FIGURE 2.
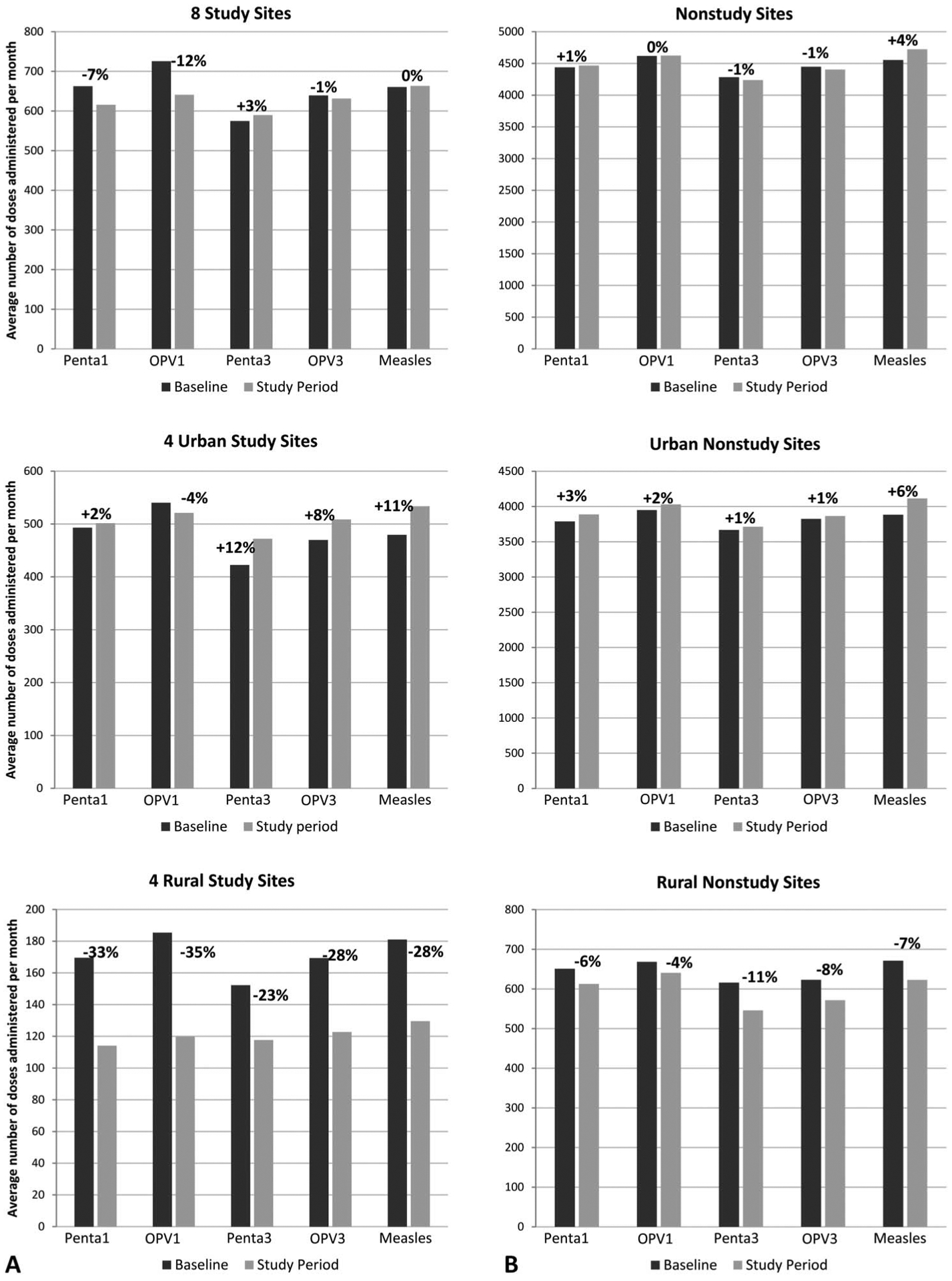
Average number of vaccine* doses administered per month during study period (April 1, 2009 to March 31, 2010) and baseline period (April 1, 2006 to March 31, 2008) at study sites (A) and nonstudy sites** in the same districts (B). Percentages indicate change in vaccine doses administered from baseline period to study period. *Penta1—pentavalent diphtheria, tetanus toxoid, whole cell pertussis, haemophilus influenzae type B, and hepatitis B vaccination at 4 weeks of age; OPV1—oral polio vaccination at 4 weeks of age, Penta3—pentava-lent diphtheria, tetanus toxoid, whole cell pertussis, haemophilus influenzae type B, and hepatitis B vaccination at 12 weeks of age; OPV3—oral polio vaccination at 4 weeks of age 12 weeks of age; Measles—measles vaccination at 9 months of age. **At the start of the baseline period and of the study period, the number of nonstudy sites was 146 and 148, urban nonstudy sites was 93 and 93, and rural non-study sites was 53 and 55.
