Abstract
Biopolymers are promising environmentally benign materials applicable in multifarious applications. They are especially favorable in implantable biomedical devices thanks to their excellent unique properties, including bioactivity, renewability, bioresorbability, biocompatibility, biodegradability and hydrophilicity. Additive manufacturing (AM) is a flexible and intricate manufacturing technology, which is widely used to fabricate biopolymer-based customized products and structures for advanced healthcare systems. Three-dimensional (3D) printing of these sustainable materials is applied in functional clinical settings including wound dressing, drug delivery systems, medical implants and tissue engineering. The present review highlights recent advancements in different types of biopolymers, such as proteins and polysaccharides, which are employed to develop different biomedical products by using extrusion, vat polymerization, laser and inkjet 3D printing techniques in addition to normal bioprinting and four-dimensional (4D) bioprinting techniques. This review also incorporates the influence of nanoparticles on the biological and mechanical performances of 3D-printed tissue scaffolds. This work also addresses current challenges as well as future developments of environmentally friendly polymeric materials manufactured through the AM techniques. Ideally, there is a need for more focused research on the adequate blending of these biodegradable biopolymers for achieving useful results in targeted biomedical areas. We envision that biopolymer-based 3D-printed composites have the potential to revolutionize the biomedical sector in the near future.
Keywords: 3D printing, Biopolymers, Biomedical, Tissue engineering, Sustainable biomaterials, Additive manufacturing
Graphical abstract
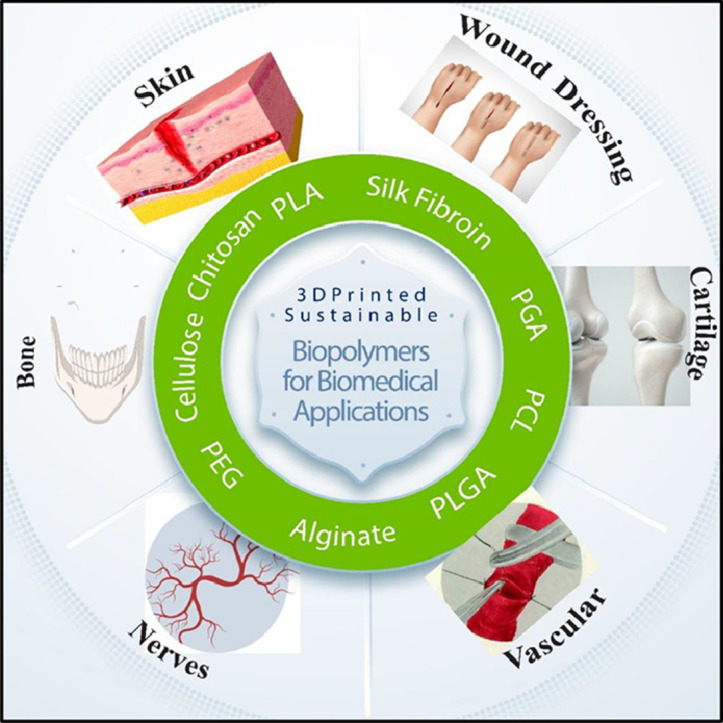
1. Introduction
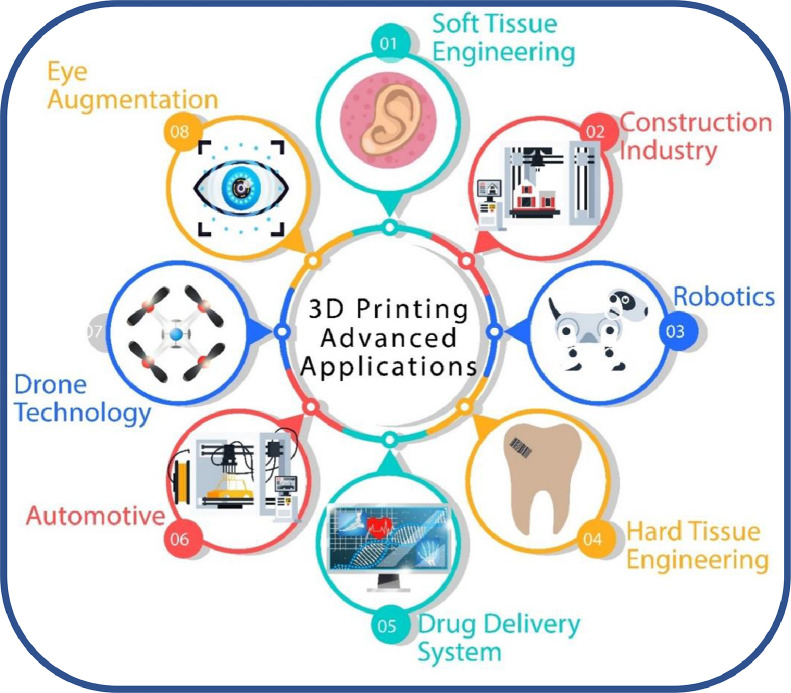
Scientific progresses in novel manufacturing approaches especially in the additive manufacturing (AM), alias three-dimensional (3D) printing areas, have laid the foundations for many engineering and biomedical applications thanks to its efficiency, precision and accuracy [1], as illustrated in Fig. 1. The AM technology uses imaging techniques or computer-aided design (CAD) software to fabricate 3D customized objects like patient-specific implants, without the need for molds or machining [2], [3], [4]. This technology is highly appropriate to develop intricate structures by using different materials, in contrast to conventional manufacturing processes [5], [6], [7]. Over the years, this technology has found its potential in myriad manufacturing areas including, but not limited to, automotive, aerospace, construction, rapid prototyping, jewelry and biomedical fields [8], [9], [10], [11].
Fig. 1.
Recent scientific progresses in various fields of engineering.
Since the beginning of the 21st century, the 3D printing technique has been extensively applied in the biomedical sector for developing personalized prosthetics, dental implants, organ and tissue fabrications, anatomical models, and pharmaceutical products [12], [13], [14]. Some studies also illustrate the utilization of this novel technology for producing exoskeletons, ears, stem cells, bones and microvascular networks [15], [16], [17]. The technology utilizes different biomaterials including metals, powders, liquids, ceramics, polymers and living cells to develop intricate structures with excellent mechanical characteristics, which cannot be attained through conventional manufacturing techniques [18], [19], [20]. Biomaterials used for the development of such implants and human organs can be classified into three types of materials, i.e., metals, polymers and ceramics [21]. Despite the high strength, hardness, fracture toughness and corrosion resistance of inert metallic implants such as stainless steel (SS), these 3D-printed components may adversely impact on the human body because of their non-biodegradability [22], [23], [24], [25]. However, metallic implants exhibit high elastic moduli that result in stress shielding. Furthermore, toxic effects appeared due to the release of ions from the metallic implants limiting their use in biomedical applications [26].
Other non-biodegradable alloy scaffolds such as chromium-cobalt(Cr–Co) alloys also exhibit limited advantages, e.g., they can support tissues but simultaneously cause inflammation and allergic reactions at the implantation sites [27]. Most of these alloys contain free ions in their structures, which are responsible for these problems. It is worth mentioning that these are relatively expensive materials as well. To circumvent the aforementioned drawbacks, sustainable biomaterials have been formulated for a wide range of applications [28].
Renewable resources are the most attractive sources of raw material in terms of green environment and planetary health. Sustainable materials are acquired from renewable natural resources, recycling or other low-carbon feedstock, which are managed through biodegradation and recycling approaches [29]. These materials including natural, synthetic or modified bio-based polymers, are sustainable, renewable and extraordinary materials with low carbon footprints and low embodied energy levels, compared to the existing traditional stabilizers. Carbon dioxide released at the end of service time, due to biodegradation is reabsorbed by fauna and flora, which makes them carbon neutral [30]. Biopolymers follow a circular economy model, which helps their recycling at the end of life. Additionally, the accumulated plastic waste has triggered the use of these environmentally benign polymers in different industrial sectors including biomedical engineering/science [31].
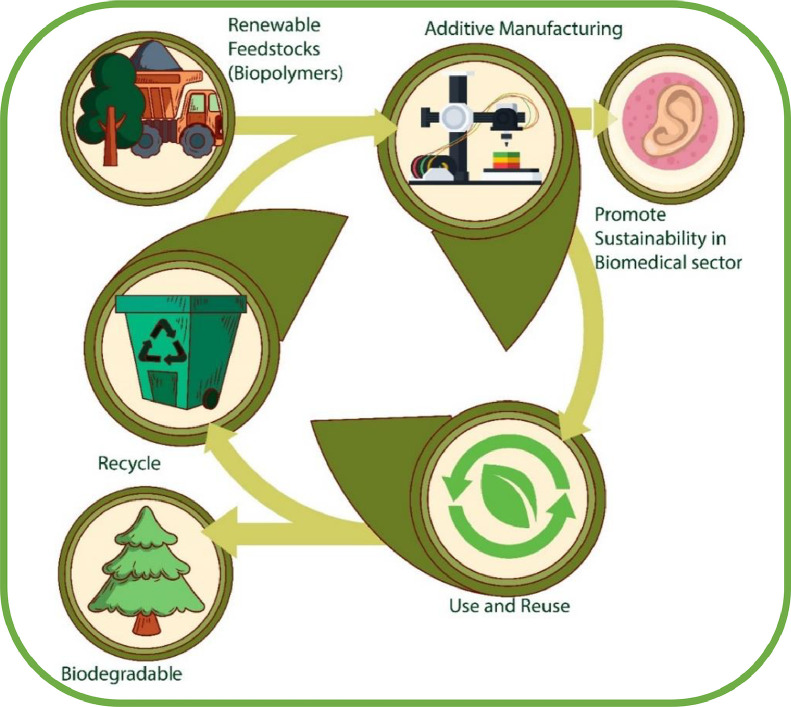
Sustainable biopolymers provide an interrelationship between renewable natural resources and biomaterials, and the world has considered the development of novel and sustainable biopolymer-based biomaterials as a feedstock for the AM technology, as illustrated in Fig. 2, thanks to their biodegradability, biocompatibility and renewability [32], [33], [34]. These types of feedstock materials promote the sustainability within the AM technology itself [35]. Sustainable biopolymers including bio-based polymers are viable raw materials, which upon formulation and modification into resins and inks offer sustainable AM solutions [36]. These polymers provide AM users the environmentally benign manufacturing options [37].
Fig. 2.
Biopolymer-based biomaterials, as feedstock materials for AM technology to promote sustainable environment. (Figure modified from [38]).
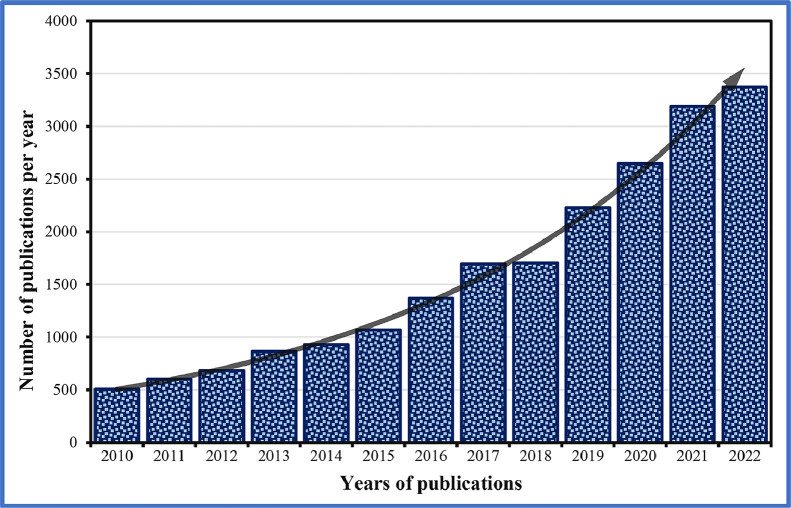
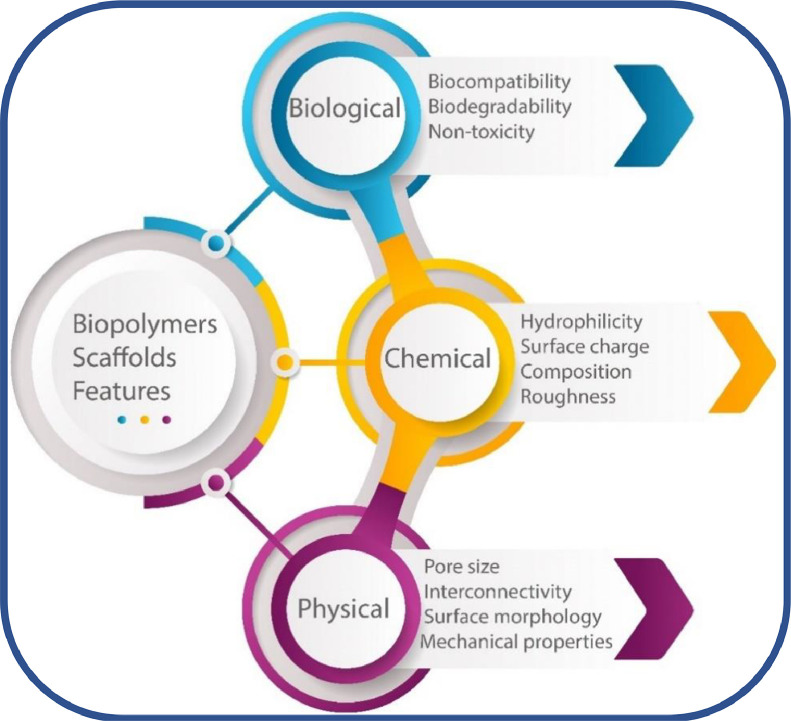
Bio-based polymeric materials like proteins, polysaccharides and aliphatic polyesters are produced from plants, animals or microbial synthesis [39]. These polymers are different from other biopolymers and can exist as biodegradable (like starch) or non-biodegradable (like bio-polyethylene) [40]. Especially polycaprolactone (PCL) and polylactide (PLA) have been vastly explored in AM to generate biodegradable and biocompatible scaffolds for the biomedical sector [41]. The applications of these biopolymer-based sustainable biomaterials have increased quite dramatically in the last decade, compared to traditional materials, as illustrated in Fig. 3. The decomposition can be adjusted precisely by developing harmless components upon the implantation of sustainable materials [42], [43], [44]. The unique features can assist constructing hard and soft tissues simultaneously by using a selected array of synthetic and natural biopolymers [45]. Furthermore, these biopolymeric materials are less costly and have matching chemical, physical and biological characteristics, as shown in Fig. 4, which are similar to certain living cells and tissues.
Fig. 3.
Number of publications related to biopolymer-based biomaterials from 2010 to 2022. (Figure drawn by using both “Biopolymers” and “Biomedical” as keywords from Scopus database).
Fig. 4.
Characteristics of 3D-printed biopolymer scaffolds (Figure drawn through the information provided by [46]).
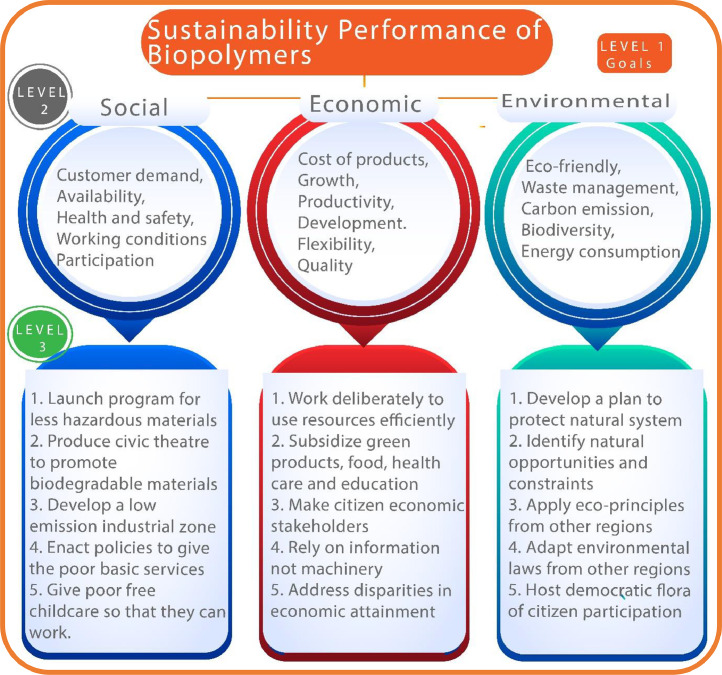
Fig. 5 depicts the socio-economic and environmental factors for evaluating the sustainability performance of biopolymeric composites. Sustainable materials have shown huge potentials in the 3D printing sector [38]. Soft biomaterials are vastly applied in different biomedical applications including tissue engineering (TE), lab-on-chip, scaffold design, nerve grating, microvascular network, wound healing and drug carrier applications [47], [48], [49], [50], [51], to mention a few. The 3D printing of biopolymeric materials is further revolutionizing healthcare systems by fabricating on-demand drug-released medical devices [52]. Different novel formulations including multi-drug combinations, controlled-release, novel design, orally disintegrating,and pediatric-friendly formulations have also been reported in the literatures [53], [54], [55].
Fig. 5.
Sustainability performances of biopolymeric-based biomaterials (Figure modified from [56]).
Amid the coronavirus disease 2019 (COVID-19) pandemic, the estimated market size of AM in the healthcare system was $1.45 billion in 2021 [57]. It is predicted that the economic growth of 3D-printed medical models and devices in the healthcare market will reach $6.21 billion by 2030 [58]. Due to enormous interest in biopolymers for the AM, it is necessary to highlight the recent progresses and the role of environmentally sustainable biomaterials in advanced healthcare systems. Herein, we highlight some of the recent advancements in 3D printing biopolymeric materials including their potential applications in the field.
2. Biopolymer-based sustainable materials
Biopolymers are derived from biological renewable resources such as animals, plants and microorganisms, which exhibit excellent biocompatibility, chemical versatility, non-toxicity, bioresorbability, bioactivity and tunable biodegradability. The use of these sustainable materials in the biomedical sector including bone, cardiac and liver regeneration, wound healing, and drug delivery systems, has been increasing day by day due to more refined and efficient treatments [59], [60], [61], [62]. Some prominent natural- and synthetic-based biopolymeric materials and their biomedical applications are provided in Table 1. The biocompatibility of bioactive materials has influenced the functional properties of additively manufactured tissues or organs. Additionally, biomaterials require adherence of the native cells to maintain adhesion, viability and interaction [63]. At present, synthetic biopolymers induce inflammatory reactions. However, it is necessary to overcome issues related to the safety and efficacy of these materials. This can be done by synthesizing composite scaffolds through chemical modifications [64].
Table 1.
Types of commonly employed biodegradable polymers for the fabrication of scaffolds, their characteristics, and recent biomedical applications.
| Biopolymer type | Biopolymer | Sustainability credentials for biomedical applications | AM technique | Advantages | Disadvantages | Degradation time | Suggested polymers and bio-ceramics to develop composites | Formulations | Biomedical applications | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Natural | Chitosan | Biodegradable Low carbon footprint |
Extrusion, SLA | Non-immunogenicity, easily metabolized, antibacterial activity, and biocompatibility | Low mechanical strength, brittle, stiff | >20 weeks | HAp, BG, alginate, or collagen | Sponge, hydrogels, composite scaffolds | Gene delivery, wound dressing, bone, nervous, skin, liver, cardiovascular, and cartilage TE | [65], [66], [67] |
| Alginate | Lower carbon footprint Biodegradable |
Extrusion | Non-immunogenicity, bioactivity, biocompatibility, and non-antigenicity | Limited toughness and mechanical strength | 80 d | BG, HAp, chitosan, or PLA | Micro/nanosphere, hydrogels | Hollow vascular channels, bone, cartilage, neural, skin regeneration, and wound healing | [68], [69], [70] | |
| Cellulose | Excellent biodegradability Low carbon footprint |
DIW, FDM, IJP | Bioactivity, excellent mechanical characteristics, and biocompatibility | Limited cell adhesion | Weeks to months | HAp, CNTs, chitosan, PLA, or PBS | Composite scaffolds | Neural, skin, tendons, muscle, cardiac, cartilage, and bone regeneration | [71], [72], [73], [74] | |
| Collagen | Biodegradable Low embodied energy level and carbon footprint |
Extrusion, IJP | High porosity, bioactivity, excellent mechanical characteristics, biocompatibility, and poor immunogenicity | Low antigenicity, low mechanical strength, and low stiffness | 12 h | HA, PLGA, BG, or HAp | Scaffolds | Drug delivery, vascular, dental, cornea, bone, cartilage, and artificial skin regeneration | [75], [76], [77] | |
| SF | Excellent biodegradability Low carbon footprint |
Micro-extrusion, SLA, IJP | Biocompatibility, excellent mechanical characteristics, high tensile strength, bioactivity, high flexibility, and low immunogenicity | Brittle, rapidly degrade | 6 weeks | Collagen, HAp, PLA, or calcium phosphate | Scaffolds | Gene delivery, wound healing, hepatic, vascular, cornea, neural, tendon, bone, cartilage, and skin regeneration | [78], [79], [80], [81] | |
| Gelatin | Biodegradable Low embodied energy level |
Extrusion, SLA | Biocompatibility, bioactivity, ECM mimicked, poor immunogenicity, and better solubility | Rapid degradation, low mechanical strength, limited solubility in concentrated solutions | 10 d | Chitosan, HAp, PLA, or PCL | Micro/nanosphere, hydrogels | Aortic valves, neovascularization, cartilage, neural, bone, and skin regeneration | [82], [83], [84] | |
| Starch | Excellent biodegradability Low carbon footprint |
Extrusion | Non-toxicity and biocompatibility | Brittle and less surface area | Several weeks | GO, BG, or PCL | Composite scaffolds | bone, skin regeneration, and drug delivery systems | [85], [86], [87], [88] | |
| HA | Biodegradable Low embodied energy level |
Extrusion | Non-toxicity, easily modified through chemical reaction, and biocompatibility | Fast degradation rate and low mechanical characteristics | 4 months | PEG, PLA, PLGA, collagen, or chitosan | Scaffolds, hydrogels | Skin and neural regeneration | [89], [90], [91] | |
| Synthetic | PLA | PLA degradation within the human body PLA copolymers, which can help in the adjustment of degradation |
Extrusion, SLA, IJP | Highly flexible and biocompatible | Highly inflammable, low cellular adhesion, porosity and bioactivity, poor rate of degradation | 20 months | HA, alginate, chitosan, PCL, HAp, or BG | Hydrogels, composite scaffolds | Suture, neural, bone, skin cartilage, cardiovascular, ligament regeneration, and drug delivery applications | [92], [93], [94] |
| PCL | Slow degradation rate Water, solvent, oil, and chlorine resistant |
Extrusion, SLA, IJP | Highly flexible, excellent mechanical characteristics, degradation and solubility, biocompatible, and minimal inflammability | Limited degradation and low cell adhesion | 6–28 months | Chitosan, PLA, BG, or HAp | Composite scaffolds, hydrogels | Dentistry, vascular, bone, retina, skin regeneration, and pharmaceutical applications | [95], [96], [97] | |
| PGA | Insoluble in water Biodegradable |
Extrusion, SLA, IJP | Excellent tensile strength, bioresorbable, and biocompatible | Limited solubility and rapid degradation | 5 months | PLA, PEG, PLGA, collagen, or chitosan | Composite scaffolds, hydrogels | Surgical sutures, bone, ligament, and cartilage reconstruction | [98], [99], [100], [101] | |
| PHB | Biodegradable | Extrusion, IJP | Excellent mechanical, barrier properties, piezoelectricity, and optical activity | Limited solubility, and low cell adhesion | 6–10 months | Chitosan or alginate | Composite scaffolds, hydrogels | Surgical implants, biomedical devices, bone, skin, cartilage regeneration, or breast augmentation | [102], [103], [104] | |
| PVA | Biodegradable Low carbon footprint |
Extrusion, IJP | Biocompatibility, non-toxicity, self-healing property, and hydrophilicity | Low cell adhesion | 16–25 d | Gelatin, chitosan, PLA, or PGA | Hydrogels, composite scaffolds | Drug delivery, wound dressing, bone, cartilage, and skin regeneration | [105], [106], [107] |
Natural bio-organisms including algae, fungi and bacteria decompose biopolymers into tiny molecules through anaerobic or aerobic techniques by forming organic H2O and CO2 products [108]. Additionally, these materials exhibit highly compatible behavior due to their resemblance with the extra-cellular matrix (ECM). ECM contains thick layers of tissues annexed together by adhesive polysaccharides or protein molecules. Moreover, it also promotes cell adhesion, interaction, proliferation and differentiation [109].
2.1. Natural biopolymers
Natural biodegradable polymers (polysaccharides and proteins) are highly versatile and used for tissue regeneration, gene delivery, controlled drug delivery, bio-actuators and other healthcare applications. These biomaterials are generally derived from plants, animals or microbes. Generally, natural biopolymers exhibit high molecular weight, which results in viscous polymer solutions that enable them to be used in the 3D printing. Consequently, processability and printability of these polymers remain a challenge [110]. Some of these polymers can be chemically modified, which improve non-toxicity, biocompatibility and biodegradability. Some natural biopolymers are chitosan, silk fibroin (SF), collagen, cellulose, gelatin, hemicellulose, alginate, hyaluronic acid (HA), lignin and starch. Despite excellent bioactivity, biocompatibility and biodegradability, natural biopolymers have some disadvantages such as poor mechanical properties, high water solubility, source instability, possible immunogenicity and denaturation during processing [111], [112], [113].
