Fig.6.
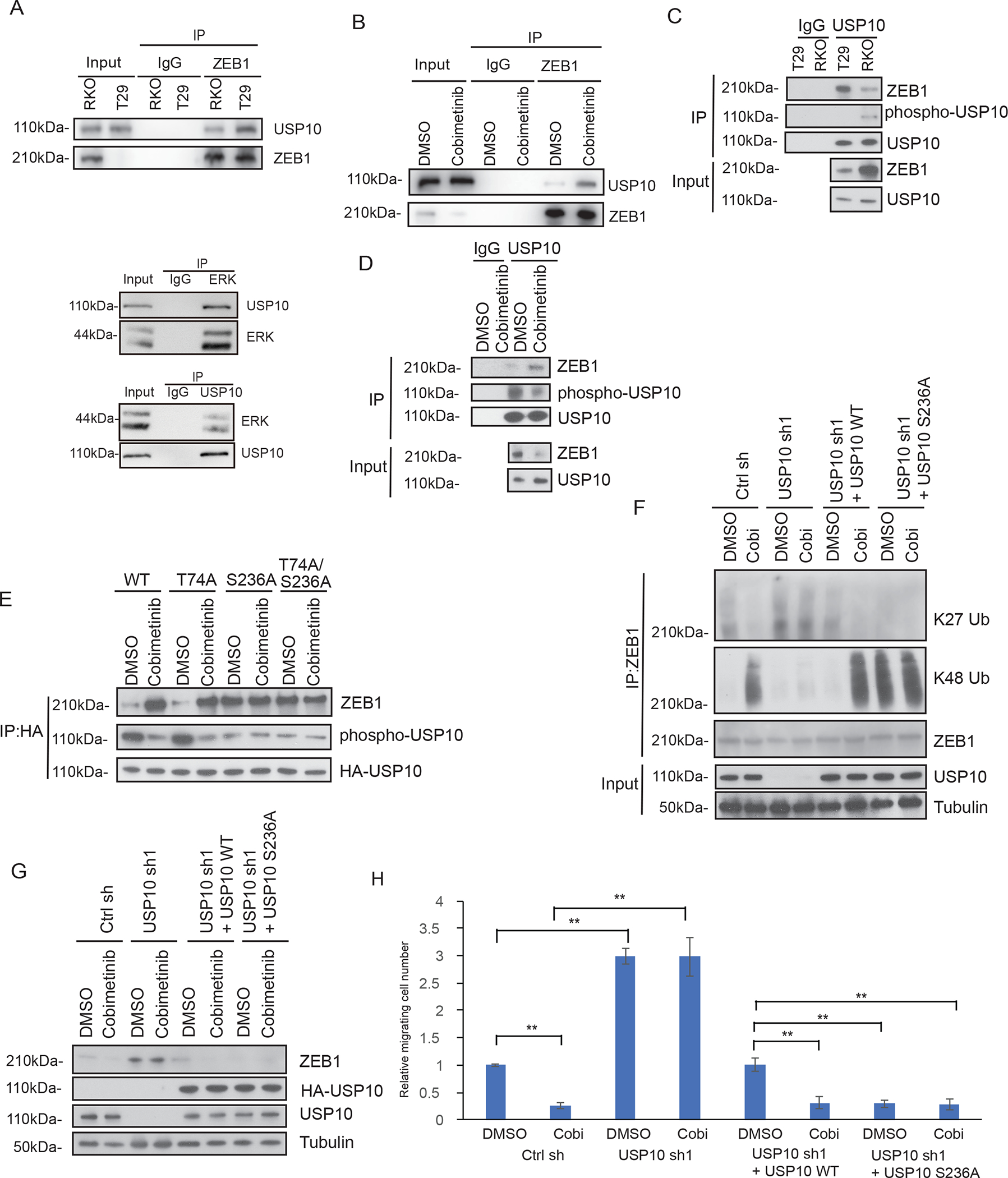
Phosphorylation of USP10 by ERK impairs its interaction with ZEB1 and results in ZEB1 stabilization. A-D, Analysis of interaction between ZEB1 and USP10 by co-immunoprecipitation in isogenic RKO CRC cells including binding between ERK and USP10 (A,C), or in RKO cells treated with vehicle or cobimetinib (B,D). E, Identification of the ERK-mediated phosphorylation site on the USP10 protein using a phospho-MAPK substrate antibody. Potential ERK phosphorylation sites, T74 and S236, were identified based on the ERK substrate motif and mutants were generated. Effect of treatment of cells vehicle (DMSO) versus the MEK inhibitor cobimetinib on USP10 phosphorylation events and the interaction of USP10 and ZEB1. F-H, Analysis of ZEB1 K27 linkage and K48-linkage chain polyubiquitination in RKO cells expressing control shRNA, USP10 shRNA, wildtype USP10 reconstituted cells or USP10 S236A mutant expressing cells in the presence or absence of vehicle (DMSO) or cobimetinib treatment (F). Analysis of ZEB1 protein expression (G) and assessment of cell migration (H) were also performed in the same RKO expressing cell lines. Migrated cells were quantified from the images (n=5) (H). Data are presented as mean ±SEM using the one-way ANOVA followed by post hoc Tukey’s test. **, p < 0.01.
