribution histogram is shown in Fig. 3a. The TEM image of the NPs at low magnification and its selected area electron diffraction (SAED) pattern are displayed in Fig. 3b. The crystalline structure of the produced nanoparticle was confirmed from the SAED pattern. The TEM image shown in Fig. 3c and d confirms the spherical shape with the hexagonal structure for the nanoparticles synthesized. The encapsulation of biosynthesized nanoparticles by the secondary metabolites of horse gram is observed in TEM images and also confirms reveals the formation of HG-AgNps with an average size of 45.22 nm.
Fig. 3.
(a) Particle size analysis of synthesized HG-AgNPs, (b) SAED pattern of HG-AgNPs, (c) TEM image of HG-AgNPs.
The observations and conclusions from the spectral characterization studies such as TEM, UV–Vis and SEM carried out for the biosynthesized AgNPs are as follows; The secondary metabolites carbohydrate and phenol present in the horse gram water extract can act as an effective reducing agent for the reduction of Ag + ions which can be attributed to their well-known antioxidant activity. This biomolecule is expected to promote the nucleation and growth of spherical silver nano particles.
3.5. In vitro anti-urolithiatic activity
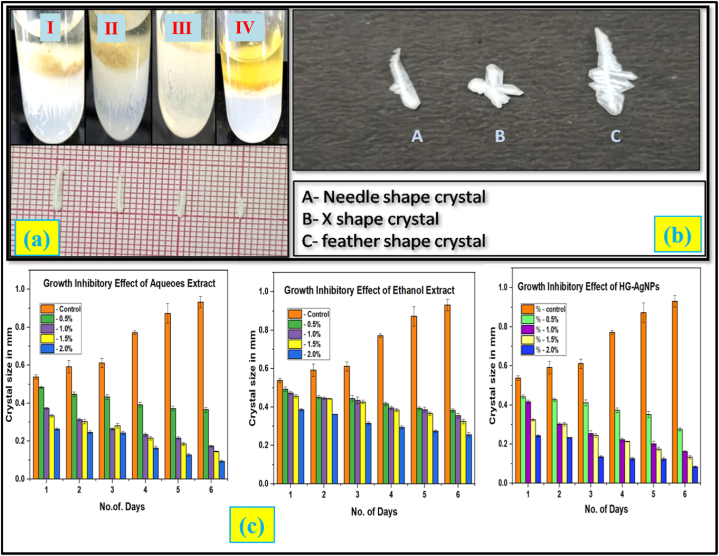
Crystal growth and its morphology: The struvite crystals that were grown with different concentrations in the gel medium with and without extracts are shown in Fig. 4a and they are labeled as I, II, III and IV. The crystals obtained resulted in the growth of struvite crystals with three morphological shapes, namely (i) needle type, (ii) X-shaped dendritic type and (iii) feather shape as shown in Fig. 4b. The presence of X-shaped crystals near the interface indicates fast development of this morphology [[29], [30], [31], [32], [33], [34]]. The struvite natures of the crystals grown were confirmed from the XRD pattern of the crystals. The XRD pattern of the crystal with the assigned plane indices matches well with the standard (JCPDS No. 96-900-7675) of struvite crystals [35,36]. The crystal growth inhibiting study was monitored for six days by treating with different concentrations of water and ethanol extracts (0.5, 1, 1.5 and 2%) and HG-AgNPs (100, 150, 200 and 250 μg) respectively (Table 3). The study reveals that the maximum inhibition concentration for both the extracts as 2% and that of HG-AgNPs (250 μg) which resulted in the considerable inhibitory impact with p value < 0.01. On comparing HG-AgNPs and HG extracts, the crystal inhibition was found to be greater with HG-AgNPs than that of HG extracts. It was also observed that the crystals began to dissolve on the sixth day, demonstrating that the HG extracts and HG-AgNPs possesses significant struvite crystal inhibitory efficacy. From the study it is found that the HG extracts showed a significant role in the crystal growth as a natural inhibitor by attaching itself to the crystals growth site and thus prevent the crystals from growing and aggregating further. This is expected to slow down the growth of the struvite crystals [5]. The bioactive constituents present in Horse gram water extract, such as; carbohydrate, phenols, terpenoid, and sterol, is expected to inhibit the formation of struvite crystals by forming a compound with magnesium ions and therefore preventing crystal growth as reported [24,25]. The inhibitory function could possibly be attributed to the active components which can influence on pathogenic organisms that can prevent struvite crystal formation [26,27].