Chitosan, a polysaccharide material derived from the deacetylation of chitin, is found in crustacean skeleton and extensively applied in biomedical applications [114], [115], [116]. However, the low mechanical resistance of these materials limits their use in drug delivery applications [117], [118], [119]. Alginate, a heteropolysaccharide, which abundantly exists as an ingredient of cell walls of brown seaweed and in the capsule of bacteria pseudomonas sp. and Azotobacter sp, possesses the ability to form a gel upon the incorporation of divalent cations [120], [121], [122]. Additionally, it has also been used for preparing hydrogels through various crosslinking approaches for a wide range of applications in the biomedical area [123], [124], [125]. Collagen, a natural polymer, is a ubiquitous protein found in animals, especially in the human body. Collagen scaffolds contain the fibrous structure of principal receptors (integrins) with dimeric peptides [126]. For instance, Heo et al. [127] observed that the incorporation of umbilical vein endothelial cells (UVECs) and mesenchymal stem cells (MSCs) into collagen hydrogels significantly improved osteogenic differentiation, cell viability and vasculature ingrowth. Moreover, the blending of collagen with other natural biopolymers helps in forming fibrous polymeric scaffolds, which exhibit excellent strength and stability due to the crosslinked structure. Additionally, collagen sponges are also being used as a wound dressing material, due to porosity, structure and surface properties [128], [129], [130]. SF, a natural polymer of proteinic nature, extracted from Bombyx mori cocoons, spiders and silkworms, is highly elastic, strong and high strength-to-density ratio. The porosity of the structure can be improved by adding calcium phosphate (CaP) in silk without any noticeable changes in its compressive behavior [131], [132], [133]. Nowadays, silk-based hydrogels are employed to release potential anticancer drugs including doxorubicin. Additionally, they also help in delivering genes, growth factors, proteins and plasma molecules [134], [135], [136], [137]. Starch is a renewable polymer obtained through plants. This material is primarily deposited in tubers, seeds or roots of plants. Its structure contains amylopectin and amylose, constituting about 98%−99% dry weight of this biopolymer. Modified starch employed in acetylated, phosphate ester and grafted forms for drug release applications [138], [139], [140]. Gelatin is one of the most versatile and promising natural biopolymers derived through partial hydrolysis and denaturation of collagens. It originates from different sources including pigskin, hides, fish and cattle bones, and contains proline, glycine and hydroxyproline constituents [141], [142], [143]. Excellent viscosity, gel strength and low melting point are some of its unique characteristics that appear due to the presence of amino acids [144], [145], [146]. Cellulose, a renewable and biodegradable polysaccharide, is abundantly available in natural biological sources ranging from plants (bamboo, wood, bast and cotton) to micro-organisms (algae, bacteria, and fungi) [147]. However, cellulose shows minimal solubility in the organic solvent and difficulty in melting due to strong hydrogen bonds, which makes its processability highly cumbersome [148]. Cellulosic fibers are mostly employed to reinforce the matrices of bioactive materials, which are manufactured through the AM technology. Similarly, bioinks for the AM technology can also be prepared by using nanocellulose materials such as cellulose nanofibrils (CNF) and cellulose nanocrystals (CNC) as a reinforcing media [149], [150], [151]. HA, an emerging and versatile linear polysaccharide, naturally occurs in the body consisting of glycosaminoglycan with non-sulfated bonds [152], and plays an important role in cellular adhesion and differentiation, which makes it highly suitable for modern therapeutic formulations [153].
2.2. Synthetic biopolymers
Diverse and versatile synthetic polymers such as polyanhydrides, polyamides, poly-α-hydroxyesters, polyurethanes and poly(ortho-esters) can be applied in tissue regeneration, medical devices, drug and gene delivery systems due to their modifications or tailorable designs [154], [155], [156]. These polymers have relatively low production cost compared to natural biodegradable polymers [156], [157], [158]. Aliphatic polyesters can be used as substitutes to petrochemical polymers due to their excellent mechanical properties and biodegradability. Synthetic biodegradable polymers including PLA, polyhydroxybutyrate (PHB), polyvinyl alcohol (PVA), polyethylene glycol (PEG), poly(lactide-co-glycolide) (PLGA), poly(glycerol sebacate) (PGS), polybutylene succinate (PBS), PCL, and polyglycolic acid (PGA) have gained considerable attention in healthcare systems.
Nowadays, synthetic biopolymers are considered attractive alternatives for the biomedical sector. These polymers provide better control over molecular weight and chemical composition compared to their natural counterparts. Most synthetic biodegradable polymers are aliphatic polyesters like PLA, PCL, PGA and their copolymers [159], [160], [161], [162], [163]. These polymers show high biocompatibility and controlled degradation rate. Furthermore, their degradated products in vivo have not produced any toxic effects on tissues [164]. Additionally, polymers with improved mechanical properties are developed by manually controlling synthetic parameters and designs. However, some synthetic biopolymers exhibit in vivo degradation and yield acidic degradated products that lower the local pH value, thus, resulting in the acceleration of the degradation rate of grafts and triggering inflammatory foreign body reactions at the transplantation location. Compared to natural biopolymers, synthetic biopolymers lack cellular adhesion; however, the chemical modifications of these biopolymers can help in improving cell adhesion [165]. These biopolymers are highly beneficial in the biomedical sector and their characteristics can be tuned for tissue regeneration applications [166].
PLA, an eco-friendly synthetic biopolymer, is one of the most promising sustainable biomaterials used in healthcare systems [167]. Lactic acid can be acquired through sugar fermentation, which is derived from renewable resources like corns and sugarcanes [168]. Some limitations like hydrophobicity, slow degradation rate, and low impact resistance associated with PLA polymer. The blending of PLA with other polymers helps in improving its mechanical properties [169]. PCL is an aliphatic semi-crystalline, biocompatible, easily accessible, hydrophobic nature, and biodegradable polyester, which is widely applied for tissue regeneration and wound healing applications [170], [171], [172]. PCL exhibits tailorable biological properties, mechanical strength, and physiochemical conditions. It also exhibits excellent permeability to deliver therapeutic molecules in TE, however, undesired burst release and low encapsulation limit its utilization in drug delivery applications. Additionally, the properties of PCL can be improved by developing copolymers through the combination of PCL with other poly(α‑hydroxy esters) like poly(d, l-lactic acid-co-ε-caprolactone) (PDLLACL) and poly(l-lactic acid-co-ε-caprolactone) (PLCL) [173]. PGA, semi-crystalline aliphatic polyester similar in biochemistry to PLA, is a well-known bioresorbable tissue-engineered polymer, which is extensively explored for the bioengineering field. Additionally, the fast-degrading nature of PGA makes it a good candidate for short-term tissue scaffolds [174], [175], [176].
3. Conventional manufacturing techniques
It is difficult to control pore parameters as well as incorporate intricate architectural details, while ensuring reproducibility through conventional manufacturing techniques like gas foaming, freeze drying, powder forming, solvent casting, solvent casting/particulate leaching, sol-gel method, electrospinning, and thermally induced phase separation [177], [178], [179]. These conventional techniques are unable to generate fully interconnected and uniform pores in tissue scaffolds [180]. Additionally, it is almost impossible to avoid deviation during the conventional fabricating processes, which may result in the failure of the developed tissue constructs [181]. Table 2 summaries the key advantages and disadvantages of different conventional manufacturing techniques. 3D printing technology has led to the implementation of AM technology, which precisely controls the porosity as well as can distribute them uniformly throughout the tissue scaffolds [182].
Table 2.
Advantages and disadvantages of conventional manufacturing techniques applied for developing biomedical products.
| Conventional techniques | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Freeze drying |
(i) Suitable technique to develop interconnected pores (ii) Low temperature (iii) Distinct leaching is not necessary |
(i) Irregular and small pores (ii) Time consuming process |
[183] |
| Gas foaming |
(i) Porous scaffolds (ii) Do not use organic solvents |
(i) Pore geometry cannot be controlled (ii) Require excessive heat (iii) Non-interconnected pore structures |
[184] |
| Electrospinning |
(i) Controlled porosity, fiber diameter and pore size (ii) Micro- to nano-sized diameter scaffolds (iii) Highly porous scaffolds |
(i) Use organic solvents (ii) Low mechanical strength (iii) Pore size is reduced with fiber thickness |
[185], [186], [187] |
| Thermally induced phase separation |
(i) Highly porous 3D scaffolds (ii) Excellent mechanical properties |
(i) Small pores (<200 µm) (ii) Use of organic solvent, which are harmful to cells |
[188] |
| Solvent casting |
(i) Expensive equipment is not required (ii) Ease of fabrication |
(i) Develop simple shape scaffolds only (ii) Use residual solvents |
[189] |
| Solvent casting /particulate leaching |
(i) Expensive equipment is not required (ii) Ease of fabrication |
(i) Protein denaturation (ii) Lack of control on the interconnectivity of pores (iii) Only form simple shape scaffolds (iv) Residual solvent is harmful to cells |
[190] |
| Powder forming |
(i) Scaffolds with high porosity (ii) Tailorable pore size |
(i) Use organic solvents | [191] |
| Sol-gel method | (i) Develop scaffolds by using different types of ceramics | (i) Low mechanical strength of scaffolds | [192] |
4. Additive manufacturing techniques
AM technology has been widely explored by biomedical engineers to manufacture a variety of customized products for healthcare systems. The technology is highly beneficial to develop patient-specific anatomic models and medical implants by using an appropriate 3D printing process [193], [194], [195]. The transformation of reasonable AM and biopolymer availability are significant elements for their selection in biomedical applications [196], [197], [198]. Biopolymers for the 3D printing should ideally possess good printability, processability, structural stability, and high-shape fidelity, as well as precise and accurate 3D plotting of polymers [199], [200], [201].
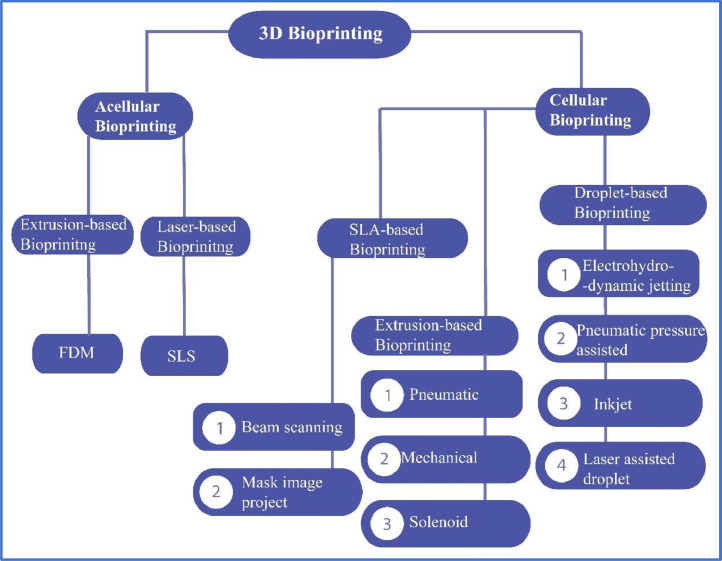
Fig. 6 depicts the general classification of 3D bioprinting processes as per the American Society for Testing and Materials (ASTM) International. Among these processes, extrusion-based printing (fused deposition modeling (FDM) and direct ink writing (DIW)), inkjet printing (IJP)/binder jetting (BJ), stereolithography (SLA) and digital light processing (DLP) are vastly applied for the 3D bioprinting of sustainable polymeric materials [202], [203], [204], [205]. Each of the 3D printing techniques has its advantages and limitations. Table 3 describes the schematic diagram and key aspects of some AM processes, which are generally adopted in the 3D manufacturing of biopolymer-based scaffolds and TE applications.
Fig. 6.
Classification of 3D bioprinting processes commonly applied in biomedical applications.
Table 3.
Description about general additive manufacturing processes.
| AM technologies | 3D printing | Key Aspects | Resolution | Materials for 3D printing | Processing parameter | Biomedical applications | Cell viability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Extrusion | FDM |
(i) High mechanical strength (ii) Freedom in the selection of materials |
100–700 µm | Nanocellulose, PLA, PCL, PLGA, PEG, and HA | Physical crosslinking, freeze-drying | Prostheses, orthoses, bone, cartilage, and vascular TE |
80%−96% | [206], [207], [208] |
| DIW |
(i) High resolution (ii) Complex porous scaffolds |
100–600 µm | Viscous biopolymer such as CNC, starch, gelatin, alginate, maize protein, k-carrageenan | Freeze drying | Bone TE, drug delivery systems, and personalized medicine | 80%−96% | [209], [210], [211] | |
| Vat Polymerization | DLP |
(i) Higher accuracy and good surface finish (ii) High printing speed |
15–100 µm | PEG, PEGDA, PDLLA, and PCL | UV or visible light | TE, drug delivery systems, and complex organ structures | > 90% | [212], [213], [214] |
| SLA |
(i) High precision and resolution (ii) Can be used for cell patterning and growth factors |
5–100 µm | Photo-polymerizable resins of CNCs, silk, and alginate | UV or visible light | Prostheses, surgical instruments, bone and cartilage TE |
> 90% | [215,216] | |
| Laser-based printing | SLS |
(i) High processing temperature (ii) Difficult to print biological materials or cell structures |
10–120 µm | PVA, PLGA, PCL-based biopolymer composites | Laser action | Prostheses, orthoses, bone, and cartilage TE | N/A | [217], [218], [219] |
| SLM |
(i) Rough surface (ii) Material wastage |
30–150 µm | PP, PU, metals, and alloys | Laser action | Prostheses, orthoses, and bone TE | N/A | [220], [221], [222] | |
| Inkjet printing /binder jetting | IJP |
(i) Multi-cell heterogenous constructs (ii) Cell aggregation (iii) Low resolution |
20–200 µm | Less viscous materials including alginate, SF, nanocellulose, PEG, and PEGDMA |
Liquid binding agent | Personalized medicine, liver, skin, bone regeneration, and drug delivery systems | 85%−98% | [223], [224], [225] |
4.1. Extrusion-based printing
Extrusion-based printing was one of the earliest technologies that was previously applied to develop prototypes by using metal or plastic as a feedstock material [226]. Even today, the extrusion-based 3D printing technique is the most common, relatively straightforward, and cost-effective AM process applied for prototyping biopolymers [227]. Different feeding mechanisms including piston-, pneumatic- or screw-type used to extrude viscous materials with a viscosity between 30 mPa.s to 6 × 107 mPa.s [228]. Irrespective of the type of extrusion mechanism, the ink will be extruded continuously to perform a layer-by-layer deposition on the print bed, which solidifies to develop 3D objects, as illustrated in Fig. 7A. Biomedical applications usually employ micro-extrusion techniques to print highly dense cellular structures in a controlled fashion. The extrusion-based printing is further divided into FDM and DIW, based on the printing temperature [229], [230], [231]. FDM is considered a highly suitable strategy for the printing of biopolymers, in which thermoplastic filaments are heated into a molten state or semi-liquid state and extruded through an orifice onto a printing platform [232]. However, this strategy usually extrudes only viscous polymeric materials at room temperature and low resolution is achieved during the process [233]. In contrast, DIW is an extrusion-based printing technique exhibiting the ability to extrude biopolymeric-based viscoelastic ink in the liquid or heated form to generate fibers at ambient temperature. The deposition of these fibers into a specific pattern helps to produce scaffolds for tissue regeneration [234]. This technique can help in developing multipolymer-based tissue constructs. The manufactured bioinks must possess appropriate rheological characteristics, extraordinary shape retention ability, and high storage modulus [235]. Furthermore, bioinks should be able to hold their shape without depending upon the drying or solidification of raw materials. Bioink materials containing high-storage modulus make them highly suitable materials for developing bone regeneration scaffolds [236].
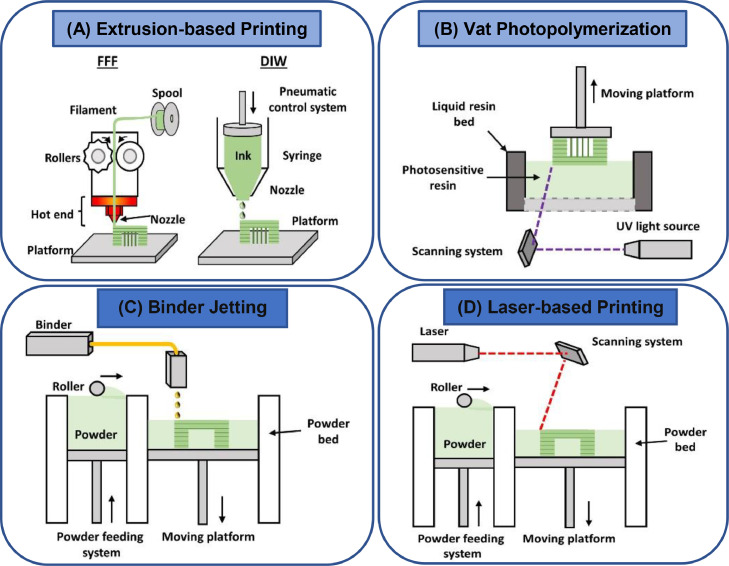
Fig. 7.
Schematic illustration of different AM processes; (A) FDM and DIW printing techniques; (B) Vat photopolymerization; (C) BJ/IJP; (D) SLM (adapted with permission from [237], copyright 2021, Elsevier Inc.).
4.2. Vat photopolymerization
Vat photopolymerization is another effective technique used for the processing of biopolymeric composites. In this technique, 3D objects are formed by exposing photo-sensitive polymers to light or ultraviolet (UV) radiation [238]. Here, UV light is used to trigger a reactive species or catalyst for the radical photopolymerization of methacrylates. Such a technique is highly attractive due to its printing speed and high resolution. Based on the variation of curing source, this technology is further categorized into SLA and DLP [239].
SLA, a fascinating 3D printing process, uses selectively cross-linked materials including elastomers, thermosetting plastics, ceramic-based resins, and bioink materials in the presence of UV or visible light to develop patterned structures [240]. It is widely applied for producing biopolymer-based porous scaffolds and intricate constructs both for hard and soft tissue regeneration applications [241]. This technique facilitates the high printing resolution of values up to 20 µm and is considered one of the most accurate 3D printing techniques. Therein, the curing is triggered through the degradation of photo-initiators upon exposure to a light source [242], as illustrated in Fig. 7B. Thermoset resins in the SLA technique usually exhibit limited degradability under the action of the light source. Therefore, the combination of biodegradable polymers including diethyl fumarate (DEF), poly(propylene) fumarate (PPF), poly(trimethylene carbonate) (PTMC) and poly(d,l-lactic acid) (PDLLA) was applied for developing tissue biodegradable scaffolds [243]. However, SLA is not a suitable technique for the simultaneous printing of living cells due to the insolubility of photo-initiators in water solution which makes cells highly toxic. Furthermore, cells are traumatized due to the action of the UV light upon curing. Due to this reason, cells are incorporated after the development of scaffolds [244].
DLP technique is a rapid 3D printing process, which has gained significant attraction in the TE field due to its customizability and high precision [245]. In this process, the curing laser beam is controlled through a digital mirror device (DMD). The DMD contains an array of micro-mirrors that regulates the laser beam [246]. It can cure a complete layer simultaneously, thus, reducing printing time significantly compared to the traditional SLA process [247]. This process is highly suitable to develop intricate ceramic products with high accuracy and resolution, along with desirable mechanical characteristics. The variation in photocurable resin formulations affects the end-use characteristics of the printed scaffolds [248]. Thus, this process helps in developing 3D-printed scaffolds with specific characteristics and functionalities through the regulation of resin formulations [249].
4.3. Laser-based printing
Laser-based printing approaches consist of two printing techniques i.e., SLM and SLS for the processing of biopolymeric powders, which use laser light to fuse the material [250]. In the SLM technique, polymeric granules are completely melted, whereas, SLS permits heating below melting temperature just to fuse materials [251]. This approach uses a heater to preheat the powdered feedstock into the build cavity and a heating source (laser radiation) to fuse (sintering or melting) different cross-sections, as illustrated in Fig. 7D. This layer-by-layer melting and followed by a solidification process develops 3D objects. SLS/SLM approach develops accurate 3D-printed products compared to other processes like FDM or SLA [252]. Biomaterials such as biopolymers and ceramics are mainly applied in the SLS technique. This approach uses a variety of biopolymeric sustainable composite materials like PCL, PLA, PDLLA, poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV), PVA, PCL/HAp, PLA/PCL/HAp to print scaffolds for BTE, cardiac TE, and cartilage TE applications [253]. For instance, Patel et al. [254] developed PHBV-based biodegradable scaffolds for BTE by using the SLS method and observed degradation mechanism and comparable mechanical properties.
4.4. Inkjet printing
IJP technique provides rapid prototyping by depositing tiny photopolymerized ink suspension/solution onto the substrate to develop 3D models and scaffolds, as illustrated in Fig. 7C. It is a powerful tool to deposit biomolecules, polymers, and living cells with high resolution and efficiency [255]. In this technique, for most cases, the viscosity of the bioink should be lower than 10 mPa▪s for effective printing. Additionally, this technique offers low cell densities and high fabrication speed compared to other 3D printing processes [256].
IJP has mainly two working modes; drop-on-demand (DOD) IJP and continuous inkjet (CIJ) printing [257]. DOD, a non-contact 3D printing technique, uses tiny ink droplets of diameter (25–50 mm) that are developed on-demand, and direct the binder droplets with the help of pressure or voltage pulses. This technique is mostly applied to develop scaffolds for TE applications [258]. It possesses excellent control over droplet directionality, uniformity and size. Additionally, the quality of printing depends upon the positional accuracy of ink droplets. In CIJ printing, less viscous bioink materials are converted into a continuous droplet flow after passing through a nozzle (or a set of nozzles), to fabricate 3D objects. The spacing and size of binder droplets are regulated through a pressure wave pattern [259]. IJP is used to develop scaffolds by using both ceramics and biopolymer-based biomaterials like HAp, BG, PLA, PCL, PGA, etc. There is a variety of applications of IJP in other biomedical applications like personalized medicine, controlled drug delivery and protheses [260].
4.5. Bioprinting
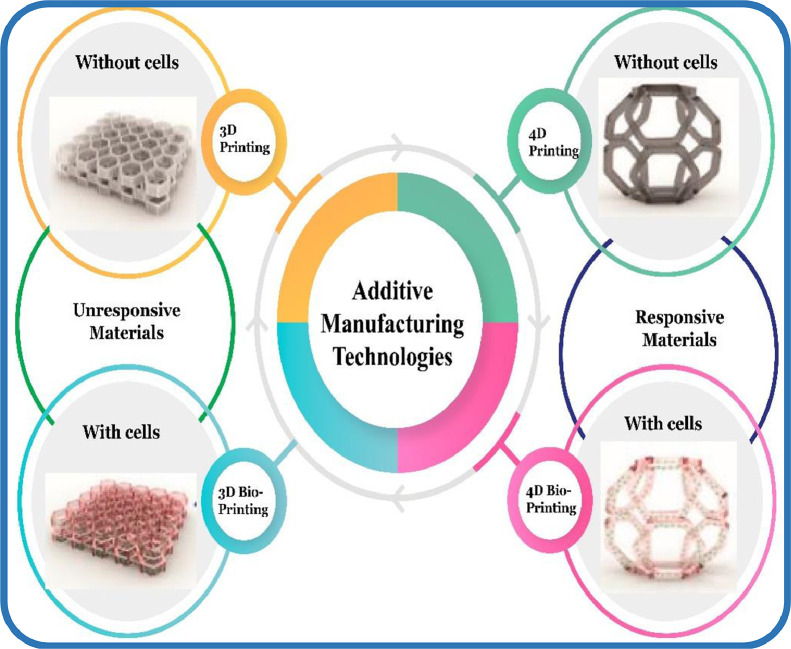
Bioactive materials are natural or engineered materials that interact with the living tissues without producing any adverse effects and ensure treatment, augmentation or substitution of organs [261]. In other words, the advancement in 3D printing technology has resulted in the development of 3D commercial bioprinters that include BioBots, Aether, Regenhu, and Cellink [262], [263], [264], [265]. 3D bioprinting, an emerging and innovative technology, is derived from AM technology and incorporates the viable cells with bioactive materials iteratively to fabricate biomedical components (shown in Fig. 8) that have revolutionized the TE, bone regeneration and pharmaceutical sectors [266], [267], [268], [269].
Fig. 8.
Schematic diagram illustrating the difference between 3D/4D bioprinting and 3D/4D printing (adapted with permission from [273], copyright 2022 Elsevier B.V.).
Bioinks, as feedstock materials for the 3D bioprinting help to develop intricate and heterogeneous architectures like vasculatures, enhance cell adhesion, growth and differentiation with native tissues [270]. In comparison to traditional 3D printing technologies, the development of artificial tissues is more challenging in 3D bioprinting due to the selection of cell growth, types, differentiation factors, construction and functionalities of tissues [271]. Nowadays, 3D bioprinting is fulfilling the demands of traumas, cancers, tooth extraction and accidents by modulating porosity and their uniform dispersion during human interaction. However, there is a need to address some challenges including cell incorporation problems, structural activities and feedstock requirements, in this approach [272].
4.6. 4D printing
Four-dimensional (4D) printing, an innovative technology, involves the combination of stimuli-responsive materials and a 3D printing technology to develop dynamic patient-specific scaffolds [274]. This technology uses stimuli-responsive polymers as a feedstock material, which can change to a temporary state or return to their original state upon exposure to external stimuli, as illustrated in Fig. 8. It was initially introduced by Tibbits in 2013 and has gained tremendous attraction in the biomedical field, due to its ability to produce tissue scaffolds with a dynamic environment [275]. 4D bioprinting, mostly an extension-based 4D printing, is extensively applied in healthcare systems, which involves the maturation of living cells after 3D printing [273]. During the maturation process, cell-incorporated 3D-printed scaffolds self-transform themselves in the presence of stimuli like light, humidity, heat, magnetic field, electric field, ultrasound, pH, etc. [276].
