Abstract
The SARS-CoV-2 spike S1 subunit (S1) can cross the blood–brain barrier and elicit neuroinflammatory response independent of viral infection. Here we examined whether S1 influences blood pressure (BP) and sensitizes the hypertensive response to angiotensin (ANG) II by enhancing neuroinflammation and oxidative stress in hypothalamic paraventricular nucleus (PVN), a key brain cardiovascular regulatory center. Rats received central S1 or vehicle (VEH) injection for 5 days. One week after injection, ANG II or saline (control) was subcutaneously delivered for 2 weeks. S1 injection induced greater increases in BP, PVN neuronal excitation and sympathetic drive in ANG II rats but had no effects in control rats. One week after S1 injection, mRNA for proinflammatory cytokines and oxidative stress marker were higher but mRNA of Nrf2, the master regulator of inducible antioxidant and anti-inflammatory responses, was lower in the PVN in S1-injected rats than in VEH-injected rats. Three weeks after S1 injection, mRNA for proinflammatory cytokines and oxidative stress marker, microglia activation and reactive oxygen species in the PVN were comparable between S1 and VEH treated control rats but were elevated in two groups of ANG II rats. Notably, ANG II-induced elevations in these parameters were exaggerated by S1. Interestingly, ANG II increased PVN Nrf2 mRNA in VEH-treated rats but not in S1-treated rats. These data suggest that S1 exposure has no effect on BP, but post-S1 exposure increases susceptibility to ANG II-induced hypertension by downregulating PVN Nrf2 to promote neuroinflammation and oxidative stress and augment sympathetic excitation.
Keywords: COVID-19, SARS-CoV-2 spike protein, Hypertension, Brain, Sensitization
Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS coronavirus-2 (SARS-CoV-2) has now raged worldwide for more than 3 years and remains a major threat to public health. As of August 28. 2022, 600 million cases have been confirmed, leading to over 6.4 million deaths worldwide [Word Health Organization, COVID-19 Dashboard, https://covid19.who.int/]. Despite COVID-19 is primarily a respiratory disease, it also affects other systems, such as the central nervous system and the cardiovascular system [1–4]. COVID-19 patients with cardiovascular disorder (CVD) develop a more severe clinical course with a significantly increased risk of death than non-CVD patients [1]. Moreover, emerging evidence reveals that COVID-19 patients without a history of pre-existing cardiovascular disorders have a greater risk of developing cardiovascular disease after COVID-19 recovery [3].
Hypertension is a major risk factor for a variety of cardiovascular diseases, including stroke, coronary artery disease, heart failure, and renal disease [5]. Several clinical studies showed that arterial blood pressure was significantly elevated in COVID-19 patients after recovery from infection [3, 6]. These observations suggest that hypertension might be a sequela of COVID-19 infection, but the mechanisms underlying elevated blood pressure in the post-acute phase of COVID-19 infection are unclear.
It has long been established from both animal and human studies that excessive activation of the sympathetic nervous system plays a primary role in the development and progression of the essential hypertension [7–9]. Inflammation and oxidative stress in the brain, particularly in the hypothalamic paraventricular nucleus (PVN), a cardiovascular regulatory center in the brain that integrates neural and humoral signals driving the sympathetic nervous system, are key factors in regulating sympathetic nerve activity and blood pressure [10, 11]. Previous studies have indicated that the presence of several central inflammatory challenges can enhance hypertensive response to subsequent angiotensin (ANG) II administration by promoting inflammation in the brain to increase sympathetic excitation [9, 12–14]. The S1 subunit of the SARS-CoV-2 spike protein (S1) has been shown to cross the blood–brain barrier (BBB) and cause inflammatory response in the brain independent of SARS-CoV-2 cellular infection [15, 16]. However, whether S1 protein has an impact on blood pressure is unknown. In the present study, we examined whether S1 protein exposure influences blood pressure and sensitizes ANG II-induced hypertensive response and if so, whether post-S1 protein exposure promotes inflammatory response and oxidative stress in the PVN and augments sympathetic excitation.
Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee and performed in accordance with the guidelines of the Animal Care and Use Committee at Shandong University. Male Sprague–Dawley rats (Beijing Laboratory Animal Research Center, Beijing, China) that weighed between 220 and 250 g on arrival to the laboratory were housed in temperature- and humidity-controlled rooms (22 ± 1 °C; 50 ± 5%) under a 12–12 h light–dark cycle with standard rat chow and water ad libitum. All rats were allowed ≥ 1 week of habituation to the laboratory before the start of experiments.