Fig. 4.
(a) Development of struvite crystals in gel medium with different concentration of HG-AgNPs (I-100 μg, II-150 μg, III-200 μg and IV-250 μg) (b) struvite crystals grown and their morphology (c) struvite crystal growth inhibitory effect of extracts and its nanoparticles.
Table 3.
Struvite crystal growth inhibitory effects of Aquoues, Ethanol Extracts and HG-AgNps.
| No of days | Control | HGW-Extract |
HGE- Extract |
HG-AgNps |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5% | 1% | 1.5% | 2% | 0.5% | 1% | 1.5% | 2% | 100 μg | 150 μg | 200 μg | 250 μg | ||
| 1 | 0.539 ± 0.01 | 0.48 ± 0.05 | 0.374 ± 0.03 | 0.335 ± 0.02 | 0.264 ± 0.01 | 0.493 ± 0.06 | 0.474 ± 0.03 | 0.456 ± 0.02 | 0.387 ± 0.01 | 0.443 ± 0.02 | 0.415 ± 0.01 | 0.324 ± 0.02 | 0.243 ± 0.02 |
| 2 | 0.591 ± 0.03 | 0.448 ± 0.04 | 0.315 ± 0.01 | 0.304 ± 0.04 | 0.248 ± 0.06 | 0.452 ± 0.02 | 0.446 ± 0.01 | 0.443 ± 0.04 | 0.365 ± 0.02 | 0.429 ± 0.04 | 0.305 ± 0.03 | 0.304 ± 0.04 | 0.235 ± 0.03 |
| 3 | 0.613 ± 0.02 | 0.431 ± 0.03 | 0.264 ± 0.02 | 0.28 ± 0.01 | 0.241 ± 0.03 | 0.447 ± 0.04 | 0.438 ± 0.04 | 0.427 ± 0.01 | 0.317 ± 0.02 | 0.410 ± 0.02 | 0.254 ± 0.01 | 0.243 ± 0.01 | 0.134 ± 0.01 |
| 4 | 0.772 ± 0.01 | 0.391 ± 0.05 | 0.232 ± 0.06 | 0.214 ± 0.05 | 0.163 ± 0.02 | 0.417 ± 0.02 | 0.397 ± 0.03 | 0.386 ± 0.03 | 0.296 ± 0.01 | 0.373 ± 0.03 | 0.221 ± 0.04 | 0.212 ± 0.03 | 0.123 ± 0.018 |
| 5 | 0.875 ± 0.04 | 0.375 ± 0.03 | 0.217 ± 0.03 | 0.184 ± 0.01 | 0.129 ± 0.01 | 0.395 ± 0.01 | 0.384 ± 0.02 | 0.366 ± 0.02 | 0.275 ± 0.04 | 0.354 ± 0.01 | 0.201 ± 0.02 | 0.176 ± 0.01 | 0.110 ± 0.03 |
| 6 | 0.931 ± 0.02 | 0.365 ± 0.01 | 0.174 ± 0.02 | 0.143 ± 0.03 | 0.095 ± 0.02 | 0.381 ± 0.03 | 0.355 ± 0.04 | 0.325 ± 0.02 | 0.256 ± 0.03 | 0.278 ± 0.02 | 0.165 ± 0.02 | 0.132 ± 0.01 | 0.086 ± 0.03 |
3.6. In vitro anti-diabetic activity
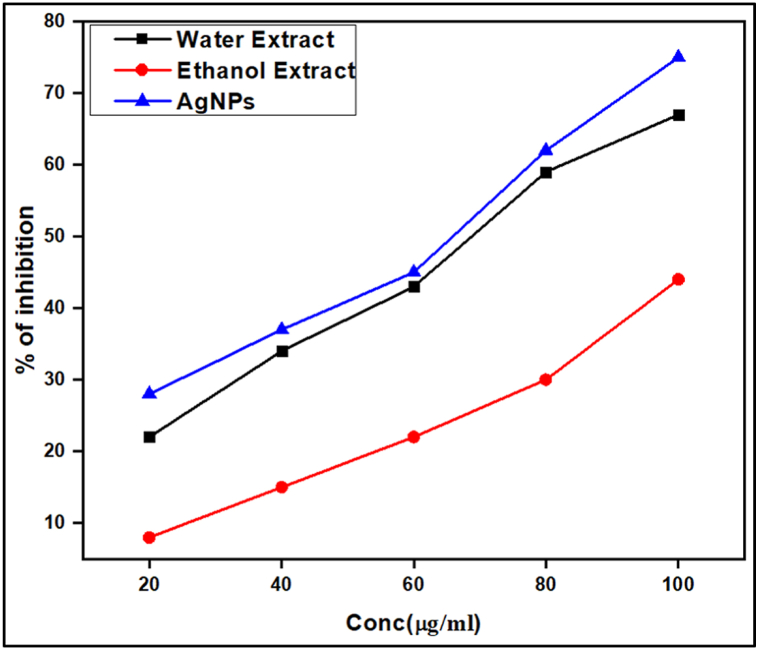
The inhibitory effects of alpha-amylase test were used to investigate antidiabetic efficacy in, in-vitro model for the extracts and the HG-AgNps. Diabetes mellitus is a set of metabolic illnesses characterized by persistently elevated blood sugar levels. Inhibiting carbohydrate digesting enzymes, which prevents the breakdown of carbohydrates into monosaccharides, is the major