Table 4 summarizes different stimuli-responsive biopolymers, which are well-suited for biomedical applications, especially TE applications. Beside stimuli-responsive polymers, lipids and hydrogels have been vastly applied as feedstock materials for the 4D bioprinting [277], [278], [279]. Additionally, different types of smart hydrogels including peptide, natural and synthetic hydrogels have found their applications in the biomedical sector. These hydrogels develop architectures with tailorable porosity and excellent cell interconnectivity [280].
Table 4.
Stimuli-responsive biopolymers used to develop smart materials for healthcare system.
| Stimuli-responsive biopolymeric composites | AM technology | Stimulus | Applications | Ref. |
|---|---|---|---|---|
| PLA/Fe3O4 | FDM | Magnetic | Tracheal stents | [281] |
| PLA-PCL copolymer | FDM | Temperature | Elbow protection | [282] |
| PCL/Fe3O4/BG | FDM | Magnetic | Bone tissue scaffolds | [283] |
| Collagen/agarose/iron NPs | DIW | Magnetic | Cartilage tissue scaffolds | [284] |
| Gelatin/chitosan | Extrusion | Temperature | Tissue vascularization | [285] |
| PCL/Fe3O4 | SLA | Magnetic | Tissue scaffolds | [286] |
| PEGDA | SLA | Light | Optogenetic muscle | [287] |
| Methacrylated alginate & Methacrylated HA | Extrusion | Humidity | Tissue vascularization | [288] |
| PLA | FDM | Temperature | Protective visors frame | [289] |
| Alginate/glycerin | Extrusion | pH | Skin dressing | [290] |
| PLA/Fe3O4/benzophenone | DIW | Magnetic | Cardiovascular implant | [291] |
| Collagen fibers | Extrusion | Temperature | Left atrial appendage occlusion devices | [292] |
5. Biopolymeric nanocomposites
Biopolymer-based tissue constructs exhibit poor barrier properties and low thermal stability along with low mechanical characteristics [293]. In contrast, biopolymeric nanocomposites incorporate nanosized materials, which improve the mechanical characteristics of biopolymers [294]. These nanocomposites exist in the form of nano-filament composites, nano-layer composites or nano-particulate composites [295]. Table 5 incorporates some of the recent biopolymeric-based nanocomposites used to develop scaffolds in tissue regeneration.
Table 5.
Summary of nanocomposites which are obtained by incorporating NPs into biopolymers developed through various AM techniques.
| Biopolymers | NPs | AM process | Applications | Ref. |
|---|---|---|---|---|
| Alginate | HAp | Bioprinting | Bone TE | [296] |
| PVA/sodium alginate/CNF | HAp | Extrusion | Bone TE | [297] |
| PLA/GelMA | Gold NPs | FDM | Bone TE | [298] |
| PCL | Mesoporous BGs | Bioprinting | Bone TE | [299] |
| PCL/PEG | β-TCP | Extrusion | Bone TE | [300] |
| PCL | HAp | SLS | Bone TE | [301] |
| PLLA/PHBV | CaP | SLS | Bone tissue regeneration | [302] |
| PCL | Zn/HAp/GO | Micro-extrusion | Bone TE | [303] |
| PCL/PEG | HAp | Extrusion | Bone tissue regeneration | [304] |
| GelMA | HAp | DLP | Bone TE | [305] |
| PCL/PLA | Halloysite | FDM | Bone tissue regeneration | [306] |
| PCL | Strontium/HAp | Extrusion | Bone TE | [307] |
| Alginate | BGs | Extrusion | Bone tissue regeneration | [308] |
| PCL | GO | Extrusion | Bone TE | [309] |
| PEGDA | CNCs | SLA | Soft TE | [310] |
| Alginate | MWCNTs | Extrusion | Vascular tissue regeneration | [311] |
| PCL | MWCNTs | Extrusion | Cardiac TE | [312] |
| PCL | CNFs | FDM | Drug eluting cardiovascular scaffolds | [313] |
| Chitosan/alginate | HAp | Hybrid 3D printing | Cartilage tissue regeneration | [314] |
| Alginate/thymoquinone | Halloysite | Extrusion | Cartilage tissue repairing | [315] |
| Xanthan gum | CNC | DIW | Liver TE | [316] |
| PLA | Halloysite | Extrusion | Soft TE | [317] |
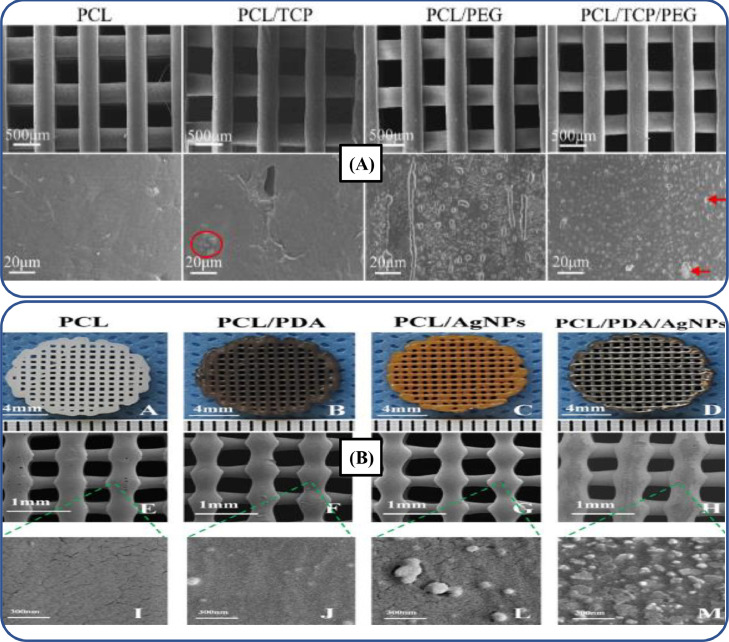
The characterization of the polymer nanocomposites is an analytical approach, which helps to evaluate their size, structure, physical and chemical properties. The incorporation of nanoparticles (NPs) into biopolymers provides better control on size, morphology and dimensions of nano-constructs. Nevertheless, proper dispersion and integration of NPs into biopolymeric matrices are necessary for cell proliferation, adhesion and infiltration within scaffolds. For instance, Liu et al. [300] incorporated tricalcium phosphate (TCP)-based nanomaterials into PCL/PEG-based 3D-printed composite bone scaffolds for improving the mechanical properties. Fig. 9A depicts the scanning electron microscope (SEM) analysis, which showed that composite scaffolds contain uniformly dispersed TCP. Such an uniform dispersion of TCP into biopolymer matrices improved the mechanical properties and cell viability.
Fig. 9.
(A) Surface characterization of composite scaffolds, where PCL/PEG/TCP-based composite scaffold showed excellent dispersion of the NPs (adapted with permission from [300], copyright 2022, Elsevier Ltd.); (B) Surface morphology and microstructure of 3D-printed bone scaffolds (adapted with permission from [318], copyright 2019, Elsevier Ltd.).
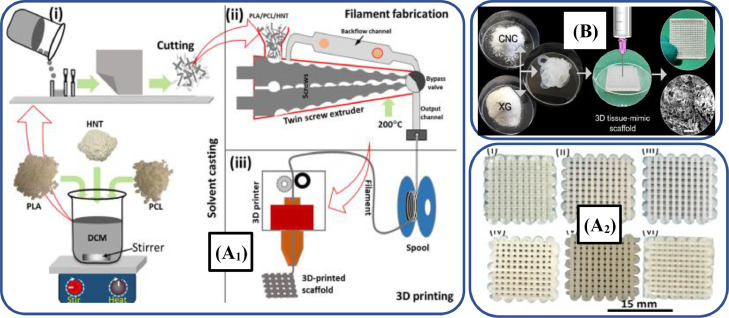
Nanocelluloses in the form of CNCs and CNFs are vastly employed, as fillers to develop tissue scaffolds [319]. For instance, Baniasadi et al. [316] developed 3D-printed scaffolds for soft tissue regeneration by using xanthan gum (XG)/CNC, as illustrated in Fig. 10B. The authors reported excellent swelling ratio, porosity and mechanical properties of scaffolds, which can be applied for soft tissue regenerations. Additionally, these scaffolds showed better attachment, differentiation and proliferation of liver cancer cells.
Fig. 10.
(A1) Schematic illustration of 3D-printed PCL/PLA/halloysite scaffolds; (A2) Optical photographs of 3D-printed scaffolds printed by varying halloysite (adapted from [306], under the Creative Commons Attribution License 4.0); (B) CNC, as a filler to develop XG/CNC-based scaffolds for repairing soft tissues (adapted with permission from [316], copyright 2022 Elsevier B.V.).
Biopolymers are often used in a mixture with some other inorganic fillers such as ceramic or metal NPs, nano-fibers, graphene, carbon nanotubes (CNTs) as well as living cells which are obtained from some living materials [320], [321], [322], [323]. Among these inorganic materials, CNTs are highly effective for developing 3D-printed biopolymeric-based nanocomposite scaffolds for bone, cardiac and neuronal tissue regeneration, due to their extraordinary electrical conductivity, mechanical properties and distinct dimensional features [324]. CNTs also help to improve strength, flexibility and biocompatibility, along with the reduction of thrombosis and induction of angiogenesis during tissue regeneration [325], [326], [327]. For instance, Lee et al. [328] developed porous PEGDA/multi-walled carbon nanotube (MWCNT)-based nerve scaffolds through the SLA technique. The results indicated that the incorporation of MWCNTs promoted the growth and proliferation of neuronal cells, thus, it is a highly effective strategy for developing scaffolds for nerve TE applications. However, these materials exhibit non-resorbable behavior upon in vivo experimentation [329]. In another study, Alam et al. [306] developed PCL/PLA/halloysite scaffolds by using FDM technique, as illustrated in Fig. 10A. The results revealed that halloysite-incorporated scaffolds exhibited cellular adhesion, cytocompatibility and biodegradation rate. Thus, these scaffolds have promising applications for bone regeneration.
Anti-microbial properties of biopolymer composites can be enhanced by using metal-based micro- and NPs like bronze, copper and silver. These anti-bacterial properties in the biopolymer composites are essential for tissue scaffolds [330], [331], [332], [333]. For instance, Sang et al. [334] coated gold NPs on the surface of PCL-based scaffolds developed through 3D printing. Such NPs enhanced osteogenic differentiation and anti-microbial properties of 3D-printed bone scaffolds. Likewise, Li et al. [318] printed anti-microbial dual functional PCL-based scaffolds with self-assembly micro-nano surface, PDA and silver NPs manufactured through the FDM technique, as illustrated in Fig. 9B. The NP-incorporated scaffolds exhibited excellent cytocompatibility, anti-bacterial, and mechanical properties. These scaffolds demonstrated their excellent potential for bone tissue regeneration.
There is a great demand for bioactive materials like TCP, hydroxyapatite (HAp) and bioactive glass (BG) in TE and regenerative medicine, due to non-toxicity, biocompatibility and better interaction with the human body, which accelerates the healing mechanism [335], [336], [337]. HAp, an inorganic component, is highly suitable for developing biopolymer-based nanocomposites for bone tissue regeneration that provides excellent cell adhesion, proliferation, and differentiation [338]. BG is a commercially available micro-sized filler and pure BG cannot be employed for developing tissue scaffolds, due to lose of its amorphous characteristics at a high sintering temperature [339]. Similarly, BG/biopolymer composites are special type biomaterials, which are used in healthcare systems for various applications ranging from surgical implants to tissue regeneration scaffolds [340]. However, bioactive reinforced-biopolymer scaffolds possess excellent biocompatibility, bio-functionality, biodegradability, and mechanical properties [341]. Aráoz et al. [342] incorporated BG into PHBV to fabricate 3D-printed scaffolds for bone tissue regeneration with biological and mechanical properties similar to ECM of trabecular bone.
6. Scopes of biopolymeric composites in healthcare system
Biopolymeric composites are widely used in many clinical and biomedical applications [343]. These sustainable materials have also addressed the demands of environmental toxicological and public health studies, due to inherent properties including biodegradability, non-toxicity, biocompatibility, flexibility,and renewability [344]. A wide range of natural and synthetic biopolymers are now under extensive consideration for many applications such as 3D anatomical models, TE, surgical equipment, scaffold design and artificial implants [345]. Particularly, these composites have myriad of scopes in both hard and soft TE [346]. This section illustrates some of the key applications of 3D-printed biopolymeric composite materials.
6.1. Tissue engineering
Biodegradable polymer-based porous scaffolds developed through the 3D printing processes are vastly applied as artificial ECMs to support native tissues, which help in regenerating and reconstructing tissues [347]. Sometimes, biologically active molecules or cells are incorporated to promote tissue regeneration. Depending upon the type of application, these porous scaffolds should possess excellent biocompatibility, cytotoxicity, porosity, optimal pore size and interconnectivity. Furthermore, porous scaffolds have a significant role in the application of drug delivery systems, the development of biomedical devices and surgical instruments, and the encapsulation of human and animal cells [348], [349], [350].
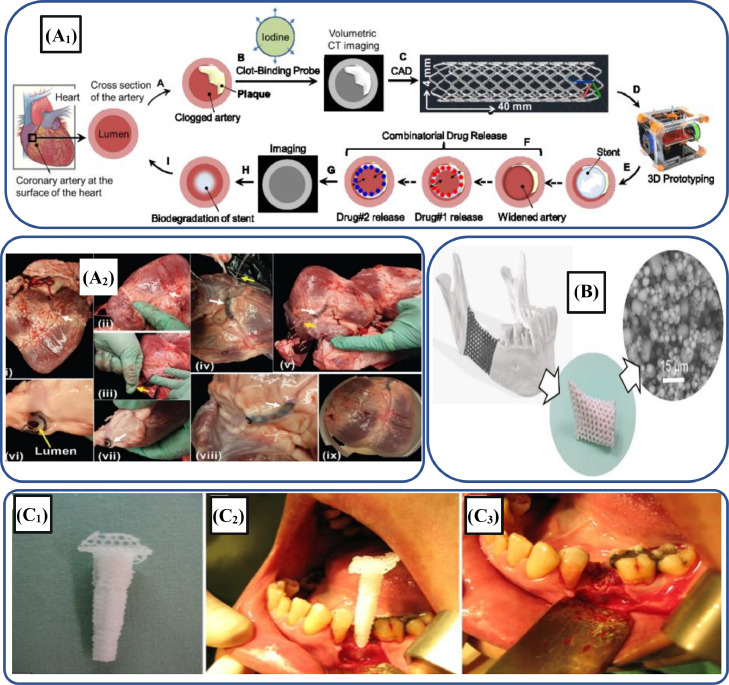
Additionally, 3D-printed human organs, stents, medical devices and drug delivery systems have been developed using biodegradable polymers [351], [352], [353]. For instance, Misra et al. [353] developed a multi-drug eluting 3D-printed stent by incorporating graphene nano-platelets into the biodegradable PCL-based polymer through an extrusion-based process. This printed stent was deployed in a pig heart, as shown in Fig. 11A. The improved mechanical properties, as well as in vitro results, depicted that these novel biodegradable stents can be employed for treating heart patients suffering from blocked coronary arteries. Table 6 provides the summary of different 3D-printed biopolymeric composites employed in different soft and hard tissue regeneration applications.
Fig. 11.
(A1) Schematic illustration depicting different steps for fabricating micro-stents; (A2) Sequential demonstration of 3D-printed stent PCL-based polymer composite implanted in the heart of the pork (adapted with permission from [353], copyright 2017 WILEY‐VCH); (B) Implantation of 3D-printed PMTC-based scaffolds into the human jawbone manufactured through SLA strategy (adapted with permission from [354], copyright 2019 American Chemical Society); (C1) 3D-printed PCL-based polymer scaffold; (C2) Insertion of scaffold into the tooth socket of human mouth; (C3) Trimming of the excess scaffold (adapted with permission from [355], copyright 2014 John Wiley & Sons Ltd)
Table 6.
Biopolymeric scaffolds manufactured through various 3D printing techniques.
| Type of tissue | Target tissue | Biopolymeric material(s) |
Printing technique | In vitro study | Structure | Ref. |
|---|---|---|---|---|---|---|
| Hard | Bone | PEG/Silk/PCL | Extrusion-based 3D printing | BMSCs | Crypt-like structures | [356] |
| Bone | Gel/PVA | Extrusion-based 3D printing | MG63 cells | – | [357] | |
| Bone | PLA | FDM | hBMSCs | – | [358] | |
| Bone | PVA/BC | FDM | Human osteoblast cells | – | [359] | |
| Soft | Cartilage | PCL/PLA/PEG | FDM | hBMSCs | Layer by layer-based honeycomb structure | [360] |
| Cartilage | SF/PEG | Extrusion-based 3D printing | Chondrocytes | Disk/meniscus-shaped scaffold: | [361] | |
| Cartilage | SF/Gelatin | Extrusion-based 3D printing | hMSCs | Layer-based 3D structure | [362] | |
| Nasal cartilage | Collagen | Extrusion-based 3D printing | Human chondrocytes | Microporous structure | [363] | |
| Nerve | Alginate/CMC/ agarose | DIW | Human iPSC-derived glial cells | Layered porous structure | [364] | |
| Nerve | PCL | Electrohydrodynamic jet-based 3D printing | PC12 | Tubular multi-layered complex | [365] | |
| Skin | Keratin/glycol chitosan methacrylate |
Extrusion-based 3D printing | hASCs | “NTU”-based 3D model | [366] | |
| Skin | PEG/SF | DLP | NIH/3T3 | 3D lattice structure containing thin keratin layer | [367] | |
| Cornea | GelMA | Extrusion-based 3D printing | Human keratocytes | Complex porous | [368] | |
| Liver | SF/Gelatin | Extrusion-based 3D printing | Hepatocytes, Huh7 | Six-layered-based scaffolds | [369] | |
| Lung | SF/CNF | Extrusion-based 3D printing | Lung epithelial stem cells | Two crossing layers | [370] |
6.1.1. Hard tissue engineering
Natural and synthetic 3D-printed biodegradable polymers have huge potential to be used for hard tissue (bone) regeneration due to their biocompatibility and cytotoxicity [371]. Furthermore, these 3D printing processes have the flexibility to provide any complex shape using biopolymers along with satisfactory biological, physical as well as mechanical properties [372], [373], [374].
Bone, a naturally regenerative tissue, may suffer significant trauma due to accidents, thus, hindering its normal regeneration, which causes bone defects [375]. Bone defects require artificial scaffold support during the healing process and bone growth. Since the inception of 3D printing techniques, myriad of biomedical researchers tried to develop scaffolds for bone tissue engineering (BTE) applications, as making scaffolds as this technology is simple and easy. For instance, Dienel et al. [354] employed the SLA technique to fabricate biodegradable implants for a bone generation. In this study, the 3D-printed scaffolds were manufactured by incorporating 51 wt% of β-tricalcium phosphate (β-TCP) into PTMC to get high resolution and best quality implant. Fig. 11B depicts 3D-printed porous scaffolds embedded into the human jaw. Similarly, Ben and Tan [355] employed PCL-based biodegradable material for the fabrication of scaffolds to heal the socket of human tooth, as depicted in Fig. 11C. For this purpose, a 3D printing technique was used to fabricate the PCL scaffold that could be used in the bone healing of the human tooth. The 3D-printed scaffold was inserted into the teeth socket of the human without using the filler and observed the results after 6 months. The results depicted that the insertion of a biodegradable PCL-based scaffold significantly healed the bone.
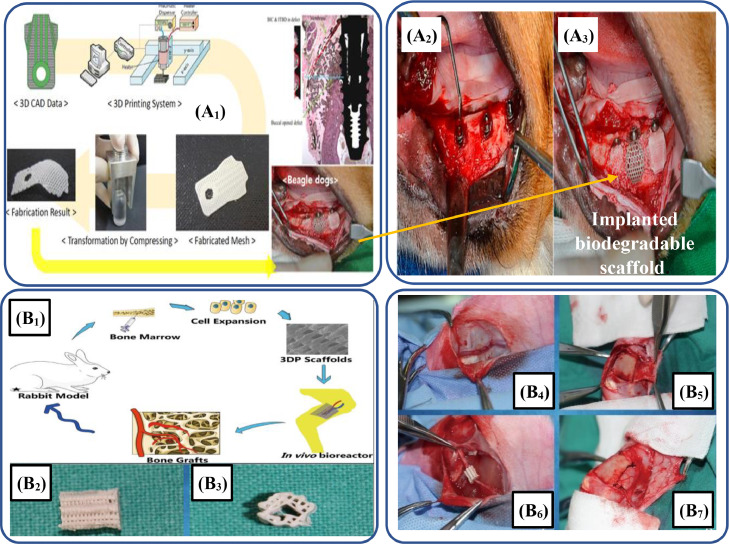
In another study, Choi et al. [376] developed PLA-based biodegradable polymers that could be used effectively in the formation of bone scaffolds. For this, a FDM-based technique was used to fabricate the specimens by incorporating a chain extender and a chemical foaming agent and observed the improvement in the morphology, porosity, and melting properties. Similarly, Shim et al. [377] studied the effect of PLGA/PCL/β-TCP-based scaffolds through FDM for bone regeneration and osteointegration of dog tooth. Fig. 12A1 depicts the sequential procedure of this work adopted by the authors to manufacture 3D-printed scaffolds. For in vitro examination, the developed 3D-printed membrane was implanted into the dog's mouth, as shown in Fig. 12A2. Both in vivo and in vitro results further help to print scaffolds for applications.
Fig. 12.
(A1) Sequential procedure starting from CAD design of membrane to the implantation of membrane into the edentulous mandibular alveolar ridge; (A2) Implantation of implants into the edentulous mandibular alveolar ridge; (A3) Used the grafting material to compromise and fill the defects and then membrane was implanted (adapted from [377] under the Creative Commons Attribution License 4.0); (B1) Schematic illustration showing the experimentational procedure of in vitro vascularized tissue generation of the bone; (B2) 3D-printed PLA/HAp-based composite scaffold in lateral and (B3) front view; (B4) Figure showing the saphenous arteriovenous blood bundles; (B5) Periosteum was displayed on the surface for surgery; (B6) Implantation of PLA/HAp-based composite scaffold; (B7) Scaffold was rolled in the form of capsule (adapted from [378] under the Creative Commons Attribution License 4.0).
Zhang et al. [378] explored a strategy to repair bone defects using the PLA/HAp-based biodegradable scaffold. The preparation of these scaffolds was performed by the vascularized BTE of the rabbit using an in vivo bioreactor. Fig. 12B1 depicts the experimental procedure adopted by the authors to completely analyze the in vitro behavior of the fabricated scaffold. In this methodology, the tibial periosteum capsule was filled with PLA/HAp composite scaffolds and rabbit bone marrow cells, as depicted in Fig. 12B6. After 8 weeks, the results depicted that these scaffolds are helpful in generating vascularized bone tissues. The mechanical properties of biopolymer-based scaffolds are matchable to the properties of the targeted hard tissues. Additionally, the degradation rate of the scaffolds is the same as that of the replacement rate of cells. It helps in the replacement and remodeling of natural ECM. Table 7 provides most remarkable, recently fabricated 3D-printed biopolymeric composite-based bone scaffolds, their properties, morphologies, and research highlights.
Table 7.
