Abstract
Water is essential for the survival of humans, animals and plants. Numerous research has been conducted on the prevalence and antibiotic resistance of Escherichia coli (E. coli) in water from various African countries, however, there is lack of comprehensive analysis of published literature. We conducted a systematic review and meta-analysis following the PRISMA guidelines where articles published in English language between January 2000 and March 2022 were searched from ScienceDirect, PubMed, Google Scholar, Scopus, African Journal Online (AJO), and Africa Index Medicus (AIM). Comprehensive Meta-Analysis (CMA) Ver 3.0 software was used to analyze the data. The pooled prevalence estimate (PPE) with 95% confidence interval was calculated using the random-effects model (CI). The overall PPE and antimicrobial resistance trends of E. coli isolated from water was screened from 4009 isolates which were isolated from 2586 samples. We extracted data from 17 studies including drinking water (n = 6), rivers (n = 5), wastewaters (n = 4) and wastewater/river (n = 1) which are all covering 27 countries in Africa with 3438 isolates. The PPE of E. coli in water was 71.7% (0.717; 95% CI: 0.562–0.833). The highest PPE antibiotic resistance was against penicillin followed by erythromycin, and ampicilin with resistance rates of 93.4%, 92.3%, and 69.4%, respectively. This systematic review provides critical evidence of E. coli consolidated prevalence and antibiotic resistance profiles, as well as regions where future studies and enhanced reporting could be beneficial in the African continent.
Keywords: Escherichia coli, Systematic review, meta-Analysis, Antibiotic resistance, Water, Africa
1. Introduction
Water is necessary for all living organisms and is a basic human right (WHO/UNICEF, 2005). Some of the most serious health dangers are produced by microorganisms such as bacteria which can live, reproduce, and spread in water systems [1]. Unfortunately, neither wastewater nor drinking water treatment techniques completely remove antibiotic-resistant bacteria (ARB) [2,3]. Enteric bacterial pathogens such as E. coli isolated from water sources are regarded as a major public health danger to consumers [4].
Escherichia coli (E. coli) is an anaerobic Gram-negative, rod-shaped bacterium that belongs to the Enterobacteriaceae family found in the human guts, warm-blooded animals, cold-blooded animals, as well as in different environments [5,6]. It can further be found in the environment such as in water [7]. Amongst the strains are diverse pathogens that can cause variety of diseases and a majority of them are difficult to treat [8]. Some of these strains are also a primary cause of foodborne outbreaks in people as well as animals [9]. Human diarrhoeal outbreaks and other dangerous waterborne diseases are caused by diarrhoeagenic Escherichia coli (DEC) strains [10,11]. Five types of DEC reported are Enterohaemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), and enteroinvasive E. coli (EIEC) [11,12].
Expression of antibiotic resistance genes (ARGs) in bacteria is becoming a major problem to public health as a result of developing resistance to routinely used antimicrobial agents [13]. The ARGs in Enterobacteriaceae are a major public health concern, particularly in underdeveloped countries [14]. Antibiotic resistance is thought to be spread by wastewater and wastewater treatment plants amongst other sources [3]. Drug resistance is characterized as intrinsic if it exists prior to therapy and acquired if it occurs during treatment [13, 15]. Antibiotic usage in human and veterinary medicine is common, however incorrect use such as underdosing as well as residues in the environment contributes to the global rise in antimicrobial resistance. Interaction of animals, humans and environment contribute to the fast spread of antimicrobial resistance in surface and subsurface waters, either directly or indirectly [16]. The recent findings by Sonola et al. [17] reported high antibiotic resistance levels of E. coli isolates from animals, humans and the environment. A range of acquired virulence genes increase the pathogenicity of E. coli strains [8] and if this is linked to resistance genes then treatment of infections is jeopordised.
Methods for synthesizing research information such as systematic reviews and meta-analyses, are routinely employed in numerous areas to formally evaluate intervention studies [[18], [19], [20]]. There are numereous studies conducted in Africa that showed pooled prevalence of E. coli in foods of animal origin [21], antibiotic resistance in food animals in Africa [22] as well as in under-five year old children with diarrhea [23]. In the last decade several systematic reviews and meta-analyses have been perfomed in Africa on antibiotic ressistence to E. coli in humans [[24], [25], [26]] animals and humans [27], as well as in food animals [22]. However, there is no data on the pooled prevalence and antibiotic resistance of E. coli isolates from water in Africa. Thus, this study used a systematic review and meta-analysis as a step-by-step approach to analyze and summarize the pooled prevalence and antibiotic resistance profiles of E. coli isolated from different water sources using published data in the African continent.
2. Methods
2.1. Study design and systematic review protocol
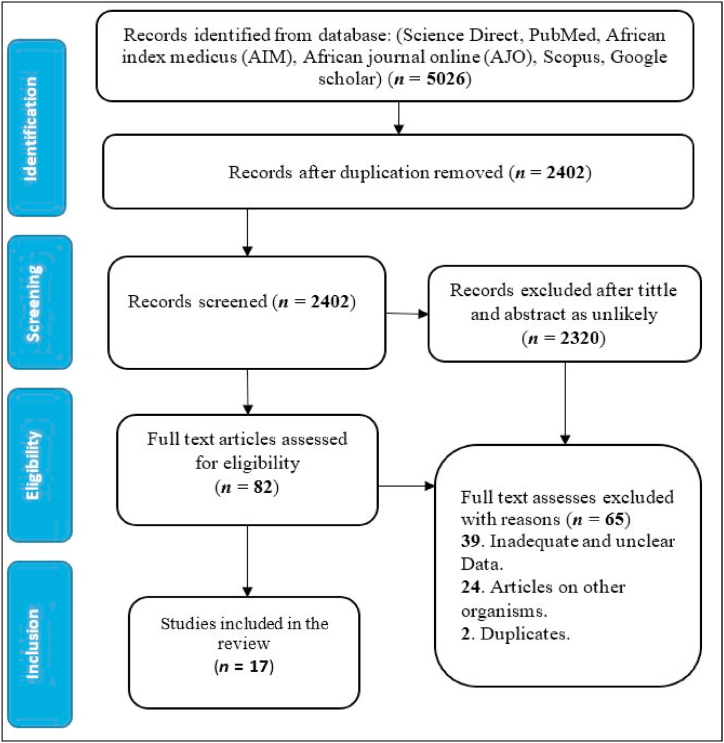
Using published literature, this study was conducted to determine the prevalence of E. coli isolates from water in Africa. This systematic review was performed following the Systematic Reviews and Meta-analyses (PRISMA) standards [28]. The article search approach is presented on a flow chart in Fig. 1.
Fig. 1.
The PRISMA flow diagram for the selection of articles.