cause of hyperglycemia. Hence inhibiting digestive enzymes like α-glucosidase is proved to be an effective treatment to treat T2 diabetes [37]. In the current study it is observed that identifying drugs that can block carbohydrate hydrolyzing enzymes could be a valuable strategy to manage diabetes. In the present study it was observed that α-amylase is strongly inhibited by HG-AgNps in a dose-dependent manner. The results show that when the concentration of HG-AgNps increased, the enzyme activity levels decreased dramatically, yielding IC50 values of 65.2 and 53.8 g/ml, respectively, which were much higher than the extracts. As a result, the biomolecules from Horse gram extracts have increased the anti-diabetic potential of the produced NPs. In the present study α-amylase inhibitory effects were observed in HGE, HGW and HG-AgNp's is in increasing order.
The inhibitory effects of extracts and their NPs on alpha amylase were found to be dosage dependent, as shown by the standard curve in Fig. 5. The HG-AgNps had the maximum inhibitory activity of 75.36 ± 0.34% at a concentration of 100 μg/ml, compared to water extract at the same concentration which is found to be 68.58 ± 0.38%. At concentrations of 20 μg/ml, 40 μg/ml, 60 μg/ml, 80 μg/ml, and 100 μg/ml, the HG-AgNps and water extract showed inhibition values as 28.02 ± 0.40%, 34.42 ± 0.26%, 43.82 ± 0.32%, 59.33 ± 0.46%, 75.36 ± 0.44% and 22.02 ± 0.49%, 37.84 ± 0.76%, 44.79 ± 0.43%, 62.45 ± 0.43%, 67.18 ± 0.16% respectively. For the different concentrations of ethanol extracts the inhibition values of 8.12 ± 0.49%, 15.84 ± 0.76%, 22.17 ± 0.43%, 30.45 ± 0.43%, and 44.29 ± 0.16% at the same selected concentrations. The IC50 value for HG-AgNPs, water extract and ethanol extract was computed using the standard curve and found to be 67.13, 81.19 and 113.41 respectively. As evident in the quantitative analysis the carbohydrate and phenols present in the extract is expected to contribute remarkably to the biological activities that are tested. Further the silver metal also found to have excellent biological activities which are evident in the biosynthesized nanomaterial.
Fig. 5.
Antidiabetic activity of HG Extracts and HG-AgNPs in different concentrations.