Experiment Protocols
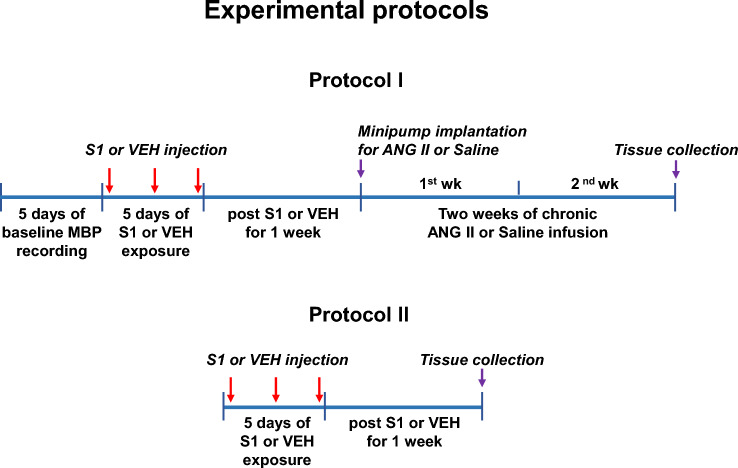
The protocols for experiments are presented in Fig. 1. In protocol 1, we examined whether S1 protein exposure would alter blood pressure and sensitize ANG II-induced hypertension. Rats were randomly assigned to 4 groups: (1) rats treated with intra-cisterna magna (ICM) injection of vehicle (VEH, artificial cerebrospinal fluid) and subcutaneous (SC) infusion of saline (VEH + Saline); (2) rats treated with ICM injection of S1 protein and SC infusion of saline (S1 + Saline); (3) rats treated with ICM injection of VEH and SC infusion of ANG II (VEH + ANG II); (4) rats treated with ICM injection of S1 protein and SC infusion of ANG II (S1 + ANG II). Recombinant SARS-CoV-2 full-length S1 protein were obtained from RayBiotech (GA, USA). Some animals from each group (n = 7 rats/per group) received a telemetry implant for continuous recording daily mean blood pressure (MBP) and heart rate (HR). After 1 week recovery from surgery, baseline MBP and HR were measured over 5 days to ensure stable MBP and HR recordings. VEH or S1 protein was then given by ICM injection once every other day for 5 days (three applications total). The dose of S1 protein and the route of administration used here were based on a previous study in rats demonstrating the neuroinflammatory effects of S1 protein in several brain areas including hypothalamus [16]. We wanted to determine the direct effects of the S1 protein in the brain, and this route of administration avoids the peripheral inflammatory effects that would be caused by an intravenous injection and therefore eliminates a potential confounder. One week after final S1 protein injection was completed, animals were implanted with subcutaneous osmotic mini-pumps that delivered a slow-pressor dose of ANG II (120 ng/kg/min in a volume of 0.5 µL/h) over 14 days. One day prior to sacrifice, ganglionic blockade was produced by intraperitoneal injection of hexamethonium bromide (30 mg/kg) to evaluate the sympathetic contribution to BP and HR, as previously described [17]. The animals were then euthanized to collect blood and brain tissues for molecular studies. Additional animals from each group without telemetry probe implantation were treated identically. At the end of the experiment, animals were perfused transcardially with 4% paraformaldehyde (n = 6 rats/per group) or directly euthanized to collect brain (n = 6 rats/per group) for immunohistochemical studies. Protocol 2 was conducted to determine whether S1 protein exposure would have an effect on molecular signaling in the PVN 1 week after completion of S1 injection (before ANG II infusion). Rats were randomly assigned to VEH and S1 protein exposure groups (n = 7 rats/per group). VEH or S1 protein was administrated identically to protocol 1; however, telemetry devices were not implanted. Animals were euthanized one week after final S1 protein injection and the brains were collected for molecular studies.
Fig. 1.
Schematic diagram of the experimental protocol
Telemetry Probe Implantation and Measurement of BP and HR
Arterial BP and HR in individual animals were directly recorded using a telemetry probe (HD-S10, Data Sciences International, (DSI® 89), St. Paul, MN) as described previously [17]. Briefly, rats were anesthetized with a mixture of ketamine and xylazine (90% ketamine and 10% xylazine, IP) and a ventral incision was made to assess the femoral artery. The right femoral artery was isolated, and the catheter of the telemetric device was inserted into the right femoral artery. Through the same ventral incision, a pocket along the right flank was formed. The body of the telemetric device was placed in the pocket and secured with tissue adhesive (Vetbond, 3 M Animal Care Products, St Paul, MN, USA). The ventral incision was then closed with suture. All animals were allowed 1 week for recovery before being used in experiments.
ICM Injection
ICM injection was performed as described previously [16]. Briefly, animals were anaesthetized with isoflurane (5% for induction, 3% for maintenance) in oxygen. The dorsal aspect of the skull was shaved and disinfected with iodine solution and 70% ethanol. A sterile 27-gauge needle attached via sterile PE50 tubing to a 25 μl Hamilton syringe was inserted into the cisterna magna. After verification by withdrawing 2 μl of clear CSF, the S1 protein or VEH was injected over a 30 s period. After the injection was completed, the needle was left in place for 30 s to allow for diffusion of drug.
Measurement of mRNA Expression in the PVN
mRNA levels for proinflammatory cytokines interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, and NAD(P)H oxidase subunits NOX2 and NOX4 in the hypothalamic PVN were assessed using real-time PCR as described previously [17–19]. Briefly, the PVN tissues were micropunched from the forebrain and RNA was isolated from the PVN using the RNeasy plus mini kit (QIAGEN China Co. Ltd., Shanghai, China). The RNA was then reverse transcribed into cDNA using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Inc, Hercules, CA, USA). The primers used for real-time PCR are presented in Table 1. cDNA was amplified and analyzed using the QuantStudio 3 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Changes in mRNA expression levels were normalized to β-actin levels and calculated using the comparative cycle threshold method. Results are expressed as fold change relative to VEH + Saline or VEH group.
Table 1.
Sequences for primers
| Gene | Primers | Sequences |
|---|---|---|
| IL-1β |
Forward primer: Reverse primer: |
5′-CACCTCTCAAGCAGAGCACAG-3′ 5′-GGGTTCCATGGTGAAGTCAAC-3′ |
| IL-6 |
Forward primer: Reverse primer: |
5′-TCCTACCCCAACTTCCAATGCTC-3′ 5′-TTGGATGGTCTTGGTCCTTAGCC-3′ |
| TNF-α |
Forward primer: Reverse primer: |
5′-AAATGGGCTCCCTCTCATCAGTTC-3′ 5′-TCTGCTTGGTGGTTTGCTACGAC-3′ |
| NOX2 |
Forward primer: Reverse primer: |
5′-CAAGATGGAGGTGGGACAGT-3′ 5′-GCTTATCACAGCCACAAGCA-3′ |
| NOX4 |
Forward primer: Reverse primer: |
5′-GGATCACAGAAGGTCCCTAGC-3′ 5′-AGAAGTTCAGGGCGTTCACC-3′ |
| Nrf2 |
Forward primer: Reverse primer: |
5′-GCCAGCTGAACTCCTTAGAC-3′ 5′-GATTCGTGCACAGCAGCA-3′ |
| β-actin |
Forward primer: Reverse primer: |
5′-CAGGGTGTGATGGTGGGTATGG-3′ 5'-AGTTGGTGACAATGCCGTGTTC-3′ |
Immunofluorescence and Immunohistochemistry
Rats were anesthetized and perfused with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformadlehyde in PBS. Brains were removed and placed in 4% paraformadlehyde overnight and then in 30% sucrose for 2 days. Brains were sectioned into several 20-μm transverse sections using a cryostat microtome and stored at − 20 °C until processed for immunohistochemistry. Slices were washed in 0.1 M PBS for 3 times, incubated in 1% hydrogen peroxide for 30 min to block endogenous peroxidase, and blocked with 3% goat serum for 1 h. The sections were then incubated with mouse monoclonal primary antibodies against CD11b (clone OX-42, Chemicon, Temecula, USA) or against Fra-LI (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 days at 4 °C. Subsequently, the sections were incubated in an anti-mouse Alex Fluor 488 (Invitrogen, Carlsbad, CA, United States) or anti-mouse biotinylated IgG for 1 h at room temperature. After washing in PBS, sections were coverslipped with mounting medium and images were taken with a Zeiss LSM 710 confocal microscope or an Olympus BX51 microscope. The number of Fra-LI positive neurons, total and activated microglia in the PVN were manually counted as previously described [20]. Activated microglia were presented as a percentage of the total number of microglia.