2.2. Search strategy for relevant studies
A comprehensive systematic literature search from databases: ScienceDirect (https://www.sciencedirect.com/from February 16, 2022 to February 17, 2022); PubMed (https://pubmed.ncbi.nlm.nih.gov/, from February 19, 2022); Google scholar (https://scholar.google.com/from February 25, 2021 to March 02, 2022); Africa Index Medicus (AIM) (https://indexmedicus.afro.who.int/, March 12, 2022), Scopus (https://www.scopus.com/, March 14, 2022 to March 16, 2022), and African Journal Online (AJO) (https://www.ajol.info/index.php/ajol/, March 19, 2022) were accessed using the following search keywords: E. coli OR water OR river OR dam OR seawater OR sewage OR wastewater OR canal OR ocean OT tap OR borehole OR groundwater OR Africa OR congo OR coted'ivoire OR ivory coast OR democratic republic of the congo OR zaire OR djibouti OR egypt OR malawi OR mali OR mauritania OR mauritius OR mayotte OR morocc) OR mozambique OR algeria OR angola OR benin OR botswana OR burkina faso OR burundi OR cameroon OR cape verde OR central african republic OR chad OR comoros OR equatorial guinea OR eritrea OR ethiopia OR gabon OR gambia OR ghana OR guinea OR guinea-bissau OR kenya OR lesotho OR liberia OR libya OR madagascar OR namibia OR niger OR nigeria OR reunion OR rwanda OR saint helena OR sao tome and principe OR senegal OR seychelles OR sierra leone OR somalia OR south africa OR south sudan OR sudan OR swaziland OR tanzania OR togo OR tunisia OR uganda OR zambia OR zimbabwe. Following the search process, suitable journal article titles and abstracts were scanned and downloaded. The last search took place on March 16, 2022.
2.3. Selection process and data extraction
Reports found through electronic searches were first reviewed for eligibility by two authors (TR, KL) independent reviewers using titles and abstracts to make a preliminary selection of reports potentially fulfilling the selection criteria. A third reviewer (OT) was ready to give a definitive judgement on any outstanding concerns if there were any disagreements during the review process. Following a comprehensive analysis, the following information was extracted and summarized from each article: first author's last name, year of publication, country, continent, total analysed samples, sample source, detection technique, volume of water used for analysis, and number of positive samples. If the number of positive E. coli isolates identified exceeds the sample size due to culturing, the number was recorded at 100% prevalence.
2.4. Quality assessment of included studies
The Joanna Briggs Institute (JBI) Critical Appraisal checklist for prevalence studies 2007 for studies including prevalence data was used to assess the quality of each article included in the study [29]. After evaluating each study against these criteria, studies with a score of 5 or higher were included. Two writers independently assessed the quality of each study (T.R and K.L). Discussion with the third independent reviewer resolved the discrepancy (O.T). This JBI instrument consists of nine criteria, of which details are available (Supplementary Table S1).
2.5. Inclusion criteria
The following criteria were used to select the studies for inclusion in the meta-analysis: 1) Did the study report the proportion of water collected from river, dam, sewage, wastewater, canal, ocean containing E. coli? 2) Did it report on antibiotic profile in Africa as well? 3) Did the article clearly report on the isolation of E. coli in water samples by culture or detection via molecular methods? 4) Is the journal article published in English language? 5) Study reporting sample size? 6) Did it report the number of isolates as well? 7) The availability of the full texts, and its reported primary data; 8) Journal articles published between January 2000 and March 2022.
2.6. Exclusion criteria
-
1)
Studies with unclear sample information [no number of samples screened, no number of isolates, no antibiotic resistant] were excluded from this review; 2) Studies not conducted in Africa were omitted; 3) Additionally, studies not written in English, not peer-reviewed, and were published before 2000.
2.7. Meta-analysis
To assess the relative risk, we included articles reporting the prevalence and antibiotic resistance in this meta-analysis. Studies were grouped on the basis of country, the source, years, detection methods, and antibiotic resistance. All statistical analyses were carried out using comprehensive meta-analysis (CMA) Version 3.0 b y Biostat (Englewood, NJ, USA). The 95% confidence interval (CI) and weighted pooled prevalence estimate (PPE) were calculated. The data generated was visualized using forest plots. The Cochrane Q test was used to calculate Cochran's heterogeneity (Q) among the included studies, as well as the percentage inverse variation (I2). If I2 was ≤25%, 50% or ≥75%, then heterogeneity was classified as low, moderate, or high, respectively. The publication bias was assessed using funnel plots [30] with ocular examination and the Begg and Mazumdar rank correlation test [31]. All pooled estimates were arrived at using a random-effects model. Heterogeneity with a value less than 0.05 was considered as statistically significant.
2.8. Countries from which published studies were conducted
The following is the country from which the articles originated: seven articles from South Africa [[32], [33], [34], [35], [36], [37], [38]], three articles from each of Ethiopia [[39], [40], [41]] and Nigeria [14,42,43] were added. One article from each of Tanzania [44], Morocco [45], Kenya [46], and Ghana [47] was included.
3. Results
3.1. Descriptive results of eligible studies
This review covered studies from seven African countries namely; South Africa, Ethiopia, Nigeria, Tanzania, Morocco, Kenya, and Ghana. All the articles included in this study were peer-reviewed and published between January 2000 until March 16, 2022. A total of 5026 studies were initially identified across ScienceDirect, PubMed, Google scholar, Scopus, African Journal Online (AJO), and Africa Index Medicus (AMI) databases. There were 2402 papers available for title and abstract screening after duplicate articles were removed. Eighty-two (n = 82) articles out of 2402 met the eligibility criteria for full-text review, and sixty five (n = 65) were eligible for inclusion after full text review. Ultimately, seventeen (n = 17) articles from 7 countries that reported the prevalence and antibiotic resistance of E. coli from water were included in this review. Majority of the studies were conducted in South Africa (n = 7), Ethiopia (n = 3), and Nigeria (n = 3).
Out of the seventeen (n = 17) eligible peer-reviewed studies, 6 were conducted from drinking water [14,38,[43], [44], [45],47] and included a total of 1383 samples, 5 were from rivers [32,33,35,40,46] and included 796 samples, 4 studies were from wastewater [34,39,41,42] and included 234 samples, whereas one study included 66 samples collected from both wastewater and river [37]. On the other hand one study included 107 samples collected from a beach and a canal [36]. For each study, the number of samples ranged from 33 to 520. The prevalence amongst the overall studies ranged between 56.2% and 83.3%. Culture and biochemical diagnostic techniques, polymerase chain reaction (PCR) and Colilert-18/Quanti-Tray were utilized to isolate and identify bacterial species from the eligible investigations. A total of 10/17 (58.8%) studies used culture-based and biochemical tests for isolation and identification of E. coli, meanwhile six (6/17, 35.3%) studies only used PCR for E. coli identification. Lastly, one (5.9%) study used Colilert-18/Quanti-Tray for E. coli identification.