4. Conclusions
The presence of secondary metabolites of the HG extracts generated by the Ultra sonic assisted approach reveal that the extract contains considerable amount of carbohydrate and phenol. The other constituents such as terpenoids, sterol, and protein were also found to be present in a significant amount in both the extract. The green synthesis of AgNPs using the natural product horse gram is demonstrated in the study with its characterization by spectroscopic studies. The study confirms the formation of nanoparticle which is representing spherical morphology with average particle size of 45.22 nm. The antiurolithiatic property of the extracts and the HG-AgNPs were studied with the respect to the growth of struvite crystals in the gel medium. It was observed that the growth of the crystals was effectively inhibited by HG extracts and its silver nanoparticles. The treatment of the crystals treated with the HG extracts prevented crystal nucleation and also dissolved the crystals that were formed in the gel medium, with the inhibitory activity of the HG-AgNps being even stronger. Both HGW and its HG- AgNPs inhibited the development of struvite stones effectively. The urolithiasis-inhibiting action of the HG could be attributed to its high bioactive contents of phenol and terpenoid. The results from the antidiabetic activity of horse gram extracts and HG-AgNPs showed better antidiabetic activity. According to the findings, Horse gram water extract and HG-AgNPs with its duo properties can be used as an alternative therapy for the prevention of urolithiatic conditions and diabetics. Further the pharmacognostical properties substantiates its usage in drug formulation which could be a potential herbal drug and also the horse gram seed flour could be a value added ingredient in the dietary formulations.
Author contribution statement
S Sudha: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mary Saral: Conceived and designed the experiments; Analyzed and interpreted the data; Reviewed and edited the manuscipt.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank VIT management for providing a seed grant and research fellowship to one of the author. The SAIF of VIT is duly acknowledged for the cgarcterisation studies.
References
- 1.Qurat U., Rizvi Eain Hyder, Kumar Krishan, Ahmed Naseer, Chauhan Divya, Thakur Priyanka, Jan Sumaira, Sheikh Imran. Effect of processing treatments on nutritional, anti-nutritional and bioactive characteristics of horse gram (Macrotyloma uniflorum L.) J. Postharvest Technol. 2022:10. [Google Scholar]
- 2.Ramalingam Malarvizhi, Sali Veeresh K., Bhardwaj Meenakshi, Mani Sugumar, Hannah Vasanthi R. Inhibition of cyclooxygenase enzyme by bioflavonoids in horse gram seeds Alleviates pain and inflammation. Comb. Chem. High Throughput Screen. 2020:23. doi: 10.2174/1386207323666200127114551. [DOI] [PubMed] [Google Scholar]
- 3.Trautwein Elke A., Vermeer Mario A., Hiemstra Harry, Ras Rouyanne T. LDL-cholesterol Lowering of Plant Sterols and Stanols. Nutrients. 2018:10. doi: 10.1016/j.lwt.2022.113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujihata Masao. Mechanism of calcium oxalate renal stone formation and renal tubular cell injury. Int. J. Urol. 2008;15:115–120. doi: 10.1111/j.1442-2042.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- 5.Velu Vinodhini, Das Moonjit, Nambi Raj Arunai, Dua Kamal, Malipeddi Himaja. Evaluation of in vitro and in vivo anti-urolithiatic activity of silver nanoparticles containing aqueous leaf extract of Tragia involucrate. Drug Delivery Transl. Res. 2017;7 doi: 10.1007/s13346-017-0363-x. [DOI] [PubMed] [Google Scholar]