Detection of Reactive Oxygen Species (ROS) Production
The nonfixed sections were used for to detect superoxide in situ in the PVN by laser scanning confocal microscope as described previously [18, 19]. Briefly, the brain was removed and immediately frozen at –80 °C for 1 h, blocked in the coronal plane, and cut at − 20 °C using a cryostat microtome at 30 μm. The sections at the level of the PVN were mounted on microscope slides and incubated with DHE (Molecular Probes, Eugene, OR, USA) for 30 min at 37 °C in a light-protected humidified chamber. The stock solution of DHE was made by dissolving 5 mg of the dye in 1 mL of water; for staining, it was diluted to 5 µmol/L in PBS. Images were visualized and DHE fluorescence in the PVN were analyzed by NIH image software.
Biochemical Assay
Trunk blood was collected at the time of euthanasia for biochemical assay. Plasma levels of norepinephrine (NE) were measured with a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturers’ instructions.
Statistical Analysis
All data are presented as the mean ± S.E.M. Kolmogorov–Smirnov test and Levene’s test were applied to verify normal distributions and equal variances, respectively. Statistical analyses were performed with Student’s t-test or two-way ANOVA followed by Tukey’s post hoc test for multiple group comparisons, using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
Results
Effects of S1 Protein Exposure on BP and HR
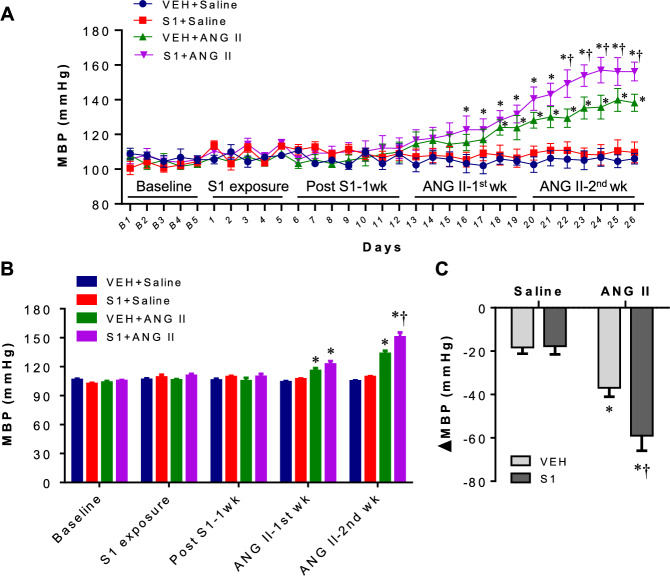
Figure 2A displays average daily MBP during baseline recordings (day B1-5), S1 protein exposure (days 1–5), one week post S1 protein or VEH exposure (days 6–12), first week of ANG II or saline infusion (days 13–19) and second week of ANG II or saline infusion (days 20–26). There was no significant difference in MBP during baseline recordings, S1 protein exposure and one week post S1 protein exposure across 4 experimental groups. After ANG II or saline infusion, VEH + ANG II rats experienced a gradual increase in MBP compared with VEH + Saline rats and a significant elevation in MBP began on day 18, whereas S1 + ANG II rats showed a more rapid elevation in MBP during ANG II infusion and a significant elevation in MBP began on day 16. Furthermore, ANG II-induced increase in MBP was greater in S1 + ANG II rats than that in VEH + ANG II rats on days 22–26. MBP in S1 + Saline rats did not differ from that in VEH + Saline rats during saline or ANG II infusion. Figure 2B shows MBP blocked by phase. Compared with VEH + Saline rats, MBP was significantly higher in both groups of ANG II-infused rats in the first and second phases of ANG II infusion. However, S1 + ANG II rats showed a greater increase in MBP than VEH + ANG II rats in the second phase of ANG II infusion. Average daily HR (Fig. 3A) and HR blocked by phase (Fig. 3B) were comparable among 4 experimental groups throughout the experimental protocol.
Fig. 2.
A Daily mean blood pressure (MBP) during baseline recordings, SARS-CoV-2 spike protein S1 (S1) or vehicle (VEH) exposure, 1 week post S1 or VEH exposure, first week of angiotensin II (ANG II) or saline infusion, and second week of ANG II or saline infusion in each group. B MAP blocked by phase in each group. C The peak changes (Δ) in MBP in response to ganglionic blockade at the end of the study in each group. Values are mean ± SEM (n = 7 for each group). *P < 0.05 vs. VEH + saline; †P < 0.05 vs. VEH + ANG II
Fig. 3.
A Daily mean heart rate (HR) during baseline recordings, SARS-CoV-2 spike protein S1 (S1) or vehicle (VEH) exposure, 1 week post S1 or VEH exposure, first week of ANG II or saline infusion, and second week of ANG II or saline infusion in each group. B Mean HR blocked by phase in each group. Values are mean ± SEM (n = 7 for each group). *P < 0.05 vs. VEH + Saline; †P < 0.05 vs. VEH + ANG II
To evaluate the contributions of the sympathetic nervous system activation to BP, ganglionic blocker hexamethonium bromide was administered at the end of the study. Although hexamethonium bromide caused a significant reduction in MBP in all 4 groups (Fig. 2C), the depressor response was significantly bigger in VEH + ANG II and S1 + ANG II rats than VEH + Saline rats. When compared with VEH + ANG II rats, S1 + ANG II rats had a significantly greater depressor response. S1 + Saline rats had a similar depressor response to VEH + Saline rats.