Recently adopted different 3D-printed biodegradable polymer composite-based scaffolds for tissue regeneration.
| AM process | Biopolymeric composites | Mechanical characteristics | Morphology | Highlights | Ref. |
|---|---|---|---|---|---|
| FDM | PLGA/HA/HACC | Compressive strength: 31.3 MPa Tensile strength: 22.7 MPa Elastic modulus: 1.9 GPa |
 |
The results of the in vivo study showed that the biodegradation of the scaffolds was influenced by the bone infection and helped in the repairing of the bone. | [379] |
| Extrusion | Alginate/gelatin/CNC | Storage modulus: ∼150,000 MPa at 100 rad/s |  |
Rapid bone grafting has been noted in the rat CCD-1 defects model in the presence of the biopolymers-based scaffolds after 21 d of the transplantation. | [380] |
| FDM | PLA/HAp | Nozzle diameter: 0.2 mm Layer thickness: 50 μm |
 |
Young's modulus, similar to the modulus of the cancellous bone when 50 wt% of HAp were used. | [381] |
| Extrusion | MWCNTs/PCL | Melting temperature: 90 °C Air pressure: 6 bar Deposition velocity: 20 mm/s |
 |
The in vitro study depicted that implanted scaffold containing 3 wt.% of MWCNTs significantly repaired the bone tissues. | [382] |
| Micro-extrusion | Alginate/gelatin/GO/chondroitin sulfate | Compressive modulus: 100 kPa |  |
The incorporation of GO in biopolymer-based scaffolds exhibited excellent cell proliferation, adhesion, and proliferation. In vitro analysis showed excellent bioactivity, cytotoxicity, and biocompatibility. These scaffolds are excellent candidates for TE. | [383] |
| Extrusion | Silica NPs/oxidized alginate | Yield stress: 79 Pa with 2 wt.% of NPs |  |
The results exhibited that incorporation of silica NPs enhanced mechanical stability, shear-thinning properties, and high fidelity. | [384] |
| DLP | PGSA | Feature thickness: 80 μm Elastic modulus: 3668.7 kPa Ultimate tensile strength: 919.1 kPa |
 |
The results revealed that PGSA-based biodegradable tubular scaffold exhibited excellent mechanical properties and degradation kinetics. Thus, it has the potentiality to be applied for tissue regeneration applications including vascular grafting. | [385] |
| DLP | β-TCP/PCL | Compressive strength: 11 ± 4 MPa |  |
The experimental results indicated that 3D-printed hybrid scaffolds exhibited excellent compressive strength. Thus, these rigid bioactive scaffolds have the potential to be applied for BTE applications. | [386] |
| DLP | PCL/PEG/GelMA | Diameter: 1.5 mm, 2 mm, and 2.5 mm Wall thickness: 0.75 mm, 1 mm, and 1.5 mm |
 |
3D-printed scaffolds exhibited excellent biocompatibility and mechanical properties. Hence, these composite scaffolds will be highly suitable for nerve repair. | [387] |
| SLS | PCL | Laser power: 0.3 – 0.7 W Laser beam diameter: 260 μm, 390 μm Elastic modulus: 11.3 ± 0.5 MPa |
 |
PCL-based porous scaffolds have depicted excellent biocompatibility and comparable elastic modulus. Therefore, these scaffolds can be applied for bone regeneration. | [252] |
| FDM | PCL/PGA/yarn fiber | Tensile strength: 79.7 MPa Elastic modulus: 3.5 GPa |
 |
Stiffness and tensile strength of 3D-printed biodegradable scaffolds were enhanced, significantly with the incorporation of yarn fibers. Additionally, these scaffolds exhibited excellent biocompatibility and cytotoxicity. These scaffolds have the potential to be applied for bone regeneration. | [388] |
| FDM | PLA | Yield strength: 60 MPa Young's modulus: 4 GPa |
 |
3D-printed vascular stent exhibited excellent self-expandable and thermal properties. The synergetic combination of these properties makes this 3D-printed product a promising candidate for solving complications of cardiovascular disease. | [389] |
| FDM | PCL | Compressive strength: 0.65 MPa Compressive modulus: 10.60 MPa |
 |
Build envelope temperature, nozzle temperature, material volume and deposition speed are important parameter for determining the fidelity of PCL lattice scaffold structures. | [390] |
6.1.2. Soft tissue engineering
3D-printed biopolymeric composites are promising candidates for mimicking native soft tissues, as illustrated in Fig. 13. Several soft tissues including cartilage, urethra, nerve, skin, tendon, liver, ligament, intestine, and vascular are continuously performing their function in the human body. In comparison to hard tissues, soft tissues exhibit distinct properties including compliant modulus of elasticity, flexibility, and weak mechanical properties, therefore, semi-crystalline biopolymeric materials are not considered for these soft-tissue applications [391], [392], [393], [394]. Additionally, the composition and structural characteristics of these scaffolds should be matchable to ECM tissues for helping in cell growth, proliferation, and differentiation. Similarly, biocompatibility, porosity, and nutrient transportation of these scaffolds are other essential attributes for soft tissue regeneration [395]. Table 7 also includes some of biopolymeric composite-based scaffolds fabricated through 3D printing techniques for soft tissue regeneration applications.
Fig. 13.
Various parts of the human body where 3D-printed biopolymeric composites can be used to regenerate soft tissues.
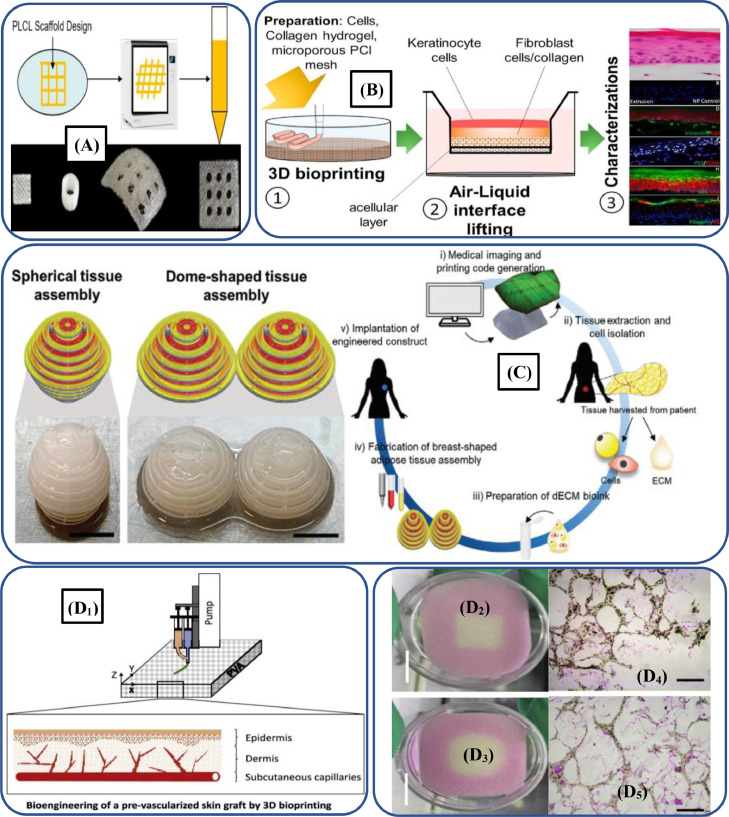
Different natural biopolymers (α-keratin, chitosan, HA, alginate and collagen) and synthetic biopolymers (PCL, PGS, PEG, PLA and their copolymers) can be employed to print scaffolds for soft TE applications [396]. For instance, Liu et al. [397] regulated the elastic modulus and stiffness of the PLLA by incorporating PCL-based biopolymer and noted that PLCL-copolymer scaffolds Fig. 14 Aexhibited good biocompatibility and mechanical properties. Thus, copolymerized PLCL-based scaffolds show promising potential for the regeneration and repairing of muscle, cardiac, tendon and skin tissues. Similarly, customized bioinks and PCL-based biopolymer were employed by Cho et al. [398] to develop biocompatible dome and spherical-shaped adipose tissue assemblies, as illustrated in Fig. 14C. Thus, the research has shown great promise for regenerating breast tissues.
Fig. 14.
(A) PLCL-based 3D-printed scaffolds which exhibited tailorable elasticity, stiffness as well as excellent biocompatibility (adapted with permission from [397], copyright 2020 Royal Society of Chemistry); (B) Schematic process illustration depicting the collagen/PCL-based scaffolds and their characterizations (adapted with permission from [400], copyright 2020 Elsevier B.V.); (C) PCL-based biodegradable polymer used to develop dome and spherical-shaped adipose tissue assemblies, which depicts its potential utilization for developing breast-replicating soft tissue repairing (adapted from with permission [398], copyright 2020 Wiley‐VCH GmbH);(D1) Biopolymer-based 3D-printed hybrid skin constructs; (D2-D3) Multi-layered PVA-based porous cell-laden scaffolds; (D4-D5) Hematoxylin and Eosin stain images depicting the distribution of cells in multi-layered 3D-printed scaffolds (adapted with permission from [402], copyright 2020 Elsevier B.V.).
Various fibrous materials including collagens through the 3D printing techniques produce scaffolds for wound dressing and skin regenerative therapies [399]. For instance, Ramasamy et al. [400] printed collagen/PCL-based biodegradable scaffolds through a extrusion-based process, as shown in Fig. 14B, and observed excellent cell differentiation, viability and reproducibility.
3D-printed artificial skin tissues contain different bioactive materials, growth factors and cells [27]. Several researchers have 3D-printed skin constructs by incorporating stem cells, antimicrobial particles and growth factors. For instance, Afghah et al. [401] developed poly(propylene) succinate (PPS)/PCL-based scaffolds by incorporating anti-microbial silver granules and human dermal fibroblast (HDF) cells. 3D-printed skin constructs exhibited excellent antimicrobial characteristics and degradation behavior, thus, considered as a potential candidate for skin TE applications. In another study, Zhang et al. [402] developed 3D hybrid cell-laden skin constructs by using PVC-based biodegradable polymers and poly(N-isopropyl acrylamide-co-acrylic acid) (PNIPAAm-AA)-based hydrogels, as illustrated in Fig. 14D. The in vitro experimentation revealed that cell-laden constructs exhibited excellent superficial cornification, splitting, and sprouting of the subcutaneous ECs. These artificial tissues have the potential to be applied for wound healing applications.
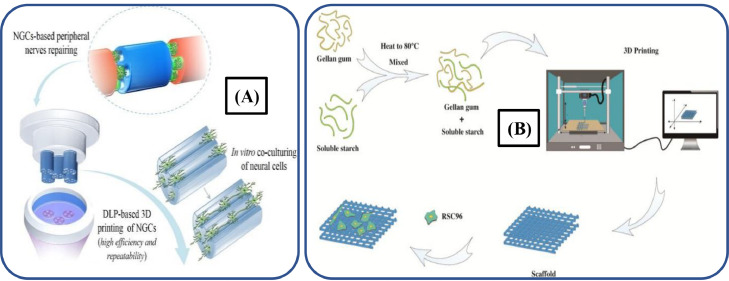
Peripheral nerve injury (PNI) cannot be self-healed and requires neural grafting or end-to-end suturing for its remedy. 3D printing is a highly suitable and versatile approach for developing patient-specific branched or unbranched conduits with high resolutions, better features and native scale dimensions [403]. Biopolymer-derived sustainable biomaterials exhibit incomparable biological properties, which are equivalent to ECM. Thus, they provide sites for biological cues and protein binding that regulate the cell behavior. Recently, different biopolymer scaffolds including CNFs, alginate, gelatin, starch and collagen have gained attraction for TE and are used to develop the next-generation conduits for neural tissue regeneration [404]. The rapid development in the nerve regeneration yields multiform biopolymer-based nerve scaffolds with different micro/nano-scaled structures, which possess excellent biological characteristics, cues, and appropriate mechanical strength to fulfill the nerve regeneration requirements. The mechanical properties and internal microstructures of nerve guidance conduits (NGCs) may be determinants in promoting axonal regeneration and remyelination. Yoo et al. [405] combined electrospun PLCL and 3D-printed collagen hydrogel to develop a single-lumen nerve conduit to repair PNI. The results indicated that developed NGCs significantly promoted myelin regeneration, axonal growth, and nerve function recovery.
In another study, Ye et al. [406] employed the DLP process to fabricate NGCs by using GelMA-based hydrogels, as illustrated in Fig. 15A. These 3D-printed NGCs depicted excellent support for the differentiation, migration, proliferation and survival of neural cells along the longitudinal channel. Likewise, Zhang et al. [407] developed starch/gellan gum-based composite scaffolds via an extrusion-based 3D printing, as depicted in Fig. 15B. These porous structured scaffolds exhibited excellent biodegradability, biocompatibility, printability and cytotoxicity, which can permit their use for treating PNI.
Fig. 15.
(A) Schematic illustration of GelMA-based hydrogels NGCs fabricated through DLP (adapted from [406], Creative Commons Attribution 4.0 International License); (B) Schematic diagram depicting the cell-laden starch/gellan gum-based composite scaffold for PNI treatment (adapted from [407] Creative Commons Attribution 4.0 International License).
6.2. Pharmaceutical and other biomedical applications
The applications of 3D-printed biopolymer composites vary from nose reconstruction to dental manufacturing, human ear construction to bone regeneration, and surgical instruments manufacturing to developing human hand models. Fig. 16 depicts some of the applications of biopolymeric composites in the healthcare system. Controlled drug delivery systems are important to improve the therapeutic efficiency of drugs. Delivery rates of drugs must meet the physiological conditions [408], [409], [410], [411], [412], [413], [414], [415].
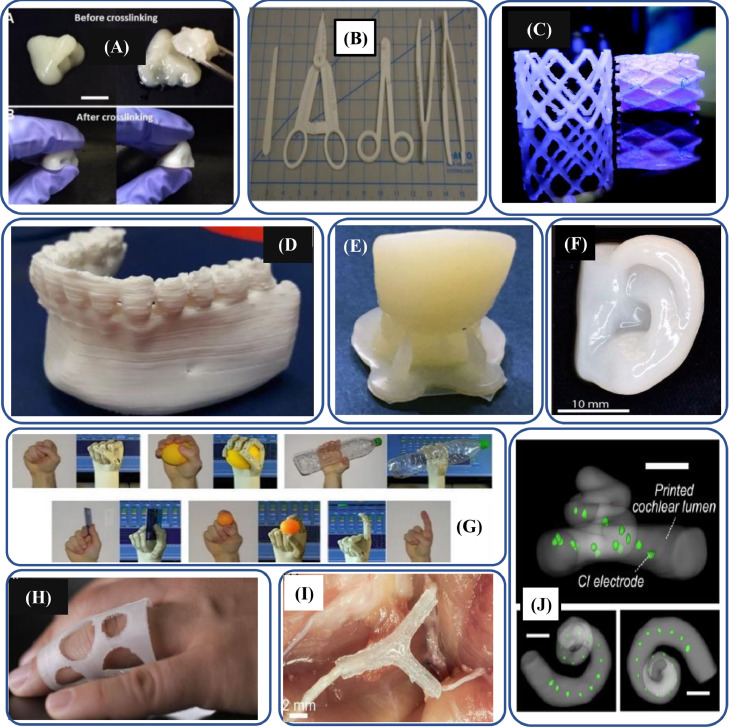
Fig. 16.
3D-printed biopolymer-based manufactured parts for healthcare systems; (A) CNF/GelMA-based composite used to develop 3D-printed nose structure (adapted with permission from [408]); (B) 3D-printed surgical instruments including scalpel handle, hemostats, needle drivers, forceps nylon surgical set (adapted with permission from [409], copyright 2016 Société Internationale de Chirurgie, part of Springer Nature); (C) 3D-printed biopolymer stent (adapted with permission from [410], copyright 2017, Mary Ann Liebert, Inc.); (D) 3D-printed anatomical models PDLLA-based mandibular model (adapted with permission from [411], copyright 2020 Elsevier Ltd.); (E) 3D-printed denture containing HA-loaded PEGDA resins (adapted with permission from [412], copyright 2021, Biomedical Engineering Society part of Springer Nature); (F) 3D-printed alginate/CNF-based human ear model (adapted with permission from [413], copyright 2015 American Chemical Society); (G) Exo-prostheses: multifunctional prosthetic 3D-printed hand prototype fabricated through fiber-reinforced nylon (adapted with permission from [414], copyright 2014, Springer Science Business Media Dordrecht); (H) Personalized medical 3D-printed PHBH/CNC-based device for finger dislocation (adapted with permission from [416], copyright 2020 American Chemical Society); (I) 3D-printed implanted nerve scaffold NGF gradient for sensory path signals and GDNF gradient for motor path signals in the scaffold (adapted with permission from [415], copyright 2015 WILEY‐VCH Verlag GmbH); (J) 3D-printed biomimetic cochleae (adapted from [417], under the Creative Commons Attribution License 4.0).
3D printing is an emerging technology that uses biopolymers to fabricate drug dosage forms in different intricate shapes. These polymers modulate the drug release rate and provide physical stability to active drug ingredients [418]. However, the usage of 3D-printed biopolymer dosage must fulfill regulatory requirements, in terms of safety and quality standards for human use. FDM and IJP are the most preferred 3D printing techniques used in pharmaceutics and drug delivery applications. These techniques provide high accuracy, patient-customized drugs, quick drug release, and high dosage loading of drugs [419]. However, they suffer from low productivity, compared to conventional fabrication techniques of biomedicine. Different biopolymers have been developed as delivery mediums by using 3D printing technologies. For example, Tappa et al. [420] fabricated 3D-printed biomedical implants and medical devices using PCL-based biodegradable polymer and incorporating estrogen or progesterone. The printed samples were surgical meshes, subdermal rods, medical devices, and pessaries. This study also gives a feasible concept for the application of drug delivery systems. Moreover, 4D printing has also gained significant attraction in the pharmaceutical industry [421]. For instance, Melocchi et al. [422] used water-responsive PVA-based polymer for intravesical drug delivery systems manufactured through FDM.
7. Future perspectives of 3D-printed biopolymeric composites
3D printing technology has shown significant advancement through biopolymers for constructing 3D-hybrid tissues with tunable mechanical properties and controllable biological characteristics. Despite extraordinary advancements in the 3D printing of biopolymeric composites for a wide range of applications, further research is needed to address the remaining challenges. The new generation of printing technologies construct tissue scaffolds through the combination of hydrogels, synthetic and natural-based biopolymers [423]. For instance, Morris et al. [424] developed PEGDA/chitosan-based hybrid scaffolds through the SLA technology. To use its full potentials, it is essential to develop nanometer-to-millimeter hierarchical biopolymer-based architectures. Advanced hybrid manufacturing (i.e., traditional manufacturing processes with 3D/4D printing) technologies can be employed to fabricate intricate constructs. For instance, an artificial collagen/fibrin hydrogel with electrospun PCL and animal chondrocytes was employed for the construction of cartilaginous tissues through an electrospinning/hybrid IJP system [425].
Indeed, 3D-printed biodegradable-based biopolymers have transformed the design and manufacturing landscapes of scaffolds. These biopolymers are successfully employed in the fabrication of synthetic bone models through the FDM technology [426]. However, over the technology possesses low printing resolution which is especially true for the 3D bioprinting of trabecular bone architecture. Hence, there is a further need to investigate 3D printing and hybrid technologies other than the FDM technology for the fabrication of 3D-printed biopolymer-based bone scaffolds.
Biodegradable polymers should fulfill the safety standards, which require long-term and rigorous efforts. Furthermore, there is a need to modulate the degradation rate of the developed scaffolds for providing appropriate mechanical support to the regenerated tissues. The use of biopolymers for soft and hard tissues requires collaborative efforts of material scientists and researchers of relevant fields. There is a need to further explore a few perspectives for 3D-printed biodegradable polymers including mechanical properties and smart mechanisms for their degradability in the complex natural micro-environment. For instance, a largely material extrusion-based technique has been widely employed for scaffold manufacturing [427]. However, this technique is not flexible enough to load fillers. Hence, it cannot achieve the required mechanical characteristics and biocompatibility. These novel avenues require insightful exploration for developing efficient tissue scaffolds.
Biopolymer composites can contribute to healthcare systems, due to their improved biodegradability, biocompatibility, renewability, sustainability and bioresorbability [428]. Furthermore, insightful comprehension of the composition of biopolymers along with in vitro and in vivo analyses will be helpful in producing novel hierarchical scaffolds. Fig. 17 shows the future of 3D printing biopolymers in different biomedical applications. Currently, many 3D printing technologies are employed to develop biodegradable-based polymer scaffolds [429], however, their actual utilization on the commercial scale depends on the fulfillment of different scaffolds criteria including mechanical integrity, thermal stability, chemical composition and biological characteristics. Additionally, the biological cell growth or adhesions with scaffolds is imperative to boost their clinical applications. Therefore, it is essential to further investigate and characterize additively manufactured biopolymer-based scaffolds with a focus on establishing their clinical role in BTE and other biomedical applications.
Fig. 17.
Glimpse of future of 3D-printed biopolymer composite in different applications.
There is a need to develop biopolymer-based composite tissue grafts for addressing the issues of tissue interfaces including tendon-to-bone, ligament-to-bone, and cartilage-to-bone. Sustainable gradient biomaterials with anisotropic structural properties will help to reestablish tissue connectivity, and function as well as improve long-term clinical outcomes. Machine learning and artificial intelligence may also help to adjust the chemical structure of biopolymer composites for developing gradient tissue constructs.
The availability of appropriate biopolymers for the 3D printing is still limited compared to the materials available for traditional fabrication techniques. There is a need to evaluate further biomaterials for achieving more feasible combinations. By overcoming this challenge, the utilization of 3D printing technology will be increased in the pharmaceutical industry. Additionally, shape-recovery polymers and hydrogels also exhibited tremendous potentials for the pharmaceutical sector. 3D bioprinting technologies are extensively applied to develop intricate tissue structures through a controlled and automated approach [430]. However, the dynamic behavior of tissues cannot be precisely imitated by the presently manufactured structures. Additionally, tissue structures may undergo conformational changes during tissue repair and regeneration [431]. Consequently, time-dependent stimuli response can be employed in the 3D-printed tissues to ensure their structural transformations.
In addition to this, a novel 4D bioprinting technology has been devised by researchers via cell traction forces and stimuli-responsive bioactive materials especially smart biopolymers that help to construct tissues of dynamic nature [432]. This technology is highly suitable to develop intricate dynamic structures, smart medical devices or complex human organs. However, the concept of 4D bioprinting is still in the nascent stage and its realization in clinical applications is limited. Moreover, it is extremely difficult to predict the deformation of 4D printing due to the lack of computational modeling. Similarly, there is a need to develop bioink materials for 4D printing by considering their biocompatibility and stiffness. Furthermore, 4D bioprinting requires further research on multiple-responsive stimuli, as in vivo environments might possess more than one stimulus. Additionally, valiant efforts are worth devoting to developing biopolymer-based products for the biomedical sector through 4D printing in certain conditions where unresponsiveness of the 4D-printed parts is required for certain stimuli including temperature and pH.
To conclude, both natural and synthetic biopolymers have exceptional utilizations in the 3D printing, due to their biodegradability, renewability and biocompatibility. These polymers can be used to repair/develop ears, bones, heart valves, stents and organs, as well as can help to produce medical equipment. Furthermore, the implants exhibit the necessary deformable and soft characteristics to perfectly align with the native tissues. The integration of bioelectronics with the human body will take this world towards a cybernetic future, as illustrated in Fig. 18. Biopolymer-based scaffolds will help to treat patients with organ or tissue malfunction, due to different factors and road accidents, cancers, injuries, trauma, burn diseases, metabolic disorders and war injuries. Artificial parts have the same biological and mechanical properties as organs, which are employed as life saviors in the case of a shortage of donors at a crucial time.
Fig. 18.
Recent developments in sustainable biomaterials are leading towards a cybernetic future (adapted with permission from [433], copyright 2018 Wiley‐VCH GmbH).
Conficts of interest
The authors declare no conflict of interest.
Contributor Information
Zia Ullah Arif, Email: chzia980@gmail.com.
Muhammad Yasir Khalid, Email: 100062624@ku.ac.ae.
Seeram Ramakrishna, Email: seeram@nus.edu.sg.