3.2. Subgroup of analyses
3.2.1. Source of E. coli
The subgroup analysis based on source indicated that the highest PPE of E. coli was in wastewater 84.5% (0.845; 95% CI: 0.421–0.976, I2 = 93.5, p < 0.248) followed by rivers 70.5% (0.705; 95% CI: 0.288–0.934, I2 = 98.0, p < 0.071). In contrast, the lowest PPE of E. coli was observed in drinking water 61.9% (0.619; 95% CI: 0.377–0.813, I2 = 97.4, p < 0.174). The wastewater/river and beach/canal were not included in meta-analysis because of low number of studies (Table 1).
Table 1.
Pooled prevalence of E. coli from water, screening methods, study year and sampling sites.
| Risk factors | Number of studies | Pooled estimates |
Measure of heterogeneity |
Publication bias |
||||
|---|---|---|---|---|---|---|---|---|
| Samplesize | Number of isolates | I2 (95%CI) | Q Value | I2 | Q-P | Begg and Mazumdar rank P-value | ||
| Overall study | 17 | 2586 | 3438 | 71.7 (56.2–83.3) | 504.160 | 96.826 | 0.007 | 0.217 |
| River | 5 | 796 | 433 | 70.5 (28.8–93.4) | 204.337 | 98.042 | 0.336 | 0.071 |
| Wastewater | 4 | 234 | 168 | 84.5 (42.1–97.6) | 46.622 | 93.565 | 0.099 | 0.248 |
| Drinking water | 6 | 1383 | 647 | 61.9 (37.7–81.3) | 195.977 | 97.449 | 0.336 | 0.174 |
| Wastewater/river | 1 | 66 | 66 | – | – | – | – | – |
| Beach/canal | 1 | 107 | 73 | – | – | – | – | – |
| Study year | ||||||||
| 2000–2010 | 1 | 33 | 21 | – | – | – | – | – |
| 2010–2021 | 16 | 2553 | 3988 | 72.4 (56.4–84.2) | 496.859 | 96.981 | 0.008 | 0.343 |
| Diagnostic technique | ||||||||
| PCR | 6 | 629 | 576 | 95.3 (84.2–98.7) | 39.842 | 87.450 | 0.000 | 0.287 |
| Culture and | 10 | 1777 | 656 | 44.3 (28.9–60.9) | 251.565 | 96.422 | 0.505 | 0.076 |
| Biochemical test | ||||||||
| Colilert-18/Quanti-Tray | 1 | 180 | 165 | – | – | – | – | – |
| Countries | ||||||||
| South Africa | 7 | 693 | 934 | 94.0 (82.9–98.1) | 56.407 | 89.363 | 0.440 | |
| Ethopia | 3 | 220 | 187 | 45.0 (16.5–77.3) | 18.157 | 88.985 | 0.784 | 0.301 |
| Nigeria | 3 | 528 | 300 | 59.5 (39.9–76.5) | 34.516 | 94.206 | 0.341 | 0.301 |
| Tanzania | 1 | 155 | 155 | – | ||||
| Morocco | 1 | 152 | 48 | – | – | – | – | – |
| Kenya | 1 | 318 | 53 | – | – | – | – | – |
| Ghana | 1 | 520 | 97 | – | – | – | – | – |
| Antibiotic test methods | ||||||||
| DDA | 11 | 1997 | 881 | 58.6 (39.5–75.4) | 421.833 | 97.629 | 0.883 | 0.218 |
| MIC | 5 | 495 | 483 | 98.6 (79.1–99.9) | 36.470 | 89.032 | 0.004 | 0.312 |
| VITEK® 2 AST card | 1 | 94 | 23 | – | – | – | – | – |
MIC: Minimal inhibitory concentration; DDM: Disk Diffusion Assay; PCR: Polymerase Chain Reaction.
3.2.2. Study years
A subgroup analysis based on the years showed that the highest PPE of E. coli appeared in period 2010 to 2021 with 72.4% (0.724; 95% CI: 0.564–0.842, I2 = 96.9, p < 0.343). One study was conducted between 2000 and 2010 (Table 1), hence, meta-analysis was not conducted.
3.2.3. Countries where E. coli has been reported
A subgroup analysis was conducted based on the country of origin (Table 1). The highest PPE of E. coli was found in South Africa with 94.0% (0.940; 95% CI: 0.829–0.981, I2 = 89.3, p < 0.440), followed by Nigeria 59.5% (0.595; 95% CI: 0.399–0.765, I2 = 94.2, p < 0.301) and Ethiopia with PPE of 45.0% (0.450; 95% CI: 0.165–0.773, I2 = 88.9, p < 0.301). Tanzania, Morocco, Kenya, and Ghana were not included in the meta-analysis due to few numbers of studies conducted (one study per country).
3.2.4. Diagnostic methods
Articles with the highest PPE of E. coli are those that used PCR 95.3% (0.953; 95% CI: 0.842–0.987 p < 0.287), with a very high level of high heterogeneity (I2 = 98%). The PPE for culture and biochemical tests was 44.3% (0.443; 95% CI: 0.289–0.609, I2 = 96.4, p < 0.076). Colilert-18/Quanti-Tray was not included on meta-analyses due to insufficient studies conducted.
3.2.5. Antibiotic resistance detection methods
The PPE of the minimum inhibitory concentration (MIC) was 98.6% (0.986; 95% CI: 0.971–0.999, I2 = 89.0, p < 0.312), and 58.6% (0.586; 95% CI: 0.395–0.754, p < 0.218) for disk diffusion assays (DDA), with a high level of heterogeneity (I2 = 97.6%) (Table 1).