Effect of S1 Protein Exposure on Sympathetic Excitation
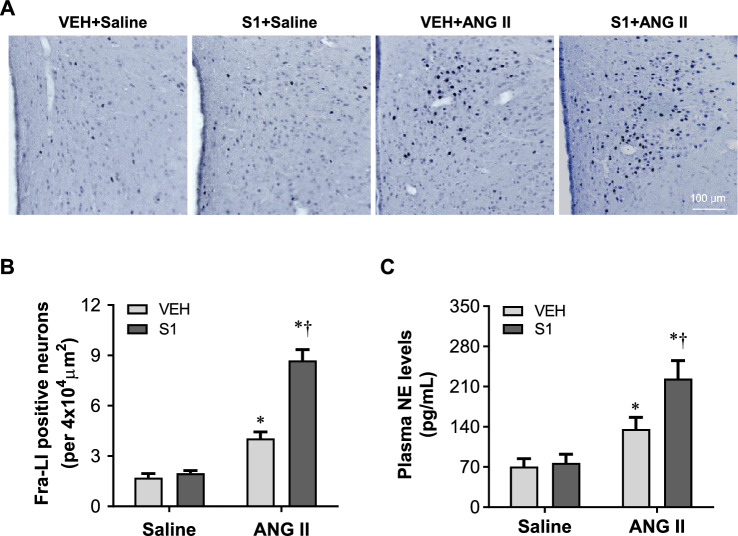
We next assessed effects of S1 protein exposure on sympathetic excitation by measuring Fra-LI positive PVN neurons (a marker of chronic neuronal excitation) and plasma levels of NE (an indicator of global sympathetic excitation). As shown in Fig. 4, VEH + Saline or S1 + Saline rats had few Fra-LI positive neurons in the PVN 2 weeks after saline infusion and there was no difference between two groups in the number of Fra-LI positive neurons (Fig. 4A and B). Compared with VEH + Saline rats, the number of Fra-LI positive neurons in the PVN was markedly higher in both VEH + ANG II and S1 + ANG II rats. Notably, S1 + ANG II rats had more Fra-LI positive neurons in the PVN than VEH + ANG II rats.
Fig. 4.
A Representative photomicrographs showing Fra-LI (a marker of chronic neuronal excitation) positive PVN neurons in the PVN in rats 3 weeks after completion of S1 or VEH exposure and two weeks after chronic ANG II or saline infusion. Dark dots indicate Fra-LI positive neurons. B Quantification of Fra-LI positive neurons in the PVN in each group. C Plasma levels of norepinephrine (NE), an indicator of global sympathetic excitation, in each group. Values are mean ± SEM (n = 6 for each group). *P < 0.05 vs. VEH + Saline; †P < 0.05 vs. VEH + ANG II
There were no differences between VEH + Saline and S1 + Saline rats in plasma levels of NE (Fig. 4C). Plasma levels of NE were significantly increased in both VEH + ANG II and S1 + ANG II rats, but the increases were greater in S1 + ANG II rats than VEH + ANG II rats.
Short-Term Effects of S1 Protein Exposure on Neuroinflammation and Oxidative Stress in the PVN
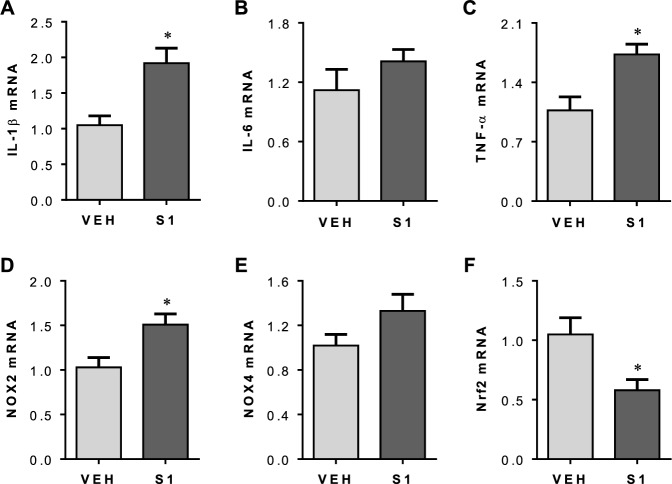
One week after completion of S1 protein or VEH injection, brains were collected to assess neuroinflammation and oxidative stress in the PVN. Real-time PCR showed that mRNA expression for inflammatory cytokines IL-1β (Figs. 5A) and TNF-a (Figs. 5C), and mRNA expression of oxidative stress marker NOX2 (Figs. 5D) were significantly increased in the PVN in rats treated with S1 protein compared with rats treated with VEH. There were no differences between groups in mRNA expression of inflammatory cytokine IL-6 (Figs. 5B) or oxidative stress marker NOX4 (Figs. 5E).
Fig. 5.
mRNA expression for proinflammatory cytokines IL-1β (A), IL-6 (B) and TNF-α (C), oxidative stress markers NOX2 (D) and NOX4 (E), and mRNA expression of Nrf2 (F), the master regulator of inducible antioxidant and anti-inflammatory responses, in the PVN in rats one week after completion of S1 or VEH exposure. Values are mean ± SEM (n = 7 for each group). Real-time PCR results are expressed as a fold change relative to VEH. *P < 0.05 vs. VEH
mRNA expression of nuclear factor erythroid 2-related factor (Nrf2), a key transcription factor that plays a critical role in the regulation of the antioxidant and anti-inflammatory responses, was decreased in the PVN in rats treated with S1 protein as compared to rats treated with VEH (Fig. 5F).