References
- 1.Zhu Y., Joralmon D., Shan W., Chen Y., Rong J., Zhao H., et al. 3D printing biomimetic materials and structures for biomedical applications. Bio-Design Manuf. 2021;4:405–428. [Google Scholar]
- 2.Arif Z.U., Khalid M.Y., ur Rehman E. Laser-aided additive manufacturing of high entropy alloys: processes, properties, and emerging applications. J Manuf Process. 2022;78:131–171. [Google Scholar]
- 3.Touri M., Kabirian F., Saadati M., Ramakrishna S., Mozafari M. Additive manufacturing of biomaterials − the evolution of rapid prototyping. Adv Eng Mater. 2019;21 [Google Scholar]
- 4.Singh S., Ramakrishna S. Biomedical applications of additive manufacturing: present and future. Curr Opin Biomed Eng. 2017;2:105–115. [Google Scholar]
- 5.Liu G., Zhang X., Chen X., He Y., Cheng L., Huo M., et al. Additive manufacturing of structural materials. Mater Sci Eng R Rep. 2021;145 [Google Scholar]
- 6.Javaid M., Haleem A. Additive manufacturing applications in medical cases: a literature based review. Alexandria J Med. 2018;54:411–422. [Google Scholar]
- 7.Li J., Wu C., Chu P.K., Gelinsky M. 3D printing of hydrogels: rational design strategies and emerging biomedical applications. Mater Sci Eng R Rep. 2020;140 [Google Scholar]
- 8.Hao W., Zheng Z., Zhu L., Pang L., Ma J., Zhu S., et al. 3D printing-based drug-loaded implanted prosthesis to prevent breast cancer recurrence post-conserving surgery. Asian J Pharm Sci. 2021;16:86–96. doi: 10.1016/j.ajps.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nesic D., Durual S., Marger L., Mekki M., Sailer I., Scherrer S.S. Could 3D printing be the future for oral soft tissue regeneration? Bioprinting. 2020;20:e00100. [Google Scholar]
- 10.Vanaei S., Parizi M.S., Vanaei S., Salemizadehparizi F., Vanaei H.R. An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng Regen. 2021;2:1–18. [Google Scholar]
- 11.Lamichhane S., Bashyal S., Keum T., Noh G., Seo J.E., Bastola R., et al. Complex formulations, simple techniques: can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J Pharm Sci. 2019;14:465–479. doi: 10.1016/j.ajps.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezvani Ghomi E., Khalili S., Nouri Khorasani S., Esmaeely Neisiany R., Ramakrishna S. Wound dressings: current advances and future directions. J Appl Polym Sci. 2019;136:47738. [Google Scholar]
- 13.Ramakrishna S., Mayer J., Wintermantel E., Leong K.W. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol. 2001;61:1189–1224. [Google Scholar]
- 14.Al Rashid A., Khan SA G., Al-Ghamdi S., Koç M. Additive manufacturing: technology, applications, markets, and opportunities for the built environment. Autom Constr. 2020;118 [Google Scholar]
- 15.Tümer E.H., Erbil H.Y. Extrusion-based 3D printing applications of PLA composites: a review. Coatings. 2021:11. [Google Scholar]
- 16.Tan H.W., Choong Y.Y.C. Additive manufacturing in COVID-19: recognising the challenges and driving for assurance. Virtual Phys Prototyp. 2021;16:498–503. [Google Scholar]
- 17.Steyrer B., Neubauer P., Liska R., Stampfl J. Visible light photoinitiator for 3D-printing of tough methacrylate resins. Materials. 2017;10:1–11. doi: 10.3390/ma10121445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szymczyk-Ziółkowska P., Łabowska M.B., Detyna J., Michalak I., Gruber P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern Biomed Eng. 2020;40:624–638. [Google Scholar]
- 19.Radmanesh S., Shabangiz S., Koupaei N., Hassanzadeh-Tabrizi S.A. 3D printed bio polymeric materials as a new perspective for wound dressing and skin tissue engineering applications: a review. J Polym Res. 2022;29:50. [Google Scholar]
- 20.Wasyłeczko M., Krysiak Z.J., Łukowska E., Gruba M., Sikorska W., Kruk A., et al. Three-dimensional scaffolds for bioengineering of cartilage tissue. Biocybern Biomed Eng. 2022;42:494–511. [Google Scholar]
- 21.Huang Z., Shao G., Li L. Micro/nano functional devices fabricated by additive manufacturing. Prog Mater Sci. 2023;131 [Google Scholar]
- 22.Zhang Y., Lim C.T., Ramakrishna S., Huang Z.-.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci Mater Med. 2005;16:933–946. doi: 10.1007/s10856-005-4428-x. [DOI] [PubMed] [Google Scholar]
- 23.Li N., Qiao D., Zhao S., Lin Q., Zhang B., Xie F. 3D printing to innovate biopolymer materials for demanding applications: a review. Mater Today Chem. 2021;20 [Google Scholar]
- 24.Alizadeh-Osgouei M., Li Y., Wen C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact Mater. 2019;4:22–36. doi: 10.1016/j.bioactmat.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azuraini M.J., Vigneswari S., Huong K.-H., Khairul W.M., AK H.P.S., Ramakrishna S., et al. Surface modification of sponge-like porous poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/gelatine blend scaffolds for potential biomedical applications. Polymers. 2022;14 doi: 10.3390/polym14091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver AA, Guillory RJ, Flom KL, Morath LM, Kolesar TM, Mostaed E, et al. analysis of vascular inflammation against bioresorbable Zn − Ag- based alloys 2020. [DOI] [PMC free article] [PubMed]
- 27.Qiu S., Zhou Y., Waterhouse G.I.N., Gong R., Xie J., Zhang K., et al. Optimizing interfacial adhesion in PBAT/PLA nanocomposite for biodegradable packaging films. Food Chem. 2021;334 doi: 10.1016/j.foodchem.2020.127487. [DOI] [PubMed] [Google Scholar]
- 28.Giubilini A., Bondioli F., Messori M., Nyström G., Siqueira G. Advantages of additive manufacturing for biomedical applications of polyhydroxyalkanoates. Bioeng. 2021;8 doi: 10.3390/bioengineering8020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbasian M., Massoumi B., Mohammad-Rezaei R., Samadian H., Jaymand M. Scaffolding polymeric biomaterials: are naturally occurring biological macromolecules more appropriate for tissue engineering? Int J Biol Macromol. 2019;134:673–694. doi: 10.1016/j.ijbiomac.2019.04.197. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty A.K., Wu F., Mincheva R., Hakkarainen M., Raquez J.-.M., Mielewski D.F., et al. Sustainable polymers. Nat Rev Methods Prim. 2022;2:46. [Google Scholar]
- 31.Biswas M.C., Jony B., Nandy P.K., Chowdhury R.A., Halder S., Kumar D., et al. Recent advancement of biopolymers and their potential biomedical applications. J Polym Environ. 2021 [Google Scholar]
- 32.Han S., Kim C.M., Jin S., Kim T.Y. Study of the process-induced cell damage in forced extrusion bioprinting. Biofabrication. 2021;13:35048. doi: 10.1088/1758-5090/ac0415. [DOI] [PubMed] [Google Scholar]
- 33.Duan B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann Biomed Eng. 2017;45:195–209. doi: 10.1007/s10439-016-1607-5. [DOI] [PubMed] [Google Scholar]
- 34.Khalid M.Y., Arif Z.U., Ahmed W., Arshad H. Recent trends in recycling and reusing techniques of different plastic polymers and their composite materials. Sustain Mater Technol. 2022;31:e00382. [Google Scholar]
- 35.Ravichandran R., Sundarrajan S., Venugopal J.R., Mukherjee S., Ramakrishna S. Advances in polymeric systems for tissue engineering and biomedical applications. Macromol Biosci. 2012;12:286–311. doi: 10.1002/mabi.201100325. [DOI] [PubMed] [Google Scholar]
- 36.Khalid M.Y., Arif Z.U. Novel biopolymer-based sustainable composites for food packaging applications: a narrative review. Food Packag Shelf Life. 2022;33 [Google Scholar]
- 37.Amulya K., Katakojwala R., Ramakrishna S., Venkata Mohan S. Low carbon biodegradable polymer matrices for sustainable future. Compos Part C Open Access. 2021;4 [Google Scholar]
- 38.Sanchez-Rexach E., Johnston T.G., Jehanno C., Sardon H., Nelson A. Sustainable materials and chemical processes for additive manufacturing. Chem Mater. 2020;32:7105–7119. [Google Scholar]
- 39.Samir A., Ashour F.H., Hakim A.A.A., Bassyouni M. Recent advances in biodegradable polymers for sustainable applications. Npj Mater Degrad. 2022;6:68. [Google Scholar]
- 40.Brizga J., Hubacek K., Feng K. The unintended side effects of bioplastics: carbon, land, and water footprints. One Earth. 2020;3:45–53. [Google Scholar]
- 41.Babu R.P., O'Connor K., Seeram R. Current progress on bio-based polymers and their future trends. Prog Biomater. 2013;2:8. doi: 10.1186/2194-0517-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma B., Sharma S., Jain P. Leveraging advances in chemistry to design biodegradable polymeric implants using chitosan and other biomaterials. Int J Biol Macromol. 2021;169:414–427. doi: 10.1016/j.ijbiomac.2020.12.112. [DOI] [PubMed] [Google Scholar]
- 43.Xu X., Awad A., Robles-Martinez P., Gaisford S., Goyanes A., Basit A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J Control Release. 2021;329:743–757. doi: 10.1016/j.jconrel.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Gao N., Lu D.H., Zhao Y.Y., Liu X.W., Liu G.H., Wu Y., et al. Strengthening of a CrMnFeCoNi high-entropy alloy by carbide precipitation. J Alloys Compd. 2019;792:1028–1035. [Google Scholar]
- 45.Baptista R., Guedes M. Porosity and pore design influence on fatigue behavior of 3D printed scaffolds for trabecular bone replacement. J Mech Behav Biomed Mater. 2021;117 doi: 10.1016/j.jmbbm.2021.104378. [DOI] [PubMed] [Google Scholar]
- 46.Adel I.M., ElMeligy M.F., Elkasabgy N.A. Conventional and recent trends of scaffolds fabrication: a superior mode for tissue engineering. Pharm. 2022:14. doi: 10.3390/pharmaceutics14020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yadav L.R., Chandran S.V., Lavanya K., Selvamurugan N. Chitosan-based 3D-printed scaffolds for bone tissue engineering. Int J Biol Macromol. 2021;183:1925–1938. doi: 10.1016/j.ijbiomac.2021.05.215. [DOI] [PubMed] [Google Scholar]
- 48.Noroozi R., Mashhadi Kashtiban M., Taghvaei H., Zolfagharian A., Bodaghi M. 3D-printed microfluidic droplet generation systems for drug delivery applications. Mater Today Proc. 2022 [Google Scholar]
- 49.Olmos-Juste R., Guaresti O., Calvo-Correas T., Gabilondo N., Eceiza A. Design of drug-loaded 3D printing biomaterial inks and tailor-made pharmaceutical forms for controlled release. Int J Pharm. 2021;609 doi: 10.1016/j.ijpharm.2021.121124. [DOI] [PubMed] [Google Scholar]
- 50.Wei Y., Wang F., Guo Z., Zhao Q. Tissue-engineered vascular grafts and regeneration mechanisms. J Mol Cell Cardiol. 2022;165:40–53. doi: 10.1016/j.yjmcc.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Jain P., Kathuria H., Dubey N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121639. [DOI] [PubMed] [Google Scholar]
- 52.Zare M., Ghomi E.R., Venkatraman P.D., Ramakrishna S. Silicone-based biomaterials for biomedical applications: antimicrobial strategies and 3D printing technologies. J Appl Polym Sci. 2021;138:50969. [Google Scholar]
- 53.Ponnamma D., Yin Y., Salim N., Parameswaranpillai J., Thomas S., Hameed N. Recent progress and multifunctional applications of 3D printed graphene nanocomposites. Compos Part B Eng. 2021;204 [Google Scholar]
- 54.Jeong K.-.H., Park D., Lee Y.-.C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: a mini-review. J Polym Res. 2017;24:112. [Google Scholar]
- 55.Rett J.P., Traore Y.L., Ho E.A. Sustainable materials for fused deposition modeling 3D printing applications. Adv Eng Mater. 2021;23 [Google Scholar]
- 56.Moshood T.D., Nawanir G., Mahmud F., Mohamad F., Ahmad M.H., AbdulGhani A. Biodegradable plastic applications towards sustainability: a recent innovations in the green product. Clean Eng Technol. 2022;6 [Google Scholar]
- 57.Kumar K.P.A., Pumera M. 3D-printing to mitigate COVID-19 pandemic. Adv Funct Mater. 2021;31 doi: 10.1002/adfm.202100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bin Lee Y, O Jeon, Lee S.J., Ding A., Wells D., Alsberg E. Induction of four-dimensional spatiotemporal geometric transformations in high cell density tissues via shape-changing hydrogels. Adv Funct Mater. 2021;31 doi: 10.1002/adfm.202010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang Y.-.H., Um T., Ahn G.-.N., Kim D-P, Lee H. Robust and scalable production of emulsion-templated microparticles in 3D-printed milli-fluidic device. Chem Eng J. 2022;431 [Google Scholar]
- 60.Tan X.H., Liu L., Mitryashkin A., Wang Y., Goh J.C.H. Silk fibroin as a bioink – a thematic review of functionalization strategies for bioprinting applications. ACS Biomater Sci Eng. 2022;8:3242–3270. doi: 10.1021/acsbiomaterials.2c00313. [DOI] [PubMed] [Google Scholar]
- 61.Peydayesh M., Bagnani M., Soon W.L., Mezzenga R. Turning food protein waste into sustainable technologies. Chem Rev. 2022;123:2112–2154. doi: 10.1021/acs.chemrev.2c00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Mu W., Zhang Y., He X., Wang Y., Ma H., et al. Recent advances in cardiac patches: materials, preparations, and properties. ACS Biomater Sci Eng. 2022;8:3659–3675. doi: 10.1021/acsbiomaterials.2c00348. [DOI] [PubMed] [Google Scholar]
- 63.Ghomi ER, Shakiba M, Ardahaei AS, Kenari MA, Faraji M, Ataei S, et al. Innovations in drug delivery for chronic wound healing. Curr Pharm Des. 2022;28:340–351. doi: 10.2174/1381612827666210714102304. [DOI] [PubMed] [Google Scholar]
- 64.Williams D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front Bioeng Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suo H., Zhang J., Xu M., Wang L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater Sci Eng C. 2021;123 doi: 10.1016/j.msec.2021.111963. [DOI] [PubMed] [Google Scholar]
- 66.Loo H.L., Goh B.H., Lee L-H, Chuah L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian J Pharm Sci. 2022;17:299–332. doi: 10.1016/j.ajps.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heidarian P., Kouzani A.Z., Kaynak A., Paulino M., Nasri-Nasrabadi B., Zolfagharian A., et al. Dynamic plant-derived polysaccharide-based hydrogels. Carbohydr Polym. 2020;231 doi: 10.1016/j.carbpol.2019.115743. [DOI] [PubMed] [Google Scholar]
- 68.Li J., Liu X., Crook J.M., Wallace G.G. 3D printing of cytocompatible graphene/alginate scaffolds for mimetic tissue constructs. Front Bioeng Biotechnol. 2020;8:824. doi: 10.3389/fbioe.2020.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad Raus R., Wan Nawawi W.M.F., Nasaruddin R.R. Alginate and alginate composites for biomedical applications. Asian J Pharm Sci. 2021;16:280–306. doi: 10.1016/j.ajps.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li C., Wu J., Shi H., Xia Z., Sahoo J.K., Yeo J., et al. Fiber-based biopolymer processing as a route toward sustainability. Adv Mater. 2022;34 doi: 10.1002/adma.202105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Q., Sun J., Yao Q., Ji C., Liu J., Zhu Q. 3D printing with cellulose materials. Cellulose. 2018;25:4275–4301. doi: 10.1007/s10570-018-1888-y. [DOI] [Google Scholar]
- 72.Khalid M.Y., Al Rashid A., Arif Z.U., Ahmed W., Arshad H. Recent advances in nanocellulose-based different biomaterials: types, properties, and emerging applications. J Mater Res Technol. 2021;14:2601–2623. [Google Scholar]
- 73.Zinge C., Kandasubramanian B. Nanocellulose based biodegradable polymers. Eur Polym J. 2020;133 doi: 10.1016/j.eurpolymj.2020.109758. [DOI] [Google Scholar]
- 74.Deng J., Song Q., Liu S., Pei W., Wang P., Zheng L., et al. Advanced applications of cellulose-based composites in fighting bone diseases. Compos Part B Eng. 2022;245 [Google Scholar]
- 75.Marques C.F., Diogo G.S., Pina S., Oliveira J.M., Silva T.H., Reis R.L. Collagen-based bioinks for hard tissue engineering applications: a comprehensive review. J Mater Sci Mater Med. 2019;30:32. doi: 10.1007/s10856-019-6234-x. [DOI] [PubMed] [Google Scholar]
- 76.Stanisz M., Klapiszewski Ł., Jesionowski T. Recent advances in the fabrication and application of biopolymer-based micro- and nanostructures: a comprehensive review. Chem Eng J. 2020;397 doi: 10.1016/j.cej.2020.125409. [DOI] [Google Scholar]
- 77.Calori I.R., Braga G., de Jesus P, da C.C., Bi H., Tedesco A.C. Polymer scaffolds as drug delivery systems. Eur Polym J. 2020;129 [Google Scholar]
- 78.Sun W., Gregory D.A., Tomeh M.A., Zhao X. Silk fibroin as a functional biomaterial for tissue engineering. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bose S., Koski C., Vu A.A. Additive manufacturing of natural biopolymers and composites for bone tissue engineering. Mater Horiz. 2020;7:2011–2027. doi: 10.1039/D0MH00277A. [DOI] [Google Scholar]
- 80.Guo J., Goh M., Zhu Z., Lee X., Nai M.L.S., Wei J. On the machining of selective laser melting CoCrFeMnNi high-entropy alloy. Mater Des. 2018;153:211–220. [Google Scholar]
- 81.Rippon J.A., Evans D.J. Elsevier Ltd; 2020. Improving the Properties of Natural Fibres by Chemical Treatments. [DOI] [Google Scholar]
- 82.Yazdanian M., Tabesh H., Houshmand B., Tebyanian H., Soufdoost R.S., Tahmasebi E., et al. Fabrication and properties of βTCP/Zeolite/Gelatin scaffold as developed scaffold in bone regeneration: in vitro and in vivo studies. Biocybern Biomed Eng. 2020;40:1626–1637. [Google Scholar]
- 83.Saroia J., Yanen W., Wei Q., Zhang K., Lu T., Zhang B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Design Manuf. 2018;1:265–279. [Google Scholar]
- 84.Liu L., Gao Q., Lu X., Zhou H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J Pharm Sci. 2016;11:673–683. [Google Scholar]
- 85.Yan Q., Dong H., Su J., Han J., Song B., Wei Q., et al. A review of 3D printing technology for medical applications. Engineering. 2018;4:729–742. [Google Scholar]
- 86.Qamruzzaman M., Ahmed F., Mondal M.I.H. An overview on starch-based sustainable hydrogels: potential applications and aspects. J Polym Environ. 2022;30:19–50. [Google Scholar]
- 87.DeBari M.K., Keyser M.N., Bai M.A., Abbott R.D. 3D printing with silk: considerations and applications. Connect Tissue Res. 2020;61:163–173. doi: 10.1080/03008207.2018.1553959. [DOI] [PubMed] [Google Scholar]
- 88.Nanda S., Patra B.R., Patel R., Bakos J., Dalai A.K. Innovations in applications and prospects of bioplastics and biopolymers: a review. Environ Chem Lett. 2022;20:379–395. doi: 10.1007/s10311-021-01334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collins M.N., Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—a review. Carbohydr Polym. 2013;92:1262–1279. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 90.Ding Y.-.W., Zhang X.-.W., Mi C.-.H., Qi X.-.Y., Zhou J., Wei D.-X. Recent advances in hyaluronic acid-based hydrogels for 3D bioprinting in tissue engineering applications. Smart Mater Med. 2023;4:59–68. [Google Scholar]
- 91.Prajapati S.K., Jain A., Jain A., Jain S. Biodegradable polymers and constructs: a novel approach in drug delivery. Eur Polym J. 2019;120 [Google Scholar]
- 92.Liu Z., Wang Y., Wu B., Cui C., Guo Y., Yan C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int J Adv Manuf Technol. 2019;102:2877–2889. [Google Scholar]
- 93.Park S.-.B., Lih E., Park K.-.S., Joung Y.K., Han D.K. Biopolymer-based functional composites for medical applications. Prog Polym Sci. 2017;68:77–105. [Google Scholar]
- 94.Wei S., Ma J.-.X., Xu L., Gu X.-.S., Ma X.-.L. Biodegradable materials for bone defect repair. Mil Med Res. 2020;7:54. doi: 10.1186/s40779-020-00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gupta S., Bissoyi A., Bit A. A review on 3d printable techniques for tissue engineering. Bionanoscience. 2018;8:868–883. [Google Scholar]
- 96.Raina N., Pahwa R., Khosla J.K., Gupta P.N., Gupta M. Polycaprolactone-based materials in wound healing applications. Polym Bull. 2022;79:7041–7063. [Google Scholar]
- 97.George A., Sanjay M.R., Srisuk R., Parameswaranpillai J., Siengchin S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int J Biol Macromol. 2020;154:329–338. doi: 10.1016/j.ijbiomac.2020.03.120. [DOI] [PubMed] [Google Scholar]
- 98.Ceccarelli G., Presta R., Benedetti L., Cusella De Angelis M.G., Lupi S.M., Rodriguez y Baena R. Emerging perspectives in scaffold for tissue engineering in oral surgery. Stem Cells Int. 2017;2017 doi: 10.1155/2017/4585401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Long L., Wu C., Hu X., Wang Y. Biodegradable synthetic polymeric composite scaffold-based tissue engineered heart valve with minimally invasive transcatheter implantation. Polym Adv Technol. 2020;31:2422–2432. [Google Scholar]
- 100.Jem K.J., Tan B. The development and challenges of poly (lactic acid) and poly (glycolic acid) Adv Ind Eng Polym Res. 2020;3:60–70. [Google Scholar]
- 101.Low Y.J., Andriyana A., Ang B.C., Zainal Abidin N.I. Bioresorbable and degradable behaviors of PGA: current state and future prospects. Polym Eng Sci. 2020;60:2657–2675. [Google Scholar]
- 102.Pandey A., Adama N., Adjallé K., Blais J.-.F. Sustainable applications of polyhydroxyalkanoates in various fields: a critical review. Int J Biol Macromol. 2022;221:1184–1201. doi: 10.1016/j.ijbiomac.2022.09.098. [DOI] [PubMed] [Google Scholar]
- 103.Soleymani Eil Bakhtiari S., HR Bakhsheshi-Rad, Karbasi S., Razzaghi M., Tavakoli M., Ismail A.F., et al. 3-Dimensional printing of hydrogel-based nanocomposites: a comprehensive review on the technology description, properties, and applications. Adv Eng Mater. 2021;23 [Google Scholar]
- 104.Yeo J.C.C., Muiruri J.K., Thitsartarn W., Li Z., He C. Recent advances in the development of biodegradable PHB-based toughening materials: approaches, advantages and applications. Mater Sci Eng C. 2018;92:1092–1116. doi: 10.1016/j.msec.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 105.Chakrapani G., Zare M., Ramakrishna S. Intelligent hydrogels and their biomedical applications. Mater Adv. 2022;3:7757–7772. [Google Scholar]
- 106.Kamoun E.A., Loutfy S.A., Hussein Y., Kenawy E.-.R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: a review. Int J Biol Macromol. 2021;187:755–768. doi: 10.1016/j.ijbiomac.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 107.Dhanraj N.D., Hatha A.A.M., Jisha M.S. Biodegradation of petroleum based and bio-based plastics: approaches to increase the rate of biodegradation. Arch Microbiol. 2022;204:258. doi: 10.1007/s00203-022-02883-0. [DOI] [PubMed] [Google Scholar]
- 108.Shiroud Heidari B., Ruan R., Vahabli E., Chen P., De-Juan-Pardo E.M., Zheng M., et al. Natural, synthetic and commercially-available biopolymers used to regenerate tendons and ligaments. Bioact Mater. 2023;19:179–197. doi: 10.1016/j.bioactmat.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagarajan S., Radhakrishnan S., Kalkura S.N., Balme S., Miele P., Bechelany M. Overview of protein-based biopolymers for biomedical application. Macromol Chem Phys. 2019;220 [Google Scholar]
- 110.Maharjan B., Park J., Kaliannagounder V.K., Awasthi G.P., Joshi M.K., Park C.H., et al. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr Polym. 2021;251 doi: 10.1016/j.carbpol.2020.117023. [DOI] [PubMed] [Google Scholar]
- 111.Farokhi M., Mottaghitalab F., Reis R.L., Ramakrishna S., Kundu S.C. Functionalized silk fibroin nanofibers as drug carriers: advantages and challenges. J Control Release. 2020;321:324–347. doi: 10.1016/j.jconrel.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 112.Biswal T. Biopolymers for tissue engineering applications: a review. Mater Today Proc. 2021;41:397–402. [Google Scholar]
- 113.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49:780–792. [Google Scholar]
- 114.Malik S., Sundarrajan S., Hussain T., Nazir A., Ayyoob M., Berto F., et al. Sustainable nanofibers in tissue engineering and biomedical applications. Mater Des Process Commun. 2021;3:e202. [Google Scholar]
- 115.Mukhtar M., Fényes E., Bartos C., Zeeshan M., Ambrus R. Chitosan biopolymer, its derivatives and potential applications in nano-therapeutics: a comprehensive review. Eur Polym J. 2021;160 [Google Scholar]
- 116.Bedian L., Villalba-Rodríguez A.M., Hernández-Vargas G., Parra-Saldivar R., Iqbal H.M.N. Bio-based materials with novel characteristics for tissue engineering applications – a review. Int J Biol Macromol. 2017;98:837–846. doi: 10.1016/j.ijbiomac.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 117.Yao X., Yang Y., Zhou Z. Non-mulberry silk fiber-based composite scaffolds containing millichannels for auricular cartilage regeneration. ACS Omega. 2022;7:15064–15073. doi: 10.1021/acsomega.2c00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moghaddaszadeh A., Seddiqi H., Najmoddin N., Abbasi Ravasjani S., Klein-Nulend J. Biomimetic 3D-printed PCL scaffold containing a high concentration carbonated-nanohydroxyapatite with immobilized-collagen for bone tissue engineering: enhanced bioactivity and physicomechanical characteristics. Biomed Mater. 2021;16:65029. doi: 10.1088/1748-605X/ac3147. [DOI] [PubMed] [Google Scholar]