- 6.McAteer James A., Evan Andrew P. The acute and long-term adverse effects of shock wave lithotripsy. Semin. Nephrol. 2008;28(2):200–213. doi: 10.1016/j.semnephrol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan C.K., Joshi M.J. Growth inhibition of Struvite crystals in the presence of juice of Citrus medica Linn. Urol. Res. 2008;36:265–273. doi: 10.1007/s00240-008-0154-4. [DOI] [PubMed] [Google Scholar]
- 8.Barros M.E., Boim M.A. Effects of an aqueous extract from Phyllanthus niruri on calcium oxalate crystallization in vitro. Urol. Res. 2003;30:374–379. doi: 10.1007/s00240-002-0285-y. [DOI] [PubMed] [Google Scholar]
- 9.Pantha Rubee, Pandey Jitendra, Joshi Neeraj, Budathoki Rajesh, Ghimire Sangita, Pokhrel Tara, Joshi Dirgha Raj, Rokaya Rabindra Kumar, Khadka Ram Bahadur, Aryal Pramod, Bhandari Ravin. Anti-urolithiatic property of Crataeva nurvala root and bark from nepal on ethylene glycol induced urolithiatic mice. J. Pharmaceut. Sci. Res. 2020;12(5):658–662. [Google Scholar]
- 10.Gautam M., Datt N., Chahota R.K. Assessment of calcium oxalate crystal inhibition potential, antioxidant activity and amino acid profiling in horse gram (Macrotyloma uniflorum): high altitude farmer's varieties. 3 Biotech. 2020;10(9):402. doi: 10.1007/s13205-020-02394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satpal Netrapal Sharma, Bisht Singh, Gupta Sanjay, Rana Mahendra, Kumar Ajay, Pathak Rajesh. In-silico study for antiurolithiatic activities of compounds extracted from horse gram (Macrotyloma uniflorum) collected from Uttarakhand. Int. J. Recent Sci. Res. 2019;10 doi: 10.24327/ijrsr.2019.1007.3711. [DOI] [Google Scholar]
- 12.Swathy Bekkeri. A review on metallic silver nanoparticles. IOSR J. Pharm. 2014;4(7):38–44. [Google Scholar]
- 13.Gour Aman, Jain Narendra Kumar. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019;47:844–851. doi: 10.1080/21691401.2019.1577878. [DOI] [PubMed] [Google Scholar]
- 14.Vinodhini S., Scholastica Mary Vithiya B., Augustine Arul Prasad T. Green synthesis of silver nanoparticles by employing the Allium fistulosum, Tabernaemontana divaricate and Basella alba leaf extracts for antimicrobial applications. J. King Saud Univ. Sci. 2022;34 doi: 10.1016/j.jksus.2022.101939. [DOI] [Google Scholar]
- 15.Reshmi Agnes Preethi D., Philominal A. Antimicrobial and antiurolithiatic activities of pure and silver doped copper oxide nanoparticles using Moringa Oleifera leaf extract on struvite urinary stones. Appl. Surf. Sci. Adv. 2022;12 doi: 10.1016/j.apsadv.2022.100351. [DOI] [Google Scholar]
- 16.Suchithra M.R., Bhuvaneswari S., Sampathkumar P., Dineshkumar R., Chithradevi K., Beevi farhana noor M., Madhumitha R., Kavisri M. In vitro study of antioxidant, antidiabetic and antiurolithiatic activity of synthesized silver nanoparticles using stem bark extracts of Hybanthus enneaspermus. Biocatal. Agric. Biotechnol. 2021;38 doi: 10.1016/j.bcab.2021.102219. [DOI] [Google Scholar]
- 17.Pradeepa R., Mohan V. Epidemiology of type 2 diabetes in India. Indian J. Ophthalmol. 2021;69:2932–2938. doi: 10.4103/ijo.IJO162721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindappa M., Hemashekhar B., Arthikala Manoj-Kumar, Ravishankar Rai V., Ramachandra Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018;9:400–408. [Google Scholar]
- 19.Silvana R., Oliveira A. Jose, Gomes Neto, Nobrega Joaquim A., Jones Bradley T. Determination of macro- and micronutrients in plant leaves by high-resolution continuum source flame atomic absorption spectrometry combining instrumental and sample preparation strategies. Spectrochim. Acta, Part B. 2010;65:316–320. [Google Scholar]
- 20.Monika T., Ilavarasi L., Abinaya T., Saravanadevi M.D., Karolin Daisy Rani R., Meenakumari R. Standardization of A Classical Siddha poly Herbal formulation “Nannari Mathirai” through organoleptic character. Physiochem. Phytochem. Anal. Eur. J. Pharm. Med. Res. 2020:7. [Google Scholar]
- 21.Phytohemical Methods, J.B.Horborne, second ed..
