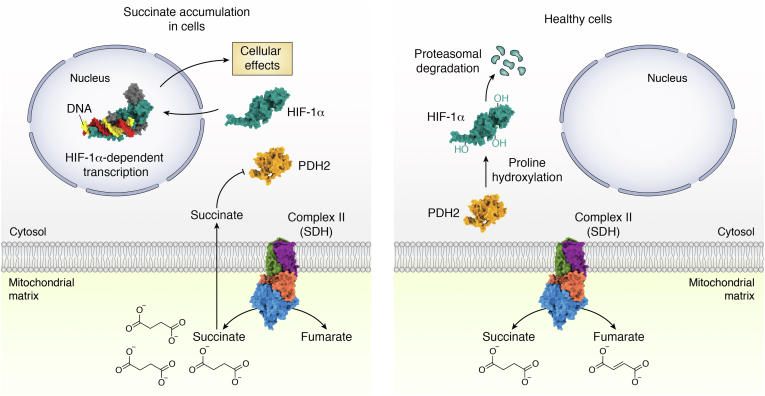
Figure 6.
Control of HIF1α activity through substrate-level inhibition of its degradation. Healthy cells under normoxia use oxidative phosphorylation to synthesize the majority of ATP. During succinate accumulation, cells can redirect metabolism to generate lactate via glycolysis, which is less efficient in producing ATP. One metabolic switch involves changes in complex II (1ZOY (75)) activity, resulting in the accumulation of cellular succinate. Some of the biological situations where succinate might accumulate are hypoxia, during complex II inhibition, in patients with reduced functional complex II, during reverse electron transfer, or during cancer. One theory is that succinate accumulation controls the quantity of the transcription factor HIF1α (modified from 4ZPR (235)). In healthy cells under aerobic conditions, PHD2 (2CGN (177)) covalently attaches hydroxides to proline residues in HIF1α. The end effect is targeting of the HIF1α transcription factor for degradation by the proteasome. Cellular dicarboxylates, including succinate, inhibit prolyl hydroxylase-2. When HIF1α accumulates, it binds to DNA (4ZPR (235)) and induces the transcription of a range of proteins used in the hypoxic response. This shunts respiration away from oxidative phosphorylation and toward glycolysis and the biosynthesis of nucleotides and lipids. When this occurs under aerobic conditions in cancer cells, it is also termed the Warburg effect. HIF1α, hypoxia-inducible factor 1α; SDH, succinate dehydrogenase; PHD2, prolyl hydroxylase-2.