- 119.Gunes O.C., Kara A., Baysan G., Bugra Husemoglu R., Akokay P., Ziylan Albayrak A., et al. Fabrication of 3D printed poly(lactic acid) strut and wet-electrospun cellulose nano fiber reinforced chitosan-collagen hydrogel composite scaffolds for meniscus tissue engineering. J Biomater Appl. 2022 doi: 10.1177/08853282221109339. [DOI] [PubMed] [Google Scholar]
- 120.Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amin Noroozi Mohammad, mahmoudi Reza, Zolfagharian Ali, Asgari Fatemeh, Mousavizadeh Ali, Bodaghi Mahdi, Hadi amin, Haghighipour Nooshin R, S In vitro static and dynamic cell culture study of novel bone scaffolds based on 3D-printed PLA and cell-laden alginate hydrogel. Biomed Mater. 2022 doi: 10.1088/1748-605X/ac7308. [DOI] [PubMed] [Google Scholar]
- 122.Yang J., Yu H., Wang L., Liu J., Liu X., Hong Y., et al. Advances in adhesive hydrogels for tissue engineering. Eur Polym J. 2022;172 [Google Scholar]
- 123.You C., Ning L., Wu H., Huang C., Wang F. A biocompatible and pH-responsive nanohydrogel based on cellulose nanocrystal for enhanced toxic reactive oxygen species generation. Carbohydr Polym. 2021;258 doi: 10.1016/j.carbpol.2021.117685. [DOI] [PubMed] [Google Scholar]
- 124.Bose S., Ke D., Sahasrabudhe H., Bandyopadhyay A. Additive manufacturing of biomaterials. Prog Mater Sci. 2018;93:45–111. doi: 10.1016/j.pmatsci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malekmohammadi S., Sedghi Aminabad N., Sabzi A., Zarebkohan A., Razavi M., Vosough M., et al. Smart and biomimetic 3D and 4D printed composite hydrogels: opportunities for different biomedical applications. Biomed. 2021;9 doi: 10.3390/biomedicines9111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rezvani Ghomi E., Nourbakhsh N., Akbari Kenari M., Zare M., Ramakrishna S. Collagen-based biomaterials for biomedical applications. J Biomed Mater Res Part B Appl Biomater. 2021;109:1986–1999. doi: 10.1002/jbm.b.34881. [DOI] [PubMed] [Google Scholar]
- 127.Heo D.N., Hospodiuk M., Ozbolat I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019;95:348–356. doi: 10.1016/j.actbio.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 128.Chen K., Hu H., Zeng Y., Pan H., Wang S., Zhang Y., et al. Recent advances in electrospun nanofibers for wound dressing. Eur Polym J. 2022;178 [Google Scholar]
- 129.Ge L., Xu Y., Li X., Yuan L., Tan H., Li D., et al. Fabrication of antibacterial collagen-based composite wound dressing. ACS Sustain Chem Eng. 2018;6:9153–9166. [Google Scholar]
- 130.Li Z., Du T., Ruan C., Niu X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact Mater. 2021;6:1491–1511. doi: 10.1016/j.bioactmat.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim S.H., Hong H., Ajiteru O., Sultan M.T., Lee Y.J., Lee J.S., et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat Protoc. 2021;16:5484–5532. doi: 10.1038/s41596-021-00622-1. [DOI] [PubMed] [Google Scholar]
- 132.Wani S.U.D., Gautam S.P., Qadrie Z.L., Gangadharappa H.V. Silk fibroin as a natural polymeric based bio-material for tissue engineering and drug delivery systems-a review. Int J Biol Macromol. 2020;163:2145–2161. doi: 10.1016/j.ijbiomac.2020.09.057. [DOI] [PubMed] [Google Scholar]
- 133.Farokhi M., Mottaghitalab F., Fatahi Y., Saeb M.R., Zarrintaj P., Kundu S.C., et al. Silk fibroin scaffolds for common cartilage injuries: possibilities for future clinical applications. Eur Polym J. 2019;115:251–267. [Google Scholar]
- 134.Thomas S., Gopi S., Amalraj A. Elsevier; 2020. Biopolymers and their industrial applications: from plant, animal, and marine sources, to functional products. [Google Scholar]
- 135.Reddy M.S., Ponnamma D., Choudhary R., Sadasivuni K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polym. 2021;13 doi: 10.3390/polym13071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Maria C., Chiesa I., Morselli D., Ceccarini M.R., Bittolo Bon S., Degli Esposti M., et al. Biomimetic tendrils by four dimensional printing bimorph springs with torsion and contraction properties based on bio-compatible graphene/silk fibroin and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Adv Funct Mater. 2021;31 [Google Scholar]
- 137.Li Y., Shi Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt Laser Technol. 2021;134 [Google Scholar]
- 138.Jin G., Prabhakaran M.P., Kai D., Annamalai S.K., Arunachalam K.D., Ramakrishna S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials. 2013;34:724–734. doi: 10.1016/j.biomaterials.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 139.Zheng L., Yu Y., Tong Z., Zou Q., Han S., Jiang H. The characteristics of starch gels molded by 3D printing. J Food Process Preserv. 2019;43:e13993. [Google Scholar]
- 140.Ratheesh G., Venugopal J.R., Chinappan A., Ezhilarasu H., Sadiq A., Ramakrishna S. 3D fabrication of polymeric scaffolds for regenerative therapy. ACS Biomater Sci Eng. 2017;3:1175–1194. doi: 10.1021/acsbiomaterials.6b00370. [DOI] [PubMed] [Google Scholar]
- 141.Basa B., Jakab G., Kállai-Szabó N., Borbás B., Fülöp V., Balogh E., et al. Evaluation of biodegradable PVA-based 3D printed carriers during dissolution. Mater. 2021:14. doi: 10.3390/ma14061350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lyu Y., Zhao H., Wen X., Lin L., Schlarb A.K., Shi X. Optimization of 3D printing parameters for high-performance biodegradable materials. J Appl Polym Sci. 2021;138:50782. [Google Scholar]
- 143.Lodoso-Torrecilla I., van den Beucken J.J.J.P., Jansen J.A. Calcium phosphate cements: optimization toward biodegradability. Acta Biomater. 2021;119:1–12. doi: 10.1016/j.actbio.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 144.Echave M.C., Hernáez-Moya R., Iturriaga L., Pedraz J.L., Lakshminarayanan R., Dolatshahi-Pirouz A., et al. Recent advances in gelatin-based therapeutics. Expert Opin Biol Ther. 2019;19:773–779. doi: 10.1080/14712598.2019.1610383. [DOI] [PubMed] [Google Scholar]
- 145.Il Kang J, KM Park. Advances in gelatin-based hydrogels for wound management. J Mater Chem B. 2021;9:1503–1520. doi: 10.1039/d0tb02582h. [DOI] [PubMed] [Google Scholar]
- 146.Li L., Qin S., Peng J., Chen A., Nie Y., Liu T., et al. Engineering gelatin-based alginate/carbon nanotubes blend bioink for direct 3D printing of vessel constructs. Int J Biol Macromol. 2020;145:262–271. doi: 10.1016/j.ijbiomac.2019.12.174. [DOI] [PubMed] [Google Scholar]
- 147.Khalid M.Y., Al Rashid A., Arif Z.U., Ahmed W., Arshad H., Zaidi A.A. Natural fiber reinforced composites: sustainable materials for emerging applications. Results Eng. 2021;11 [Google Scholar]
- 148.Pang M., Huang Y., Meng F., Zhuang Y., Liu H., Du M., et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur Polym J. 2020;122 [Google Scholar]
- 149.Vincent S., Kandasubramanian B. Cellulose nanocrystals from agricultural resources: extraction and functionalisation. Eur Polym J. 2021;160 [Google Scholar]
- 150.Janmohammadi M., Nazemi Z., Salehi A.O.M., Seyfoori A., John J.V., Nourbakhsh M.S., et al. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact Mater. 2023;20:137–163. doi: 10.1016/j.bioactmat.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eichhorn S.J., Etale A., Wang J., Berglund L.A., Li Y., Cai Y., et al. Current international research into cellulose as a functional nanomaterial for advanced applications. J Mater Sci. 2022;57:5697–5767. [Google Scholar]
- 152.Saravanakumar K., Park S., Santosh S.S., Ganeshalingam A., Thiripuranathar G., Sathiyaseelan A., et al. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: a review. Int J Biol Macromol. 2022 doi: 10.1016/j.ijbiomac.2022.10.055. [DOI] [PubMed] [Google Scholar]
- 153.Dovedytis M., Liu Z.J., Bartlett S. Hyaluronic acid and its biomedical applications: a review. Eng Regen. 2020;1:102–113. [Google Scholar]
- 154.Saadi M.A.S.R., Maguire A., Pottackal N., Thakur M.S.H., Ikram M.M., Hart A.J., et al. Direct ink writing: a 3D printing technology for diverse materials. Adv Mater. 2022 doi: 10.1002/adma.202108855. n/a. [DOI] [PubMed] [Google Scholar]
- 155.Babaei M., Jamshidi N., Amiri F., Rafienia M. Effects of low-intensity pulsed ultrasound stimulation on cell seeded 3D hybrid scaffold as a novel strategy for meniscus regeneration: an in vitro study. J Tissue Eng Regen Med. 2022;16:812–824. doi: 10.1002/term.3331. [DOI] [PubMed] [Google Scholar]
- 156.Vyas A., Bandhu Ghosh S., Bandyopadhyay-Ghosh S., Agrawal A.K., Khare D., Dubey A.K. Digital light processing mediated 3D printing of biocomposite bone scaffolds: physico-chemical interactions and in-vitro biocompatibility. Polym Compos. 2022;43:3175–3188. [Google Scholar]
- 157.Sharma S., Gupta V., Mudgal D. Current trends, applications, and challenges of coatings on additive manufacturing based biopolymers: a state of art review. Polym Compos. 2022 n/a. [Google Scholar]
- 158.Arif Z.U., Khalid M.Y., Sheikh M.F., Zolfagharian A., Bodaghi M. Biopolymeric sustainable materials and their emerging applications. J Environ Chem Eng. 2022;10 [Google Scholar]
- 159.Herath B., Suresh S., Downing D., Cometta S., Tino R., Castro N.J., et al. Mechanical and geometrical study of 3D printed Voronoi scaffold design for large bone defects. Mater Des. 2021;212 [Google Scholar]
- 160.Hakan C., Olcay D.N., Ceren Y.I., Nur M.M., Umut K.Z., Metin S. 3D printed personalized magnetic micromachines from patient blood–derived biomaterials. Sci Adv. 2021;7:eabh0273. doi: 10.1126/sciadv.abh0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lagreca E., Onesto V., Di Natale C., La Manna S., Netti P.A., Vecchione R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog Biomater. 2020;9:153–174. doi: 10.1007/s40204-020-00139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tsachouridis K., Christodoulou E., Zamboulis A., Michopoulou A., Barmpalexis P., Bikiaris D.N. Evaluation of poly(lactic acid)/and poly(lactic-co-glycolic acid)/ poly(ethylene adipate) copolymers for the preparation of paclitaxel loaded drug nanoparticles. J Drug Deliv Sci Technol. 2022 [Google Scholar]
- 163.Choubar E.G., Nasirtabrizi M.H., Salimi F., Sohrabi-gilani N., Sadeghianamryan A. Fabrication and in vitro characterization of novel co-electrospun polycaprolactone/collagen/polyvinylpyrrolidone nanofibrous scaffolds for bone tissue engineering applications. J Mater Res. 2022 [Google Scholar]
- 164.Tian H., Tang Z., Zhuang X., Chen X., Jing X. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci. 2012;37:237–280. [Google Scholar]
- 165.Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog Polym Sci. 2011;36:1254–1276. [Google Scholar]
- 166.Amini S., Salehi H., Setayeshmehr M., Ghorbani M. Natural and synthetic polymeric scaffolds used in peripheral nerve tissue engineering: advantages and disadvantages. Polym Adv Technol. 2021;32:2267–2289. [Google Scholar]
- 167.Arif Z.U., Khalid M.Y., Noroozi R., Sadeghianmarayn A., Jalalvand M., Hossain M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int J Biol Macromol. 2022;218:930–968. doi: 10.1016/j.ijbiomac.2022.07.140. [DOI] [PubMed] [Google Scholar]
- 168.Singhvi M.S., Zinjarde S.S., Gokhale D.V. Polylactic acid: synthesis and biomedical applications. J Appl Microbiol. 2019;127:1612–1626. doi: 10.1111/jam.14290. [DOI] [PubMed] [Google Scholar]
- 169.Fazal F., Diaz Sanchez F.J., Waqas M., Koutsos V., Callanan A., Radacsi N. A modified 3D printer as a hybrid bioprinting-electrospinning system for use in vascular tissue engineering applications. Med Eng Phys. 2021;94:52–60. doi: 10.1016/j.medengphy.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 170.Shen Y., Tang C., Sun B., Zhang Y., Sun X., EL-Newehy M., et al. 3D printed personalized, heparinized and biodegradable coronary artery stents for rabbit abdominal aorta implantation. Chem Eng J. 2022;450 [Google Scholar]
- 171.Siddiqui N., Kishori B., Rao S., Anjum M., Hemanth V., Das S., et al. Electropsun polycaprolactone fibres in bone tissue engineering: a review. Mol Biotechnol. 2021;63:363–388. doi: 10.1007/s12033-021-00311-0. [DOI] [PubMed] [Google Scholar]
- 172.Yang J., Zhang H., Hu T., Xu C., Jiang L., Shrike Zhang Y., et al. Recent advances of microneedles used towards stimuli-responsive drug delivery, disease theranostics, and bioinspired applications. Chem Eng J. 2021;426 [Google Scholar]
- 173.Mahmoud Salehi A.O., Heidari Keshel S., Sefat F., Tayebi L. Use of polycaprolactone in corneal tissue engineering: a review. Mater Today Commun. 2021;27 [Google Scholar]
- 174.Budak K., Sogut O., Aydemir Sezer U. A review on synthesis and biomedical applications of polyglycolic acid. J Polym Res. 2020;27:208. [Google Scholar]
- 175.Jazayeri H.E., Lee S.-.M., Kuhn L., Fahimipour F., Tahriri M., Tayebi L. Polymeric scaffolds for dental pulp tissue engineering: a review. Dent Mater. 2020;36:e47–e58. doi: 10.1016/j.dental.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 176.Pal A.K., Mohanty A.K., Misra M. Additive manufacturing technology of polymeric materials for customized products: recent developments and future prospective. RSC Adv. 2021;11:36398–36438. doi: 10.1039/d1ra04060j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huang Z.-.M., Zhang Y.-.Z., Kotaki M., Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63:2223–2253. [Google Scholar]
- 178.Roseti L., Parisi V., Petretta M., Cavallo C., Desando G., Bartolotti I., et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C. 2017;78:1246–1262. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 179.Rezvani Ghomi E., Khosravi F., Neisiany R.E., Shakiba M., Zare M., Lakshminarayanan R., et al. Advances in electrospinning of aligned nanofiber scaffolds used for wound dressings. Curr Opin Biomed Eng. 2022;22 [Google Scholar]
- 180.Wang Z., Wang C., Li C., Qin Y., Zhong L., Chen B., et al. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: a review. J Alloys Compd. 2017;717:271–285. [Google Scholar]
- 181.Roshandel M., Dorkoosh F. Cardiac tissue engineering, biomaterial scaffolds, and their fabrication techniques. Polym Adv Technol. 2021;32:2290–2305. [Google Scholar]
- 182.Lan W., Huang X., Huang D., Wei X., Chen W. Progress in 3D printing for bone tissue engineering: a review. J Mater Sci. 2022 [Google Scholar]
- 183.Serrano-Aroca Á., Cano-Vicent A., Sabater i Serra R., El-Tanani M., Aljabali A., Tambuwala M.M., et al. Scaffolds in the microbial resistant era: fabrication, materials, properties and tissue engineering applications. Mater Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Z., Feng Y., Wang L., Liu D., Qin C., Shi Y. A review of preparation methods of porous skin tissue engineering scaffolds. Mater Today Commun. 2022;32 [Google Scholar]
- 185.Karimi Afshar S., Abdorashidi M., Dorkoosh F.A., Akbari Javar H. Electrospun fibers: versatile approaches for controlled release applications. Int J Polym Sci. 2022;2022 [Google Scholar]
- 186.Rahmati M., Mills D.K., Urbanska A.M., Saeb M.R., Venugopal J.R., Ramakrishna S., et al. Electrospinning for tissue engineering applications. Prog Mater Sci. 2021;117 [Google Scholar]
- 187.Poomathi N., Singh S., Prakash C., Subramanian A., Sahay R., Cinappan A., et al. 3D printing in tissue engineering: a state of the art review of technologies and biomaterials. Rapid Prototyp J. 2020;26:1313–1334. [Google Scholar]
- 188.Mitra S., Mateti T., Ramakrishna S., Laha A. A review on curcumin-loaded electrospun nanofibers and their application in modern medicine. JOM. 2022 doi: 10.1007/s11837-022-05180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bigham A., Foroughi F., Rezvani Ghomi E., Rafienia M., Neisiany R.E., Ramakrishna S. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio-Design Manuf. 2020;3:281–306. [Google Scholar]
- 190.Vasudevan A., Tripathi D.M., Sundarrajan S., Venugopal J.R., Ramakrishna S., Kaur S. Evolution of electrospinning in liver tissue engineering. Biomimetics. 2022;7 doi: 10.3390/biomimetics7040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ansari M.A.A., Golebiowska A.A., Dash M., Kumar P., Jain P.K., Nukavarapu S.P., et al. Engineering biomaterials to 3D-print scaffolds for bone regeneration: practical and theoretical consideration. Biomater Sci. 2022;10:2789–2816. doi: 10.1039/d2bm00035k. [DOI] [PubMed] [Google Scholar]
- 192.Sultana A., Zare M., Thomas V., Kumar T.S.S., Ramakrishna S. Nano-based drug delivery systems: conventional drug delivery routes, recent developments and future prospects. Med Drug Discov. 2022;15 [Google Scholar]
- 193.Mani M.P., Sadia M., Jaganathan S.K., Khudzari A.Z., Supriyanto E., Saidin S., et al. A review on 3D printing in tissue engineering applications. J Polym Eng. 2022 [Google Scholar]
- 194.Chen Y., Dong X., Shafiq M., Myles G., Radacsi N., Mo X. Recent advancements on three-dimensional electrospun nanofiber scaffolds for tissue engineering. Adv Fiber Mater. 2022;4:959–986. [Google Scholar]
- 195.Imran R., Al Rashid A., Koç M. Review on computational modeling for the property, process, product and performance (PPPP) characteristics of additively manufactured porous magnesium implants. Bioprinting. 2022;28:e00236. [Google Scholar]
- 196.Gonzalez G., Roppolo I., Pirri C.F., Chiappone A. Current and emerging trends in polymeric 3D printed microfluidic devices. Addit Manuf. 2022;55 [Google Scholar]
- 197.Zhang C., Li Y., Kang W., Liu X., Wang Q. Current advances and future perspectives of additive manufacturing for functional polymeric materials and devices. SusMat. 2021;1:127–147. [Google Scholar]
- 198.Mabrouk M., Beherei H.H., Das D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater Sci Eng C. 2020;110 doi: 10.1016/j.msec.2020.110716. [DOI] [PubMed] [Google Scholar]
- 199.Udayakumar G.P., Muthusamy S., Selvaganesh B., Sivarajasekar N., Rambabu K., Banat F., et al. Biopolymers and composites: properties, characterization and their applications in food, medical and pharmaceutical industries. J Environ Chem Eng. 2021;9 [Google Scholar]
- 200.Li N., Huang S., Zhang G., Qin R., Liu W., Xiong H., et al. Progress in additive manufacturing on new materials: a review. J Mater Sci Technol. 2019;35:242–269. [Google Scholar]
- 201.Miechowicz S., Wojnarowska W., Majkut S., Trybulec J., Pijanka D., Piecuch T., et al. Method of designing and manufacturing craniofacial soft tissue prostheses using Additive Manufacturing: a case study. Biocybern Biomed Eng. 2021;41:854–865. [Google Scholar]
- 202.Al Rashid A., Khan SA G., Al-Ghamdi S., Koç M. Additive manufacturing of polymer nanocomposites: needs and challenges in materials, processes, and applications. J Mater Res Technol. 2021;14:910–941. [Google Scholar]
- 203.Pahlevanzadeh F., Emadi R., Valiani A., Kharaziha M., Poursamar S.A., Bakhsheshi-Rad H.R., et al. Three-dimensional printing constructs based on the chitosan for tissue regeneration: state of the art, developing directions and prospect trends. Mater. 2020;13 doi: 10.3390/ma13112663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kholgh Eshkalak S., Rezvani Ghomi E., Dai Y., Choudhury D., Ramakrishna S. The role of three-dimensional printing in healthcare and medicine. Mater Des. 2020;194 [Google Scholar]
- 205.Ghasemi-Mobarakeh L., Kolahreez D., Ramakrishna S., Williams D. Key terminology in biomaterials and biocompatibility. Curr Opin Biomed Eng. 2019;10:45–50. [Google Scholar]
- 206.Yarali E., Baniasadi M., Zolfagharian A., Chavoshi M., Arefi F., Hossain M., et al. Magneto-/electro-responsive polymers toward manufacturing, characterization, and biomedical/ soft robotic applications. Appl Mater Today. 2022;26 [Google Scholar]
- 207.Sandanamsamy L., Harun W.S.W., Ishak I., Romlay F.R.M., Kadirgama K., Ramasamy D., et al. A comprehensive review on fused deposition modelling of polylactic acid. Prog Addit Manuf. 2022 doi: 10.1007/s40964-022-00356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Luhar S., Suntharalingam T., Navaratnam S., Luhar I., Thamboo J., Poologanathan K., et al. Sustainable and renewable bio-based natural fibres and its application for 3D printed concrete: a review. Sustain. 2020:12. [Google Scholar]
- 209.Narendra Babu Y., Venkateswara Rao M., Gopala Krishna A. Role of reinforcement on mechanical and thermal characteristics of polymer nano composites: a review. Mater Today Proc. 2021;44:2125–2130. [Google Scholar]
- 210.Jiang D., Ning F., Wang Y. Additive manufacturing of biodegradable iron-based particle reinforced polylactic acid composite scaffolds for tissue engineering. J Mater Process Technol. 2021;289 [Google Scholar]
- 211.Sakthiabirami K., Kang J.-.H., Jang J.-.G., Soundharrajan V., Lim H.-.P., Yun K.-.D., et al. Hybrid porous zirconia scaffolds fabricated using additive manufacturing for bone tissue engineering applications. Mater Sci Eng C. 2021;123 doi: 10.1016/j.msec.2021.111950. [DOI] [PubMed] [Google Scholar]
- 212.Paredes C., Martínez-Vázquez F.J., Elsayed H., Colombo P., Pajares A., Miranda P. Evaluation of direct light processing for the fabrication of bioactive ceramic scaffolds: effect of pore/strut size on manufacturability and mechanical performance. J Eur Ceram Soc. 2020:13–15. [Google Scholar]
- 213.Zhang B., Li H., Cheng J., Ye H., Sakhaei A.H., Yuan C., et al. Mechanically robust and UV-curable shape-memory polymers for digital light processing based 4D printing. Adv Mater. 2021;33 doi: 10.1002/adma.202101298. [DOI] [PubMed] [Google Scholar]
- 214.Xue Y., Qi L., Niu Y., Huang H., Huang F., Si T., et al. Integration of electrospray and digital light processing for freeform patterning of porous microstructures. Adv Mater Technol. 2020;5:1–10. [Google Scholar]
- 215.Moreno Madrid A.P., Vrech S.M., Sanchez M.A., Rodriguez A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater Sci Eng C. 2019;100:631–644. doi: 10.1016/j.msec.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 216.Song M., Zhou R., Gu J., Wang Z., Ni S., Liu Y. Nitrogen induced heterogeneous structures overcome strength-ductility trade-off in an additively manufactured high-entropy alloy. Appl Mater Today. 2020;18 [Google Scholar]
- 217.DiNoro J.N., Paxton N.C., Skewes J., Yue Z., Lewis P.M., Thompson R.G., et al. Laser sintering approaches for bone tissue engineering. Polymers. 2022;14 doi: 10.3390/polym14122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nouri A., Rohani Shirvan A., Li Y., Wen C. Additive manufacturing of metallic and polymeric load-bearing biomaterials using laser powder bed fusion: a review. J Mater Sci Technol. 2021;94:196–215. [Google Scholar]
- 219.Daikuara L.Y., Chen X., Yue Z., Skropeta D., Wood F.M., Fear M.W., et al. 3D bioprinting constructs to facilitate skin regeneration. Adv Funct Mater. 2022;32 doi: 10.1002/adfm.202105080. [DOI] [Google Scholar]
- 220.Rafiee M., Farahani R.D., Therriault D. Multi-material 3D and 4D printing: a survey. Adv Sci. 2020;7 doi: 10.1002/advs.201902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Han T., Kundu S., Nag A., Xu Y. 3D printed sensors for biomedical applications: a review. Sensors. 2019:19. doi: 10.3390/s19071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nguyen T.T., Kim J. 4D-printing — fused deposition modeling printing and PolyJet printing with shape memory polymers composite. Fibers Polym. 2020;21:2364–2372. [Google Scholar]
- 223.Pavan Kalyan B.G., Kumar L. 3D printing: applications in tissue engineering, medical devices, and drug delivery. AAPS PharmSciTech. 2022;23:92. doi: 10.1208/s12249-022-02242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mahmud Z., Hassan M., Hasan A., Gomes V.G. 3D printed nanocomposites for tailored cardiovascular tissue constructs: a minireview. Materialia. 2021;19 [Google Scholar]
- 225.Andrew J.J., Dhakal H.N. Sustainable biobased composites for advanced applications: recent trends and future opportunities – a critical review. Compos Part C Open Access. 2022;7 [Google Scholar]
