Figure 1.
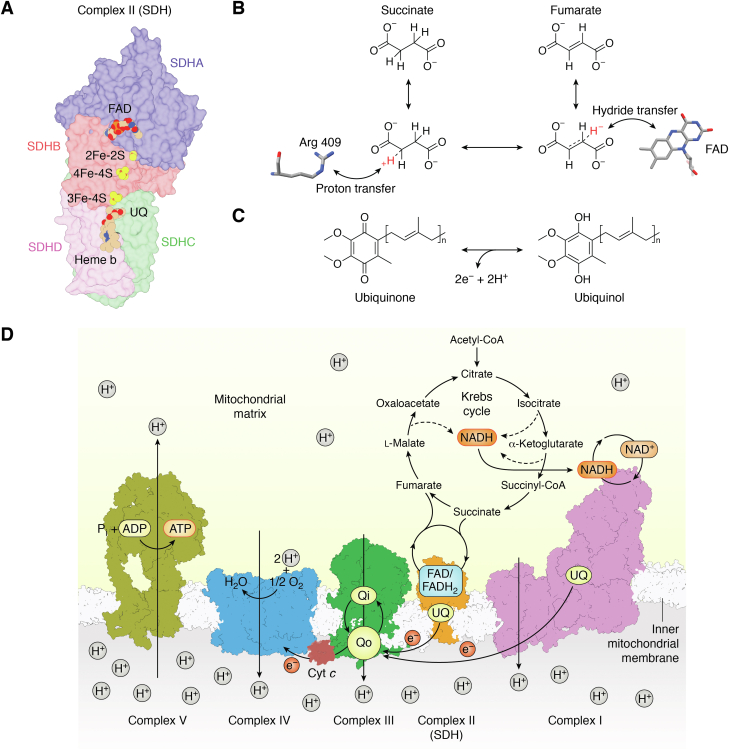
Mitochondrial complex II and its reactions in mitochondrial aerobic respiration.A, architecture of mitochondrial complex II. Mitochondrial complex II (PDB 1ZOY (75)) contains four subunits: SDHA, SDHB, SDHC, and SDHD. SDHA (blue) is also called the flavoprotein (Fp) subunit; it houses the covalent FAD and the succinate oxidation site. SDHB (red) is also called the iron–sulfur (Ip) subunit; it houses the three Fe-S clusters that support electron transfer between the two active sites. The SDHC (green) and SDHD (purple) subunits are membrane-embedded, contain b-type heme, and house the ubiquinone reduction site. B and C, complex II couples two redox reactions: succinate/fumarate interconversion and quinone/quinol interconversion. The reactions are believed to be dependent as electrons that are coproducts of one reaction are transferred to the second active site to be used as cosubstrates. B, Succinate/fumarate interconversion. The mechanism of succinate/fumarate interconversion is better studied in the fumarate reduction direction. For fumarate reduction, substrate binding involves two active site histidines (His 232 and His 355, from the E. coli QFR) and one active site arginine (Arg 390 of E. coli QFR, which is equivalent to Arg 409 of human SDHA) (58). Catalysis requires an initial transfer of a hydride from FAD to fumarate via a neutral flavin semiquinone (71). This is followed by the transfer of a proton by an active site arginine (Arg 287, E. coli QFR numbering) (72, 207). Supporting the reaction through the transition state is a hydrogen bond between substrate carboxylate and a threonine side chain hydroxyl (Thr 244, E. coli QFR numbering) (73). Because this catalytic threonine is on a different domain than the binding residues, interdomain motion changes the position of this hydrogen-bond donor, which twists the substrate during the reaction. Both a diode effect and the presence of a different flavin intermediate suggest that intermediates of succinate oxidation and fumarate reduction differ (71, 202). C, quinone/quinol interconversion. The second chemical reaction housed by complex II is the 2H+/2e− interconversion of quinone and quinol in the membrane. In mitochondrial aerobic respiration, this involves the electrons harvested from succinate and uses ubiquinone as the quinone. Different types of respiration may use quinones with different potentials, which affects the driving force of the reaction. D, aerobic respiration usually relies upon oxidative phosphorylation to synthesize ATP because this is the most efficient way to convert energy to a biologically useful form. In animals, oxidative phosphorylation involves four membrane-spanning complexes of the electron transfer chain. The figure uses ovine complex I (pink, PDB 7ZD6, (208)), porcine complex II (orange, PDB 1ZOY (75)), bovine complex III (green, PDB 1NTZ (209)), and bovine complex IV (blue, PDB 2OCC (210)). These complexes guide the transfer of electrons down small, energetically favorable electron steps until they reach O2, which is reduced to H2O in complex IV. The strong oxidizing power of oxygen, ∼800 mV, drives the entire process. The overarching theme of all respiration is the coupling of these electron transfer steps with the formation of a transmembrane electrochemical gradient, shown as a proton gradient here. Complex V (mustard yellow, PDB 6ZPO (211)), also called the ATP synthase, converts the energy stored in the electrochemical gradient to a more biologically useful form. To do this, complex V couples the transfer of a proton back along its gradient with conformational changes that promote the formation of a bond between ADP and inorganic phosphate and that release the product, ATP. SDH, succinate dehydrogenase; QFR, quinol:fumarate reductase.