- 22.Ghorai Narayan, Chakraborty Sondipon, Gucchait Shamik, Saha Samir Kumar, Biswas Suman. Bioassay directed isolation and characterization of bioactive compounds from fruit pulp of Annona Muricata L. World J. Eng. Res. Technol. 2017:4. [Google Scholar]
- 23.Roghini R., Vijayalakshmi K. Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of citrus paradisi. Int. J. Pharm. Sci. Res. 2018:9. [Google Scholar]
- 24.Banerjee Soumitra, Haldar Swarrna, Reddy Narendra, Reddy Roopa, Nagananda G.S., Mitra Jayeeta. Under-utilized germinated horse gram (Macrotyloma uniflorum) protein – extraction, process optimization, characterization and its use in cookies fortification. Food Sci. Technol. 2022:160. doi: 10.1016/j.lwt.2022.113276. [DOI] [Google Scholar]
- 25.AbiramiValli S., Uma Gowri S. Characterization of phytopharmaceuticals from fresh and dried sprouts of Macrotyloma uniflorum (Lam.) Verdc. Int. J. Chemtech. Res. 2017:10. [Google Scholar]
- 26.Vijayalakshmi Mahadevan, Rameshkumar Ganesan, Rajagopal Thangavel, Thangapandian Veerapandiyan, Ponmanickam Ponnirul. Phytofabrication of silver nanoparticles using horse gram (Dolichos biflorus L.) seed extract and assessment of its bactericidal and antioxidant activities. Thai J. Pharm. Sci. 2015:39. [Google Scholar]
- 27.Chakraborty Gayatri, Manna Kuntal, Debnath Bikash, Singh Waikhom Somraj, Goswami Sanchari, Maiti Debasis. Phytochemical analysis, anti-oxidant and cytotoxic activity of seed coat of Macrotyloma uniflorum in different solvents. Nat. Prod. Chem. Res. 2018:6. doi: 10.4172/2329-6836.1000335. [DOI] [Google Scholar]
- 28.Marimuthu M., Krishnamoorthi K. Nutrients and functional properties of horse gram (Macrotyloma uniflorum), an underutilized south Indian food legume. J. Chem. Pharm. Res. 2013:5. [Google Scholar]
- 29.Kaleeswaran B., Rama Devi S., Murugesan R., Srigopalram, Suman T. Evaluation of anti-urolithiatic potential of ethyl acetate extract of Pedalium murex on struvite crystal (kidney stone) J. Tradit. Complement. Med. 2017;9 doi: 10.1016/j.jtcme.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel Vaibhavkumar Baldavbhai, Acharya Niyati. Effect of Macrotyloma uniflorum in ethylene glycol induced urolithiasis in rats. Ind. J. Pharm. Educ. Res. 2020:54. doi: 10.1016/j.heliyon.2020.e04253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deka Kangkan, Kakoti Bibhuti Bhusan, Das Moonjit. Antiurolithiatic activity of leaf extracts of Syzygium Jambos (L.) Alston and its zinc nanoparticles: an in-vitro and in-vivo approach. Int. J. Pharmaceut. Sci. Res. 2021:12. doi: 10.13040/IJPSR.0975-8232.12(1).336-46. [DOI] [Google Scholar]
- 32.Sai Harshita P., Soma Yasaswi P., Rajeshwari M., Jyothi Vemuri, Sonali Kanchan. Anti-urolithiasis activity of Vaccinium macrocarpon fruits: an in vitro study. J. Med. Plant Stud. 2020:8. doi: 10.22271/plants.2020.v8.i5a.1191. [DOI] [Google Scholar]
- 33.Phatak Rohan Sharadanand, Hendre Anup Subhash. In-vitro antiurolithiatic activity of Kalanchoe pinnata extract. Ind. J. Pharmacogn. Phytochem. Res. 2015:7. [Google Scholar]
- 34.Rathod N., Chitme Hr, Chandra R. In vivo and in vitro models for evaluating anti-urolithiasis activity of herbal drugs. Int. J. Pharm. Res. Bio Sci. 2014:3. [Google Scholar]
- 35.Bawari Sweta, Negi Sah Archana, Tewari Devesh. Antiurolithiatic activity of Daucus carota: an in vitro study. Pharmacogn. J. 2018:10. doi: 10.5530/pj.2018.5.148. [DOI] [Google Scholar]
- 36.Dhanya Raj C.T., Palaninathan Vivekanandan, James Rathinam Arthur. Anti-uropathogenic, antioxidant and struvite crystallization inhibitory potential of fresh and fermented coconut water. Biocatal. Agric. Biotechnol. 2023;47 doi: 10.1016/j.bcab.2022.102555. [DOI] [Google Scholar]
- 37.Das Gitishree, Kumar Patra Jayanta, Debnath Trishna, Ansari Abuzar, Shin Han-Seung. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus. PLoS One. 2019:7. doi: 10.1371/journal.pone.0220950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.