- 226.Rashid A.A., Koç M. Fused filament fabrication process: a review of numerical simulation techniques. Polym. 2021;13 doi: 10.3390/polym13203534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Duty C., Ajinjeru C., Kishore V., Compton B., Hmeidat N., Chen X., et al. What makes a material printable? A viscoelastic model for extrusion-based 3D printing of polymers. J Manuf Process. 2018;35:526–537. [Google Scholar]
- 228.Singh S., Singh G., Prakash C., Ramakrishna S. Current status and future directions of fused filament fabrication. J Manuf Process. 2020;55:288–306. [Google Scholar]
- 229.Placone J.K., Engler A.J. Recent advances in extrusion-based 3d printing for biomedical applications. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cisneros-López E.O., Pal A.K., Rodriguez A.U., Wu F., Misra M., Mielewski D.F., et al. Recycled poly(lactic acid)–based 3D printed sustainable biocomposites: a comparative study with injection molding. Mater Today Sustain. 2020;7-8 [Google Scholar]
- 231.Jeantet L., Regazzi A., Taguet A., Pucci M.F., Caro-Bretelle A.S., Quantin J.-.C. Biopolymer blends for mechanical property gradient 3D printed parts. Express Polym Lett. 2021;15:137–152. [Google Scholar]
- 232.Waheed S., Cabot J.M., Macdonald N.P., Lewis T., Guijt R.M., Paull B., et al. 3D printed microfluidic devices: enablers and barriers. Lab Chip. 2016;16:1993–2013. doi: 10.1039/c6lc00284f. [DOI] [PubMed] [Google Scholar]
- 233.Davoodi E., Sarikhani E., Montazerian H., Ahadian S., Costantini M., Swieszkowski W., et al. Extrusion and microfluidic-based bioprinting to fabricate biomimetic tissues and organs. Adv Mater Technol. 2020;5 doi: 10.1002/admt.201901044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hossain N., Chowdhury M.A., Shuvho M.B.A., Kashem M.A., Kchaou M. 3D-printed objects for multipurpose applications. J Mater Eng Perform. 2021;30:4756–4767. doi: 10.1007/s11665-021-05664-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Song G., Zhao H.Q., Liu Q., Fan Z. A review on biodegradable biliary stents: materials and future trends. Bioact Mater. 2022 doi: 10.1016/j.bioactmat.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tortorella S., Greco P., Valle F., Barbalinardo M., Foschi G., Lugli F., et al. Laser Assisted Bioprinting of laminin on biodegradable PLGA substrates: effect on neural stem cell adhesion and differentiation. Bioprinting. 2022;26:e00194. [Google Scholar]
- 237.Park S., Shou W., Makatura L., Matusik W., Fu K., (Kelvin) 3D printing of polymer composites: materials, processes, and applications. Matter. 2022;5:43–76. [Google Scholar]
- 238.Al Rashid A., Ahmed W., Khalid M.Y., Koç M. Vat photopolymerization of polymers and polymer composites: processes and applications. Addit Manuf. 2021;47 [Google Scholar]
- 239.Ng W.L., Lee J.M., Zhou M., Chen Y.-.W., Lee K.-.X.A., Yeong W.Y., et al. Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges. Biofabrication. 2020;12:22001. doi: 10.1088/1758-5090/ab6034. [DOI] [PubMed] [Google Scholar]
- 240.Kumar P., Rajak D.K., Abubakar M., Ali S.G.M., Hussain M. 3D printing technology for biomedical practice: a review. J Mater Eng Perform. 2021;30:5342–5355. [Google Scholar]
- 241.Manapat J.Z., Chen Q., Ye P., Advincula R.C. 3D printing of polymer nanocomposites via stereolithography. Macromol Mater Eng. 2017;302 [Google Scholar]
- 242.Kang J.-.H., Sakthiabirami K., Jang K.-.J., Jang J.-.G., Oh G.-.J., Park C., et al. Mechanical and biological evaluation of lattice structured hydroxyapatite scaffolds produced via stereolithography additive manufacturing. Mater Des. 2022;214 [Google Scholar]
- 243.Bahati D., Bricha M., El Mabrouk K. Vat photopolymerization additive manufacturing technology for bone tissue engineering applications. Adv Eng Mater. 2022 n/a. [Google Scholar]
- 244.Palucci Rosa R., Rosace G. Nanomaterials for 3D printing of polymers via stereolithography: concept, technologies, and applications. Macromol Mater Eng. 2021;306 [Google Scholar]
- 245.Teng C.-.L., Chen J.-.Y., Chang T.-.L., Hsiao S.-.K., Hsieh Y.-.K., Villalobos Gorday K., et al. Design of photocurable, biodegradable scaffolds for liver lobule regeneration via digital light process-additive manufacturing. Biofabrication. 2020;12:35024. doi: 10.1088/1758-5090/ab69da. [DOI] [PubMed] [Google Scholar]
- 246.Singh S., Ramakrishna S., Berto F. 3D printing of polymer composites: a short review. Mater Des Process Commun. 2020;2:e97. [Google Scholar]
- 247.Voet V.S.D., Guit J., Loos K. Sustainable photopolymers in 3D printing: a review on biobased, biodegradable, and recyclable alternatives. Macromol Rapid Commun. 2021;42 doi: 10.1002/marc.202000475. [DOI] [PubMed] [Google Scholar]
- 248.Wu G.-.H., Hsu S. Review: polymeric-based 3d printing for tissue engineering. J Med Biol Eng. 2015;35:285–292. doi: 10.1007/s40846-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang J., Hu Q., Wang S., Tao J., Gou M. Digital light processing based three-dimensional printing for medical applications. Int J Bioprinting. 2019;6:242. doi: 10.18063/ijb.v6i1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Valino A.D., Dizon J.R.C., Espera A.H., Chen Q., Messman J., Advincula R.C. Advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog Polym Sci. 2019;98 [Google Scholar]
- 251.Arif Z.U., Khalid M.Y., Al Rashid A., ur Rehman E., Atif M. Laser deposition of high-entropy alloys: a comprehensive review. Opt Laser Technol. 2022;145 [Google Scholar]
- 252.Tortorici M., Gayer C., Torchio A., Cho S., Schleifenbaum J.H., Petersen A. Inner strut morphology is the key parameter in producing highly porous and mechanically stable poly(ε-caprolactone) scaffolds via selective laser sintering. Mater Sci Eng C. 2021;123 doi: 10.1016/j.msec.2021.111986. [DOI] [PubMed] [Google Scholar]
- 253.Yazdanpanah Z., Johnston J.D., Cooper D.M.L., Chen X. 3D bioprinted scaffolds for bone tissue engineering: state-of-the-art and emerging technologies. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.824156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Patel R., Monticone D., Lu M., Grøndahl L., Huang H. Hydrolytic degradation of porous poly(hydroxybutyrate-co-hydroxyvalerate) scaffolds manufactured using selective laser sintering. Polym Degrad Stab. 2021;187 [Google Scholar]
- 255.Hassan M., Dave K., Chandrawati R., Dehghani F., Gomes V.G. 3D printing of biopolymer nanocomposites for tissue engineering: nanomaterials, processing and structure-function relation. Eur Polym J. 2019;121 [Google Scholar]
- 256.Jiao T., Lian Q., Zhao T., Wang H., Li D. Preparation, mechanical and biological properties of inkjet printed alginate/gelatin hydrogel. J Bionic Eng. 2021;18:574–583. [Google Scholar]
- 257.Ligon S.C., Liska R., Stampfl J., Gurr M., Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev. 2017;117:10212–10290. doi: 10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bisht B., Hope A., Mukherjee A., Paul M.K. Advances in the fabrication of scaffold and 3D printing of biomimetic bone graft. Ann Biomed Eng. 2021;49:1128–1150. doi: 10.1007/s10439-021-02752-9. [DOI] [PubMed] [Google Scholar]
- 259.Wang S., Zhao S., Yu J., Gu Z., Zhang Y. Advances in translational 3D printing for cartilage, bone, and osteochondral tissue engineering. Small. 2022;18 doi: 10.1002/smll.202201869. [DOI] [PubMed] [Google Scholar]
- 260.Kholghi Eshkalak S., Chinnappan A., Jayathilaka W.A.D.M., Khatibzadeh M., Kowsari E., Ramakrishna S. A review on inkjet printing of CNT composites for smart applications. Appl Mater Today. 2017;9:372–386. [Google Scholar]
- 261.Naghieh S., Chen X. Printability–a key issue in extrusion-based bioprinting. J Pharm Anal. 2021;11:564–579. doi: 10.1016/j.jpha.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang S., Bilal M., Zdarta J., Cui J., Kumar A., Franco M., et al. Biopolymers and nanostructured materials to develop pectinases-based immobilized nano-biocatalytic systems for biotechnological applications. Food Res Int. 2021;140 doi: 10.1016/j.foodres.2020.109979. [DOI] [PubMed] [Google Scholar]
- 263.Montero Y., Souza A.G., Oliveira É.R., Rosa D dos S. Nanocellulose functionalized with cinnamon essential oil: a potential application in active biodegradable packaging for strawberry. Sustain Mater Technol. 2021;29:e00289. [Google Scholar]
- 264.Yang G.H., Kim W., Kim J., Kim G. A skeleton muscle model using GelMA-based cell-aligned bioink processed with an electric-field assisted 3D/4D bioprinting. Theranostics. 2021;11:48–63. doi: 10.7150/thno.50794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tong A., Pham Q.L., Abatemarco P., Mathew A., Gupta D., Iyer S., et al. Review of low-cost 3D bioprinters: state of the market and observed future trends. SLAS Technol Transl Life Sci Innov. 2021;26:333–366. doi: 10.1177/24726303211020297. [DOI] [PubMed] [Google Scholar]
- 266.Cano-Vicent A., Tambuwala M.M., Hassan S.S., Barh D., Aljabali A.A.A., Birkett M., et al. Fused deposition modelling: current status, methodology, applications and future prospects. Addit Manuf. 2021;47 doi: 10.1016/J.ADDMA.2021.102378. [DOI] [Google Scholar]
- 267.Bertana V., Catania F., Cocuzza M., Ferrero S., Scaltrito L., Pirri C.F. Medical and biomedical applications of 3D and 4D printed polymer nanocomposites. 3D 4D Print Polym Nanocomposite Mater Process Appl Challenges. 2019:325–366. [Google Scholar]
- 268.Singh S., Prakash C., Ramakrishna S. 3D printing of polyether-ether-ketone for biomedical applications. Eur Polym J. 2019;114:234–248. [Google Scholar]
- 269.Patil N.A., Kandasubramanian B. Functionalized polylysine biomaterials for advanced medical applications: a review. Eur Polym J. 2021;146 [Google Scholar]
- 270.Fazal F., Raghav S., Callanan A., Koutsos V., Radacsi N. Recent advancements in the bioprinting of vascular grafts. Biofabrication. 2021;13:32003. doi: 10.1088/1758-5090/ac0963. [DOI] [PubMed] [Google Scholar]
- 271.Higgins M., Leung S., Radacsi N. 3D printing surgical phantoms and their role in the visualization of medical procedures. Ann 3D Print Med. 2022;6 [Google Scholar]
- 272.Rezvani Ghomi E., Khosravi F., Neisiany R.E., Singh S., Ramakrishna S. Future of additive manufacturing in healthcare. Curr Opin Biomed Eng. 2021;17 [Google Scholar]
- 273.Arif Z.U., Khalid M.Y., Zolfagharian A., Bodaghi M. 4D bioprinting of smart polymers for biomedical applications: recent progress, challenges, and future perspectives. React Funct Polym. 2022 [Google Scholar]
- 274.Khalid M.Y., Arif Z.U., Noroozi R., Zolfagharian A., Bodaghi M. 4D printing of shape memory polymer composites: a review on fabrication techniques, applications, and future perspectives. J Manuf Process. 2022 [Google Scholar]
- 275.Khalid M.Y., Arif Z.U., Ahmed W. 4D printing: technological and manufacturing renaissance. Macromol Mater Eng. 2022 [Google Scholar]
- 276.Khalid M.Y., Arif Z.U., Ahmed W., Umer R., Zolfagharian A., Bodaghi M. 4D printing: technological developments in robotics applications. Sensors Actuators A Phys. 2022 [Google Scholar]
- 277.Bodaghi M., Noroozi R., Zolfagharian A., Fotouhi M., Norouzi S. 4D printing self-morphing structures. Mater. 2019:12. doi: 10.3390/ma12081353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Liu H., Wang F., Wu W., Dong X., Sang L. 4D printing of mechanically robust PLA/TPU/Fe3O4 magneto-responsive shape memory polymers for smart structures. Compos Part B Eng. 2023;248 [Google Scholar]
- 279.Noroozi R., Zolfagharian A., Fotouhi M., Bodaghi M. In: Addit. Manuf. Mater. Technol. Bodaghi M, Zolfagharian ABT-SM, editors. Elsevier; 2022. 7 - 4D-printed shape memory polymer: modeling and fabrication; pp. 195–228. in AM. [Google Scholar]
- 280.Narupai B., Smith P.T., Nelson A. 4D printing of multi-stimuli responsive protein-based hydrogels for autonomous shape transformations. Adv Funct Mater. 2021;31 [Google Scholar]
- 281.Zhang F., Wen N., Wang L., Bai Y., Leng J. Design of 4D printed shape-changing tracheal stent and remote controlling actuation. Int J Smart Nano Mater. 2021:1–15. [Google Scholar]
- 282.Cheng C, Xie H, Xu Z, Li L, Jiang M, Tang L, et al. 4D printing of shape memory aliphatic copolyester via UV-assisted FDM strategy for medical protective devices 2020;396.
- 283.Zhang J., Zhao S., Zhu M., Zhu Y., Zhang Y., Liu Z., et al. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J Mater Chem B. 2014;2:7583–7595. doi: 10.1039/c4tb01063a. [DOI] [PubMed] [Google Scholar]
- 284.Betsch M., Cristian C., Lin Y.-.Y., Blaeser A., Schöneberg J., Vogt M., et al. Incorporating 4D into bioprinting: real-time magnetically directed collagen fiber alignment for generating complex multilayered tissues. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800894. [DOI] [PubMed] [Google Scholar]
- 285.Wen H., Li J., Payne G.F., Feng Q., Liang M., Chen J., et al. Hierarchical patterning via dynamic sacrificial printing of stimuli-responsive hydrogels. Biofabrication. 2020;12:35007. doi: 10.1088/1758-5090/ab7e74. [DOI] [PubMed] [Google Scholar]
- 286.De Santis R., D'Amora U., Russo T., Ronca A., Gloria A., Ambrosio L. 3D fibre deposition and stereolithography techniques for the design of multifunctional nanocomposite magnetic scaffolds. J Mater Sci Mater Med. 2015;26:250. doi: 10.1007/s10856-015-5582-4. [DOI] [PubMed] [Google Scholar]
- 287.Raman R., Cvetkovic C., Uzel S.G.M., Platt R.J., Sengupta P., Kamm R.D., et al. Optogenetic skeletal muscle-powered adaptive biological machines. Proc Natl Acad Sci. 2016;113 doi: 10.1073/pnas.1516139113. 3497 LP –3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kirillova A., Maxson R., Stoychev G., Gomillion C.T., Ionov L. 4D biofabrication using shape-morphing hydrogels. Adv Mater. 2017;29 doi: 10.1002/adma.201703443. [DOI] [PubMed] [Google Scholar]
- 289.Ji Q., Wang X.V., Wang L., Feng L. Customized protective visors enabled by closed loop controlled 4D printing. Sci Rep. 2022;12:7566. doi: 10.1038/s41598-022-11629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mirani B., Pagan E., Currie B., Siddiqui M.A., Hosseinzadeh R., Mostafalu P., et al. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201700718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wei H., Zhang Q., Yao Y., Liu L., Liu Y., Leng J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl Mater Interfaces. 2017;9:876–883. doi: 10.1021/acsami.6b12824. [DOI] [PubMed] [Google Scholar]
- 292.Lin C., Liu L., Liu Y., Leng J. 4D printed programmable shape memory left atrial appendage occlusion device. Proc SPIE. 2022;12041 [Google Scholar]
- 293.Lopez de Armentia S., del Real J.C., Paz E., Dunne N. Advances in biodegradable 3D printed scaffolds with carbon-based nanomaterials for bone regeneration. Mater. 2020;13 doi: 10.3390/ma13225083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sánchez-Salcedo S., Nieto A., Vallet-Regí M. Hydroxyapatite/β-tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem Eng J. 2008;137:62–71. doi: 10.1016/j.cej.2007.09.011. [DOI] [Google Scholar]
- 295.Simorgh S., Alasvand N., Khodadadi M., Ghobadi F., Malekzadeh Kebria M., Brouki Milan P., et al. Additive manufacturing of bioactive glass biomaterials. Methods. 2022 doi: 10.1016/j.ymeth.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 296.Iglesias-Mejuto A., García-González C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater Sci Eng C. 2021;131 doi: 10.1016/j.msec.2021.112525. [DOI] [PubMed] [Google Scholar]
- 297.Abouzeid R.E., Khiari R., Salama A., Diab M., Beneventi D., Dufresne A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int J Biol Macromol. 2020;160:538–547. doi: 10.1016/j.ijbiomac.2020.05.181. [DOI] [PubMed] [Google Scholar]
- 298.Heo D.N., Castro N.J., Lee S.-.J., Noh H., Zhu W., Zhang L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale. 2017;9:5055–5062. doi: 10.1039/c6nr09652b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pant S., Subramanian S., Thomas S., Loganathan S., Valapa R.B. Tailoring of mesoporous bioactive glass composite scaffold via thermal extrusion based 3D bioprinting and scrutiny on bone tissue engineering characteristics. Microporous Mesoporous Mater. 2022;341 [Google Scholar]
- 300.Liu K., Sun J., Zhu Q., Jin X., Zhang Z., Zhao Z., et al. Microstructures and properties of polycaprolactone/tricalcium phosphate scaffolds containing polyethylene glycol fabricated by 3D printing. Ceram Int. 2022 [Google Scholar]
- 301.Du Y., Liu H., Shuang J., Wang J., Ma J., Zhang S. Microsphere-based selective laser sintering for building macroporous bone scaffolds with controlled microstructure and excellent biocompatibility. Colloids Surfaces B Biointerfaces. 2015;135:81–89. doi: 10.1016/j.colsurfb.2015.06.074. [DOI] [PubMed] [Google Scholar]
- 302.Duan B., Wang M., Zhou W.Y., Cheung W.L., Li Z.Y., Lu W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010;6:4495–4505. doi: 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 303.Maleki-Ghaleh H., Hossein Siadati M., Fallah A., Zarrabi A., Afghah F., Koc B., et al. Effect of zinc-doped hydroxyapatite/graphene nanocomposite on the physicochemical properties and osteogenesis differentiation of 3D-printed polycaprolactone scaffolds for bone tissue engineering. Chem Eng J. 2021;426 [Google Scholar]
- 304.Cao C., Huang P., Prasopthum A., Parsons A.J., Ai F., Yang J. Characterisation of bone regeneration in 3D printed ductile PCL/PEG/hydroxyapatite scaffolds with high ceramic microparticle concentrations. Biomater Sci. 2022;10:138–152. doi: 10.1039/d1bm01645h. [DOI] [PubMed] [Google Scholar]
- 305.Song P., Li M., Zhang B., Gui X., Han Y., Wang L., et al. DLP fabricating of precision GelMA/HAp porous composite scaffold for bone tissue engineering application. Compos Part B Eng. 2022;244 [Google Scholar]
- 306.Alam F., Verma P., Mohammad W., Teo J., Varadarajan K.M., Kumar S. Architected poly(lactic acid)/poly(ε-caprolactone)/halloysite nanotube composite scaffolds enabled by 3D printing for biomedical applications. J Mater Sci. 2021;56:14070–14083. [Google Scholar]
- 307.Liu D., Nie W., Li D., Wang W., Zheng L., Zhang J., et al. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem Eng J. 2019;362:269–279. [Google Scholar]
- 308.Luo G., Ma Y., Cui X., Jiang L., Wu M., Hu Y., et al. 13-93 bioactive glass/alginate composite scaffolds 3D printed under mild conditions for bone regeneration. RSC Adv. 2017;7:11880–11889. [Google Scholar]
- 309.Seyedsalehi A., Daneshmandi L., Barajaa M., Riordan J., Laurencin C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci Rep. 2020;10:22210. doi: 10.1038/s41598-020-78977-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Palaganas N.B., Mangadlao J.D., de Leon A.C.C., Palaganas J.O., Pangilinan K.D., Lee Y.J., et al. 3D printing of photocurable cellulose nanocrystal composite for fabrication of complex architectures via stereolithography. ACS Appl Mater Interfaces. 2017;9:34314–34324. doi: 10.1021/acsami.7b09223. [DOI] [PubMed] [Google Scholar]
- 311.Dolati F., Yu Y., Zhang Y., De Jesus AM, Sander E.A., Ozbolat I.T. In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology. 2014;25 doi: 10.1088/0957-4484/25/14/145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ho C.M.B., Mishra A., Lin P.T.P., Ng S.H., Yeong W.Y., Kim Y.-.J., et al. 3D printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromol Biosci. 2017;17 doi: 10.1002/mabi.201600250. [DOI] [PubMed] [Google Scholar]
- 313.Jeong Y.-.J., Jeong S., Kim S., Kim H.J., Jo J., Shanmugasundaram A., et al. 3D-printed cardiovascular polymer scaffold reinforced by functional nanofiber additives for tunable mechanical strength and controlled drug release. Chem Eng J. 2023;454 [Google Scholar]
- 314.Sadeghianmaryan A., Naghieh S., Yazdanpanah Z., Alizadeh Sardroud H., Sharma N.K., Wilson L.D., et al. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Int J Biol Macromol. 2022;204:62–75. doi: 10.1016/j.ijbiomac.2022.01.201. [DOI] [PubMed] [Google Scholar]
- 315.Zineh B.R., Roshangar L., Meshgi S., Shabgard M. 3D printing of alginate/thymoquinone/halloysite nanotube bio-scaffolds for cartilage repairs: experimental and numerical study. Med Biol Eng Comput. 2022;60:3069–3080. doi: 10.1007/s11517-022-02654-5. [DOI] [PubMed] [Google Scholar]
- 316.Baniasadi H., Kimiaei E., Polez R.T., Ajdary R., Rojas O.J., Österberg M., et al. High-resolution 3D printing of xanthan gum/nanocellulose bio-inks. Int J Biol Macromol. 2022;209:2020–2031. doi: 10.1016/j.ijbiomac.2022.04.183. [DOI] [PubMed] [Google Scholar]
- 317.Wu F., Zheng J., Li Z., Liu M. Halloysite nanotubes coated 3D printed PLA pattern for guiding human mesenchymal stem cells (hMSCs) orientation. Chem Eng J. 2019;359:672–683. [Google Scholar]
- 318.Li J., Li L., Zhou J., Zhou Z., Wu X., Wang L., et al. 3D printed dual-functional biomaterial with self-assembly micro-nano surface and enriched nano argentum for antibacterial and bone regeneration. Appl Mater Today. 2019;17:206–215. [Google Scholar]
- 319.Tiskaya M., Shahid S., Gillam D., Hill R. The use of bioactive glass (BAG) in dental composites: a critical review. Dent Mater. 2021;37:296–310. doi: 10.1016/j.dental.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 320.Shi H., Dai Z., Sheng X., Xia D., Shao P., Yang L., et al. Conducting polymer hydrogels as a sustainable platform for advanced energy, biomedical and environmental applications. Sci Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147430. [DOI] [PubMed] [Google Scholar]
- 321.Lopes Nalesso P.R., Wang W., Hou Y., Bagne L., Pereira A.T., Helaehil J.V., et al. In vivo investigation of 3D printed polycaprolactone/graphene electro-active bone scaffolds. Bioprinting. 2021;24:e00164. [Google Scholar]
- 322.Huang B., Vyas C., Byun J.J., El-Newehy M., Huang Z., Bártolo P. Aligned multi-walled carbon nanotubes with nanohydroxyapatite in a 3D printed polycaprolactone scaffold stimulates osteogenic differentiation. Mater Sci Eng C. 2020;108 doi: 10.1016/j.msec.2019.110374. [DOI] [PubMed] [Google Scholar]
- 323.Kaith B.S., Singh A., Sharma A.K., Sud D. Hydrogels: synthesis, classification, properties and potential applications—a brief review. J Polym Environ. 2021;29:3827–3841. [Google Scholar]
- 324.Shokrani H., Shokrani A., Sajadi S.M., Khodadadi Yazdi M., Seidi F., Jouyandeh M., et al. Polysaccharide-based nanocomposites for biomedical applications: a critical review. Nanoscale Horizons. 2022;7:1136–1160. doi: 10.1039/d2nh00214k. [DOI] [PubMed] [Google Scholar]
- 325.Ghilan A., Chiriac A.P., Nita L.E., Rusu A.G., Neamtu I., Chiriac V.M. Trends in 3D printing processes for biomedical field: opportunities and challenges. J Polym Environ. 2020;28:1345–1367. doi: 10.1007/s10924-020-01722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sireesha M., Jagadeesh Babu V., Kranthi Kiran A.S., Ramakrishna S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites. 2018;4:36–57. [Google Scholar]
- 327.Kim H., Jeon H., Shin G., Lee M., Jegal J., Hwang S.Y., et al. Biodegradable nanocomposite of poly(ester-co-carbonate) and cellulose nanocrystals for tough tear-resistant disposable bags. Green Chem. 2021;23:2293–2299. [Google Scholar]
- 328.Lee S.-.J., Zhu W., Nowicki M., Lee G., Heo D.N., Kim J., et al. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J Neural Eng. 2018;15:16018. doi: 10.1088/1741-2552/aa95a5. [DOI] [PubMed] [Google Scholar]
- 329.Guo Z., Poot A.A., Grijpma D.W. Advanced polymer-based composites and structures for biomedical applications. Eur Polym J. 2021;149 [Google Scholar]
- 330.Sheridan M., Winters C., Zamboni F., Collins M.N. Biomaterials: antimicrobial surfaces in biomedical engineering and healthcare. Curr Opin Biomed Eng. 2022;22 doi: 10.1016/j.cobme.2022.100373. [DOI] [Google Scholar]
- 331.Sonatkar J., Kandasubramanian B. Bioactive glass with biocompatible polymers for bone applications. Eur Polym J. 2021;160 [Google Scholar]
- 332.Zhang L., Forgham H., Shen A., Wang J., Zhu J., Huang X., et al. Nanomaterial integrated 3D printing for biomedical applications. J Mater Chem B. 2022;10:7473–7490. doi: 10.1039/d2tb00931e. [DOI] [PubMed] [Google Scholar]
- 333.Mostafavi E., Dubey A.K., Walkowiak B., Kaushik A., Ramakrishna S., Teodori L. Antimicrobial surfaces for implantable cardiovascular devices. Curr Opin Biomed Eng. 2022;23 [Google Scholar]