3.2.6. Antibiotic resistance
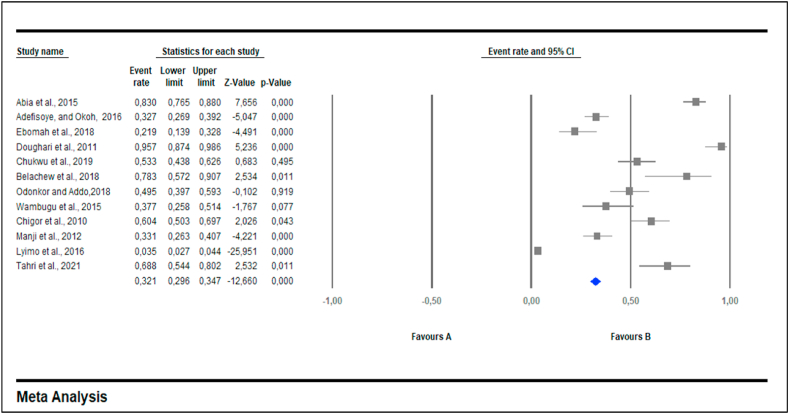
Antibiotic resistance subgroup study revealed that the highest PPE of antibiotic resistance was against penicillin 93.4% (0.934; 95% CI: 0.258–99.8, I2 = 95.6%) followed by erythromycin 92.3% (0.923; 95% CI: 0.130–99.9, I2 = 0.951%), ampicillin 69.4% (0.694; 95% CI: 0.485–84.5, I2 = 0.984%), cefaxitin 44.1% (0.441; 95% CI: 31.1–58.0, I2 = 70.9%), tetracycline 40.2% (0.402; 95% CI: 0.194–0.652, I2 = 98.3%), amoxycillin-clavulanic acid 40.1% (0.401; 95% CI: 0.020–0.641, I2 = 98.2%), nitrofurantoin 33.5% (0.335; 95% CI; 7.5–75.7, I2 = 97.2%), cephalothin 33.1% (0.331; 95% CI; 0.060–0.794, I2 = 96.7%), cefuroxime 30.5% (0.305; 95% CI; 0.680–0.725, I2 = 96.2%), cefotaxime 25.3% (0.253; 95% CI; 0.062–0.633, I2 = 96.6%), nalidixic acid 22.9% (0.229; 95% CI; 0.114–0.407, I2 = 95.1%), ceftazidime 21.8% (0.218; 95% CI; 0.150–0.834, I2 = 97.5%), amikacin 15.9% (0.159; 95% CI; 0.032–0.516, I2 = 97.8%), gentamycin 15.4% (0.154; 95% CI; 0.054–0.367, I2 = 96.6%), ciprofloxacin 13.1% (0.131; 95% CI: 0.580–0.271, I2 = 96.3%), chloramphenicol 8.2% (0.082; 95% CI; 0.039–0.163, I2 = 84.6%), and streptomycine 9.4% (0.940; 95% CI; 0.030–0.259, I2 = 96.9%).
3.2.7. Multidrug resistance
Twelve out of 17 (70.6%) published articles reported detection of multidrug resistant (MDR) E. coli isolates. A total of 642 isolates had a PPE of 25.3% (CI = 6.2–63.3%) and were resistant to more than three antimicrobial agents [multidrug resistance] (Table 2). Subgroup analysis performed based on MDR had a PPE of 50.7% (0.507; 95% CI; 0.286–0.726, I2 = 98.3%) (Table 2). Fig. 2 shows a funnel plot with asymmetric distribution of MDR studies conducted in different water sources. Antibiotics such as cefuroxime, colistin sulfate, nitrofurantoin, norfloxacin, polymyxin B, aztreonam, sulphomethaxazole-trimethoprim, cotrimoxazole, amoxycillin and rifampicin were not included in meta-analysis due to low number of studies (less than 3 studies).
Table 2.
Pooled prevalence rate and 95% CI of antibiotic resistance of E. coli based on meta-analysis.
| Subgroup (Antibiotics) | Number of studies | Number of Ioslates | Prevalence % (95%CI) | I2 (%) | P-value |
|---|---|---|---|---|---|
| Ampicillin | 15 | 1186 | 0.694 (0.485–0.845) | 98.451 | 0.402 |
| Streptomycin | 7 | 122 | 0.094 (0.030–0.259) | 96.984 | 0.440 |
| Ceftazidime | 3 | 132 | 0.218 (0.015–0.834) | 97.553 | 0.301 |
| Cephalothin | 4 | 63 | 0.331 (0.060–0.794) | 96.735 | 0.500 |
| Penicillin | 3 | 349 | 0.934 (0.258–0.998) | 95.633 | 0.059 |
| Tetracycline | 12 | 500 | 0.402 (0.194–0.652) | 98.393 | 0.392 |
| Ciprofloxacin | 13 | 223 | 0.131 (0.058–0.271) | 96.360 | 0.232 |
| Cefuroxime | 3 | 101 | 0.305 (0.068–0.725) | 96.203 | 0.301 |
| Gentamicin | 9 | 204 | 0.154 (0.054–0.367) | 96.631 | 0.149 |
| Chloramphenicol | 7 | 83 | 0.082 (0.039–0.163) | 84.653 | 0.147 |
| Erythromycin | 3 | 168 | 0.923 (0.130–0.999) | 95.101 | 0.301 |
| Cefotaxime | 5 | 92 | 0.253 (0.062–0.633) | 96.679 | 0.164 |
| Cefaxitin | 4 | 88 | 0.441 (0.311–0.580) | 70.947 | 0.248 |
| Amikacin | 6 | 168 | 0.159 (0.032–0.516) | 97.865 | 0.425 |
| Nalidixic acid | 7 | 231 | 0.229 (0.114–0.407) | 95.158 | 0.325 |
| Amoxycillin-clavulanic acid | 6 | 213 | 0.402 (0.202–0.641) | 96.296 | 0.019 |
| Nitrofurantoin | 3 | 145 | 0.335 (0.075–0.757) | 97.239 | 0.301 |
| MDR | 12 | 642 | 0.507 (0.286–0.726) | 98.390 | 0.027 |
MDR: Multidrug resistant.
Fig. 2.
Forest plot showing MDR prevalence in E. coli from Africa between 2000 and 2021. Random effects model: I squared = 98.390; tau = 0.424; Q value = 683.202; df = 10. The diamond at the base indicates the pooled estimates from the overall studies.
The following antibiotic-resistance genes: atrA [34], aadA [32,34], tetA [[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]], tetB [34,44], tet D, tetK, tetM, blaTEM, cmlA1, catI, tetC [34], blaTEM-1 [44], blaDHA, blaCMY [38], blaCTX-M [38,44], blaSHV-1 [38,44] have been reported by some studies included in this review.
3.3. Publication bias
Begg and Mazumdar Rank Correlation Test: For almost all parameters, the Begg and Mazumdar rank correlation test revealed no substantial publication bias. The Kendall's tau b is 0.13333, with a one-tailed p-value of 0.22921 or a two-tailed p-value of 0.45841. This value compares the effect size and variance with the tau value and the value closes to 1, correlates to signify the publication bias.
Egger's Test of the Intercept: Egger's regression test was used to confirm the presence of publication bias. In this case the intercept (B0) is 5.11412, 95% CI (−0.88949–9.3874), with t = 2.58023, df = 15. The 1-tailed p-value is 0.01045, and the 2-tailed p-value is 0.02091.