Effects of Post-S1 Protein Exposure on ANG II-Induced Neuroinflammation and Oxidative Stress in the PVN
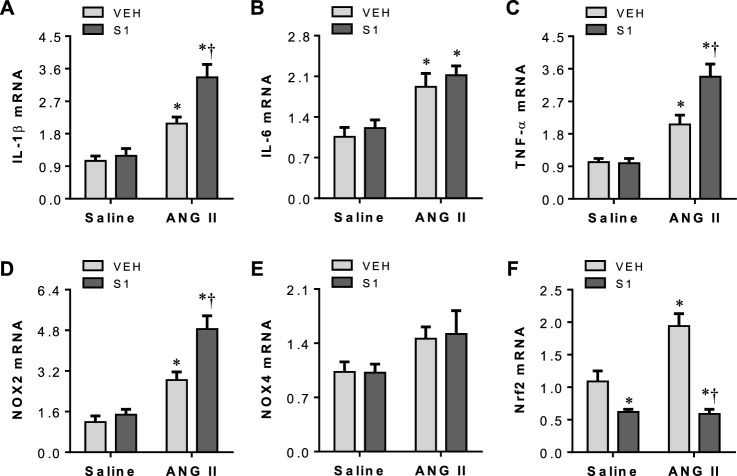
Three weeks after VEH or S1 protein exposure and 2 weeks after saline or ANG II infusion, brains were collected to assess neuroinflammation and oxidative stress in the PVN. As shown in Fig. 6, levels of mRNA for all measured proinflammatory cytokines and oxidative stress markers in the PVN were comparable between S1 + Saline and VEH + Saline rats. Both VEH + ANG II and S1 + ANG II rats had significant increase in mRNA expression of IL-1β (Fig. 6A), IL-6 (Fig. 6B), TNF-α (Fig. 6C) and NOX2 (Fig. 6D) compared with VEH + Saline rats, but mRNA expression of IL-1β, TNF-α, and NOX2 was higher in S1 + ANG II rats compared with VEH + ANG II rats. There was no difference in mRNA expression of NOX4 in the PVN across groups (Fig. 6E).
Fig. 6.
mRNA expression for proinflammatory cytokines IL-1β (A), IL-6 (B) and TNF-α (C), oxidative stress markers NOX2 (D) and NOX4 (E), and mRNA expression of Nrf2 (F) in the PVN in rats three weeks after completion of S1 or VEH exposure and two weeks after chronic ANG II or saline infusion. Values are mean ± SEM (n = 7 for each group). Real-time PCR results are expressed as a fold change relative to VEH + saline. Values are mean ± SEM (n = 7 for each group). *P < 0.05 vs. VEH + saline; †P < 0.05 vs. VEH + ANG II
mRNA expression of Nrf2 was significantly lower in the PVN in S1 + Saline or S1 + ANG II rats, compared with VEH + Saline rats, but was higher in VEH + ANG II rats (Fig. 6F). There was no difference between S1 + Saline and S1 + ANG II rats in Nrf2 mRNA expression in the PVN.
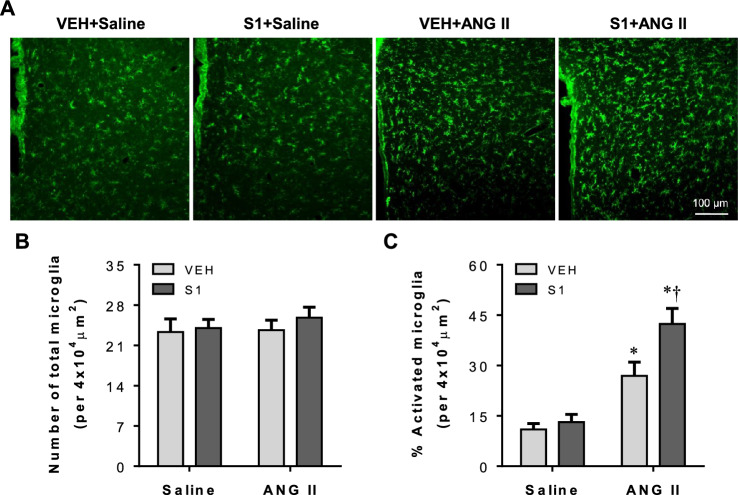
Microglia are the resident immune cells of the brain and have been considered as the primary source of proinflammatory cytokines within the brain [21]. Given that post-S1 protein exposure promoted ANG II-induced proinflammatory cytokines in the PVN, we assessed microglia activation using immunofluorescence study. As presented in Fig. 7, there was no difference in the number of total microglia in the PVN among 4 experimental groups at 2 weeks after saline or ANG II infusion (Fig. 7A and B). Very few activated microglia characterized by strong CD11b immunoreactivity, an enlarged soma, fewer and shorter processes, were found in the PVN in VEH + Saline or S1 + Saline rats. By contrast, activated microglia were clearly observed in the PVN in both VEH + ANG II and S1 + ANG II rats at this time point. Furthermore, the number of activated microglia was significantly greater in S1 + ANG II rats when compared with VEH + ANG II rats (Fig. 7A and C).
Fig. 7.
A Representative photomicrographs showing CD11b-immunoreactive microglia in the PVN in each group three weeks after completion of S1 or VEH exposure and two weeks after chronic ANG II or saline infusion. B and C Quantitative analysis of total and activated microglia in the PVN in each group. Values are mean ± SEM (n = 6 for each group). *P < 0.05 vs. VEH + saline; †P < 0.05 vs. VEH + ANG II
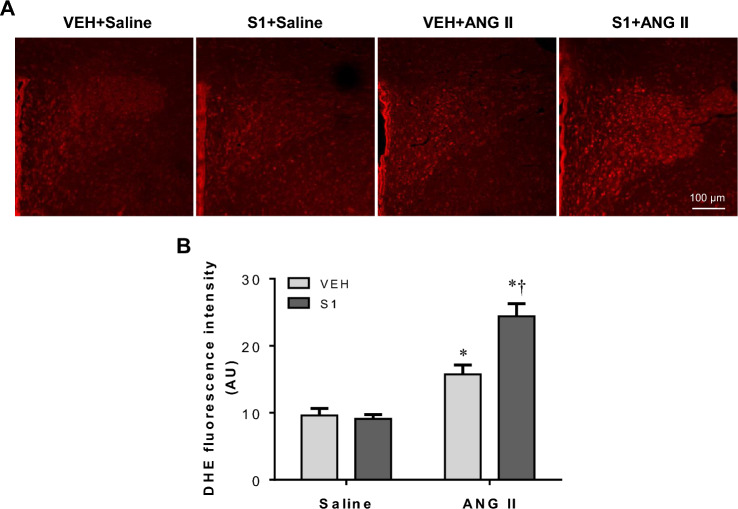
To further confirm the effect of post-S1 protein exposure on ANG II-induced oxidative stress in the PVN, we measured intracellular ROS production using DHE staining. No difference in the intensity of DHE fluorescence in the PVN was observed between VEH + Saline and S1 + Saline rats 2 weeks after Saline or ANG II infusion (Fig. 8). The intensity of DHE fluorescence in the PVN was significantly increased in both VEH + ANG II and S1 + ANG II rats, and the increase in the intensity of DHE fluorescence was higher in S1 + ANG II rats than VEH + ANG II rats.