- 334.Lee S.J., Lee H.-.J., Kim S.-.Y., Seok J.M., Lee J.H., Kim W.D., et al. In situ gold nanoparticle growth on polydopamine-coated 3D-printed scaffolds improves osteogenic differentiation for bone tissue engineering applications: in vitro and in vivo studies. Nanoscale. 2018;10:15447–15453. doi: 10.1039/c8nr04037k. [DOI] [PubMed] [Google Scholar]
- 335.Hasanin M.S., Moustafa G.O. New potential green, bioactive and antimicrobial nanocomposites based on cellulose and amino acid. Int J Biol Macromol. 2020;144:441–448. doi: 10.1016/j.ijbiomac.2019.12.133. [DOI] [PubMed] [Google Scholar]
- 336.Levingstone T.J., Herbaj S., Dunne N.J. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nanomaterials. 2019;9 doi: 10.3390/nano9111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Christy P.N., Basha S.K., Kumari V.S., Bashir A.K.H., Maaza M., Kaviyarasu K., et al. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications – a review. J Drug Deliv Sci Technol. 2020;55 [Google Scholar]
- 338.Mahendiran B., Muthusamy S., Sampath S., Jaisankar S.N., Popat K.C., Selvakumar R., et al. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: a review. Int J Biol Macromol. 2021;183:564–588. doi: 10.1016/j.ijbiomac.2021.04.179. [DOI] [PubMed] [Google Scholar]
- 339.Dukle A., Murugan D., Nathanael A.J., Rangasamy L., Oh T.-.H. Can 3D-printed bioactive glasses be the future of bone tissue engineering? Polymers (Basel) 2022;14 doi: 10.3390/polym14081627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Silva M., Ferreira F.N., Alves N.M., Paiva M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J Nanobiotechnology. 2020;18:23. doi: 10.1186/s12951-019-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Yang D-L, Faraz F., Wang J.-.X., Radacsi N. Combination of 3D printing and electrospinning techniques for biofabrication. Adv Mater Technol. 2022 n/a. [Google Scholar]
- 342.Aráoz B., Karakaya E., González Wusener A., Detsch R., Bizzotto J., Gueron G., et al. 3D printed poly(hydroxybutyrate-co-hydroxyvalerate)—45S5 bioactive glass composite resorbable scaffolds suitable for bone regeneration. J Mater Res. 2021;36:4000–4012. [Google Scholar]
- 343.Wei P., Xu Y., Zhang H., Wang L. Continued sustained insulin-releasing PLGA nanoparticles modified 3D-Printed PCL composite scaffolds for osteochondral repair. Chem Eng J. 2021;422 [Google Scholar]
- 344.Oladapo B.I., Zahedi S.A., Ismail S.O., Olawade D.B. Recent advances in biopolymeric composite materials: future sustainability of bone-implant. Renew Sustain Energy Rev. 2021;150 [Google Scholar]
- 345.Zhu Y., Sun L., Fu X., Liu J., Liang Z., Tan H., et al. Engineering microcapsules to construct vascularized human brain organoids. Chem Eng J. 2021;424 [Google Scholar]
- 346.McGivern S., Boutouil H., Al-Kharusi G., Little S., Dunne N.J., Levingstone T.J. Translational application of 3d bioprinting for cartilage tissue engineering. Bioengineering. 2021;8 doi: 10.3390/bioengineering8100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wojnicz W., Augustyniak M., Borzyszkowski P. Mathematical approach to design 3D scaffolds for the 3D printable bone implant. Biocybern Biomed Eng. 2021;41:667–678. [Google Scholar]
- 348.Huang B., Vyas C., Roberts I., Poutrel Q.-.A., Chiang W.-.H., Blaker J.J., et al. Fabrication and characterisation of 3D printed MWCNT composite porous scaffolds for bone regeneration. Mater Sci Eng C. 2019;98:266–278. doi: 10.1016/j.msec.2018.12.100. [DOI] [PubMed] [Google Scholar]
- 349.Zanini N., Carneiro E., Menezes L., Barud H., Mulinari D. Palm fibers residues from agro-industries as reinforcement in biopolymer filaments for 3D-printed scaffolds. Fibers Polym. 2021;22:2689–2699. [Google Scholar]
- 350.Bochove B Van, Grijpma D.W. Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J Biomater Sci Polym Ed. 2019;30:77–106. doi: 10.1080/09205063.2018.1553105. [DOI] [PubMed] [Google Scholar]
- 351.Bandyopadhyay A., Ghosh S., Boccaccini A.R., Bose S. 3D printing of biomedical materials and devices. J Mater Res. 2021;36:3713–3724. [Google Scholar]
- 352.Sadeghianmaryan A., Naghieh S., Alizadeh Sardroud H., Yazdanpanah Z., Afzal Soltani Y., Sernaglia J., et al. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int J Biol Macromol. 2020;164:3179–3192. doi: 10.1016/j.ijbiomac.2020.08.180. [DOI] [PubMed] [Google Scholar]
- 353.Misra S.K., Ostadhossein F., Babu R., Kus J., Tankasala D., Sutrisno A., et al. 3D-Printed Multidrug-Eluting Stent from Graphene-Nanoplatelet-Doped Biodegradable Polymer Composite. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201700008. [DOI] [PubMed] [Google Scholar]
- 354.Dienel KEG, Bochove B Van, Seppa JV. Additive manufacturing of bioactive poly (trimethylene carbonate)/β -tricalcium phosphate composites for bone regeneration. Biomacromolecules 2020;21:366–375 [DOI] [PubMed]
- 355.Ben D, Tan P. Novel 3D polycaprolactone scaffold for ridge preservation – a pilot randomised controlled clinical trial. Clin. Oral Impl. Res. 2014;26:271–277. doi: 10.1111/clr.12486. [DOI] [PubMed] [Google Scholar]
- 356.Heichel D.L., Tumbic J.A., Boch M.E., Ma A.W.K., Burke K.A. Silk fibroin reactive inks for 3D printing crypt-like structures. Biomed Mater. 2020;15:55037. doi: 10.1088/1748-605X/ab99d4. [DOI] [PubMed] [Google Scholar]
- 357.Kim H., Yang G.H., Choi C.H., Cho Y.S., Kim G. Gelatin/PVA scaffolds fabricated using a 3D-printing process employed with a low-temperature plate for hard tissue regeneration: fabrication and characterizations. Int J Biol Macromol. 2018;120:119–127. doi: 10.1016/j.ijbiomac.2018.07.159. [DOI] [PubMed] [Google Scholar]
- 358.Grémare A., Guduric V., Bareille R., Heroguez V., Latour S., L'heureux N., et al. Characterization of printed PLA scaffolds for bone tissue engineering. J Biomed Mater Res Part A. 2018;106:887–894. doi: 10.1002/jbm.a.36289. [DOI] [PubMed] [Google Scholar]
- 359.Aki D., Ulag S., Unal S., Sengor M., Ekren N., Lin C.-.C., et al. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater Des. 2020;196 [Google Scholar]
- 360.Urtaza Olatz, Gorroñogoitia Izar, Zubiarrain-Laserna Ana, Muiños-López Emma, Granero-Moltó Froilán, Lamo de Espinosa J.M., López-Martínez Tania, Vega Mazo, Manuel M., Prosper Felipe, Zaldua Ane Miren, Anakabe Jon U, G 3D printed bioresorbable scaffolds for articular cartilage tissue engineering: a comparative study between neat Polycaprolactone (PCL) and Poly (lactide-b-ethylene glycol) (PLA-PEG) block copolymer. Biomed Mater. 2022 doi: 10.1088/1748-605X/ac78b7. [DOI] [PubMed] [Google Scholar]
- 361.Li Z., Zhang X., Yuan T., Zhang Y., Luo C., Zhang J., et al. Addition of platelet-rich plasma to silk fibroin hydrogel bioprinting for cartilage regeneration. Tissue Eng Part A. 2020;26:886–895. doi: 10.1089/ten.TEA.2019.0304. [DOI] [PubMed] [Google Scholar]
- 362.Sharma A., Desando G., Petretta M., Chawla S., Bartolotti I., Manferdini C., et al. Investigating the role of sustained calcium release in silk-gelatin-based three-dimensional bioprinted constructs for enhancing the osteogenic differentiation of human bone marrow derived mesenchymal stromal cells. ACS Biomater Sci Eng. 2019;5:1518–1533. doi: 10.1021/acsbiomaterials.8b01631. [DOI] [PubMed] [Google Scholar]
- 363.Lan X., Liang Y., Erkut E.J.N., Kunze M., Mulet-Sierra A., Gong T., et al. Bioprinting of human nasoseptal chondrocytes-laden collagen hydrogel for cartilage tissue engineering. FASEB J. 2021;35:e21191. doi: 10.1096/fj.202002081R. [DOI] [PubMed] [Google Scholar]
- 364.Gu Q., Tomaskovic-Crook E., Lozano R., Chen Y., Kapsa R.M., Zhou Q., et al. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv Healthc Mater. 2016;5:1429–1438. doi: 10.1002/adhm.201600095. [DOI] [PubMed] [Google Scholar]
- 365.Vijayavenkataraman S., Zhang S., Thaharah S., Sriram G., Lu W.F., Fuh J.Y.H. Electrohydrodynamic jet 3D printed nerve guide conduits (NGCs) for peripheral nerve injury repair. Polymers (Basel) 2018;10 doi: 10.3390/polym10070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Yu K.-.F., Lu T.-.Y., Li Y.-.C.E., Teng K.-.C., Chen Y.-.C., Wei Y., et al. Design and synthesis of stem cell-laden keratin/glycol chitosan methacrylate bioinks for 3D bioprinting. Biomacromolecules. 2022;23:2814–2826. doi: 10.1021/acs.biomac.2c00191. [DOI] [PubMed] [Google Scholar]
- 367.Kwak H., Shin S., Lee H., Hyun J. Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing. J Ind Eng Chem. 2019;72:232–240. [Google Scholar]
- 368.Kilic Bektas C., Hasirci V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater Sci. 2020;8:438–449. doi: 10.1039/c9bm01236b. [DOI] [PubMed] [Google Scholar]
- 369.Sharma A., Rawal P., Tripathi D.M., Alodiya D., Sarin S.K., Kaur S., et al. Upgrading hepatic differentiation and functions on 3d printed silk–decellularized liver hybrid scaffolds. ACS Biomater Sci Eng. 2021;7:3861–3873. doi: 10.1021/acsbiomaterials.1c00671. [DOI] [PubMed] [Google Scholar]
- 370.Huang L., Yuan W., Hong Y., Fan S., Yao X., Ren T., et al. 3D printed hydrogels with oxidized cellulose nanofibers and silk fibroin for the proliferation of lung epithelial stem cells. Cellulose. 2021;28:241–257. doi: 10.1007/s10570-020-03526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Alonzo M., Alvarez Primo F., Anil Kumar S., Mudloff J.A., Dominguez E., Fregoso G., et al. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr Opin Biomed Eng. 2021;17 doi: 10.1016/j.cobme.2020.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Abdal-hay A., Raveendran N.T., Fournier B., Ivanovski S. Fabrication of biocompatible and bioabsorbable polycaprolactone/magnesium hydroxide 3D printed scaffolds: degradation and in vitro osteoblasts interactions. Compos Part B Eng. 2020;197 [Google Scholar]
- 373.Zhang B., Chung S.H., Barker S., Craig D., Narayan R.J., Huang J. Direct ink writing of polycaprolactone /polyethylene oxide based 3D constructs. Prog Nat Sci Mater Int. 2021;31:180–191. [Google Scholar]
- 374.McCarthy A., John J.V., Saldana L., Wang H., Lagerstrom M., Chen S., et al. Electrostatic flocking of insulative and biodegradable polymer microfibers for biomedical applications. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202100766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Razali M.S., Khimeche K., Melouki R., Boudjellal A., Vroman I., Alix S., et al. Preparation and properties enhancement of poly(lactic acid)/calcined-seashell biocomposites for 3D printing applications. J Appl Polym Sci. 2022;139:51591. [Google Scholar]
- 376.Jun W, Seob K, Jun H, Lee C, Hwa C, Hee T, et al. Materials science & engineering C rapid development of dual porous poly (lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold sapplications 2020:110–110693. [DOI] [PubMed]
- 377.Shim J, Won J, Sung S, Lim D, Yun W. Comparative efficacies of a 3D-printed PCL /PLGA / β -TCP membrane and a titanium membrane for guided bone regeneration in beagle dogs 2015:2061–77.
- 378.Zhang H., Mao X., Zhao D., Jiang W., Du Z., Li Q. Three dimensional printed polylactic acid-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone : an in vivo bioreactor model. Sci Rep. 2017;7:15255. doi: 10.1038/s41598-017-14923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Yang Y., Chu L., Yang S., Zhang H., Qin L., Guillaume O., et al. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018;79:265–275. doi: 10.1016/j.actbio.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 380.Dutta S.D., Hexiu J., Patel D.K., Ganguly K., Lim K.T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int J Biol Macromol. 2021;167:644–658. doi: 10.1016/j.ijbiomac.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 381.Dubinenko G., Zinoviev A., Bolbasov E., Kozelskaya A., Shesterikov E., Novikov V., et al. Highly filled poly(l-lactic acid)/hydroxyapatite composite for 3D printing of personalized bone tissue engineering scaffolds. J Appl Polym Sci. 2021;138:49662. [Google Scholar]
- 382.e Silva E.P., Huang B., Helaehil J.V., Nalesso P.R.L., Bagne L., de Oliveira M.A., et al. In vivo study of conductive 3D printed PCL/MWCNTs scaffolds with electrical stimulation for bone tissue engineering. Bio-Design Manuf. 2021;4:190–202. [Google Scholar]
- 383.Olate-Moya F., Arens L., Wilhelm M., Mateos-Timoneda M.A., Engel E., Palza H. Chondroinductive alginate-based hydrogels having graphene oxide for 3D printed scaffold fabrication. ACS Appl Mater Interfaces. 2020;12:4343–4357. doi: 10.1021/acsami.9b22062. [DOI] [PubMed] [Google Scholar]
- 384.Lee M., Bae K., Levinson C., Zenobi-Wong M. Nanocomposite bioink exploits dynamic covalent bonds between nanoparticles and polysaccharides for precision bioprinting. Biofabrication. 2020;12:25025. doi: 10.1088/1758-5090/ab782d. [DOI] [PubMed] [Google Scholar]
- 385.Wu Y.L., D'amato A.R., Yan A.M., Wang R.Q., Ding X., Wang Y. Three-dimensional printing of poly(glycerol sebacate) acrylate scaffolds via digital light processing. ACS Appl Bio Mater. 2020 doi: 10.1021/acsabm.0c00804. [DOI] [PubMed] [Google Scholar]
- 386.Paredes C., Martínez-Vázquez F.J., Elsayed H., Colombo P., Pajares A., Miranda P. Using ductile cores for enhancing the mechanical performance of hollow strut β-TCP scaffolds fabricated by digital light processing. Ceram Int. 2021;47:10163–10173. doi: 10.1016/j.ceramint.2020.12.165. [DOI] [Google Scholar]
- 387.Tao J., Zhang J., Du T., Xu X., Deng X., Chen S., et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019;90:49–59. doi: 10.1016/j.actbio.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 388.Hedayati S.K., Behravesh A.H., Hasannia S., Bagheri Saed A., Akhoundi B. 3D printed PCL scaffold reinforced with continuous biodegradable fiber yarn: a study on mechanical and cell viability properties. Polym Test. 2020;83 [Google Scholar]
- 389.Jia H., Gu S.-.Y., Chang K. 3D printed self-expandable vascular stents from biodegradable shape memory polymer. Adv Polym Technol. 2018;37:3222–3228. [Google Scholar]
- 390.Paetzold R., Coulter F.B., Singh G., Kelly D.J., O'Cearbhaill E.D. Fused filament fabrication of polycaprolactone bioscaffolds: influence of fabrication parameters and thermal environment on geometric fidelity and mechanical properties. Bioprinting. 2022;27:e00206. [Google Scholar]
- 391.Mondschein R.J., Kanitkar A., Williams C.B., Verbridge S.S., Long T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials. 2017;140:170–188. doi: 10.1016/j.biomaterials.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 392.Kim J.E., Kim S.H., Jung Y. Current status of three-dimensional printing inks for soft tissue regeneration. Tissue Eng Regen Med. 2016;13:636–646. doi: 10.1007/s13770-016-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Xu Y., Meng Q., Jin X., Liu F., Yu J. Biodegradable scaffolds for urethra tissue engineering based on 3D printing. ACS Appl Bio Mater. 2020;3:2007–2016. doi: 10.1021/acsabm.9b01151. [DOI] [PubMed] [Google Scholar]
- 394.Fazal F., Melchels F.P.W., McCormack A., Silva A.F., Callanan A., Koutsos V., et al. A vertical additive-lathe printing system for the fabrication of tubular constructs using gelatin methacryloyl hydrogel. J Mech Behav Biomed Mater. 2023;139 doi: 10.1016/j.jmbbm.2023.105665. [DOI] [PubMed] [Google Scholar]
- 395.Jammalamadaka U., Tappa K. Recent advances in biomaterials for 3D printing and tissue engineering. J Funct Biomater. 2018;9 doi: 10.3390/jfb9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Praveen S., Kim H.S. High-entropy alloys: potential candidates for high-temperature applications – an overview. Adv Eng Mater. 2018;20 [Google Scholar]
- 397.Liu W., Feng Z., Ou-Yang W., Pan X., Wang X., Huang P., et al. 3D printing of implantable elastic PLCL copolymer scaffolds. Soft Matter. 2020;16:2141–2148. doi: 10.1039/c9sm02396h. [DOI] [PubMed] [Google Scholar]
- 398.Cho W.-.W., Kim B.S., Ahn M., Ryu Y.H., Ha D-H, Kong J.S., et al. Flexible adipose-vascular tissue assembly using combinational 3D printing for volume-stable soft tissue reconstruction. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202001693. [DOI] [PubMed] [Google Scholar]
- 399.Govindharaj M., Roopavath U.K., Rath S.N. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J Clean Prod. 2019;230:412–419. [Google Scholar]
- 400.Ramasamy S., Davoodi P., Vijayavenkataraman S., Teoh J.H., Thamizhchelvan A.M., Robinson K.S., et al. Optimized construction of a full thickness human skin equivalent using 3D bioprinting and a PCL/collagen dermal scaffold. Bioprinting. 2021;21:e00123. [Google Scholar]
- 401.Afghah F., Ullah M., Seyyed Monfared Zanjani J., Akkuş Süt P., Sen O., Emanet M., et al. 3D printing of silver-doped polycaprolactone-poly propylene succinate composite scaffolds for skin tissue engineering. Biomed Mater. 2020 doi: 10.1088/1748-605X/ab7417. [DOI] [PubMed] [Google Scholar]
- 402.Zhang J., Yun S., Karami A., Jing B., Zannettino A., Du Y., et al. 3D printing of a thermosensitive hydrogel for skin tissue engineering: a proof of concept study. Bioprinting. 2020;19:e00089. [Google Scholar]
- 403.Liu K., Yan L., Li R., Song Z., Ding J., Liu B., et al. 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv Sci. 2022;9 doi: 10.1002/advs.202103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Rodríguez-Sánchez D.N., Pinto G.B.A., Cartarozzi L.P., de Oliveira A.L.R., Bovolato A.L.C., de Carvalho M., et al. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res Ther. 2021;12:303. doi: 10.1186/s13287-021-02315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Yoo J., Park J.H., Kwon Y.W., Chung J.J., Choi I.C., Nam J.J., et al. Augmented peripheral nerve regeneration through elastic nerve guidance conduits prepared using a porous PLCL membrane with a 3D printed collagen hydrogel. Biomater Sci. 2020;8:6261–6271. doi: 10.1039/d0bm00847h. [DOI] [PubMed] [Google Scholar]
- 406.Ye W., Li H., Yu K., Xie C., Wang P., Zheng Y., et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater Des. 2020;192 [Google Scholar]
- 407.Zhang L., Zheng T., Wu L., Han Q., Chen S., Kong Y., et al. Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth. Nanotechnol Rev. 2021;10:50–61. [Google Scholar]
- 408.Shin S., Park S., Park M., Jeong E., Na K., Youn H.J., et al. Cellulose nanofibers for the enhancement of printability of low viscosity gelatin derivatives. Bioresour Vol. 2017;12(2) [Google Scholar]
- 409.George M., Aroom K.R., Hawes H.G., Gill B.S., Love J. 3D Printed Surgical Instruments: the Design and Fabrication Process. World J Surg. 2017;41:314–319. doi: 10.1007/s00268-016-3814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Cabrera M.S., Sanders B., Goor O.J.G.M., Driessen-Mol A., Oomens C.W.J., Baaijens F.P.T. Computationally designed 3D printed self-expandable polymer stents with biodegradation capacity for minimally invasive heart valve implantation: a proof-of-concept study. 3D Print Addit Manuf. 2017;4:19–29. doi: 10.1089/3dp.2016.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Puppi D., Chiellini F. Biodegradable polymers for biomedical additive manufacturing. Appl Mater Today. 2020;20 [Google Scholar]
- 412.Sharma D., Mathur V.P., Satapathy B.K. Biodegradable and biocompatible 3D constructs for dental applications: manufacturing options and perspectives. Ann Biomed Eng. 2021;49:2030–2056. doi: 10.1007/s10439-021-02839-3. [DOI] [PubMed] [Google Scholar]
- 413.Markstedt K., Mantas A., Tournier I., Martínez Ávila H., Hägg D., Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 414.Andrianesis K., Tzes A. Development and control of a multifunctional prosthetic hand with shape memory alloy actuators. J Intell Robot Syst. 2015;78:257–289. [Google Scholar]
- 415.Johnson B.N., Lancaster K.Z., Zhen G., He J., Gupta M.K., Kong Y.L., et al. 3D printed anatomical nerve regeneration pathways. Adv Funct Mater. 2015;25:6205–6217. doi: 10.1002/adfm.201501760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Giubilini A., Siqueira G., Clemens F.J., Sciancalepore C., Messori M., Nyström G., et al. 3D-printing nanocellulose-poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biodegradable composites by fused deposition modeling. ACS Sustain Chem Eng. 2020;8:10292–10302. [Google Scholar]
- 417.Lei I.M., Jiang C., Lei C.L., de Rijk S.R., Tam Y.C., Swords C., et al. 3D printed biomimetic cochleae and machine learning co-modelling provides clinical informatics for cochlear implant patients. Nat Commun. 2021;12:6260. doi: 10.1038/s41467-021-26491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Gheorghita R., Anchidin-Norocel L., Filip R., Dimian M., Covasa M. Applications of biopolymers for drugs and probiotics delivery. Polymers (Basel) 2021;13 doi: 10.3390/polym13162729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Syed M.H., Zahari M.A.K.M., Khan M.M.R., Beg M.D.H., Abdullah N. An overview on recent biomedical applications of biopolymers: their role in drug delivery systems and comparison of major systems. J Drug Deliv Sci Technol. 2023;80 [Google Scholar]
- 420.Tappa K, Jammalamadaka U, Ballard DH, Bruno T, Israel R, Vemula H, et al. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study 2017:1–17. [DOI] [PMC free article] [PubMed]
- 421.Durga Prasad Reddy R., Sharma V. Additive manufacturing in drug delivery applications: a review. Int J Pharm. 2020;589 doi: 10.1016/j.ijpharm.2020.119820. [DOI] [PubMed] [Google Scholar]
- 422.Melocchi A., Inverardi N., Uboldi M., Baldi F., Maroni A., Pandini S., et al. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): design concept and 4D printing feasibility. Int J Pharm. 2019;559:299–311. doi: 10.1016/j.ijpharm.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 423.Lee H., Ahn S., Bonassar L.J., Kim G. Cell(MC3T3-E1)-printed poly(ϵ-caprolactone)/alginate hybrid scaffolds for tissue regeneration. Macromol Rapid Commun. 2013;34:142–149. doi: 10.1002/marc.201200524. [DOI] [PubMed] [Google Scholar]
- 424.Morris V.B., Nimbalkar S., Younesi M., McClellan P., Akkus O. Mechanical properties, cytocompatibility and manufacturability of chitosan:PEGDA hybrid-gel scaffolds by stereolithography. Ann Biomed Eng. 2017;45:286–296. doi: 10.1007/s10439-016-1643-1. [DOI] [PubMed] [Google Scholar]
- 425.Xu T., Binder K.W., Albanna M.Z., Dice D., Zhao W., Yoo J.J., et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5:15001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 426.Kumar M., Sharma V. Additive manufacturing techniques for the fabrication of tissue engineering scaffolds: a review. Rapid Prototyp J. 2021;27:1230–1272. [Google Scholar]
- 427.Dong L., Bu Z., Xiong Y., Zhang H., Fang J., Hu H., et al. Facile extrusion 3D printing of gelatine methacrylate/Laponite nanocomposite hydrogel with high concentration nanoclay for bone tissue regeneration. Int J Biol Macromol. 2021;188:72–81. doi: 10.1016/j.ijbiomac.2021.07.199. [DOI] [PubMed] [Google Scholar]
- 428.Jin S., Xia X., Huang J., Yuan C., Zuo Y., Li Y., et al. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127:56–79. doi: 10.1016/j.actbio.2021.03.067. [DOI] [PubMed] [Google Scholar]
- 429.Wee C.Y., Yang Z., Thian E.S. Past, present and future development of microspheres for bone tissue regeneration: a review. Mater Technol. 2021;36:364–374. [Google Scholar]
- 430.Jariwala S.H., Lewis G.S., Bushman Z.J., Adair J.H., Donahue H.J. 3D printing of personalized artificial bone scaffolds. 3D Print Addit Manuf. 2015;2:56–64. doi: 10.1089/3dp.2015.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Mahmud M.A.P., Tat T., Xiao X., Adhikary P., Chen J. Advances in 4D-printed physiological monitoring sensors. Exploration. 2021;1 doi: 10.1002/EXP.20210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Arif Z.U., Khalid M.Y., Ahmed W., Arshad H. A review on four-dimensional bioprinting in pursuit of advanced tissue engineering applications. Bioprinting. 2022:e00203. [Google Scholar]
- 433.Mehrali M., Bagherifard S., Akbari M., Thakur A., Mirani B., Mehrali M., et al. Blending electronics with the human body: a pathway toward a cybernetic future. Adv Sci. 2018;5 doi: 10.1002/advs.201700931. [DOI] [PMC free article] [PubMed] [Google Scholar]