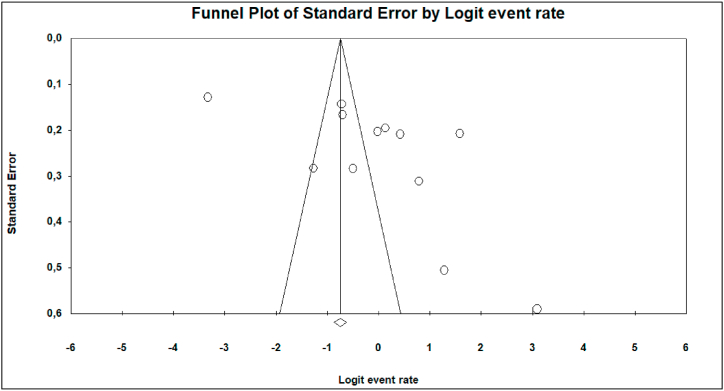
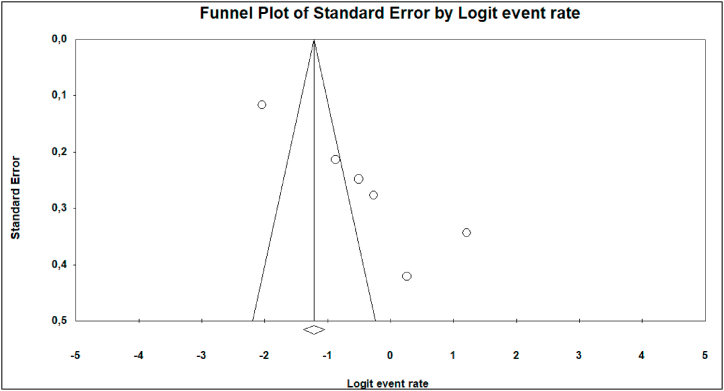
The Funnel plot, as well as Egger's linear regression test, revealed publication bias from a few subgroup (Antibiotic resistance) analyses; Amoxilline-clavulanic acid (Fig. 3, Z = −14.33, p = 0.019), and MDR (Fig. 4, Z = −12.66, p = 0.027).
Fig. 3.
Funnel plot of Amoxillin-clavulanic acid studies included in the meta-analysis.
Fig. 4.
Funnel plot of studies including the MDR studies in our meta-analysis.
4. Discussion
South Africa (41.2%), Ethiopia (17.6%), and Nigeria (17.6%) had higher number of studies owing to their higher socioeconomic status in the region and thus their ability to invest well for research and facilities. However, Tanzania, Morocco, Kenya and Ghana had on average fewer studies (one study for each). In this meta-analysis, the region representativeness was low, as a result, we did not have any regional sub-categories to analyze.
The number of studies published between 2010 and 2021 was significantly higher [72.4% (56.4–84.2), p = 0.343] than the previous decade. This could be due to the availability of new and sophisticated detection methods in recent years. Furthermore, experts are becoming more conscious of the threat of E. coli in water.
The data obtained from 17 published studies showed an overall PPE of 71.7% for E. coli in water which is higher than the findings conducted in review studies conducted in Ethiopia which reported the PPE of E. coli at 15% foods of animal origin, and 25% from human [21,23]. However, this is lower in coparison to similar systematic review and meta-analysis conducted in China where 84.6% were from humans [48]. In the current study, the E. coli isolates were more prevalent among samples from drinking water and rivers as compared to samples from wastewater, beach/canal and wastewater/river.
The number and quality of studies has increased in recent years as a result of the use of latest diagnostic tools such as molecular methods particularly PCR [21]. Culture-based approaches were utilized in about 58.8% of the articles included in this review. Culturing and plating are traditional microbiological methods which are considered as gold standards since they efficiently enable the identification of several bacterial species [[49], [50], [51]]. Using molecular approach such as PCR to identify bacterial infection has also been found to be more effective than traditional culture-based methods [49,52]. In this analysis, we discovered that PCR was also used to detect E. coli in about 6 studies detecting prevalence of 95.3% with over 629 samples tested.
When considering the ARGs detected in the studies included in this meta-analysis, it was observed that multidrug resistance was potentially due to efflux pump systems. Multidrug efflux systems are the most common mechanism of bacterial resistance to antimicrobial drugs [53,54]. Efflux pumps have been linked as one of the mechanism responsible to increase antibiotic resistance in bacteria biofilm structures as they allow faster diffusion rate of antibiotics [55]. Antibiotics' extensive usage, particularly those with a broad spectrum of activity, encourages microorganisms to develop specialized drug defence methods [53,56]. Antibiotic resistance has evolved quickly in recent decades to become one of the most serious public health issues of the twenty-first century [53].
There is inconsistancy between the phenotype and genotype traits from antibitotic resistant isolates. Some isolates are phenotypically resistant without detection of antimicrobial resistance (AMR) genes, whilst others can be phenotypically susceptible and express resistance genes [6]. This could mean that phenotypic resistance to an antibiotic could be due intrinsic factors and not necessary due to triggered expression/mutation of a specific gene. On the other hand the presence of ARGs that does not relate to a resistance phenotype could mean that the ARG is not expressed in the specific isolate. These observations are not uncommon [57,58].
There is now substantial evidence that excessive and incorrect drug administration to farm animals without knowledge of the consequences leads to an increase in antibiotic-resistant bacteria [59]. AMR among E. coli may be caused by intrinsic and acquired resistance mechanisms [60]. Resistance genes can be acquired by E. coli strains mostly by horizontal gene transfer which is a key mechanism for the fast spread of antibiotic resistance genes among gram-negative bacteria (GNB) [61,62]. Providing accurate picture of E. coli drug-resistance patterns across Africa can help to limit the spread of antibiotic resistance. This meta-analysis investigated the incidence of antibiotic resistance in E. coli isolated from water samples from the year 2000–2021.
In the current review, the prevalence of ciprofloxacin-resistant E. coli bacteria isolated from water samples was found to be 13.1%, which is consistent with previous systematic review (7.1%) conducted by Pormohammad et al. [63] on E. coli from humans, animals, food, and the environmental samples. Ciprofloxacin, aminoglycosides and sulphamethoxazole-trimethoprim are used as therapeutics in human medicine for simple urinary track infections (UTIs) [64,65], they have also been used for the production of food for animals, growth promotion and disease prevention [66,67]. The prevalence of E. coli isolates with resistance to amoxicillin-clavulanic, trimethoprim-sulfamethoxazole and nitrofurantoin were 40.2%, 38.4% and 33.5%, respectively. Moreover, the prevalence of resistence to ampicilin, amikacin which are used to treat E. coli infection was 69.4% and 15.9%, respectively. Cefuroxime is a second-generation cephalosporin antibiotic that is efficient against Enterobacteriaceae bacteria [68]. It is also among the clinically used drugs for treatment of E. coli infection (Acute uncomplicated cystitis) [69]. The overall prevalence of MDR E. coli water isolates was 50.7%, according to the meta-analysis results obtained in this study. The results of the publication bias analysis led us to believe that a variety of factors, including sample size and diagnostic techniques have resulted in a large disparity in results.
4.1. Limitations
This systematic review and meta-analysis has provided an overview on E. coli prevalence and its antibiotic resistance profiles from different water sources in Africa. There is scarcity of published research studies on E. coli from water sources from most countries of the African continent. Our findings revealed that antibiotic resistant E. coli strains are present in several water sources in African countries. However, it is not clear as to whether these antibiotic resitant strains originate from animal or human sources.