Fig. 8.
In situ detection of superoxide by DHE fluorescence. A Representative photomicrographs showing DHE fluorescence in the PVN in each group three weeks after completion of S1 or VEH exposure and two weeks after chronic ANG II or saline infusion. B Quantitative comparison of DHE fluorescence intensity in the PVN in each group. Values are mean ± SEM (n = 6 for each group). *P < 0.05 vs. VEH + saline; †P < 0.05 vs. VEH + ANG II
These immunofluorescence observations are consistent with mRNA data, indicating that S1 protein exposure does not cause long-lasting neuroinflammation and oxidative stress in the PVN, but post-S1 protein exposure exaggerates ANG II-induced neuroinflammation and oxidative stress in the PVN.
Discussion
The major findings of the present study are: (1) S1 protein exposure doesn't alter BP, but post-S1 protein exposure causes enhanced pressure response to a slow-pressor dose of ANG II by promoting sympathetic nerve activity; (2) S1 protein exposure induces short-term elevations in neuroinflammation and oxidative stress and a long-lasting downregulation of Nrf2, the master regulator of inducible antioxidant and anti-inflammatory responses, in the PVN; (3) post-S1 protein exposure leads to exaggerations of neuroinflammation and oxidative stress in the PVN in response to ANG II. Taken together, these data suggest that S1 protein exposure alone has no effect on BP, but a history of S1 protein exposure may make individuals more susceptible to developing hypertension by exaggerating central neuroinflammation and oxidative stress to promote sympathetic excitation.
It is well established that neuroinflammation and oxidative stress in the brain cardiovascular regulatory centers including the PVN contribute to the progression of hypertension by augmenting sympathetic nerve activity [18, 19]. Neuroinflammation can cause oxidative stress and reduces cellular antioxidant capacity; oxidative stress can in turn activate a variety of transcription factors to generate more pro-inflammatory cytokines and chemokines, facilitating neuroinflammatory response [22]. These two components create a vicious cycle of neuroinflammation and oxidative stress enhancing each other and so leading to neuronal excitation and sympathetic overdrive. SARS-CoV-2 infection can cause neuroinflammation and oxidative stress as well as neurological disorders, which can persist for an extended period after resolution of the infection [16, 23]. Recent studies demonstrated that S1 protein derived from SARS-CoV-2 can enter the brain and function as a pathogen-associated molecular pattern to elicit neuroinflammatory immune responses independent of viral infection, and thus play a role in the complications of COVID-19 [15, 16]. To date, however, no studies have examined the influence of S1 protein on BP. In the present study, we found that BP was not altered during or after central S1 protein injection in S1 + Saline rats when compared with VEH + Saline rats. Importantly, we observed that post-S1 protein exposure induced a significantly enhanced hypertensive response to a slow pressor infusion of ANG II when compared to post-VEH exposure. It is widely recognized that there are at least two distinct phases of hypertension induced by a slow pressor infusion of ANG II. First, an initial increase in pressure is associated with an ANGII-mediated systemic vasoconstriction. Then, this phase is replaced by a second, long-term, neurogenic component [9, 24, 25]. In the present studies, the initial increase in BP response to systemic ANG II administration during the first week was comparable between rats pretreated with S1 protein and rats pretreated with VEH. During the second week, ANG II-induced increase in BP accelerated in rats pretreated with S1 protein compared with rats pretreated with VEH. This suggests that sensitization occurs during the time when neurogenic mechanisms are contributing to the progression of increased BP. In support of this hypothesis, we assessed BP response to ganglionic blocker hexamethonium bromide, Fra-LI positive PVN neurons, and plasma levels of NE. Our data showed that S1 + ANG II rats, compared with VEH + ANG II rats, had a significantly greater depressor response to hexamethonium bromide, much more Fra-LI positive PVN neurons, and greater increases in plasma levels of NE. These results demonstrate that S1 protein exposure alone has no effect on BP, but it leads to sensitization of ANG II-induced hypertension by augmenting sympathetic nerve activity.
We next verified neuroinflammation and oxidative stress in the PVN. We found that S1 protein exposure induced neuroinflammation and oxidative stress in the PVN at 1 week after completion of S1 protein injection as indicated by increased mRNA expression for proinflammatory cytokines and oxidative stress marker, which are consistent with previous report showing that central administration of S1 protein induced neuroinflammation in the hypothalamus that could last for up to 7 days [16]. Three weeks after completion of VEH or S1 protein injection, mRNA expression of proinflammatory cytokines, number of activated microglia that are major source of proinflammatory cytokines in the brain, mRNA expression of oxidative stress marker, and production of ROS were comparable between S1 + Saline and VEH + Saline groups. These data suggest that the S1 protein exposure induces a short-term, low-grade neuroinflammatory response and oxidative stress in the PVN that are insufficient to elevate BP and can decrease and resolve after 3 weeks. However, a 2 week ANG II infusion, starting 1 week after completion of VEH or S1 protein injection, induces marked increases in mRNA expression for proinflammatory cytokines and oxidative stress marker, number of activated microglia, and ROS production in the PVN in both rats pretreated with VEH and rats pretreated with S1 protein, but the increases were significantly greater in rats pretreated with S1 protein. These observations indicate that post-S1 protein exposure leads to exaggerations of neuroinflammation and oxidative stress in the PVN in response to ANG II and subsequently greater elevation in BP.