5. Conclusion
Data obtained in this study revealed that the E. coli is commonly isolated from water in Africa. The pooled prevalence estimate of E. coli. Was 71.1% based on published studies. This study reveals the great knowledge gap on E. coli prevalence from water in Africa. There are significant gaps in surveillance and lack of published studies on the prevalence of E. coli in some countries. Our findings revealed the occurrence of antibiotic resistance amongst E. coli isolates from water sources. According to our data analysis, the most tested antibiotics against E. coli isolates are ampicillin, ciprofloxacin, tetracycline, gentamicin, chloramphenicol, streptomycin, and amikacin. Disk diffusion was the commonly used method for identifying antibiotic resistance profiles of E. coli isolates. MDR prevention and management require careful monitoring relevant strains and early detection of these isolates utilizing phenotypic and genotypic laboratory approaches. In addition, it is recommended that antimicrobials should be closely monitored. There is a need for future E. coli prevalence research from all representative regions of the continent.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made iavailable on request.
Additional information
No additional information is available for this paper.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Consent for publication
All authors consent to the publication of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16123.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Nurliyana M.R., Sahdan M.Z., Wibowo K.M., Muslihati A., Saim H., Ahmad S.A., Sari Y., Mansor Z. The detection method of Escherichia coli in water resources: a review. J. Phys. Conf. Se. 2018;995 [Google Scholar]
- 2.Uluseker C., Kaster K.M., Thorsen K., Basiry D., Shobana S., Jain M., Kumar G., Kommedal R., Pala-Ozkok I. A review on occurrence and spread of antibiotic resistance in wastewaters and in wastewater treatment plants: mechanisms and perspectives. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.717809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Şahin S., Akpinar I., Sivri N. An alternative material for an effective treatment technique proposal in the light of bibliometric profile of global scientific research on antibiotic resistance and Escherichia coli. Environ. Monit. Assess. 2020;192:1–10. doi: 10.1007/s10661-020-08678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abd El Shakour E.H., Mostafa A. Antimicrobial resistance profiles of Enterobacteriaceae isolated from rosetta branch of river nile, Egypt. World Appl. Sci. J. 2012;19:1234–1243. [Google Scholar]
- 5.Ramos C.P., Santana J.A., Morcatti Coura F., Xavier R.G.C., Leal C.A.G., Junior Oliveira C.A., Heinemann M.B., Lage A.P., Lobato F.C.F., Silva R.O.S. Identification and characterization of Escherichia coli, Salmonella spp., Clostridium perfringens, and C. difficile isolates from reptiles in Brazil. BioMed Res. Int. 2019;(2019) doi: 10.1155/2019/9530732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelwahab G.E., Ishag H.Z.A., Al Hammadi Z.M., Al Yammahi S.M.S., Mohd Yusof M.F.B., Al Yassi M.S.Y., Al Mansoori A.M.A., Al Hamadi F.H.A., Al Hamadi I.A.S., Hosani M.A.A.A., Mohammed Al Muhairi S.S. Antibiotics resistance in Escherichia coli isolated from livestock in the emirate of abu dhabi, UAE, 2014–2019. Internet J. Microbiol. 2022:2022. doi: 10.1155/2022/3411560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolukaoto J.Y., Singh A., Alfinete N., Barnard T.G. Occurrence of hybrid diarrhoeagenic Escherichia coli associated with multidrug resistance in environmental water, Johannesburg, South Africa. Microorganisms. 2021;9:2163. doi: 10.3390/microorganisms9102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonola V.S., Katakweba A., Misinzo G., Matee M.I. Molecular epidemiology of antibiotic resistance genes and virulence factors in multidrug-resistant Escherichia coli isolated from rodents, humans, chicken, and household soils in Karatu, Northern Tanzania. Int. J. Environ. Res. Publ. Health. 2022;19:5388. doi: 10.3390/ijerph19095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makhado U.G., Foka F.E.T., Tchatchouang C.D.K., Ateba C.N., Manganyi M.C. Detection of virulence gene of Shiga toxin-producing Escherichia coli (STEC) strains from animals with diarrhoea and water samples in the North-West Province, South Africa. Gen. Rep. 2022;2022 [Google Scholar]
- 10.Bolukaoto J.Y., Singh A., Alfinete N., Barnard T.G. Occurrence of hybrid diarrhoeagenic Escherichia coli associated with multidrug resistance in environmental water, Johannesburg, South Africa. Microorganisms. 2021;9(10):2163. doi: 10.3390/microorganisms9102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J., Kim J.S., Kim S., Shin E., Oh K.H., Kim Y., Kim C.H., Hwang M.A., Jin C.M., Na K., Lee J. A waterborne outbreak of multiple diarrhoeagenic Escherichia coli infections associated with drinking water at a school camp. Int. J. Infect. Dis. 2018;66:45–50. doi: 10.1016/j.ijid.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Chique C., Hynds P., Burke L.P., Morris D., Ryan M.P., O'Dwyer J. Contamination of domestic groundwater systems by verotoxigenic Escherichia coli (VTEC), 2003–2019: a global scoping review. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116496. [DOI] [PubMed] [Google Scholar]
- 13.Racewicz P., Majewski M., Biesiada H., Nowaczewski S., Wilczyński J., Wystalska D., Kubiak M., Pszczoła M., Madeja Z.E. Prevalence and characterisation of antimicrobial resistance genes and class 1 and 2 integrons in multiresistant Escherichia coli isolated from poultry production. Sci. Rep. 2022;12:1–13. doi: 10.1038/s41598-022-09996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chigor V.N., Umoh V.J., Smith S.I., Igbinosa E.O., Okoh A.I. Multidrug resistance and plasmid patterns of Escherichia coli O157 and other E. coli isolated from diarrhoeal stools and surface waters from some selected sources in Zaria, Nigeria. Int. J. Environ. Res. Publ. Health. 2010;7:3831–3841. doi: 10.3390/ijerph7103831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madurantakam Royam M., Ramesh R., Shanker R., Sabarimurugan S., Kumarasamy C., Ramesh N., Gothandam K.M., Baxi S., Gupta A., Krishnan S., Jayaraj R. Mirna predictors of pancreatic cancer chemotherapeutic response: a systematic review and meta-analysis. Cancers. 2019;11:900. doi: 10.3390/cancers11070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ateba C.N., Tabi N.M., Fri J., Bissong M.E.A., Bezuidenhout C.C. Occurrence of antibiotic-resistant bacteria and genes in two drinking water treatment and distribution systems in the North-West Province of South Africa. Antibiotics. 2020;9(11):745. doi: 10.3390/antibiotics9110745. 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonola V.S., Katakweba A.S., Misinzo G., Matee M.I. Occurrence of multi-drug-resistant Escherichia coli in chickens, humans, rodents and household soil in Karatu, Northern Tanzania. Antibiotics. 2021;10(9):1137. doi: 10.3390/antibiotics10091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greig J.D., Waddell L., Wilhelm B., Wilkins W., Bucher O., Parker S., Rajić A. The efficacy of interventions applied during primary processing on contamination of beef carcasses with Escherichia coli: a systematic review-meta-analysis of the published research. Food Control. 2012;27(2):385–397. [Google Scholar]
- 19.Kamali Dolatabadi R., Feizi A., Halaji M., Fazeli H., Adibi P. The prevalence of adherent-invasive Escherichia coli and its association with inflammatory bowel diseases: a systematic review and meta-analysis. Front. Med. 2021;8 doi: 10.3389/fmed.2021.730243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halaji M., Fayyazi A., Rajabnia M., Zare D., Pournajaf A., Ranjbar R. Phylogenetic group distribution of uropathogenic Escherichia coli and related antimicrobial resistance pattern: a meta-analysis and systematic review. Front. Cell. Infect. Microbiol. 2022;12:126. doi: 10.3389/fcimb.2022.790184. Front. Cell. Infect. Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assefa A., Bihon A. A systematic review and meta-analysis of prevalence of Escherichia coli in foods of animal origin in Ethiopia. Heliyon. 2018;4(8) doi: 10.1016/j.heliyon.2018.e00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Founou L.L., Amoako D.G., Founou R.C., Essack S.Y. Antibiotic resistance in food animals in Africa: a systematic review and meta-analysis. Microb. Drug Resist. 2018;24(5):648–665. doi: 10.1089/mdr.2017.0383. [DOI] [PubMed] [Google Scholar]
- 23.Zenebe T., Mitiku M., Alem Y. Prevalence of Escherichia coli in under-five children with diarrhea in Ethiopia: a systematic review and meta-analysis. Internet J. Microbiol. 2020:2020. doi: 10.1155/2020/8844294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunduki G.K., Heinz E., Phiri V.S., Noah P., Feasey N., Musaya J. Virulence factors and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from urinary tract infections: a systematic review and meta-analysis. BMC Infect. Dis. 2021;21(1):1–13. doi: 10.1186/s12879-021-06435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernabe K.J., Langendorf C., Ford N., Ronat J.B., Murphy R.A. Antimicrobial resistance in West Africa: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2017;50(5):629–639. doi: 10.1016/j.ijantimicag.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Tadesse B.T., Ashley E.A., Ongarello S., Havumaki J., Wijegoonewardena M., González I.J., Dittrich S. Antimicrobial resistance in Africa: a systematic review. BMC Infect. Dis. 2017;17(1):1–17. doi: 10.1186/s12879-017-2713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escher N.A., Muhummed A.M., Hattendorf J., Vonaesch P., Zinsstag J. Systematic review and meta‐analysis of integrated studies on antimicrobial resistance genes in Africa—a One Health perspective. Trop. Med. Int. Health. 2021;26(10):1153–1163. doi: 10.1111/tmi.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021;10(1):1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buccheri R.K., Sharifi C. Critical appraisal tools and reporting guidelines for evidence‐based practice. Worldviews Evidence-Based Nurs. 2017;14(6):463–472. doi: 10.1111/wvn.12258. [DOI] [PubMed] [Google Scholar]
- 30.Venâncio I., Luís Â., Domingues F., Oleastro M., Pereira L., Ferreira S. The prevalence of arcobacteraceae in aquatic environments: a systematic review and meta-analysis. Pathogens. 2022;11(2):244. doi: 10.3390/pathogens11020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nontongana N., Sibanda T., Ngwenya E., Okoh A.I. Prevalence and antibiogram profiling of Escherichia coli pathotypes isolated from the Kat River and the Fort Beaufort abstraction water. Int. J. Environ. Res. Publ. Health. 2014;11(8):8213–8227. doi: 10.3390/ijerph110808213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abia A.L.K., Ubomba-Jaswa E., Momba M.N.B. High prevalence of multiple-antibiotic-resistant (MAR) Escherichia coli in river bed sediments of the Apies River, South Africa. Environ. Monit. Assess. 2015;187(10):1–13. doi: 10.1007/s10661-015-4879-6. [DOI] [PubMed] [Google Scholar]
- 34.Adefisoye M.A., Okoh A.I. Identification and antimicrobial resistance prevalence of pathogenic Escherichia coli strains from treated wastewater effluents in Eastern Cape, South Africa. Microbiol. 2016;5(1):143–151. doi: 10.1002/mbo3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mariano V., McCrindle C.M.E., Cenci-Goga B., Picard J.A. Case-control study to determine whether river water can spread tetracycline resistance to unexposed impala (Aepyceros melampus) in Kruger National Park (South Africa) Appl. Environ. Microbiol. 2009;75(1):113–118. doi: 10.1128/AEM.01808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebomah K.E., Adefisoye M.A., Okoh A.I. Pathogenic Escherichia coli strains recovered from selected aquatic resources in the eastern cape, South Africa, and its significance to public health. Int. J. Environ. Res. Publ. Health. 2018;15(7):1506. doi: 10.3390/ijerph15071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doughari H.J., Ndakidemi P.A., Human I.S., Benade S. Virulence factors and antibiotic susceptibility among verotoxic non O157: H7 Escherichia coli isolates obtained from water and wastewater samples in Cape Town, South Africa. Afr. J. Biotechnol. 2011;10(64):14160–14168. [Google Scholar]
- 38.Chukwu M.O., Abia A.L.K., Ubomba-Jaswa E., Obi L.C., Dewar J.B. Antibiotic resistance profile and clonality of E. coli isolated from water and paediatric stool samples in the north-west, province South Africa. J. Pure Appl. Microbiol. 2019;13:517–530. [Google Scholar]
- 39.Moges F., Endris M., Belyhun Y., Worku W. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, Northwest Ethiopia. BMC Res. Notes. 2014;7(1):1–6. doi: 10.1186/1756-0500-7-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belachew T., Mihret A., Legesse T., Million Y., Desta K. High level of drug resistance by gram-negative bacteria from selected sewage polluted urban rivers in Addis Ababa, Ethiopia. BMC Res. Notes. 2018;11(1):1–6. doi: 10.1186/s13104-018-3622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teshome A., Alemayehu T., Deriba W., Ayele Y. Antibiotic resistance profile of bacteria isolated from wastewater systems in Eastern Ethiopia. Environ. Public Health. 2020;2020 doi: 10.1155/2020/2796365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chigor C.B., Ibangha I.A.I., Nweze N.O., Ozochi C.A., Onuora V.C., Titilawo Y., Chernikova T.N., Golyshin P.N., Chigor V.N. bioRxiv; 2020. Multidrug Resistance and High Prevalence of Class 1 Integrons in Escherichia coli Isolated from Waters and Vegetables in Nsukka and Enugu, Nigeria; p. 2020. [Google Scholar]