S1 protein contains a receptor-binding domain that can specifically bind to angiotensin-converting enzyme 2 (ACE2) receptor [26]. ACE2 is widely expressed throughout the brain including PVN and colocalizes with neurons, astrocytes, and endothelial cells [27]. However, expression in microglia was not found [16]. Additionally, there is no evidence that S1 signaling via ACE2 induces inflammatory mediators. A recent study shows that S1 protein directly induces a proinflammatory response in primary microglia [16]. Microglia express several toll-like receptor [28], thus S1 protein might promote inflammatory response in microglia through toll-like receptor but not ACE2.
Inflammation and oxidative stress can be regulated by Nrf2, which is a master regulator of the antioxidant cellular defense system and expressed in most tissues including brain [29, 30]. Under physiological conditions, Nrf2 binds to Kelch-like ECH-associated protein 1 (keap1) in the cytoplasm. Upon exposure to ROS, Nrf2 is released from keap1 in the cytoplasm and translocates to the nucleus, where it upregulates downstream antioxidant target genes to prevent oxidative stress. Nrf2 also suppresses activation of the transcription factor nuclear factor-kappa B to inhibit the production of proinflammatory cytokines [29, 30]. Deficiency of Nrf2 has been shown to cause the failure to upregulate downstream antioxidant target genes in the brain in response to acrylamide, resulting in exaggerated microglia activation, oxidative stress, neuroinflammation and enhanced neurotoxicity in mice [31]. In contrast, pharmacologic activation of Nrf2 reduces activation of microglia and neuroinflammation, alleviates neurotoxicity, and improves synaptic and mitochondrial function in animals following lipopolysaccharide challenge [32, 33]. Recent studies have shown that levels of Nrf2 and its antioxidant target genes in the plasma and tissues are significantly lower in COVID-19 patients than control subjects and that a lower levels of Nrf2 are negatively correlated with the levels of proinflammatory markers [23, 34, 35]. In the present study, we found that mRNA expression of Nrf2 was decreased in the PVN 1 week after completion of S1 protein injection and remained significantly lower 3 weeks later. Interestingly, mRNA expression of Nrf2 in the PVN was significantly increased in rats pretreated with VEH but not in rats pretreated with S1 protein in response to a 2 week ANG II infusion. These findings suggest that S1 protein-induced downregulation of Nrf2 results in the failure to trigger anti-inflammatory and antioxidant defense system in response to ANG II, leading to exaggerated neuroinflammation and oxidative stress in the PVN.
In the present study, S1 protein was directly delivered to the brain by ICM injection. Although the dose of S1 protein used in our study was tenfold lower than the dose used in a previous study in mice by intravenous injection, we could not exclude the possibility that S1 protein might promote ANG II-induced hypertension in part by impairing peripheral blood vessels to enhance ANG II-mediated systemic vasoconstriction. Additionally, S1 protein exposure followed by ANG II infusion (S1 + ANG II) was conducted to examine whether S1 protein exposure could influence the development of ANG II-dependent hypertension. Because some patients might already have high levels of plasma ANG II before COVID-19 infection, further studies are warranted to determine whether ANG II infusion followed by S1 protein exposure can replicate the results of S1 + ANG II obtained in the present study.
In conclusion, the present study demonstrates that S1 protein exposure has no effect on BP, but post-S1 protein exposure enhances the hypertensive response to ANG II by promoting PVN neuroinflammation and oxidative stress and augmenting sympathetic nerve activity. S1 protein-induced downregulation of Nrf2 in the PVN might account for exaggerated neuroinflammation and oxidative stress in response to ANG II. Our findings suggest that post-acute phase of COVID-19 infection might enhance susceptibility to developing hypertension by augmenting sympathetic excitation in individuals who have some disorders with high levels of circulating ANG II, such as obesity or diabetes mellitus. Activation of Nrf2 in the brain might provide a novel potential strategy to prevent the development of hypertension in individuals with high levels of circulating ANG II after recover from COVID-19 infection.
Author Contributions
Conceived and designed the experiments: QS and FQ; Performed the experiments: QS, LL, FJ, YL, BY, WM and ZZ; Analyzed the data: QS, LL and FJ; Wrote the paper: QS and FQ.
Funding
The present study was supported by Qilu Hospital of Shandong University.