- 43.Manji P.L., Antai S.P., Jacob I.O. Incidence of Staphylococcus aureus, coliforms and antibiotic resistant strains of Escherichia coli in rural water supplies in Calabar South Local Government Area. J. Publ. Health Epidemiol. 2012;4(9):230–237. [Google Scholar]
- 44.Lyimo B., Buza J., Subbiah M., Smith W., Call D.R. Comparison of antibiotic resistant Escherichia coli obtained from drinking water sources in northern Tanzania: a cross-sectional study. BMC Microbiol. 2016;16(1):1–10. doi: 10.1186/s12866-016-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tahri L., Hafiane F.Z., Fekhaoui M. Prevalence and antibiotic resistance of the Escherichia coli in the groundwater (Tadla-Morocco) Groundw. Sustain. Dev. 2021;13 [Google Scholar]
- 46.Wambugu P., Habtu M., Impwi P., Matiru V., Kiiru J. Antimicrobial susceptibility profiles among Escherichia coli strains isolated from Athi River water in Machakos County, Kenya. Adv. Microbiol. 2015;5:711–719. [Google Scholar]
- 47.Odonkor S.T., Addo K.K. Prevalence of multidrug-resistant Escherichia coli isolated from drinking water sources. Internet J. Microbiol. 2018:2018. doi: 10.1155/2018/7204013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao F., Yang H., Bi D., Khaledi A., Qiao M. A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections. Microb. Pathog. 2020;144 doi: 10.1016/j.micpath.2020.104196. [DOI] [PubMed] [Google Scholar]
- 49.Monyama M.C., Onyiche E.T., Taioe M.O., Nkhebenyane J.S., Thekisoe O.M. Bacterial pathogens identified from houseflies in different human and animal settings: a systematic review and meta‐analysis. Vet. Med. Sci. 2022;8(2):827–844. doi: 10.1002/vms3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotsilkov K., Popova C., Boyanova L., Setchanova L., Mitov I. Comparison of culture method and real-time PCR for detection of putative periodontopathogenic bacteria in deep periodontal pockets. Biotechnol. Biotechnol. Equip. 2015;29(5):996–1002. [Google Scholar]
- 51.Ramatla T., Tawana M., Onyiche T.E., Lekota K.E., Thekisoe O. Prevalence of antibiotic resistance in Salmonella serotypes concurrently isolated from the environment, animals, and humans in South Africa: a systematic review and meta-analysis. Antibiotics. 2021;10(12):1435. doi: 10.3390/antibiotics10121435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adzitey F., Huda N., Ali G.R.R. Molecular techniques for detecting and typing of bacteria, advantages and application to foodborne pathogens isolated from ducks. 3. Biotec. 2013;392:97–107. doi: 10.1007/s13205-012-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wójcicki M., Świder O., Daniluk K.J., Średnicka P., Akimowicz M.M.Ł., Roszko, Sokołowska B., Juszczuk-Kubiak E. Transcriptional regulation of the multiple resistance mechanisms in Salmonella — a review. Pathogens. 2021;10(7):801. doi: 10.3390/pathogens10070801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramatla T., Mileng K., Ndou R., Tawana M., Mofokeng L., Syakalima M., Lekota K.E., Thekisoe O. Campylobacter jejuni from slaughter age broiler chickens: genetic characterization, virulence, and antimicrobial resistance genes. Internet J. Microbiol. 2022;2022 doi: 10.1155/2022/1713213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soto S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013;4(3):223–229. doi: 10.4161/viru.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Połaska M., Sokołowska B. Bacteriophages—a new hope or a huge problem in the food industry. AIMS microbiol. 2019;5(4):324. doi: 10.3934/microbiol.2019.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van C.N., Zhang L., Thanh T.V.T., Son H.P.H., Ngoc T.T., Huang Q., Zhou R. Association between the phenotypes and genotypes of antimicrobial resistance in Haemophilus parasuis isolates from swine in quang binh and thua thien hue provinces. Vietnam Eng. 2020;6(1):40–48. [Google Scholar]
- 58.Lanz R., Kuhnert P., Boerlin P. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 2003;91(1):73–84. doi: 10.1016/s0378-1135(02)00263-8. [DOI] [PubMed] [Google Scholar]
- 59.Karimi Dehkordi M., Halaji M., Nouri S. Prevalence of class 1 integron in Escherichia coli isolated from animal sources in Iran: a systematic review and meta-analysis. Trp. Med. Health. 2020;48(1):1–7. doi: 10.1186/s41182-020-00202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galindo-Méndez M. Antimicrobial resistance in Escherichia coli. E. Coli infections-importance of early diagnosis and efficient treatment. Antimicrob. Resist. Infect. 2020;2020:1–20. [Google Scholar]
- 61.Chamosa L.S., Álvarez V.E., Nardelli M., Quiroga M.P., Cassini M.H., Centrón D. Lateral antimicrobial resistance genetic transfer is active in the open environment. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-00600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leungtongkam U., Thummeepak R., Tasanapak K., Sitthisak S. Acquisition and transfer of antibiotic resistance genes in association with conjugative plasmid or class 1 integrons of Acinetobacter baumannii. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0208468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pormohammad A., Nasiri M.J., Azimi T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: a systematic review and meta-analysis. Infect. Drug Resist. 2019;12:1181. doi: 10.2147/IDR.S201324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goncuoglu M., Bilir Ormanci F.S., Ayaz N.D., Erol I. Antibiotic resistance of Escherichia coli O157: H7 isolated from cattle and sheep. Ann. Microbiol. 2010;60(3):489–494. [Google Scholar]
- 65.McKinnell J.A., Stollenwerk N.S., Jung C.W., Miller L.G. Nitrofurantoin compares favorably to recommended agents as empirical treatment of uncomplicated urinary tract infections in a decision and cost analysis. Mayo Clin. Proc. 2011;86(6):480–488. doi: 10.4065/mcp.2010.0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibrahim M., Ahmad F., Yaqub B., Ramzan A., Imran A., Afzaal M., Mirza S.A., Mazhar I., Younus M., Akram Q., Taseer M.S.A. Elsevier; 2020. Trends of antimicrobials used in food animals and aquaculture; pp. 39–69. (Antibiotics and Antimicrobial Resistance Genes in the Environment). 2020. [Google Scholar]
- 67.Cromwell G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002;13(1):7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- 68.Chang U.I., Kim H.W., Wie S.H. Use of cefuroxime for women with community-onset acute pyelonephritis caused by cefuroxime-susceptible or-resistant Escherichia coli. Korean J. Intern. Med. 2016;31(1):145. doi: 10.3904/kjim.2016.31.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venkatesh S., Chauhan Gadpayle L.S.A.K., Jain T.S., Ghafur A., Wattal C. National Centre For Disease Control, MOHFW, Government of India; India: 2011. National Treatment Guidelines for Antimicrobial Use in Infectious Diseases; pp. 1–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made iavailable on request.