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pelisek J, Reutersberg B, Greber UF, Zimmermann A. Vascular dysfunction in COVID-19 patients: update on SARS-CoV-2 infection of endothelial cells and the role of long non-coding RNAs. Clin Sci (Lond) 2022;136(21):1571–1590. doi: 10.1042/CS20220235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biancolella M, Colona VL, Mehrian-Shai R, Watt JL, Luzzatto L, Novelli G, Reichardt JKV. COVID-19 2022 update: transition of the pandemic to the endemic phase. Hum Genomics. 2022;16(1):19. doi: 10.1186/s40246-022-00392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrona M, Skrypnik D. New-onset diabetes mellitus, hypertension, dyslipidaemia as sequelae of COVID-19 infection-systematic review. Int J Environ Res Public Health. 2022 doi: 10.3390/ijerph192013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobler DL, Pruzansky AJ, Naderi S, Ambrosy AP, Slade JJ. Long-term cardiovascular effects of COVID-19: emerging data relevant to the cardiovascular clinician. Curr Atheroscler Rep. 2022;24(7):563–570. doi: 10.1007/s11883-022-01032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381(3):243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 6.Wasim D, Alme B, Jordal S, Lind Eagan TM, Tadic M, Mancia G, Guttormsen AB, Saeed S. Characteristics of 24-hour ambulatory blood pressure monitoring in a COVID-19 survivor. Future Cardiol. 2021;17(8):1321–1326. doi: 10.2217/fca-2020-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seravalle G, Grassi G. Sympathetic nervous system and hypertension: new evidences. Auton Neurosci. 2022;238:102954. doi: 10.1016/j.autneu.2022.102954. [DOI] [PubMed] [Google Scholar]
- 8.Grassi G. The sympathetic nervous system in hypertension: roadmap update of a long journey. Am J Hypertens. 2021;34(12):1247–1254. doi: 10.1093/ajh/hpab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue B, Johnson AK. Sensitization of hypertension: the impact of earlier life challenges: excellence award for hypertension research 2021. Hypertension. 2023;80(1):1–12. doi: 10.1161/HYPERTENSIONAHA.122.18550. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets. 2008;12(6):717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyenet PG, Stornetta RL, Souza G, Abbott SBG, Brooks VL. Neuronal networks in hypertension: recent advances. Hypertension. 2020;76(2):300–311. doi: 10.1161/HYPERTENSIONAHA.120.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue B, Zhang Y, Johnson AK. Interactions of the brain renin-angiotensin-system (RAS) and inflammation in the sensitization of hypertension. Front Neurosci. 2020;14:650. doi: 10.3389/fnins.2020.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AK, Xue B. Central nervous system neuroplasticity and the sensitization of hypertension. Nat Rev Nephrol. 2018;14(12):750–766. doi: 10.1038/s41581-018-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension. 2016;67(1):163–170. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24(3):368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank MG, Nguyen KH, Ball JB, Hopkins S, Kelley T, Baratta MV, Fleshner M, Maier SF. SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: evidence of PAMP-like properties. Brain Behav Immun. 2022;100:267–277. doi: 10.1016/j.bbi.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue B, Cui JL, Guo F, Beltz TG, Zhao ZG, Zhang GS, Johnson AK. Voluntary exercise prevents hypertensive response sensitization induced by angiotensin II. Front Neurosci. 2022;16:848079. doi: 10.3389/fnins.2022.848079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai J, Yu XJ, Liu KL, Wang FF, Jing GX, Li HB, Zhang Y, Huo CJ, Li X, Gao HL, Qi J, Kang YM. Central administration of tert-butylhydroquinone attenuates hypertension via regulating Nrf2 signaling in the hypothalamic paraventricular nucleus of hypertensive rats. Toxicol Appl Pharmacol. 2017;333:100–109. doi: 10.1016/j.taap.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Wang FF, Ba J, Yu XJ, Shi XL, Liu JJ, Liu KL, Fu LY, Su Q, Li HB, Kang KB, Yi QY, Wang SQ, Gao HL, Qi J, Li Y, Zhu GQ, Kang YM. Central Blockade of E-prostanoid 3 receptor ameliorated hypertension partially by attenuating oxidative stress and inflammation in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Cardiovasc Toxicol. 2021;21(4):286–300. doi: 10.1007/s12012-020-09619-w. [DOI] [PubMed] [Google Scholar]
- 20.Rana I, Stebbing M, Kompa A, Kelly DJ, Krum H, Badoer E. Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res. 2010;1326:96–104. doi: 10.1016/j.brainres.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumus H, Erat T, Ozturk I, Demir A, Koyuncu I. Oxidative stress and decreased Nrf2 level in pediatric patients with COVID-19. J Med Virol. 2022;94(5):2259–2264. doi: 10.1002/jmv.27640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorbea-Oppliger VJ, Melaragno MG, Potter GS, Petit RL, Fink GD. Time course of losartan blockade of angiotensin II hypertension versus blockade of angiotensin II fast pressor effects. J Pharmacol Exp Ther. 1994;271(2):804–810. [PubMed] [Google Scholar]
- 25.Cox BF, Bishop VS. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am J Physiol. 1991;261(4 Pt 2):H1284–H1291. doi: 10.1152/ajpheart.1991.261.4.H1284. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez VS, Zetter MA, Guerra EC, Hernandez-Araiza I, Karuzin N, Hernandez-Perez OR, Eiden LE, Zhang L. ACE2 expression in rat brain: implications for COVID-19 associated neurological manifestations. Exp Neurol. 2021;345:113837. doi: 10.1016/j.expneurol.2021.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J Neuroimmunol. 2019;332:16–30. doi: 10.1016/j.jneuroim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Zinovkin RA, Grebenchikov OA. Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochemistry (Mosc) 2020;85(7):833–837. doi: 10.1134/S0006297920070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhandari R, Khanna G, Kaushik D, Kuhad A. Divulging the intricacies of crosstalk between NF-Kb and Nrf2-Keap1 pathway in neurological complications of COVID-19. Mol Neurobiol. 2021;58(7):3347–3361. doi: 10.1007/s12035-021-02344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekuban FA, Zong C, Takikawa M, Morikawa K, Sakurai T, Ichihara S, Itoh K, Yamamoto M, Ohsako S, Ichihara G. Genetic ablation of Nrf2 exacerbates neurotoxic effects of acrylamide in mice. Toxicology. 2021;456:152785. doi: 10.1016/j.tox.2021.152785. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg M, Patil J, D'Angelo B, Weber SG, Mallard C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend BE, Johnson RW. Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp Gerontol. 2016;73:42–48. doi: 10.1016/j.exger.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olagnier D, Farahani E, Thyrsted J, Blay-Cadanet J, Herengt A, Idorn M, Hait A, Hernaez B, Knudsen A, Iversen MB, Schilling M, Jorgensen SE, Thomsen M, Reinert LS, Lappe M, Hoang HD, Gilchrist VH, Hansen AL, Ottosen R, Nielsen CG, Moller C, van der Horst D, Peri S, Balachandran S, Huang J, Jakobsen M, Svenningsen EB, Poulsen TB, Bartsch L, Thielke AL, Luo Y, Alain T, Rehwinkel J, Alcami A, Hiscott J, Mogensen TH, Paludan SR, Holm CK. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun. 2020;11(1):4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mihic D, Loinjak D, Maricic L, Smolic R, Sahinovic I, Steiner K, Viland S, Seric V, Duvnjak M. The relationship between Nrf2 and HO-1 with the severity of COVID-19 disease. Medicina (Kaunas) 2022;58(11):1658. doi: 10.3390/medicina58111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.